Abstract
Light is a uniquely powerful tool for controlling molecular events in biology. No other external input (e.g., heat, ultrasound, magnetic field) can be so tightly focused or so highly regulated as a clinical laser. Drug delivery vehicles that can be photonically activated have been developed across many platforms, from the simplest “caging” of therapeutics in a prodrug form, to more complex micelles and circulating liposomes that improve drug uptake and efficacy, to large-scale hydrogel platforms that can be used to protect and deliver macromolecular agents including full-length proteins. In this Review, we discuss recent innovations in photosensitive drug delivery and highlight future opportunities to engineer and exploit such light-responsive technologies in the clinical setting.
Keywords: Photochemistry, photoactivated, light-mediated delivery, in vivo drug delivery, photodegradable hydrogels, photoresponsive nanoparticles, prodrugs, dual-action, photoswitches
Graphical Abstract
1. Introduction
Engineering photoresponsive behavior into drug delivery vehicles has progressed remarkably since the first literature expounded on the benefits of light-based therapies. Early treatments of lupus vulgaris exploited ultraviolet (UV) light, a therapy option that in 1903 won Niels Ryberg Finsen one of the first awarded Nobel Prizes in Medicine [1]. Since this discovery, light has found consistent use in the delivery and activation of drugs in the body [2]. Despite challenges associated with tissue opacity, light’s surprising tenacity over decades of drug delivery advancements can be attributed to the uniquely enabling spatiotemporal control that it affords (Figure 1a).
Figure 1.
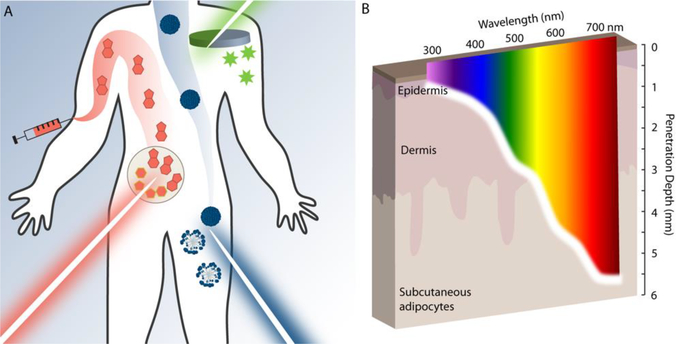
A. methods for incorporation of photoresponse in drug delivery. Small-molecule prodrugs (red), nanostructures (blue), and hydrogel biomaterials (green) have all been modified with photoactivators to deliver drug cargo. B. Differently colored light affords different penetration into skin. Image depicts the maximum depth into human skin that >1% of incident photons will reach as a function of wavelength.
The first and primary challenge facing the clinical application of photoresponsive drug delivery vehicles is the poor penetration depth of low-energy electromagnetic radiation. While X-rays or radio waves may pass through the body relatively easily, visible, UV, and infrared (IR) light experience variable and high levels of absorption/scattering by living tissue. As such, the greatest depth of penetration is achieved by low-energy IR light, with 750 nm light penetrating ~5 mm below the skin’s surface (Figure 1b). Due to high amounts of light scattering as well as high absorption of hemoglobin and melanin, higher-energy light fares much worse with 350 nm UV light penetrating less than a millimeter before complete attenuation [3,4]. This increased attenuation of high-energy over low-energy light provides for the optical window for in vivo photoregulation, the range of most effectively penetrating wavelengths in the visible-IR regions, generally accepted to be between 650 – 900 nm [5,6].
Another consideration when using light to trigger biological events is the energy flux through tissue to the location desired. Though most organic and inorganic chromophores have molar absorptivities (104-105 M−1 cm−1 at 500 nm) that exceed those of biological chromophores such as human rhodopsin (~104 M−1 cm−1 at 500 nm), the comparatively high concentration of the latter can necessitate fairly large light dosages to phototrigger engineered material changes. Since quantum yields are often lower than desired for many traditional photochemical reactions, the flux of light required may near the range of thermal tissue damage, especially in the use of high-energy light. For more excellent commentary on photothermal tissue damage, we direct the reader to this excellent review [1].
While challenges persist in its clinical application, light is unique among all other endogenous and exogenous triggers in important respects: (1) Light as electromagnetic radiation occurs in a wide range of efficacious and relatively safe energies – nominally UV, visible, and IR light – that can be used to achieve molecular changes to activate and/or release drugs in a fully cytocompatible manner [7]. These energies can be accessed selectively using different wavelengths of light, allowing researchers to potentially trigger multiple events separately and/or sequentially, compounding the efficacy of drug therapies. (2) Light can be highly focused to yield localized response, permitting precise spatiotemporal control over therapeutic release. Light-responsive drug constructs can be dispersed throughout the body but activated only within the targeted tissue or organ using a variety of readily available light sources (Box 1).
Box 1. Light sources for laboratory light delivery.
There are several options for expanding in-house access to light sources at a wide variety of wavelengths. The most readily available are listed below with their wavelength ranges.
| Mercury Arc lamps | 250–600 nm | Broad-spectra mercury-arc lamps are available from several suppliers (e.g., Excelitas) whose output can be refined to desired wavelengths with bandpass filters (typically to capture major outputs at 254, 365, 405, 436, 546, and/or 579 nm). These light sources are comparatively inexpensive and high powered. |
| LEDs | 250–700 nm | A wide variety of wavelengths and intensities are available from several manufacturers. ThorLabs offers multiwavelength arrays of LEDs for use in microscopy that can be easily adapted for light delivery on the benchtop. One can also purchase individual LEDs from companies (e.g., LED Engin) for user control over wavelength and power. While LEDs offer changeable intensity, their emission can be broad, with the full-width half maximum (FWHM) value extending ±10–20 nm beyond the reported wavelength. |
| Lasers | 250–900 nm | Laser pointers may be repurposed for laboratory research, and are often desirable for their highly focused beam and narrow bandwidth; lasers are often centered within ±1 nm of the reported wavelength. Zbolt offers a selection of high powered, constant-on laser pointers that are useful for benchtop experiments and the irradiation of a small area. Though highly focused, laser pointers cannot change intensity, so experiments testing variable light intensities are impossible. |
Owing to these intrinsic features, light has persisted as one of the most popular external triggers for therapeutic application to date. In this regard, photomediated reactive oxygen generation, small-molecule activation, micelle disruption, and material degradation has dominated the literature for the past several decades [8–12]. Photodynamic therapy (PDT), where small molecules are designed to generate reactive oxygen species (ROSs) upon irradiation, is well represented in the literature [13,14]. The entry of several PDT drugs into clinical trials and FDA approval [15] has led to innovations in light application devices in the clinic, enabling light to be delivered to any region in the body with varying levels of invasiveness [1]. “Caging” small molecules by blocking their activity until irradiation liberates the bioactive molecule has prompted the elucidation of new neuronal circuits and advancements in epigenetics [16,17].
Embedding light-responsive nanoparticles or highly conjugated organic molecules in micelles and liposomes has rendered these popular nanostructures photosensitive, combining their potential for solubilizing hydrophobic drugs with the possibility of targeting drug delivery. Other advances using light to create and/or anisotropically modify biologic-presenting materials has launched an entirely new field of spatiotemporally governing cell fate, as scientists work towards the ultimate goal of growing organoids and tissues on the benchtop. Biomaterials spanning several orders of magnitude in size have been used to protect sensitive protein cargo, in an age when fully folded proteins are poised as the next generation of therapeutics.
This Review surveys common approaches within photomediated drug delivery, highlighting recent reports and innovative applications using systems across a wide range of size scales including small molecules, nanostructures, and hydrogels. Recurring chemical moieties that render material photoresponsiveness will be discussed, including new ruthenium-based small-molecule photosensitizers and innovations in the use of ortho-nitrobenzyl (oNB) groups, inorganic nanoparticles, and conjugated organic porphyrins (Table 1). We offer our near- and long-term vision of the field’s future, identifying technical innovations that will need to be developed along the way so as to maximize utility and clinical translation.
Table 1.
Common Photochemical Reactions for Targeted Drug Delivery
2. Circulating Drug Carriers: Micelles, Liposomes, and Small-Molecule Prodrugs
The primary motivation for the design of photoregulatable circulating drug carriers is the minimization of off-targeting effects; ubiquitous delivery through the circulatory system but local activation with light affords precise control over therapeutic function. This benefit is particularly attractive in cancer treatment, where chemotherapeutics are cytotoxic by design and likely to harm healthy tissue if misdelivered. In this regard, several photosensitizers for PDT have reached the clinic, with photoactivatable biomolecules following closely behind. Photocaged species have been used extensively in vitro, where achieving light penetration is less of an issue, and selectively in vivo, particularly in small animal models including zebrafish embryos. Abundant literature is available on the use of large, photoresponsive caging groups for neurotransmitters that have helped drive a revolution in neurobiology and the study of neuronal signaling on the benchtop [17,35,36].
“Prodrugs” have also been widely explored for localized therapeutic regulation. Such small molecules have little to no activity until they encounter a specific stimulus (e.g., light), gaining activity through a conformational change or generation of other reactive species. Of these, the generation of ROS is the most common mode of action and is particularly effective when cell destruction is desired. Many metal-based chemotherapeutics utilize light to yield a structural change, revealing species that can bind to DNA and trigger apoptosis [37,38].
Other circulating, light-responsive designs include the incorporation of photoactivators in micellar and liposomal structures leading to their triggerable disruption and associated cargo release. These nanostructures are often useful at solubilizing hydrophobic drugs, taking advantage of current photoresponsive technologies both organic and inorganic in nature, and maintaining appreciable therapeutic levels in the body while avoiding rapid clearance by the kidneys. Micelles and liposomes incorporating hydrophilic or hydrophobic photoactivators to create or destroy structure upon light irradiation have been reported, some of which have successfully utilized in mouse models [39,40]. These in vivo studies demonstrate long circulation times on the order of 2–4 hours and accumulation in the liver and kidneys, but with little off-target activation or side effects.
In this section, we will discuss past and present efforts in photoregulating circulating carrier activity, highlighting unique examples and pointing the reader to other references as appropriate (Figure 2).
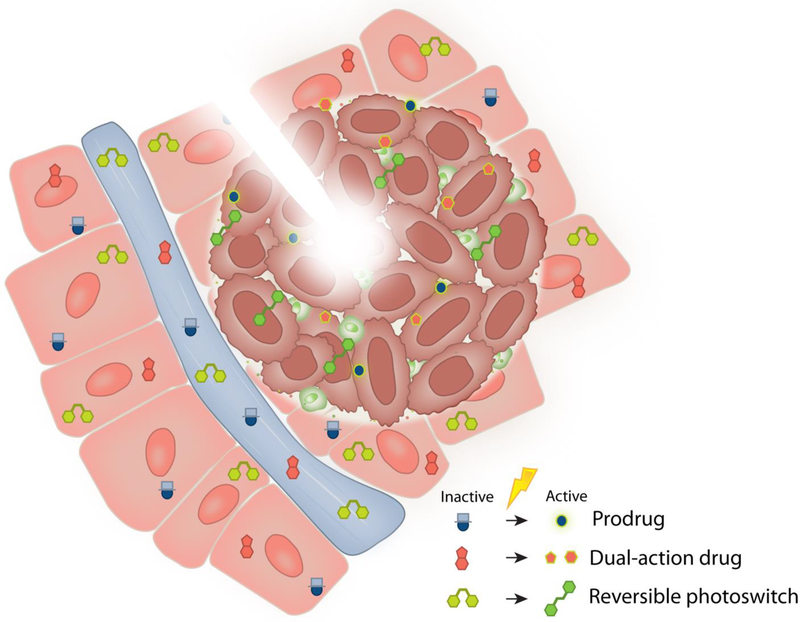
Figure 2.
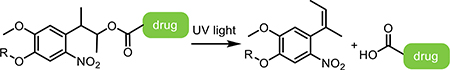
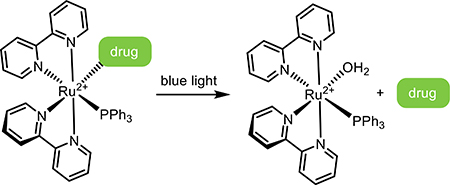
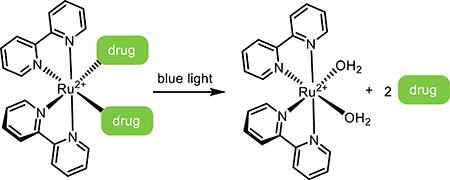
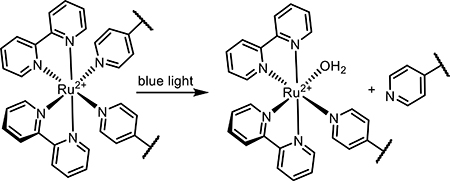
Light-responsive small molecules have distinct benefits in terms of ease of dosing, wide circulation, as well as spatiotemporally targeted activation. Light-activated designs rely on the circulatory system for delivery into local tissue, where the active form is revealed through targeted irradiation. Common strategies exploit prodrugs, dual-action drugs, and reversible photoswitches.
2.1. Small-Molecule Prodrugs
Prodrugs, or caged small molecules, have brought innovation to several areas of drug delivery, from targeted chemotherapeutics to directed gene expression. These small molecules can be introduced to cells in culture by inclusion in the media, or by injection into various animal models. Frequently these designs are not targeted to a specific cell type, but see uptake in all cells, followed by spatially specific activation or uncaging. Here we will discuss various molecular designs and their applications in recent literature, from caging neurotransmitters to targeted gene expression profiles.
2.1.1. Photocaged Small Molecules for In Vitro Discovery
Controlled in vitro drug delivery or activation has led to an increased understanding of neuronal systems and intercellular communication. Triggering a physical change in a small molecule to reveal an active form with high spatiotemporal control is one of the most straightforward applications of light-based technologies. For over 200 years, the study of ion channels and neurophysiology has been facilitated by application of electrodes to cultured neuronal tissue slices. With the advent of light-activated neurotransmitters and optogenetic proteins, light has slowly taken over the field as the most predominant interrogator of these complex networks of cells [41]. The Ellis-Davies research group was one of the early pioneers of this field, using photocaged neurotransmitters (e.g., glutamine) for the detailed study of neuronal ion channels and receptors [35].
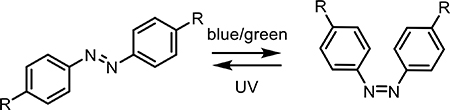
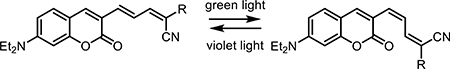
In this field, two major classifications of photoactivated small molecules has emerged, characterized by whether modulation occurs reversibly or irreversibly. Reversible photoswitches based on azobenzene or coumarin-diene moieties can control membrane potential [42] and the structure of peptides/proteins [18]. Since 1971, reversible molecular switches have dramatically altered the study of molecular interactions inside and between cells [43–46]. Recently, the Ellis-Davis group reported the use of the incredibly thermostable tetrafluoroazobenzene (4FAB) for the modulation of voltage-gated ion channels in living neurons [47]. By appending two quaternary amines onto a 4FAB structure, their molecular structure was able to modulate ion channel flow in cultured brain tissue slices only when in the light-activated cis conformation. The same group has also reported the development of other molecular photoswitches derived from coumarin [20].
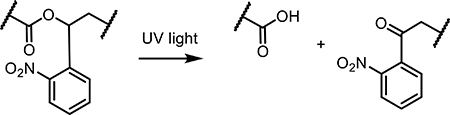
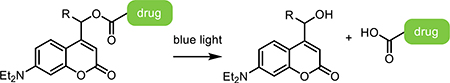
Irreversible photoresponsive molecules have been primarily used to cage bioactive small molecules, dramatically reducing their biological activity through attachment of a large photoresponsive group (i.e., putting the bioactive molecule in a “cage”). As interest in the photocages originated in the field of neuroscience, γ-aminobutyric acid (GABA) and glutamate were among the first molecules to be caged. As the predominant chemical messengers in the brain and eliciting opposite responses, GABA and glutamate remain the most popular photocaged neurotransmitters in modern-day literature. Glutamate is an excitatory neurotransmitter that activates a large family of glutamate receptors, while GABA is an inhibitory neurotransmitter that binds ligand-gated chloride channels and G-protein-coupled receptors [48]. GABA has been caged by a variety of light-sensitive species, each with their own advantages and disadvantages; oNB- [49,50], (methoxy-)nitroindolino- [51,52], 7-diethylaminocoumarin- [53], and ruthenium- [54] based photocages have each been reported. Glutamate has also been caged with many of the same photosensitive groups [55–57].
Photocaging neurotransmitters and other small molecules is not always an easy task. Caging methodologies are well reported for several functional moieties (e.g., amines, alcohols) but not all, and may prove challenging when many such groups are present on a single species. Additionally, in some cases, bulky caging groups must be employed to eliminate activity in the dark state. In one example, the Ellis-Davies group used a large dendrimer to fully cloak GABA’s activity before irradiation [58]. This technique may prove to be necessary in more systems than currently described, as researchers further advance their work with caged neurotransmitters and other small molecules. In many cases, these model compounds have not made it beyond initial in vitro studies on cells cultured on the benchtop; stability and dark activity in an in vivo setting have yet to be determined for many of these constructs.
In vitro discovery extends beyond caged neurotransmitters to other techniques for monitoring RNA levels and the transcriptome in living tissue samples. Lovatt et al. demonstrated the use of a sterically caged Transcriptome In Vivo Analysis tag composed of a poly-U RNA oligomer for hybridizing messenger RNA (mRNA) poly-A tails, two oNB photocleavable linkers, two fluorophores to track location and uncaging, and a biotin tag for pulldown [59]. Upon irradiation with single-photon UV or multiphoton red light, the crosslinkers are cleaved and blocking strands melt off the construct to reveal the poly-U binding tag. The tagged mRNA is isolated from irradiated cells (e.g., neurons in cultured brain slices) and analyzed for gene expression. This probe has been iteratively improved upon, and currently studies are underway using it in more complex in vivo environments [60].
2.1.2. Caged Prodrugs for Targeted Delivery
With innovations in light sources and in photoresponsive molecular design, an increasing number of small molecules have been caged for on-demand activation in vivo. Interestingly, many prodrugs designed for use in vivo have more than one target or purpose. Here, we see the advent of dual-action agents where both the caging group and the caged molecule have biological activity, as well as the expansion of a new field of drug delivery – theranostics – where the same small molecule can both diagnose and treat disease.
2.1.2.1. Transition-Metal Complexes as Photocages and Dual-Action Prodrugs
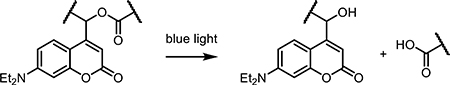
Ruthenium complexes have been recently developed as versatile cages for many different types of biologically active molecules. While early work in the design of ruthenium coordination complexes focused on caging primary amines [54,61,62], their utilization and scope rapidly expanded to include control of more complex biomolecules. Ruthenium polypyridyl complexes have been employed to cage a broad spectrum of biomolecules including nicotine [63], thioethers [64], and enzyme inhibitors [65,66]. Small-molecule ruthenium complexation has permitted caging of previously uncageable moieties, particularly nitrile and pyridine-based groups [66]. While not as common in neurotransmitters, nitrile and pyridine moieties are common in inhibitors for metalloenzymes, as they are excellent metal-binding motifs that bind reversibly to iron and copper-based active sites. Using a metal-based photocaging group to bind the active pyridine or nitrile moiety has been shown to successfully knock down or fully eliminate background activity of these enzyme inhibitors [66]. Inhibitors targeting cathepsins and cytochrome P450 have been successfully caged following a relatively simple synthetic pathway and photoreleased in vitro [65].
Ruthenium complexes as a class of biologically active drug targets are not new to the scene of targeted drug delivery. The more common application of these inorganic complexes is triggered cell apoptosis via irreversible DNA binding and generation of ROS [67,68]. As such, ruthenium photocages can also be designed as a dual-action drug, where all photoproducts are bioactive [69]. This dual therapy is purported to have high efficacy against drug resistant cancers and complex tumor environments. Dual-action Ru(II) complexes have been published from several groups, with a focus on the release of enzyme-binding molecules as well as light-mediated DNA damage and cell death. Upon release of the bioactive molecule inside the cell, the Ru(II) core is free to migrate to the nucleus and bind DNA, a process which leads to some toxicity in the dark, and is compounded upon light irradiation and generation of ROS. Delivery of chemotherapy agent 5-cyanouracil via Ru polypyridyl complex as a dual-action design has successfully degraded DNA and slowed HeLa cell growth [64,67]. The Glazer group has studied the combination of cytochrome P450 inhibition with Ru(II)-mediated 1O2 production in vitro [31]. Arora et al. demonstrated this technique in 2D and 3D cell culture, targeting surface cathepsin B with a photoreleased inhibitor, then tracking uptake of the Ru(II) metal center into the cell, leading to photomediated cell death [70]. While examples of Ru-biomolecule dual-action therapies abound [71–73], none have progressed to in vivo trials. While these reports suggest these complexes are stable in cell culture medium and serum, effective tumor targeting has not been reported for these small molecules, whether administered intraperitoneally or intravenously.
Conversely, simpler prodrugs may rely on a conformational or molecular change to reveal a reactive moiety or drug once inside the cell. These prodrugs are common in the literature, ranging from activating common inorganic cisplatin-like derivatives to revealing a chelating ligand. Significant work has been done in the Sadler group to generate stable Pt(IV)-based prodrugs that are reduced to bioactive Pt(II) upon irradiation, and/or exchange stabilizing ligands for water, activating the Pt-based drug in a similar way to cisplatin. These complexes can be designed to generate ROS in response to low-power red light [74] and to bind DNA [75]. Other reviews on this extensive area of research have been written in recent years [76–78].
2.1.2.2. Small-Molecule Light-Activated Theranostic Approaches
Several photoactivatable small molecules have been reported that can serve both diagnostic and therapeutic roles in the treatment of disease. These “theranostic” species often take advantage of elements at the center of the periodic table for either the imaging moiety or the phototoxic prodrug. In one example, Chaudhuri et al. recently reported the phototriggered release of a copper-binding ligand that, when chelated with endogenous copper, effectively turns on Cu’s apoptotic properties [79]. Coupling targeted release of this chelator with the sequestration of Cu in cancer cells of the breast, prostate, large intestine, lungs, and brain could lead to an effective method of shutting down cancer cell migration and initializing apoptosis [80]. In another theranostic approach, Li et al. linked a europium-based fluorophore and a ruthenium DNA-binding prodrug to image and trigger release of the Ru DNA-binding phototoxic core [81]. Imaging with low- (700 nm) and activating with higher-energy light (488 nm) permitted selectivity in this approach, with efficacy demonstrated in vivo in a mouse tumor model. Biswas et al. leveraged coumarin’s native fluorescence to synthesize a coumarin-doxorubicin (DOX) chemotherapeutic prodrug. Based on progressive structural changes of the coumarin fluorophore in response to light and local nitric oxide concentrations, the design facilitated imaging cellular uptake, detecting elevated levels of nitric oxide, and subsequent release DOX upon irradiation [82].
2.1.2.3. Photoregulating Gene Expression
As therapeutic targets move progressively towards large complex biomolecules (e.g., full proteins, oligonucleotides), efforts to control efficacy of these therapeutics with light have increased. Specifically, directing gene function with light has proved useful in spatiotemporally regulating gene expression [83]. Temporary gene knockdown in vivo is often achieved through the use of single-stranded antisense oligonucleotides (ASOs) or double-stranded short interfering RNA (siRNA). ASOs may be modified at their backbone to be more stable to deoxyribonucleases present in serum and prolong their half-life; common modifications include exchanging the phosphodiester linkage for a morpholine-based linker or thiolating the backbone. ASOs bind to mRNA, and can be designed to block any number of translational steps as transcribed RNA is spliced and read by the ribosome [84].
Caging ASOs can be achieved through stochastic decoration of individual nucleotide bases with photocaging groups [85,86] or by enforcing a secondary structure which blocks hybridization. The latter approach involves only one photocaging group, compared to multiple cages required for the first design, and can give extended serum stability due to the secondary structure of the oligonucleotide. Both morpholine-modified (“morpholinos”) and unmodified ASOs have been used in vivo and ex vivo. The Chen group has long pioneered this method, even using multiple crosslinkers to circularize two different morpholino ASOs. In their recent report, the group multiplexed oNB and coumarin photocrosslinkers (photoresponsive molecules used to crosslink two parts of a molecule together) to give selective gene silencing with two wavelengths of light [22,24]. The Dmochowski group has also recently published examples improving upon this design, using a ruthenium-based photocleavable crosslinker for in vivo gene knockdown [87] and incorporating a non-coding stem to further decrease activity in the dark [88].
siRNA is more popular than single-stranded ASOs, primarily for its extended stability and ease of synthesis [89,90]. Caging or deactivating siRNA often relies on sterically blocking the binding of the RNA-Induced silencing complex (RISC) to the siRNA [91], either through including azobenzenes to change the structure of the antisense strand [92], incorporating photocaging molecules on individual bases [93,94], or modifying the 5’ terminal phosphate [95]. Interestingly, serum and cellular proteins can also be harnessed to cage siRNA, as shown in recent work by Ji et al, who used a vitamin E modification at the 5’ terminus of the sense strand coupled with an oNB group to give photoactivity. They found that extensive recruitment of vitamin E-binding proteins helped block extraneous activity. These recruited proteins and bulky lipid factors were released upon irradiation with UV light, restoring activity and successfully knocking down two genes of interest [96].
2.2. Nanoparticle Delivery Vehicles: Micelles, Liposomes, and Nanoparticles
Nanoparticle drug delivery systems, including those based on covalent polymer networks and micellar structures, have revolutionized the drug delivery industry. These platforms have led to dramatic improvements in improved and targeted delivery of poorly water soluble drugs, transcytosis across cellular membranes and tight endothelial layers, and theranostics, wherein drug delivery and efficacy can be observed in real time [97,98]. The ability to trigger cargo delivery from these systems using light further establishes their utility in targeted drug delivery.
Micelles and liposomes are common drug carriers: their readily accessible syntheses and surface modification provides flexible handles for targeting their cellular uptake, and the combination of hydrophobic and hydrophilic regions enable efficient loading of chemically distinct drugs. Polymer-based micelles have already entered into clinical trials as delivery vehicles for hydrophobic chemotherapeutics including paclitaxel (PTX), DOX, and others [99]. Incorporating light responsiveness into nanostructures is an effective way to increase targeting to tumors, and is compatible with other targeting methods [e.g., surface functionalization, modulating size/geometry, protecting circulating drugs by PEG-ylation with poly(ethylene glycol)] [100]. Both organic photosensitizers and inorganic small molecule and nanoparticle photosensitizers have been incorporated to endow photoresponsiveness into polymeric nanostructures [101].
2.2.1. Disrupting Nanostructures with Organic Photosensitizers
Organic photosensitizers take many forms and can interact with light in several ways. To build this functionality into a liposome or micelle, photosensitizers have been leveraged to break bonds and disrupt the hydrophobic/hydrophilic nature of the polymers (in the case of micelles) or disrupt intermolecular interactions between hydrophobic polymers (for liposomes). In one interesting example, Poelma et al. developed a donor-acceptor Stenhouse adduct that switched from hydrophobic to hydrophilic upon green light irradiation (530 – 570 nm). In its hydrophobic form, the photosensitizer induced micelle formation around PTX. When the photosensitizer was converted to a hydrophilic molecule through irradiation, the micelle was rendered unstable and the PTX was released [102].
Linking hydrophilic and hydrophobic polymers with a cleavable linker has also shown promise in disrupting micellar structures to release drug cargo. oNB-based photocleavable crosslinkers have been used in micelle formulations to break the junction point between hydrophobic and hydrophilic polymers [25]. In their material design, Pei et al. formed polymer-based micelles around ROS-generating chlorin e6 (Ce6), attaching doxorubicin to the polymer via an ROS-sensitive thioketal bond [103]. As Ce6 is sensitive to red light (660 nm), localized DOX release was demonstrated in tumors in a mouse model. Incorporating ROS-sensitive bonds between hydrophobic and hydrophilic polymers in micelle structures has also been demonstrated in vivo. In their design, Uthaman et al. synthesized a poly(ethylene glycol)-stearamine(C18) amphiphilic construct linked with a thioketal linker and loaded the resultant micelles with DOX and a photosensitizer pheophorbide A [104]. Upon accumulation in tumors, red light (670 nm) was used to generate ROSs and break the micelle structure, releasing the DOX and killing tumor cells. Positive results for mouse studies demonstrated the clinical relevance of this technique, while also laying the groundwork for other light-activated nanoparticulate systems usage in vivo.
Photodisrupting liposome structure follows a slightly different methodology, with fewer examples of cleaving covalent bonds and more examples with larger, conjugated porphyrin molecules that yields membrane destabilization upon irradiation. This can lead to better drug release from endosomes following endocytosis by prompting fusion of the liposome with the endosome membrane, as well as direct liposome structural breakdown. In one example, a malachite green porphyrin was attached to the outside cell membrane of a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine liposome, which upon UV irradiation dramatically improved DOX delivery to the cytosol and nucleus [105]. Similarly, a polymer-based system carrying Zn2+ and Ca2+ ions was disrupted by the incorporation of a fused porphinato-Zn construct in the hydrophobic region that upon blue light irradiation (488 nm) underwent a conformational change. This hydrophobic bilayer disruption successfully released a variety of cargos, including large fluorescent conjugates [106] and cations (e.g., Zn2+, Ca2+) in vitro and in vivo [107].
Combining different modes of action helps increase the specificity of targeting these nanostructures to a more localized region. One such design takes advantage of hypoxic environments in tumors and a light-absorbing conjugated polymer to act as a multifunctional polymersome construct. The incorporated 2-nitroimidazole group generates ROS under green light irradiation, followed by a change in polarity triggered by elevated ROS concentrations and hypoxic conditions. With the loss of hydrophobicity upon irradiation, the polymersome is disrupted, releasing DOX to the intercellular environment [108]. A theranostic liposome has also been developed by Liu et al., combining a gadolinium magnetic resonance imaging (MRI) sensitizer with a liposome structure linked by oNB groups to both image localization and release DOX once localized and identified [109]. The utility of this construct was also demonstrated in vivo in a targeted mouse study, establishing the viability of this MRI-guided light-mediated therapy in a unique combination of electromagnetic interactions in the body.
2.2.2. Disrupting Nanostructures with Inorganic Photosensitizers
The use of inorganic nanoparticles as photosensitizers is extensively reported in the literature. Gold nanoparticles, nanoshells, and nanorods have been used for decades as photothermal sensitizers that generate local heating upon irradiation with red and near-IR (NIR) light. Such localized heating has been leveraged to target cancerous tumors and local tissue in several applications separate from drug delivery. Combining gold-based nanomaterials with thermosensitive polymers is one widely reported method to trigger on-demand drug release from micelles or liposomes, often by eliciting a phase transition within the lipid mono- or bi-layer [110,111]. As this field is extensive and beyond the scope of this article, the reader is directed to other excellent reviews on the subject [112–114].
Upconverting nanoparticles (UCNPs) have also seen wide use for drug delivery. When coupled with existing photosensitizing technologies, UCNPs have the potential to extend light-mediated drug delivery deeper into tissue. Researchers are working to use UCNPs to improve drug delivery in the brain [115] and to drug-resistant tumors [116], in addition to many other targets [117]. Taking advantage of their NIR fluorescence to image localization as well as deliver drug cargo, UCNPs have been utilized as theranostic agents in vitro [118,119]. The sensitivity of UCNPs to IR light opens the possibility to achieve drug delivery in vivo due to the light’s enhanced tissue penetration. UCNPs have been thoroughly studied and reviewed elsewhere; for a more detailed discussion on their uses in vivo and other effective designs, we recommend the reader to several excellent reviews on this topic [117,120,121].
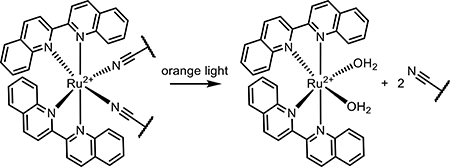
The use of photoactive inorganic coordination compounds as integral parts of micelle drug delivery systems has increased as well, as more transition metal-based therapeutics enter clinical trials. One design incorporates the cisplatin precursor Pt(IV)(NH3)2(N3)2 into the hydrophobic polymer, which upon irradiation with sub-500 nm light ruptured the polymer to release bioactive Pt(II) [122]. The efficacy of their material design was also demonstrated in vivo in a mouse tumor model. In a similar vein, Sun et al. published the design of a ruthenium-core micelle, taking advantage of the charged Ru(II) prodrug species to form a micelle structure and deliver the small molecule intracellularly [123]. The Ru(H2O)2 species is released from the polymer upon visible light irradiation (530 nm) and is freed to bind DNA and generate ROS [124].
While inorganic photosensitizers may be instrumental in increasing sensitivity to longer wavelengths of light, concerns over their toxicity, especially for lanthanide-doped UCNPs, continue to persist. However, several biomaterial designs using UCNPs have demonstrated efficacy in mouse models with limited background toxicity, suggesting they may present a viable path forward.
3. Drug-loaded Depots: Soft Biomaterials as Drug Delivery Platforms
Circulating drug delivery vehicles have driven significant innovation in improving drug solubility, circulation time, and targeting. In certain instances, however, a large, non-circulating depot containing a therapeutic is preferable to oral or intravenous drug administration [125,126]. In the brain, for example, using a drug-loaded polymer material that is surgically implanted can dramatically increase and prolong drug delivery by side-stepping the blood-brain barrier. Larger therapeutics including full-length proteins can more easily be administered to local tissue via an injectable or surgically implanted drug depot, as the material design can protect sensitive cargo. A stationary, implanted drug depot also opens the possibility of more complex release profiles and designs that incorporate live cells for real-time responsiveness to biological cues, such as protected implants of islet cells for sensing blood glucose levels.
For the protection of complex therapeutics and encapsulation of live cells, hydrogels have come to the forefront of these drug depot designs. Hydrogels are made up of 90–99% water, with a 3D structure maintained by a hydrophilic polymer network. These biomaterials can be designed using any number of polymers, with a variety of mesh sizes for controlled cargo release, and a wide range of crosslinking density and stiffness to improve the body’s response to their implantation.
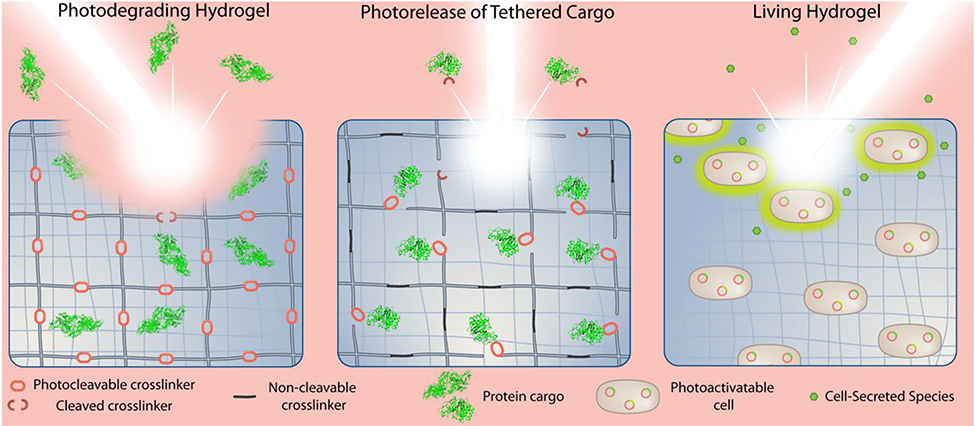
Hydrogel biomaterials can be modified for light-triggered drug delivery in several ways (Figure 3). Directly tethering drug cargo (large or small) to the hydrogel matrix with a photocleavable crosslinker has effectively sequestered and then released drug cargo in several designs. A hydrogel may also be modified in their polymer backbone with a photocleavable crosslinker for degradation of the structure of the material and subsequent release of cargo by opening broad pores in the material. Hydrogels may be rendered fully degradable by irradiation via this method, an outcome that may be desired to eliminate the need to retrieve the depot after all the drug has been released.
Figure 3.
Different photoactivatable hydrogel designs with engineered light-responsive triggers for the release of bioactive therapeutics.
In this section, we will discuss recent examples demonstrating drug release from macroscale hydrogels, as well as several examples exploiting photoresponsive hydrogels to probe and direct cell fate on the benchtop.
3.1. Phototriggered Drug Release from Hydrogels
Hydrogel depots are most commonly reported as protective drug delivery vehicles for large, complex therapeutics, with the appropriately sized and comparatively inexpensive globular protein bovine serum albumin (BSA) frequently serving as a model drug. Delivering large peptides and full-length proteins while maintaining native bioactivity remains a challenge in targeted drug delivery. In addition to the challenges associated with working with inherently fragile species, therapeutic proteins must be protected from the harsh conditions of the stomach and blood; direct delivery to local tissue is often desired.
3.1.1. Photodegradable Hydrogels for Drug Delivery
In some cases, complete degradation of the hydrogel depot represents a desirable trait. In this case, a photodegradable material may be designed to release cargo by breaking up the polymer matrix, opening pore sizes to allow for uninhibited diffusion of proteins from the material. Generally, attaching photoresponsive moieties to a polymer backbone as part of the crosslinking network forming the hydrogel can render a hydrogel photodegradable. For example, modifying hyaluronic acid (HA) polymers with azobenzene or beta-cyclodextrin (βCD) leads to the formation of photoresponsive crosslinks between polymers: when the azobenzene is in the trans isomeric form it can form a hydrophobic “host-guest” interaction with βCD, forming strong crosslinks. Upon irradiation, the azobenzene shifts to the cis isomer and can no longer interact with βCD, breaking the crosslinks and opening pores in the hydrogel. This interaction is reversible, yielding a material that can give varying doses of cargo upon subsequent doses of light. Azobenzene-βCD chemistry has been employed in several formulations. Rosales et al. demonstrated an HA-based hydrogel for the on-demand release of BSA with UV light [19]. Subsequent irradiation with visible (405 nm) light returned a fully crosslinked hydrogel, demonstrating controlled dosing of large protein cargo. Similarly, Wang et al. synthesized a poly(acrylic acid)-based hydrogel modified with βCD and methoxy-substituted azobenzene for a red-shifted response [127]. Their BSA-loaded hydrogel responded to red light, releasing the protein cargo in bolus or in dose-dependent manners.
Photodegradable hydrogels containing oNB moieties within their polymeric backbone have also been used to release siRNA. In multiple reports using similar material designs, the siRNA is trapped within the hydrogel until UV irradiation; upon photorelease, the active siRNA is able to knock down expression of model proteins (e.g., green fluorescent protein, luciferase) in cultured HeLa cells [26] and to direct osteogenesis of human mesenchymal stem cells [27].
Inorganic nanoparticles have also been incorporated into degradable hydrogel matrices for on-demand drug delivery. These material approaches again fall into two use categories: taking advantage of thermal responsivity in the hydrogel or coupling common photocleavable crosslinkers with upconverting nanoparticles. Platinum nanoparticles were incorporated into a supramolecular hydrogel with thermally responsive interactions between PEG sidechains and α-cyclodextrin to render a NIR-responsive hydrogel [128]. This hydrogel design was injectable (due to the supramolecular interactions) and “melted” in response to NIR irradiation, delivering various small-molecule fluorophores both in vitro and in a proof-of-concept in vivo trial injecting the hydrogel into a tumor.
3.1.2. Photocleavable Linkers to Release Tethered Cargo
In cases where complete degradation of the hydrogel material is not desired, cargos can be tethered directly to a stable material through a photocleavable linker. In these instances, covalent attachment of the cargo to the hydrogel material can preserve its activity while minimizing untriggered release. Methods to covalently attach molecules have drawbacks, most frequently in terms of activity loss accompanying chemically unspecified tethering, though recent innovations and alternative modes of site-specifically tethering proteins to hydrogels have worked to overcome these disadvantages [129,130].
Taking advantage of reactive handles available as part of a protein’s native amino acid side chains is a popular method for covalent crosslinking protein cargo to hydrogels. Primary amines and carboxylic acid groups on various amino acids (e.g., lysine, glutamic acid, aspartic acid) are reactive through a variety of chemistries and are often solvent-accessible. In some cases, these residues can be covalently linked to the hydrogel matrix without significant loss to protein function, demonstrated by Grim et al. using transferrin and transforming growth factor beta-1 (TGF-β1) [131]. The authors used an allyl sulfide that could undergo reversible fragmentation chain-transfer with surface thiol installed by N-hydroxysuccinimide (NHS) chemistries of several protein constructs (e.g., transferrin, ovalbumin, TGF-β1) to pattern in and out of a PEG-based hydrogel. Tethered TGF-β1 stimulated downstream signaling, indicating that the patterned protein retained some biological activity. Rapp et al. used a ruthenium-based photocleavable crosslinker to simultaneously crosslink an HA-based hydrogel as well as to tether model enzyme beta-lactamase (bLA) to the matrix [33]. Their Ru-based crosslinker was modified with aldehydes to serve as a crosslinker for hydrazine-modified HA, as well as linking protein cargo (in this case beta-lactamase) via surface lysine residues to the hydrogel matrix, though they observed significant loss of bLA activity upon release. Additionally, work by Sridhar et al. has shown that immobilizing proteins in hydrogels can help preserve enzyme activity even under thermal stress, maintaining activity of otherwise temperature-sensitive proteins for weeks at 60 °C [132].
While there are some examples in which random attachment of a protein to a hydrogel results in minimal disturbance of activity, the vast majority of these approaches have a severely detrimental effect on protein activity. Using NHS chemistry to stochastically attach reactive groups (such as azides or alkynes for subsequent click reactions) onto the surface of a protein can completely eliminate enzyme activity. This is suggested to be a result of over-modification of a protein to ensure that the reactive group has been successfully installed. To eliminate this challenge, several recent reports have used recombinant protein techniques to site-specifically modify proteins with a chemical or protein handle that can be used to attach a protein of interest (POI) to a hydrogel. In one such example, Shadish et al. used a chemoenzymatic transamidation-based strategy, expressing POIs with the recognition sequence of a sortase enzyme at the C terminus [29]. When POIs are incubated with sortase and a peptide containing an N-terminal triglycine, the peptide is enzymatically attached to the recognition sequence at the C terminus of the protein. Using this approach, the authors successfully modified bLA, epidermal growth factor (EGF), and several fluorescent proteins with azides, aldehydes, and oNB-based photocleavable crosslinkers. The site-specific modification yielded proteins with native bioactivity, and photoreleasable growth factors were used to control signaling with subcellular resolution. Following up on this work, Shadish et al. tethered proteins to hydrogel through a photocleavable protein and demonstrated their spatiotemporal release using 405 nm light [133].
Site-specific modification of proteins for incorporation into hydrogels is becoming the preferred method of attaching protein cargo to a hydrogel depot. In another example of a reversible protein-hydrogel interaction, Hammer et al. generated a recombinant protein construct taking advantage of the phototriggered, reversible protein conformational change in light-oxygen-voltage-sensing domain 2 (LOV2) and the strong binding affinity of Zdark to its dark-state conformation [134]. POIs were genetically fused to the Zdark domain, demonstrating repeated loading and photorelease of model fluorescent proteins from a hydrogel modified with LOV2.
Combining photodegradable crosslinkers with linkers that cleave under other internal triggers such as local enzyme population or a reducing environment can improve targeting of such therapeutics to a highly localized area [134]. Targeting specific tumor or organ microenvironments may only be possible by rendering pro-drug formulations accessible only via multiple inputs. For example, while tumors usually have a lower pH than healthy tissue, so does the stomach and certain organelles within cells. To avoid drug uptake and off-target effects in these areas, an ideal material drug delivery platform may require input from a combination of external and internal triggers. In 2018, Badeau et al. introduced a generalizable strategy to program hydrogel degradation in response to well-defined combinations of external inputs (including light) following Boolean YES/OR/AND logic [30]. Since this initial report, the DeForest group has extended these concepts in using topologically defined molecular crosslinks to control release of small molecules [135] and full-length proteins [136] from stable gels.
3.2. Directing Cell Growth In Vitro with Light
Not only do hydrogels show promise in the safe sequestration and delivery of sensitive drug cargo, they can be designed as platforms for encapsulating cells, differentiation of stem cells, and cell-mediated therapeutic response. Many types of hydrogels have been used for 3D cell culture, optimizing cell growth conditions on the benchtop, and as supportive scaffolds for the implantation of healthy cells into the body. These cell therapies rely on the protective hydrogel material to sustain a healthy cell population while also providing access to physiological cues from the body when implanted. In many cases, researchers direct patient-derived stem cell growth on the benchtop to form organs or organoids for future implantation. This benchtop control of cell fate and structure has driven many advancements in the use of light-responsive cues, either of signaling proteins attached to the matrix via a photocleavable linker, or in the modulation of material stiffness to drive cell fate; in these many case, photochemistry is used exploited to deliver cues to encapsulated cells to alter function.
Hydrogels for the study of cellular development on the benchtop have used light successfully in many applications to direct cell growth, stem cell differentiation, and cellular release for further study and classification. Hydrogel matrix stiffness can be changed under irradiation, directing cell growth towards softer regions. These photomodulated hydrogels have enabled significant additions to our knowledge of organ growth [137], and especially towards the growth of living tissues on the benchtop for improved drug efficacy outcomes [138]. Recent reviews on the subject can be found in the literature [138].
After the initial work by the Anseth lab in directing cell growth by both hydrogel softening and patterning adherent peptides [139], this method of directing cell growth, migration, and identity has spread to many other applications. Attempts to eliminate challenges presented by slow diffusion rates through hydrogels has led to innovations in immobilizing caged peptides or signals in a hydrogel that can be activated instantly upon irradiation. In one such example, Farrukh et al. demonstrated the power of caging a lysine residue on a laminin adhesive peptide which enabled them to direct neuron growth through a hydrogel [140,141]. The del Campo group has shown the broad flexibility of photocaged peptide modifications in hydrogels, successfully directing cell growth through an oNB-caged alpha5beta1 integrin-binding peptide [142]. Caging cyclic RGD peptides similarly directed cell growth into fibers, and continues to be an active area of research [23]. Caged RGD peptides have proved very interesting in the study of immune response to implanted biomaterials, when a hydrogel bearing islet cells was decorated with caged RGD and implanted. Activating the RGD peptide on the surface of the hydrogel after a timepoint of 24 hours led to an improved immune response and prolonging the lifetime of transplanted islet cells [143].
3.3. Light-responsive Living Hydrogels
“Living” hydrogels with embedded cells highlight the potential for continuous delivery of bioactive compounds while minimizing design complexity. This method of drug delivery also falls under cell therapy – using patient-derived or otherwise sourced cells to manufacture and release protein therapeutics. When looking to engineer these living biomaterials with a photoresponse, the del Campo lab has leveraged photoinduced protein expression and secretion of proteins from Escherichia coli on and within gels. In their first such demonstration of these living hydrogels, the del Campo lab exploited a photoactivatable isopropyl β-d-1-thiogalactopyranoside (PA-IPTG) applied externally to trigger protein expression in E. coli immobilized on the surface of a hydrogel [144]. ClearColi® BL21(DE3) bacteria genetically modified to produce a lipopolysaccharides that do not trigger endotoxic response in human cells were immobilized to the gel surface using electrostatic interactions between the negative cell membrane and positive Poly-D-Lysine (PDL) polymer. Upon treatment of the system with PA-IPTG and light, surface adhesion protein RGD was expressed, leading to mammalian cell adhesion and growth.
del Campos’ unique approach was expanded up in a subsequent report using embedded ClearColi® in a hydrogel to deliver deoxyviolacein (dVio), a tryptophan derivative with antifungal, antibacterial, and antitumor properties [145]. In this case, optogenetic control of enzyme expression was incorporated using vioABCE as an upstream phototrigger of expression, eliminating the need for exogenous PA-IPTG. Sustained, pulsatile, and spatially resolved expression and release of dVio was achieved in this system, demonstrating the power of this technique. Future studies should include in vivo studies confirming biocompatibility of these hydrogels.
4. Perspectives
Light will always retain its place as one of the most ubiquitous external triggers in biology and drug delivery. The excellent and precise control it offers clinicians and researchers is unparalleled, and the broad range of photosensitizers makes the engineering of light-responsive technologies accessible to labs possessing different skillsets. Recent literature suggests that innovations in modern methods and molecular design will soon be able to address most if not all concerns in the field. Light affords focusability and opportunities for multiplexed delivery and response extending well beyond that achieved using other triggers.
Because of this incredible flexibility and focusability, researchers continue to work towards the development of ideal photoresponsive small molecules, nanoparticles, and hydrogels: response to low energy light, minimal dark activity, and demonstration of improved efficacy in vivo. Many new reports in the literature outline a pathway to low-energy light responsive biomaterials and photoactivatable prodrugs, some demonstrated in vivo using small animal models. Despite these encouraging results, additional characterization is required to elucidating the true stability and dark activity of many of these constructs prior to clinical translation.
With the advent of advanced photosensitizers that respond to lower energy light, it is increasingly possible to photochemically trigger multiple events orthogonally within a single material design. As a critical step towards capturing and capitalizing on biology’s complexity, the capability to trigger two or more activating events with one biomaterial or drug cocktail represents a powerful innovation. There are several potential application spaces for such multiplexed biomaterials, including sequential release of proteins to direct an immune response [146] and supporting the regeneration of blood vessels by sequential release of growth factors [147]. Bringing thermally stable low energy-responsive photosensitizers into biomaterials and prodrug designs is currently inhibited by synthetic challenges, through researchers are actively exploring several promising avenues researchers to bring this technology forward.
The last great hurdle for many of these innovative designs is the journey from 2D cell culture into animal models. Some of the technologies mentioned here have been demonstrated with varying levels of success in mouse models, suggesting their efficacy in complex tissues as well as their response to light through skin, muscle, and fat. To continue bolstering this field of photomediated drug delivery, the most successful approaches that will be developed in coming years must demonstrate design efficacy in vivo.
Photomediated approaches continues to dominate the drug delivery literature, with exciting new molecular and material designs reported each year. With demonstrated application in probing fundamental biological understanding to near-instantaneously triggering therapeutic release from biomaterials, possibilities for in vitro applications are endless and ever expanding. With the concerted push to utilize higher wavelengths of light, photocontrolled drug delivery will soon flourish in vivo, bringing materials into the clinic and broadening the horizons of scientific discovery.
5. Acknowledgements
This work was supported by a Faculty Early Career Development CAREER Award (DMR 1652141, C.A.D.) and a grant (DMR 1807398, C.A.D.) from the National Science Foundation, a Maximizing Investigators’ Research Award (R35GM138036, C.A.D.) from the National Institutes of Health, and a Postdoctoral Fellowship from the Washington Research Foundation (T.L.R.).
Abbreviations:
- 4FAB
tetrafluoroazobenzene
- ASO
antisense oligonucleotide
- βCD
beta-cyclodextrin
- bLA
beta-lactamase
- BSA
bovine serum albumin
- Ce6
chlorin e6
- DOX
doxorubicin
- dVio
deoxyviolacein
- EGF
epidermal growth factor
- GABA
γ-aminobutyric acid
- HA
hyaluronic acid
- IR
infrared
- LOV2
light-oxygen-voltage-sensing domain 2
- MRI
magnetic resonance imaging
- mRNA
messenger RNA
- NHS
N-hydroxysuccinimide
- NIR
near-infrared
- NMT
N-myristoyltransferase
- oNB
ortho-nitrobenzyl
- PA-IPTG
photoactivatable isopropyl β-d-1-thiogalactopyranoside
- PDT
photodynamic therapy
- PEG
poly(ethylene glycol)
- POI
protein of interest
- PTX
paclitaxel
- RISC
RNA-Induced silencing complex
- ROS
reactive oxygen species
- siRNA
short interfering RNA
- TGF-β1
transforming growth factor beta-1
- UCNP
upconverting nanoparticle
- UV
ultraviolet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Yun SH, Kwok SJJ, Light in diagnosis, therapy and surgery, Nat. Biomed. Eng. 1 (2017) 0008. 10.1038/s41551-016-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stochel G, Wanat A, Kuliś E, Stasicka Z, Light and metal complexes in medicine, Coord. Chem. Rev. 171 (1998) 203–220. 10.1016/s0010-8545(98)90033-9. [DOI] [Google Scholar]
- [3].Ash C, Dubec M, Donne K, Bashford T, Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods, Lasers Med. Sci. 32 (2017). 10.1007/s10103-017-2317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rapp TL, DeForest CA, Visible Light-Responsive Dynamic Biomaterials: Going Deeper and Triggering More, Adv. Healthc. Mater. 9 (2020) 1901553. 10.1002/adhm.201901553. [DOI] [PubMed] [Google Scholar]
- [5].Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR, Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring, Semin. Cutan. Med. Surg. 32 (2013) 41–52. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4126803/ (accessed September 28, 2020). [PMC free article] [PubMed] [Google Scholar]
- [6].Sainter AW, King TA, Dickinson MR, Effect of target biological tissue and choice of light source on penetration depth and resolution in optical coherence tomography, J. Biomed. Opt. 9 (2004) 193–199. 10.1117/1.1628243. [DOI] [PubMed] [Google Scholar]
- [7].Ruskowitz ER, DeForest CA, Proteome-wide Analysis of Cellular Response to Ultraviolet Light for Biomaterial Synthesis and Modification, ACS Biomater. Sci. Eng. 5 (2019) 2111–2116. 10.1021/acsbiomaterials.9b00177. [DOI] [PubMed] [Google Scholar]
- [8].Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, Khurshid A, Hasan T, Development and applications of photo-triggered theranostic agents, Adv. Drug Deliv. Rev. 62 (2010) 1094–1124. 10.1016/j.addr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Qiao Y, Wan J, Zhou L, Ma W, Yang Y, Luo W, Yu Z, Wang H, Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 11 (2019) e1527. 10.1002/wnan.1527. [DOI] [PubMed] [Google Scholar]
- [10].Jones CD, Steed JW, Gels with sense: Supramolecular materials that respond to heat, light and sound, Chem. Soc. Rev. 45 (2016) 6546–6596. 10.1039/c6cs00435k. [DOI] [PubMed] [Google Scholar]
- [11].Karimi M, Sahandi Zangabad P, Baghaee-Ravari S, Ghazadeh M, Mirshekari H, Hamblin MR, Smart Nanostructures for Cargo Delivery: Uncaging and Activating by Light, J. Am. Chem. Soc. 139 (2017) 4584–4610. 10.1021/jacs.6b08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ruskowitz ER, DeForest CA, Photoresponsive biomaterials for targeted drug delivery and 4D cell culture, Nat. Rev. Mater. 3 (2018) 17087. 10.1038/natrevmats.2017.87. [DOI] [Google Scholar]
- [13].Li X, Kwon N, Guo T, Liu Z, Yoon J, Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy, Angew. Chemie - Int. Ed. 57 (2018) 11522–11531. 10.1002/anie.201805138. [DOI] [PubMed] [Google Scholar]
- [14].Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O, Kotowski K, Kulbacka J, Photodynamic therapy – mechanisms, photosensitizers and combinations, Biomed. Pharmacother. 106 (2018) 1098–1107. 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- [15].Baskaran R, Lee J, Yang S-G, Clinical development of photodynamic agents and therapeutic applications, Biomater. Res. 22 (2018) 25. 10.1186/s40824-018-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ellis-Davies GCR, Useful Caged Compounds for Cell Physiology, Acc. Chem. Res. 53 (2020) 1593–1604. 10.1021/acs.accounts.0c00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ellis-Davies GCR, Caged compounds: Photorelease technology for control of cellular chemistry and physiology, Nat. Methods. 4 (2007) 619–628. 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hoersch D, Let there be light: How to use photoswitchable cross-linker to reprogram proteins, Biochem. Soc. Trans. 45 (2017) 831–837. 10.1042/BST20160386. [DOI] [PubMed] [Google Scholar]
- [19].Rosales AM, Rodell CB, Chen MH, Morrow MG, Anseth KS, Burdick JA, Reversible Control of Network Properties in Azobenzene-Containing Hyaluronic Acid-Based Hydrogels, Bioconjug. Chem. 29 (2018) 905–913. 10.1021/acs.bioconjchem.7b00802. [DOI] [PubMed] [Google Scholar]
- [20].Richers MT, Du Tran D, Wachtveitl J, Ellis-Davies GCR, Coumarin-diene photoswitches for rapid and efficient isomerization with visible light, Chem. Commun. 54 (2018) 4983–4986. 10.1039/c8cc01091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fan L, Lewis RW, Hess GP, Ganem B, A new synthesis of caged GABA compounds for studying GABAA receptors, Bioorg. Med. Chem. Lett. 19 (2009) 3932–3933. 10.1016/J.BMCL.2009.03.065. [DOI] [PubMed] [Google Scholar]
- [22].Yamazoe S, Liu Q, McQuade LE, Deiters A, Chen JK, Sequential Gene Silencing Using Wavelength-Selective Caged Morpholino Oligonucleotides, Angew. Chemie Int. Ed. 53 (2014) 10114–10118. 10.1002/anie.201405355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Salierno MJ, García-Fernandez L, Carabelos N, Kiefer K, García AJ, del Campo A, Phototriggered fibril-like environments arbitrate cell escapes and migration from endothelial monolayers, Biomaterials. 82 (2016) 113–123. 10.1016/j.biomaterials.2015.12.001. [DOI] [PubMed] [Google Scholar]
- [24].Yamazoe S, Shestopalov IA, Provost E, Leach SD, Chen JK, Cyclic Caged Morpholinos: Conformationally Gated Probes of Embryonic Gene Function, Angew. Chemie Int. Ed. 51 (2012) 6908–6911. 10.1002/anie.201201690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhao C, Shuai S, Zhou S, Liu Y, Huo W, Zhu H, Rao Z, Li Y, Zhou K, Ge W, Hao J, Synthesis and characterization of photo-responsive flower-like copolymer micelles with o-nitrobenzyl as the junction point, Mater. Lett. 261 (2020) 127151. 10.1016/j.matlet.2019.127151. [DOI] [Google Scholar]
- [26].Huynh CT, Nguyen MK, Tonga GY, Longé L, Rotello VM, Alsberg E, Photocleavable Hydrogels for Light-Triggered siRNA Release, Adv. Healthc. Mater. 5 (2016) 305–310. 10.1002/adhm.201500778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huynh CT, Zheng Z, Nguyen MK, McMillan A, Yesilbag Tonga G, Rotello VM, Alsberg E, Cytocompatible Catalyst-Free Photodegradable Hydrogels for Light-Mediated RNA Release to Induce hMSC Osteogenesis, ACS Biomater. Sci. Eng. 3 (2017) 2011–2023. 10.1021/acsbiomaterials.6b00796. [DOI] [PubMed] [Google Scholar]
- [28].Arakawa CK, Badeau BA, Zheng Y, DeForest CA, Multicellular Vascularized Engineered Tissues through User-Programmable Biomaterial Photodegradation, Adv. Mater. 29 (2017) 1703156. 10.1002/adma.201703156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shadish JA, Benuska GM, DeForest CA, Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials, Nat. Mater. 18 (2019) 1005–1014. 10.1038/s41563-019-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Badeau BA, Comerford MP, Arakawa CK, Shadish JA, DeForest CA, Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery, Nat. Chem. 10 (2018) 251–258. 10.1038/nchem.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zamora A, Denning CA, Heidary DK, Wachter E, Nease LA, Ruiz J, Glazer EC, Ruthenium-containing P450 inhibitors for dual enzyme inhibition and DNA damage, Dalt. Trans. 46 (2017) 2165–2173. 10.1039/C6DT04405K. [DOI] [PubMed] [Google Scholar]
- [32].Garner RN, Gallucci JC, Dunbar KR, Turro C, [Ru(bpy) 2 (5-cyanouracil) 2 ] 2+ as a Potential Light-Activated Dual-Action Therapeutic Agent, Inorg. Chem. 50 (2011) 9213–9215. 10.1021/ic201615u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rapp TL, Highley CB, Manor BC, Burdick JA, Dmochowski IJ, Ruthenium-Crosslinked Hydrogels with Rapid, Visible-Light Degradation, Chem. - A Eur. J. 24 (2018) 2328–2333. 10.1002/chem.201704580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rapp TL, Wang Y, Delessio MA, Gau MR, Dmochowski IJ, Designing Photolabile Ruthenium Polypyridyl Crosslinkers for Hydrogel Formation and Multiplexed, Visible-light Degradation, RSC Adv. 9 (2019) 4942–4947. 10.1039/C8RA09764J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Paoletti P, Ellis-Davies GCRR, Mourot A, Optical control of neuronal ion channels and receptors, Nature Publishing Group, 2019. 10.1038/s41583-019-0197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ellis-Davies GCR, Two-Photon Uncaging of Glutamate, Front. Synaptic Neurosci. 10 (2019) 48. 10.3389/fnsyn.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].White JK, Schmehl RH, Turro C, An overview of photosubstitution reactions of Ru(II) imine complexes and their application in photobiology and photodynamic therapy, Inorganica Chim. Acta. 454 (2017) 7–20. 10.1016/j.ica.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tian J, Huang B, Nawaz MH, Zhang W, Recent advances of multi-dimensional porphyrin-based functional materials in photodynamic therapy, Coord. Chem. Rev. 420 (2020) 213410. 10.1016/j.ccr.2020.213410. [DOI] [Google Scholar]
- [39].Tao Y, Chan HF, Shi B, Li M, Leong KW, Light: A Magical Tool for Controlled Drug Delivery, Adv. Funct. Mater. (2020). 10.1002/adfm.202005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lee HP, Gaharwar AK, Light-Responsive Inorganic Biomaterials for Biomedical Applications, Adv. Sci. 7 (2020) 2000863. 10.1002/advs.202000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Scanziani M, Häusser M, Electrophysiology in the age of light, Nature. 461 (2009) 930–939. 10.1038/nature08540. [DOI] [PubMed] [Google Scholar]
- [42].Bartels E, Wassermann NH, Erlanger BF, Photochromic activators of the acetylcholine receptor., Proc. Natl. Acad. Sci. U. S. A. 68 (1971) 1820–1823. 10.1073/pnas.68.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Szymański W, Beierle JM, Kistemaker HAV, Velema WA, Feringa BL, Reversible photocontrol of biological systems by the incorporation of molecular photoswitches, Chem. Rev. 113 (2013) 6114–6178. 10.1021/cr300179f. [DOI] [PubMed] [Google Scholar]
- [44].Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A, Light-controlled tools, Angew. Chemie - Int. Ed. 51 (2012) 8446–8476. 10.1002/anie.201202134. [DOI] [PubMed] [Google Scholar]
- [45].Bléger D, Hecht S, Visible-Light-Activated Molecular Switches, Angew. Chemie Int. Ed. 54 (2015) 11338–11349. 10.1002/anie.201500628. [DOI] [PubMed] [Google Scholar]
- [46].Kathan M, Hecht S, Photoswitchable molecules as key ingredients to drive systems away from the global thermodynamic minimum, Chem. Soc. Rev. 46 (2017) 5536–5550. 10.1039/c7cs00112f. [DOI] [PubMed] [Google Scholar]
- [47].Passlick S, Thapaliya ER, Chen Z, Richers MT, Ellis-Davies GCR, Optical probing of acetylcholine receptors on neurons in the medial habenula with a novel caged nicotine drug analogue, J. Physiol. 596 (2018) 5307–5318. 10.1113/JP276615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Stanton-Humphreys MN, Taylor RDT, McDougall C, Hart ML, Brown CTA, Emptage NJ, Conway SJ, Wavelength-orthogonal photolysis of neurotransmitters in vitro, Chem. Commun. 48 (2012) 657–659. 10.1039/c1cc15135e. [DOI] [PubMed] [Google Scholar]
- [49].Gee KR, Wieboldt R, Hess GP, Synthesis and Photochemistry of a New Photolabile Derivative of GABA. Neurotransmitter Release and Receptor Activation in the Microsecond Time Region, J. Am. Chem. Soc. 116 (1994) 8366–8367. 10.1021/ja00097a054. [DOI] [Google Scholar]
- [50].Molnár P, Nadler JV, γ-Aminobutyrate, α-carboxy-2-nitrobenzyl ester selectively blocks inhibitory synaptic transmission in rat dentate gyrus, Eur. J. Pharmacol. 391 (2000) 255–262. 10.1016/S0014-2999(00)00106-0. [DOI] [PubMed] [Google Scholar]
- [51].Canepari M, Nelson L, Papageorgiou G, Corrie JET, Ogden D, Photochemical and pharmacological evaluation of 7-nitroindolinyl-and 4-methoxy-7-nitroindolinyl-amino acids as novel, fast caged neurotransmitters., J. Neurosci. Methods. 112 (2001) 29–42. 10.1016/S0165-0270(01)00451-4. [DOI] [PubMed] [Google Scholar]
- [52].Matsuzaki M, Hayama T, Kasai H, Ellis-Davies GCR, Two-photon uncaging of i 3-aminobutyric acid in intact brain tissue, Nat. Chem. Biol. 6 (2010) 255–257. 10.1038/nchembio.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fan L, Lewis RW, Hess GP, Ganem B, A new synthesis of caged GABA compounds for studying GABAA receptors, Bioorganic Med. Chem. Lett. 19 (2009) 3932–3933. 10.1016/j.bmcl.2009.03.065. [DOI] [PubMed] [Google Scholar]
- [54].Zayat L, Noval MG, Campi J, Calero CI, Calvo DJ, Etchenique R, A New Inorganic Photolabile Protecting Group for Highly Efficient Visible Light GABA Uncaging, ChemBioChem. 8 (2007) 2035–2038. 10.1002/cbic.200700354. [DOI] [PubMed] [Google Scholar]
- [55].Matsuzaki M, Ellis-Davies GCR, Nemoto T, Miyashita Y, Iino M, Kasai H, Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons, Nat. Neurosci. 4 (2001) 1086–1092. 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Olson JP, Kwon HB, Takasaki KT, Chiu CQ, Higley MJ, Sabatini BL, Ellis-Davies GCRR, Optically selective two-photon uncaging of glutamate at 900 nm, J. Am. Chem. Soc. 135 (2013) 5954–5957. 10.1021/ja4019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fino E, Araya R, Peterka DS, Salierno M, Etchenique R, Yuste R, RuBi-Glutamate: Two-Photon and Visible-Light Photoactivation of Neurons and Dendritic spines., Front. Neural Circuits. 3 (2009) 2. 10.3389/neuro.04.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Richers MT, Amatrudo JM, Olson JP, Ellis-Davies GCR, Cloaked Caged Compounds: Chemical Probes for Two-Photon Optoneurobiology, Angew. Chemie Int. Ed. 56 (2017) 193–197. 10.1002/anie.201609269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lovatt D, Ruble BK, Lee J, Dueck H, Kim TK, Fisher S, Francis C, Spaethling JM, Wolf JA, Grady MS, V Ulyanova A, Yeldell SB, Griepenburg JC, Buckley PT, Kim J, Sul J-Y, Dmochowski IJ, Eberwine J, Transcriptome in vivo analysis (TIVA) of spatially defined single cells in live tissue, Nat. Methods. 11 (2014) 190–196. 10.1038/nmeth.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yeldell SB, Yang L, Lee J, Eberwine JH, Dmochowski IJ, Oligonucleotide Probe for Transcriptome In Vivo Analysis (TIVA) of Single Neurond with Minimal Background, ACS Chem. Biol. (2020). 10.1021/acschembio.0c00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zayat L, Salierno M, Etchenique R, Ruthenium(II) bipyridyl complexes as photolabile caging groups for amines, Inorg. Chem. 45 (2006) 1728–1731. 10.1021/ic0512983. [DOI] [PubMed] [Google Scholar]
- [62].Zayat L, Filevich O, Baraldo LM, Etchenique R, Ruthenium polypyridyl phototriggers: from beginnings to perspectives., Philos. Trans. A. Math. Phys. Eng. Sci. 371:201203 (2013) 10.1098/rsta.2012.0330. 10.1098/rsta.2012.0330. [DOI] [PubMed] [Google Scholar]
- [63].Filevich R, Oscar; Salierno, Marcello; Etchenique, A caged nicotine with nanosecond range kinetics and visible light sensitivity, J. Inorg. Biochem. 104 (2010) 1248–1251. 10.1016/J.JINORGBIO.2010.08.003. [DOI] [PubMed] [Google Scholar]
- [64].Garner RN, Joyce LE, Turro C, Effect of Electronic Structure on the Photoinduced Ligand Exchange of Ru(II) Polypyridine Complexes, Inorg. Chem. 50 (2011) 4384–4391. 10.1021/ic102482c. [DOI] [PubMed] [Google Scholar]
- [65].Huisman M, White JK, Lewalski VG, Podgorski I, Turro C, Kodanko JJ, Caging the uncageable: using metal complex release for photochemical control over irreversible inhibition, Chem. Commun. 52 (2016) 12590–12593. 10.1039/C6CC07083C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li A, Turro C, Kodanko JJ, Ru(II) polypyridyl complexes as photocages for bioactive compounds containing nitriles and aromatic heterocycles, Chem. Commun. 54 (2018) 1280–1290. 10.1039/C7CC09000E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sgambellone MA, David A, Garner RN, Dunbar KR, Turro C, Cellular Toxicity Induced by the Photorelease of a Caged Bioactive Molecule : Design of A Potential Dual-Action Ru ( II ) Complex, J. Am. Chem. Soc. 135 (2013) 11274–11282. 10.1021/ja4045604. [DOI] [PubMed] [Google Scholar]
- [68].Zeng L, Gupta P, Chen Y, Wang E, Ji L, Chao H, Chen ZS, The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials, Chem. Soc. Rev. 46 (2017) 5771–5804. 10.1039/c7cs00195a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Palmer AM, Peña B, Sears RB, Chen O, El Ojaimi M, Thummel RP, Dunbar KR, Turro C, Cytotoxicity of cyclometallated ruthenium complexes: the role of ligand exchange on the activity., Philos. Trans. A. Math. Phys. Eng. Sci. 371 (2013) 20120135. 10.1098/rsta.2012.0135. [DOI] [PubMed] [Google Scholar]
- [70].Arora K, Herroon M, Al-Afyouni MH, Toupin NP, Rohrabaugh TN, Loftus LM, Podgorski I, Turro C, Kodanko JJ, Catch and Release Photosensitizers: Combining Dual-Action Ruthenium Complexes with Protease Inactivation for Targeting Invasive Cancers, J. Am. Chem. Soc. 140 (2018) 14367–14380. 10.1021/jacs.8b08853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Loftus LM, White JK, Albani BA, Kohler L, Kodanko JJ, Thummel RP, Dunbar KR, Turro C, New Ru (II) Complex for Dual Activity: Photoinduced Ligand Release and 1O2 Production, Chem. - A Eur. J. 22 (2016) 3704–3708. 10.1002/chem.201504800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Albani BA, Peña B, Dunbar KR, Turro C, New cyclometallated Ru(II) complex for potential application in photochemotherapy?, Photochem. Photobiol. Sci. 13 (2014) 272–80. 10.1039/c3pp50327e. [DOI] [PubMed] [Google Scholar]
- [73].Lameijer LN, Hopkins SL, Brevé TG, Askes SHC, Bonnet S, D- Versus L-Glucose Conjugation: Mitochondrial Targeting of a Light-Activated Dual-Mode-of-Action Ruthenium-Based Anticancer Prodrug, Chem. - A Eur. J. 22 (2016) 18484–18491. 10.1002/chem.201603066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wang Z, Wang N, Cheng SC, Xu K, Deng Z, Chen S, Xu Z, Xie K, Tse MK, Shi P, Hirao H, Ko CC, Zhu G, Phorbiplatin, a Highly Potent Pt(IV) Antitumor Prodrug That Can Be Controllably Activated by Red Light, Chem. 5 (2019) 3151–3165. 10.1016/j.chempr.2019.08.021. [DOI] [Google Scholar]
- [75].Shi H, Imberti C, Sadler PJ, Diazido platinum(IV) complexes for photoactivated anticancer chemotherapy, Inorg. Chem. Front. 6 (2019) 1623–1638. 10.1039/c9qi00288j. [DOI] [Google Scholar]
- [76].Ciesienski KL, Franz KJ, Keys for Unlocking Photolabile Metal-Containing Cages, Angew. Chemie Int. Ed. 50 (2011) 814–824. 10.1002/anie.201002542. [DOI] [PubMed] [Google Scholar]
- [77].Shi H, Clarkson GJ, Sadler PJ, Dual action photosensitive platinum(II) anticancer prodrugs with photoreleasable azide ligands, Inorganica Chim. Acta. 489 (2019) 230–235. 10.1016/j.ica.2019.02.016. [DOI] [Google Scholar]
- [78].Imran M, Ayub W, Butler IS, Zia-ur-Rehman, Photoactivated platinum-based anticancer drugs, Coord. Chem. Rev. 376 (2018) 405–429. 10.1016/j.ccr.2018.08.009. [DOI] [Google Scholar]
- [79].Chaudhuri A, Mengji R, Venkatesh Y, Jana A, Pradeep Singh ND, An improved tumor-specific therapeutic strategy by the spatio-temporally controlled in situ formation of a Cu(II) complex, leading to prompt cell apoptosis via photoactivation of a prodrug, Chem. Commun. 56 (2020) 4559–4562. 10.1039/d0cc00667j. [DOI] [PubMed] [Google Scholar]
- [80].Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C, Advances in copper complexes as anticancer agents, Chem. Rev. 114 (2014) 815–862. 10.1021/cr400135x. [DOI] [PubMed] [Google Scholar]
- [81].Li H, Xie C, Lan R, Zha S, Chan CF, Wong WY, Ho KL, Chan BD, Luo Y, Zhang JX, Law GL, Tai WCS, Bünzli JCG, Wong KL, A Smart Europium-Ruthenium Complex as Anticancer Prodrug: Controllable Drug Release and Real-Time Monitoring under Different Light Excitations, J. Med. Chem. 60 (2017) 8923–8932. 10.1021/acs.jmedchem.7b01162. [DOI] [PubMed] [Google Scholar]
- [82].Biswas S, Rajesh Y, Barman S, Bera M, Paul A, Mandal M, Pradeep Singh ND, A dual-analyte probe: Hypoxia activated nitric oxide detection with phototriggered drug release ability, Chem. Commun. 54 (2018) 7940–7943. 10.1039/c8cc01854e. [DOI] [PubMed] [Google Scholar]
- [83].Kowalik L, Chen JK, Illuminating developmental biology through photochemistry, Nat. Chem. Biol. 13 (2017) 587–598. 10.1038/nchembio.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bennett CF, Therapeutic Antisense Oligonucleotides Are Coming of Age, Annu. Rev. Med. 70 (2019) 307–321. 10.1146/annurev-med-041217-010829. [DOI] [PubMed] [Google Scholar]
- [85].Kröck L, Heckel A , Photoinduced Transcription by Using Temporarily Mismatched Caged Oligonucleotides, Angew. Chemie Int. Ed. 44 (2005) 471–473. 10.1002/anie.200461779. [DOI] [PubMed] [Google Scholar]
- [86].Young DD, Lusic H, Lively MO, Yoder JA, Deiters A, Gene Silencing in Mammalian Cells with Light-Activated Antisense Agents, ChemBioChem. 9 (2008) 2937–2940. 10.1002/cbic.200800627. [DOI] [PubMed] [Google Scholar]
- [87].Griepenburg JC, Rapp TL, Carroll PJ, Eberwine J, Dmochowski IJ, Ruthenium-caged antisense morpholinos for regulating gene expression in zebrafish embryos, Chem. Sci. 6 (2015) 2342–2346. 10.1039/C4SC03990D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yang L, Kim H-B, Sul J-Y, Yeldell SB, Eberwine JH, Dmochowski IJ, Efficient synthesis of light-triggered circular antisense oligonucleotides targeting cellular protein expression, ChemBioChem. 19 (2018) 1250–1254. 10.1002/cbic.201800012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T, Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells, Nature. 411 (2001) 494–498. 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- [90].Braasch DA, Jensen S, Liu Y, Kaur K, Arar K, White MA, Corey DR, RNA interference in mammalian cells by chemically-modified RNA, Biochemistry. 42 (2003) 7967–7975. 10.1021/bi0343774. [DOI] [PubMed] [Google Scholar]
- [91].Casey JP, Blidner RA, Monroe WT, Caged siRNAs for spatiotemporal control of gene silencing, Mol. Pharm. 6 (2009) 669–685. 10.1021/mp900082q. [DOI] [PubMed] [Google Scholar]
- [92].Hammill ML, Isaacs-Trépanier C, Desaulniers J-P, siRNAzos: A New Class of Azobenzene-Containing siRNAs that Can Photochemically Regulate Gene Expression, ChemistrySelect. 2 (2017) 9810–9814. 10.1002/slct.201702322. [DOI] [Google Scholar]
- [93].Monroe WT, McQuain MM, Chang MS, Alexander JS, Haselton FR, Targeting expression with light using caged DNA, J. Biol. Chem. 274 (1999) 20895–20900. 10.1074/jbc.274.30.20895. [DOI] [PubMed] [Google Scholar]
- [94].Ando H, Furuta T, Tsien RY, Okamoto H, Photo-mediated gene activation using caged RNA/DNA in zebrafish embryos, Nat. Genet. 28 (2001) 317–325. 10.1038/ng583. [DOI] [PubMed] [Google Scholar]
- [95].Nguyen QN, Chavli RV, Marques JT, Conrad PG, Wang D, He W, Belisle BE, Zhang A, Pastor LM, Witney FR, Morris M, Heitz F, Divita G, Williams BRG, McMaster GK, Light controllable siRNAs regulate gene suppression and phenotypes in cells, Biochim. Biophys. Acta - Biomembr. 1758 (2006) 394–403. 10.1016/j.bbamem.2006.01.003. [DOI] [PubMed] [Google Scholar]
- [96].Ji Y, Yang J, Wu L, Yu L, Tang X, Photochemical Regulation of Gene Expression Using Caged siRNAs with Single Terminal Vitamin E Modification, Angew. Chemie Int. Ed. 55 (2016) 2152–2156. 10.1002/anie.201510921. [DOI] [PubMed] [Google Scholar]
- [97].Farokhzad OC, Langer R, Impact of nanotechnology on drug delivery, ACS Nano. 3 (2009) 16–20. 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- [98].Mishra D, Hubenak JR, Mathur AB, Nanoparticle systems as tools to improve drug delivery and therapeutic efficacy, J. Biomed. Mater. Res. Part A. 101 (2013) 3646–3660. 10.1002/jbm.a.34642. [DOI] [PubMed] [Google Scholar]
- [99].Zhou Q, Zhang L, Yang TH, Wu H, Stimuli-responsive polymeric micelles for drug delivery and cancer therapy, Int. J. Nanomedicine. 13 (2018) 2921–2942. 10.2147/IJN.S158696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Shim G, Ko S, Kim D, Le QV, Park GT, Lee J, Kwon T, Choi HG, Kim YB, Oh YK, Light-switchable systems for remotely controlled drug delivery, J. Control. Release. 267 (2017) 67–79. 10.1016/j.jconrel.2017.09.009. [DOI] [PubMed] [Google Scholar]
- [101].Li Y, Zhang Y, Wang W, Phototriggered targeting of nanocarriers for drug delivery, Nano Res. 11 (2018) 5424–5438. 10.1007/s12274-018-2132-7. [DOI] [Google Scholar]
- [102].Poelma SO, Oh SS, Helmy S, Knight AS, Burnett GL, Soh HT, Hawker CJ, Read De Alaniz J, Controlled drug release to cancer cells from modular one-photon visible light-responsive micellar system, Chem. Commun. 52 (2016) 10525–10528. 10.1039/c6cc04127b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Pei P, Sun C, Tao W, Li J, Yang X, Wang J, ROS-sensitive thioketal-linked polyphosphoester-doxorubicin conjugate for precise phototriggered locoregional chemotherapy, Biomaterials. 188 (2019) 74–82. 10.1016/j.biomaterials.2018.10.010. [DOI] [PubMed] [Google Scholar]
- [104].Uthaman S, Pillarisetti S, Mathew AP, Kim Y, Bae WK, Huh KM, Park IK, Long circulating photoactivable nanomicelles with tumor localized activation and ROS triggered self-accelerating drug release for enhanced locoregional chemo-photodynamic therapy, Biomaterials. 232 (2020) 119702. 10.1016/j.biomaterials.2019.119702. [DOI] [PubMed] [Google Scholar]
- [105].Hayashi K, Watanabe M, Iwasaki T, Shudou M, Uda RM, Endosomal escape by photo-activated fusion of liposomes containing a malachite green derivative: A novel class of photoresponsive liposomes for drug delivery vehicles, Photochem. Photobiol. Sci. 18 (2019) 1471–1478. 10.1039/c8pp00495a. [DOI] [PubMed] [Google Scholar]
- [106].Kamat NP, Robbins GP, Rawson J, Therien MJ, Dmochowski IJ, Hammer DA, A generalized system for photoresponsive membrane rupture in polymersomes, Adv. Funct. Mater. 20 (2010) 2588–2596. 10.1002/adfm.201000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Griepenburg JC, Sood N, Vargo KB, Williams D, Rawson J, Therien MJ, Hammer DA, Dmochowski IJ, Caging metal ions with visible light-responsive nanopolymersomes, Langmuir. 31 (2015) 799–807. 10.1021/la5036689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Qian C, Yu J, Chen Y, Hu Q, Xiao X, Sun W, Wang C, Feng P, Shen Q-D, Gu Z, Light-Activated Hypoxia-Responsive Nanocarriers for Enhanced Anticancer Therapy, Adv. Mater. 28 (2016) 3313–3320. 10.1002/adma.201505869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Liu C, Ewert KK, Wang N, Li Y, Safinya CR, Qiao W, A multifunctional lipid that forms contrast-agent liposomes with dual-control release capabilities for precise MRI-guided drug delivery, Biomaterials. 221 (2019) 119412. 10.1016/j.biomaterials.2019.119412. [DOI] [PubMed] [Google Scholar]
- [110].Zhan C, Wang W, McAlvin JB, Guo S, Timko BP, Santamaria C, Kohane DS, Phototriggered Local Anesthesia, Nano Lett. 16 (2016) 177–181. 10.1021/acs.nanolett.5b03440. [DOI] [PubMed] [Google Scholar]
- [111].Zhan C, Wang W, Santamaria C, Wang B, Rwei A, Timko BP, Kohane DS, Ultrasensitive Phototriggered Local Anesthesia, Nano Lett. 17 (2017) 660–665. 10.1021/acs.nanolett.6b03588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Liu Y, Bhattarai P, Dai Z, Chen X, Photothermal therapy and photoacoustic imaging: Via nanotheranostics in fighting cancer, Chem. Soc. Rev. 48 (2019) 2053–2108. 10.1039/c8cs00618k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Riley RS, Day ES, Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment, Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 9 (2017). 10.1002/wnan.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zou L, Wang H, He B, Zeng L, Tan T, Cao H, He X, Zhang Z, Guo S, Li Y, Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics, Theranostics. 6 (2016) 762–772. 10.7150/thno.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Fu L, Chung R, Shi B, Upconversion nanoparticle-based strategy for crossing the blood-brain barrier to treat the central nervous system disease, in: Methods Mol. Biol, Humana Press Inc., 2019: pp. 263–282. 10.1007/978-1-4939-9769-5_17. [DOI] [PubMed] [Google Scholar]
- [116].Yao C, Wang P, Li X, Hu X, Hou J, Wang L, Zhang F, Near-Infrared-Triggered Azobenzene-Liposome/Upconversion Nanoparticle Hybrid Vesicles for Remotely Controlled Drug Delivery to Overcome Cancer Multidrug Resistance, Adv. Mater. 28 (2016) 9341–9348. 10.1002/adma.201503799. [DOI] [PubMed] [Google Scholar]
- [117].Jalani G, Tam V, Vetrone F, Cerruti M, Seeing, Targeting and Delivering with Upconverting Nanoparticles, J. Am. Chem. Soc. 140 (2018) 10923–10931. 10.1021/jacs.8b03977. [DOI] [PubMed] [Google Scholar]
- [118].Zhang Z, Jayakumar MKG, Shikha S, Zhang Y, Zheng X, Zhang Y, Modularly Assembled Upconversion Nanoparticles for Orthogonally Controlled Cell Imaging and Drug Delivery, ACS Appl. Mater. Interfaces. 12 (2020) 12549–12556. 10.1021/acsami.0c00672. [DOI] [PubMed] [Google Scholar]
- [119].Jalani G, Naccache R, Rosenzweig DH, Haglund L, Vetrone F, Cerruti M, Photocleavable Hydrogel-Coated Upconverting Nanoparticles: A Multifunctional Theranostic Platform for NIR Imaging and On-Demand Macromolecular Delivery, J. Am. Chem. Soc. 138 (2016) 1078–1083. 10.1021/jacs.5b12357. [DOI] [PubMed] [Google Scholar]
- [120].Liu Q, Zhan C, Kohane DS, Phototriggered drug delivery using inorganic nanomaterials, Bioconjug. Chem. 28 (2017) 98–104. 10.1021/acs.bioconjchem.6b00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Bagheri A, Arandiyan H, Boyer C, Lim M, Lanthanide-Doped Upconversion Nanoparticles: Emerging Intelligent Light-Activated Drug Delivery Systems, Adv. Sci. 3 (2016) 1500437. 10.1002/advs.201500437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhou D, Guo J, Kim GB, Li J, Chen X, Yang J, Huang Y, Simultaneously Photo-Cleavable and Activatable Prodrug-Backboned Block Copolymer Micelles for Precise Anticancer Drug Delivery, Adv. Healthc. Mater. 5 (2016) 2493–2499. 10.1002/adhm.201600470. [DOI] [PubMed] [Google Scholar]
- [123].Sun W, Thiramanas R, Slep LD, Zeng X, Mailänder V, Wu S, Photoactivation of Anticancer Ru Complexes in Deep Tissue: How Deep Can We Go?, Chem. - A Eur. J. 23 (2017) 10832–10837. 10.1002/chem.201701224. [DOI] [PubMed] [Google Scholar]
- [124].Sun W, Wen Y, Thiramanas R, Chen M, Han J, Gong N, Wagner M, Jiang S, Meijer MS, Bonnet S, Butt H-J, Mailänder V, Liang X-J, Wu S, Red-Light-Controlled Release of Drug-Ru Complex Conjugates from Metallopolymer Micelles for Phototherapy in Hypoxic Tumor Environments, Adv. Funct. Mater. 28 (2018) 1804227. 10.1002/adfm.201804227. [DOI] [Google Scholar]
- [125].Hoare TR, Kohane DS, Hydrogels in drug delivery: Progress and challenges, Polymer (Guildf). 49 (2008) 1993–2007. 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- [126].Dimatteo R, Darling NJ, Segura T, In situ forming injectable hydrogels for drug delivery and wound repair, Adv. Drug Deliv. Rev. 127 (2018) 167–184. 10.1016/j.addr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wang D, Wagner M, Butt H-J, Wu S, Supramolecular hydrogels constructed by red-light-responsive host-guest interactions for photo-controlled protein release in deep tissue., Soft Matter. 11 (2015) 7656–62. 10.1039/c5sm01888a. [DOI] [PubMed] [Google Scholar]
- [128].Wang X, Wang C, Zhang Q, Cheng Y, Near infrared light-responsive and injectable supramolecular hydrogels for on-demand drug delivery, Chem. Commun. 52 (2016) 978–981. 10.1039/C5CC08391E. [DOI] [PubMed] [Google Scholar]
- [129].Shadish JA, DeForest CA, Site-Selective Protein Modification: From Functionalized Proteins to Functional Biomaterials, Matter. 2 (2020) 50–77. 10.1016/j.matt.2019.11.011. [DOI] [Google Scholar]
- [130].Grim JC, Marozas IA, Anseth KS, Thiol-ene and photo-cleavage chemistry for controlled presentation of biomolecules in hydrogels, J. Control. Release. 219 (2015) 95–106. 10.1016/j.jconrel.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Grim JC, Brown TE, Aguado BA, Chapnick DA, Viert AL, Liu X, Anseth KS, A Reversible and Repeatable Thiol-Ene Bioconjugation for Dynamic Patterning of Signaling Proteins in Hydrogels, ACS Cent. Sci. 4 (2018) 909–916. 10.1021/acscentsci.8b00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sridhar BV, Janczy JR, Hatlevik Ø, Wolfson G, Anseth KS, Tibbitt MW, Thermal Stabilization of Biologics with Photoresponsive Hydrogels, Biomacromolecules. 19 (2018) 740–747. 10.1021/acs.biomac.7b01507. [DOI] [PubMed] [Google Scholar]
- [133].Shadish JA, Strange AC, DeForest CA, Genetically Encoded Photocleavable Linkers for Patterned Protein Release from Biomaterials, J. Am. Chem. Soc. 141 (2019) 15619–15625. 10.1021/jacs.9b07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hammer JA, Ruta A, West JL, Using Tools from Optogenetics to Create Light-Responsive Biomaterials: LOVTRAP-PEG Hydrogels for Dynamic Peptide Immobilization, Ann. Biomed. Eng. (2019) 1–10. 10.1007/s10439-019-02407-w. [DOI] [PubMed] [Google Scholar]
- [135].Ruskowitz ER, Comerford MP, Badeau BA, DeForest CA, Logical stimuli-triggered delivery of small molecules from hydrogel biomaterials, Biomater. Sci. 7 (2019) 542–546. 10.1039/c8bm01304g. [DOI] [PubMed] [Google Scholar]
- [136].Gawade PM, Shadish JA, Badeau BA, DeForest CA, Logic-Based Delivery of Site-Specifically Modified Proteins from Environmentally Responsive Hydrogel Biomaterials, Adv. Mater. 31 (2019) 1902462. 10.1002/adma.201902462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Hushka EA, Yavitt FM, Brown TE, Dempsey PJ, Anseth KS, Relaxation of Extracellular Matrix Forces Directs Crypt Formation and Architecture in Intestinal Organoids, Adv. Healthc. Mater. 9 (2020) 1901214. 10.1002/adhm.201901214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bahlmann LC, Fokina A, Shoichet MS, Dynamic bioengineered hydrogels as scaffolds for advanced stem cell and organoid culture, MRS Commun. 7 (2017) 472–486. 10.1557/mrc.2017.72. [DOI] [Google Scholar]
- [139].DeForest CA, Anseth KS, Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions, Nat. Chem. 3 (2011) 925–931. 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Farrukh A, Paez JI, del Campo A, 4D Biomaterials for Light-Guided Angiogenesis, Adv. Funct. Mater. 29 (2019) 1807734. 10.1002/adfm.201807734. [DOI] [Google Scholar]
- [141].Farrukh A, Fan W, Zhao S, Salierno M, Paez JI, del Campo A, Photoactivatable Adhesive Ligands for Light-Guided Neuronal Growth, ChemBioChem. 19 (2018) 1271–1279. 10.1002/cbic.201800118. [DOI] [PubMed] [Google Scholar]
- [142].Nair RV, Farrukh A, del Campo A, A Photoactivatable α 5 β 1 -Specific Integrin Ligand, ChemBioChem. 19 (2018) 1280–1287. 10.1002/cbic.201800180. [DOI] [PubMed] [Google Scholar]
- [143].Lee TT, García JR, Paez JI, Singh A, Phelps EA, Weis S, Shafiq Z, Shekaran A, Del Campo A, García AJ, Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials., Nat. Mater. 14 (2015) 352–60. 10.1038/nmat4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Sankaran S, Zhao S, Muth C, Paez J, del Campo A, Toward Light-Regulated Living Biomaterials, Adv. Sci. 5 (2018) 1800383. 10.1002/advs.201800383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Sankaran S, Becker J, Wittmann C, del Campo A, Optoregulated Drug Release from an Engineered Living Material: Self-Replenishing Drug Depots for Long-Term, Light-Regulated Delivery, Small. 15 (2019) 1804717. 10.1002/smll.201804717. [DOI] [PubMed] [Google Scholar]
- [146].Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G, Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds, Biomaterials. 37 (2015) 194–207. https://www-sciencedirect-com.offcampus.lib.washington.edu/science/article/pii/S0142961214010643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Rambhia KJ, Ma PX, Controlled drug release for tissue engineering, J. Control. Release. 219 (2015) 119–128. 10.1016/j.jconrel.2015.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]