Abstract
Kaposi’s sarcoma (KS) is a malignancy of vascular origin. It is caused by the Kaposi’s sarcoma-associated herpes virus (KSHV). Immune dysregulation is a key feature in the development and progression of KS. The main aim of this study was to determine and compare circulating CD4+ and CD8+ T cell subsets including their expression of CD40 ligand (CD40L) and programmed cell death protein 1 (PD1), natural killer (NK) cells, and NK T cells between individuals with active HIV-associated KS versus those in remission.
We found that the proportion of CD4+ T cells was significantly higher in individuals in remission compared to those with active KS (26.3% vs 13.9%; p=0.01). We also observed that the proportion of CD4+ T cells and central memory CD4+ T cells expressing CD40L was significantly higher in individuals with active KS versus those in remission, (10.6% vs 5.4%; p=0.03) and (14.8% vs 5.9%; p=0.01) respectively. There was no significant difference in proportion of CD4+ and CD8+ naïve, central memory, effector memory, and terminal effector cells between the two groups. In addition, there was no difference in expression of PD1 on the T cell subsets between the two groups. Furthermore, the proportion of NK cells and NK T cells were not differential between individuals with active disease versus those in remission. CD40L expression is higher in individuals with active HIV-associated KS compared to those in remission. The proportion of CD4+ T cells is higher in individuals in remission compared to those with active HIV-associated KS.
Keywords: Kaposi’s sarcoma, HIV, T cell subsets, CD40L, PD1, Natural killer cells
Introduction
The vascular malignancy Kaposi’s sarcoma (KS) is highly prevalent among HIV-infected individuals [1]. It is caused by the Kaposi’s sarcoma-associated herpesvirus (KSHV), also known as human herpes virus type 8 [2]. There are 4 major types of KS including endemic KS, epidemic (HIV-associated) KS, classical KS, and iatrogenic KS. Epidemic KS is by far the most common type of KS. Epidemic KS incidence dramatically increased during the early years of the HIV pandemic. With the introduction and wide-availability of antiretroviral therapy, there was a dramatic decrease in the incidence of epidemic KS, especially in developed countries [3]. However, in some low and middle-income settings including ours, there is still a high prevalence of HIV-associated KS in the ART era [4, 5].
Immune dysregulation and immunosuppression appear to be key in the development of most types of KS. For instance, iatrogenic KS is a result of immunosuppressive therapy, while epidemic KS results from HIV-induced immunosuppression [6, 7]. For epidemic KS, low CD4 count and high HIV viral loads are associated with a high risk of KS tumorigenesis [8]. Subsets of CD4 and CD8 T cells have significance in predicting the development and/or progression of diseases including cancer [9]. In addition, these cells can express molecules such as PD1 that can inhibit T cells from identifying and killing tumor cells, and CD40L which is a co-stimulatory glycoprotein that may have pro-apoptotic or anti-apoptotic effects [10]. Also, natural killer (NK) cells are important in killing tumor cells and viral-infected cells [7]. Whether T cell subsets, PD1 and CD40L expression, and/or NK cells are differential in individuals with active KS versus KS in remission has not been extensively studied.
The main objective of this study was to determine and compare CD4 and CD8 T cell subsets and markers including programmed death ligand 1 (PD1) and CD40 ligand (CD40L) on these subsets, Natural Killer (NK) cells, and Natural Killer T (NK T) cells between individuals in remission versus those who had active KS disease.
Materials and Methods
This was a cross-sectional study. We compared individuals with active HIV-associated KS to those in remission for HIV-associated KS. Individuals with active KS disease were chemotherapy-naïve and newly-diagnosed with KS. Individuals in remission for KS had been treated previously with chemotherapy (Doxorubicin, Bleomycin, and Vincristine) and were in complete remission. At the time of recruitment, individuals in remission had been off chemotherapy for at least one month or more. All the study participants in both groups were on ART.
This study compared T cell subsets, NK cells, and NK T cells between individuals that had active HIV-associated KS versus those who were in remission. We stained whole blood for 30 minutes with the following antibodies: CD3 APC-H7, CD4 PE, CD8 PE-Cy7, CD45RO APC, CCR7 BB515, CD40L BB700, PD1 BB700, CD16 APC, and CD56 BB515. The antibodies were obtained from BD Biosciences (Belgium). Flow cytometry was performed on a 6-color BD FacsVerse instrument. Fluorescence-minus-one (FMO) controls were used to identify and gate cell populations. Analysis of the flow data was done using Flow Jo version 10. The gating strategies used are shown on S1 Fig.
The Wilcoxon Rank-sum test was used to compare differences in T cell subsets, NK cells, and NK T cells between individuals in remission compared to those with active KS disease.
Results
We recruited and compared 10 individuals who were in remission to 8 individuals that had active HIV-associated KS disease [Table 1]. The proportion of CD4+ T cells was significantly higher among individuals who were in remission compared to those with active KS disease. The proportion of CD4+ T cells expressing CD40L was significantly lower in individuals who were in remission compared to those with active KS disease. CD4+ T cell subsets including naïve, central memory, effector memory, and terminal effector cells were not significantly different between individuals in remission versus those with active KS disease. Expression of CD40L on these subsets was only significantly different for central memory cells where it was significantly higher among those with active disease than individuals in remission (Figure 1). The expression of PD1 on CD4+ T cells and all the subsets was not significantly different between individuals in remission versus those with active disease (Table 2). There was no difference in proportion of CD8+ T cells, their subsets, and expression of CD40L or PD1 between individuals in remission versus those with active disease (Table 3). In addition, there was no difference in NK cells or NK T cells in individuals in remission versus those with active disease (Table 4).
Table 1.
Characteristics of Study Participants by Disease Status
| KS in Remission (N=10) |
Active KS (N=8) |
|
|---|---|---|
| Age in Years | 40 [29-43] | 38 [30.5-41.5] |
| Males | 6 (60%) | 5 (62.5%) |
| Smoking | 1 (10%) | 1 (12.5%) |
| Alcohol | 4 (40%) | 0 (0%) |
| HIV Positive | 10 (100%) | 8 (100%) |
| CD4 Count (cells/μl) | 275 [223-422] | 305 [172-377] |
Figure 1.
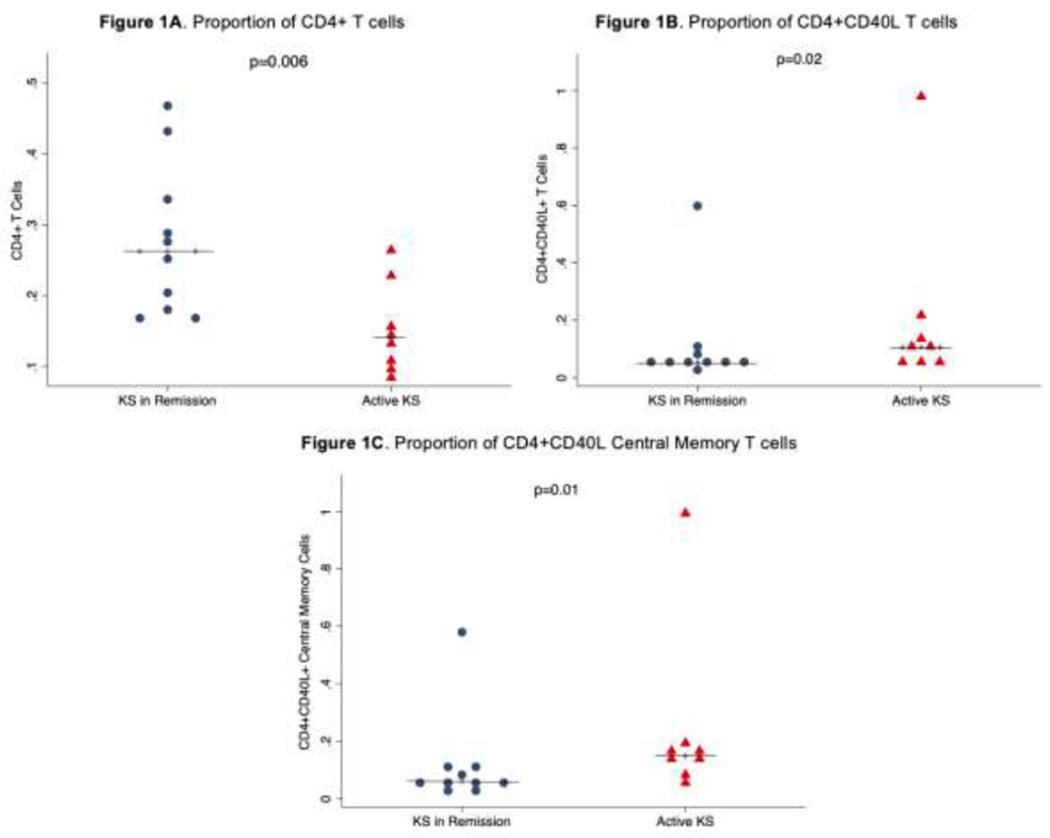
A) The proportion of CD4+ T cells was higher in individuals in KS remission compared to those with active KS; B) The proportion of CD4+ T cells expressing CD40L was significantly higher in individuals with active KS compared to those in remission; C) The proportion of central memory CD4+ T cells expressing was significantly higher in individuals with active KS compared to those in remission.
Table 2.
CD4 T cell subsets in Active KS versus KS in Remission
| KS in Remission | Active KS | P value | |
|---|---|---|---|
| All CD4 T cells | 26.3% [18-33.3] | 13.9% [10.2-19.1] | 0.01 |
| CD40 Ligand | 5.4% [4.4-7.2] | 10.6% [6.5-17.8] | 0.03 |
| PD1 | 27.2% [22.2-35] | 28.1% [20.2-49.8] | 0.79 |
| Naïve T cells | 30.5% [18.6-39.9] | 27.3% [10.3-45.5] | 1.00 |
| CD40 Ligand | 3.3% [2.9-4.0] | 7.1% [4.8-18.1] | 0.05 |
| PD1 | 2.1% [1.1-3.6] | 1.8% [1-3.4] | 0.89 |
| Central Memory Cells | 37.7% [35.1-42.9] | 41.5% [32.5-45.6] | 0.86 |
| CD40 Ligand | 5.9% [4.5-11.4] | 14.8% [11.4-18] | 0.01 |
| PD1 | 31.8% [26-35.3] | 40.8% [22.8-60] | 0.53 |
| Effector Memory Cells | 27.9% [16.8-30.1] | 27.7% [20.8-43.1] | 0.53 |
| CD40 Ligand | 5.3% [4-7.7] | 6.7% [4.4-12.2] | 0.72 |
| PD1 | 56.2% [44.7-63.1] | 77.4% [57.4-85.7] | 0.06 |
| Terminal Effector Cells | 1.2% [0.8-2.4] | 0.6% [0.3-1.5] | 0.18 |
| CD40 Ligand | 8.4% [0-25%] | 5% [0-18.8] | 0.68 |
| PD1 | 27.9% [16.7-45] | 30% [0-72] | 0.69 |
Table 3.
CD8 T cell subsets in Active KS versus KS in Remission
| KS in Remission | Active KS | P value | |
|---|---|---|---|
| All CD8 T cells | 21% [14.2-26.7] | 21.9% [12.2-26.8] | 0.79 |
| CD40 Ligand | 1.9% [1.1-6.2] | 4.9% [3.4-8.6] | 0.25 |
| PD1 | 23.4% [12.6-30.3] | 23.6% [11.9-33.2] | 0.76 |
| Naïve T cells | 24.1% [7.3-32.6] | 12.3% [11.1-24] | 0.72 |
| CD40 Ligand | 3.1% [1.8-5.9] | 9.2% [2.4-14.5] | 0.40 |
| PD1 | 6.8% [2.4-8.9] | 13% [4.7-18.9] | 0.16 |
| Central Memory Cells | 1.6% [0.9-2.2] | 1.1% [0.8-2.6] | 0.72 |
| CD40 Ligand | 7% [0-12.5] | 11.3% [0-22.7] | 0.52 |
| PD1 | 28.1% [11.1-33.3] | 26.1% [8.6-48.9] | 0.62 |
| Effector Memory Cells | 10.7% [9.7-12] | 13.8% [9.2-21.3] | 0.72 |
| CD40 Ligand | 1.8% [0.2-7.8] | 5.8% [1.3-11.3] | 0.42 |
| PD1 | 44.3% [24.7-50.9] | 38% [22.1-53.5] | 0.93 |
| Terminal Effector Cells | 62.8% [56.3-70.4] | 64.8% [56.5-73.1] | 0.86 |
| CD40 Ligand | 1% [0.5-4.1] | 3.8% [2.3-4.8] | 0.21 |
| PD1 | 25.7% [16.7-38.1] | 18.9% [8.5-36.1] | 0.93 |
Table 4.
NK and NK T cells in Active KS versus KS in Remission
| KS in Remission | Active KS | P value | |
|---|---|---|---|
| CD3-CD16+ Cells | 9.6% [4.7-16.8] | 11.1% [8.1-13.9] | 0.66 |
| CD3-CD56+ Cells | 5.5% [3.0-8.7] | 4.2% [1.2-6.6] | 0.33 |
| CD3-CD16+CD56+ Cells | 16.1% [11.2-23.8] | 12.4% [2.6-17.8] | 0.25 |
| CD3+CD8+CD16+ Cells | 0.7% [0.5-0.8] | 1.5% [0.7-3.8] | 0.10 |
| CD3+CD8+CD56+ Cells | 7.8% [3.9-10.7] | 11.8% [5.7-19.6] | 0.13 |
| CD3+CD8+CD16+CD56+ Cells | 0.1% [0.0-0.2] | 0.1% [0.0-0.5] | 0.89 |
Discussion
This was a study on immunophenotyping T and NK cells in individuals with active HIV-associated KS versus those in remission. When we compared individuals in remission to those with active KS disease, the proportion of CD4 T cells was significantly higher among those in remission than those with active KS disease. Previous studies have also observed that CD4 percentage is a better predictor of the occurrence of AIDS-related events than CD4 counts [11]. This suggests that proportion of CD4 T cells is a better predictor of KS disease status than absolute CD4 T cell counts.
The CD4 and CD8 T cell subsets including naïve, central memory, effector memory, and effector cells were not differential between individuals with active disease versus those in remission. Expression of the immune checkpoint PD1 on these subsets was also not significantly different between individuals with active disease and those in remission. However, expression of CD40L was significantly higher among all CD4 T cells and among the central memory CD4 T cells in individuals with active HIV-associated KS compared than those who were in remission. CD40 ligand is a co-stimulatory glycoprotein that is predominantly and transiently expressed on activated CD4+ T cells, and binds to CD40 which is expressed by antigen presenting cells and tumors [12]. When expressed by a subset of CD4+ T cells called T follicular helper cells (TFH), CD40L binds to CD40 expressed on B cells and promotes B cell maturation, and thereby promotes immunoglobulin class switching and affinity maturation [13]. In cancer, CD40 is known to be expressed in many types of malignancies, and the CD40-CD40L interaction can lead to expression of pro-apoptotic or anti-apoptotic proteins which can result in either regression or proliferation of malignancies [10, 14]. In KS, the CD40 expression on tumor cells and its interaction with CD40L has been observed to promote the growth, survival, and neovascularization of KS tumors [15, 16]. In addition, CD40L has been shown to activate KSHV-infected B cells which leads to replication of KSHV in B cells [17, 18]. These observations may partly explain the activation and anti-KSHV antibody production of KSHV-infected B cells in individuals with active KS as has been observed previously [7, 19]. Hence, CD40L expression by CD4+ T cells in KS patients may have more of a tumor-promoting effect and may be a potential therapeutic target for HIV-associated KS.
Conclusion
Major subsets of CD4 and CD8 T cells, NK cells, and NK T cells are not significantly different between individuals with active versus inactive HIV-associated KS. CD40 ligand expression is higher on CD4+ T cells of individuals with active HIV-associated KS compared to those in remission. The proportion of CD4+ T cells is higher in individuals in remission than those with active KS.
Supplementary Material
Highlights.
The proportion of CD4+ T cells expressing CD40L is significantly higher in individuals with active HIV-associated KS versus in remission.
The proportion of CD4+ T cells is significantly higher in individuals in remission for HIV-associated KS compared to those with active disease.
There is no significant difference in naïve, central memory, effector memory, and/or terminal effector cells between individuals with active HIV-associated KS compared to those in remission.
There is no significant difference in natural killer and/or natural killer T cells between individuals with active HIV-associated KS compared to those in remission.
Acknowledgments
Funding
This work was supported by the Fogarty International Center, National Cancer Institute, and National Institutes of Health under award numbers K43TW011095 to ON, D43TW010354 and U54CA221204. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
Ethics Statement
All study participants gave informed consent before recruitment into the study. Ethical approval was obtained from the University of Zambia Biomedical Research Ethics Committee (Ref. No. 019-07-18).
References
- 1.Mremi A, Mswima J, Mlay MG, Bartholomew H, Alloyce JP, Mmbaga BT, et al. Cancer spectrum in HIV-infected patients: A zonal hospital experience in Tanzania. Cancer Treat Res Commun. 2020;25:100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Innis-Whitehouse W, Wang X, Restrepo N, Salas C, Moreno K, Restrepo A, et al. Kaposi sarcoma incidence in females is nearly four-fold higher in the Lower Rio Grande Valley compared to the Texas average. Cancer Treat Res Commun. 2018;16:45–52. [DOI] [PubMed] [Google Scholar]
- 3.Chang E, Mapakshi SR, Mbang P, El-Mallawany NK, Kramer JR, White DL, et al. Impact of Protease Inhibitors on HIV-Associated Kaposi Sarcoma Incidence: A Systematic Review. J Acquir Immune Defic Syndr. 2018;79(2):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngalamika O, Minhas V, Wood C. Kaposi’s sarcoma at the University Teaching Hospital, Lusaka, Zambia in the antiretroviral therapy era. Int J Cancer. 2015;136(5):1241–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 6.Lazzarini R, Lopes AS, Lellis RF, Brasil F. Iatrogenic Kaposi’s sarcoma caused by corticosteroids. An Bras Dermatol. 2016;91(6):867–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngalamika O, Munsaka S. Cells of the Innate and Adaptive Immune Systems in Kaposi’s Sarcoma. J Immunol Res. 2020;2020:8852221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasti G, Martellotta F, Berretta M, Mena M, Fasan M, Di Perri G, et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98(11):2440–6. [DOI] [PubMed] [Google Scholar]
- 9.Chraa D, Naim A, Olive D, Badou A. T lymphocyte subsets in cancer immunity: Friends or foes. J Leukoc Biol. 2019;105(2):243–55. [DOI] [PubMed] [Google Scholar]
- 10.Baxendale AJ, Dawson CW, Stewart SE, Mudaliar V, Reynolds G, Gordon J, et al. Constitutive activation of the CD40 pathway promotes cell transformation and neoplastic growth. Oncogene. 2005;24(53):7913–23. [DOI] [PubMed] [Google Scholar]
- 11.Pirzada Y, Khuder S, Donabedian H. Predicting AIDS-related events using CD4 percentage or CD4 absolute counts. AIDS Res Ther. 2006;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawabe T, Matsushima M, Hashimoto N, Imaizumi K, Hasegawa Y. CD40/CD40 ligand interactions in immune responses and pulmonary immunity. Nagoya J Med Sci. 2011;73(3-4):69–78. [PMC free article] [PubMed] [Google Scholar]
- 13.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229(1):152–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong AW, Papayoti MH, Netto G, Armstrong DT, Ordonez G, Lawson JM, et al. Growth-inhibitory effects of CD40 ligand (CD154) and its endogenous expression in human breast cancer. Clin Cancer Res. 2001;7(3):691–703. [PubMed] [Google Scholar]
- 15.Biancone L, Cantaluppi V, Boccellino M, Del Sorbo L, Russo S, Albini A, et al. Activation of CD40 favors the growth and vascularization of Kaposi’s sarcoma. J Immunol. 1999;163(11):6201–8. [PubMed] [Google Scholar]
- 16.Cantaluppi V, Deregibus MC, Biancone L, Deambrosis I, Bussolati B, Albini A, et al. The expression of CD154 by Kaposi’s sarcoma cells mediates the anti-apoptotic and migratory effects of HIV-1-TAT protein. Int J Immunopathol Pharmacol. 2006;19(1):81–96. [PubMed] [Google Scholar]
- 17.Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, et al. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J Virol. 2008;82(10):4793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowlton ER, Rappocciolo G, Piazza P, Lepone LM, Nadgir SV, Bullotta A, et al. Human herpesvirus 8 induces polyfunctional B lymphocytes that drive Kaposi’s sarcoma. mBio. 2014;5(5):e01277–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P, Kuwa NY, Minhas V, Marimo C, Shea DM, Kankasa C, et al. Higher levels of neutralizing antibodies against KSHV in KS patients compared to asymptomatic individuals from Zambia. PLoS One. 2013;8(8):e71254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


