Abstract
Introduction
Tear trough deformity (TTD) is currently a major concern for many individuals that seek periorbital rejuvenation. Among the different options currently available for treating TTD, hyaluronic acid (HA) filler injections have become increasingly popular.
Purpose
To provide a dual approach, direct and indirect strategies for treating TTD with HA fillers according to patient facial structure.
Methods
The current paper combined the authors’ experience with the currently available scientific evidence.
Results
The current study presents the authors clinical experience regarding TTD treatment and serves as a guide on the best therapeutic approach with HA fillers. For achieving good aesthetic outcomes, especially in TTD, it is crucial to have a detailed understanding of both facial anatomy and the individual characteristics of the HA fillers. Proper management of full-face facial rejuvenation should have into consideration three main pillars: structure, contour, and refinement.
Conclusion
Treatment of TTD should be addressed from a comprehensive perspective, including potential lack of structural support, as well as interventions on the dynamic processes involved in the problem. Additionally, HA fillers can be used to modulate mechanically muscle movement by either facilitating their action or decreasing contractility by reducing their movement. Clinicians can benefit from ongoing guidance on the use of these products in order to tailor and optimize treatments to patient’s requirements. Although HA filler injections have low rates of side effects, TTD was listed as the most challenging area to treat with HA. Therefore, TTD treatment should be performed only by well-trained and experienced specialists.
Keywords: tear trough deformity, hyaluronic acid, MD Codes, MD Dyna Codes, cohesivity, periorbital region
Introduction
Periorbital lipoatrophy may be defined as a natural consequence of the anatomic attachments of the periorbital tissues.1 It consists of a physiological process of supraperiosteal and subcutaneous fat loss that is noticeable as a result of inherited anatomical differences and aging.1
Despite being of an important cosmetic concern, the exact etiology of the tear trough deformity (TTD) has not been well defined. However, different factors, such as gender (there are differences between men and women), congenital or age-related maxillary hypoplasia, laxity of the retaining ligaments of the periorbital area, cutaneous elastosis, structural changes in deep and/or superficial fat compartments, and a certain genetic predisposition have been proposed.1–3
Multiple classification systems have been introduced to provide an objective means of evaluating the TTD and to aid the aesthetic medicine specialists in choosing appropriate treatment options.4–8
Over the last several years, there has been considerable interest in nonsurgical treatment of the TTD using injectable products, such as hyaluronic acid (HA).9 Among the different therapeutic strategies, the MD Codes® (Codes) represent specific anatomical subunits for injection of HA fillers.10 Additionally, HA fillers can be used to modulate mechanically muscle movement by either facilitating their action, via a lever or pulley effect, or decreasing contractility by reducing their movement.11
The purpose of this paper is to provide two different treatment strategies for addressing tear trough deformity, with HA fillers, depending on patient’s facial structure.
Methods
The current study presents the clinical experience of the authors regarding TTD treatment, as well as an overview of the current evidence.
Patients signed written informed consent before treatment. Patients were fully informed about procedure, including side effects and potential complications. All procedures were carried out in accordance with the tenets of the Declaration of Helsinki. Study protocol was approved by the ethics committee of Instituto Médico Miramar.
Classification of TTD
Different classification systems have been proposed with the purpose of providing an objective method for assessing the TTD and selecting the best treatment options.4–8
Barton et al,4 in an effort to analyze objectively their postoperative results, proposed a grading system based on anatomic analysis (Table 1).
Table 1.
Tear Trough Deformity Classification Systems.
| Barton et al4 | Hirmand5 | Belhaouari et al7 |
|---|---|---|
|
|
|
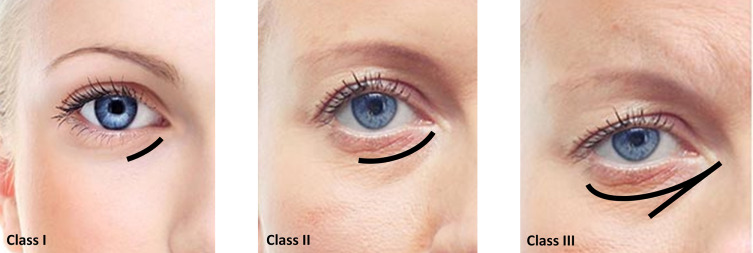
Hirmand proposed a classification system of the TTD based on clinical evaluation. According to this classification, the TTD might be addressed into three different types: Class I, II, and III (Table 1, Figure 1).5
Figure 1.
Hirmand classification of tear trough deformity. Data from Hirmand.5
There are currently two classification systems that evaluate the type of TTD and guide us on the best therapeutic approach with HA fillers.4,7
Belhaouari et al7 proposed a classification that integrated both the semiological analysis and the therapeutic strategy (Tables 1 and 2). According to this classification, the TTD might be addressed into two ways: Direct one, which is indicated in the stages 2A and 3A; and an Indirect one that was proposed for the stages 1A, 1B, 2B, and 3B.7,8
Table 2.
Belhaouari et al9 Tear Trough Classification System According to the Volume and the Ptosis with the Treatment Strategy with Hyaluronic Acid Filler
| Tear Trough Classification System and Treatment Strategy |
|---|
|
Abbrevaition: VYC, Vycross technology.
Classification systems are mainly based on anatomic analysis (Figure 1, Tables 1 and 2).
Treatment
Treatment strategy is based on the MD Codes®, which were developed by Mauricio de Maio.10,11
Myomodulation makes reference to the mechanic effect of HA on muscle function.10–12 It is based on myotatic reflex, which causes muscle contraction after the muscle is stretched.10–12 According to this theory, if there is a structural deficiency (either congenital or aging-related) and muscle contraction is weakened, HA filler placed under the muscle provides support and improves tensile strength by increasing distance between the origin and insertion, which increases mechanical advantage and facilitates the action of the muscle.10–12 On the contrary, if the muscle action is excessive, injecting HA filler above the muscle or directly into or beneath the muscle near its origin or insertion creates a mechanical obstacle to the muscle action.10–12
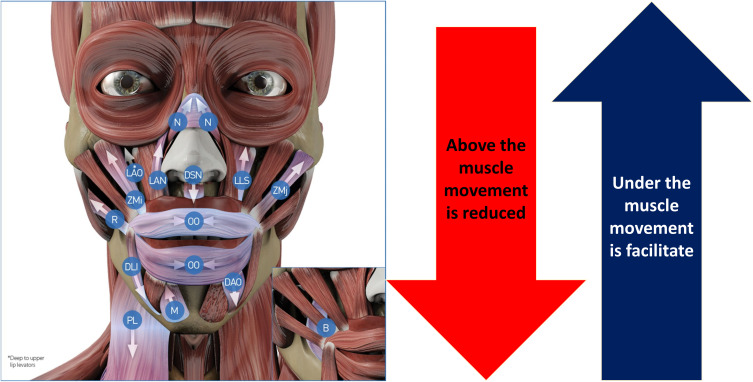
An overview of the action of HA fillers on the action of the different facial muscles is summarized in Figure 2. Additionally, Table 3 shows the mechanic myomodulation effect with HA filler on the different treatment points.
Figure 2.
Overview of the hyaluronic acid (HA) injectable filler on the muscle activity. Data from de Maio.11 Image courtesy from and reprinted with permission from Allergan plc, Dublin, Ireland.
Abbreviation: N, nasalis; LAN, levator labii superioris alaeque nasi; LLS, levator labii superioris; LAO, levator anguli oris; ZMj, zygomaticus major; ZMi, zygomaticus minor; DSN, depressor septi nasi; DAO, depressor anguli oris; DLI, depressor labi inferiores; B, buccinator; R, risorius; PL, plastisma; OO, orbicularis oris; M, mentalis.
Table 3.
Mechanic Myomodulation Effect with Hyaluronic Acid (HA) Filler on the Different Treatment Points.
| Treated Point | Location | Action |
|---|---|---|
| T1 | Under | To increase tightening in temporal-orbicular and temporal-cutaneous true retaining ligaments, which cause elevation of the tail of the eyebrow. |
| Ck1 | Under | Myomodulation on the zygomaticus major muscle and tension of the zygomatic-cutaneous-orbicular and orbicular true retaining ligaments, with canthopexy effect. |
| Ck2 | Under | Myomodulation on the zygomaticus minor, orbicular, and levator upper lip muscles and tightening of the zygomatic-cutaneous-orbicular and orbital-malar true retaining ligaments, causing a canthopexy effect |
| Ck3 | Undera | Myomodulation on the zygomaticus minor and levator upper lip muscles, and tightening of the zygomatic-cutaneous-orbicular and orbital-malar true retaining ligaments, which cause anterior/superior projection of the cheekbone. |
| Ck4 | Above | Myomodulation on zygomaticus major muscle and tightening of true orbicularis retaining ligament. |
| O1 | Under | Myomodulation on orbicular muscle and tightening of true orbicularis retaining ligament. |
| O2 | Under | Myomodulation on orbicular muscle and tightening of true orbicularis retaining ligament. |
| O3 | Under | Myomodulation on orbicular muscle and tightening of true orbicularis retaining ligament. |
| Tt1b | Underc | Myomodulation on orbicular muscle. |
| Tt2b | Underc | Myomodulation on orbicular muscle. |
| Tt3b | Underc | Myomodulation on orbicular muscle. |
Notes: aInto the deep and supraperiosteal fat compartment at the medial level of the malar. bThe direct approach to this area is indicated when patients present negative snap-tests and have a normal or excessive suborbicularis oculi fat pad (SOOF) volume. cBolus of hyaluronic acid filler in front of the orbital septum, at supraperiosteal level, between the orbicularis retaining ligament and the zygomatic cutaneous ligament. Data from these studies.10–12
Abbreviations: T1, anterior temple; T2, posterior temple; Ck1, zygomatic arch; Ck2, zygomatic eminence; Ck3, anteromedial cheek; Ck4, lateral lower cheek/parotid area; O1, central lateral orbital; O2, lower lateral orbital; O3, upper lateral orbital; Tt1, central infraorbital; Tt2, lateral infraorbital; Tt3, medial infraorbital.
Table 4 summarizes different HA injectable fillers used for the aesthetic management of the TTD in this article.
Table 4.
Overview of the Different Hyaluronic Acid (HA) Fillers Used in Tear Trough Deformity Treatment and Their Main Characteristics
| VYC-25L* | VYC-20L* | VYC-17.5L* | VYC-15L* | VYC-12L* | |
|---|---|---|---|---|---|
| Indication | Restore and create volume of the face. | Facial volume restoration. | Treatment of deep skin depressions, face contouring and volume restoration to correct facial structural defects, contour deformities volume loss in the lips, cheeks, chin, lower face. | Treatment of fine lines and medium-sized skin depressions, enhancement and pouting of the lips to correct structural defects, contour deformities and volume loss. | Treatment of superficial cutaneous depressions such as fine lines and for improvement of skin quality attributes such as hydration and elasticity. |
| Concentration of HA | 25 mg/mL | 20 mg/mL | 17.5 mg/mL | 15 mg/mL | 12 mg/mL |
| HA molecular weight | Low & high | Low & high | Low & high | Low & high | Low & high |
| Duration | Up to18 months | Up 2 years | Up to 18 months | Up 1 year | Up to 9 months |
Note: *With lidocaine.
Abbreviations: VYC-25L, Volux® (Allergan plc, Dublin, Ireland); VYC-20L, Voluma® (Allergan plc, Dublin, Ireland); VYC-17.5L, Volif® (Allergan plc, Dublin, Ireland); Volbella® (Allergan plc, Dublin, Ireland); VYC-12L, Volite® (Allergan plc, Dublin, Ireland); HA, hyaluronic acid.
Therapeutic Strategy
TTD treatment should not be restricted to the groove itself. It would be advisable to contemplate a comprehensive approach of the problem, which includes potential lack of structural support, as well as interventions on the dynamic processes involved in the problem.
Based on Hirmand,5 Belhaouari7 classifications, and facial lipoatrophy,13 we attempt to do a comprehensive approach for treating TTD that may help clinicians to treat this challenging area.
Table 5 shows different recommendations of the authors.
Table 5.
Authors Recommendations for Treating Tear Trough
| Recommendations |
|
A proper diagnosis of the TTD is essential to address the right treatment strategy.
From a clinical point of view, the recommended therapeutic strategy of the tear trough deformity would be (Table 6):
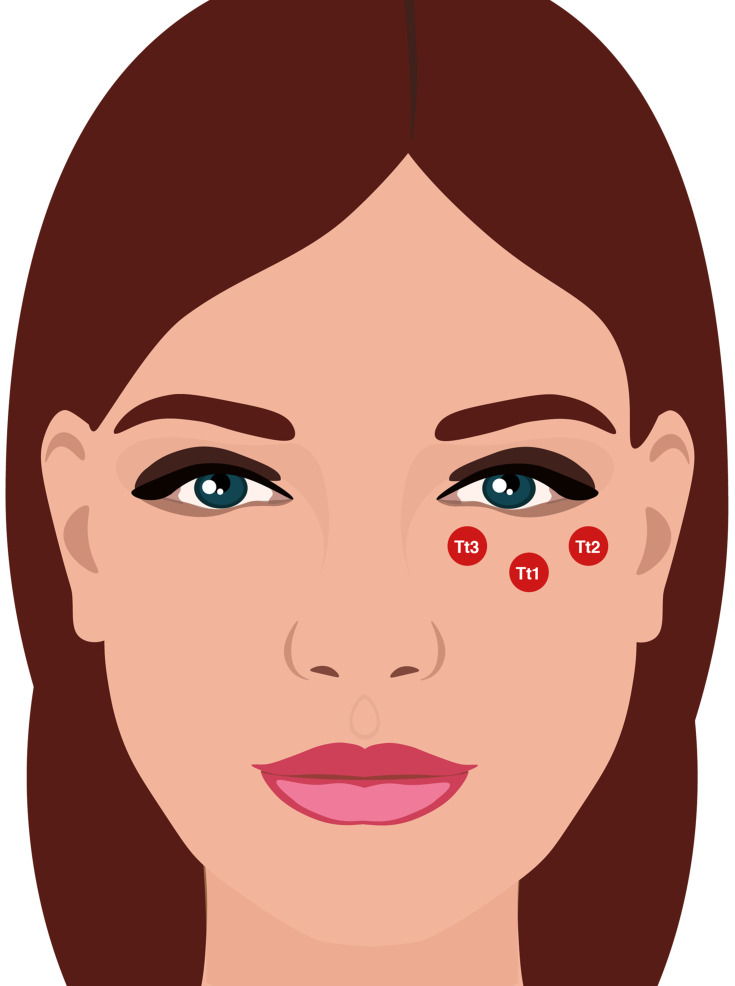
Direct approach (stages 2A and 3A of the Belhaouari classification system): Central infraorbital (Tt1) + Lateral infraorbital (Tt2) + medial infraorbital (Tt3) (Figure 3).
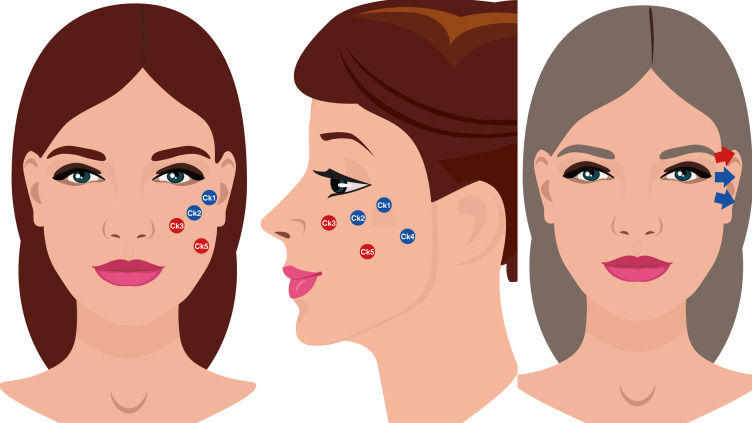
Indirect approach (stages 1A,1B, 2B, and 3B of the Belhaouari classification system): Anterior temple (T1) + Zygomatic arch (Ck1) + Zygomatic eminence (Ck2) + Anteromedial cheek (Ck3) + Lateral lower cheek/parotid area (Ck4) + Submalar/buccal area (Ck5) + Tt1 + Tt2 + Tt3 + Central lateral orbital (O1) + Lower lateral orbital (O2) + Upper lateral orbital (O3) (Figure 4).
Table 6.
Overview of the Authors’ Recommendations for Treating Tear Trough Deformity with Hyaluronic Acid (HA) Filler According to the MD Codes®.
| Code | HA Filler | Amount | Administration | Level |
|---|---|---|---|---|
| T1+T2 | VYC-20L/VYC-25L | 0.5 to 0.7 mL | 27G needle | Supraperiosteal |
| Ck1a | VYC-20L/VYC-25L | 0.3 mL | 27–30 needle | Supraperiosteal |
| Ck2 | VYC-20L | 0.1 to 0.2 mL | 27–30G needle | Supraperiosteal |
| Ck3b | VYC-20L | 0.2 to 0.4 mL | 27G and 30G needle | Supraperiosteal |
| VYC-20L+VYC-17.5L | 0.3 to 0.6 mL | 25G-27G blunt cannula | Supraperiosteal | |
| Ck4 | VYC-20L | 0.5 to 0.7 mL | 25–27 G blunt cannula | Deep subcutaneous |
| Ck5 | VYC-20L or VYC-25Lc | 0.4 to 0.7 mL | 25–27 G blunt cannula | Deep subcutaneous |
| O1d | VYC-15L or VYC-20L | 0.1–0.15 mL | 27–30G needle | Supreperiosteal |
| O2d | VYC-15L or VYC-20L | 0.1–0.15 mL | 27–30G needle | Supreperiosteal |
| O3d | VYC-15L or VYC-20L | 0.1–0.15 mL | 27–30G needle | Supreperiosteal |
| Tt1 | VYC-15L | 0.1–0.2 mL | 27 G blunt cannula | Supreperiosteal |
| Tt2 | VYC-15L | 0.1–0.2 mL | 27 G blunt cannula | Supreperiosteal |
| Tt3 | VYC-15L | 0.1–0.1 mL | 27 G blunt cannula | Supreperiosteal |
Notes: aIt is possible to inject 3 microboluses of 0.1 mL (one in the suture notch and the other two ones to the sides). bTreatment is performed in two phases. Phase 1: To inject 0.2 to 0.4 mL of a HA filler of 20 mg/mL (VYC-20L), at the supraperiosteal, by means 27G and 30G needles. Phase 2: Injecting 0.3 to 0.6 mL (with a fan technique) of VYC-20L, by means25-27G cannulas, and injecting afterwards in the same area a more superficial amount of HA (either VYC-25L or VYC-20L, according to the patient needs) under the orbicularis muscle. cThe injection of the bolus of VYC-20L or VYC-25L depends on the skin thickness and bone structure of the patient. dIn some patients with excessive contractility of the orbicularis muscle, it is necessary to inject, at the superficial subdermal level, a HA filler (VYC-15L) for correcting the horizontal wrinkles. Data from these studies.10–12
Abbreviations: T1, anterior temple; T2, posterior temple; Ck1, zygomatic arch; Ck2, zygomatic eminence; Ck3, anteromedial cheek; O1, central lateral orbital; O2, lower lateral orbital; O3, upper lateral orbital; Tt1, central infraorbital; Tt2, lateral infraorbital; Tt3, medial infraorbital; VYC-15L, Volift® (Allergan plc, Dublin, Ireland); VYC-17.5L, Volbella® (Allergan plc, Dublin, Ireland); VYC-20L, Voluma® (Allergan plc, Dublin, Ireland); VYC-25L, Volux® (Allergan plc, Dublin, Ireland).
Figure 3.
Overview of the different codes involved in the tear trough deformity direct approach treatment. Image courtesy from and reprinted with permission from Allergan plc, Dublin, Ireland. Codes have been adapted from de Maio.10,11
Abbreviations: Tt1, central infraorbital; Tt2, lateral infraorbital; Tt3, medial infraorbital.
Figure 4.
Overview of the different Codes involved in the tear trough deformity indirect approach treatment. Data from Peng et al6 and de Maio.10 Image courtesy from and reprinted with permission from Allergan plc, Dublin, Ireland.
Abbreviations: Ck1, zygomatic arch; Ck2, zygomatic eminence; Ck3, anteromedial cheek; Ck4, lateral lower cheek/parotid area; Ck5, submalar/buccal area; O1, central lateral orbital; O2, lower lateral orbital; O3, upper lateral orbital.
Figures 3 and 4 summarize the different treatment strategies.
Results
Direct Approach
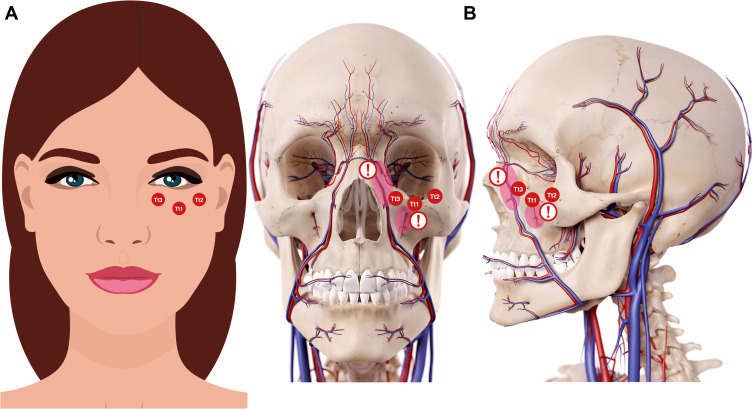
The proper technique for a direct approach of tear trough area and palpebro-malar area is to use HA fillers of mid to low density, elasticity, and cohesiveness, and additionally, with a mid to low water retention capacity. HA should be administered in small boluses with a 25/27 G blunt cannula, performing always an aspiration maneuver before injecting the filler6,10,11 (Figure 5).
Figure 5.
Codes involved in the direct approach strategy for treating tear trough deformity. Tear trough and orbital codes should be reserved for specialists specifically trained in this technique and those who have a sound knowledge of the anatomy and physiology for this particular area. Data from Peng et al6 and de Maio.10 Image courtesy from and reprinted with permission from Allergan plc, Dublin, Ireland. (A) Imaging representing the frontal view. (B) Imaging representing the anatomical structures. Red circle under Tt1 and exclamation mark: Be aware of the infraorbital artery branches. Red circle near to Tt3 and exclamation mark: Be aware of the angular artery and vein. Codes have been adapted from de Maio.10,11
Abbreviations: Tt1, central infraorbital; Tt2, lateral infraorbital; Tt3, medial infraorbital.
The direct approach to this area is indicated when patients present negative snap-tests and have a normal or excessive suborbicularis oculi fat pad (SOOF) volume. In other words, those subjects classified as stages 2 and 3 in the Belhaouari classification or Class I in the Hirmand one (Figure 1 and Table 2).
The treatment approach consists in a bolus of HA filler in front of the orbital septum (a thin fibrous layer arising from the periosteum along the inferior and superior orbital rims), at supraperiosteal level, between the orbicularis retaining ligament and the zygomatic cutaneous ligament6,11 (Figure 5).
The specialist must be aware of the infraorbital artery branches and of the angular artery and vein. It is important to take into account that tear trough and orbital codes should be reserved for well-trained and experienced specialists, who had received specific training in this technique and those who have an exhaustive knowledge of the anatomy and physiology for this particular area.10,11
Indirect Approach
This treatment strategy is based on the sequence: Structure-Contour-Refinement.
Structure
Temporal Region
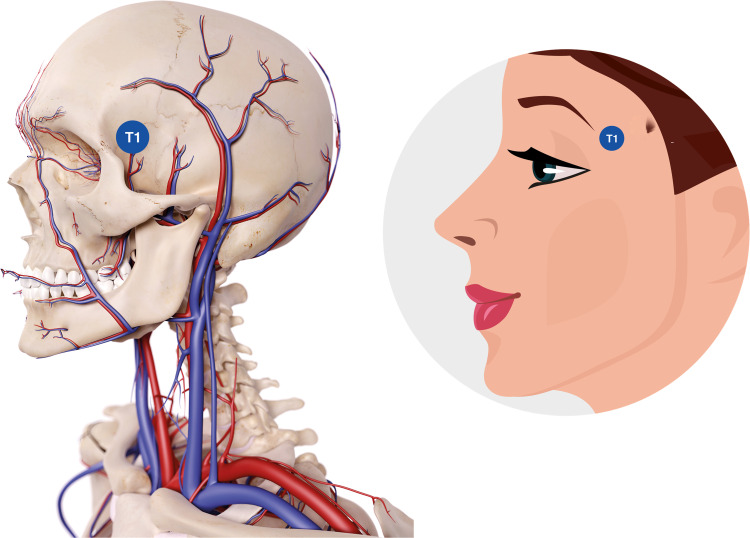
The point that should be treated is T1, at the supraperiosteal level, accessing from T1 located at the fronto-sphenoidal junction, at the beginning of the temporal fossa (Figure 6).
Figure 6.
Temporal point to be treated. Data from de Maio.10 Image courtesy from and reprinted with permission from Allergan plc, Dublin, Ireland.
Abbreviation: T1, anterior temple.
A HA filler presenting a G’ and high cohesivity, such as VYC-25L (Volux®; Allergan plc, Dublin, Ireland) or VYC 20L (Voluma®; Allergan plc, Dublin, Ireland), in thin skins, may be used. Injection is made by using with a 27G needle, performing a supraperiosteal bolus of approximately 0.5–0.7 mL. A soft massage, for redistributing the product, is subsequently done.
The clinical effect obtained is the correction of the temporal fossa, at the supraperiosteal level and below the temporal muscle, with elevation of the eyebrow tail, which causes tension in orbicular muscle.
Special attention should be paid to the temporal arteries, which are located at the posterior supraperiosteal level, below the temporal muscle. HA filler injection at point T1 should be located anterior to the vessels. The location of point T1 at the level of the fronto-sphenoidal junction is performed by asking the patient to open the mouth. Before injecting, aspiration should be performed as a prophylactic measure and a new needle without filler should be used prior to deep bolus injections.14,15
Cheek Region
In some cases, TTD may be addressed by treating the lateral cheek.
Three different points: Ck1, Ck2, and Ck3 are treated (see Figure 7).
Figure 7.
Structural treatment of tear trough deformity. Image courtesy from and reprinted with permission from Allergan plc, Dublin, Ireland. aDo not inject into the cartilage or into the bone, but rather at the level of the cartilage or the level of the bone.
Abbreviations: Ck, cheek; Ck1, zygomatic arch; Ck2, zygomatic eminence; Ck3, anteromedial cheek.
For treating Ck1, a 27 needle, at supraperiosteal level, should be used. A HA filler, like VYC-20L or VYC-25L, in a 0.3 mL bolus or 3 microboluses of 0.1 mL (one in the suture notch and the other two ones to the sides). The 3 microboluses approach provides greater tension than the 0.3 mL bolus one.
The clinical effect of this Code would be tension at the supraperiosteal level of the zygomaticus major muscle, orbicular and zygomaticus-cutaneous-orbicular retaining ligaments (with canthopexy effect), and myomodulation of the zygomaticus major and orbicularis muscles.
Therapeutic approach of Ck2 point is made at supraperiosteal level by means a 27G needle, applying a 0.1–0.2 mL bolus of a HA filler (VYC-20L) (Figure 7).
We should be aware of the zygomaticofacial artery. At this point, aspiration is highly recommended before injecting the filler.10
The clinical effect on this point would be tautness the muscles zygomaticus minor and levator labii superioris. Additionally, it tightens the zygomatic-cutaneous-orbicularis and the orbitomalar retaining ligaments (with canthopexy effect), as well as myomodulation of the zygomaticus minor, orbicularis, and lip levator muscles inducing latero-facial tension.10,11
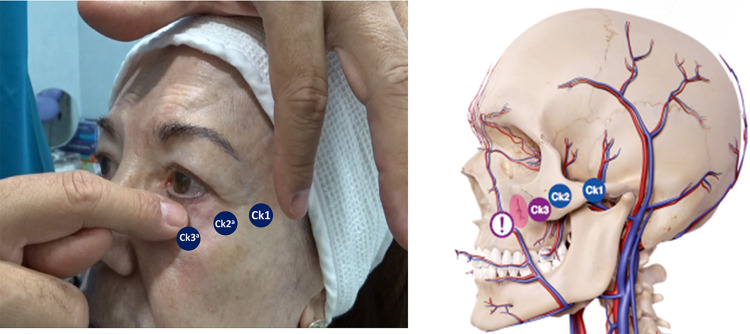
Ck3 treatment should be done before treating the nasojugal groove.6 Its treatment can be performed with 25G blunt cannula and with 27G needle.
The treatment of this point is carried out in two phases. The first one is to inject 0.2 mL to 0.4 mL of a HA filler of 20 mg/mL (VYC-20L), at the supraperiosteal level and performing aspiration before injecting the filler. Subsequently, through the same point, we will access to the deep-medial inferior SOOF, by means a 25/27G blunt cannula, first at the supraperiosteal level, injecting 0.3 to 0.6 mL (with a fan technique) of VYC-20L, and injecting afterwards in the same area a more superficial amount of HA either VYC-17.5L (Volbella®; Allergan plc, Dublin, Ireland) or VYC-20L, according to the patient needs, under the orbicularis muscle.6,10
Injection of VYC-20L or VYC-17.5L depends on the skin thickness and bone structure of the patient. It is important to bear in mind that injecting HA fillers superficially, above the orbicularis muscle, without having provided previously structure to the deep ones, may cause a “squirrel” appearance, which will be increased with facial mimicry.16,17
Treatment of this point would induce tautness by increasing volume, at supraperiosteal level, of the zygomaticus minor and levator labii superioris muscles and the zygomatic-cutaneous-orbicularis and orbitomalar retaining ligaments, which cause anterosuperior projection of the cheekbone. Additionally, the treatment of the Ck3 point induces muscle myomodulation of the zygomaticus minor, orbicularis, and levator labii superioris muscles.
Specialists should be aware of the infraorbital neurovascular bundle, which emerges onto the face through the infraorbital foramen. It is located above the zygomatic process of the maxilla, about 2–3 cm from the median vertical line of the face. Therefore, aspiration is highly recommended when injecting a HA filler at this point.6,10,11
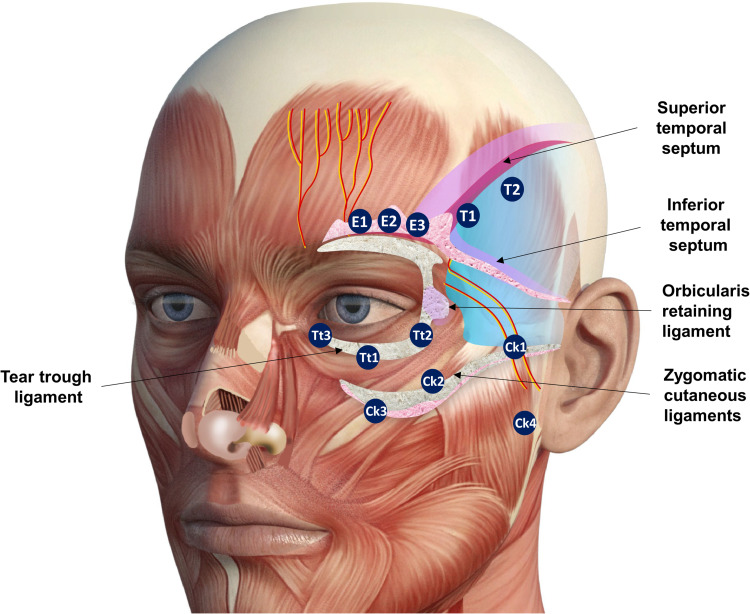
If nasojugal sulcus needs to be treated, the SOOF approach is crucial. Figure 8 shows an overview of different anatomical structures and the Codes of the periorbital region.
Figure 8.
Different anatomical structures and the codes of the periorbital region. Data from de Maio.10 Image courtesy from and reprinted with permission from Allergan plc, Dublin, Ireland.
Abbreviations: E1, eyebrow tail; E2, eyebrow center; E3, eyebrow head; T1, anterior temple; T2, posterior temple; Ck1, zygomatic arch; Ck2, zygomatic eminence; Ck3, anteromedial cheek; Ck4, lateral lower cheek/parotid area; Tt1, central infraorbital; Tt2, lateral infraorbital; Tt3, medial infraorbital.
Contour
Once the treatment of facial structure has been addressed, the second step is to reestablish those facial contours that were lost throughout the facial aging process.
The therapeutic approach of the contours requires special actions on certain static and dynamic facial Codes that, in turn, are based on the structure management performed during the first phase of the treatment.
Once the structural codes at the cheekbones, Ck1, Ck2, and Ck3 have been addressed (see Structure section), it is time to integrate the “structural anatomical corrections” into a comprehensive approach more visual and understandable for the patients (Figure 4).
Treatment is usually performed with a 25–27 G blunt cannula, with a fan technique, by using a 20 mg/mL HA filler (VYC-20L). Special attention should be paid to the small preauricular and superficial zygomatic vessels, which are frequent in this region.6,10,18
Therapeutic approach to Ck5 Code is subcutaneous, with either VYC-25L or VYC-20L (in patients with thin skin), by means a 25–27G blunt cannula, with a fan technique, from the oral commissure and upwards towards Ck4.6,10 The amount of filler ranges from 0.4 mL to 0.7 mL depending on patient’s needs. The clinical effect is to provide volume to a lipoatrophic area that becomes evident when the patient sucks or pouts their lips. The HA is injected deep subcutaneous, above the buccinator muscle and under the risorius one, inducing myomodulation in both muscles.6,11
The management of these Codes will provide a more contoured appearance of the cheek region, without “steps” in the zygomatic arch area, trying to integrate the restored/created structure in the facial contour. In men, it is advisable to reinforce the anterior projection of the cheek bone a little bit more. Whereas, in women, the posterior anterolateral projection will determine the beauty.
Refinement
Refinement is the third step of this comprehensive therapeutic approach and represents its “final touch”, once the structure and contour processes have been carried out.
Orbicular Region
The main purpose of volumizing this area is to recover the supraperiosteal deep fat pockets, which, along with loss of the orbital bone rim, determine the subcutaneous structure of the lateral orbit (Figure 4).
In many patients, TTD causes sagging and superficial dynamic wrinkles due to both a hypercontractility of the orbicularis muscles and a laxity of the orbicular retaining ligament.
The treatment is performed, by administering a supraperiosteal bolus (0.1–0.15mL, at each point O1, O2, O3), of a 15 mg/mL HA filler (VYC-15L; Volift ®; Allergan plc, Dublin, Ireland), by means a 30G needle.6,10,11 The objective is to induce myomodulation of the orbicularis muscle and increase the tension of the orbicular retaining ligament, causing a slight canthopexy effect and correcting horizontal wrinkles.
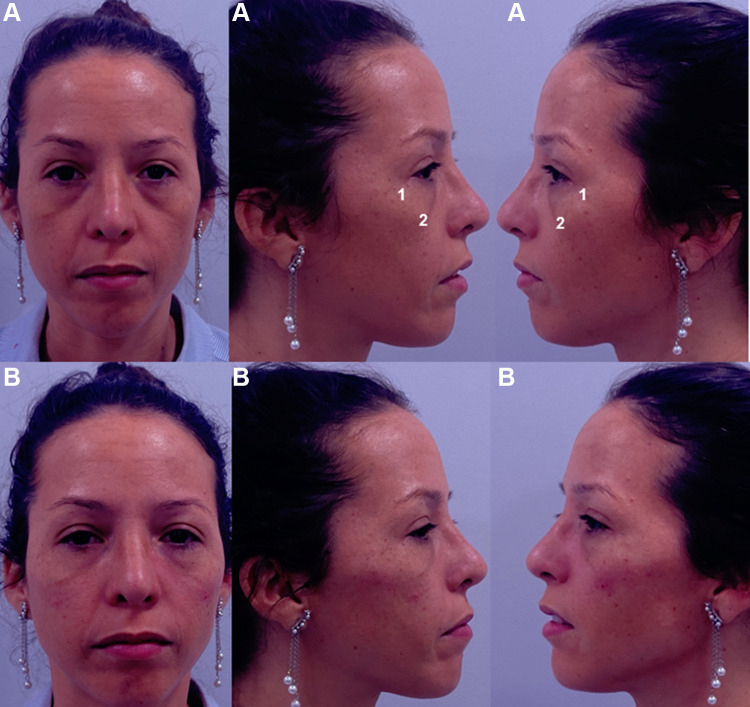
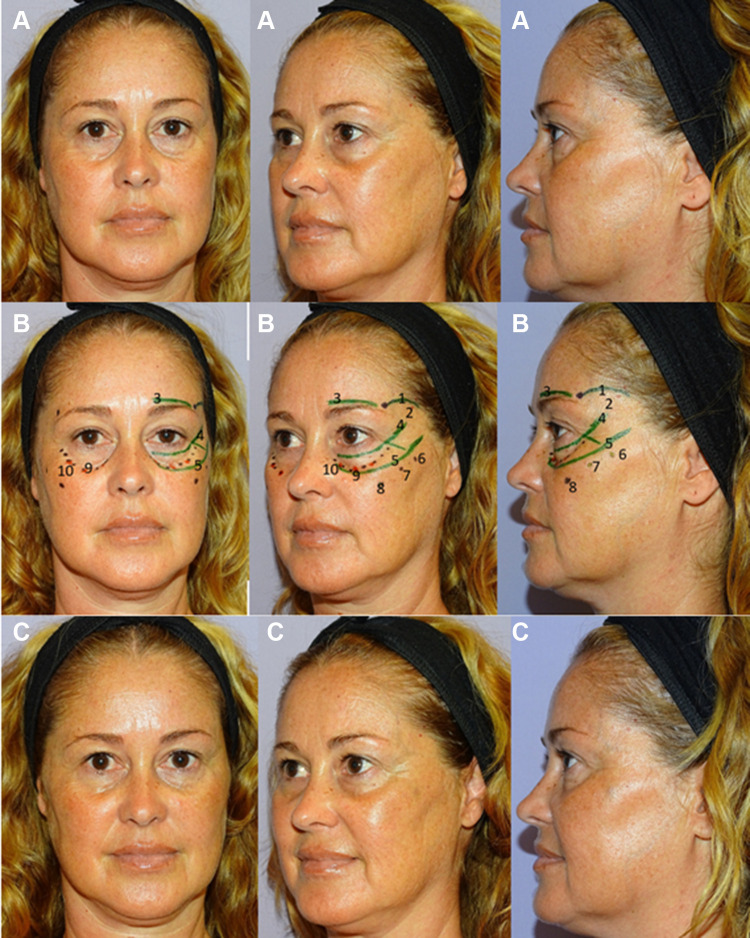
Clinical results, assessed by means photographs, have shown a significant aesthetic improvement (Figures 9 and 10).
Figure 9.
Frontal, right, and left projection of a 40-year-old patient face before (A) and after (B) being treated. The patient provided their consent for the use of their image in this publication. 1. Zygomatic arch (Ck1): 0.3 mL per side of Hyaluronic acid filler (VYC-20L). 2. Anteromedial cheek (Ck3): 0.7 mL per side of Hyaluronic acid filler (VYC-20L). Image courtesy with permission from Dr Farollch. Codes have been adapted from de Maio.10
Figure 10.
Forty-five years old women with a tear trough deformity (according to Belhaouari classification: 1B (see reference 7)) before treatment (A); that shows the treatment plan (B); and after treatment (C). The patient provided their consent for the use of their image in this publication. 1. Temporo-orbicular cutaneous retaining ligament; 2. Anterior temple (T1); 3. Palpebral ligament; 4. Orbicular retaining ligament; 5. Zygomatic-cutaneous-orbicularis retaining ligament; 6. Zygomatic arch (Ck1); 7. Zygomatic eminence (Ck2); 8. Anteromedial cheek (Ck3); 9. Central infraorbital (Tt1); 10. Medial infraorbital (Tt3). T1 (2): 0.5 mL per side of Hyaluronic acid (HA) filler (VYC-25L; Volux®; Allergan plc, Dublin, Ireland). Ck1 (6): 3 microboluses of 0.1 mL (one in the suture notch and the other two ones to the sides) of HA filler (VYC-20L). Ck2 (7): 0.1 mL of HA filler (VYC-20L). Ck3 (8): 0.2 mL lateral + 0.25 mL medial of HA filler (VYC-20L). Tt1 (9): 0.2 mL of HA filler (VYC-20L). Tt3 (10): 0.2 mL of HA filler (VYC-20L). Image courtesy with permission from courtesy of Dr Urdiales-Gálvez. Codes have been adapted from de Maio.10
There are some Codes that merit a special consideration (Table 7).
Table 7.
Different Aspects That Need to Be Considered When Injecting Hyaluronic Acid (HA) Fillers for Treating Tear Trough Deformity. Adapted from de Maio
| MD Code® | Special Consideration |
|---|---|
| T1 | Pay attention to the superficial frontal artery and the deep temporal arteries. |
| Ck1 | None |
| Ck2 | Pay attention to the zygomaticofacial artery |
| Ck3 | Pay attention to the infraorbital artery |
| Tt1 | Pay attention to the infraorbital artery branches |
| Tt2 | None |
| Tt3 | Pay attention to the angular artery and vein |
As previously mentioned, aspiration is highly recommended when performing injections at a deep level. Additionally, the TTD treatment should be performed only by well-trained and experienced specialists, who have a sound knowledge of the anatomy of this region.10,11
Safety
Adverse events can be divided into early events (occurring up to several days post-treatment) or delayed events (occurring from weeks to years post-treatment). Early events include pain, erythema, etc., while delayed events include orange-brown staining, Tyndall effect, etc.19–23 (Tables 8 and 9).
Table 8.
Overview of the Adverse Events Associated with the Use of Dermal Fillers. Adapted from Funt and Pavicic
| Early Adverse Eventsa | Delayed Adverse Eventsb | |
|---|---|---|
| Injection site reactionsc | Erythema Edema Pain/tenderness Bruising Itching |
Erythema Edema Pain/tenderness Nodule/abscess Systemic responses Biofilm |
| Infection | Erythema Edema Pain/tenderness Acne papule formation Nodule/abscess |
Foreign body granulomad |
| Hypersensitivity | Erythema Edema Pain/tenderness Non-fluctuant nodules |
Migration of filler material |
| Technical and placement errors | Lumps Asymmetries Contour irregularities Dysesthesias, paresthesias, and anesthesia |
Immune reactions Dysesthesias, paresthesias, and anesthesia |
| Skin discoloration | Redness Whiteness Hyperpigmentation |
Persistent discoloration Persistent scarring |
| Vascular compromisee | Blurred vision Loss of vision |
Tissue necrosis |
Table 9.
Overview of the Early and Delayed Adverse Events in Patients Who Underwent Tear Trough Deformity Treatment with Hyaluronic Acid (HA) Fillers
| Early Adverse Events | |
| Event | Prevention/Treatment |
| Pain | To use HA fillers with lidocaine |
| Erythema | N.A. |
| Swelling/Bruising | To avoid all blood-thinning medications. Fillers that incorporate lidocaine and epinephrine (adrenaline) may reduce the amount of postinjection bruising. To apply firm pressure and ice packs before and after the treatment session. |
| Asymmetry | N.A. |
| Migraine | N.A. |
| Delayed Adverse Events | |
| Event | Prevention/Treatment |
| Orange-brown staining | Preinjection ice application. Proper depth of injection. Discontinuation of anticoagulants at least seven days before injection. |
| Postinflammatory hyperpigmentation | It is usually associated with dark skin types due to bruising and hematoma. It can be difficult to treat |
| Puffiness | Its incidence may be reduced by proper patient and filler selection. |
| Infections | Filler injections should not be performed if there is an infection in the adjacent site. It can be treated with antibiotics active against frequent skin bacteria including Staphylococcus epidermidis or Propionibacterium acnes. |
| The Tyndall effect | It occurs when particulate HA fillers are inappropriately implanted into the superficial dermis or epidermis. Hyaluronidase should be the initial approach to treatment. |
| Nodules | They can be treated with local massage, aspiration or incision and drainage of the product. Hyaluronidase can be used to dissolve a nodule or a focus of overcorrection. |
| Blindnessa | To limit the amount of filler bolus injected in one site. One way to do this is to use blunt cannulas. To apply minimal pressure on the syringe, which decreases the risk of retrograde flow into the retinal arteries. |
More serious and true complications with fillers include vascular occlusion and necrosis.19–25 In the authors’ practice, no serious complications, such as arterial embolic accident or necrosis, have occurred.
Discussion
Since facial aging involves interactions between changes in various anatomic structures (bone, ligaments, muscles, adipose tissue, and skin),3 knowledge of age-related anatomy changes is, therefore, crucial for achieving elegant and aesthetically balanced results.
Tear trough deformity is one of the most challenging areas in facial rejuvenation, which requires a comprehensive treatment approach for addressing the different age-related changes that occur at that level.18 In younger people TTD is mainly restricted to the tear trough. However, during aging TTD may affect the entire region under the eye.
Many different strategies have been used for addressing TTD treatment. Nevertheless, there is growing interest in techniques that provide a tailored approach towards specific anatomic abnormalities.
Despite HA fillers have been successfully used for correcting periorbital volume loss, their role in addressing TTD combined with eye bags is a relatively new practice. It has been reported that HA fillers may be a valuable option for treating not only periorbital hollowness but also concealed the bulge of infraorbital fat pads.18,26 Additionally, it was recently proposed a treatment strategy with HA fillers that had into consideration different anatomic aspects of TTD.27
We propose two patient-tailored treatment approaches, according to their facial structure. Both, direct and indirect treatment approaches, are based on the Codes.10
Direct approach is mainly focus in volumizing tear trough and palpebromalar areas (stages 2A and 3A of the Belhaouari classification system and Class I in the Hirmand one). It is indicated in patients who present negative snap-tests and have a normal or excessive SOOF volume.
The indirect approach is indicated in those cases who require a comprehensive strategy and is based on the sequence: Structure-Contour-Refinement.
Regarding structure, therapeutic strategies should be focused on providing an adequate correction of the age-related structure loss.
When thinking about contour, it is essential to take into consideration the sexual dimorphism in human facial form, which involves both size and shape variations of the soft tissue structures.28 These contours will require special actions on certain facial areas.10 However, it should be considered that previous actions on structure could have been done, which might have already modified the facial contour.
Once the structure and contours have been restored, refinement represents the “final touch” of the treatment. At this time, those “details” that were not corrected when treating structure and contour should be addressed. A proper correction of the small details is going to have a great impact on the aesthetic outcomes and patient satisfaction, even greater than those achieved with the structural or contouring approach.11
Structure, contour, and refinement have different Codes assigned to each of them, which will facilitate the systematization of the treatment approaches throughout the process.
The Codes represents precise anatomical sites and procedures for the injection of HA fillers, which might easily serve as a platform of communication for clinicians worldwide.10
The Codes were designed to address aging changes that are visible at rest.10 Their purpose is to improve structure, contour, and refinement.
On the other hand, the MD Dyna Codes® were designed to address dynamic aging changes by mechanical modulation of the muscle and providing a natural appearance during muscle activity.11
The lack or the loss of structural support can yield alterations in facial muscles, which affects the balance in activity between them.11,12 HA filler treatment may be used to modulate muscle activity, either supporting its movement or reducing its overaction, regardless unbalance is due to a structural deficiency or a loss of volume in aging.11,12 Mechanical myomodulation refers to the effect that the HA fillers have on the function of facial mimetic muscles.11,12
Codes of the upper and midface are involved in the treatment of the TTD.29,30
A proper selection of HA filler is a crucial point of these treatment approaches. Different manufacturing-related factors, including HA concentration, polymer chain length, crosslinking degree, or cross-linking technology are going to influence significantly on different filler properties, such as requisite needle size; particle size; duration; extrusion force; and elastic Modulus (G’), which will critically influence product selection and indication.31–33 For example, VYC-25L, VYC-20L, and VYC-17.5L, for their rheologic characteristics, are specially indicated for addressing structural deficiencies,27 whereas VYC-15L is more indicated for refining.26,27
Regarding safety, as indications for HA fillers expand, the number and spectrum of adverse events will increase. Most adverse events of HA fillers are mild and transient. However, more serious adverse events can occur, leaving patients with long-lasting or permanent functional and aesthetic defects.15,19–21,23
Correction of a TTD with nonsurgical techniques presents unique challenges. Unlike other facial hollows (such as the nasolabial folds, which are easily camouflaged), the tear trough requires more technically demanding treatment due to the breadth of the hollow, skin quality changes (thinning), and the presence of adjacent orbital fat pads.2,14
As limitation, need to be mentioned that the current article presents the personal experience of the authors on a specific subject and this fact should be considered when interpreting data from this paper. Additionally, all the treatments have been done with Vycross® technology products. Appropriate caution is therefore recommended when extending the results to other HA fillers.
Conclusions
The treatment of TTD should be addressed from a comprehensive perspective, including potential lack of structural support, as well as interventions on the dynamic processes involved in the problem.
Additionally, it should be taken into consideration that TTD was listed as the most challenging area to treat with HA. That is why TTD treatment should be performed only by well-trained and experienced specialists, who have a sound knowledge of the anatomy of this region.
Acknowledgments
Medical writing and editorial assistant services have been provided by Ciencia y Deporte S.L. and covered by a grant from Allergan. Support for this assistance was funded by Allergan Aesthetics, an AbbVie company.
Funding Statement
Medical writing services has been provided by Allergan Aesthetics, an AbbVie company. Allergan did not participate in either data collection, analysis or redaction of the manuscript. Neither honoraria nor payments were made for authorship.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr Urdiales-Gálvez has received a grant from Allergan Aesthetics, an AbbVie company, for covering the medical writing services. Dr Farollch-Prats has received research grants from Allergan Aesthetics, an AbbVie company.
References
- 1.Jiang J, Wang X, Chen R, Xia X, Sun S, Hu K. Tear trough deformity: different types of anatomy and treatment options. Postep Derm Alergol. 2016;33(4):303–308. doi: 10.5114/ada.2016.61607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stutman RL, Codner MA. Tear trough deformity: review of anatomy and treatment options. Aesthet Surg J. 2012;32(4):426–440. doi: 10.1177/1090820X12442372 [DOI] [PubMed] [Google Scholar]
- 3.Cotofana S, Fratila AA, Schenck TL, Redka-Swoboda W, Zilinsky I, Pavicic T. The anatomy of the aging face: a Review. Facial Plast Surg. 2016;32(3):253–260. doi: 10.1055/s-0036-1582234 [DOI] [PubMed] [Google Scholar]
- 4.Barton FE Jr, Ha R, Awada M. Fat extrusion and septal reset in patients with the tear trough triad: a critical appraisal. Plast Reconstr Surg. 2004;113(7):2115–2121;discussion 2122–2113. doi: 10.1097/01.PRS.0000122409.00716.34 [DOI] [PubMed] [Google Scholar]
- 5.Peng L, Peng J. Treating the tear trough: a new classification system, a 6-step evaluation procedure, hyaluronic acid injection algorithm, and treatment sequences. J Cosmet Dermatol. 2018;17(3):333–339. doi: 10.1111/jocd.12514 [DOI] [PubMed] [Google Scholar]
- 6.Belhaouari L, Gassia V, Lauwers F. J. Dynamique et embellissement du mid-faceMéd. Esth Et Chir Derm. 2014;XXXXI(164):203–209. [Google Scholar]
- 7.Authors no listed. The international study on aesthetic/cosmetic procedures performed in 2018. Available from: https://www.isaps.org/wp-content/uploads/2019/12/ISAPS-Global-Survey-Results-2018-new.pdf. Last accessed January7, 2021.
- 8.de Maio M. MD Codes™: a methodological approach to facial aesthetic treatment with injectable hyaluronic acid fillers. Aesthetic Plast Surg. 2020;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Maio M. Myomodulation with injectable fillers: an innovative approach to addressing facial muscle movement. Aesthetic Plast Surg. 2018;42(3):798–814. doi: 10.1007/s00266-018-1116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125(2):699–708. doi: 10.1097/PRS.0b013e3181c82f90 [DOI] [PubMed] [Google Scholar]
- 11.Belhaouari L, Quinodoz P, Prevot A. Myomodulation et acide hyaluronique. J Méd Esth Et Chir Derm. 2019;XXXXVI(183):219–225. [Google Scholar]
- 12.de Maio M. Myomodulation with injectable fillers: an update. Aesthetic Plast Surg. 2020;44(4):1317–1319. doi: 10.1007/s00266-020-01768-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ascher B, Coleman S, Alster T, et al. Full scope of effect of facial lipoatrophy: a framework of disease understanding. Dermatol Surg. 2006;32(8):1058–1069. doi: 10.1111/j.1524-4725.2006.32230.x [DOI] [PubMed] [Google Scholar]
- 14.Cotofana S, Steinke H, Schlattau A, et al. The anatomy of the facial vein: implications for plastic, reconstructive, and aesthetic procedures. Plast Reconstr Surg. 2017;139(6):1346–1353. doi: 10.1097/PRS.0000000000003382 [DOI] [PubMed] [Google Scholar]
- 15.Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Preventing the complications associated with the use of dermal fillers in facial aesthetic procedures: an expert group consensus Report. Aesthetic Plast Surg. 2017;41(3):667–677. doi: 10.1007/s00266-017-0798-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carruthers J, Carruthers A, Tezel A, Kraemer J, Craik L. Volumizing with a 20-mg/mL smooth, highly cohesive, viscous hyaluronic acid filler and its role in facial rejuvenation therapy. Dermatol Surg. 2010;36(Suppl 3):1886–1892. doi: 10.1111/j.1524-4725.2010.01778.x [DOI] [PubMed] [Google Scholar]
- 17.Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(Suppl 1):S284–292. doi: 10.1097/DSS.0000000000000541 [DOI] [PubMed] [Google Scholar]
- 18.Sharad J. Treatment of the tear trough and infraorbital hollow with hyaluronic acid fillers using both needle and cannula. Dermatol Ther. 2020;33(3):e13353. doi: 10.1111/dth.13353 [DOI] [PubMed] [Google Scholar]
- 19.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;Dec(6):295–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Plast Surg Nurs. 2015;35(1):13–32. doi: 10.1097/PSN.0000000000000087 [DOI] [PubMed] [Google Scholar]
- 21.Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498–510. doi: 10.1007/s00266-017-1063-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharad J. Dermal fillers for the treatment of tear trough deformity: a review of anatomy, treatment techniques, and their outcomes. J Cutan Aesthet Surg. 2012;5(4):229–238. doi: 10.4103/0974-2077.104910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philipp-Dormston WG, Goodman GJ, De Boulle K, et al. Global approaches to the prevention and management of delayed-onset adverse reactions with hyaluronic acid-based fillers. Plast Reconstr Surg Glob Open. 2020;8(4):e2730. doi: 10.1097/GOX.0000000000002730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peter S, Mennel S. Retinal branch artery occlusion following injection of hyaluronic acid (Restylane). Clin Experiment Ophthalmol. 2006;34(4):363–364. doi: 10.1111/j.1442-9071.2006.01224.x [DOI] [PubMed] [Google Scholar]
- 25.Beleznay K, Humphrey S, Carruthers JD, Carruthers A. Vascular compromise from soft tissue augmentation: experience with 12 cases and recommendations for optimal outcomes. J Clin Aesthet Dermatol. 2014;7(9):37–43. [PMC free article] [PubMed] [Google Scholar]
- 26.Goel A, Sethi P. Concealing of under eye orbital fat pads with hyaluronic acid filler: a case report. J Cosmet Dermatol. 2020;19(4):820–823. doi: 10.1111/jocd.13097 [DOI] [PubMed] [Google Scholar]
- 27.Peng HP, Peng JH. Treating the tear trough-eye bag complex: treatment targets, treatment selection, and injection algorithms with case studies. J Cosmet Dermatol. 2020;19(9):2237–2245. doi: 10.1111/jocd.13622 [DOI] [PubMed] [Google Scholar]
- 28.Ferrario VF, Sforza C, Schmitz JH, Miani A Jr, Taroni G. Fourier analysis of human soft tissue facial shape: sex differences in normal adults. J Anat. 1995;187(Pt 3):593–602. [PMC free article] [PubMed] [Google Scholar]
- 29.de Maio M, Swift A, Signorini M, Fagien S. Aesthetic leaders in facial aesthetics consensus committee. Facial assessment and injection guide for botulinum toxin and injectable hyaluronic acid fillers: focus on the upper face. Plast Reconstr Surg. 2017;140(2):265e–276e. doi: 10.1097/PRS.0000000000003544 [DOI] [PubMed] [Google Scholar]
- 30.de Maio M, DeBoulle K, Braz A, Rohrich RJ. Alliance for the Future of aesthetics consensus committee. Facial assessment and injection guide for botulinum toxin and injectable hyaluronic acid fillers: focus on the midface. Plast Reconstr Surg. 2017;140(4):540e–550e. doi: 10.1097/PRS.0000000000003716 [DOI] [PubMed] [Google Scholar]
- 31.Micheels P, Sarazin D, Tran C, Salomon D. Effect of different crosslinking technologies on hyaluronic acid behavior: a visual and microscopic study of seven hyaluronic acid gels. J Drugs Dermatol. 2016;15(5):600–606. [PubMed] [Google Scholar]
- 32.Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(5 Suppl):139S–148S. doi: 10.1097/PRS.0000000000001734 [DOI] [PubMed] [Google Scholar]
- 33.Pierre S, Liew S, Bernardin A. Basics of dermal filler rheology. Dermatol Surg. 2015;41(Suppl1):S120–126. doi: 10.1097/DSS.0000000000000334 [DOI] [PubMed] [Google Scholar]