Abstract
Background
Coronavirus-19 (SARS-CoV-2) is constantly changed through mutation, and new stains of this virus are detected throughout the world such as B.1.1.7 (UK), B.1.351 (South Africa), and P.1 (Brazil). These strains seem to be more easily transmissible than other variants, which may lead to more cases and more deaths. Currently, there are many vaccines for SARS-CoV-2 available in the market but without full clinical data beside. Despite the existence of these vaccines, the numbers of outpatients are still increasing in many countries around the world, and the reliability of these vaccines still remains elusive. It is well known that trace element deficiencies increase the individual susceptibility to immune dysfunction and lead to global health problem. In this context, improving the immune defense system to combats this pandemic is absolutely necessary. The purpose of this review is to establish the probable relation between trace elements supplementation and COVID-19.
Main body
Several clinical studies confirmed that Cu, Se, and Zn insufficiencies alter the immune system and increase the vulnerability to viral infections. Based on antiviral and anti-inflammatory effects of these micronutrients, it seems logical that dietary supplementations of these components might enhance human immune system and lower the graveness of COVID-19 infection.
Conclusion
Based on available data, we hypothesize that the clinical use of some essential trace element supplementations such as copper, selenium, and zinc might be a preventive and promising option to enhance human immunity against the new pandemic COVID-19 and its new strains.
Graphical abstract
Keywords: COVID-19, Essential trace elements, Human health, Immune system, New strains, SARS-CoV-2
Background
The common name “coronavirus” was used the first time in 1968 to classify a new family of viruses called Coronaviridae associated to single-stranded RNA genomes that cause respiratory diseases including influenza (common cold), severe acute respiratory syndrome (SARS-CoV), and middle east respiratory syndrome (MERS-CoV) [10]. There are 04 subfamilies of Coronaviridae including the alpha (α), beta (β), gamma (γ), and delta (δ) coronaviruses. These kinds of viruses signifying “crown” or “wreath”, which belong to the family of Coronaviridae, are spherical form enclosed in a triple envelopes (lipid and protein envelopes, and nucleocapsid) [20]. The diameter of the virus ranged between 80 and 120 nm with molecular weight of 40,000 kDa [18]. The external surface was covered by shaped spikes, from which its name comes [8].
According to the International Committee on Taxonomy of Viruses (ICTV), more than 40 kinds of coronaviruses were identified, with the vast majority detected in mammals. Seven coronaviruses can infect humans and among them three types of coronaviruses belong to the β-coronavirus subfamily and cause a high mortality rate: SARS-CoV, MERS-CoV, and SARS-CoV-2 (COVID-19) [10].
Coronavirus-19 (severe acute respiratory syndrome coronavirus) is a new grave pneumonic syndrome extended rapidly all over the world. This disease has a severe impact especially in elderly patients with hypertension, diabetes, and pneumatic diseases [36]. There are many vaccines trials for COVID-19 available in the market, with some positive but a reliable vaccine is not available yet. The SARS-CoV-2 is continually changing into novel emerging variants decreasing the efficiency of recent vaccines against virus in many countries in the world [12].
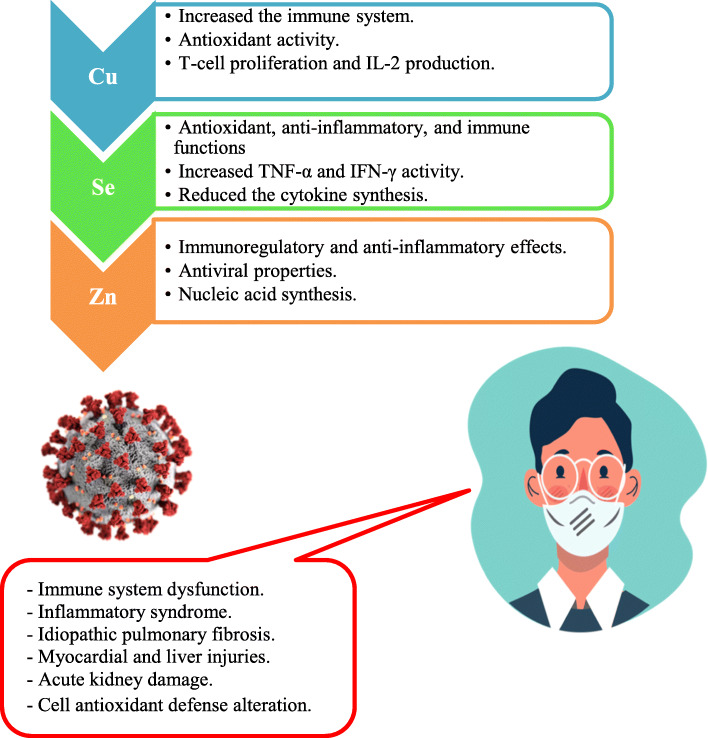
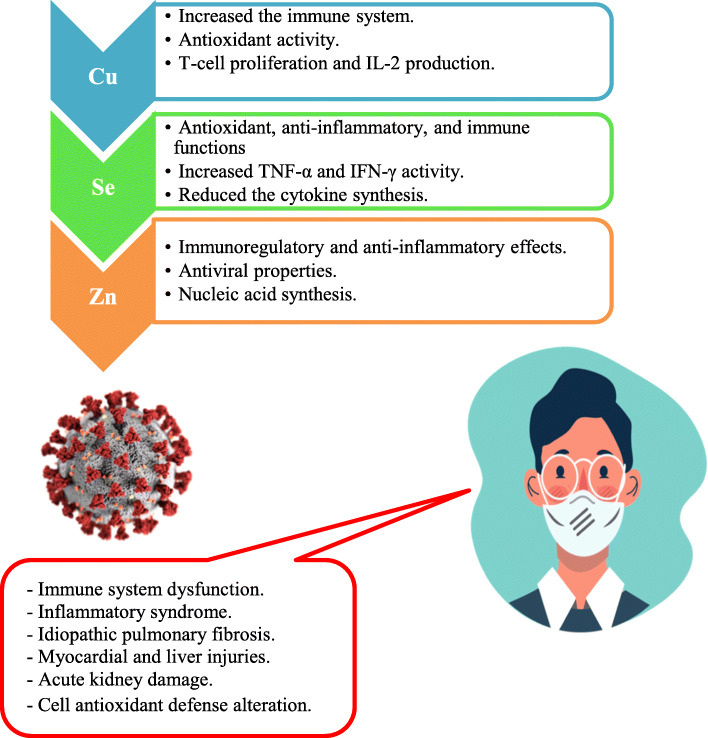
Currently, we have enough clinical information about the effect of pharmaceutical supplementation of trace elements concerning COVID-19 [9, 19, 27]. Dietary trace element supplementations might enhance human immune system and lower the graveness of the viral infection (Fig. 1). It should be mentioned that these low-cost and readily obtainable trace elements to treat viral infections have been largely ignored, due to scarce knowledge about their clinical aspects.
Fig. 1.
COVID-19 disorders and potential benefits of Cu, Se, and Zn supplementations
Main text
Copper (Cu) plays a significant role in the immune system maintenance. This trace element is closely related to the blood immune cell functions such as natural killer (Nk) and T helper (Th) cells required in the viral infection elimination and antibodies synthesis. The recommended daily ingestion (RDI) of Cu is 0.90 mg/day [22] (Table 1). Cu acts as a cofactor of superoxide dismutase (SOD) involved in the cell defense from oxidative injury [16]. The antiviral activities of Cu in lytic virus replication by damaging RNA genome was approved by Ishida [14]. In the case of Cu shortage, the body shows an exceptional vulnerability to viral infections. Thus, Percival [23] demonstrated that immune dysfunction appear most pronounced in young and elderly patients with dietary Cu deficiency. Many viruses such as bronchitis virus, poliovirus, and virus type 1(HIV-1) were inactivated by Cu supplementation [26]. Hopkins and Failla [13] reported that Cu lack in human diet decreases the interleukin-2 (IL-2) synthesis and IL-2 mRNA of T-lymphocytes. According to Noyce et al. [21] the active viral particles of human influenza A virus decreased in number from 500,000 to 500 after incubation on Cu surface for 6 h. Warnes et al. [32] demonstrated that coronavirus 229E virus was morphologically affected (envelope degeneration, surface spikes disintegration, and genomic DNA damage) and remains inactive by Cu contact surfaces. In this way, recently, van Doremalen et al. [30] found that novel SARS-CoV-2 (COVID-19) is more sensitive to the Cu surface contact compared to SARS-CoV-1.
Table 1.
Dietary sources, recommended daily intake (RDI), daily permissible limit (DPL), immune functions, and supplementation effects of Cu, Se, and Zn
| Trace elements | Dietary sourcea | Contenta | RDIb | DPLc | Immune functions | Deficiency | Supplementation effects | References |
|---|---|---|---|---|---|---|---|---|
| Cu | Beef meat | 12.5 mg/100 g | 0.9 mg/day | 10 mg/day | - Supported macrophage function and natural killer (Nk) cell activity | -Decreased interleukin-2 (IL-2) production and IL-2 mRNA in T-lymphocytes |
- Inhibited NF-kB activation - Increased IL-2 synthesis |
- Percival [23] - Hopkins and Failla [13] |
| Oysters, shellfish | 4.9 mg/100 g | |||||||
| Mushroom | 1 mg/100 g | |||||||
| Nuts | 2 mg/100 g | |||||||
| Se | Tuna | 100 μg/100 g | 55 μg/day | 300 μg/day |
- Increased T and B lymphocyte function - Increased the antioxidant enzyme activity |
- Decreased Nk cell activity - Reduced the GSH-Px activity - Increased the expression of the inflammatory cytokine, IL-6, and TNF-α |
-Improved immune function - Induced up-regulation of the IL-2 - Stimulated T-cell proliferation - Increased the TNF-α and IFN-γ |
- Guillin et al. [11] - Alexander et al. [1] - Zhang et al. [35] |
| Sardines | 90 μg/100 g | |||||||
| Shellfish | 85 μg/100 g | |||||||
| Chicken | 25 μg/100 g | |||||||
| Eggs | 20 μg/100 g | |||||||
| Nuts | 1700 μg/100 g | |||||||
| Cereals | 19 μg/100 g | |||||||
| Zn | Oysters | 61 mg/100 g | 11 mg/day | 40 mg/day |
- Antiviral function - RNA polymerase inhibition - Enhanced the Nk cells activity - Activation of antibody production |
- Induced viral infection vulnerability - Risk factor for pneumonia - Increased inflammation |
- Reduced common cold severity - Increased the T-cell production |
- Barnett et al. [2] - Read et al. [25] - de Almeida Brasiel [4] |
| Beef | 11 mg/100 g | |||||||
| Chicken | 2 mg/100 g | |||||||
| Wheat | 17 mg/100 g | |||||||
| Beans, lentils | 1 mg/100 g |
Selenium (Se) acts as cofactor of many enzymes such as glutathione peroxidase (GPX) involved in the cell protection from oxidative damage (ROS). Otten [22] suggested that 55 μg of Se/day is sufficient dietary intake for human nutrition (Table 1). Poor Se status leads to increases the vulnerability to influenza virus infection [3]. The antiviral proprieties of selenite (Se+4) are related to antioxidant capacity of this trace element. Generally, the protein disulfide isomerase (PDI) of virus was blocked by the presence of selenite and leads to avoid virus to penetrate in healthy human cells [6]. Ivory et al. [15] demonstrated that Se supplementation has beneficial effects on immunity to influenza in older adults and enhanced T lymphocyte multiplication. Recently, Moghaddam et al. [19] showed that non-survivor SARS-CoV-19 patients have a pronounced deficit in total serum Se and SELENOP concentrations compared to surviving patients.
Zinc (Zn) is an essential trace element required for many metabolism activities. This component plays a substantial role in antiviral immunity, decreases the risk of viral infection, and possesses anti-inflammatory proprieties [24, 25]. Maares and Haase [17] shown that Zn regulates the lymphocyte apoptosis by regulating the cytokine activity. For human nutrition, optimal daily intake concentration of Zn is 11 mg/day [22]. Zn deficiency may be frequent for COVID-19 elderly patients [5]. te Velthuis et al. [28] revealed that Zn supplementation prevents the arterivirus RNA polymerase activity and blocks the coronavirus proliferation in vitro cell culture. In the USA, Barnett et al. [2] found that nursing home elderly have a significant T lymphocyte proliferation when supplemented by 30 mg Zn/day (Table 1). Also, Wang and Song [31] showed that Zn administration as an adjunct reduced the incidence and mortality of patients with severe pneumonia. In a recent clinical study, significant improvement was shown in 04 confirmed SARS-CoV-19 outpatients (26–63 years old) when treated with 115–184 mg Zn/day for 10 to 14 days [9]. In another clinical report, three SARS-CoV-19 patients (38–74 years old) recovered when they received 220 mg of Zn for 5 days, combined with hydroxychloroquine (HCQ) [27]. Contrary in observational study, Yao et al. [34] examined the remedy effects of zinc sulfate supplementation in 196 SARS-CoV-19 patients and indicated that Zn had a minimal effect on the survival of hospitalized patients.
Many countries throughout the world started clinical use of many vaccines such as Sputnik V, Pfizer-BioNTech, and Moderna and drugs like chloroquine (CQ), HCQ, remdesivir, lopinavir, and ritonavir to treat COVID-19, but without full clinical data beside. Numerous clinical investigations confirmed that trace element insufficiencies alter the immune system and increase the vulnerability to viral infections. For better enhancement of immunity including blood immune cell proliferation (lymphocyte apoptosis, natural killer (Nk) cells, T helper (Th) cells) and cytokine synthesis, sufficient daily intakes of essential trace element were required. We hypothesize that Cu, Se, and Zn supplementation can enhance immunity and reduced the risk of COVID-19 infection. Such information is essential to identification of new clinical trials to battle this pandemic.
Conclusion
The new acute pulmonary infection (COVID-19) is usually associated with the decrease of human immune defense system leading to severe pneumonic inflammation and death. In view of the characteristics revealed above and the evidence of antiviral and anti-inflammatory effects of trace elements, it seems logical to assume that Se, Zn, and Cu could represent suitable components to strengthen immunity against viral infections including COVID-19 and its new emerging variants.
Acknowledgements
The author is very grateful to anonymous reviewers for their comments, which helped us tremendously to improve the quality of this manuscript.
Abbreviations
- COVID-19
Coronavirus disease-2019
- CQ
Chloroquine
- DPL
Daily permissible limit
- GSH-Px
Glutathione peroxidase
- HCQ
Hydroxychloroquine
- IFN-γ
Interferon-γ
- IL-2
Interleukin-2
- IL-6
Interleukin-6
- MERS-CoV
Middle east respiratory syndrome
- Nk
Natural killer cells
- PDI
Protein disulfide isomerase
- RDI
Recommended daily ingestion
- ROS
Reactive oxygen species
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SOD
Superoxide dismutase
- Th
T helper cells
- TNF-α
Tumor necrosis factor-α
Author’s contributions
BN performed the work, collected data, and wrote and approved the final manuscript.
Funding
The author greatly acknowledges the financial assistance provided by the Algerian Ministry of Higher Education and Scientific Research through PRFU Project # D04N01UN170120200003 entitled “Trace element determination in some vegetable species by XRF analysis: Phytoremediation and implications for human health”.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alexander J, Tinkov A, Strand TA, Alehagen U, Skalny A, Aaseth J. Early nutritional interventions with zinc, selenium and vitamin d for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12(8):2358. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett JB, Dao MC, Hamer DH, Kandel R, Brandeis G, Wu D, Dallal GE, Jacques PF, Schreiber R, Kong E, Meydani SN. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2016;103(3):942–951. doi: 10.3945/ajcn.115.115188. [DOI] [PubMed] [Google Scholar]
- 3.Beck MA, Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001;15(8):1481–1483. doi: 10.1096/fj.00-0721fje. [DOI] [PubMed] [Google Scholar]
- 4.de Almeida Brasiel PG. The key role of zinc in elderly immunity: a possible approach in the COVID-19 crisis. Clin Nutr ESPEN. 2020;38:65–66. doi: 10.1016/j.clnesp.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derwand R, Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today’s battle against COVID-19? Med Hypotheses. 2020;142:109815. doi: 10.1016/j.mehy.2020.109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diwaker D, Mishra KP, Ganju L. Potential role of protein disulfide isomerase in viral infections. Acta Virol. 2013;57:293–304. [PubMed] [Google Scholar]
- 7.EFSA (2020) https://www.efsa.europa.eu/en/topics/topic/dietary-reference-valuesand-dietary-guidelines. Accessed 4 Jan 2021.
- 8.Fan Y, Zhao K, Shi ZL, Zhou P. Bat coronaviruses in China. Viruses. 2019;11(3):210. doi: 10.3390/v11030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int J Infect Dis. 2020;99:307–309. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, Poon LLM, Samborskiy DV, Sidorov IA, Sola I, Ziebuhr J. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillin OM, Vindry C, Ohlmann T, Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11(9):2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hewawaduge C, Senevirathne A, Jawalagatti V, Kim JW, Lee JH. Copper-impregnated three-layer mask efficiently inactivates SARS-CoV2. Environ Res. 2021;196:110947. doi: 10.1016/j.envres.2021.110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopkins RG, Failla ML. Copper deficiency reduces interleukin-2 (IL-2) production and IL-2 mRNA in human T-lymphocytes. J Nutr. 1997;127(2):257–262. doi: 10.1093/jn/127.2.257. [DOI] [PubMed] [Google Scholar]
- 14.Ishida T. Antiviral activities of Cu2+ ions in viral prevention replication, RNA degradation, and for antiviral efficacies of lytic virus, ROS-mediated virus, copper chelation. World Sci News. 2018;99:148–168. [Google Scholar]
- 15.Ivory K, Prieto E, Spinks C, Armah CN, Goldson AJ, Dainty JR, Nicoletti C. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin Nutr. 2017;36(2):407–415. doi: 10.1016/j.clnu.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, Culotta VC. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci USA. 2015;112(38):E5336–E5342. doi: 10.1073/pnas.1513447112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maares M, Haase H. Zinc and immunity: an essential interrelation. Arch Biochem Biophys. 2016;611:58–65. doi: 10.1016/j.abb.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moghaddam A, Heller RA, Sun Q, Seelig J, Cherkezov A, Seibert L, Hackler J, Seemann P, Diegmann J, Pilz M, Bachmann M, Minich WB, Schomburg L. Selenium deficiency is associated with mortality risk from covid-19. Nutrients. 2020;12(7):2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuman BW, Kiss G, Kunding AH, Bhella D, Baksh MF, Connelly S, Droese B, Klaus JP, Makino S, Sawicki SG, Siddell SG, Stamou DG, Wilson IA, Kuhn P, Buchmeier MJ. A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol. 2011;174(1):11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noyce JO, Michels H, Keevil CW. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl Environ Microbiol. 2007;73(8):2748–2750. doi: 10.1128/AEM.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otten JJ, Hellwig JP, Meyers LD. Dietary reference intakes: the essential guide to nutrient requirements. Washington, D.C.: National Academies Press; 2006. [Google Scholar]
- 23.Percival SS. Copper and immunity. Am J Clin Nutr. 1998;67(5):1064S–1068S. doi: 10.1093/ajcn/67.5.1064S. [DOI] [PubMed] [Google Scholar]
- 24.Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care. 2009;12(6):646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- 25.Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sagripanti JL, Routson LB, Lytle CD. Virus inactivation by copper or iron ions alone and in the presence of peroxide. Appl Environ Microbiol. 1993;59(12):4374–4376. doi: 10.1128/AEM.59.12.4374-4376.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sattar Y, Connerney M, Rauf H, Saini M, Ullah W, Mamtani S, Syed U, Luddington S, Walfish A. Three cases of COVID-19 disease with colonic manifestations. Am J Gastroenterol. 2020;115(6):948–950. doi: 10.14309/ajg.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.te Velthuis AJW, van den Worm SHE, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.USDA (2020) https://www.nal.usda.gov/fnic/food-composition. Accessed 4 Jan 2021.
- 30.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Song Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: a meta-analysis of randomized, double-blind and placebo-controlled trials. Clin Respir J. 2018;12(3):857–864. doi: 10.1111/crj.12646. [DOI] [PubMed] [Google Scholar]
- 32.Warnes SL, Little ZR, Keevil CW. Human coronavirus 229E remains infectious on common touch surface materials. mBio. 2015;6(6):e01697–e01615. doi: 10.1128/mBio.01697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO/FAO . 53rd meeting, Rome, Italy. 1999. Expert committee on food additives, summary and conclusions. [Google Scholar]
- 34.Yao JS, Paguio JA, Dee EC, Tan HC, Moulick A, Milazzo C, Jurado J, Penna ND, Celi LA. The minimal effect of zinc on the survival of hospitalized patients with COVID-19: an observational study. Chest. 2021;159(1):108–111. doi: 10.1016/j.chest.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Saad R, Taylor EW, Rayman MP. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37:101715. doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.