Abstract
Leber congenital amaurosis due to CEP290 ciliopathy is being explored by treatment with an antisense oligonucleotide (AON) sepofarsen. One individual who was part of a larger cohort (ClinicalTrials.gov no. NCT03140969) was studied for 15 months after a single intravitreal sepofarsen injection. Concordant measures of visual function and retinal structure reached a substantial efficacy peak near 3 months post-injection. At 15 months, there was sustained efficacy even though there was evidence of reduction from peak response. Efficacy kinetics can be explained by the balance of AON-driven new CEP290 protein synthesis and a slow natural rate of CEP290 protein degradation in human foveal cone photoreceptors.
Keywords: Cone photoreceptors, intravitreal injection, LCA10, Leber congenital amaurosis, NPHP6, QR-110, retinal degeneration
Editor summary:
Post-hoc analyses and follow up of a patient with Leber congenital amaurosis treated with the antisense oligonucleotide sepofarsen as part of a clinical trial indicates sustained improvement of vision 15 months after a receiving a single dose of the drug
Inherited retinal diseases (IRDs) are a group of 300+ monogenic causes of visual disability with a worldwide prevalence of 1:10001. Only one IRD form is currently treatable, but scores of genetic therapies are being evaluated for the remaining IRDs with the ultimate goal of improving vision and doing so durably with an acceptable number of therapeutic interventions and optimized patient burden. One of the most severe forms of IRD is Leber congenital amaurosis (LCA), a childhood-onset blindness. The primary molecular pathology in one third of all LCA is due to mutant ciliary transition zone proteins controlling the traffic between inner and outer segments (IS and OS) of photoreceptors. The most common LCA-ciliopathy is caused by mutations in CEP290 (Centrosomal protein 290)2. A growing body of research suggests that retained but dysfunctional foveal cone photoreceptors in CEP290-LCA maintain sufficient post-receptoral connections over a wide window of ages to subserve improved vision from successful intervention strategies3-9.
A novel therapeutic approach was proposed using antisense oligonucleotides (AONs) to correct a commonly occurring deep intronic c.2991+1655A>G (p.Cys998*) CEP290 mutation by splice modulation. A 17-mer 2'-O-methyl modified phosphorothioate RNA AON, sepofarsen, was designed10, and a clinical trial was conducted (NCT03140969). The dosing regimen for the clinical trial, based on data derived from normal animals and cultured cells from patients, was to administer repeated injections of the sepofarsen into the vitreous every 3 months. Preliminary results in the first 10 patients suggested efficacy7. The eleventh and last patient (P11) to be enrolled in the trial, a homozygote for the intronic mutation, experienced subjective visual improvement and declined repeat dosing to avoid potential lenticular adverse events7; continued monitoring, however, was accepted. Details of the result of the single treatment and the subsequent 15 months of careful phenotyping provided a unique opportunity to determine the longevity and kinetics of the sepofarsen efficacy in vivo. Results of P11 could be compared to 5 other patients (P1-P5) who received the same initial dose and received subsequent doses at 3 months and later time points according to the protocol7.
At pre-injection baseline, P11 had a retinal structure phenotype that was typical for CEP290-LCA: an elliptical central retinal island of retained RPE melanization and retained photoreceptor nuclei symmetrically in both eyes (Extended Data Fig.1a). Visual acuities of P11 were better than P1-P5 (Extended Data Fig.1b) but within the range of acuities previously reported in CEP290-LCA3-5,11; color vision was normal in both eyes. Light sensitivity was measured with chromatic full-field sensitivity tests (FST). Red and blue FSTs of P11 were mediated by the cone system, symmetric, and were near the higher range of P1-P5 (Extended Data Fig.1c,d). Visual acuities and FST sensitivities tend to be correlated in CEP290-LCA4,5. Three months after intravitreal injection of 160 ug of sepofarsen, visual acuity of P11 in the treated eye had improved to a clinically meaningful extent by 0.2 log MAR which was comparable to the peak improvement seen in some other trial patients after their first injection (Extended Data Fig.1e). Light sensitivity improvements of P11 of 0.9 and 0.7 log cd.m−2 for red (Extended Data Fig.1f) and blue (Extended Data Fig.1g) stimuli, respectively, were comparable to those in other trial participants. P11 did not report development of photoaversion. Interocular FST differences increased to 0.9 (Extended Data Fig.1i) and 0.5 log (Extended Data Fig.1j) for red and blue stimuli, respectively, favoring the treated eyes. At 3 months post-injection when the study patient was due for a maintenance dose per protocol, P11 declined all further planned maintenance doses but remained in the study for long-term observation.
A wide spectrum of visual function measures of P11 demonstrated concordant and substantial improvements following the sepofarsen injection, and vision remained better than baseline at 15 months post-injection. Importantly, temporal changes of vision measures allowed better understanding of the time course of initial improvement, peak timing and the rate of decline that followed. Visual acuities that were equal before treatment became asymmetric by 2 months with a 0.2 log MAR improvement in the treated eye, and this asymmetry remained throughout the study duration (Fig.1a; Extended Data Fig.2). Under low luminance conditions, the asymmetry between the eyes was substantially larger with the treated eye being 0.4 log MAR better (reading 20 letters more) than the untreated eye; there was a slow reduction of the low luminance acuity asymmetry over the next 12 months (Fig.1a; Extended Data Fig.2). Light sensitivity with dark-adapted FST reached peak improvement 2-3 months post-injection, remaining mostly unchanged until 7 months (Extended Data Fig.3a,c). Thereafter there was evidence of a slow decline for the next 8 months. Under light-adapted conditions, FST thresholds appeared to improve slower reaching a peak 6-9 months post-injection and showed decline thereafter (Extended Data Fig.3b,d). Sensitivity gain and loss over the 15 months were also apparent in interocular differences displaying the kinetics of improvement in the treated eye (Fig.1b; Extended Data Fig.3e,f). The development of sensitivity asymmetry after treatment likely explained the substantial asymmetry in low luminance visual acuity. Visual function changes were well correlated with functional vision evaluated with mobility testing which showed improvements by 1 month and remained better than baseline throughout the following 14 months (Extended Data Fig.4). To supplement subjective measures of visual function and functional vision, we also evaluated an objective measure of visual function using the pupillary light reflex9. P11 showed acceleration of the pupillary response latency by 3 months and the effect was partially retained to 9 months (Fig.1c; Extended Data Fig.5).
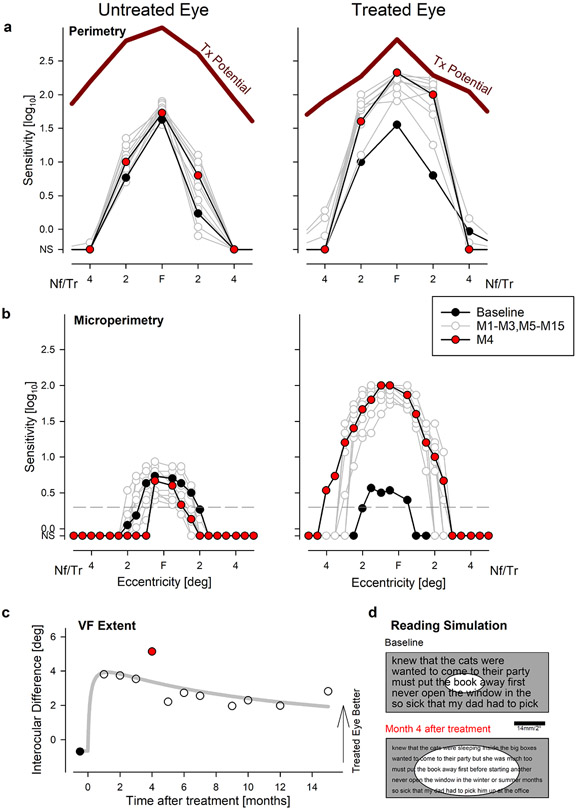
Figure 1: Time Course of Vision Improvement and Durability Over Fifteen Months After a Single Treatment.
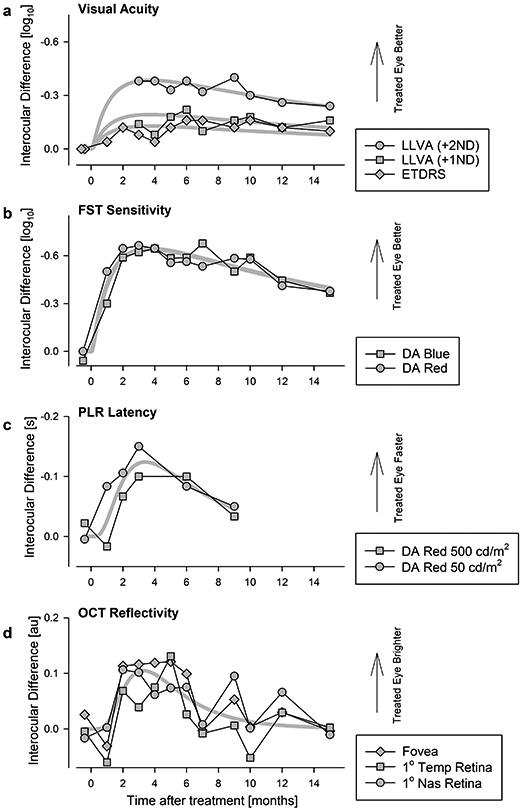
a, b, Interocular difference in visual acuity (a) and FST light sensitivity (b). Peak improvement occurs near month 3 followed by a slow decline over 12 months. At 15 months, all measures remain better than baseline. Visual acuities measured under standard (ETDRS) and two low-luminance (LLVA) conditions. Symbols in panel a are individual data points. Symbols in panel b are differences of means from 6-20 replicates. c, Pupil constriction latency as an objective measure of visual function demonstrates an interocular difference that peaks at 3 months and declines thereafter. Symbols are differences of means from 1-3 replicates. d, Magnitude of OCT reflectivity near the photoreceptor ciliary transition zone showing a transient peak at 3-5 months followed by decline to baseline by 15 months. Symbols are differences of means from 3 replicates. Thick gray lines represent the 3-parameter log-normal fits to data in individual sets (a) or in combination (b-d).
During the 15-months following the single sepofarsen dose, P11 had no clinical evidence of retinal changes. To confirm, retinal pigment epithelium health was quantitatively evaluated and there was no evidence of RPE melanization changes both in terms of the extent of the retained central island as well as the intensity of the near-infrared autofluorescence near the fovea (Extended Data Fig.6). Qualitative evaluation of the retinal cross-sectional images with OCT showed no obvious changes (Extended Data Fig.7). Considering the molecular defect, quantitative features of the photoreceptors were evaluated (Extended Data Fig.8a) at 7 locations across the central 6° diameter region. The thickness of the outer nuclear layer (ONL) containing photoreceptor nuclei showed no changes from baseline over 15 months in the treated eye that was greater than the expected test-retest variability (Extended Data Fig.8b). The IS layer of photoreceptors is where proteins are synthesized and trafficked to the cilia. The thickness of the IS layer was mostly unchanged from baseline in both eyes except for a transient increase at the fovea and a neighboring location near 4-months post-injection (Extended Data Fig.8c, arrows). The thickness of the OS layer where phototransduction occurs showed no changes over 15 months in both eyes (Extended Data Fig.8d).
CEP290 protein is located at the photoreceptor ciliary transition zone between the IS and OS. A major backscatter signal on OCT originating from near the IS/OS junction was previously shown to have abnormally low intensity in CEP290-LCA4. In P11, IS/OS intensity was also low but showed a transient increase in the treated eye only between 2 and 4 months followed by a decline to baseline (Extended Data Fig.8e, arrows). The time-courses of the interocular difference of IS/OS intensity at the fovea and two neighboring locations (Fig.1d) were concordant with those of vision measures (Fig.1a-c) implying a correlation between structure and function.
Retained oculomotor control in P11 with foveal fixation and a small amplitude nystagmus (Extended Data Fig.9) allowed the opportunity to evaluate the spatial distribution of light sensitivity. With free-viewing perimetry, there were distinct improvements in sensitivity across the central 4° of the treated eye only (Fig.2a). Foveal and 2° eccentric locations reached nearly their treatment potential (Fig.2a) estimated with artificial intelligence from the retinal structure8. To better understand the changes in central visual field, microperimetry was performed at higher spatial density with tracking and compensation of eye movements in real time (Fig.2b). In the treated eye, sensitivity increased by more than 1 log unit across the central retina. Estimated extent of the visual field at baseline was 2.6° and 3.3° in treated and untreated eyes, respectively (Fig.2b). Four months after treatment, the visual field extent widened to 7° in the treated eye but reduced to 1.8° in the untreated eye. Interocular difference of visual field extent showed a peak 1-4 months post-injection (Fig.2c). Unprompted, P11 reported substantially improved ability to read after the treatment. Combined effects of visual acuity and visual field extent measured at baseline and month 4 was used in a quantitative simulation to provide an explanation of the possible changes in reading performance. According to this simulation, the study patient would be expected to see smaller font (8 points instead of 14 points) and larger number of words (5 words instead of 1 word) after treatment compared to baseline (Fig.2d).
Figure 2: Improvement of Foveal Sensitivity, Expansion of Visual Field and Predicted Effect on Reading Performance.
a, b, Visual field sensitivities across the central 8° diameter region with free-viewing perimetry (a) and gaze controlled microperimetry (b) at baseline (black), at 4 months (red), and other times (gray) during 15 months of followup. Maximum treatment (Tx) potential predicted from artificial intelligence is shown as thick red lines. In the untreated eye, there are no changes. In the treated eye, there are major improvements within the central 4° starting at month 1 reaching a peak at month 4 and remaining well above baseline by month 15. Gray horizontal dashed line (b) is the sensitivity criterion at which visual field extent was estimated. Nf, Tr refer to nasal visual field and temporal retina, respectively. NS, not seen. All symbols represent individual data points. c, Interocular difference of visual field extent demonstrating the peak effect and durability. d, Simulation of readability of text at baseline and month 4 in the treated eye taking into account visual acuity improvement (smaller font) and the expansion of the visual field (elliptical windows). Calibration bar would subtend 2° when text is appropriately enlarged and held at the standard 40 cm reading distance.
Inherited retinal diseases have been at the forefront of the development of innovative gene-specific treatments12. Two such approaches – AAV-vectored genomic editing and naked oligonucleotide therapeutics - are ongoing in clinical trials for the single most common mutation causing childhood blindness7,13. In general, a genomic editing approach is thought to require a single (albeit surgical) administration for potentially lifelong benefit, whereas RNA therapy using naked AONs are thought to require repetitive but less invasive IVT injections. Both approaches have broad appeal for applicability to scores of other monogenic diseases and the potential for becoming breakthrough therapeutics14,15. The efficacy or durability of the genomic editing approach has not been demonstrated in humans to date. Preliminary clinical results with sepofarsen suggest efficacy with repeat dosing7 but the durability of the effect after a single dose administration is unknown.
Following a single sepofarsen injection, the study patient P11 demonstrated substantial and spatiotemporally concordant improvements in all recorded subjective and objective measurements of visual function as well as retinal structure. Efficacy was detectable by one month, peaked near three months, and unexpectedly, remained better than pre-treatment values by all functional measures to at least 15 months. The onset and peak of efficacy would be expected to depend on the kinetics of additional normal CEP290 protein synthesis driven by sepofarsen which is estimated to be rapidly taken up into rabbit photoreceptors and remain with a 2-month half-life10,16. Once sepofarsen concentration in photoreceptors declines and the new CEP290 synthesis ceases, long term durability of the efficacy would be dominated by the natural degradation of CEP290 protein already inserted at the cilial transition zone. The largest compartments of photoreceptor cilia are outer segments which show extreme turnover by being rebuilt every 10 days17. All outer segment proteins must traffic through the ciliary transition zone where CEP290 is located18,19 but synthesis and degradation rates of transition zone proteins are mostly unknown, and likely to be different between rod and cone photoreceptors. We now know that efficacy in the study patient was spatially located to the highly specialized foveal cone photoreceptors of the retina. Thus, our results place a lower limit of 12 months (or longer) to the natural degradation rate of ciliary CEP290 protein at the foveal cone photoreceptors and provide a patient-centric approach for optimizing administration frequency of naked AONs.
The c.2991+1655A>G allele is thought to be hypomorphic with the splicing defect occurring in some, but not all, mRNA transcripts in vitro2,10. It may be hypothesized that patients homozygous for the deep intronic allele, including P11, should demonstrate a milder phenotype compared to most of the patients who are compound heterozygotes with a second allele that has an inactivating mutation. However, retinal structure phenotype of P11 was identical to the stereotypical findings in nearly all CEP290-LCA patients consisting of retained central photoreceptors surrounded by severe retina-wide degeneration3-8. In terms of foveal function, relatively retained baseline visual acuity of P11 was within the range of 20/25 to 20/400 acuities reported in 30% of homozygotes and 28% of compound heterozygotes3-5,20-25. Thus, current evidence does not support P11 to have an exceptionally mild phenotype before treatment, but it remains to be seen whether the durability of the visual improvement after a single dose in P11 could also be applicable to compound heterozygous patients.
Methods
Study medication and trial design.
This study complies with all relevant ethical regulations and was approved by the institutional review board of University of Pennsylvania. The proband case provided informed written assent with the parents providing informed written consent.
Sepofarsen (aka. QR-110) is a 17-mer single-stranded 2'-O-methyl modified phosphorothioate RNA antisense oligonucleotide (AON) designed for potential treatment of the vision loss experienced by subjects with Leber congenital amaurosis (LCA) due to the CEP290 c.2991+1655A>G p.(Cys998*) mutation. Sepofarsen is thought to bind to the exonic splicing enhancer sequence at intron 26 of the CEP290 pre-mRNA and modulate the RNA splicing process, blocking access to the active cryptic splicing site, and restoring preference for the wildtype splicing sites. A resulting increase of wildtype mRNA transcript is predicted to lead to an increase of functional CEP290 protein. Details have been previously described7,10.
An open-label study was designed to evaluate the safety and tolerability of sepfarsen administered via unilateral intravitreal (IVT) injection every three months for up to 1 year (ClinicalTrials.gov no. NCT03140969). The study was conducted according to the Declaration of Helsinki as well as according to the principles of Good Clinical Practice. There were three sites (Iowa City, US; Philadelphia, US; and, Ghent, Belgium) and Institutional Review Boards of the three institutions approved the studies. Preliminary results from the first 10 treated patients have been reported7. The current report describes a previously unreported eleventh patient (P11) who was treated at age 14 with a single IVT injection of 160 μg sepofarsen in 50 μL in the worse-seeing eye and followed without any further intervention for 15 months. The better-seeing eye was not treated. P11 was homozygous for the c.2991+1655A>G mutation, whereas the other 10 subjects previously reported were compound heterozygous.
Safety evaluations.
Ocular safety was assessed with standard eye examinations, including gradings of the anterior and posterior segment, and of the lens. Systemic safety was evaluated with physical examinations at screening and post dosing visits. Routine hematology, serum chemistry, prothrombin time (with international normalized ratio) were performed at screening and post dosing. All safety and efficacy data from all eleven patients completing the clinical trial will be reported separately. For the current report, there were no treatment-emergent adverse events recorded for P11. Regarding general and systemic parameters, there were no clinically significant changes in hepatic and renal functions, assessed by liver enzymes and glomerular filtration rate, respectively. There was no intra-ocular inflammation and there were no lenticular or retinal changes throughout the 15-month observation period following the single 160 ug injection.
Outcome measures of visual function, functional vision, and retinal structure.
Subjective and objective measures of visual function and functional vision together with imaging of the retinal and RPE microstructure were performed per clinical trial protocol. Importantly, all pre-treatment measures were duplicated at two visits ~30 days apart. In addition, P11 was a patient of our center for hereditary retinal degenerations and was evaluated two years earlier with detailed non-invasive assessments of visual function and retinal structure (P8 in ref5; P21 in ref8). Contemporaneous with the clinical trial, P11 was enrolled in additional research studies that had been approved by the University of Pennsylvania Institutional Review Board. These specialized non-invasive assessments provided further details of visual function and retinal structure that expanded the findings from the clinical trial protocol.
Standard (ETDRS) visual acuity.
Best corrected visual acuity (BCVA) was measured using Early Treatment Diabetic Retinopathy Study (ETDRS) methodology26 at the screening and the baseline visit and all post-injection visits starting at month 1 through month 15 per clinical trial protocol. BCVA was scored as the number of letters correctly read at 4 m and expressed as logarithm of the minimum angle of resolution (log10 MAR). Results are presented in Fig.2a, and Extended Data Figs.1,2.
Low-luminance visual acuity (LLVA).
Prompted by patient feedback, measured increases in dark-adapted sensitivity at the fovea and the long-known relationship between visual acuity and ambient light levels, LLVA was measured as a specialized assessment with 1ND and 2ND filters in a darkened room27. LLVA measures started at month 3 and were continued through month 15. There were no LLVA measures at baseline or months 1 and 2. Results are presented in Fig.1a and Extended Data Fig.2.
Full-field Stimulus Testing (FST).
Sensitivity to chromatic light flashes with full-field stimulus testing (FST) was developed specifically for patients with severe vision loss and oculomotor instability28-30. At all timepoints, three types of FST were performed. First, per clinical trial protocol, FST was performed dark-adapted with the commercial software using a binary thresholding algorithm and an unconstrained response-acceptance window31,32. Results from P11 and P1-P5 are presented in Extended Data Fig.1. In addition, chromatic FSTs were performed in P11 as a specialized assessment under dark-adapted and light-adapted conditions using a 4/2 dB staircase with two response reversals and a limited response-acceptance window to minimize the effect of extraneous responses not synchronized with the stimulus presentation. Results are presented in Fig.1b and Extended Data Fig.3.
Cross-sectional retinal imaging.
Spectral-domain Optical Coherence Tomography (OCT) was used to obtain cross-sectional imaging of the retina (RTVue-100; Optovue, Fremont, CA) per clinical trial protocol. All OCT images were aligned by straightening the major RPE reflection. Quantitative analyses of the retinal sub-lamination thicknesses and the backscatter intensity of the peak originating near the junction of inner and outer segments was performed using longitudinal reflectively profiles (LRP)4,7,33. In brief, groups of 34 neighboring LRPs were averaged at 7 locations centered at the fovea, and 1, 2, 3° eccentricity on horizontal scans. Peaks corresponding to outer plexiform layer (OPL), external limiting membrane (ELM), IS/OS junction, and OS/RPE junction were manually defined on three independent sets of data obtained in each eye at each visit. Layer thicknesses of ONL (OPL to ELM), IS (ELM to IS/OS), OS (IS/OS to OS/RPE) were calculated. IS/OS relative reflectivity was measured as the ratio of IS/OS peak backscatter value to the OS/RPE peak value. Test-retest variability for each of the four measures was estimated from the untreated eye, and used to determine potentially significant changes in the treated eye. Results are presented in Fig.1d and Extended Data Figs.1,7,8. In addition, treatment potential was estimated from the OCT scans using an artificial intelligence approach previously published8. Results on vision potential are presented in Fig.2a.
En face retinal imaging.
Near-infrared autofluorescence (NIR-AF) imaging was performed per clinical trial protocol using a confocal scanning laser ophthalmoscope (Spectralis HRA, Heidelberg Engineering, Heidelberg, Germany) as previously described3-5,34. For each eye, NIR-AF images from all visits were spatially aligned to the first baseline image (MATLAB, Mathworks, Natick, MA). Across all aligned images, NIR-AF signal intensity profile was calculated along 0.58°-wide bands centered on the fovea along the horizontal and vertical meridians35,36. Each profile was normalized by the average autofluorescence intensity within ±3° eccentricity of the fovea to compare the extents of centrally retained elliptical regions over the follow up visits to baseline. Results are shown in Extended Data Fig.6.
Pupillary light reflex (PLR).
Pupillometric recordings were performed per protocol after at least 40 min of binocular dark adaptation (RETIport; Roland Consult Stasche & Finger GmbH, Brandenburg an.der Havel, Germany). An infra-red sensitive video camera (uEye, IDS, Obersulm, Germany) acquired the ‘direct’ pupil constriction videos (in the stimulated eye with the contralateral eye patched) at 30 frames per second, starting from 1 s before the stimulus onset (pre-stimulus baseline) for a total duration of 15 s. Pupil magnification was fixed (cornea to camera distance 0.3 m, 0.05 mm/pixel). The pupil boundaries were detected and tracked automatically by the commercial software from the video record (Roland Consult Ver.1017.2.0.6). Post-acquisition quantitative assessments of pupil response latency were performed for responses from each eye evoked by two luminance levels (50 and 500 cd.m−2) of red (peak 630 nm) full-field brief (1 s) stimuli9. Latency was defined as the time to reach 0.3 mm constriction; 2 to 4 repetitions of each response were available and quantified. Recordings at months 12 and 15 were not analyzed because at those visits the patient was on sertraline with known side effects on pupillary function37,38. Results are shown in Fig.1c and Extended Data Fig.5.
Eye movement and fixation monitoring.
Since the study patient had comparatively good visual acuity, eye movements and location of fixation were recorded by video imaging of the retina under near-infrared (NIR) illumination as an additional assessment (MP1; Nidek Technologies America, Inc., Greensboro, NC, USA)39. Monocular fixation epochs of 10 s duration were recorded with a bright red stationary target subtending 1°. The reference retinal image obtained at the start of each epoch and the data defining the translation of the reference image during the recording epoch were exported and analyzed. An overall fixation instability measure based on the upper limit (mean+2 SD) radial extent was calculated. Results are shown in Extended Data Fig.9.
Perimetry.
Dark-adapted free-viewing static perimetry (Humphrey Field Analyzer, HFA-750i analyzer, Zeiss-Humphrey, Dublin, CA, USA) with monochromatic red (650 nm) stimulus (Goldmann V, 200 ms) was performed as a specialized assessment along the horizontal meridian8 considering P11 retained foveal fixation with fine nystagmus. Treatment potential was estimated from the retinal structure using an artificial intelligence approach8. A single red fixation was used to obtain sensitivities at parafoveal locations of 2, 4, and 6° eccentric. Fixation to the center of four lights forming a small diamond was used to obtain sensitivity of the fovea. Perimetric sensitivities and treatment potential are presented in Fig.2a. To obtain better correspondence between light sensitivity and defined retinal locations, a retina-tracking microperimeter (MP1, Nidek Inc, Fremont, CA) was used as a specialized assessment40. White stimuli (max luminance=127 cd/m2, Goldmann V, 200 ms) were presented on a red background (1 cd/m2). Fixation was a red cross and sensitivities were sampled every 0.5° between 0.5 and 10° eccentric along the horizontal meridian. A criterion sensitivity of half of the maximum luminance was used to estimate the extent of the visual field. Results are shown in Fig.2b-d.
Mobility.
The visual navigation challenge (ORA-VNC™) was used to assess mobility performance per protocol7. There were four courses, and each course had several lighting conditions defining 20 levels. For each level there were several combinations of obstacle placements (chosen randomly) to avoid learning effects. A level of 0 represented failing to pass successfully any of the courses at any light level, and a level of 19 corresponded to passing the most difficult course under dimmest light conditions. For each eye and each visit, the highest level course passed was recorded. Results are shown in Extended Data Fig.4.
Literature review.
To better understand the relation between genotype and visual acuity, we reviewed the CEP290-LCA literature where individual-level data with c.2991+1655A>G genotype and visual acuities were reported. There were 70 patients reported in seven publications5,20-25. 47 patients (67%) were compound heterozygotes, and 23 patients (33%) were homozygotes. 13 of 47 (28%) compound heterozygotes and 7 of 23 (30%) homozygotes had 20/400 or better vision; vision in the remaining patients in both groups were mostly LP (light perception only).
Statistical & Reproducibility.
Descriptive statistics (mean and standard deviation) for technical replicates were calculated with Excel (Microsoft, Redmond, WA) or Sigmaplot (Systat, San Jose, CA). No statistical method was used to predetermine sample size for the open-label non-randomized clinical trial. The investigators were not masked to the eye that received the intervention during outcome assessments.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Extended Data
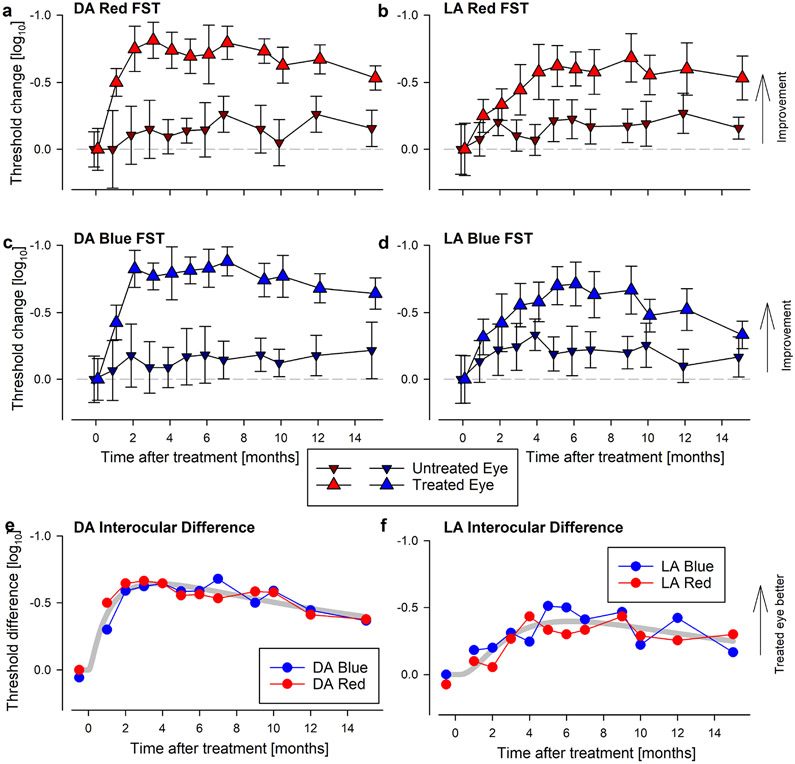
Extended Data Fig. 1. Retinal Structure, Visual Acuity and Light Sensitivity of the Study Patient P11 at Baseline and Three Months After Treatment.
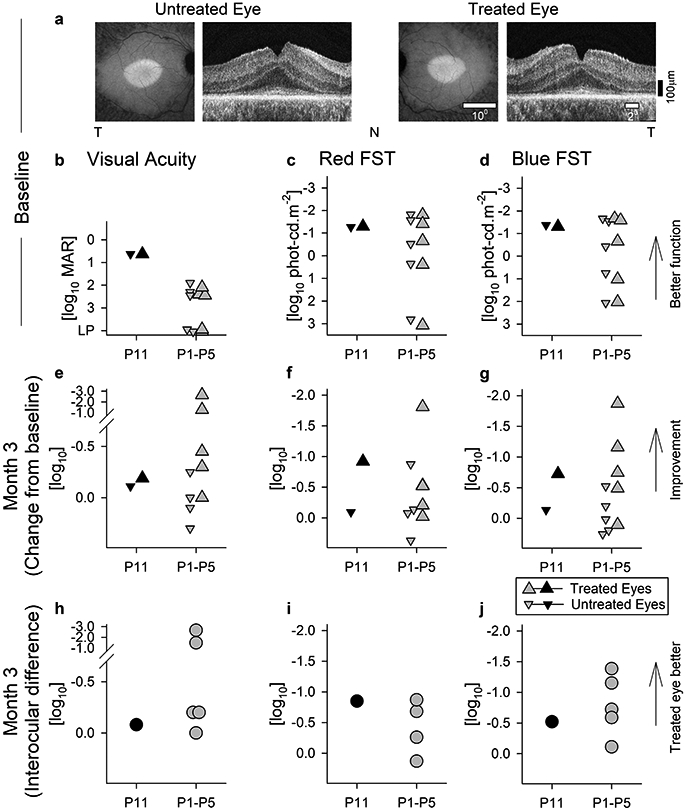
a, P11 retained a macular island of RPE and photoreceptor cells in both eyes before treatment as demonstrated by near-infrared (melanin) autofluorescence (left) and cross-sectional OCT imaging (right). T, temporal retina, N, nasal retina. b-d, Visual acuity and light sensitivity (red and blue FSTs) for both eyes of P11 compared to five others (P1-P5) at baseline. e-g, Visual acuity and sensitivity changes from baseline at 3 months after intravitreal injection of 160 μg sepofarsen into one eye. Treated eyes are larger up triangles, untreated eyes are smaller down triangles. h-j, Interocular difference of visual acuity and sensitivity at 3 months. b-j, Black symbols are data from P11, whereas gray symbols are from P1-P5 who received the same initial dose. After 3 months, P11 was continued to be followed without intervention whereas P1-P5 were redosed. Symbols in panels c and d are the mean value of 6-20 replicates. Symbols in panels b, e, and h are individual data points. Symbols in f, g, i and j are differences of means from 6-20 replicates.
Extended Data Fig. 2. Evaluation of visual acuity.
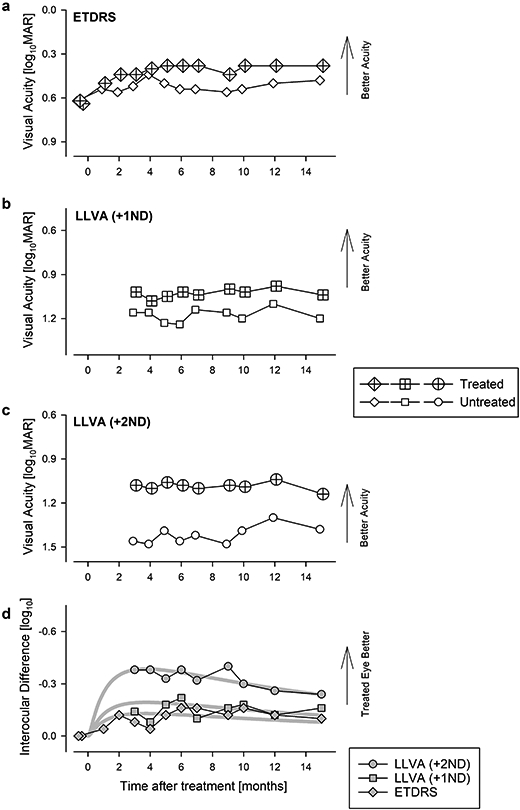
a, Change in best corrected ETDRS acuity from baseline where both eyes were symmetric. After treatment, there was a larger improvement in the treated eye compared to untreated eye. Acuity in the treated eye remained asymmetric and better than baseline at 15 months. b, c, Specialized assessments of low-luminance visual acuity (LLVA) starting at 3 months after treatment using neutral density filters, +1ND (b) and +2ND (c). Symbols in panels a,b, and c are individual data points. d, Interocular difference of different acuity measures. Thick gray lines represents the 3-parameter log Normal fit to data. Symbols in panel d are differences between individual data points from each eye. Panel d is duplicated in Fig.1a.
Extended Data Fig. 3. Evaluation of light sensitivity with FST.
a-d, Change in dark adapted (DA, a, c) and light adapted (LA, b, d) FST thresholds from baseline for red and blue stimuli. There is substantial improvement in the treated eye (larger triangles) by all four measures. DA sensitivities peak at 2-3 months and show slow decline after ~7 months. LA sensitivities peak later between 5-9 months and show a slow decline thereafter. All four measures remain above baseline at 15 months. Gray dashed line represents no change from baseline. Symbols and error bars represent mean±1SD from 6-20 technical replicates at each time point for each test. e, f, Interocular difference of DA (e) and LA (f) FST thresholds for red (red symbols) and blue (blue symbols) stimuli. Kinetics show peak improvement followed by slow decline. Symbols in panels e and f are differences of means from 6-20 replicates. Thick gray line represents the 3-parameter log Normal fit to data. Panel e is duplicated in Fig. 1b.
Extended Data Fig. 4. Changes to functional vision evaluated with visual navigation challenge (VNC) obstacle course.
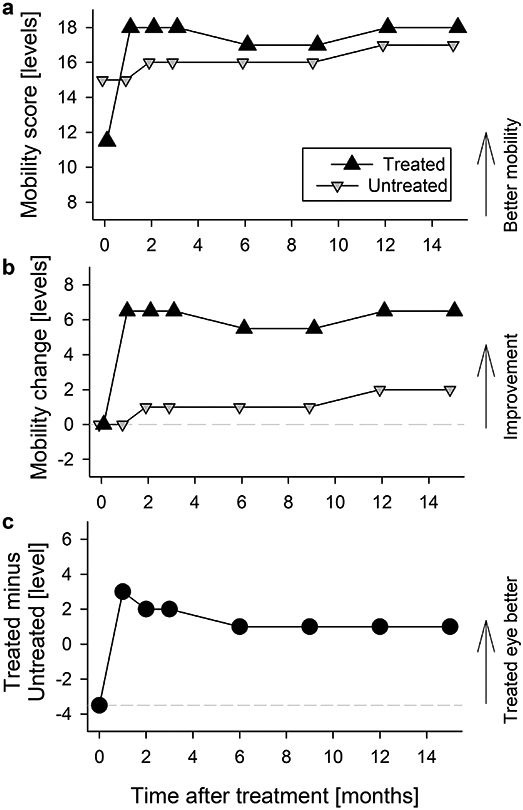
a, b, Best mobility levels passed in the treated eye (up triangle) and in the untreated eye (down triangle). There is a rapid improvement with treatment peaking at month 1 and sustained through month 15. The raw score representing number of course levels that was navigable (a) and change of navigable levels from baseline performance (b) are shown for both treated and untreated eyes. Symbols are individual data points. c, The interocular difference number of course levels that was navigable. Treated eye that was initially the worse eye becomes the better eye. Symbols are the differences between individual data points from each eye.
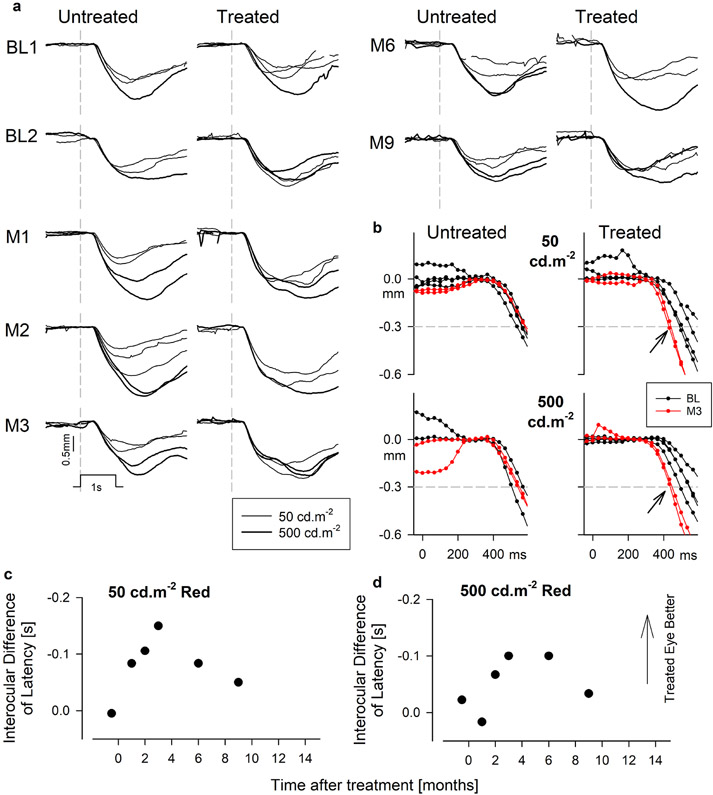
Extended Data Fig. 5. Changes in objective measures of visual function with dark adapted pupillometry.
a, Raw traces of pupillary diameter as a function of time for brief (1s) red flashes of 50 (thinner traces) and 500 (thicker traces) phot-cd.m−2 luminance at different visits. Vertical gray dashed lines represent the onset of the flash. Stimulus marker and scale bar are shown. b, Magnified view showing the early phases of constriction in the untreated and treated eyes at baseline (black) and month 3 (red) for the two stimuli. Horizontal dashed lines mark the criteria of 0.3 mm constriction used to define latency. Arrows demonstrate the acceleration of the response. c,d, Interocular differences of latency as a function of time after treatment for both red stimuli. Results show pupil constrictions accelerating to a peak at month 3 in the treated eye. Symbols are the differences of means from 1-3 replicates. Panels are duplicated in Fig.1c.
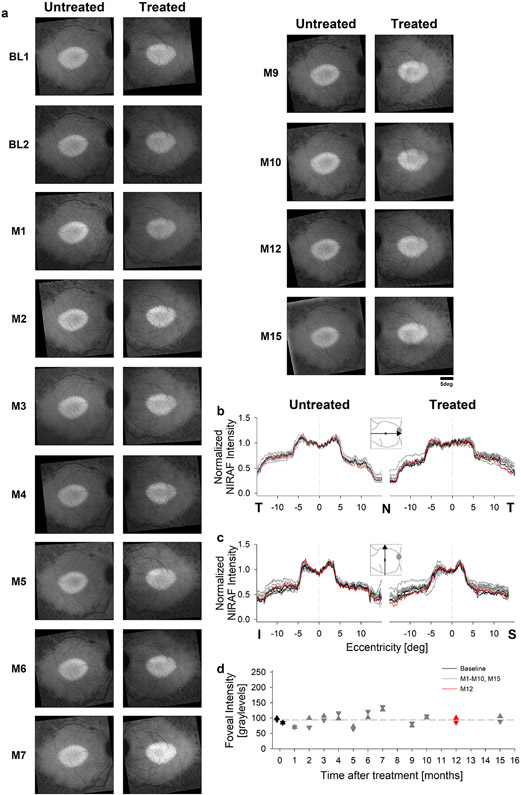
Extended Data Fig. 6. Lack of discernable changes in RPE melanization.
a, Near-infrared excited autofluorescence (NIRAF) images of the macular region in the untreated and treated eyes showing an elliptical region of preserved RPE melanization in both eyes qualitatively unchanged through month 15. b, c Normalized NIRAF intensity profiles as a function of retinal eccentricity in both horizontal (b) and vertical meridian (c). Month 12 (red traces) and baseline visit (black traces) are highlighted to demonstrate lack of change. Vertical dashed black line represents the fovea. d, Foveal NIRAF intensity used for normalization of the traces in panels b and c. Symbols are individual data points.
Extended Data Fig. 7. Retinal cross-sectional structure.
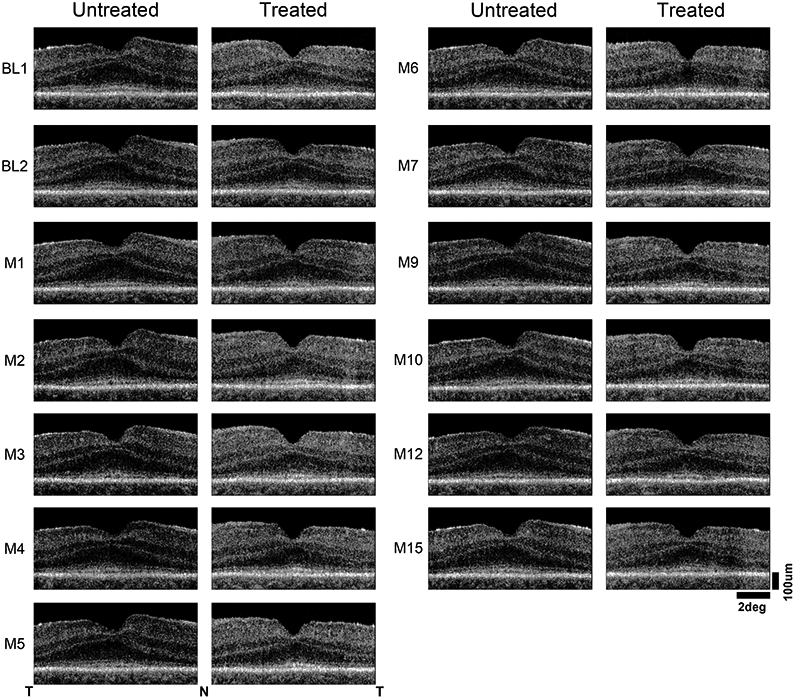
OCT scans along the horizontal meridian crossing the fovea illustrating the laminar architecture observed through month 15 in the treated and untreated eyes. Nasal (N), temporal retina (T) are marked and scale bars are provided.
Extended Data Fig. 8. Quantification of the photoreceptor sublaminae for 15 months following treatment.
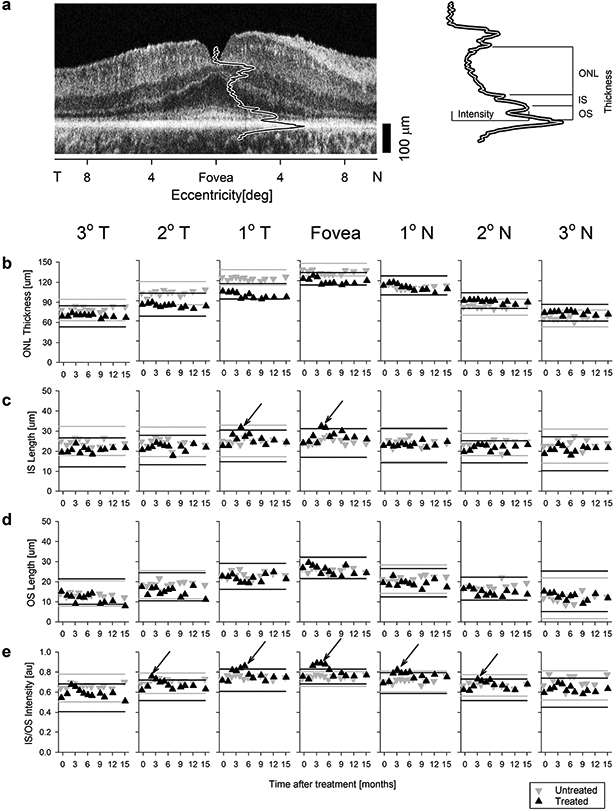
a, Representative OCT scan showing the foveal longitudinal reflectivity profile (LRP) and the three thickness parameters (ONL thickness, IS length, and OS length) and one intensity parameter (IS/OS intensity) quantified. b-e, Photoreceptor sublaminae parameters consisting of outer nuclear layer (ONL) thickness (b), inner segment (IS) length (c), outer segment (OS) length (d), and IS/OS intensity (e) are shown. Seven panels on each row represent data from 7 retinal locations as a function of time after treatment. Gray and black lines delimit test-retest variability in the untreated and treated eye, respectively. Black arrows point to transient changes in IS length and IS/OS intensity between months 2 and 5 in the treated eye (black up triangle). T, temporal retina; N, nasal retina. Each symbol in panels b-e represents the average of three replicates. Interocular differences of the IS/OS intensity at fovea and 1° T and N eccentricities shown in panel e are plotted in Fig.1d.
Extended Data Fig. 9. Fixational stability.
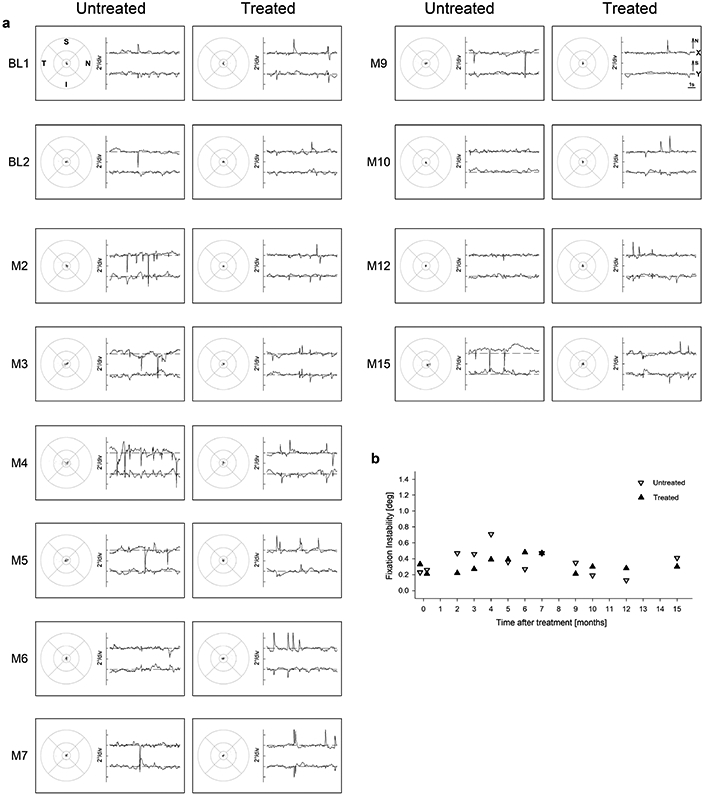
a, Eye movement data during fixation to a large visible red target are shown in spatial (left) and spatiotemporal (right) coordinates. Spatial distribution of fixation clouds is shown on standard circles (radii at 1.65°, 5°, and 10°) representing the macular region centered on the anatomical foveal depression. Spatiotemporal distribution of eye movements is shown on chart records for x and y directions; up is nasal retina for x and superior retina for y. b, Fixation instability as a function of time after treatment is unchanged in both the treated (up-triangle) and untreated eye (down-triangle). Symbols are individual data points.
Acknowledgements
This work was supported by a clinical trial contract from ProQR to A.V.C administered by the University of Pennsylvania.
Footnotes
Competing interests
M.R.S. and A.G. are employees of ProQR. All other authors have no competing interests.
Data Availability
All relevant patient-level data are displayed in Figures. All requests for data will be reviewed by ProQR Therapeutics and the University of Pennsylvania to verify whether the request is subject to any intellectual property or confidentiality obligations. Patient-related data may be subject to confidentiality. Any data that can be shared will be released.
References
- 1.Hanany M, Rivolta C, Sharon D. Worldwide carrier frequency and genetic prevalence of autosomal recessive inherited retinal diseases. Proc Natl Acad Sci U S A. 2020;117(5):2710–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.den Hollander AI, Koenekoop RK, Yzer S, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79(3):556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cideciyan AV, Aleman TS, Jacobson SG, et al. Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: implications for therapy of Leber congenital amaurosis. Hum Mutat. 2007;28(11):1074–1083. [DOI] [PubMed] [Google Scholar]
- 4.Cideciyan AV, Rachel RA, Aleman TS, et al. Cone photoreceptors are the main targets for gene therapy of NPHP5 (IQCB1) or NPHP6 (CEP290) blindness: generation of an all-cone Nphp6 hypomorph mouse that mimics the human retinal ciliopathy. Hum Mol Genet. 2011;20(7):1411–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson SG, Cideciyan AV, Sumaroka A, et al. Outcome measures for clinical trials of Leber congenital amaurosis caused by the intronic mutation in the CEP290 gene. Invest Ophthalmol Vis Sci. 2017;58(5):2609–2622. [DOI] [PubMed] [Google Scholar]
- 6.Cideciyan AV, Jacobson SG. Leber congenital amaurosis (LCA): Potential for improvement of vision. Invest Ophthalmol Vis Sci. 2019;60(5):1680–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cideciyan AV, Jacobson SG, Drack AV et al. Effect of an intravitreal antisense oligonucleotide on vision in Leber congenital amaurosis due to a photoreceptor cilium defect. Nat Med 2019;25:225–228. [DOI] [PubMed] [Google Scholar]
- 8.Sumaroka A, Garafalo AV, Semenov EP, et al. Treatment potential for macular cone vision in Leber congenital amaurosis due to CEP290 or NPHP5 mutations: Predictions from artificial intelligence. Invest Ophthalmol Vis Sci. 2019;60(7):2551–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnan AK, Jacobson SG, Roman AJ, et al. Transient pupillary light reflex in CEP290- or NPHP5-associated Leber congenital amaurosis: Latency as a potential outcome measure of cone function. Vision Res. 2020;168:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulla K, Aguila M, Lane A, et al. Splice-modulating oligonucleotide QR-110 restores CEP290 mRNA and function in human c.2991+1655A>G LCA10 models. Mol Ther Nucleic Acids. 2018;12:730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walia S, Fishman GA, Jacobson SG, et al. Visual acuity in patients with Leber's congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology. 2010;117(6):1190–1198. [DOI] [PubMed] [Google Scholar]
- 12.Trapani I, Auricchio A. Seeing the light after 25 years of retinal gene therapy. Trends Mol Med. 2018;24(8):669–681. [DOI] [PubMed] [Google Scholar]
- 13.Maeder ML, Stefanidakis M, Wilson CJ, et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat Med. 2019;25(2):229–233. [DOI] [PubMed] [Google Scholar]
- 14.Doudna JA. The promise and challenge of therapeutic genome editing. Nature. 2020;578(7794):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoch KM, Miller TM. Antisense oligonucleotides: Translation from mouse models to human neurodegenerative diseases. Neuron. 2017;94(6):1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry SP, Miner RC, Drew WL, et al. Antiviral activity and ocular kinetics of antisense oligonucleotides designed to inhibit CMV replication. Invest Ophthalmol Vis Sci. 2001;42(11):2646–2651. [PubMed] [Google Scholar]
- 17.Kocaoglu OP, Liu Z, Zhang F, Kurokawa K, Jonnal RS, Miller DT. Photoreceptor disc shedding in the living human eye. Biomed Opt Express. 2016;7(11):4554–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baehr W, Hanke-Gogokhia C, Sharif A, Reed M, Dahl T, Frederick JM, Ying G. Insights into photoreceptor ciliogenesis revealed by animal models. Prog Retin Eye Res. 2019;71:26–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potter VL, Moye AR, Robichaux MA, Wensel TG. Superresolution microscopy reveals photoreceptor-specific subciliary location and function of Cep290. BioRxiv preprint server. 10.1101/2020.10.28.357806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Littink KW, Pott JW, Collin RW, et al. A novel nonsense mutation in CEP290 induces exon skipping and leads to a relatively mild retinal phenotype. Invest Ophthalmol Vis Sci. 2010;51(7):3646–3652. [DOI] [PubMed] [Google Scholar]
- 21.Pasadhika S, Fishman GA, Stone EM, et al. Differential macular morphology in patients with RPE65-, CEP290-, GUCY2D-, and AIPL1-related Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2010;51(5):2608–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yzer S, Hollander AI, Lopez I, et al. Ocular and extra-ocular features of patients with Leber congenital amaurosis and mutations in CEP290. Mol Vis. 2012;18:412–425. [PMC free article] [PubMed] [Google Scholar]
- 23.Collison FT, Park JC, Fishman GA, McAnany JJ, Stone EM. Full-field pupillary light responses, luminance thresholds, and light discomfort thresholds in CEP290 Leber congenital amaurosis patients. Invest Ophthalmol Vis Sci. 2015;56(12):7130–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheck L, Davies WIL, Moradi P, et al. Leber congenital amaurosis associated with mutations in CEP290, clinical phenotype, and natural history in preparation for trials of novel therapies. Ophthalmology. 2018;125(6):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldhaus B, Weisschuh N, Nasser F, et al. CEP290 mutation spectrum and delineation of the associated phenotype in a large German cohort: A monocentric study. Am J Ophthalmol. 2020;211:142–150. [DOI] [PubMed] [Google Scholar]
- 26.Ferris FL 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. [PubMed] [Google Scholar]
- 27.Sunness JS, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(9):1480–1488.e14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roman AJ, Schwartz SB, Aleman TS, et al. Quantifying rod photoreceptor-mediated vision in retinal degenerations: dark-adapted thresholds as outcome measures. Exp Eye Res. 2005;80(2):259–272. [DOI] [PubMed] [Google Scholar]
- 29.Roman AJ, Cideciyan AV, Aleman TS, Jacobson SG. Full-field stimulus testing (FST) to quantify visual perception in severely blind candidates for treatment trials. Physiol Meas. 2007;28(8):N51–N56. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson SG, Cideciyan AV, Peshenko IV, et al. Determining consequences of retinal membrane guanylyl cyclase (RetGC1) deficiency in human Leber congenital amaurosis en route to therapy: residual cone-photoreceptor vision correlates with biochemical properties of the mutants. Hum Mol Genet. 2013;22(1):168–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein M, Birch DG. Psychophysical assessment of low visual function in patients with retinal degenerative diseases (RDDs) with the Diagnosys full-field stimulus threshold (D-FST). Doc Ophthalmol. 2009;119(3):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collison FT, Fishman GA, McAnany JJ, Zernant J, Allikmets R. Psychophysical measurement of rod and cone thresholds in Stargardt disease with full-field stimuli. Retina. 2014;34(9):1888–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cideciyan AV, Hufnagel RB, Carroll J, et al. Human cone visual pigment deletions spare sufficient photoreceptors to warrant gene therapy. Hum Gene Ther. 2013;24(12):993–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cideciyan AV, Swider M, Aleman TS, et al. Reduced-illuminance autofluorescence imaging in ABCA4-associated retinal degenerations. J Opt Soc Am A. 2007;24(5):1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrera W, Aleman TS, Cideciyan AV, et al. Retinal disease in Usher syndrome III caused by mutations in the clarin-1 gene. Invest Ophthalmol Vis Sci. 2008;49(6):2651–2660. [DOI] [PubMed] [Google Scholar]
- 36.Gibbs D, Cideciyan AV, Jacobson SG, Williams DS. Retinal pigment epithelium defects in humans and mice with mutations in MYO7A: imaging melanosome-specific autofluorescence. Invest Ophthalmol Vis Sci. 2009;50(9):4386–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saletu B, Grünberger J. Drug profiling by computed electroencephalography and brain maps, with special consideration of sertraline and its psychometric effects. J Clin Psychiatry. 1988;49 Suppl:59–71. [PubMed] [Google Scholar]
- 38.Granholm E, Morris S, Galasko D, et al. Tropicamide effects on pupil size and pupillary light reflexes in Alzheimer's and Parkinson's disease. Int J Psychophysiol. 2003;47:95–115. [DOI] [PubMed] [Google Scholar]
- 39.Cideciyan AV, Aguirre GK, Jacobson SG, et al. Pseudo-fovea formation after gene therapy for RPE65-LCA. Invest Ophthalmol Vis Sci. 2014;56(1):526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cideciyan AV, Swider M, Aleman TS, et al. Macular function in macular degenerations: repeatability of microperimetry as a potential outcome measure for ABCA4-associated retinopathy trials. Invest Ophthalmol Vis Sci. 2012;53(2):841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant patient-level data are displayed in Figures. All requests for data will be reviewed by ProQR Therapeutics and the University of Pennsylvania to verify whether the request is subject to any intellectual property or confidentiality obligations. Patient-related data may be subject to confidentiality. Any data that can be shared will be released.