Abstract
Purpose:
Fumarate hydratase-deficient renal cell carcinoma (FH-RCC) is a rare, aggressive form of RCC associated with hereditary leiomyomatosis and RCC syndrome (HLRCC). Evidence for systemic therapy efficacy is lacking.
Methods:
We studied clinical and genomic characteristics of FH-RCC, including response (ORR) to systemic therapies and next-generation sequencing (NGS). Patients with metastatic FH-RCC, defined by presence of pathogenic germline or somatic FH mutation plus immunohistochemical evidence of FH-loss, were included.
Results:
28/32 included patients (median age 46; range 20–74; M:F, 20:12) underwent germline testing; 23 (82%) harbored a pathogenic FH germline variant. 5 (16%) were negative for germline FH mutations; all had biallelic somatic FH loss. Somatic NGS (31/32 patients) revealed co-occurring NF2 mutation most frequently (n=5). Compared to clear cell RCC, FH-RCC had lower mutation count (median 2 vs 4; p<0.001) but higher fraction of genome altered (18.7 vs 10.3%; p=0.001).
26 patients were evaluable for response to systemic therapy: mTOR/VEGF combination (n=18, ORR 44%), VEGF monotherapy (n=15, ORR 20%), checkpoint inhibitor therapy (n=8, ORR 0%) and mTOR monotherapy (n=4, ORR 0%). No complete responses were seen. Median overall and progression-free survival were 21.9 months (95% CI: 14.3, 33.8) and 8.7 months (95% CI: 4.8, 12.3), respectively.
Conclusion:
Although most FH-RCC tumors are due to germline FH alterations, a significant portion result from biallelic somatic FH loss. Both somatic and germline FH-RCC have similar molecular characteristics, with NF2 mutations, low TMB, and high fraction of genome altered. Although immunotherapy alone produced no objective responses, combination mTOR/VEGF therapy showed encouraging results.
Keywords: renal cell carcinoma, fumarate hydratase-deficient renal cell carcinoma, HLRCC, fumarate hydratase, Outcomes, Genomics
Introduction:
Fumarate hydratase (FH)-deficient renal cell carcinoma (RCC) is a type of RCC associated with hereditary leiomyomatosis renal cell cancer syndrome (HLRCC), an autosomal dominant disorder characterized by uterine and cutaneous leiomyomas and increased predisposition to an aggressive form of RCC.(1) The syndrome is caused by heterozygous mutations to the FH gene, which encodes fumarate hydratase (FH), a critical component of the Krebs cycle.(2, 3) The lifetime renal cancer risk for FH mutation carriers is estimated to be 15%.(4) These RCCs typically occur in younger patients (median age: 39–45 years) and median survival with advanced/metastatic disease is poor, between 18–24 months in prior series.(5–7)
Since 2016, the WHO classification of tumors includes HLRCC syndrome–associated RCC as a distinct entity (8). This term, however, is a misnomer and does not include histologically indistinguishable tumors that can arise from biallelic somatic loss of FH, with absence of the germline mutation and without the associated syndromic features of HLRCC. While the clinical behavior of biallelic somatic FH loss RCC and HLRCC-associated RCC are suspected to be similar, the incidence of biallelic somatic-only FH-RCC are unknown. Collectively, FH-RCC tumors demonstrate a broad range of morphologic features, although often containing a papillary component and exhibiting prominent nucleoli with perinucleolar clearing in tumor cells, their distinction from other subtypes of RCC typically relies on immunohistochemical (IHC) evidence of FH-deficiency.(7–12)
In FH-RCC, there is an accumulation of the Krebs cycle intermediate fumarate, which functions as an oncometabolite, activating a complex variety of oncogenic cascades and causing metabolic dysregulation.(13) Among these, fumarate accumulation leads to hypoxia inducible factor (HIF) stabilization with subsequent effects on HIF targets and disrupted function of multiple proteins by succination.(13–15) By contrast, the most well-studied canonical pathways involved in the pathogenesis of clear cell RCC (ccRCC) include the VHL tumor suppressor/HIF pathway [including HIF targets, such as vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), epidermal growth factor (EGF)] and the mammalian target of rapamycin (mTOR) pathways. It is unknown whether the available “targeted” therapies in RCC, aimed at intervention in the VHL/HIF pathway involved in ccRCC, are effective for FH-RCC.
Clinically, FH-RCC is particularly difficult to manage because of its highly aggressive course, therefore information on clinical presentation, response to therapies and potential molecular drivers of disease are critical. To date, preliminary data from a single phase II study evaluating treatment outcomes for HLRCC patients has been reported, showing promising results with combination bevacizumab and erlotinib, however, other therapeutic strategies will undoubtedly be required in this population.(6) Due to lack of other retrospective or prospective data, treatment options are often extrapolated from studies of ccRCC. In the past decade, several new therapies have been approved for advanced RCC, including the anti-VEGF and multi-targeted tyrosine kinase inhibitors sunitinib, pazopanib, cabozantinib and lenvatinib, the immune checkpoint inhibitors ipilimumab, nivolumab and pembrolizumab, and VEGF/checkpoint blockade combination therapies.(16–20) Although these agents are often used for any patient with metastatic RCC, their efficacy in FH-RCC has not been established. In this study, we aimed to retrospectively assess patients with FH-RCC for clinical characteristics, treatment outcomes, molecular correlates, and differences between germline and somatic carriers.
Patients and Methods:
Study Population
Patients were retrospectively identified from an institutional database that includes all pathology reports from 1993 to present, and the MSK integrated mutation profiling of actionable cancer targets (MSK-IMPACT) database, which started enrolling patients in 2012, with data cutoff of August 2nd, 2019. Patients with metastatic FH-RCC, genomically defined by presence of FH germline pathogenic or likely pathogenic variant were included. Patients with metastatic RCC without a confirmed germline FH mutation but with a somatic FH mutation and IHC evidence of FH-deficiency [FH loss and/or 2-succino-cysteine (2SC) positive immunoreactivity], were also eligible and included. Electronic medical records were then queried for clinical data. All patients were included in the descriptive population demographics and genomic analyses. Treatment received in other institutions and treatment received as part of ongoing or unpublished clinical trials were excluded from the outcome analyses. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Memorial Sloan Kettering Cancer Center (MSKCC) institutional review board (IRB), including waiver of consent.
Immunohistochemical Analysis
All samples were reviewed by a genitourinary pathologist (Y.B.C.) to confirm diagnosis. Immunohistochemistry for FH and 2-succino-cysteine (2SC) [FH loss and/or 2SC gain] was performed in 5-mm FFPE tissue sections where tissue was available. Immunohistochemistry for FH was performed using a mouse monoclonal antibody (clone J-13, Santa Cruz Biotechnology) as previously described.(21) An absence of FH staining in the neoplastic cells, in the presence of positive internal control (cytoplasmic, granular staining in non-neoplastic cells), was interpreted as lost or FH-deficient status. Immunohistochemical staining for S-(2-succino)-cysteine (2SC) was performed using a polyclonal antibody described previously.(10, 22) Briefly, 4-μm-thick sections from representative formalin-fixed, paraffin-embedded tissue blocks were processed using the Ventana Discovery XT system with antigen retrieval (CC1 solution, 60 min), primary antibody (1:2000), and OptiView DAB IHC detection steps (Ventana). The presence of diffuse, nuclear and cytoplasmic staining was interpreted as positive.
Genomic Analyses
Germline and somatic FH mutations were identified either by matched tumor-normal next-generation sequencing (NGS) using the MSK-IMPACT platform or via other CLIA-approved commercial laboratories. The MSK-IMPACT platform sequences paraffin-embedded tumor and blood from patients and utilizes a capture-based NGS assay to assess 341 cancer-associated genes in the first iteration and 468 in the more recent iteration, as described previously.(23) After alignment to the reference human genome, somatic alterations (missense mutations, small insertions and deletions, structural rearrangements) were identified using a bioinformatics pipeline, as described previously.(23) Tumor purity and allele specific copy number estimates were obtained using the FACETS algorithm.(24) FACETS output was integrated with mutation calls to assign mutation clonality and mutation-specific copy number, including loss-of-heterozygosity (LOH) and amplification. Tumor mutational burden (TMB) and fraction of the genome altered (FGA) in the FH-deficient cohort were compared to an institutional cohort of clear cell RCC (ccRCC) samples. Somatic alterations were annotated using OncoKB (http://oncokb.org), a curated precision oncology knowledge base describing therapeutic implications of individual gene alterations in a tumor type–specific manner.(25) Germline analysis of the FH gene was performed through MSK-IMPACT (n=25) as previously described or at a CLIA-approved laboratory (n=3).(26) Only pathogenic or likely pathogenic variants were considered to be deleterious and are included in this analysis.
Response to Therapy and Clinical Outcomes
Information on systemic therapy treatment was collected from electronic health records (EHR), including type of therapy, line of therapy and dates of administered doses. Patients treated on clinical studies had response assessments according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 guidelines as per the study protocol.(27) All other imaging studies were performed per standard of care at MSKCC, with RECIST 1.1 criteria assessed for all patients by a single genitourinary radiologist (I.N.), blinded to treatment type. Patients were considered to have progression of disease if there was either progression according to RECIST criteria or if they discontinued therapy because of worsening symptoms or decline in performance status (clinical progression).
The primary outcome measure was best objective response rate (ORR). Key secondary outcome measure was disease control rate (DCR), defined as complete response (CR) + partial response (PR) + stable disease (SD) by RECIST v1.1. Other secondary outcome measures included overall survival (OS), progression free survival (PFS), duration of treatment, identification of patterns of metastatic spread and an exploratory analysis on secondary mutations that might confer prognostic or predictive value.
Statistical Analysis
Baseline characteristics and treatment received were summarized descriptively. OS and PFS were calculated using Kaplan Meier estimates from diagnosis of metastatic disease until death or date of progression, respectively. Only patients who received first-line treatment at our institution were included in the PFS analysis. Duration of treatment was calculated using Kaplan Meier estimates from treatment start date to last administered dose of treatment. Patients who were still alive or continuing treatment at the data cutoff (August 2nd, 2019) were censored at that timepoint. Patients enrolled in ongoing clinical trials which have not reported results were excluded from response and survival analyses. Exploratory analyses were performed to assess for factors associated with OS and PFS including treatment type, secondary NF2 mutation status and germline vs. somatic biallelic FH mutation status. TMB and FGA were compared to an institutional cohort of >500 ccRCC samples using a Wilcoxon rank sum test to compare the two groups. Statistical analysis was performed using SAS v9.4 (Cary, NC).
Results:
Patient Characteristics
32 patients (median age 46; range 20–74; M:F, 20:12) with metastatic FH-RCC were identified, with first diagnosis in 2005 (median year of diagnosis, 2014). Patient and tumor characteristics are summarized in Table 1. Within the cohort, 23 patients had a confirmed FH germline mutation while 9 patients had a confirmed FH somatic mutation and FH-deficiency by IHC. Most patients (63%) presented with de novo metastatic disease. For patients who presented with localized disease, median time from nephrectomy to diagnosis of metastatic disease was 9.0 months. 10/12 (83%) of women had a personal history of uterine fibroids. Of the two female patients without fibroids, one had an FH germline mutation, but was 20 years of age at time of analysis, while the other did not have a germline FH mutation. Only 1 patient (germline FH positive) had documented cutaneous leiomyomas prior to RCC diagnosis. 5/23 (22%) of germline FH positive patients had a family history of RCC, 8/23 (35%) had a family history of uterine leiomyomas and 2 (9%) had a family history of skin leiomyomas.
Table 1.
Patient demographics and tumor characteristics. FH: Fumarate Hydratase, IHC: Immunohistochemistry, LOH: Loss of Heterozygosity, 2SC: S-(2-succino)-cysteine, IMDC: International Metastatic RCC Database Consortium.
| All N (%) |
Germline FH N (%) |
Somatic FH N (%) |
Germline not assessed N (%) |
|
|---|---|---|---|---|
| Total Number of Patients | 32 (100) | 23 (72) | 5 (16) | 4 (13) |
|
Age at Diagnosis, Yrs
Median (Range) |
46 (20–74) |
46 (20–73) |
42 (25–57) |
51 (30–74) |
| Sex | ||||
| Male | 20 (63) | 14 (61) | 3 (60) | 3 (75) |
| Female | 12 (37) | 9 (39) | 2 (40) | 1 (25) |
| Female Patients with Uterine Fibroids | 10/12 (83) | 8/9 (89) | 1/2 (50) | 1/1 (100) |
| Pathogenic/likely pathogenic FH variant: | ||||
| FH variant present | 23 (72) | 23 (100) | 0 | - |
| No germline FH variant present | 5 (16) | 0 | 5 (100) | - |
| Germline not assessed | 4 (13) | 0 | 0 | 4 (100) |
| FH Immunohistochemical (IHC) Analysis: | ||||
| FH IHC assessed | 32 (100) | 23 (100) | 5 (100) | 4 (100) |
| FH loss confirmed by IHC | 30 (94) | 22 (96) | 4 (80) | 4 (100) |
| 2SC IHC assessed | 28 (88) | 20 (87) | 4 (80) | 4 (100) |
| 2SC positive confirmed by IHC | 28/28 | 20/20 | 4/4 | 4/4 |
| Race/Ethnicity | ||||
| White | 19 (59) | 13 (57) | 3 (60) | 3 (75) |
| African American/Black | 7 (22) | 6 (26) | 0 | 1 (25) |
| Hispanic | 3 (9) | 2 (9) | 1 (20) | 0 |
| Asian | 1 (3) | 0 | 1 (20) | 0 |
| Other/Unknown/Declined to Answer | 2 (6) | 2 (9) | 0 | 0 |
| Family History of Cancer | 20 (63) | 13 (57) | 4 (80) | 3 (75) |
| RCC | 8 (25) | 5 (22) | 2 (40) | 1 (25) |
| Uterine Leiomyomas | 12 (38) | 8 (35) | 2 (40) | 2 (50) |
| Skin Leiomyomas | 3 (9) | 2 (9) | 0 | 1 (25) |
| Non-HLRCC-related cancer | 19 (59) | 12 (52) | 4 (80) | 3 (75) |
| Stage at RCC Diagnosis | ||||
| I | 3 (9) | 1 (4) | 1 (20) | 1 (25) |
| II | 1 (3) | 1 (4) | 0 | 0 |
| III | 8 (25) | 4 (17) | 2 (40) | 2 (50) |
| IV | 20 (63) | 17 (74) | 2 (40) | 1 (25) |
| Nephrectomy | ||||
| Yes | 26 (81) | 17 (74) | 5 (100) | 4 (100) |
| No | 6 (19) | 6 (26) | 0 | 0 |
| Kidney Primary | ||||
| Left | 19 (59) | 14 (61) | 2 (40) | 3 (75) |
| Right | 13 (41) | 9 (39) | 3 (60) | 1 (25) |
| Kidney Primary Size | ||||
| < 5cm | 8 (25) | 4 (17) | 2 (40) | 2 (50) |
| 5–10cm | 12 (38) | 11 (48) | 0 | 1 (25) |
| > 10cm | 12 (38) | 8 (35) | 3 (60) | 1 (25) |
| IMDC Risk Group | ||||
| Favorable | 3 (9) | 1 (4) | 1 (20) | 1 (25) |
| Intermediate | 26 (81) | 20 (87) | 3 (60) | 3 (75) |
| Poor | 3 (9) | 2 (9) | 1 (20) | 0 |
Twenty-six patients (81%) underwent nephrectomy, with a slight majority (54%) carried out in the metastatic disease/cytoreduction setting, see Supplementary Table S1 for additional surgical details. Involvement of abdominal lymph nodes was the most common site of metastasis at the time of diagnosis of metastatic disease (81%), followed by lung (50%) and thoracic lymph nodes (38%). Liver, bone and adrenal metastases were each seen at diagnosis of metastatic disease in 31% of cases. High rates of intraabdominal spread with peritoneal/omental seeding was seen, with radiographic evidence of disease seen in 69% at last follow up. Similarly, at last follow-up, abdominal lymph node (88%), lung (72%), thoracic lymph node (66%), liver (59%) and bone (53%) were all commonly involved, but no patient had been diagnosed with brain metastasis, Supplementary Table S2.
Histologic and Genomic Results
Median tumor size was 8.0cm (Range, 2.5 – 18.7) with the majority of tumors showing lymphovascular invasion (68%) and renal vein invasion (52%), see Supplementary Table S1. Tumors exhibited high grade nuclear features and frequently a mixture of papillary, tubulocystic, tubulopapillary, cribriform, cystic, and solid growth patterns (Supplementary Figure S1). Twenty-eight patients had FH loss by IHC, 2 patients had either focal or heterogenous FH loss, and 2 patients had retained FH but positive 2SC staining by IHC, Figure 1. In total, 28/32 patients had testing for 2SC, with all 28 of these patients showing positive 2SC staining.
Figure 1.
Oncoprint figure showing patient and tumor characteristics, immunohistochemical analyses, germline and somatic FH alterations, loss of heterozygosity and co-occurring gene alterations.
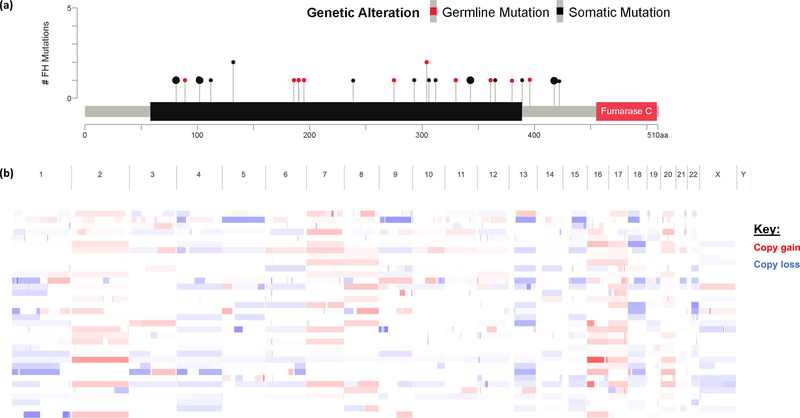
30/32 patients had somatic next generation sequencing (NGS) (Figure 1). Of 28 patients who underwent germline FH analysis, 22 (79%) had a germline pathogenic FH mutation, and 1 patient had a variant of unknown significance (VUS) but with a suspicious family history of cutaneous leiomyomas. LOH/somatic mutation of second allele was confirmed for 17/22 patients with germline FH mutations and the patient with germline VUS. All patients (5/5) who had no germline FH mutations had confirmed somatic FH mutation and LOH in the second allele. The majority of germline and somatic FH mutations (87%) were in the lyase domain (Figure 2a).
Figure 2.
(a) Lollipop plot showing FH somatic and germline alterations and (b) unsupervised clustering of whole genome copy number alteration.
The most common co-occurring alteration was NF2, seen in 5/30 (17%) cases; no VHL mutations were seen (Figure 1). Using OncoKB actionable biomarker definitions, 4 patients had level of evidence 4 oncogenic mutations (PTEN, MTOR, KRAS p.G12D, CDKN2A). No oncogenic mutations with a higher level of evidence were identified. Three patients had more than one tumor or metastatic site sequenced; second FH somatic alteration were early events, and NF2 alterations had a higher variant allele frequency in metastatic sites compared to primary (Supplementary Figure S2). One patient had FANCA and BRCA2 pathogenic germline alterations in addition to their FH germline alteration. No other patients had other actionable or pathogenic germline alterations identified.
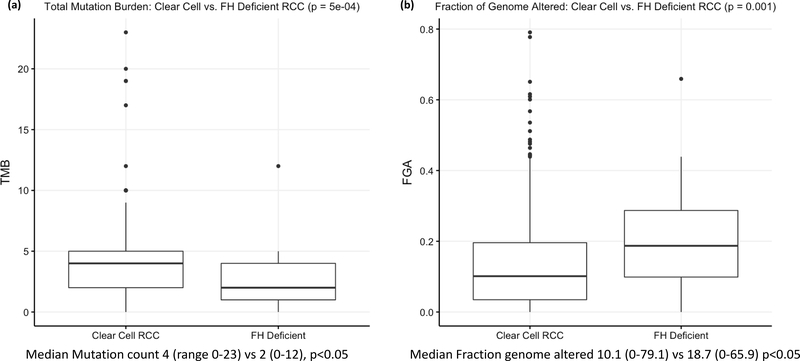
21/32 patients were evaluable for microsatellite instability (MSI), tumor mutation burden (TMB) and fraction of the genome altered (FGA). All patients were microsatellite stable (MSS), with a median MSI score of 0.33 (Range: 0–1.56). Compared to an institutional cohort of >500 patients with clear cell RCC, FH-deficient tumors had a lower mutation count (median 2 vs 4, p=0.0005) but a higher fraction of the genome altered (18.7 vs 10.1%; p=0.001), Figure 3. Broad copy number alterations were reviewed with copy number loss seen in Chromosome 1, where FH is located, but also in in chromosomes 4 and 13, while copy number gain was seen in chromosomes 2, 7, 8, 16, and 17 (Figure 2b), similar to what has previously been reported elsewhere.(12)
Figure 3.
Boxplots displaying (a) tumor mutational burden and (b) fraction of genome altered compared to an institutional cohort of ccRCC.
Treatment Response
46 treatment lines from 26 patients were evaluable for response by RECIST v1.1, Table 2. Patients received a median of 2 treatment lines (Range: 1–5), Supplementary Tables S3 & S4. Combined ORR to first (n=26), second (n=14) and third-line (n=6) therapy was 38.5%, 7% and 17%, respectively, with no complete responses seen. Combined DCR to first, second and third-line therapy was 65%, 50% and 50%, respectively. Combination therapy targeting both mTOR and VEGF was the most common treatment (Total, N=18; bevacizumab/everolimus, N=16; lenvatinib/everolimus, N=2) and showed the highest ORR (44%) and DCR (77%). VEGF monotherapy (N=15, ORR 20%, DCR 53%), checkpoint inhibitor monotherapy (N=8, ORR 0%, DCR 38%) and mTOR monotherapy (N=4, ORR 0%, DCR 25%) had lower response rates. Although numbers were small, there were no differences in DCR by FH status and treatment type, see Supplementary Table S5.
Table 2.
Best overall response by RECIST 1.1 criteria in evaluable patients (26/32), median duration of treatment and Disease Control Rate (DCR) by line of therapy. DCR, disease control rate = complete response + partial response + stable disease; mTOR, mammalian target of rapamycin inhibitor; VEGF, vascular endothelial growth factor receptor inhibitor; IO, Checkpoint inhibitor therapy.
| Overall Response | Disease Control Rate (DCR) by Line of therapy | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Partial or Complete Response (N, %) | Stable Disease (N, %) | Progressive Disease (N, %) | Duration of Treatment (Median, mo) | 1st line, DCR N/total | 2nd line, DCR N/total | 3rd line, DCR N/total | |
| Combination mTOR/VEGF | 18 | 8 (44%) | 6 (33%) | 4 (22%) | 8.4 | 12/15 | 1/2 | 1/1 |
| bevacizumab + everolimus | 16 | 7 (44%) | 5 (31%) | 4 (25%) | 9.3 | 12/15 | 0/1 | - |
| lenvatinib + everolimus | 2 | 1 (50%) | 1 (50%) | 0 (0%) | 2.0 | - | 1/1 | 1/1 |
| VEGF Inhibition | 15 | 3 (20%) | 5 (33%) | 7 (47%) | 5.5 | 3/5 | 5/8 | 0/2 |
| cabozantinib | 5 | 0 (0%) | 2 (40%) | 3 (60%) | 2.2 | 0/1 | 2/3 | 0/1 |
| sunitinib | 4 | 1 (25%) | 2 (50%) | 1 (25%) | 5.6 | - | ¾ | - |
| pazopanib | 3 | 1 (33%) | 1 (33%) | 1 (33%) | 8.1 | 2/3 | - | - |
| axitinib | 2 | 0 (0%) | 0 (0%) | 2 (100%) | 2.5 | - | 0/1 | 0/1 |
| bevacizumab + sunitinib | 1 | 1 (100%) | 0 (0%) | 0 (0%) | 6.5 | 1/1 | - | - |
| Checkpoint Inhibitor Therapy | 8 | 0 (0%) | 3 (38%) | 5 (62%) | 2.1 | 0/2 | 1/3 | 1/3 |
| ipilimumab + nivolumab | 2 | 0 (0%) | 0 (0%) | 2 (100%) | 3.5 | 0/2 | - | - |
| atezolizumab + investigational anti-CD27 agent | 2 | 0 (0%) | 1 (50%) | 1 (50%) | 4.2 | - | 1/2 | - |
| nivolumab | 4 | 0 (0%) | 2 (50%) | 2 (50%) | 1.7 | - | 0/1 | 1/3 |
| mTOR Monotherapy | 4 | 0 (0%) | 1 (25%) | 3 (75%) | 2.3 | 1/3 | 0/1 | - |
| everolimus | 3 | 0 (0%) | 1 (33%) | 2 (67%) | 3.8 | 1/2 | 0/1 | - |
| temsirolimus | 1 | 0 (0%) | 0 (0%) | 1 (100%) | 0.3 | 0/1 | - | - |
| Combination IO/ VEGF | 1 | 1 (100%) | 0 (0%) | 0 (0%) | 25.5 | 1/1 | - | - |
| lenvatinib + pembrolizumab | 1 | 1 (100%) | 0 (0%) | 0 (0%) | 25.5 | 1/1 | - | - |
Median duration on treatment was longest for the mTOR/VEGF combination (bevacizumab/everolimus, N=16; lenvatinib/everolimus, N=2) at 8.4 months, followed by VEGF monotherapy (5.5 months), mTOR monotherapy (2.3 months) and checkpoint inhibitor therapy (2.1 months) [Table 2]. One patient receiving mTOR/VEGF combination therapy in the first-line setting was continuing mTOR monotherapy with a PR at the time of study cut off (35.1 months); the VEGF inhibitor was discontinued due to proteinuria. One patient was evaluable for VEGF/IO combination therapy (lenvatinib/pembrolizumab) in the first-line setting, achieving a PR and remaining on treatment for a 25-month period.(28)
8 patients who received checkpoint inhibitor therapy were evaluable for treatment response. 2 patients received ipilimumab/nivolumab as first-line treatment, 4 patients received single agent nivolumab (1 second-line, 3 in third-line setting), and 2 patients received atezolizumab in combination with an investigational anti-CD27 monoclonal antibody in the second-line setting as part of a clinical trial (NCT02543645). Median duration of therapy for checkpoint inhibitors (N=8) was 2.1 months. 3 patients (37.5%) had stable disease and 5 (62.5%) had progressive disease; no responses were seen.
Survival Outcomes
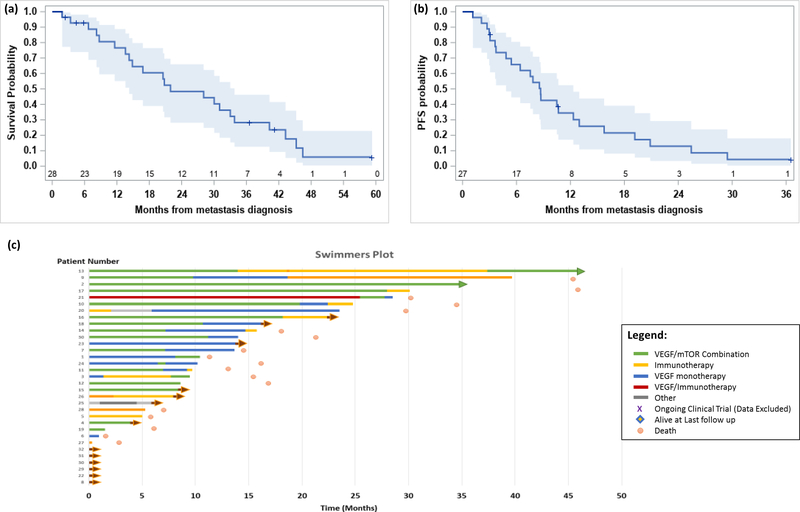
27 patients (84%) were evaluable for progression free survival (PFS) in the first-line setting; 24 progressed or died and 3 were censored at last follow-up. Median PFS was 8.7 months (95% CI: 4.8, 12.3) from time of diagnosis of metastasis (Figure 4). Six and 12-month progression-free survival rates are 65.8% (44.5, 80.6) and 34.4% (17.1, 52.5). Median PFS for the mTOR/VEGF combination (10.7 months) was longer than VEGF monotherapy (7.8 months), mTOR monotherapy (6.4 months) or checkpoint inhibition with ipilimumab/nivolumab (4.5 months).
Figure 4.
(a) Kaplan-Meier curve showing overall survival probability and 95% confidence intervals, (b) Kaplan-Meier curve showing progression free survival probability and 95% confidence intervals, (c) Swimmers plot showing duration of treatment.
28 patients (88%) were included in the overall survival (OS) analysis, 22 died and 6 were censored at last follow-up. Median OS, from diagnosis of metastatic disease, for the entire cohort was 21.9 months (95% CI: 14.3, 33.8) [Figure 4]. Twelve and 24-month survival rates are 76.6% (95% CI: 55.1, 88.8) and 48.4% (95% CI: 28.1, 66.0). Median follow-up time for survivors is 21.1 months (range: 2.3, 59.2). 1 patient remained on first-line therapy at the time of data cut off.
Median OS for evaluable patients with a confirmed germline FH mutation (N=21) was 28.1 months. Median OS for evaluable patients with confirmed biallelic somatic FH mutation, i.e. germline tested but no germline FH mutation identified, (N=3) was 13.6 months. Median OS for patients with a co-occurring NF2 mutation (N=4; Median OS: 23.9mo; 95% CI: 6.7–Not Estimable) was numerically shorter than for those without an NF2 mutation (N=19; Median OS: 28.1mo; 95% CI: 13.6–40.3) however this was not statistically significant. Median OS by first line treatment class was longest with the mTOR/VEGF combination (N=13; Median OS: 33.0mo; 95% CI: 14.3–46.5), which was longer than checkpoint inhibitors (N=2; Median OS: 30.0mo), VEGF monotherapy (N=6; Median OS: 13.2mo) and mTOR monotherapy (N=3; Median OS: 8.2mo).
Discussion:
FH-deficient RCC is an aggressive disease, and to date, a limited number of studies have described its genomic characteristics, and the response rate to systemic therapies used for RCC is unknown. Furthermore, almost all studies have described FH-RCC in the context of known germline HLRCC syndrome, however, the prevalence of non-germline (biallelic somatic FH loss) FH-deficient RCC, and whether these are genomically or clinically similar to HLRCC has not been explored.(11, 29, 30) Through comprehensive germline and somatic next-generation sequencing we show that a significant portion of FH-RCC is due to somatic “double hits” without germline mutations. In each of these cases, a somatic FH mutation and loss of heterozygosity in the second allele was identified, further strengthening the evidence for the two-hit hypothesis in these patients. Overall, tumors across each genomic subgroup were indistinguishable histologically based on morphology and IHC, while patients across each genomic subgroup showed broadly similar baseline characteristics, sites and patterns of metastatic disease, and response to therapy. This information is essential given its potential relevance for clinical and therapeutic consideration and its importance for genetic counselling.
HLRCC is thought to be rare, however several recent studies suggest it may be more prevalent than previously thought. Using genomic population databases, Shuch et al. estimated incidence of 1 in 1000 individuals.(31) Of those with HLRCC who develop RCC, our group and others have shown that when broad IHC or genomic testing is used, many cases of previously unsuspected non-clear cell RCC are found to have germline FH mutations.(11, 32)
This is, to our knowledge, the first study to assess response to therapy in FH-deficient RCC, regardless of germline or somatic nature of the FH mutation. In our cohort, the most commonly used treatment was combination mTOR/VEGF, with the majority of patients treated with bevacizumab and everolimus (n=16/18). This combination showed a promising ORR of 44% and PFS of 8.4 months. Ten of these patients were treated on a previously reported clinical trial, in which an ORR of 35% overall (unclassified RCC with papillary features, ORR 43%; papillary RCC, ORR 23%), was seen.(33, 34) Our results are consistent with these previously reported ORR results for unclassified RCC with papillary features. In a separate preliminary report of a single institution phase II clinical trial, involving 43 HLRCC patients, the combination of bevacizumab and the EGFR inhibitor erlotinib showed a ORR of 72.1% and a median PFS of 21.1 months.(6) While this study was in a highly selected population and most patients were treated in the first-line setting, the results are very promising. The mechanism by which FH-deficient RCC responds to bevacizumab and mTOR/EGFR targeted therapy still needs further elucidation. FH deficiency leads to fumarate accumulation and stabilization of the HIF-1alpha complex, as well as disruption of the TCA cycle with a shift to glycolysis. It may be that FH loss results in changes to angiogenesis and metabolism pathways that are targeted by bevacizumab and everolimus/erlotinib, respectively.(11, 15) In conglomerate, these two trials suggest a potential role for bevacizumab in combination with mTOR or EGFR targeted therapy as showing antitumor activity in this patient population, and the NCCN guidelines recommend both regimens for use in certain circumstances in non-clear cell RCC.
While participation in clinical trials is the preferred approach for patients with nccRCC, including FH-deficient RCC, clinical trials are not available for many patients and determining the efficacy of standard RCC therapies in FH-RCC is vital to provide timely therapeutic approaches for patients. In our cohort, VEGF monotherapy (N=15) had clinical activity with an ORR 20% and DCR 53%, however this analysis is limited given that these patients were treated with a range of agents with differing targets, including sunitinib, pazopanib, cabozantinib, and axitinib, and most patients received VEGF therapy in the second or later line setting. More importantly, there were no responses seen to checkpoint inhibitor therapy (N=8, ORR 0%, DCR 38%) and the PD rate was 62.5%. mTOR monotherapy (N=4, ORR 0%, DCR 25%, PD rate 75%) did not show benefit in our cohort. The small sample size and treatment in later lines of therapy (predominantly second and third line, see Supplementary Table S3) may have contributed to these findings, however the results are not promising. Response to VEGF/IO combination therapy was evaluable in only 1 patient; they achieved a partial response, remaining on treatment for a 25-month period. Similarly, 2 patients received lenvatinib+everolimus, with 1 with partial response and 1 with stable disease. Although very small numbers, given that both VEGF/mTOR inhibitors and VEGF/IO combinations are now FDA-approved in RCC and available to patients, these combinations merit consideration and study in FH-deficient RCC
To identify potential somatic alterations which would serve as biomarkers of response to therapies, we used OncoKB, a database which assigns levels of evidence on therapeutic actionability to individual genes. We only found a handful of cases with alterations of Level 4, the lowest level, indicating compelling biological evidence, but no alterations with clinical or standard care evidence. One promising potential line of therapy includes poly(ADP)-ribose polymerase (PARP) inhibitors, given evidence that fumarate suppresses the homologous recombination (HR) DNA-repair pathway.(35, 36) Clinical trials with PARP-inhibitors in this disease are ongoing [NCT04068831]. No patients had elevated microsatellite instability or tumor mutation burden to predict for checkpoint inhibitor response.(37) Tumor mutation burden was lower than an institutional cohort of >500 ccRCC patients (median 2 vs 4, p=0.0005) however, interestingly we noted a higher fraction of the genome altered (18.7 vs 10.1%; p=0.001), Figure 3. Broad copy number alterations were reviewed with copy number loss seen in Chromosome 1, where FH is located, but also in in chromosomes 4, 13, 15, 18 & 22, while copy number gain was seen in chromosomes 2, 7, 8, 16, 17 & 20 (Figure 4b). These are broadly similar patterns to those described for type II papillary RCC in the comprehensive molecular characterization of papillary RCC paper published by the cancer genome atlas (TCGA) group, further strengthening our findings, although further research is required to determine the impact of these findings.(38)
The most commonly co-occurring mutation was in NF2, which in previous studies of unclassified RCC has been associated with worse prognosis.(29) Here, NF2 was not associated with a worse prognosis although numbers were limited. NF2 encodes a key regulator of the Hippo signaling pathway, which controls cell proliferation, and loss of NF2 results in aberrant YAP1 activation.(39) Preclinical studies in NF2-deficient RCC models have shown that targeting YAP1 results in reduced tumor growth.(40, 41) Given the prevalence of NF2 somatic mutations in our FH-deficient RCC cohort, targeting of the Hippo pathway could be considered in FH-deficient RCC models.
This study has several limitations. Although 39% of treatments were administered in the context of clinical trials (Supplementary Table S3), the majority of patients received off protocol care. While this would reflect real-world outcomes, the lack of randomization, blinding or other prospective assessment might confound our findings, although the RECIST assessments were all performed by a single radiologist, blinded to treatment type, in order to limit the potential for any bias as a confounder to our results. Additionally, given the small numbers, we analyzed all VEGF TKIs together in this study, including novel multi-targeted kinases such as cabozantinib, even though they may have differing effects.
In conclusion, these findings show that FH-RCC is an aggressive form of RCC, which can occur as part of the HLRCC syndrome or sporadically. Sporadic cases, with biallelic somatic FH loss occurred in 16% of our cohort, and exhibited a similar clinical course to those with germline FH alterations. The entire FH-RCC population has a relatively specific disease course with distinct patterns of metastasis from ccRCC and high rates of locoregional spread. Responses to systemic therapy were highest with VEGF/mTOR combination and VEGF monotherapy while no responses were seen to checkpoint inhibitor therapy. Further studies are required to help guide treatment selection and uncover potential novel therapies.
Supplementary Material
Supplementary Figure S1. Representative histologic images of FH-deficient RCC. (a) Tubular and cystic elements in a case harboring germline FH mutation (FH07). (b) Predominantly papillary architecture in a case with FH somatic mutation (FH22).
Supplementary Figure S2. Representative tumor evolution figure from patients with multiple tumor samples, with variant allele frequency (VAF) heatmap.
Statement of Translational Relevance.
Fumarate Hydratase (FH) deficiency predisposes to an aggressive form of RCC, as loss-of-function mutations of FH results in a complex pro-oncogenic state resulting from the accumulation of fumarate and 2-succino-cysteine (2SC). Patients with FH-deficient RCC develop early onset, aggressive disease and evidence for systemic therapy options is generally extrapolated from other RCC subtypes. An improved understanding of the underlying genomics of this unique disease and correlation with systemic therapy outcomes is essential to advance the therapeutic options in this cohort. Here, we explore the genomic changes in FH-deficient RCC, we identified 16% of patients with biallelic somatic loss of FH and show that in our population the best responses were observed with the VEGF/mTOR combination therapy, while no responses were seen to immunotherapy.
Acknowledgments:
Memorial Sloan Kettering Cancer Center receives research funding through the Core Grant (P30 CA008748) as part of MSK’s Cancer Center Support Grant (CCSG) which is awarded by the National Cancer Institute (NCI). Maria I. Carlo is also supported by the Harold Amos Faculty Development Award.
Abbreviations:
- FH
fumarate hydratase
- RCC
renal cell carcinoma
- FH-RCC
fumarate hydratase-deficient renal cell carcinoma
- HLRCC
hereditary leiomyomatosis and RCC syndrome
- NGS
next-generation sequencing
- MSK
Memorial Sloan Kettering
- MSK-IMPACT
MSK integrated mutation profiling of actionable cancer targets
- ORR
overall response rate
- RECIST
response evaluation criteria in solid tumors
- mTOR
mammalian target of rapamycin inhibitor
- VEGF
vascular endothelial growth factor inhibitor
- HIF
hypoxia inducible factor
- IHC
immunohistochemistry
- TMB
tumor mutational burden
Footnotes
Conflict of Interest Disclosure Statement: The authors declare no potential conflicts of interest.
References:
- 1.Launonen V, Vierimaa O, Kiuru M, Isola J, Roth S, Pukkala E, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98(6):3387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehtonen HJ, Kiuru M, Ylisaukko-Oja SK, Salovaara R, Herva R, Koivisto PA, et al. Increased risk of cancer in patients with fumarate hydratase germline mutation. J Med Genet 2006;43(6):523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomlinson IPM, Alam NA, Rowan AJ, Barclay E, Jaeger EEM, Kelsell D, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet 2002;30(4):406–10. [DOI] [PubMed] [Google Scholar]
- 4.Menko FH, Maher ER, Schmidt LS, Middelton LA, Aittomäki K, Tomlinson I, et al. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13(4):637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhola PT, Gilpin C, Smith A, Graham GE. A retrospective review of 48 individuals, including 12 families, molecularly diagnosed with hereditary leiomyomatosis and renal cell cancer (HLRCC). Fam Cancer. 2018;17(4):615–20. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan R, Gurram S, Al Harthy M, Singer EA, Sidana A, Shuch BM, et al. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. Journal of Clinical Oncology. 2020;38(15_suppl):5004-. [Google Scholar]
- 7.Ohe C, Smith SC, Sirohi D, Divatia M, de Peralta-Venturina M, Paner GP, et al. Reappraisal of Morphologic Differences Between Renal Medullary Carcinoma, Collecting Duct Carcinoma, and Fumarate Hydratase-deficient Renal Cell Carcinoma. Am J Surg Pathol 2018;42(3):279–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016;70(1):93–105. [DOI] [PubMed] [Google Scholar]
- 9.Merino MJ, Torres-Cabala C, Pinto P, Linehan WM. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol 2007;31(10):1578–85. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y-B, Brannon AR, Toubaji A, Dudas ME, Won HH, Al-Ahmadie HA, et al. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am J Surg Pathol 2014;38(5):627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trpkov K, Hes O, Agaimy A, Bonert M, Martinek P, Magi-Galluzzi C, et al. Fumarate Hydratase-deficient Renal Cell Carcinoma Is Strongly Correlated With Fumarate Hydratase Mutation and Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome. Am J Surg Pathol 2016;40(7):865–75. [DOI] [PubMed] [Google Scholar]
- 12.Pivovarcikova K, Martinek P, Grossmann P, Trpkov K, Alaghehbandan R, Magi-Galluzzi C, et al. Fumarate hydratase deficient renal cell carcinoma: Chromosomal numerical aberration analysis of 12 cases. Ann Diagn Pathol 2019;39:63–8. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt C, Sciacovelli M, Frezza C. Fumarate hydratase in cancer: A multifaceted tumour suppressor. Seminars in cell & developmental biology. 2020;98:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Soga T, Pollard P, Adam J. The emerging role of fumarate as an oncometabolite. 2012;2(85). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linehan WM, Rouault TA. Molecular pathways: Fumarate hydratase-deficient kidney cancer--targeting the Warburg effect in cancer. Clin Cancer Res 2013;19(13):3345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2015;373(19):1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015;16(15):1473–82. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2018;378(14):1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2019;380(12):1116–27. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2019;380(12):1103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SC, Trpkov K, Chen YB, Mehra R, Sirohi D, Ohe C, et al. Tubulocystic Carcinoma of the Kidney With Poorly Differentiated Foci: A Frequent Morphologic Pattern of Fumarate Hydratase-deficient Renal Cell Carcinoma. The American journal of surgical pathology. 2016;40(11):1457–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardella C, El-Bahrawy M, Frizzell N, Adam J, Ternette N, Hatipoglu E, et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. The Journal of pathology. 2011;225(1):4–11. [DOI] [PubMed] [Google Scholar]
- 23.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD. 2015;17(3):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen R, Seshan VE. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic acids research. 2016;44(16):e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandelker D, Zhang L, Kemel Y, Stadler ZK, Joseph V, Zehir A, et al. Mutation Detection in Patients With Advanced Cancer by Universal Sequencing of Cancer-Related Genes in Tumor and Normal DNA vs Guideline-Based Germline Testing. Jama. 2017;318(9):825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer (Oxford, England : 1990). 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 28.Taylor MH, Lee CH, Makker V, Rasco D, Dutcus CE, Wu J, et al. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020;38(11):1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YB, Xu J, Skanderup AJ, Dong Y, Brannon AR, Wang L, et al. Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nature communications. 2016;7:13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayley JP, Launonen V, Tomlinson IP. The FH mutation database: an online database of fumarate hydratase mutations involved in the MCUL (HLRCC) tumor syndrome and congenital fumarase deficiency. BMC Med Genet 2008;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shuch B, Li S, Risch H, Bindra RS, McGillivray PD, Gerstein M. Estimation of the carrier frequency of fumarate hydratase alterations and implications for kidney cancer risk in hereditary leiomyomatosis and renal cancer. Cancer. 2020;126(16):3657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlo MI, Hakimi AA, Stewart GD, Bratslavsky G, Brugarolas J, Chen YB, et al. Familial Kidney Cancer: Implications of New Syndromes and Molecular Insights. Eur Urol 2019;76(6):754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voss MH, Molina AM, Chen Y-B, Woo KM, Chaim JL, Coskey DT, et al. Phase II Trial and Correlative Genomic Analysis of Everolimus Plus Bevacizumab in Advanced Non-Clear Cell Renal Cell Carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(32):3846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldman DR, Ged Y, Lee C-H, Knezevic A, Molina AM, Chen Y-B, et al. Everolimus plus bevacizumab is an effective first-line treatment for patients with advanced papillary variant renal cell carcinoma: Final results from a phase II trial. Cancer. 2020;n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sulkowski PL, Sundaram RK, Oeck S, Corso CD, Liu Y, Noorbakhsh S, et al. Krebs-cycle-deficient hereditary cancer syndromes are defined by defects in homologous-recombination DNA repair. Nat Genet 2018;50(8):1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulkowski PL, Oeck S, Dow J, Economos NG, Mirfakhraie L, Liu Y, et al. Oncometabolites suppress DNA repair by disrupting local chromatin signalling. Nature. 2020;582(7813):586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020;38(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. New England Journal of Medicine. 2015;374(2):135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calses PC, Crawford JJ, Lill JR, Dey A. Hippo Pathway in Cancer: Aberrant Regulation and Therapeutic Opportunities. Trends Cancer. 2019;5(5):297–307. [DOI] [PubMed] [Google Scholar]
- 40.Sourbier C, Liao PJ, Ricketts CJ, Wei D, Yang Y, Baranes SM, et al. Targeting loss of the Hippo signaling pathway in NF2-deficient papillary kidney cancers. Oncotarget 2018;9(12):10723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White SM, Avantaggiati ML, Nemazanyy I, Di Poto C, Yang Y, Pende M, et al. YAP/TAZ Inhibition Induces Metabolic and Signaling Rewiring Resulting in Targetable Vulnerabilities in NF2-Deficient Tumor Cells. Dev Cell. 2019;49(3):425–43.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Representative histologic images of FH-deficient RCC. (a) Tubular and cystic elements in a case harboring germline FH mutation (FH07). (b) Predominantly papillary architecture in a case with FH somatic mutation (FH22).
Supplementary Figure S2. Representative tumor evolution figure from patients with multiple tumor samples, with variant allele frequency (VAF) heatmap.