Abstract
Programmed cell death is a central process in the control of tissue development, organismal physiology, and disease. Ferroptosis is a recently identified form of programmed cell death that is uniquely defined by redox-active iron-dependent hydroxy-peroxidation of polyunsaturated fatty acid (PUFA)-containing phospholipids and a loss of lipid peroxidation repair capacity. This distinctive form of lipotoxic cell death has been recently implicated in multiple human diseases, spanning ischemia-reperfusion heart injury, brain damage, acute kidney injury, cancer, and asthma. Intriguingly, settings that have been associated with ferroptosis are linked to placental physiology and trophoblast injury. Such circumstances include hypoxia-reperfusion during placental development, physiological uterine contractions or pathological changes in placental bed perfusion, the abundance of trophoblastic iron, evidence for lipotoxicity during the pathophysiology of major placental disorders such as preeclampsia, fetal growth restriction, and preterm birth, and reduced glutathione peroxidation capacity and lipid peroxidation repair during placental injury. We recently interrogated placental ferroptosis in placental dysfunction in human and mouse pregnancy, dissected its relevance to placental injury, and validated the role of glutathione peroxidase-4 in guarding placental trophoblasts against ferroptotic injury. We also uncovered a role for the phospholipase PLA2G6 (PNPLA9) in attenuating trophoblast ferroptosis. Here, we summarize current data on trophoblast ferroptosis, and the role of several proteins and microRNAs as regulators of this process. Our text offers insights into new opportunities for regulating ferroptosis as a means for protecting placental trophoblasts against lipotoxic injury.
Keywords: Ferroptosis, placenta, trophoblast, phospholipids, hydroxy-peroxidation, PLA2G6
Introduction
Regulated cell death is a key component of many physiological and pathological processes. Necrotic cell death can take place in response to stress that damages the cell membrane and organelles, resulting in an uncontrolled cascade of events that leads to death. In contrast, multicellular organisms evolved diverse, tightly controlled forms of cell death, which reflect key checkpoints during normal development, differentiated functions, tissue homeostasis, and adaptation to changes in the cellular environment. Intense research into forms of cell death has identified distinct forms of programmed cell death cascades, including apoptosis, necroptosis, pyroptosis, netosis, and entosis [1]. Ferroptosis is a recently identified form of iron-dependent cell death [2,3] instigated by the accumulation of specific hydroxy-peroxidized phospholipids (Hp-PL), where the hydroxy-peroxidized fatty acid is arachidonic acid (AA, 20:4) or adrenic acid (AdrA, 22:4), commonly bound by phosphatidylethanolamine (termed hydroxy-peroxidized phosphatidylethanolamine or Hp-PE) [4]. Hence, ferroptosis reflects either excessive production of Hp-PL, or insufficient metabolic reducing ability to metabolize Hp-PL to non-damaging forms of phospholipids. Accumulation of Hp-PL triggers a cascade of signals, defined by unique morphological, biochemical, and metabolic steps that are distinctive from other forms of cell death [5]. Ferroptosis was recently shown to play a key role in the ischemia-reperfusion that underlies brain injury, other forms of neurotoxic damage, acute renal failure, asthma, and tumor response to treatment [6–9]. Therefore, targeting ferroptosis has become a key research focus, with the aim of better defining disease pathogenesis and designing new therapeutics and disease prevention measures.
Considering the central role of the placenta in fetal development, growth, maternal-fetal communication, maternal homeostasis and pregnancy adaptation to injuries, it is not surprising that the placenta plays a central role in common and severe complications of pregnancy, such as fetal growth restriction, preeclampsia, preterm birth, and abruption [10–12]. Importantly, several lines of evidence support the notion that ferroptosis may play a key role in the placental dysfunction that underlies major diseases of pregnancy [13]: (a) the placenta is normally subject to hypoxia-reoxygenation transitions early in pregnancy [14,15] and, later, as a result of uterine contractions before and during labor [16,17]; (b) hypoxia-reoxygenation injury has been linked to the pathogenesis of placental dysfunction [18–20]; (c) iron is abundant in placental trophoblasts as it is actively transferred across the placenta to the developing fetus [21,22] (d) trophoblastic lipid peroxidation has been documented in placental injury [23,24]; and (e) lower levels of glutathione peroxidase 4 (GPX4), a key enzyme that protects cells against accumulation of damaging Hp-PL species and ferroptosis (see below), have been associated with human placental dysfunction and preeclampsia [25]. Indeed, recent research has established the role of ferroptosis in trophoblast injury and clinically relevant placental dysfunction [26,27]. In this review, we describe biochemical pathways to ferroptosis, unique aspects of placental biology that may contribute to its sensitivity to ferroptosis, and our recent work on novel regulators of placental ferroptosis.
Biochemical and molecular pathways to ferroptosis
Ferroptosis was discovered through the process of small molecule library screening and induction of non-apoptotic cell death. This approach led to the identification of Erastin and RAS-selective lethal (RSL3) as inducers of ferroptosis [28–31]. Importantly, RSL3-induced cell death was not blocked by inhibitors of apoptosis, necrosis, necroptosis, or autophagy [2]. Instead, ferroptosis could be blocked by lipophilic antioxidants, iron chelators, inhibitors of lipid peroxidation, or depletion of polyunsaturated fatty acyl phospholipids, all pivotal during the process of lipid peroxidation and ferroptosis [4,6]. Integrating these data with further molecular inquiry, as defined below, led to the classification of ferroptosis as a new form of iron-dependent, non-apoptotic programmed cell death [2].
Iron metabolism and redox activity
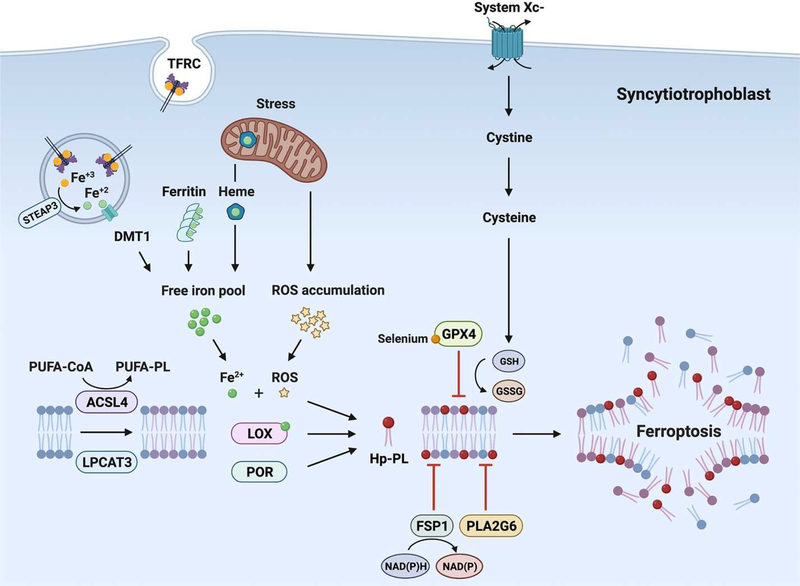
After gut uptake and release from duodenal enterocytes to the blood, iron is taken up by plasma transferrin, and delivered to target tissues [32], where it is endocytosed through the action of the broadly expressed transferrin receptor 1 (TFRC1) [33]. During pregnancy, syncytiotrophoblasts are among the transferrin targets and express a high level of TFRC1 [22,34–36]. Within cells, iron is released from transferrin and is reduced to the ferrous form by six-transmembrane epithelial antigen of prostate 3 (STEAP3, Figure 1), followed by transport into the cytosolic labile iron pool via divalent metal transporter 1 (DMT1) [37,38]. STEAP3 and STEAP4 are highly expressed in the human placenta [39–42]. To minimize the risk of cell toxicity through the formation of reactive oxygen species, iron is stored in the cells in the Ferric (Fe3+) form, bound by ferritin [43–45]. Intracellular iron mobilization and homeostasis also depend on iron regulatory proteins IRP1 (ACO1) and IRP2 (IREB2) [2,46,47]. These proteins are expressed in human trophoblasts and contribute to the mobilization of iron in response to maternal and fetal iron status [48]. Directly relevant to ferroptosis, ferrous (Fe2+) iron serves as a co-factor in the action of lipoxygenase enzymes, where they oxidize polyunsaturated fatty acids (PUFAs) [49] as described below. Both transferrin and TFRC are required for ferroptosis [2,29]. Ferritinophagy also enhances cell sensitivity to ferroptosis by increasing intracellular iron availability [50–52]. Taken together, iron uptake and its intracellular metabolism are tightly linked to ferroptosis.
Figure 1. A schematic depicting key pathways in ferroptosis, delineated in this review.
The text cites direct evidence for the accumulation of Hp-PE, a main form of pro-ferroptosis Hp-PL, in placental injury. The role of GPX4 and PLA2G6 in mitigating trophoblast ferroptosis is also highlighted. The figure also depicts key sources of trophoblastic iron pool. Additional, indirect evidence supports the presence of most ferroptotic regulators in human trophoblasts. Abbreviations: ACSL4, acyl-CoA synthetase long-chain family member 4; DMT1, divalent metal transporter 1; FSP1, ferroptosis suppressor protein 1; GPX4, glutathione peroxidase 4; GSH, glutathione; GSSG, oxidized glutathione; Hp-PE, hydroxy-peroxidized phosphatidylethanolamine; LOX, lipoxygenase; LPCAT3, lysophosphatidylcholine acyltransferase 3; NAD(P), Nicotinamide adenine dinucleotide phosphate; PLA2G6, phospholipase A2 Group VI; PUFA, polyunsaturated fatty acid; PUFA-PE, phosphatidylethanolamine-containing polyunsaturated fatty acid chain; POR, cytochrome P450 oxidoreductase; ROS, reactive oxygen species; STEAP3, six-transmembrane epithelial antigen of prostate 3; TFRC, transferrin receptor
Reactive oxygen species, lipid peroxidation and formation of Hp-PE
Reactive oxygen species (ROS) are unstable free radical molecules. Mostly derived from aerobic metabolism [53], these species include superoxide anions, hydroxyl radicals, alkoxyl radicals, organic hydroperoxides, peroxyl radicals, and hydrogen peroxide. At physiological levels, ROS act as signaling molecules that regulate many cellular processes, including cell proliferation, migration, differentiation, inflammation, and adaptation to stress [54,55]. Excessive ROS levels shift the cellular redox balance toward an oxidative state, which can attack DNA, lipids, and proteins, induce organelle damage, and lead to myriad pathologies. It is therefore clear that survival of oxygen-requiring organisms necessitates the presence of antioxidant defense systems that control ROS production and protect the cells from their harmful impact. These defense systems are divided into enzymatic and non-enzymatic antioxidants. Enzymatic antioxidants include the glutathione peroxidases (GPX), superoxide dismutases (SOD), and catalase (CAT). Non-enzymatic antioxidants, including vitamins A, C, and E, glutathione, coenzyme Q10, and β-carotene, donate electrons to radical compounds, without themselves becoming radical, thus terminating the propagation of free radicals [55–57].
Cellular ROS may also promote the peroxidation of oxidizable lipids, commonly the double bond-containing PUFAs, including arachidonic, docosatetraenoic (adrenic), linoleic, and docosahexaenoic acids. Oxidized lipids can also be synthesized in a controlled fashion by several enzymes, include lipoxygenases (LOX), cyclooxygenases (COX), and some members of the cytochrome P450 (CYP) family of proteins. Whereas excessive lipid peroxidation is associated with several cell death pathways [58], ferroptosis specifically involves LOX-dependent lipid peroxidation activity in the presence of iron [59], commonly with specific accumulation of Hp-PE species that contain these PUFA’s 15th and 17th carbons [4,9,60,61].
The family of LOXs include six isoforms of (non-heme) iron-containing enzymes that catalyze lipid peroxidation, leading to autocrine and paracrine signals that direct cell function [62]. When 15-LOX is complexed with the scaffold protein phosphatydylethanolamine binding protein 1 (PEBP1), it shifts substrate preference from free fatty acids to esterified phospholipids, culminating in the production of 15-Hp-PE-AA [4,9,61]. Pro-ferroptotic signals can also be stimulated by the action of cytochrome P450 oxidoreductase (POR), which accelerates the cycling between Fe2+ and Fe3+ in the CYP heme component, enhancing the production of pro-ferroptosis Hp-PE [63,64]. As shown in Figure 1, additional proteins, including Acyl-CoA synthetase long-chain family member 4 (ACSL4) [65] and lysophosphatidylcholine acyltransferase 3 (LPCAT3) [66] participate in Hp-PL synthesis and may directly influence cell sensitivity to ferroptosis.
Attenuation of ferroptotic signals
Ferroptosis-prone cells exhibit assorted mechanisms to lessen the ferroptotic signal and maintain function. A key player in this is GPX4, which effectively mitigates ferroptosis by converting lipid peroxides (R-OOH) to the corresponding alcohols (R-OH) with a concomitant conversion of glutathione (GSH) into oxidized glutathione (GSSG, Figure 1). Inhibition of GPX4 action using mutagenesis, knock-out or knockdown approaches, or pharmacological inhibitors, sensitizes cells to ferroptosis [7,67]. Moreover, attenuating pathways that are essential for GPX4 action also promotes ferroptosis. For example, the System Xc- is an amino acid antiporter that exchanges cystine and glutamate, a first step in the cellular synthesis of GSH. Inhibition of System Xc- (e.g., by erastin) may lead to GSH depletion that impairs GPX4 activity, culminating in ferroptosis [67]. Another important way to reduce the pro-ferroptotic signal is via the ferroptosis suppressor protein 1 (FSP1) -CoQ10-NAD(P)H pathway (Figure 1). In this pathway FSP1 functions as an oxidoreductase that restores CoQ10 (also known as ubiquinone-10) by NAD(P)H and acts as a lipophilic antioxidant that can antagonize the accumulation of lipid peroxides [68]. The myristoylation of FSP1 mediates the recruitment of this protein to its site of action at the plasma membrane [69]. By usurping ferroptosis as a means to stimulate cell death in neoplastic cells, it was found that inhibition of FSP1 may be an effective way to overcome ferroptosis-resistance in cancer [70].
Recent studies have established an intriguing link between extracellular vesicles (EVs) and defense against ferroptosis. Prominin2 is a pentaspan membrane glycoprotein that is implicated in regulation of lipid dynamics [71]. Prominin2 was recently shown to facilitate resistance to ferroptosis in mammary epithelial and breast cancer cells [72]. This action is attained through formation of ferritin-containing multivesicular bodies that lead to export of iron out of the cells though small EVs (sEVs, exosomes) [73]. Moreover, sEVs that contain miR-522 and are released from cancer-associated fibroblasts may target ALOX15 in cancer cells and thus block Hp-PL accumulation [74].
Using trophoblasts (see below) and neuronal cells [26,75], we recently showed that the calcium-independent phospholipase A2 group VI (PLA2G6, also known as PNPLA9 or iPLA2b) supports anti-ferroptotic cell defense. PLA2G6 activity is mediated by its unique ability to hydrolyze 15-Hp-AA-PE to oxidized fatty acid and lyso-PE [76]. The function of PLA2G6 was also validated through attenuation of neurodegeneration in a mouse model of Parkinson’s disease and reduced placental ferroptotic injury during mouse pregnancy [26,77]. Importantly, in the presence of intact GPX4 action, inhibition of the FSP1-COQ10-NAD(P) H pathway or PLA2G6 was not sufficient to induce ferroptosis, suggesting that these proteins provide a secondary line of defense against ferroptosis, which may be particularly important when thiol-driven protection becomes insufficient [26,78,79]. Akin to endogenous antioxidants, exogenous lipophilic antioxidants, such as ferrostatin-lor liproxstatin-1, block ferroptosis by inhibiting the propagation of oxidative damage within the membrane [7,80].
The execution of ferroptosis - from pro-ferroptotic signal to cell death
Although there is a clear association between formation of Hp-PL and ferroptotic cell death, the precise biochemical and signaling cascades that link these two pathways remain uncertain. Two different mechanisms are currently implicated in the execution of ferroptosis: (a) Following lipid peroxidation, a series of electrophilic products are generated, driving the alkylation of critical sites in survival proteins, instigating cell death. An example of one of these products is 4-hydroxy-2-nonenal (4-HNE), which accumulates in cells and tissues undergoing ferroptosis [60]. As to be expected with this mechanism, cells become more resistant to ferroptotic death by overexpressing aldo-keto reductase 1C (AKR1C), a protein involved in the detoxification of 4-HNE; (b) Hp-PL may directly affect cell membrane integrity by causing pore-like structures in the cell membrane, leading to increased membrane permeability and, eventually, cell death [81,82].
Methods to assess ferroptosis
Several methods have been used to specifically identify ferroptosis, focusing on direct or indirect methods to detect the accumulation Hp-PE or other Hp-PL species. Redox phospholipidomics based on LC-MS/MS analysis, currently used as the gold standard method for detection of ferroptosis, provide the repertoire of peroxidized phospholipids within a sample, at a resolution that distinguishes Hp-PE signals from other Hp-PLs. However, this technology is not widely available, limiting its broader application. Several fluorescent probes can detect the accumulation of cell lipid peroxidation, but not at a resolution suitable for the elucidation of phospholipid subtypes. Such probes include Liperfluo and BODIPY 581/591 C11, which are widely used to detect ferroptosis in cell culture in response to diverse stimuli [4,68]. In addition, antibodies against secondary products of lipid peroxidation, malondialdehyde (MDA) and 4-HNE, are used as markers of ferroptosis. While pointing to oxidative stress, these markers cannot distinguish between ferroptosis and other forms of oxidative stress. Finally, synthetic compounds that act as hydroperoxyl radical scavengers, such as ferrostatin-1 and liproxtatin-1 [7,83], can selectively inhibit ferroptosis and are therefore used to mark the involvement of ferroptosis in pathological processes.
Placental susceptibility to ferroptosis
Circumstances that may incite ferroptosis, including the availability of PUFA-PL substrates, free iron, physical conditions that favor lipid peroxidation, and a failure of the ferroptosis-mitigating guards, may exist during placental development and function, heightening cell sensitivity to ferroptosis. The evidence detailed here illuminates the risk of ferroptosis in trophoblasts, highlighting the significance of this process at the forefront of the placental-maternal interface.
Substrate
When compared to other cell types within the villous core, trophoblasts express relatively high levels of LPCAT3 [13] and ACSL4 [84–86], which play key roles in the formation of PUFA-PL [4,66]. Further, placental PL species are enriched by arachidonic acid [87] and are prone to hydro-peroxidation, characteristic of ferroptosis.
Iron
During pregnancy, maternal hepcidin is downregulated, facilitating absorption of dietary iron and the release of stored maternal iron to maintain serum iron concentrations [22,88]. Iron is absorbed into the placenta through the action of TFRC1 and ferroportin (FPN) [22]. While transferring, on average, 270 mg of iron to the fetus, primarily during the third trimester [89], placental trophoblasts are rich in iron, and this high level is well sustained even at the cost of fetal iron deficiency [88]. Interestingly, conditions related to dysregulation of iron have been associated with the placental dysfunction that characterizes preeclampsia [21,90,91].
Circumstances that may trigger placental ferroptosis
Tissue underperfusion and reperfusion, hypoxia-reoxygenation, and the production of ROS commonly occur during placental development and function [92,93]. ROS are critical signaling molecules during embryogenesis and early placental development [24]. Importantly, the “normal redox environment” during early pregnancy undergoes extreme changes at the most vulnerable period of early embryogenesis. During blastocyst implantation and the initial developmental transitions from totipotency and pluripotency to early trophectoderm expansion and inner cell mass differentiation, the supply of oxygen and redox buffering compounds is markedly low. Although extravillous trophoblasts invade the maternal uterine vessels and the intervillous space is formed, trophoblast cells and debris form plugs in the spiral arteries and prevent perfusion of this space, rendering the tissue severely hypoxic until 10–13 weeks of human pregnancy [94]. Initiation of perfusion at the intervillous space results in a steep increase in the partial O2 pressure, from 15–20 mm Hg to > 50mm Hg [14,95,96]. This relatively fast increase in perfusion and oxygenation predisposes the tissue to the formation of ROS and lipotoxic injury. Indeed, it is possible that abnormalities in this re-oxygenation process may underlie early pregnancy loss [18–20]. As pregnancy progresses, marked reduction in placental perfusion followed by reperfusion, occurs during antepartum uterine contractions and during labor and delivery [16,17,97]. Lastly, the placenta expresses relatively high levels of both 5- and 15-LOX [98,99], which may further promote the hydroxy-peroxidation of PUFA-PLs [23,24] and thus increase the risk of ferroptosis [7].
Enzymatic pathways that guard against placental ferroptosis
GPX4, a master repressor of ferroptosis (Figure 1), is expressed in human placental trophoblasts [86]. Therefore, reduced GPX4 expression or function are expected to sensitize trophoblasts to ferroptosis, potentially triggering placental dysfunction and ensuing pregnancy complications. Whereas Gpx4-knockout mice exhibit early fetal lethality [100], human GPX4 mutations or lower expression of GPX4 have been associated with human placental dysfunction and preeclampsia [25,27,101]. We recently showed that placental trophoblasts require GPX4 activity for survival [26]. Genetic or pharmacologic inhibition of GPX4 induced ferroptosis in human placental trophoblasts, with increased levels of Hp-PE species. In addition, sublethal activation of pro-ferroptotic signaling impaired trophoblast function. We also showed that RSL3-mediated GPX4 inhibition during murine pregnancy resulted in placental ferroptosis and a higher rate of pregnancy demise, highlighting the critical role of active anti-ferroptotic machinery that promotes feto-placental survival [26].
PLA2G6 is also highly expressed in human placental trophoblasts [26,86]. We recently identified PLA2G6 as a novel regulator of trophoblast ferroptosis [26]. When activated by oxidized phospholipids or other ROS, PLA2G6 can hydrolyze Hp-PE to lyso-PE and oxidized fatty acid, thus eliminating the ferroptotic signal [102]. We showed that genetic or pharmacologic inhibition of PLA2G6 in the BeWo trophoblast line potentiated the cells to ferroptosis, prompted by RSL3, and that the effect of GPX4 and PLA2G6 inhibition was synergistic. We further examined the role of PLA2G6 in ferroptosis during pregnancy by inhibiting GPX4 using intraperitoneal (1S,3R)-RSL3 injections at E13.5–14.5, and found increased mortality and resorption of Pla2g6KO embryos, compared to Pla2g6WT. Distinctly, in non-resorbed placentas, histological analysis showed that the placental labyrinth of RSL3-exposed Pla2g6KOwas thinner compared to Pla2g6WT, while the junctional zone was unchanged. Exposing the Pla2g6KO pregnant mice to hypoxia (E11.5–17.5) followed by reoxygenation led to significant accumulation of Hp-PE species when compared to Pla2g6WT and was associated with a higher rate of fetal demise and reduced placental size. As noted earlier, deletion of Pla2g6 was not sufficient to induce ferroptosis, signifying a role for this protein when the potent action of GPX4 is exhausted. Together, these findings support the role of PLA2G6 in placental protection against hypoxia-reoxygenation injury.
Trophoblastic macro-blebbing during ferroptosis
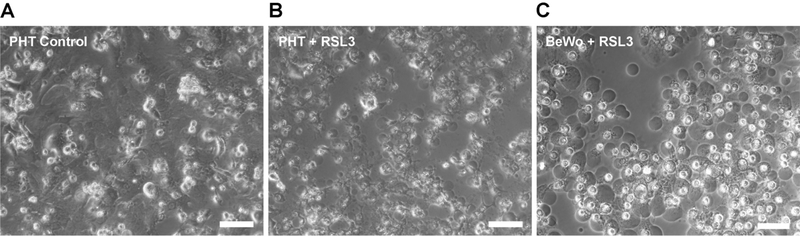
Cell membrane blebs have been described in certain physiological processes, such as cytokinesis [103], cell spreading [104], or uptake of viruses [105]. While some membrane blebbing is characteristic of apoptosis [106], we recently identified the formation of numerous, large blebs during trophoblastic ferroptosis (Figure 2), which exceeded the degree of blebbing in other cell types exposed to a similar ferroptotic signal [107]. Whereas apoptotic blebs were suggested to contain cellular content [108] or even serve to transmit pro-apoptotic signals to neighboring cells [109], we found that ferroptotic blebs were devoid of major cytoplasmic organelles, and did not transmit a pro-ferroptotic signal to neighboring cells [107]. While the fusogenic proteins syncytin-1 and −2 were not involved in the blebbing process, our modeling data suggested that cell membrane Hp-PE promoted membrane stretchability, which might promote the formation of macro-blebs [107,110].
Figure 2. Ferroptotic macro-blebbing in trophoblasts.
A phase-contrast microscopy image of ferroptotic macro-blebbing in PHT or BeWo cells, exposed to the GPX4 inhibitor RSL3 (100 nM) for 8 h. Scale bar, 50 μm.
Placental dysfunction and ferroptosis
Upregulated levels of oxidative stress, with subsequent accumulation of reactive oxygen species and lipid peroxidation, have been commonly implicated in placental dysfunction [24] and related diseases [27,111–113]. Specifically, single-nucleotide polymorphisms in the GPX4 gene (rs713041) were found to be associated with severe, and early-onset preeclampsia [25,114]. Moreover, selenium has a non-interchangeable role in GPX4 function, and may therefore inhibit ferroptosis [115]. Selenium has also been implicated in a dose-dependent reduction in the risk of preeclampsia [116]. Using LC-MS/MS for redox phospholipidomics, we recently identified the accumulation of Hp-PE in injured placentas from women with spontaneous preterm birth [26]. Importantly, ferrostatin-1 was recently shown to reduce placental MDA accumulation and attenuate a preeclampsia-like phenotype in a rat model [27]. A recent report also invoked miR-30b-5p, which is upregulated in placentas of preeclamptic patients, in placental ferroptosis [27]. The data also suggest that miR-30b-5p downregulates SLC7A11 (System Xc) antiporter and Pax3, which induces the expression of Ferroportin1, thus reducing GSH synthesis and increasing iron accumulation. Pharmacological inhibition of ferroptosis and knockdown of miR-30b-5p attenuated preeclampsia in a rat model of reduced uterine perfusion [27]. Together, these data support a link between placental ferroptosis and diseases that emanate from placental dysfunction, highlighting the need for deeper inquiry into placental ferroptosis and its role in obstetrical diseases.
Concluding remarks
Whereas hypoxia-reperfusion injury, oxidative stress, lipotoxicity, and the production of ROS have been commonly implicated in the final common pathway of numerous placental disorders, our understanding of the true meaning and significance of these processes has been severely limited. Based on the recent inquiry into ferroptosis, we can now appreciate the role of ROS, not only in tissue damage, but also in redox signaling. Thus, the discovery of ferroptosis as a discrete entity underlies the vaguely defined lipotoxic injury and may create the need for a new dimension in our definition of placental dysfunction. It may also become clear why the deployment of non-selective antioxidants for mitigating placental injury and related clinical syndromes might not lead to the desired outcome [117]. Our data, and the emerging information that link ferroptosis with placenta injury may, provide a mechanistic, biochemical, and molecular framework for better understanding of trophoblast oxidative stress and lipotoxicity and, hence, placental health. Whereas the clinical use of ferroptosis-mitigation strategies is still distant, future deployment of targeted ferroptosis therapeutics may serve to attenuate the negative aspects of oxidative stress without compromising critical redox signaling. Such therapies may usher in new means for prevention or treatment of placental dysfunction and its sequalae, including preeclampsia, fetal growth restriction, preterm birth, and pregnancy loss.
Highlights.
Iron-dependent hydroxy-peroxidation of PUFA-phospholipids characterizes ferroptosis
Placental hypoxia-reperfusion or lipotoxic injury predisposes to ferroptosis
GPX4 and PNPLA9 guard trophoblasts against ferroptotic damage
Ferroptosis may play a central role in major placenta-related obstetrical diseases
Controlling ferroptosis suggests new clinical tools for placental oxidative stress
Acknowledgements
We thank Elena Sadovsky, Julie Goff, Tiffany Coon, and Huijie Sun for technical assistance, Lori Rideout for assistance with manuscript preparation, and Bruce Campbell for editing. Figure 1 was created with BioRender.com.
Funding
The project was supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development (NIH/NICHD) grants P01HD069316, R01HD086325 and R37HD086916 (to Y.S.), the March of Dimes Prematurity Research Center at the University of Pennsylvania (to Y.S.), the 25 Club of Magee-Womens Hospital (to Y.S.), the Magee-Womens Research Institute Postdoctoral Fellowship (to O.B.), Ofek physician-scientist grant, Hadassah University Hospital, Israel (O.B), and the Jikei University School of Medicine Department of Obstetrics and Gynecology, Japan (to K.K.).
Footnotes
Competing Interests
Y. Sadovsky is a consultant at Illumina, Inc. The other authors report no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Green DR. The coming decade of cell death research: Five riddles. Cell, 177 (2019) 1094–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell, 149 (2012) 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jiang X, Stockwell BR, Conrad M. Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol Cell Biol, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kagan VE, Mao G, Qu F, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol, 13 (2017) 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xie Y, Hou W, Song X, et al. Ferroptosis: Process and function. Cell Death Differ, 23 (2016) 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell, 171 (2017) 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol, 16 (2014) 1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhao J, Dar HH, Deng Y, et al. PEBP1 acts as a rheostat between prosurvival autophagy and ferroptotic death in asthmatic epithelial cells. Proc Natl Acad Sci USA, 117 (2020) 14376–14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wenzel SE, Tyurina YY, Zhao J, et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell, 171 (2017) 628–641 e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thilaganathan B. Placental syndromes: Getting to the heart of the matter. Ultrasound Obstet Gynecol, 49 (2017) 7–9. [DOI] [PubMed] [Google Scholar]
- [11].Sibley CP. Treating the dysfunctional placenta. J Endocrinol, 234 (2017) R81–R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burton GJ, Redman CW, Roberts JM, et al. Pre-eclampsia: Pathophysiology and clinical implications. BMJ, 366 (2019) l2381. [DOI] [PubMed] [Google Scholar]
- [13].Ng SW, Norwitz SG, Norwitz ER. The impact of iron overload and ferroptosis on reproductive disorders in humans: Implications for preeclampsia. Int. J. Mol. Sci, 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Soares MJ, Iqbal K, Kozai K. Hypoxia and placental development. Birth Defects Res, 109 (2017) 1309–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Burton GJ, Watson AL, Hempstock J, et al. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab, 87 (2002) 2954–2959. [DOI] [PubMed] [Google Scholar]
- [16].Alotaibi M, Arrowsmith S, Wray S. Hypoxia-induced force increase (HIFI) is a novel mechanism underlying the strengthening of labor contractions, produced by hypoxic stresses. Proc Natl Acad Sci USA, 112 (2015) 9763–9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brar HS, Platt LD, DeVore GR, et al. Qualitative assessment of maternal uterine and fetal umbilical artery blood flow and resistance in laboring patients by Doppler velocimetry. Am J Obstet Gynecol, 158 (1988) 952–956. [DOI] [PubMed] [Google Scholar]
- [18].Hung TH, Skepper JN, Charnock-Jones DS, et al. Hypoxia-reoxygenation: A potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res, 90 (2002) 1274–1281. [DOI] [PubMed] [Google Scholar]
- [19].Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol, 218 (2018) S745–S761. [DOI] [PubMed] [Google Scholar]
- [20].Soleymanlou N, Jurisica I, Nevo O, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab, 90 (2005) 4299–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr, 106 (2017) 1567S–1574S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sangkhae V, Nemeth E. Placental iron transport: The mechanism and regulatory circuits. Free Radic Biol Med, 133 (2019) 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aouache R, Biquard L, Vaiman D, et al. Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schoots MH, Gordijn SJ, Scherjon SA, et al. Oxidative stress in placental pathology. Placenta, 69 (2018) 153–161. [DOI] [PubMed] [Google Scholar]
- [25].Peng X, Lin Y, Li J, et al. Evaluation of glutathione peroxidase 4 role in preeclampsia. Sci Rep, 6 (2016) 33300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Beharier O, Tyurin VA, Goff JP, et al. PLA2G6 guards placental trophoblasts against ferroptotic injury. Proc Natl Acad Sci USA, 117 (2020) 27319–27328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang H, He Y, Wang JX, et al. miR-30–5p-mediated ferroptosis of trophoblasts is implicated in the pathogenesis of preeclampsia. Redox Biol, 29 (2020) 101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dolma S, Lessnick SL, Hahn WC, et al. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell, 3 (2003) 285–296. [DOI] [PubMed] [Google Scholar]
- [29].Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol, 15 (2008) 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yagoda N, von Rechenberg M, Zaganjor E, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature, 447 (2007) 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wolpaw AJ, Shimada K, Skouta R, et al. Modulatory profiling identifies mechanisms of small molecule-induced cell death. Proc Natl Acad Sci USA, 108 (2011) E771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ponka P, Beaumont C, Richardson DR. Function and regulation of transferrin and ferritin. Semin Hematol, 35 (1998) 35–54. [PubMed] [Google Scholar]
- [33].Aisen P. Transferrin receptor 1. Int J Biochem Cell Biol, 36 (2004) 2137–2143. [DOI] [PubMed] [Google Scholar]
- [34].Bastin J, Drakesmith H, Rees M, et al. Localisation of proteins of iron metabolism in the human placenta and liver. Br J Haematol, 134 (2006) 532–543. [DOI] [PubMed] [Google Scholar]
- [35].Cao C, Fleming MD. The placenta: The forgotten essential organ of iron transport. Nutr Rev, 74 (2016) 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Parmley RT, Barton JC, Conrad ME. Ultrastructural localization of transferrin, transferrin receptor, and iron-binding sites on human placental and duodenal microvilli. Br J Haematol, 60 (1985) 81–89. [DOI] [PubMed] [Google Scholar]
- [37].Ohgami RS, Campagna DR, Greer EL, et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet, 37 (2005) 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature, 388 (1997) 482–488. [DOI] [PubMed] [Google Scholar]
- [39].Korkmaz CG, Korkmaz KS, Kurys P, et al. Molecular cloning and characterization of STAMP2, an androgen-regulated six transmembrane protein that is overexpressed in prostate cancer. Oncogene, 24 (2005) 4934–4945. [DOI] [PubMed] [Google Scholar]
- [40].Li YQ, Bai B, Cao XX, et al. Divalent metal transporter 1 expression and regulation in human placenta. Biol Trace Elem Res, 146 (2012) 6–12. [DOI] [PubMed] [Google Scholar]
- [41].Chong WS, Kwan PC, Chan LY, et al. Expression of divalent metal transporter 1 (DMT1) isoforms in first trimester human placenta and embryonic tissues. Hum Reprod, 20 (2005) 3532–3538. [DOI] [PubMed] [Google Scholar]
- [42].Georgieff MK, Wobken JK, Welle J, et al. Identification and localization of divalent metal transporter-1 (DMT-1) in term human placenta. Placenta, 21 (2000) 799–804. [DOI] [PubMed] [Google Scholar]
- [43].Contractor SF, Eaton BM. Role of transferrin in iron transport between maternal and fetal circulations of a perfused lobule of human placenta. Cell Biochem Funct, 4 (1986) 69–74. [DOI] [PubMed] [Google Scholar]
- [44].Brown PJ, Johnson PM, Ogbimi AO, et al. Characterization and localization of human placental ferritin. Biochem J, 182 (1979) 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Maymon R, Jauniaux E, Greenwold N, et al. Localization of p43 placental isoferritin in human maternal-fetal tissue interface. Am J Obstet Gynecol, 182 (2000) 670–674. [DOI] [PubMed] [Google Scholar]
- [46].Hentze MW, Kuhn LC. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci USA, 93 (1996) 8175–8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr, 28 (2008) 197–213. [DOI] [PubMed] [Google Scholar]
- [48].Bradley J, Leibold EA, Harris ZL, et al. Influence of gestational age and fetal iron status on IRP activity and iron transporter protein expression in third-trimester human placenta. Am J Physiol Regul Integr Comp Physiol, 287 (2004) R894–901. [DOI] [PubMed] [Google Scholar]
- [49].Kuhn H, Saam J, Eibach S, et al. Structural biology of mammalian lipoxygenases: Enzymatic consequences of targeted alterations of the protein structure. Biochem Biophys Res Commun, 338 (2005) 93–101. [DOI] [PubMed] [Google Scholar]
- [50].Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy, 12 (2016) 1425–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gao M, Monian P, Pan Q, et al. Ferroptosis is an autophagic cell death process. Cell Res, 26 (2016) 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mancias JD, Wang X, Gygi SP, et al. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature, 509 (2014) 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fukai T, Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal, 15 (2011) 1583–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J, 134 (1973) 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol, 21 (2020) 363–383. [DOI] [PubMed] [Google Scholar]
- [56].Valko M, Izakovic M, Mazur M, et al. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem, 266 (2004) 37–56. [DOI] [PubMed] [Google Scholar]
- [57].Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol, 24 (2014) R453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Su LJ, Zhang JH, Gomez H, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev, 2019 (2019) 5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Shintoku R, Takigawa Y, Yamada K, et al. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci, 108 (2017) 2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Conrad M, Pratt DA. The chemical basis of ferroptosis. Nat Chem Biol, 15 (2019) 1137–1147. [DOI] [PubMed] [Google Scholar]
- [61].Stoyanovsky DA, Tyurina YY, Shrivastava I, et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radic Biol Med, 133 (2019) 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Singh NK, Rao GN. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog Lipid Res, 73 (2019) 28–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zou Y, Li H, Graham ET, et al. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol, 16 (2020) 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Stockwell BR, Jiang X. The chemistry and biology of ferroptosis. Cell Chem Biol, 27 (2020) 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol, 13 (2017) 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dixon SJ, Winter GE, Musavi LS, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol, 10 (2015) 1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic Biol Med, 152 (2020) 175–185. [DOI] [PubMed] [Google Scholar]
- [68].Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature, 575 (2019) 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Doll S, Freitas FP, Shah R, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature, 575 (2019) 693–698. [DOI] [PubMed] [Google Scholar]
- [70].Hadian K. Ferroptosis Suppressor Protein 1 (FSP1) and Coenzyme Q10 Cooperatively Suppress Ferroptosis. Biochemistry, 59 (2020) 637–638. [DOI] [PubMed] [Google Scholar]
- [71].Florek M, Bauer N, Janich P, et al. Prominin-2 is a cholesterol-binding protein associated with apical and basolateral plasmalemmal protrusions in polarized epithelial cells and released into urine. Cell Tissue Res, 328 (2007) 31–47. [DOI] [PubMed] [Google Scholar]
- [72].Brown CW, Mercurio AM. Ferroptosis resistance mediated by exosomal release of iron. Mol Cell Oncol, 7 (2020) 1730144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Brown CW, Amante JJ, Chhoy P, et al. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell, 51 (2019) 575–586.e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhang H, Deng T, Liu R, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer, 19 (2020) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sun WY, Tyurin VA, Mikulska-Ruminska K, et al. Phospholipase iPLA2beta averts ferroptosis by eliminating a redox lipid death signal. Nat Chem Biol, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ramanadham S, Ali T, Ashley JW, et al. Calcium-independent phospholipases A2 and their roles in biological processes and diseases. J Lipid Res, 56 (2015) 1643–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mori A, Hatano T, Inoshita T, et al. Parkinson’s disease-associated iPLA2-VIA/PLA2G6 regulates neuronal functions and α-synuclein stability through membrane remodeling. Proc Natl Acad Sci USA, 116 (2019) 20689–20699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Shimada K, Skouta R, Kaplan A, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol, 12 (2016) 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kapralov AA, Yang Q, Dar HH, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol, 16 (2020) 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zilka O, Shah R, Li B, et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci, 3 (2017) 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Agmon E, Solon J, Bassereau P, et al. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci Rep, 8 (2018) 5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Agmon E, Stockwell BR. Lipid homeostasis and regulated cell death. Curr Opin Chem Biol, 39 (2017) 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Miotto G, Rossetto M, Di Paolo ML, et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol, 28 (2020) 101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cao Y, Traer E, Zimmerman GA, et al. Cloning, expression, and chromosomal localization of human long-chain fatty acid-CoA ligase 4 (FACL4). Genomics, 49 (1998) 327–330. [DOI] [PubMed] [Google Scholar]
- [85].Cao Y, Murphy KJ, McIntyre TM, et al. Expression of fatty acid-CoA ligase 4 during development and in brain. FEBS Lett, 467 (2000) 263–267. [DOI] [PubMed] [Google Scholar]
- [86].Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue-based map of the human proteome. Science, 347 (2015) 1260419. [DOI] [PubMed] [Google Scholar]
- [87].Brown SH, Eather SR, Freeman DJ, et al. A lipidomic analysis of placenta in preeclampsia: Evidence for lipid storage. PLoS One, 11 (2016) e0163972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sangkhae V, Fisher AL, Wong S, et al. Effects of maternal iron status on placental and fetal iron homeostasis. J Clin Invest, 130 (2020) 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Cao C, O’Brien KO. Pregnancy and iron homeostasis: An update. Nutr Rev, 71 (2013) 35–51. [DOI] [PubMed] [Google Scholar]
- [90].Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens Pregnancy, 21 (2002) 205–223. [DOI] [PubMed] [Google Scholar]
- [91].Lee DC, Romero R, Kim JS, et al. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: Siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am J Pathol, 179 (2011) 590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Burton GJ, Cindrova-Davies T, Yung HW, et al. Hypoxia and reproductive health: Oxygen and development of the human placenta. Reproduction, 161 (2021) F53–F65. [DOI] [PubMed] [Google Scholar]
- [93].Chang CW, Wakeland AK, Parast MM. Trophoblast lineage specification, differentiation and their regulation by oxygen tension. J Endocrinol, 236 (2018) R43–R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rodesch F, Simon P, Donner C, et al. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol, 80 (1992) 283–285. [PubMed] [Google Scholar]
- [95].Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat, 215 (2009) 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Pereira RD, De Long NE, Wang RC, et al. Angiogenesis in the placenta: The role of reactive oxygen species signaling. Biomed Res Int, 2015 (2015) 814543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update, 12 (2006) 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mitchell MD, Grzyboski CF. Arachidonic acid metabolism by lipoxygenase pathways in intrauterine tissues of women at term of pregnancy. Prostaglandins Leukot Med, 28 (1987) 303–312. [DOI] [PubMed] [Google Scholar]
- [99].Datta K, Kulkarni AP. Oxidative metabolism of aflatoxin B1 by lipoxygenase purified from human term placenta and intrauterine conceptal tissues. Teratology, 50 (1994) 311–317. [DOI] [PubMed] [Google Scholar]
- [100].Imai H, Hirao F, Sakamoto T, et al. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem Biophys Res Commun, 305 (2003) 278–286. [DOI] [PubMed] [Google Scholar]
- [101].Mistry HD, Kurlak LO, Williams PJ, et al. Differential expression and distribution of placental glutathione peroxidases 1, 3 and 4 in normal and preeclamptic pregnancy. Placenta, 31 (2010) 401–408. [DOI] [PubMed] [Google Scholar]
- [102].Balboa MA, Balsinde J. Oxidative stress and arachidonic acid mobilization. Biochim Biophys Acta, 1761 (2006) 385–391. [DOI] [PubMed] [Google Scholar]
- [103].Charras G, Paluch E. Blebs lead the way: How to migrate without lamellipodia. Nat Rev Mol Cell Biol, 9 (2008) 730–736. [DOI] [PubMed] [Google Scholar]
- [104].Norman LL, Brugues J, Sengupta K, et al. Cell blebbing and membrane area homeostasis in spreading and retracting cells. Biophys J, 99 (2010) 1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science, 320 (2008) 531–535. [DOI] [PubMed] [Google Scholar]
- [106].Coleman ML, Sahai EA, Yeo M, et al. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol, 3 (2001) 339–345. [DOI] [PubMed] [Google Scholar]
- [107].Kajiwara K, Beharier O, Chng CP, et al. Ferroptosis induces membrane blebbing in placental trophoblasts. J Cell Sci, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Charras GT, Hu CK, Coughlin M, et al. Reassembly of contractile actin cortex in cell blebs. J Cell Biol, 175 (2006) 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol, 11 (2001) 1847–1857. [DOI] [PubMed] [Google Scholar]
- [110].Chng CP, Sadovsky Y, Hsia KJ, et al. Site-specific peroxidation modulates lipid bilayer mechanics. Extreme Mechanics Letters, 42 (2021) 101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Martin A, Faes C, Debevec T, et al. Preterm birth and oxidative stress: Effects of acute physical exercise and hypoxia physiological responses. Redox Biol, 17 (2018) 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Moore TA, Ahmad IM, Zimmerman MC. Oxidative stress and preterm birth: An integrative review. Biol Res Nurs, 20 (2018) 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Basbug M, Demir I, Serin IS, et al. Maternal erythrocyte malondialdehyde level in preeclampsia prediction: A longitudinal study. J Perinat Med, 31 (2003) 469–474. [DOI] [PubMed] [Google Scholar]
- [114].Chen A, Zhao H, Wang J, et al. Haplotype analysis of candidate genes involved in inflammation and oxidative stress and the susceptibility to preeclampsia. J Immunol Res, 2020 (2020) 4683798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ingold I, Berndt C, Schmitt S, et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell, 172 (2018) 409–422 e421. [DOI] [PubMed] [Google Scholar]
- [116].Xu M, Guo D, Gu H, et al. Selenium and preeclampsia: A systematic review and meta-analysis. Biol Trace Elem Res, 171 (2016) 283–292. [DOI] [PubMed] [Google Scholar]
- [117].Roberts JM, Myatt L, Spong CY, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med, 362 (2010) 1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]