Abstract
The identification of an alternate extended form of angiotensin I composed of the first twelve amino acids at the N-terminal of angiotensinogen has generated new knowledge of the importance of noncanonical mechanisms for renin independent generation of angiotensins. The human sequence of the dodecapeptide angiotensin-(1-12) [N-Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10-Val1-Ile12-COOH] is an endogenous substrate that in the rat has been documented to be present in multiple organs including the heart, brain, kidney, gut, adrenal gland, and the bone marrow. Newer studies have confirmed the existence of Ang-(1-12) as an Ang II-forming substrate in the blood and heart of normal and diseased patients. Studies to-date document that angiotensin II generation from angiotensin-(1-12) does not require renin participation while chymase rather than angiotensin converting enzyme shows high catalytic activity in converting this substrate into angiotensin II directly.
Keywords: Renin-angiotensin system, angiotensinogen, angiotensin peptides, angiotensin-(1-12), angiotensin I, angiotensin II, renin, chymase, angiotensin-converting enzyme, renin, metabolism
Introduction
It has been widely addressed in the literature that the central role of the circulating renin-angiotensin system (RAS) is to maintain blood pressure and body fluid homeostasis (Yim and Yoo, 2008, de Souza, West, de Abreu et al., 2018). In addition to its role as an endocrine system, data demonstrates the presence of genes and proteins of the system within the interstitial spaces of the extracellular environment and the membranes, organelles and nuclei of the cells itself (Leung, 2010,Jessup, Brosnihan, Gallagher et al., 2008, Nehme, Zouein, Zayeri et al., 2019, Abadir, Walston and Carey, 2012, Kumar, Singh and Baker, 2008, Sherrod, Liu, Zhang et al., 2005). The presence of RAS proteins in tissue suggest that they are either locally synthesized and processed or represent precursors that are transported from the circulation and then processed locally (Ahmad and Ferrario, 2018, Ferrario, Ahmad, Varagic et al., 2016, Pendergrass, Averill, Ferrario et al., 2006, Whaley-Connell, Habibi, Nistala et al., 2012). Further, the processing mechanisms of the tissue RAS may differ from the circulating RAS and from one tissue type to another. For example, angiotensin II (Ang II) in the blood and the lungs is primarily generated by angiotensin converting enzyme (ACE) from angiotensin I (Ang I), whereas in tissues such as the bone marrow and the heart, chymase is the main Ang II-forming enzyme (Ahmad and Ferrario, 2018, Ferrario et al., 2016, Ahmad, Simmons, Varagic et al., 2011, Ahmad, Varagic, Groban et al., 2014, Ahmad, Varagic, VonCannon et al., 2016, Ahmad, Wei, Tallaj et al., 2013, Yamashita, Ahmad, Wright et al., 2020). The discovery of chymase as an Ang II generating enzyme originated in studies performed at the Cleveland Clinic by Bumpus and colleagues (Hirakata, Fouad-Tarazi, Bumpus et al., 1990, Kinoshita, Urata, Bumpus et al., 1991) three decades ago. Identification of angiotensin-(1-7) as a component of the RAS and the later demonstration of angiotensin converting enzyme 2 (ACE2) and the mas receptor (Mas-R) as constituents of the ACE2/Ang-(1-7)/Mas-R axis established the basis for a more insightful understanding of the system in the control of cell function and homeostasis. The current acceptance of the RAS as comprised by two intertwined biochemical arms with opposing functions [ACE/Ang II/AT1-R and ACE2/Ang-(1-7)/Mas-R axis], provides a more complete vision of how homeostasis is regulated and how an unbalance in the expression or activity of the opposing arms of the RAS can associate with or cause tissue remodeling, endothelial dysfunction, and cardiac/vascular fibrosis, as well as alter normal cell biology with attendant unregulated control of cell growth and altered immunity (McMaster, Kirabo, Madhur et al., 2015, Mikolajczyk and Guzik, 2019).
The main goal of this review is to highlight the importance of alternate non-canonical pathways in which intermediate substrates representing peptides with amino acid sequences shorter than angiotensinogen (AGT) constitute an alternate substrate for the generation of the biologically active peptides Ang II and Ang-(1-7). These alternate angiotensin substrates are -the dodecapeptide angiotensin-(1-12) [Ang-(1-12)] and ikosi pendi peptide angiotensin-(1-25) [Ang-(1-25)]. While both polypeptides were identified by Japanese researchers in Miyazaki, Japan (Nagata, Hatakeyama, Asami et al., 2013, Nagata, Kato, Sasaki et al., 2006), the bulk of the evidence for a biological role as a source of Ang II production is on Ang-(1-12) as no further data for Ang-(1-25) has been published since the original publication (Nagata et al., 2013). We also discuss the beneficial role of chymase inhibition as a novel therapy in the treatment of progressive heart and kidney diseases, given chymase importance as an Ang-(1-12) degrading enzyme (Ahmad and Ferrario, 2018, Ansary, Urushihara, Fujisawa et al., 2018, Devarajan, Yahiro, Uehara et al., 2015, Duengen, Kim, Zahger et al., 2020, Dungen, Kober, Nodari et al., 2019, Kanefendt, Thuss, Becka et al., 2019). While the mechanisms that underlie the pathological effects of chymase at the cellular level are not yet well defined, new studies yield support to the use of novel chymase inhibitors in the management of human left ventricular dysfunction and heart failure (Duengen et al., 2020, Dungen et al., 2019, Kanefendt et al., 2019).
Ang-(1-12) and Chymase Role in RAS
The intracellular presence of the AGT protein along with its’ metabolic products [Ang I, Ang II and Ang-(1-7)] are documented, even though more work needs to done in terms of understanding their intracellular compartmentalization and the specific conditions for their expression in the cell nuclei and cytosolic organelles (Abadir et al., 2012, Kumar et al., 2008,Barlucchi, Leri, Dostal et al., 2001, Gwathmey, Alzayadneh, Pendergrass et al., 2012, Sadoshima, Xu, Slayter et al., 1993, Singh, Le, Bhat et al., 2007).
Ang-(1-12) (aka proangiotensin 12) was first identified by Nagata et al. (Nagata et al., 2006) in 2006 from the blood and tissues of a japanese strain of Wistar rats. In this first study Nagata and co-workers (Nagata et al., 2006) demonstrated the ability of Ang-(1-12) to generate Ang II via angiotensin converting enzyme. In the pursuit of its potential role as an Ang II substrate, a series of studies from our laboratory showed the presence of immunoreactive (ir-) Ang-(1-12) products in the left ventricle and renal tubules of spontaneous hypertensive rats (SHR) (Jessup, Trask, Chappell et al., 2008) and rat cardio myocytes (isolated from 1-3 days old neonatal pups and adult hearts) (Ahmad et al., 2011, Ahmad, Varagic, Westwood et al., 2011). The potential contribution of Ang-(1-12) to cardiovascular regulation as an angiotensin peptide generating substrate was strengthened by additional studies showing that plasma membranes isolated from human normal left ventricular myocytes metabolized Ang-(1-12) into Ang II by chymase (Ahmad et al., 2013). Additional evidence was obtained from human biopsies of right and left atrial appendages of patients undergoing cardiac surgery for the treatment of resistant atrial fibrillation or left heart myocardial or valve disease (Ahmad et al., 2011, Wang, Varagic, Nagata et al., 2020, Wang, Varagic, Nagata et al., 2020). These studies showed a positive association among left atrial Ang-(1-12) expression, chymase gene transcripts, and chymase enzymatic activity levels in patients with enlarged left atria due to left heart disease (Wang et al., 2020a, Wang et al., 2020b). The increased activity of the Ang-(1-12)/chymase axis in these patients is in keeping with parallel demonstrations of a critical role of chymase in left atrial enlargement during volume overload (Dell’Italia, Collawn and Ferrario, 2018, Dell’Italia, Meng, Balcells et al., 1995, Powell, Wei, Fu et al., 2019) and in the evolution of primary mitral regurgitation (Butts, Ahmed, Bajaj et al., 2020).
It is generally accepted that hypertension, diabetes, aging, and oxidative stress stimulate the activity of the circulating and tissue-borne RAS (Chen, Juan and Chou, 2018, Conti, Cassis and Benigni, 2012, Ferrario, 2010, Ferrario, Ahmad, Joyner et al., 2010, Groban, Pailes, Bennett et al., 2006, Luo, Wang, Chen et al., 2015, Singh, Le, Khode et al., 2008). In keeping with these findings, intracellular levels of endogenous Ang-(1-12) are higher in neonatal myocytes isolated from 1-3 days-old pups of SHR compared to normotensive Wistar-Kyoto rats (WKY) (Ahmad et al., 2011). In addition, intact Ang-(1-12) was incorporated within cultured neonatal myocytes in a time-dependent fashion in both WKY and SHR. Importantly, the rate of Ang-(1-12) uptake was significantly higher in SHR as compared to WKY myocytes at all-time points (Ahmad et al., 2011).
Figure 1 illustrates the metabolic pathways leading to Ang-(1-12) generation and processing. While metabolism studies demonstrate the ability of angiotensin converting enzyme (ACE) to hydrolyze Ang-(1-12) into Ang I, this enzymatic pathway seems to be primarily accounting for Ang-(1-12) metabolism in the circulation during exposure to the rich ACE contained within the surface area of the vascular endothelium (Moniwa, Varagic, Simington et al., 2013). On the other hand, studies of Ang-(1-12) metabolism in tissues such as the heart and the kidneys point toward chymase as the critical enzyme metabolizing Ang-(1-12) directly into Ang II. Chymase participation in the metabolism of the Ang-(1-12) substrate was first identified in heart tissue lysate and atrial cardiomyocytes (Ahmad et al., 2011, Ahmad et al., 2016). In human atrial tissue, chymase affinity for Ang-(1-12) is 25-fold higher than for ACE (Wang et al., 2020a, Wang et al., 2020b). Ang-(1-12) preference for chymase has been further confirmed in rat bone marrow. In this tissue, chymase-mediated Ang II formation from Ang-(1-12) substrate was approximately 1,000-fold higher than that of ACE (Yamashita et al., 2020). These findings further indicate that the RAS processing enzymes are regulated differently at the cellular level. Chymase expression in tissues and its’ deleterious effect in organ damage has been the topic of a recent review (Dell’Italia et al., 2018).
Figure 1.
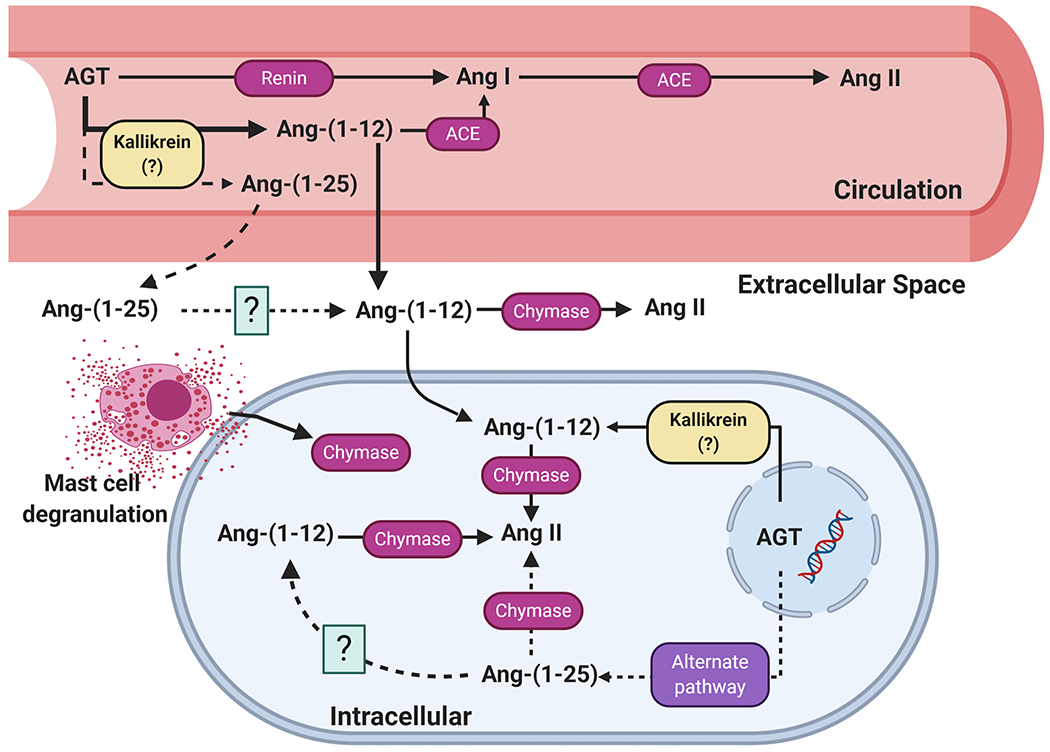
Present view of the biochemical pathways involving the metabolism of angiotensin-(1-12) and angiotensin-(1-25) in the generation of angiotensins within the blood and tissues. Abbreviations as defined in text.
Chymase participation as an Ang II forming enzyme originates in studies performed at the Cleveland Clinic thirty years ago. In pursuing the observation that Ang I exerted significant cardiac inotropism in the presence of captopril (Hirakata et al., 1990), chymase was identified as an Ang II forming enzyme from Ang I by Urata et al. (Urata, Kinoshita, Misono et al., 1990, Urata, Kinoshita, Perez et al., 1991, Urata, Nishimura and Ganten, 1995). Additional novel contributions to chymase role in cardiovascular disease were done by Husain et al. (Husain, 1993, Ju, Gros, You et al., 2001, Murakami, Karnik and Husain, 1995, Wasse, Naqvi and Husain, 2012) and Dell’Italia and colleagues (Dell’Italia et al., 2018, Butts et al., 2020, Butts, Goeddel, George et al., 2017, Dell’Italia, Meng, Balcells et al., 1997, Pat, Chen, Killingsworth et al., 2010, Wei, Lucchesi, Tallaj et al., 2003).
The rich literature concerning chymase participation as an Ang II-forming enzyme, as reviewed recently (Dell’Italia et al., 2018), remains relegated as scientists and clinicians continue to ignore accumulating evidence of a high residual risk of cardiovascular events in patients medicated with ACE inhibitors (Ferrario et al., 2016, Ferrario and Mullick, 2017, Reyes, Cheng, Roberts et al., 2019, Reyes, Varagic, Ahmad et al., 2017). Limited acceptance of a critical contribution of chymase to human cardiovascular pathology is partly influenced by the existence of multiple isoforms expressed differently in rodents and humans. Of the five more prominent chymases found in rodents, mast cell protease 5 (MCP-5) is the most structurally and phylogenetically related to the human chymase encoded by the CMA1 gene (Rao and Hoidal, 2012).
The human chymase gene (CMA1) has a significant association to immunity. A recent study showed significantly higher expression of CMA1 gene in gastric cancer tissues compared to adjacent normal tissues (Shi, Ye, Mao et al., 2020). The expression level of CMA1 (a key gene) in gastric cancer correlated with the levels of infiltrated CD4+, CD8+, neutrophils, macrophages, and dendritic cells (Shi et al., 2020). Past studies from Ferrario’s laboratory implicated the existence of an immunological imbalance during the development of experimental renal hypertension as revealed by biphasic changes in the thymus weight (Chatelain and Ferrario, 1978) and a reduced thymus T cell reactivity (Chatelain, Vessey and Ferrario, 1980). Since T lymphocytes are important regulators of immunological homeostasis, this reduction in T-cells suggested the existence of an immunological imbalance accompanying the development of experimental renal hypertension. The thymus and the bone marrow are key players in immunity in part explained by the fact that T cells migrate to the thymus to undergo further growth and differentiation (2009). Consequently the first demonstration of the existence of the genes and proteins of the RAS in the bone marrow, including evidence of de novo synthesis of Ang II by marrow stromal cells (MSC) (Strawn and Ferrario, 2008, Strawn, Richmond, Ann Tallant et al., 2004), provided initial clues as to the role of altered immunity in the pathogenesis of human hypertension. Since Ang II is expressed in the bone marrow where it functions as an autocrine-paracrine modulator of hematopoiesis, we further explored whether Ang-(1-12) and chymase may be present. This new study revealed the abundant presence of Ang-(1-12) in the bone marrow of Sprague Dawley rats; moreover, we found that chymase-mediated Ang II-formation from Ang-(1-12) was approximately 1,000-fold higher than ACE (Yamashita et al., 2020). These data are consistent with a hereto unknown role of the Ang-(1-12)/chymase axis in the modulation of hematopoiesis and inflammatory mechanisms associated with the pathogenesis of hypertension. In keeping with this interpretation, we now find a significant expression of Ang-(1-12) in the rat’s thymus (Figure 2).
Figure 2.
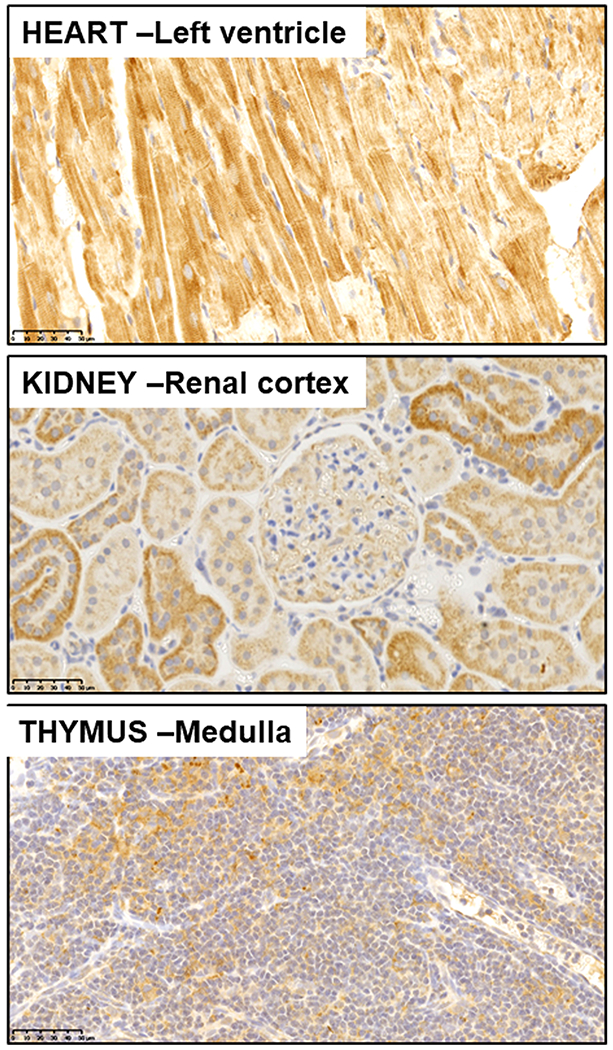
Histological composite of immunoreactive expression of angiotensin-(1-12) in cardiac, renal, and thymus from a transgenic rat expressing the human angiotensinogen gene (Ferrario et al., 2019, Ferrario et al., 2016). As documented elsewhere, Ang-(1-12) localizes within cardiac myocytes, proximal and distal renal tubules, and shows a preferential presence in epithelial cells within the thymic medulla. The Ang-(1-12) staining is achieved with a highly specific monoclonal antibody directed against the human Ang-(1-12) amino acid sequence.
Ang-(1-12) and Cellular Function
As of now, the two primary biologically active RAS components [Ang II and Ang-(1-7)] have been widely recognized for their direct cellular interactions and functionality. The most widely appreciated pathological function of the Ang II peptide is through its membrane receptor [Ang II type 1 receptor, AT1R] signaling pathways where it modulates the structural characteristic of the cells in various diseases (Mehta and Griendling, 2007, Wolf and Wenzel, 2004). Our recent compelling studies show that Ang-(1-12) functions as a tissue non-renin dependent alternate precursor for direct Ang II generation by chymase in the rodent and human heart (Ahmad et al., 2013, Ferrario, Varagic, Habibi et al., 2009, Trask, Jessup, Chappell et al., 2008). We examined that in vivo Ang-(1-12) induced alterations in global cardiac function in adult normal Sprague Dawley rats (Li, Zhang, Cheng et al., 2018), WKY (De Mello, Dell’Itallia, Varagic et al., 2016), transgenic hypertensive rats expressing the human AGT [TGR(hAGT)L1623] (Reyes et al., 2019), and SD rats with isoproterenol-induced heart failure (HF) (Li, Zhang, Zhang et al., 2020). In all cases, Ang-(1-12) induces positive inotropic responses that are the result of intracellular Ca2+ mobilization (Reyes et al., 2019) and activation of K+ currents (De Mello et al., 2016). Ang-(1-12) contractile responses are reduced in rats with isoproterenol-induced HF (Li et al., 2020) and in the hypertensive myocytes of TGR(hAGT)L1623 rats (Reyes et al., 2019). Ang-(1-12) inotropic responses in isolated cardiac myocytes are significantly inhibited in the presence of a chymase inhibitor (chymostatin). These findings suggest that the Ang-(1-12) responses are mediated by intracellular processing of the substrate by chymase, - directly generating Ang II from the Ang-(1-12) substrate.
Therapeutic Aspect of Intracellular Cardiac Chymase/ACE Inhibition
Cardiovascular diseases (CVDs) are the number one cause of death (31%) globally. Challenges remain in designing effective therapeutic agents to block intracellular sites of Ang II generation. Previous studies from Singh et al. (Singh et al., 2008) showed that neonatal rat ventricular myocytes synthesize and retain Ang II intracellularly, and that Ang II is redistributed to the nucleus under high-glucose conditions. Further, these authors noted that cardiac myocytes grown in a high-glucose environment increase intracellular concentrations of AGT and chymase. Markedly increased chymase expression in vascular smooth muscle cells in human diabetic nephropathy, and hypertensive nephropathy has been reported (Cristovam, Carmona, Arnoni et al., 2012, Huang, Chen, Truong et al., 2003). A major role of ACE-independent formation of intrarenal Ang II in diabetes (Singh et al., 2007), and the involvement of renal mast cell chymase activity has been documented in patients with autosomal dominant polycystic kidney disease (McPherson, Luo, Brown et al., 2004). These studies suggest that cellular RAS physiology is changed under high-glucose conditions, a finding that agrees with our studies in which chymase (not ACE) had a significant role in Ang-(1-12) processing to generate Ang II in human and rat heart tissues.
ACE inhibitors and AT1-R blockers are established as primary medications in the treatment of patients with hypertension and CVDs. While large well-conducted clinical trials demonstrate the beneficial effects of ACE inhibitors and ARBs in terms of blood pressure control and amelioration of target organ damage (Dusing, 2016), a more critical appraisal of their therapeutic efficacy in terms of reducing clinical events reveals an overall risk reduction of no more than 30% (Ferrario et al., 2016, Reyes et al., 2017, Ferrario, Ahmad, Nagata et al., 2014). Meta-analysis data from large clinical trials employing ACE inhibitors show a relatively small risk reduction of clinical cardiovascular endpoints (Reyes et al., 2017). In a critical reappraisal of blood pressure lowering trials in hypertension, Zanchetti et al., (Zanchetti, Thomopoulos and Parati, 2015), reported an absolute risk reduction of 18% across all trials leaving a residual cardiovascular risk of 82%. Although ACE inhibitors and ARBs may effectively control blood pressure, the residual risk for cardiovascular events remains high. The presence of such a residual risk for cardiovascular events in the face of satisfactory blood pressure control may relate to the inability of these medications to reach intracellular sites at which Ang II exerts pathological actions (Kumar et al., 2008, Ferrario et al., 2016, Ferrario and Mullick, 2017). This hypothesis is supported by the failure of ACE inhibitors and/or ARBs to reduce the tissue expression of Ang II as demonstrated in the heart of normotensive (Ferrario, Jessup, Chappell et al., 2005) and hypertensive rat models (Jessup et al., 2008, Ferrario, VonCannon, Ahmad et al., 2019, Ferrario, VonCannon, Jiao et al., 2016, Jessup, Gallagher, Averill et al., 2006, Varagic, Ahmad, VonCannon et al., 2013). In keeping with these findings, intracrine effects of Ang II on cardiac myocyte growth and hypertrophy were not inhibited by the AT1-R antagonist, losartan (Baker and Kumar, 2006). Further, these authors demonstrated that the intracellular effects of Ang II in isolated cultured myocytes is not inhibited by blocking the cell surface AT1-R. These data indicate the independence of the intracrine RAS from the external environment (Baker and Kumar, 2006, De Mello, 1998).
Chymase inhibitors have been shown to protect diabetic rats from renal lesions (Maeda, Inoguchi, Takei et al., 2010, Zhang, Huang, Bai et al., 2016), cardiac dysfunction (Pat et al., 2010), and cardiac arrhythmias (Jin, Takai, Sakaguchi et al., 2004, Tsai, Lai, Hwang et al., 2008, Yahiro, Miura, Imaizumi et al., 2013). In mice, an orally active chymase inhibitor (TEI-F00806) showed antihypertensive effects that were associated with reduced renal AGT and Ang II content as well as chymase gene transcripts (Ansary et al., 2018). In ovariectomized, middle-aged Brown-Norway X Fischer344 rats, 4 weeks of treatment with the mast cell stabilizer cromolyn sulfate improved diastolic function and mitigated the adverse effects of estrogen loss on cardiac interstitial remodeling; effects associated with a reduction in cardiac Ang II immunoreactivity and a strong propensity for lessening of cardiac chymase activity (Wang, da Silva, Alencar et al., 2016). A recently completed safety and tolerability trial on adverse cardiac remodeling after acute ST-segment-elevation myocardial infarction (STEMI) with the orally active chymase inhibitor fulacimstat did not demonstrated superiority over standard care even though the medication was well tolerated, and devoid of negative effects on blood pressure and heart rate (Duengen et al., 2020, Dungen et al., 2019, Kanefendt et al., 2019).
Summary
Exploration of alternate renin-independent mechanisms for Ang II production are shedding a more precise view of the biochemical physiology of the RAS and its role in pathology. Ang-(1-12)’s function as a source for Ang II production in cardiovascular tissues may be more relevant than currently accepted. Buttressing this possibility, Ang-(1-12) role as a biomarker of worsening outcomes of the Acute Respiratory Distress Syndrome (ARDS) has now been revealed in a recently published study by Reddy et al. (Reddy, Asante, Liu et al., 2019). This study employed a liquid chromatography-mass spectrometry-based metabolomics assay to determine how plasma angiotensins correlated with clinical and pulmonary measures in survivors and non-survivors. Median plasma Ang-(1-12) concentrations and the Ang-(1-12)/Ang I ratio were markedly elevated in patients succumbing to the disease at 72 h post admission to the intensive care unit (Reddy et al., 2019).
A robust literature underscores the uniqueness of the AGT protein as the precursor substrate for the generation of angiotensin peptides in health and disease (Celerier, Cruz, Lamande et al., 2002, Clauser, Gaillard, Wei et al., 1989, Corvol and Jeunemaitre, 1997, Corvol, Persu, Gimenez-Roqueplo et al., 1999, Jeunemaitre, Charru, Chatellier et al., 1993, Jeunemaitre, Gimenez-Roqueplo, Celerier et al., 1999). While the first 10 amino acid sequence of from the N-terminus of AGT is conserved across species, the same is not true beyond position 10 (leucine). In humans, the next four amino acids from the N-terminal of AGT are Val11-Ile12-His13-Asn14 while the same sequence in the rat is Leu11-Tyr12-Tyr13-Ser14. These differences in the N-terminal sequence of AGT amino acids explains the selective catalytic activity of human renin for human AGT (Ferrario et al., 2016,Ahmad et al., 2014, Ferrario, 2010). Moreover, little is known about the biological activity of “extended forms of Ang I”. Extended forms of Ang I that are immunologically and pharmacologically comparable to [Ile5]-Ang I, and with molecular weights ranging between 1,300 and 2,200 daltons were identified in canine cerebrospinal fluid (Husain, Bumpus, Smeby et al., 1983). The function of the remaining 98% of the AGT protein [des-(Ang I)-AGT] remains to be fully investigated. Apparent independent functions not associated with Ang I activity and acting as an anti-angiogenic factor have been reported (Celerier et al., 2002, Corvol, Lamande, Cruz et al., 2003) while Lu and colleagues (Lu, Wu, Howatt et al., 2016, Tao, Rong, Lu et al., 2019) have linked des-(Ang I)-AGT to abnormalities in carbohydrate and lipid metabolism in mice. While research in Ang-(1-12) (Ferrario et al., 2016, Ferrario, 2016) and Ang-(1-25) (Nagata et al., 2013) has partially illuminated this issue much remains to be clarified. New research into the clinical significance of the Ang-(1-12)/chymase axis is of fundamental importance given the suggestion that inhibition of hepatic AGT using antisense oligonucleotides (Ferrario and Mullick, 2017, Mullick, Yeh, Graham et al., 2017, Ravichandran, Ozkok, Wang et al., 2015, Saigusa, Dang, Mullick et al., 2016) or small interfering RNAs (siRNAs) (Uijl, Mirabito Colafella, Sun et al., 2019) may constitute a novel approach to treat hypertension. At the 2020 virtual scientific sessions of the American Heart Association, Huang et al. (Huang, Taubel, Fiore et al., 2020) summarized in a poster the outcome of suppressing hepatic AGT synthesis with a subcutaneous investigational RNAi (ALN-AGT01) on the blood pressure in hypertensive patients. The data showed that ALN-AGT01 was effective in suppressing plasma AGT and dose-related decreases in blood pressure in the absence of hypotension and side-effects over an 8-week treatment period. However, the long-term consequences of suppressing at least 96% of the hepatic AGT to achieve a reduction in plasma Ang II and blood pressure remains a concern.
Uncovering the function of the Ang-(1-12) strengthens the urgency to explore the use of chymase inhibitors in cardiovascular pathology as exemplified by recent published results obtained in normal volunteers and patients post-myocardial infarction (Duengen et al., 2020, Kanefendt et al., 2019, Okamura, Okuda, Shirai et al., 2019). A more definitive characterization of the enzymatic pathway through which Ang-(1-12) is cleaved from AGT may project this alternate peptide as a more precise target to suppress Ang II pathological actions.
Acknowledgment
This work was supported by grant from the National Heart, Lung and Blood Institute of the NIH (P01 HL-051952).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Authors declare that there are no potential conflicts of interest relevant to this article.
References
- 2009. Bone Marrow, Thymus and Blood: Changes across the Lifespan, Aging health. 5, 385–393 DOI: 10.2217/ahe.09.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadir PM, Walston JD and Carey RM, 2012. Subcellular characteristics of functional intracellular renin-angiotensin systems, Peptides. 38, 437–45 DOI: 10.1016/j.peptides.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S and Ferrario CM, 2018. Chymase inhibitors for the treatment of cardiac diseases: a patent review (2010-2018), Expert Opin Ther Pat. 28, 755–764 DOI: 10.1080/13543776.2018.1531848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC and Ferrario CM, 2011. Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue, PLoS One. 6, e28501 DOI: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Varagic J, Groban L, Dell’Italia LJ, Nagata S, Kon ND and Ferrario CM, 2014. Angiotensin-(1-12): a chymase-mediated cellular angiotensin II substrate, Curr Hypertens Rep. 16, 429 DOI: 10.1007/s11906-014-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Varagic J, VonCannon JL, Groban L, Collawn JF, Dell’Italia LJ and Ferrario CM, 2016. Primacy of cardiac chymase over angiotensin converting enzyme as an angiotensin-(1-12) metabolizing enzyme, Biochem Biophys Res Commun. 478, 559–64 DOI: 10.1016/j.bbrc.2016.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Varagic J, Westwood BM, Chappell MC and Ferrario CM, 2011. Uptake and metabolism of the novel peptide angiotensin-(1-12) by neonatal cardiac myocytes, PLoS One. 6, e15759 DOI: 10.1371/journal.pone.0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Wei CC, Tallaj J, Dell’Italia LJ, Moniwa N, Varagic J and Ferrario CM, 2013. Chymase mediates angiotensin-(1-12) metabolism in normal human hearts, J Am Soc Hypertens. 7, 128–36 DOI: 10.1016/j.jash.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansary TM, Urushihara M, Fujisawa Y, Nagata S, Urata H, Nakano D, Hirofumi H, Kitamura K, Kagami S and Nishiyama A, 2018. Effects of the selective chymase inhibitor TEI-F00806 on the intrarenal renin-angiotensin system in salt-treated angiotensin I-infused hypertensive mice, Exp Physiol. 103, 1524–1531 DOI: 10.1113/EP087209. [DOI] [PubMed] [Google Scholar]
- Baker KM and Kumar R, 2006. Intracellular angiotensin II induces cell proliferation independent of AT1 receptor, Am J Physiol Cell Physiol. 291, C995–1001 DOI: 10.1152/ajpcell.00238.2006. [DOI] [PubMed] [Google Scholar]
- Barlucchi L, Leri A, Dostal DE, Fiordaliso F, Tada H, Hintze TH, Kajstura J, Nadal-Ginard B and Anversa P, 2001. Canine ventricular myocytes possess a renin-angiotensin system that is upregulated with heart failure, Circ Res. 88, 298–304 DOI: 10.1161/01.res.88.3.298. [DOI] [PubMed] [Google Scholar]
- Butts B, Ahmed MI, Bajaj NS, Cox Powell P, Pat B, Litovsky S, Gupta H, Lloyd SG, Denney TS, Zhang X, Aban I, Sadayappan S, McNamara JW, Watson MJ, Ferrario CM, Collawn JF, Lewis C, Davies JE and Dell’Italia LJ, 2020. Reduced Left Atrial Emptying Fraction and Chymase Activation in Pathophysiology of Primary Mitral Regurgitation, JACC Basic Transl Sci. 5, 109–122 DOI: 10.1016/j.jacbts.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts B, Goeddel LA, George DJ, Steele C, Davies JE, Wei CC, Varagic J, George JF, Ferrario CM, Melby SJ and Dell’Italia LJ, 2017. Increased Inflammation in Pericardial Fluid Persists 48 Hours After Cardiac Surgery, Circulation. 136, 2284–2286 DOI: 10.1161/CIRCULATIONAHA.117.029589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier J, Cruz A, Lamande N, Gasc JM and Corvol P, 2002. Angiotensinogen and its cleaved derivatives inhibit angiogenesis, Hypertension. 39, 224–8 DOI: 10.1161/hy0202.103441. [DOI] [PubMed] [Google Scholar]
- Chatelain RE and Ferrario CM, 1978. Biphasic changes in thymus structure during evolving renal hypertension, Clin Sci Mol Med. 55, 149–56. [DOI] [PubMed] [Google Scholar]
- Chatelain RE, Vessey AR and Ferrario CM, 1980. Lymphoid alterations and impaired T lymphocyte reactivity in experimental renal hypertension, J Lab Clin Med. 95, 737–47. [PubMed] [Google Scholar]
- Chen CM, Juan SH and Chou HC, 2018. Hyperglycemia activates the renin-angiotensin system and induces epithelial-mesenchymal transition in streptozotocin-induced diabetic kidneys, J Renin Angiotensin Aldosterone Syst. 19, 1470320318803009 DOI: 10.1177/1470320318803009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauser E, Gaillard I, Wei L and Corvol P, 1989. Regulation of angiotensinogen gene, Am J Hypertens. 2, 403–10 DOI: 10.1093/ajh/2.5.403. [DOI] [PubMed] [Google Scholar]
- Conti S, Cassis P and Benigni A, 2012. Aging and the renin-angiotensin system, Hypertension. 60, 878–83 DOI: 10.1161/HYPERTENSIONAHA.110.155895. [DOI] [PubMed] [Google Scholar]
- Corvol P and Jeunemaitre X, 1997. Molecular genetics of human hypertension: role of angiotensinogen, Endocr Rev. 18, 662–77 DOI: 10.1210/edrv.18.5.0312. [DOI] [PubMed] [Google Scholar]
- Corvol P, Lamande N, Cruz A, Celerier J and Gasc JM, 2003. Inhibition of angiogenesis: a new function for angiotensinogen and des(angiotensin I)angiotensinogen, Curr Hypertens Rep. 5, 149–54 DOI: 10.1007/s11906-003-0072-3. [DOI] [PubMed] [Google Scholar]
- Corvol P, Persu A, Gimenez-Roqueplo AP and Jeunemaitre X, 1999. Seven lessons from two candidate genes in human essential hypertension: angiotensinogen and epithelial sodium channel, Hypertension. 33, 1324–31 DOI: 10.1161/01.hyp.33.6.1324. [DOI] [PubMed] [Google Scholar]
- Cristovam PC, Carmona AK, Arnoni CP, Maquigussa E, Pereira LG and Boim MA, 2012. Role of chymase in diabetic nephropathy, Exp Biol Med (Maywood). 237, 985–92 DOI: 10.1258/ebm.2012.011356. [DOI] [PubMed] [Google Scholar]
- De Mello WC, 1998. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes, Hypertension. 32, 976–82 DOI: 10.1161/01.hyp.32.6.976. [DOI] [PubMed] [Google Scholar]
- De Mello WC, Dell’Itallia LJ, Varagic J and Ferrario CM, 2016. Intracellular angiotensin-(1-12) changes the electrical properties of intact cardiac muscle, Mol Cell Biochem. 422, 31–40 DOI: 10.1007/s11010-016-2801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza AMA, West CA, de Abreu ARR, Pai AV, Mesquita LBT, Ji H, Chianca D Jr., de Menezes RCA and Sandberg K, 2018. Role of the Renin Angiotensin System in Blood Pressure Allostasis-induced by Severe Food Restriction in Female Fischer rats, Sci Rep. 8, 10327 DOI: 10.1038/s41598-018-28593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Italia LJ, Collawn JF and Ferrario CM, 2018. Multifunctional Role of Chymase in Acute and Chronic Tissue Injury and Remodeling, Circ Res. 122, 319–336 DOI: 10.1161/CIRCRESAHA.117.310978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Italia LJ, Meng QC, Balcells E, Straeter-Knowlen IM, Hankes GH, Dillon R, Cartee RE, Orr R, Bishop SP, Oparil S and et al. , 1995. Increased ACE and chymase-like activity in cardiac tissue of dogs with chronic mitral regurgitation, Am J Physiol. 269, H2065–73 DOI: 10.1152/ajpheart.1995.269.6.H2065. [DOI] [PubMed] [Google Scholar]
- Dell’Italia LJ, Meng QC, Balcells E, Wei CC, Palmer R, Hageman GR, Durand J, Hankes GH and Oparil S, 1997. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces, J Clin Invest. 100, 253–8 DOI: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan S, Yahiro E, Uehara Y, Habe S, Nishiyama A, Miura S, Saku K and Urata H, 2015. Depressor effect of chymase inhibitor in mice with high salt-induced moderate hypertension, Am J Physiol Heart Circ Physiol. 309, H1987–96 DOI: 10.1152/ajpheart.00721.2014. [DOI] [PubMed] [Google Scholar]
- Duengen HD, Kim RJ, Zahger D, Orvin K, Kornowski R, Admon D, Kettner J, Shimony A, Otto C, Becka M, Kanefendt F, Romo AI, Hasin T, Ostadal P, Rojas GC, Senni M and trial G.i.o.t.C.M., 2020. Effects of the chymase inhibitor fulacimstat on adverse cardiac remodeling after acute myocardial infarction-Results of the Chymase Inhibitor in Adverse Remodeling after Myocardial Infarction (CHIARA MIA) 2 trial, Am Heart J. 224, 129–137 DOI: 10.1016/j.ahj.2020.01.012. [DOI] [PubMed] [Google Scholar]
- Dungen HD, Kober L, Nodari S, Schou M, Otto C, Becka M, Kanefendt F, Winkelmann BR, Gislason G, Richard F, Nielsen OW, Gheorghiade M and Senni M, 2019. Safety and Tolerability of the Chymase Inhibitor Fulacimstat in Patients With Left Ventricular Dysfunction After Myocardial Infarction-Results of the CHIARA MIA 1 Trial, Clin Pharmacol Drug Dev. 8, 942–951 DOI: 10.1002/cpdd.633. [DOI] [PubMed] [Google Scholar]
- Dusing R, 2016. Mega clinical trials which have shaped the RAS intervention clinical practice, Ther Adv Cardiovasc Dis. 10, 133–50 DOI: 10.1177/1753944716644131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM and Mullick AE, 2017. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease, Pharmacol Res. 125, 57–71 DOI: 10.1016/j.phrs.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, 2010. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism, Hypertension. 55, 445–52 DOI: 10.1161/HYPERTENSIONAHA.109.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, 2016. Cardiac remodelling and RAS inhibition, Ther Adv Cardiovasc Dis. 10, 162–71 DOI: 10.1177/1753944716642677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, Ahmad S, Joyner J and Varagic J, 2010. Advances in the renin angiotensin system focus on angiotensin-converting enzyme 2 and angiotensin-(1-7), Adv Pharmacol. 59, 197–233 DOI: 10.1016/S1054-3589(10)59007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, Kon N and Dell’Italia LJ, 2014. An evolving story of angiotensin-II-forming pathways in rodents and humans, Clin Sci (Lond). 126, 461–9 DOI: 10.1042/CS20130400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, Ahmad S, Varagic J, Cheng CP, Groban L, Wang H, Collawn JF and Dell Italia LJ, 2016. Intracrine angiotensin II functions originate from noncanonical pathways in the human heart, Am J Physiol Heart Circ Physiol. 311, H404–14 DOI: 10.1152/ajpheart.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI and Gallagher PE, 2005. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2, Circulation. 111, 2605–10 DOI: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A and Sowers JR, 2009. Differential regulation of angiotensin-(1-12) in plasma and cardiac tissue in response to bilateral nephrectomy, Am J Physiol Heart Circ Physiol. 296, H1184–92 DOI: 10.1152/ajpheart.01114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, VonCannon J, Ahmad S, Wright KN, Roberts DJ, Wang H, Yamashita T, Groban L, Cheng CP, Collawn JF, Dell’Italia LJ and Varagic J, 2019. Activation of the Human Angiotensin-(1-12)-Chymase Pathway in Rats With Human Angiotensinogen Gene Transcripts, Front Cardiovasc Med. 6, 163 DOI: 10.3389/fcvm.2019.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CM, VonCannon J, Jiao Y, Ahmad S, Bader M, Dell’Italia LJ, Groban L and Varagic J, 2016. Cardiac angiotensin-(1-12) expression and systemic hypertension in rats expressing the human angiotensinogen gene, Am J Physiol Heart Circ Physiol. 310, H995–1002 DOI: 10.1152/ajpheart.00833.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW and Sonntag WE, 2006. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats, J Gerontol A Biol Sci Med Sci. 61, 28–35 DOI: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- Gwathmey TM, Alzayadneh EM, Pendergrass KD and Chappell MC, 2012. Novel roles of nuclear angiotensin receptors and signaling mechanisms, Am J Physiol Regul Integr Comp Physiol. 302, R518–30 DOI: 10.1152/ajpregu.00525.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakata H, Fouad-Tarazi FM, Bumpus FM, Khosla M, Healy B, Husain A, Urata H and Kumagai H, 1990. Angiotensins and the failing heart. Enhanced positive inotropic response to angiotensin I in cardiomyopathic hamster heart in the presence of captopril, Circ Res. 66, 891–9 DOI: 10.1161/01.res.66.4.891. [DOI] [PubMed] [Google Scholar]
- Huang SA, Taubel J, Fiore G, Dewland P, Bakris GL, Desai AS, Cheng Y, Agarwal A, Harrop J, Nguyen HV, Lu J, Foster D, Vaishnaw A and Kim JB, 2020. Dose-Related Reductions in Blood Pressure With a RNA Interference (RNAi) Therapeutic Targeting Angiotensinogen in Hypertensive Patients: Interim Results From a First-In-Human Phase 1 Study of ALN-AGT01, Circulation. 142, A14387–A14387 DOI: doi: 10.1161/circ.142.suppl_3.14387. [DOI] [Google Scholar]
- Huang XR, Chen WY, Truong LD and Lan HY, 2003. Chymase is upregulated in diabetic nephropathy: implications for an alternative pathway of angiotensin II-mediated diabetic renal and vascular disease, J Am Soc Nephrol. 14, 1738–47 DOI: 10.1097/01.asn.0000071512.93927.4e. [DOI] [PubMed] [Google Scholar]
- Husain A, 1993. The chymase-angiotensin system in humans, J Hypertens. 11, 1155–9. [PubMed] [Google Scholar]
- Husain A, Bumpus FM, Smeby RR, Brosnihan KB, Khosla MC, Speth RC and Ferrario CM, 1983. Evidence for the existence of a family of biologically active angiotensin I-like peptides in the dog central nervous system, Circ Res. 52, 460–4 DOI: 10.1161/01.res.52.4.460. [DOI] [PubMed] [Google Scholar]
- Jessup JA, Brosnihan KB, Gallagher PE, Chappell MC and Ferrario CM, 2008. Differential effect of low dose thiazides on the Renin Angiotensin system in genetically hypertensive and normotensive rats, J Am Soc Hypertens. 2, 106–15 DOI: 10.1016/j.jash.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC and Ferrario CM, 2006. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats, Am J Physiol Heart Circ Physiol. 291, H2166–72 DOI: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K and Ferrario CM, 2008. Localization of the novel angiotensin peptide, angiotensin-(1-12), in heart and kidney of hypertensive and normotensive rats, Am J Physiol Heart Circ Physiol. 294, H2614–8 DOI: 10.1152/ajpheart.91521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeunemaitre X, Charru A, Chatellier G, Dumont C, Sassano P, Soubrier F, Menard J and Corvol P, 1993. M235T variant of the human angiotensinogen gene in unselected hypertensive patients, J Hypertens Suppl. 11, S80–1. [PubMed] [Google Scholar]
- Jeunemaitre X, Gimenez-Roqueplo AP, Celerier J and Corvol P, 1999. Angiotensinogen variants and human hypertension, Curr Hypertens Rep. 1, 31–41 DOI: 10.1007/s11906-999-0071-0. [DOI] [PubMed] [Google Scholar]
- Jin D, Takai S, Sakaguchi M, Okamoto Y, Muramatsu M and Miyazaki M, 2004. An antiarrhythmic effect of a chymase inhibitor after myocardial infarction, J Pharmacol Exp Ther. 309, 490–7 DOI: 10.1124/jpet.103.061465. [DOI] [PubMed] [Google Scholar]
- Ju H, Gros R, You X, Tsang S, Husain M and Rabinovitch M, 2001. Conditional and targeted overexpression of vascular chymase causes hypertension in transgenic mice, Proc Natl Acad Sci U S A. 98, 7469–74 DOI: 10.1073/pnas.131147598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanefendt F, Thuss U, Becka M, Boxnick S, Berse M, Schultz A and Otto C, 2019. Pharmacokinetics, Safety, and Tolerability of the Novel Chymase Inhibitor BAY 1142524 in Healthy Male Volunteers, Clin Pharmacol Drug Dev. 8, 467–479 DOI: 10.1002/cpdd.579. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Urata H, Bumpus FM and Husain A, 1991. Multiple determinants for the high substrate specificity of an angiotensin II-forming chymase from the human heart, J Biol Chem. 266, 19192–7. [PubMed] [Google Scholar]
- Kumar R, Singh VP and Baker KM, 2008. The intracellular renin-angiotensin system: implications in cardiovascular remodeling, Curr Opin Nephrol Hypertens. 17, 168–73 DOI: 10.1097/MNH.0b013e3282f521a8. [DOI] [PubMed] [Google Scholar]
- Leung PS, 2010. Local RAS, Adv Exp Med Biol. 690, 69–87 DOI: 10.1007/978-90-481-9060-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zhang X, Cheng HJ, Zhang Z, Ahmad S, Varagic J, Li W, Cheng CP and Ferrario CM, 2018. Critical role of the chymase/angiotensin-(1-12) axis in modulating cardiomyocyte contractility, Int J Cardiol. 264, 137–144 DOI: 10.1016/j.ijcard.2018.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zhang Z, Zhang X, Chen Z, Cheng HJ, Ahmad S, Ferrario CM and Cheng CP, 2020. Reversal of angiotensin-(1-12)-caused positive modulation on left ventricular contractile performance in heart failure: Assessment by pressure-volume analysis, Int J Cardiol. 301, 135–141 DOI: 10.1016/j.ijcard.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Wu C, Howatt DA, Balakrishnan A, Moorleghen JJ, Chen X, Zhao M, Graham MJ, Mullick AE, Crooke RM, Feldman DL, Cassis LA, Vander Kooi CW and Daugherty A, 2016. Angiotensinogen Exerts Effects Independent of Angiotensin II, Arterioscler Thromb Vasc Biol. 36, 256–65 DOI: 10.1161/ATVBAHA.115.306740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Wang X, Chen C, Wang J, Zou X, Li C, Xu Z, Yang X, Shi W and Zeng C, 2015. Oxidative stress causes imbalance of renal renin angiotensin system (RAS) components and hypertension in obese Zucker rats, J Am Heart Assoc. 4, DOI: 10.1161/JAHA.114.001559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Inoguchi T, Takei R, Sawada F, Sasaki S, Fujii M, Kobayashi K, Urata H, Nishiyama A and Takayanagi R, 2010. Inhibition of chymase protects against diabetes-induced oxidative stress and renal dysfunction in hamsters, Am J Physiol Renal Physiol. 299, F1328–38 DOI: 10.1152/ajprenal.00337.2010. [DOI] [PubMed] [Google Scholar]
- McMaster WG, Kirabo A, Madhur MS and Harrison DG, 2015. Inflammation, immunity, and hypertensive end-organ damage, Circ Res. 116, 1022–33 DOI: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson EA, Luo Z, Brown RA, LeBard LS, Corless CC, Speth RC and Bagby SP, 2004. Chymase-like angiotensin II-generating activity in end-stage human autosomal dominant polycystic kidney disease, J Am Soc Nephrol. 15, 493–500 DOI: 10.1097/01.asn.0000109782.28991.26. [DOI] [PubMed] [Google Scholar]
- Mehta PK and Griendling KK, 2007. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system, Am J Physiol Cell Physiol. 292, C82–97 DOI: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk TP and Guzik TJ, 2019. Adaptive Immunity in Hypertension, Curr Hypertens Rep. 21, 68 DOI: 10.1007/s11906-019-0971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniwa N, Varagic J, Simington SW, Ahmad S, Nagata S, Voncannon JL and Ferrario CM, 2013. Primacy of angiotensin converting enzyme in angiotensin-(1-12) metabolism, Am J Physiol Heart Circ Physiol. 305, H644–50 DOI: 10.1152/ajpheart.00210.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick AE, Yeh ST, Graham MJ, Engelhardt JA, Prakash TP and Crooke RM, 2017. Blood Pressure Lowering and Safety Improvements With Liver Angiotensinogen Inhibition in Models of Hypertension and Kidney Injury, Hypertension. 70, 566–576 DOI: 10.1161/HYPERTENSIONAHA.117.09755. [DOI] [PubMed] [Google Scholar]
- Murakami M, Karnik SS and Husain A, 1995. Human prochymase activation. A novel role for heparin in zymogen processing, J Biol Chem. 270, 2218–23. [PubMed] [Google Scholar]
- Nagata S, Hatakeyama K, Asami M, Tokashiki M, Hibino H, Nishiuchi Y, Kuwasako K, Kato J, Asada Y and Kitamura K, 2013. Big angiotensin-25: a novel glycosylated angiotensin-related peptide isolated from human urine, Biochem Biophys Res Commun. 441, 757–62 DOI: 10.1016/j.bbrc.2013.10.124. [DOI] [PubMed] [Google Scholar]
- Nagata S, Kato J, Sasaki K, Minamino N, Eto T and Kitamura K, 2006. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system, Biochem Biophys Res Commun. 350, 1026–31 DOI: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- Nehme A, Zouein FA, Zayeri ZD and Zibara K, 2019. An Update on the Tissue Renin Angiotensin System and Its Role in Physiology and Pathology, J Cardiovasc Dev Dis. 6, DOI: 10.3390/jcdd6020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Okuda T, Shirai K and Urata H, 2019. Increase of chymase-dependent angiotensin II-forming activity in circulating mononuclear leukocytes after acute myocardial infarction chymase activity after acute myocardial infarction, Heart Vessels. 34, 1148–1157 DOI: 10.1007/s00380-019-01352-x. [DOI] [PubMed] [Google Scholar]
- Pat B, Chen Y, Killingsworth C, Gladden JD, Shi K, Zheng J, Powell PC, Walcott G, Ahmed MI, Gupta H, Desai R, Wei CC, Hase N, Kobayashi T, Sabri A, Granzier H, Denney T, Tillson M, Dillon AR, Husain A and Dell’Italia LJ, 2010. Chymase inhibition prevents fibronectin and myofibrillar loss and improves cardiomyocyte function and LV torsion angle in dogs with isolated mitral regurgitation, Circulation. 122, 1488–95 DOI: 10.1161/CIRCULATIONAHA.109.921619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergrass KD, Averill DB, Ferrario CM, Diz DI and Chappell MC, 2006. Differential expression of nuclear AT 1 receptors and angiotensin II within the kidney of the male congenic mRen2.Lewis rat, Am J Physiol Renal Physiol. 290, F1497–506 DOI: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- Powell PC, Wei CC, Fu L, Pat B, Bradley WE, Collawn JF and Dell’Italia LJ, 2019. Chymase uptake by cardiomyocytes results in myosin degradation in cardiac volume overload, Heliyon. 5, e01397 DOI: 10.1016/j.heliyon.2019.e01397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NV and Hoidal JR, 2012. Chymases, 3rd ed. Elsvier Academic Press, Amsterdam ; Boston. [Google Scholar]
- Ravichandran K, Ozkok A, Wang Q, Mullick AE and Edelstein CL, 2015. Antisense-mediated angiotensinogen inhibition slows polycystic kidney disease in mice with a targeted mutation in Pkd2, Am J Physiol Renal Physiol. 308, F349–57 DOI: 10.1152/ajprenal.00478.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R, Asante I, Liu S, Parikh P, Liebler J, Borok Z, Rodgers K, Baydur A and Louie SG, 2019. Circulating angiotensin peptides levels in Acute Respiratory Distress Syndrome correlate with clinical outcomes: A pilot study, PLoS One. 14, e0213096 DOI: 10.1371/journal.pone.0213096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S, Cheng CP, Roberts DJ, Yamashita T, Ahmad S, VonCannon JL, Wright KN, Dell’Italia LJ, Varagic J and Ferrario CM, 2019. Angiotensin-(1-12)/chymase axis modulates cardiomyocyte L-type calcium currents in rats expressing human angiotensinogen, Int J Cardiol. 297, 104–110 DOI: 10.1016/j.ijcard.2019.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S, Varagic J, Ahmad S, VonCannon J, Kon ND, Wang H, Groban L, Cheng CP, Dell’Italia LJ and Ferrario CM, 2017. Novel Cardiac Intracrine Mechanisms Based on Ang-(1-12)/Chymase Axis Require a Revision of Therapeutic Approaches in Human Heart Disease, Curr Hypertens Rep. 19, 16 DOI: 10.1007/s11906-017-0708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Xu Y, Slayter HS and Izumo S, 1993. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro, Cell. 75, 977–84 DOI: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- Saigusa T, Dang Y, Mullick AE, Yeh ST, Zile MR, Baicu CF and Bell PD, 2016. Suppressing angiotensinogen synthesis attenuates kidney cyst formation in a Pkd1 mouse model, FASEB J. 30, 370–9 DOI: 10.1096/fj.15-279299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrod M, Liu X, Zhang X and Sigmund CD, 2005. Nuclear localization of angiotensinogen in astrocytes, Am J Physiol Regul Integr Comp Physiol. 288, R539–46 DOI: 10.1152/ajpregu.00594.2004. [DOI] [PubMed] [Google Scholar]
- Shi S, Ye S, Mao J, Ru Y, Lu Y, Wu X, Xu M, Zhu T, Wang Y, Chen Y, Tang X and Xi Y, 2020. CMA1 is potent prognostic marker and associates with immune infiltration in gastric cancer, Autoimmunity. 53, 210–217 DOI: 10.1080/08916934.2020.1735371. [DOI] [PubMed] [Google Scholar]
- Singh VP, Le B, Bhat VB, Baker KM and Kumar R, 2007. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes, Am J Physiol Heart Circ Physiol. 293, H939–48 DOI: 10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- Singh VP, Le B, Khode R, Baker KM and Kumar R, 2008. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis, Diabetes. 57, 3297–306 DOI: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn WB and Ferrario CM, 2008. Angiotensin II AT1 receptor blockade normalizes CD11b+ monocyte production in bone marrow of hypercholesterolemic monkeys, Atherosclerosis. 196, 624–32 DOI: 10.1016/j.atherosclerosis.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn WB, Richmond RS, Ann Tallant E, Gallagher PE and Ferrario CM, 2004. Renin-angiotensin system expression in rat bone marrow haematopoietic and stromal cells, Br J Haematol. 126, 120–6 DOI: 10.1111/j.1365-2141.2004.04998.x. [DOI] [PubMed] [Google Scholar]
- Tao XR, Rong JB, Lu HS, Daugherty A, Shi P, Ke CL, Zhang ZC, Xu YC and Wang JA, 2019. Angiotensinogen in hepatocytes contributes to Western diet-induced liver steatosis, J Lipid Res. 60, 1983–1995 DOI: 10.1194/jlr.M093252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask AJ, Jessup JA, Chappell MC and Ferrario CM, 2008. Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart, Am J Physiol Heart Circ Physiol. 294, H2242–7 DOI: 10.1152/ajpheart.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CT, Lai LP, Hwang JJ, Chen WP, Chiang FT, Hsu KL, Tseng CD, Tseng YZ and Lin JL, 2008. Renin-angiotensin system component expression in the HL-1 atrial cell line and in a pig model of atrial fibrillation, J Hypertens. 26, 570–82 DOI: 10.1097/HJH.0b013e3282f34a4a. [DOI] [PubMed] [Google Scholar]
- Uijl E, Mirabito Colafella KM, Sun Y, Ren L, van Veghel R, Garrelds IM, de Vries R, Poglitsch M, Zlatev I, Kim JB, Hoorn EJ, Foster D and Danser AHJ, 2019. Strong and Sustained Antihypertensive Effect of Small Interfering RNA Targeting Liver Angiotensinogen, Hypertension. 73, 1249–1257 DOI: 10.1161/HYPERTENSIONAHA.119.12703. [DOI] [PubMed] [Google Scholar]
- Urata H, Kinoshita A, Misono KS, Bumpus FM and Husain A, 1990. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart, J Biol Chem. 265, 22348–57. [PubMed] [Google Scholar]
- Urata H, Kinoshita A, Perez DM, Misono KS, Bumpus FM, Graham RM and Husain A, 1991. Cloning of the gene and cDNA for human heart chymase, J Biol Chem. 266, 17173–9. [PubMed] [Google Scholar]
- Urata H, Nishimura H and Ganten D, 1995. Mechanisms of angiotensin II formation in humans, Eur Heart J. 16 Suppl N, 79–85 DOI: 10.1093/eurheartj/16.suppl_n.79. [DOI] [PubMed] [Google Scholar]
- Varagic J, Ahmad S, VonCannon JL, Moniwa N, Brosnihan KB, Wysocki J, Batlle D and Ferrario CM, 2013. Predominance of AT(1) blockade over mas-mediated angiotensin-(1-7) mechanisms in the regulation of blood pressure and renin-angiotensin system in mRen2.Lewis rats, Am J Hypertens. 26, 583–90 DOI: 10.1093/ajh/hps090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, da Silva J, Alencar A, Zapata-Sudo G, Lin MR, Sun X, Ahmad S, Ferrario CM and Groban L, 2016. Mast Cell Inhibition Attenuates Cardiac Remodeling and Diastolic Dysfunction in Middle-aged, Ovariectomized Fischer 344 x Brown Norway Rats, J Cardiovasc Pharmacol. 68, 49–57 DOI: 10.1097/FJC.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Varagic J, Nagata S, Kon ND, Ahmad S, VonCannon JL, Wright KN, Sun X, Deal D, Groban L and Ferrario CM, 2020a. Atrial angiotensin-(1-12)/chymase expression data in patient of heart diseases, Data Brief. 31, 105744 DOI: 10.1016/j.dib.2020.105744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Varagic J, Nagata S, Kon ND, Ahmad S, VonCannon JL, Wright KN, Sun X, Deal D, Groban L and Ferrario CM, 2020b. Differential Expression of the Angiotensin-(1-12)/Chymase Axis in Human Atrial Tissue, J Surg Res. 253, 173–184 DOI: 10.1016/j.jss.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasse H, Naqvi N and Husain A, 2012. Impact of Mast Cell Chymase on Renal Disease Progression, Curr Hypertens Rev. 8, 15–23 DOI: 10.2174/157340212800505007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CC, Lucchesi PA, Tallaj J, Bradley WE, Powell PC and Dell’Italia LJ, 2003. Cardiac interstitial bradykinin and mast cells modulate pattern of LV remodeling in volume overload in rats, Am J Physiol Heart Circ Physiol. 285, H784–92 DOI: 10.1152/ajpheart.00793.2001. [DOI] [PubMed] [Google Scholar]
- Whaley-Connell A, Habibi J, Nistala R, Hayden MR, Pulakat L, Sinak C, Locher B, Ferrario CM and Sowers JR, 2012. Combination of direct renin inhibition with angiotensin type 1 receptor blockade improves aldosterone but does not improve kidney injury in the transgenic Ren2 rat, Regul Pept. 176, 36–44 DOI: 10.1016/j.regpep.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G and Wenzel UO, 2004. Angiotensin II and cell cycle regulation, Hypertension. 43, 693–8 DOI: 10.1161/01.HYP.0000120963.09029.ca. [DOI] [PubMed] [Google Scholar]
- Yahiro E, Miura S, Imaizumi S, Uehara Y and Saku K, 2013. Chymase inhibitors, Curr Pharm Des. 19, 3065–71 DOI: 10.2174/1381612811319170014. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ahmad S, Wright KN, Roberts DJ, VonCannon JL, Wang H, Groban L, Dell’Italia LJ and Ferrario CM, 2020. Noncanonical Mechanisms for Direct Bone Marrow Generating Ang II (Angiotensin II) Predominate in CD68 Positive Myeloid Lineage Cells, Hypertension. 75, 500–509 DOI: 10.1161/HYPERTENSIONAHA.119.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim HE and Yoo KH, 2008. Renin-Angiotensin system - considerations for hypertension and kidney, Electrolyte Blood Press. 6, 42–50 DOI: 10.5049/EBP.2008.6.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchetti A, Thomopoulos C and Parati G, 2015. Randomized controlled trials of blood pressure lowering in hypertension: a critical reappraisal, Circ Res. 116, 1058–73 DOI: 10.1161/CIRCRESAHA.116.303641. [DOI] [PubMed] [Google Scholar]
- Zhang M, Huang W, Bai J, Nie X and Wang W, 2016. Chymase inhibition protects diabetic rats from renal lesions, Mol Med Rep. 14, 121–8 DOI: 10.3892/mmr.2016.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]


