Abstract
BACKGROUND:
Frailty is associated with numerous post-operative adverse outcomes in older adults. Current pre-operative frailty screening tools require additional data collection or objective assessments, adding expense and limiting large-scale implementation.
OBJECTIVE:
To evaluate the association of an automated measure of frailty integrated within the Electronic Health Record (EHR) with post-operative outcomes for nonemergency surgeries.
DESIGN:
Retrospective cohort study.
SETTING:
Academic Medical Center.
PARTICIPANTS:
Patients 65 years or older that underwent nonemergency surgery with an inpatient stay 24 hours or more between October 8th, 2017 and June 1st, 2019.
EXPOSURES:
Frailty as measured by a 54-item electronic frailty index (eFI).
OUTCOMES AND MEASUREMENTS:
Inpatient length of stay, requirements for post-acute care, 30-day readmission, and 6-month all-cause mortality.
RESULTS:
Of 4,831 unique patients (2,281 females (47.3%); mean (SD) age, 73.2 (5.9) years), 4,143 (85.7%) had sufficient EHR data to calculate the eFI, with 15.1% categorized as frail (eFI > 0.21) and 50.9% pre-frail (0.10 < eFI ≤ 0.21). For all outcomes, there was a generally a gradation of risk with higher eFI scores. For example, adjusting for age, sex, race/ethnicity, and American Society of Anesthesiologists class, and accounting for variability by service line, patients identified as frail based on the eFI, compared to fit patients, had greater needs for post-acute care (odds ratio (OR) = 1.68; 95% confidence interval (CI) = 1.36–2.08), higher rates of 30-day readmission (hazard ratio (HR) = 2.46; 95%CI = 1.72–3.52) and higher all-cause mortality (HR = 2.86; 95%CI = 1.84–4.44) over 6 months’ follow-up.
CONCLUSIONS:
The eFI, an automated digital marker for frailty integrated within the EHR, can facilitate pre-operative frailty screening at scale.
Keywords: frailty, preoperative assessment, healthcare utilization
INTRODUCTION
Frailty, a syndrome of vulnerability to adverse outcomes, can be identified by several validated instruments based on clinical evaluation,1,2 accumulation of deficits,3,4 or measures of resilience/physiologic reserve.5,6 Frailty consistently predicts post-operative complications, post-acute care, unplanned readmissions, and mortality, as well as increased cost and decreased health system revenue.7–12 Pre-operative frailty screening could inform decision-making and guide targeted interventions for optimizing surgical outcomes.13–15 However, routine frailty screening faces barriers to large-scale implementation given time and resource limitations. First, systematic assessments, such as comprehensive geriatric assessment, are infeasible to conduct at scale. Second, many instruments, such as the frailty phenotype,1 necessitate objective measurements such as gait speed and grip strength, rendering them infeasible to implement broadly. Brief survey instruments, such as the 14-item Risk Analysis Index (RAI), have shown encouraging prognostic ability with respect to mortality and post-operative complications.13,16 Automated approaches to risk stratification leveraging the Electronic Health Record (EHR) are still desirable for two reasons. First, survey instruments like the RAI are currently performed after referral for surgery, and so are less useful for shared decision-making concerning whether or not to refer to surgery. Second, even brief screening tools engender resource implications on a large scale, especially given multiple potential screening targets including nutrition, smoking status, and social determinants of health.
In England, frailty indices based on the theory of deficit accumulation4,17 have been adapted to leverage routinely gathered primary care data housed within the EHR; the resulting electronic Frailty Index (eFI) has been adopted by both the National Health Service and the British Geriatrics Society.18–21 In previous work, we developed an eFI and evaluated its prospective association with adverse health outcomes in primary care.22 These analyses demonstrated the feasibility of deriving an EHR frailty measure, and showed that higher eFI scores were associated with increased rates of emergency department visits and inpatient hospitalizations, injurious falls, and mortality over 1-year of follow-up. The purpose of this study was to examine the association between frailty, measured pre-operatively and pragmatically via an automated, EHR-derived eFI, and postoperative outcomes, including hospital length of stay, need for post-acute care services, 30-day hospital readmission, and all-cause mortality.
METHODS
Data Source and Study Design
We performed an analysis of patients aged 65 or more who underwent nonemergency surgery requiring 24 hours or more inpatient admission between October 8th, 2017 and June 1st, 2019 at our health system (Wake Forest Baptist Health, Winston-Salem, NC). These reference dates allowed (1) a 2-year look-back period subsequent to WFBH’s switch to ICD-10 coding, and (2) 6 months of post-operative follow-up. All data were extracted from our EHR (Epic, Verona, WI). This study was approved by the WFBH Institutional Review Board.
Calculation of the Electronic Frailty Index (eFI)
Construction of the eFI has been previously described.22 Briefly, the eFI quantifies the proportion of age-related health deficits present within a particular individual, as with other frailty indices.17 Our eFI comprises 54 total deficits, constructed from diagnosis codes, vital signs, laboratory measurements, information on smoking history and medications, and functional assessments (when available) from Medicare Annual Wellness Visits (Supplementary Figure S1). To calculate the eFI, we required that a patient had at least two outpatient encounters with a measured blood pressure in the 2 years before surgery, and 30 or more of the 54 items measured. For this analysis, we set the reference date for eFI calculation at 2 days before surgery. eFI scores range from 0 to 1, with higher scores indicating greater frailty. We categorized frailty status as fit (eFI ≤ 0.10), pre-frail (0.10 < eFI ≤ 0.21), and frail (eFI > 0.21).22,23
Outcomes
Post-operative outcomes included hospital length of stay (LOS), discharge destination, and 30-day hospital readmission. Discharge destinations were characterized as discharge to home, to home with home health services, or to a transitional care facility (inpatient rehabilitation, skilled nursing facility rehabilitation, other long-term acute care or intermediate care facility, hospice, or transfer to another hospital). We also evaluated all-cause mortality for the 6 months after surgery.
Covariates
We included age, sex, race/ethnicity, and American Society of Anesthesiologists Classification (ASA class). We also quantified multimorbidity via the Charlson Comorbidity Index for descriptive purposes.24 Because the majority of comorbidities in the Charlson index are also components of the eFI, and because these comorbidities often inform clinical determination of ASA class, we did not adjust for comorbidity in our analyses.
Statistical Analysis
We modeled the association of the eFI with LOS using robust linear mixed models to account for the skewed distribution of LOS,25 including a random effect by service line. Models were adjusted for age, sex, race/ethnicity, and ASA class. We used generalized mixed-effects models (with a logistic link function and service line random effect) to estimate the association between the eFI and discharge destination, and Cox proportional hazards regression to model the association between the eFI and mortality with the baseline hazard function stratified by service line. We also conducted stratified analyses by ASA class assigned at the time of referral for surgery. We evaluated discrimination of the eFI with respect to all outcomes except LOS using c-statistics, estimated using 10-fold cross-validation.26 All analyses were performed using the R Statistical Computing Environment (R Core Team, Vienna, Austria) or SAS v9.4 (SAS, Cary, NC).
RESULTS
Between 10/8/2017 and 6/1/2019, there were 23,479 nonemergency surgeries in patients aged 65+, of which 5,333 (22.7%) required a 24-hour or more inpatient admission (Supplementary Figure S2). After restricting to the first surgery for each patient during this time period, excluding low volume service lines, and removing a few patients with missing data, our final analytic cohort consisted of 4,831 individuals. We were able to calculate eFI in 4,143 (85.7%) patients, with 15.1% categorized as frail (eFI > 0.21) and 50.9% pre-frail (0.10 < eFI ≤ 0.21; Table 1). Patients’ mean age was 73.1 ± 5.8 (standard deviation) years, sex was 51.8% male, race was 9.8% Black or African American, and ethnicity 1.1% Hispanic (Table 1). Patients classified as frail were more likely to be older, female, and have more comorbidities (Table 1). Most surgeries occurred in the orthopedic (31.7%), neurosurgery (16.8%), or cardiothoracic (13.2%) service lines. The 10 most common surgeries within each service line are detailed in Supplementary Table S1.
Table 1.
Demographic and Clinical Variables Stratified by Frailty Status Based on the Electronic Frailty Index (eFI)
| Characteristic | Fit | Pre-Frail | Frail | P value |
|---|---|---|---|---|
| eFI ≤ 0.10 | 0.10 < eFI ≤ 0.21 | eFI >0.21 | ||
| N = 1,410 | N = 2,109 | N = 624 | ||
| Age, years, mean (SD) | 72.6 (5.5) | 73.3 (5.9) | 73.9 (6.2) | <.001 |
| Male sex, no. (%) | 764 (54.2) | 1,099 (52.1) | 282 (45.2) | .001 |
| Race/ethnicity, no. (%) | .001 | |||
| White | 1,240 (87.9) | 1844 (87.4) | 535 (85.7) | |
| Black or African-American | 115 (8.2) | 213 (10.1) | 79 (12.7) | |
| Hispanic | 17 (1.2) | 25 (1.2) | 3 (0.5) | |
| Other | 38 (2.7) | 27 (1.3) | 7 (1.1) | |
| Weighted Charlson Comorbidity Index, median (IQR) | 0 (0–2) | 2 (0–3) | 3.5 (2–5) | <.001 |
| ASA class, no. (%) | <.001 | |||
| No disease or mild systemic disease | 380 (27.0) | 283 (13.4) | 24 (3.8) | |
| Severe systemic disease | 881 (62.5) | 1,497 (71.0) | 428 (68.6) | |
| Life-threatening systemic disease | 149 (10.6) | 329 (15.6) | 172 (27.6) | |
| Operating room service line, no. (%) | <.001 | |||
| Orthopedics | 451 (32.0) | 708 (33.6) | 214 (34.3) | |
| Neurosurgery | 277 (19.6) | 332 (15.7) | 105 (16.8) | |
| Cardiothoracic | 161 (11.4) | 245 (11.6) | 94 (15.1) | |
| General surgery | 218 (15.5) | 279 (13.2) | 68 (10.9) | |
| Urology | 107 (7.6) | 179 (8.5) | 31 (5.0) | |
| Ear, nose, and throat | 88 (6.2) | 132 (6.3) | 20 (3.2) | |
| Vascular | 51 (3.6) | 157 (7.4) | 61 (9.8) | |
| Gynecology | 43 (3.0) | 56 (2.7) | 21 (3.4) | |
| Plastics | 14 (1.0) | 21 (1.0) | 10 (1.6) | |
| Length of stay, hours, median (IQR) | 70 (32–128) | 75 (35–133) | 86 (52–171) | <.001 |
Abbreviations: ASA, American Society of Anesthesiologists; IQR, interquartile range; SD, standard deviation.
Patients with insufficient data to calculate the eFI were more likely to be male and to experience a longer median LOS; they were also more likely to have fewer recorded comorbidities, despite being more likely to have an ASA classification of life-threatening systemic disease (Supplementary Table S2).
In general, we observed increasing LOS with higher eFI scores, with median LOS of 70, 75, and 86 hours for patients classified as fit (eFI ≤ 0.10), pre-frail (0.10 < eFI ≤ 0.21), and frail (eFI > 0.21), respectively (Table 2). Adjusting for age, sex, race/ethnicity, and ASA class, the mean increase in LOS comparing frailty groups was 1.2 hours (95% confidence interval (CI) = −2.7–5.2 hours) comparing pre-frail to fit, and 9.5 hours (95% CI = 3.9–15.1 hours) comparing frail to fit. For post-acute care, 26.8% of frail patients were discharged to a transitional care facility, as compared to 15.2% of pre-frail and 11.6% of fit patients. In adjusted analyses, frail patients were more likely to require formal post-acute care, such as a transitional care facility or home health (45.6% vs 26.4%; adjusted odds ratio (aOR) = 1.68, 95% CI = 1.36–2.08). Frail patients were significantly more likely to be readmitted within 30 days as compared to fit patients (12.3% compared with 5.0%; aOR = 2.46, 95% CI = 1.72–3.52).
Table 2.
Length of Stay, Discharge Destination, 30-Day Readmissions, and All-Cause Mortality After Surgery by Frailty Status Based on the Electronic Frailty Index (eFI)
| Discharge Destination | |||||||
|---|---|---|---|---|---|---|---|
| Frailty Status | No. | Length of Stay (Hours) | Died in Hospital | Facilitya | Home Health | Home | Odds Ratio (95% CI)b |
| Median (IQR) | No. (%) | ||||||
| Fit (eFI ≤ 0.10) | 1,410 | 70(32–128) | 10 (0.7) | 164 (11.6) | 208 (14.8) | 1,028 (72.9) | - |
| Pre-Frail (0.10 < eFI ≤ 0.21) | 2,109 | 75(35–133) | 13 (0.6) | 320 (15.2) | 359 (17.0) | 1,417 (67.2) | 1.10(0.94–1.29) |
| Frail (eFI > 0.21) | 624 | 86(52–171) | 14 (2.2) | 167 (26.8) | 117 (18.8) | 326 (52.2) | 1.68(1.36–2.08) |
| 30-Day Readmission | All-Cause Mortality | ||||||
| Frailty Status | No.c | No. (%) | Odds Ratio (95% CI)d | No. | 30-Day No. (Cum Inc) | 180-Day No. (Cum Inc) | Hazard Ratio (95% CI)e |
| Fit (eFI ≤ 0.10) | 1,400 | 70 (5.0) | 1,410 | 14 (1.0) | 35 (2.5) | - | |
| Pre-Frail (0.10 < eFI ≤ 0.21) | 2096 | 150 (7.2) | 1.41(1.05–1.90) | 2,109 | 23 (1.1) | 86 (4.1) | 1.46(0.98–2.17) |
| Frail (eFI > 0.21) | 610 | 75 (12.3) | 2.46(1.72–3.52) | 624 | 17 (2.8) | 56 (9.4) | 2.86(1.84–4.44) |
Abbreviations: ASA, American Society of Anesthesiologists; CI, confidence Interval; IQR, interquartile range; Cum Inc, cumulative incidence.
Facility includes inpatient rehabilitation, skilled nursing facility, hospice, other long-term acute care or intermediate care facility, or transfer to another hospital.
Adjusted odds ratio for the probability of dying in hospital, being discharged to transitional care facility, or being discharged home with home health (vs being discharged home without home health) based on a mixed-effects logistic regression model with a service line random effect, adjusting for age, sex, race/ethnicity, and ASA class.
Patients discharged alive from hospital admission after index surgery.
Adjusted odds ratio for the probability of being readmitted within 30 days based on a mixed-effects logistic regression model with a service line random effect, adjusting for age, sex, race/ethnicity, and ASA class.
Adjusted hazard ratio for all-cause mortality based on a Cox proportional hazards regression model with the baseline hazard function stratified by service line, adjusting for age, sex, race/ethnicity, and ASA class.
Figure displays the cumulative incidence of all-cause mortality by frailty category in the 6 months after surgery. Mortality risk increased with increasing eFI scores; frail patients showed significantly higher mortality as compared to fit patients (hazard ratio (HR) = 2.86, 95% CI = 1.84–4.44; Table 2). The cumulative incidence of mortality at 30 days/6 months was 2.7%/9.0% in frail patients compared with 1.0%/2.5% for fit patients. These results were consistent when we stratified analyses by ASA class (Supplementary Table S3 and Supplementary Table S4); e.g., within patients classified with severe systemic disease (ASA III), frail patients were more likely to require post-acute care services (aOR = 1.75, 95% = 1.36–2.26), readmit within 30 days (aOR = 2.21, 95% = 1.44–3.39), or to die within 180 days (HR = 2.29, 95% CI = 1.32–3.97). Finally, we examined discrimination for mortality, 30-day readmission, and requirements for post-acute care based on 10-fold cross-validation (Supplementary Table S5). Although the c-statistics were modest for the eFI alone (about 0.60), discrimination improved when the eFI was modeled as the primary predictor in conjunction with traditional covariates: demographics (age, sex, race/ethnicity), service line, and ASA class. C-statistics from those models ranged from 0.66 for 30-day readmission to 0.75 for all-cause mortality.
Figure 1.
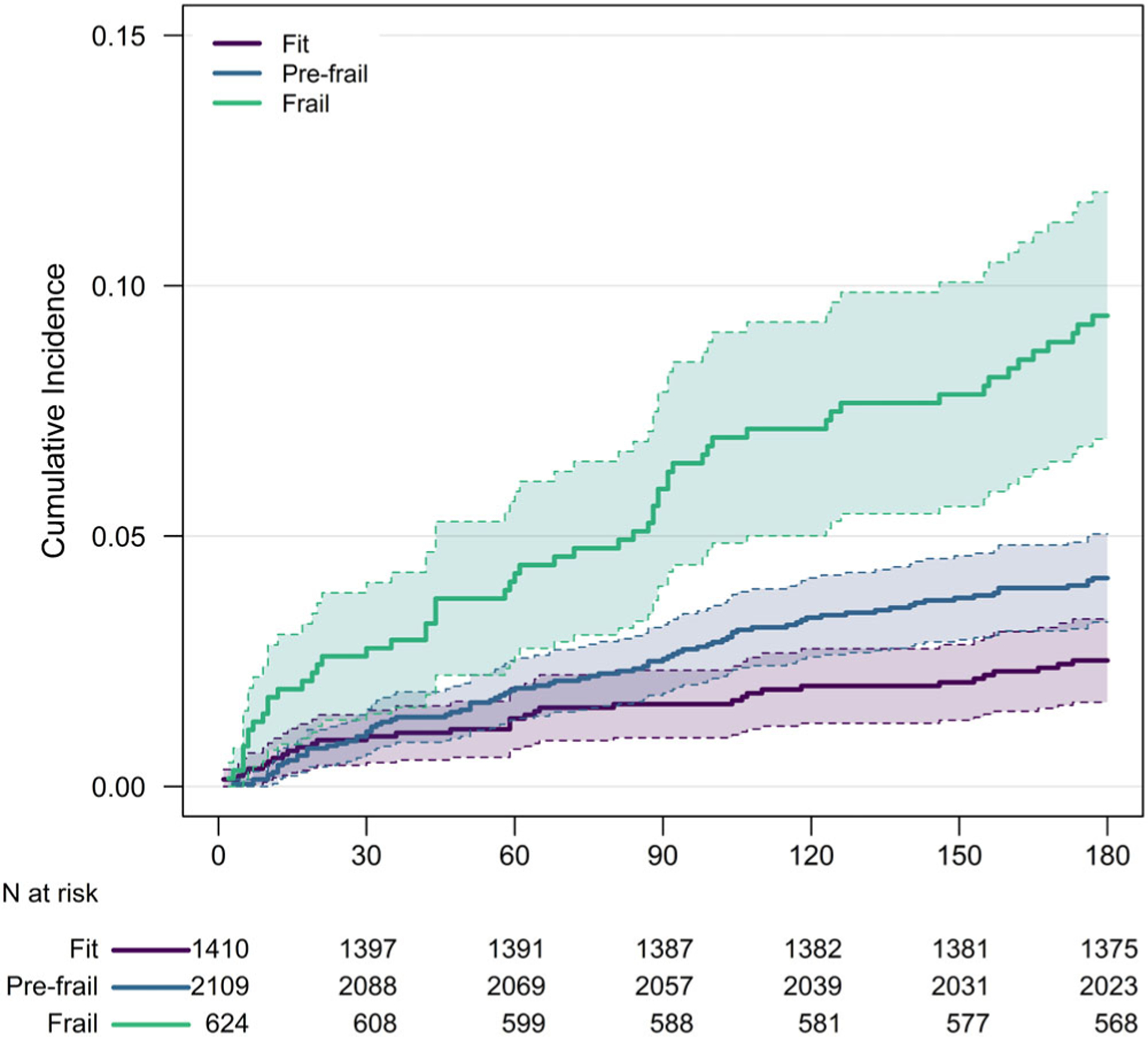
All-cause mortality after surgery by frailty status based on the electronic Frailty Index (eFI). Frailty status defined as Fit (eFI ≤ 0.10), Pre-Frail (0.10 < eFI ≤ 0.21), or Frail (eFI > 0.21). Shaded areas denote 95% pointwise confidence intervals.
DISCUSSION
The eFI is a passive marker of frailty that runs automatically in the background, using routinely-gathered EHR data. Measured pre-operatively for patients aged 65+ undergoing nonemergency surgery at a large academic medical center, the eFI was associated with increased risk for longer LOS, formal post-acute care service needs, 30-day readmissions, and all-cause mortality. When stratified by ASA class, frail patients still exhibited increased risk of adverse post-operative outcomes, reinforcing the value of the eFI beyond existing risk assessment tools. Although several studies have demonstrated a relationship between frailty and surgical outcomes,7–10 the unique value of the eFI resides in its ease of use: by leveraging existing outpatient data gathered in routine care, the eFI can inform shared medical decision-making with no additional work for busy clinicians or patients.
Surgical groups have tested a variety of frailty instruments, including the modified Frailty Index (mFI),8 the modified Hopkins frailty score,27 and the RAI,9,14,28 which, through the Frailty Screening Initiative, has shown subsequent reductions in mortality.15 However, data entry, performance-based assessments, or surveys concerning functional limitations each necessitate additional labor. The eFI adheres to deficit accumulation theory, a recognized conceptual model for frailty.4,29 In contrast, measures such as the mFI and RAI are not strictly frailty indices, as they do not ascertain a sufficiently broad set of age-related deficits (recommended to include ≥30 items)17 and are largely reflective of comorbidity. Furthermore, the eFI can be performed before a surgical appointment, enabling shared decision-making around operative or nonoperative management. Integrated into the EHR (as in our system), the eFI could be used to identify higher risk groups for pre-operative “pre-habilitation,” co-management, and expectation setting concerning goals of care, as suggested by the American College of Surgeons.30
Although this study features a large volume of patients across a variety of surgical specialties, there are limitations. First, the eFI does not specify an underlying cause of frailty, although it can identify a population more likely to benefit from pre-operative interventions focused on mobility, nutrition, and delirium prevention. Second, the eFI was not developed specifically for surgery, but rather as a consistent measure of frailty that can be used across a health system. Third, the prognostic ability of the eFI, though modest, applies to a range of outcomes beyond mortality, and could be improved with larger sample sizes and further statistical refinement. Fourth, this is a single-site study, which limited our ability to evaluate associations for specific surgical procedures and with surgical complications. Surgical complications would ideally be examined through linkage to the National Surgical Quality Improvement Program, but only ~ 10% of surgical cases are sampled for this program. Fifth, while calculated the eFI for >80% of pre-operative patients, some patients lacked sufficient outpatient data to inform the eFI. Such gaps are not unexpected, particularly for referral centers. Although the eFI may not be useful for patients with limited connection to a particular health system, it will still save labor in a two-step system, e.g., using RAI for the small number of patients with insufficient data to calculate the eFI.
We are currently pilot-testing eFI-based triage followed by streamlined geriatrics peri-operative co-management. Over time, these data may also inform future personalized pre-operative interventions and shared decision-making, with the goal of individualized risk assessment encompassing not only mortality, but also patient-centered outcomes.7,14,31,32
Supplementary Material
Supplementary Figure S1 Electronic Frailty Index components derived from the Electronic Health Record.
Supplementary Figure S2. Construction of patient cohort.
Supplementary Table S1. Top 10 Most Frequent Surgical Procedures by Service Line
Supplementary Table S2. Demographic and Clinical Variables Stratified by Availability of the Electronic Frailty Index (eFI) Within the Electronic Health Record
Supplementary Table S3. Length of Stay and Discharge Destination After Surgery by ASA Class and Frailty Status Based on the Electronic Frailty Index (eFI)
Supplementary Table S4. Thirty-day Readmissions and All-Cause Mortality After Surgery by ASA Class and Frailty Status Based on the Electronic Frailty Index (eFI)
Supplementary Table S5. Discrimination for All-Cause Mortality, 30-Day Readmission, and Need Post-Acute Care Services Based on the Electronic Frailty Index (eFI)
Key Points
The electronic Frailty Index (eFI) runs automatically in the background, distilling routinely gathered ambulatory data into a single score.
The eFI requires no additional work by clinicians, patients, or caregivers.
Frailty, as determined by the eFI, is associated with increased post-operative length of stay, 30-day readmissions, post-acute care needs, and 30- and 180-day mortality.
Why Does this Paper Matter?
Frailty has been shown to predict surgical complications, but time and personnel constraints have limited clinical implementation. The eFI, an automated passive digital marker for frailty, predicts post-operative outcomes and offers a pragmatic choice for pre-operative frailty screening at-scale.
ACKNOWLEDGMENTS
Dr. Pajewski had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. The authors wish to thank Mike Zang, MBA, Gina McRae, Renee M. Woodard, Pamela Ascano, RN, and LaShanda Brown, RN, PhD for project support.
Financial Disclosure:
The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (UL1TR001420). Dr. Callahan is supported by the Paul B. Beeson Leadership in Aging award (K76-AG059986). Additional support was provided by the Center for Healthcare Innovation at Wake Forest School of Medicine, the Claude D. Pepper Older Americans Independence Center (P30-AG21332), the J. Paul Sticht Center for Healthy Aging and Alzheimer’s Prevention, and the Office of the Dean.
Sponsor’s Role:
The NIH and remaining sponsors did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest: The authors have no conflicts.
Previous Presentation: American Geriatrics Society Annual Meeting; Portland, Oregon; May 2nd, 2019.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci. 2001;56(3):M146–M157. 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol Ser A Biol Sci Med Sci. 2007;62(7):722–727. 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 5.Tyrrell DJ, Bharadwaj MS, Jorgensen MJ, Register TC, Molina AJA. Blood cell respirometry is associated with skeletal and cardiac muscle bioenergetics: implications for a minimally invasive biomarker of mitochondrial health. Redox Biol. 2016;10:65–77. 10.1016/j.redox.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colón-Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2016;71(4): 489–495. 10.1093/gerona/glv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seib CD, Rochefort H, Chomsky-Higgins K, et al. Association of patient frailty with increased morbidity after common ambulatory general surgery operations. JAMA Surg. 2018;153(2): 160–168. 10.1001/jamasurg.2017.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panayi AC, Orkaby AR, Sakthivel D, et al. Impact of frailty on outcomes in surgical patients: a systematic review and meta-analysis. Am J Surg. 2019; 218(2):393–400. 10.1016/j.amjsurg.2018.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothenberg KA, Stern JR, George EL, et al. Association of frailty and postoperative complications with unplanned readmissions after elective outpatient surgery. JAMA Netw Open. 2019;2(5):e194330. 10.1001/jamanetworkopen.2019.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkes JG, Evans JL, Prato BS, Hess SA, MacGillivray DC, Fitzgerald TL. Frailty cost: economic impact of frailty in the elective surgical patient. J Am Coll Surg. 2019;228(6):861–870. 10.1016/j.jamcollsurg.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Sokas CM, Cowan J, Dalton MK, et al. Association between patient-reported frailty and non-home discharge among older adults undergoing surgery. J Am Geriatr Soc. 2020;68 (12): 2909–2913. 10.1111/jgs.16846. [DOI] [PubMed] [Google Scholar]
- 12.Darvall JN, Greentree K, Loth J, et al. Development of a frailty index from routine hospital data in perioperative and critical care. J Am Geriatr Soc. 2020;68:2831–2838. 10.1111/jgs.16788. [DOI] [PubMed] [Google Scholar]
- 13.Varley PR, Borrebach JD, Arya S, et al. Clinical utility of the risk analysis index as a prospective frailty screening tool within a multi-practice, multi-hospital integrated healthcare system. Ann Surg. 2020. 10.1097/SLA.0000000000003808 Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 14.George EL, Arya S. The importance of incorporating frailty screening into surgical clinical workflow. JAMA Netw Open. 2019;2(5):e193538. 10.1001/jamanetworkopen.2019.3538. [DOI] [PubMed] [Google Scholar]
- 15.Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg. 2017;152:233–240. 10.1001/jamasurg.2016.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175–182. 10.1001/jamasurg.2016.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394 (10206):1365–1375. 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 19.Lansbury LN, Roberts HC, Clift E, Herklots A, Robinson N, Sayer AA. Use of the electronic frailty index to identify vulnerable patients: a pilot study in primary care. Br J Gen Pract. 2017;67(664):e751–e756. 10.3399/bjgp17X693089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner G, Clegg A. Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing. 2014;43(6):744–747. 10.1093/ageing/afu138. [DOI] [PubMed] [Google Scholar]
- 21.Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pajewski NM, Lenoir K, Wells BJ, Williamson JD, Callahan KE. Frailty screening using the electronic health record within a medicare accountable care organization. J Gerontol—Ser A Biol Sci Med Sci. 2019;74(11):1771–1777. 10.1093/gerona/glz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Heal Reports. 2013;24(9):10–17. [PubMed] [Google Scholar]
- 24.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 25.Koller M Robustlmm: an R package for robust estimation of linear mixed-effects models. J Stat Softw. 2016;75(6):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol. 2013;13:33. 10.1186/1471-2288-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mrdutt MM, Papaconstantinou HT, Robinson BD, Bird ET, Isbell CL. Pre-operative frailty and surgical outcomes across diverse surgical subspecialties in a large health care system. J Am Coll Surg. 2019;228(4):482–490. 10.1016/j.jamcollsurg.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 28.Shinall MC, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. 2020;155: e194620. 10.1001/jamasurg.2019.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.College of Surgeons A Optimal Resources for Geriatric Surgery: 2019 Standards; 2019.
- 31.Kata A, Dutt M, Sudore RL, Finlayson E, Broering JM, Tang VL. What matters? The valued life activities of older adults undergoing elective surgery. J Am Geriatr Soc. 2019;67(11):2305–2310. 10.1111/jgs.16102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suskind AM, Finlayson E. A call for frailty screening in the preoperative setting. JAMA Surg. 2017;152(3):240–241. 10.1001/jamasurg.2016.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 Electronic Frailty Index components derived from the Electronic Health Record.
Supplementary Figure S2. Construction of patient cohort.
Supplementary Table S1. Top 10 Most Frequent Surgical Procedures by Service Line
Supplementary Table S2. Demographic and Clinical Variables Stratified by Availability of the Electronic Frailty Index (eFI) Within the Electronic Health Record
Supplementary Table S3. Length of Stay and Discharge Destination After Surgery by ASA Class and Frailty Status Based on the Electronic Frailty Index (eFI)
Supplementary Table S4. Thirty-day Readmissions and All-Cause Mortality After Surgery by ASA Class and Frailty Status Based on the Electronic Frailty Index (eFI)
Supplementary Table S5. Discrimination for All-Cause Mortality, 30-Day Readmission, and Need Post-Acute Care Services Based on the Electronic Frailty Index (eFI)


