Abstract
We performed a meta-analysis to determine safety and efficacy of tocilizumab in persons with coronavirus disease-2019 (COVID-19). We searched PubMed, Web of Science and Medline using Boolean operators for studies with the terms coronavirus OR COVID-19 OR 2019-nCoV OR SARS-CoV-2 AND tocilizumab. Review Manager 5.4 was used to analyze data and the modified Newcastle–Ottawa and Jadad scales for quality assessment. We identified 32 studies in 11,487 subjects including three randomized trials and 29 cohort studies with a comparator cohort, including historical controls (N = 5), a matched cohort (N = 12), or concurrent controls (N = 12). Overall, tocilizumab decreased risk of death (Relative Risk [RR] = 0.74; 95% confidence interval [CI], 0.59, 0.93; P = 0.008; I2 = 80%) but not of surrogate endpoints including ICU admission (RR = 1.40 [0.64,3.06]; P = 0.4; I2 = 88%), invasive mechanical ventilation (RR = 0.83 [0.57,1.22]; P = 0.34; I2 = 65%) or secondary infections (RR = 1.30 [0.97,1.74]; P = 0.08; I2 = 65%) and increased interval of hospitalization of subjects discharged alive(mean difference [MD] = 2 days [<1, 4 days]; P = 0.006; I2 = 0). RRs of death in studies with historical controls (RR = 0.28 [0.16,0.49; P < 0.001]; I2 = 62%) or a matched cohort (RR = 0.68 [0.53, 0.87]; P = 0.002; I2 = 42%) were decreased. In contrast, RRs of death in studies with a concurrent control (RR = 1.10 [0.77, 1.56]; P = 0.60; I2 = 85%) or randomized (RR = 1.18 [0.57,2.44]; P = 0.66; I2 = 0) were not decreased. A reduced risk of death was not confirmed in our analyses which questions safety and efficacy of tocilizumab in persons with COVID-19.
Subject terms: Infectious diseases, Immunotherapy
Introduction
Infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) causes coronavirus disease-2019 (COVID-19), an important pathogenetic component of which is cytokine release syndrome (CRS). CRS is mediated, at least in part, by interleukin-6 (IL-6). Tocilizumab, a humanized monoclonal antibody, selectively targets the interleukin-6 receptor (IL-6r) [1, 2] and is reported safe and effective in other settings such as after chimeric antigen receptor (CAR)-T-cell therapy, rheumatoid arthritis, and giant cell arteritis [3, 4].
Data from 11 uncontrolled studies [5–16] and most prior meta-analyses [17–24] claim tocilizumab is safe and effective in persons with COVID-19. However, data from three recent randomized controlled trials (RCTs) question this conclusion [25–27].
We conducted a systematic review and meta-analysis of 32 studies of safety and efficacy of tocilizumab in persons with COVID-19 which had a comparator cohort. Overall, we found tocilizumab decreased risk of death but not rates of intensive care unit(ICU) admission, invasive mechanical ventilation, secondary infections, and increased interval of hospitalization in persons discharged alive. However, a reduced risk of death was not confirmed in our analyses of studies with concurrent controls nor randomized trials. These data question safety and efficacy of tocilizumab in persons with COVID-19.
Methods
Search strategy and selection criteria
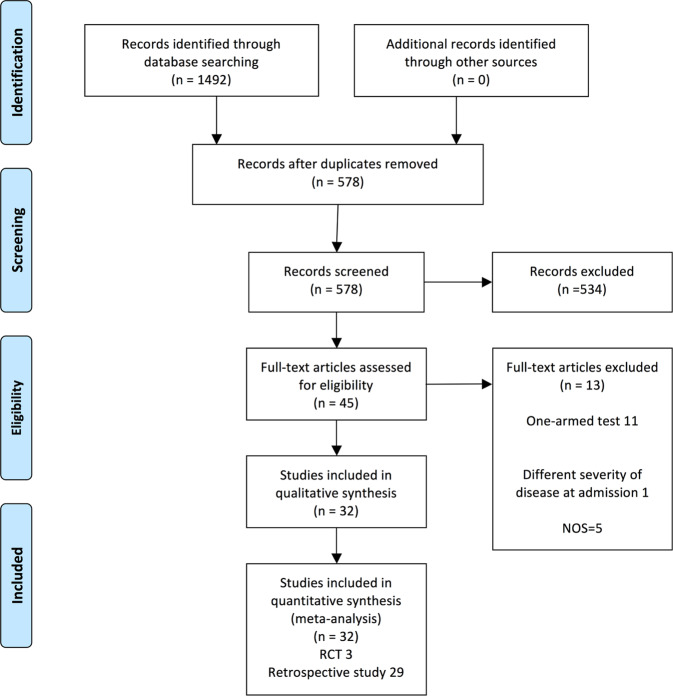
PubMed, Web of Science and Medline were searched using Boolean operators for studies with the terms coronavirus OR COVID-19 OR 2019-nCoV OR SARS-CoV-2 AND tocilizumab. Start and stop dates were 2020/1/1 and 2020/10/27. Two investigators independently reviewed abstracts of identified citations and selected articles for full review. Discordances were resolved by a third reviewer. Results were also manually searched and reviewed. We found 1492 articles excluding 914 duplicates. After further review we focused on 32 studies, 29 non-randomized comparator studies, and three RCTs [1, 2, 25–52]. A flow diagram of the search strategy and article selection is displayed in Fig. 1. Review Manager 5.4 was used to analyze data and the modified Newcastle-Ottawa score (NOS) and Jadad scale for quality assessment.
Fig. 1. Flow diagram.
Citation review process.
Inclusion and exclusion criteria
Inclusion criteria included English language reports of clinical trials and observational studies with a comparator cohort and with outcomes reporting, but not limited to, survival. Reviews and case reports were excluded as were studies with a NOS < 6.
Data extraction
For each selected article we extracted (1) first author, (2) publication year, (3) country, (4) study-design, (5) number of subjects, (6) comparator cohort, (7) baseline subject clinical and laboratory co-variates, (8) details of tocilizumab use, (9) concurrent interventions, and (10) outcomes, including: (1) survival, (2) rates of ICU admission, (3) invasive mechanical ventilation, (4) secondary infection, and (5) interval of hospitalization. The study used anonymized, published data with no requirement for Ethics Committee approval. (Table 1; Supplement Table 1)
Table 1.
Included Studies.
| Ref | Study-type/control cohort | NOS | Deaths (Tocilizumab vs. control) |
|---|---|---|---|
| [2] | Matched | 9 | 17/64 vs. 24/64 |
| [1] | Historical | 8 | 5/32 vs. 11/33 |
| [29] | Matched | 8 | 102/210 vs. 256/420 |
| [49] | Matched | 9 | 23/106 vs. 61/138 |
| [28] | Matched | 8 | 3/22 vs. 2/22 |
| [31] | Concurrent | 8 | 5/21 vs. 19/91 |
| [30] | Historical | 8 | 2/62 vs. 11/23 |
| [36] | Concurrent | 7 | 44/132 vs. 97/475 |
| [35] | Historical | 7 | 13/179 vs. 73/365 |
| [33] | Matched | 9 | 5/29 vs. 19/58 |
| [34] | Concurrent | 7 | 14/59 vs. 16/87 |
| [38] | Concurrent | 7 | 3/28 vs. 2/23 |
| [40] | Matched | 6 | 8/30 vs. 66/176 |
| [41] | Concurrent | 6 | 61/260 vs. 120/969 |
| [42] | Concurrent | 6 | 2/76 vs. 8/62 |
| [43] | Historical | 6 | 10/41 vs. 20/38 |
| [50] | Matched | 8 | 15/74 vs. 59/148 |
| [52] | Concurrent | 6 | 9/92 vs. 1/89 |
| [48] | Concurrent | 6 | 43/96 vs. 55/97 |
| [32] | Historical | 6 | 7/90 vs. 34/68 |
| [58] | Concurrent | 7 | NA |
| [46] | Matched | 9 | 2/40 vs. 12/40 |
| [47] | Matched | 9 | 0/10 vs. 1/10 |
| [45] | Matched | 9 | 12/29 vs. 8/29 |
| [44] | Matched | 9 | 2/20 vs. 3/40 |
| [39] | Concurrent | 7 | 19/54 vs. 11/57 |
| [37] | Concurrent | 7 | 119/433 vs. 1295/3491 |
| [51] | Concurrent | 7 | 14/78 vs. 27/76 |
| [57] | Matched | 7 | NA |
| [26] | RCT | Jadad 4 | 2/60 vs. 1/66 |
| [27] | RCT | Jadad 4 | 7/63 vs. 8/67 |
| [25] | RCT | Jadad 5 | 9/161 vs. 3/81 |
NOS Newcastle–Ottawa scale, RCT randomized controlled trial, NA not available.
Risk of bias assessment
Risk of bias was assessed using the Jadad scale in four domains: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants, and (4) complete reporting of withdrawals and dropouts [53]. Methodological quality of comparator studies was assessed using the modified Newcastle–Ottawa scale (NOS) [54, 55] consisting of three domains: (1) subject selection, (2) comparability of the study groups, and (3) outcomes assessment. A score of 0–9 was allocated to each relevant study. Observational studies with a NOS score <6 (N = 1) were excluded [56].
Statistics
We pooled data and utilized Relative Risks (RRs) and Confidence Intervals (CIs) to describe dichotomous outcomes, including risk of death, ICU admission, invasive mechanical ventilation, secondary infection . We used mean difference (MD) and CIs for continuous outcomes including interval of hospitalization. We grouped the cohort studies into unmatched historical controls and concurrent controls, matched and unmatched or subgroup analyses. A fixed-effects model was used when I2 ≤ 50% and the Cochran Q statistic P > 0.1 and a random-effects model when I2 > 50% and Q statistic P ≤ 0.1. Funnel plots were used to screen for potential publication bias. Statistical analyses were carried out with Review Manager 5.4 (Cochrane Collaboration)
Results
We included 11,487 subjects from 29 studies (NOS scores 6–9) with a comparator cohort including historical controls (N = 5) [1, 30, 32, 35, 43], a matched cohort (N = 12) [2, 28, 29, 33, 40, 44–47, 49, 50, 57] or concurrent controls (N = 12) [31, 34, 36–39, 41, 42, 48, 51, 52, 58] and three randomized controlled trials (Jadad scales 4–5). We excluded some studies because they were uncontrolled (N = 11) [5–16] and/or different study-entry severities of COVID-19 between treated subjects and controls (N = 1) [59]. Two studies reported only a composite endpoint (ICU admission, use of mechanical ventilation or death) [57, 58]. These were included in the systemic review but not used to estimate RRs for specific endpoints.
Survival
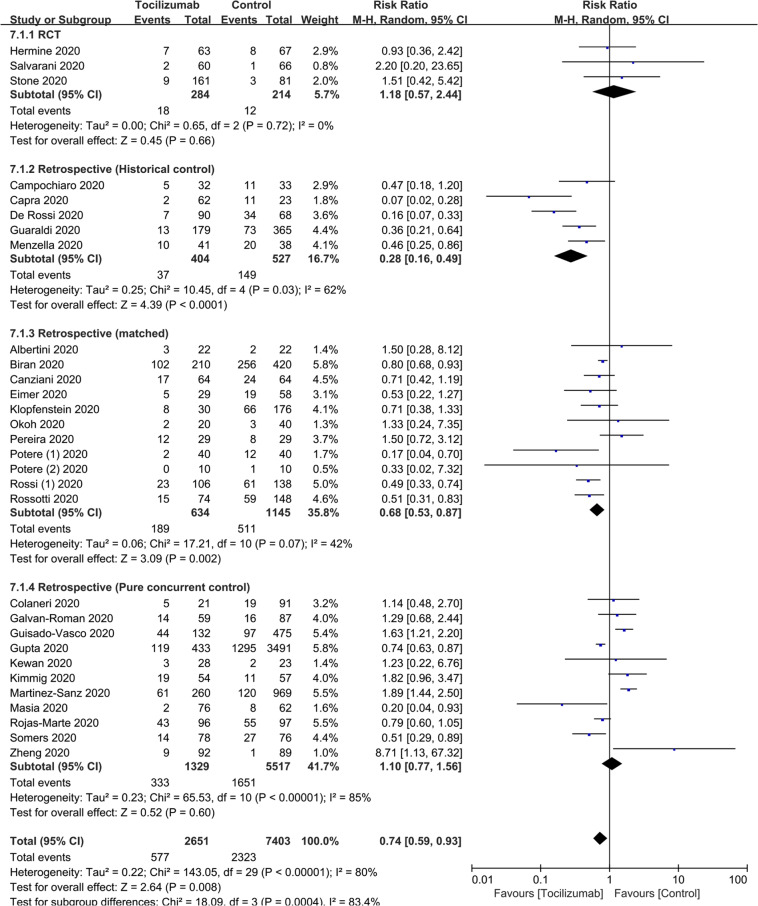
To test the impact of tocilizumab on survival we included 30 studies, three RCTs and 27 other comparator studies of 10,054 subjects. [1, 2, 25–52] Relative Risk (RR) of death = 0.74 (95% Confidence Interval [CI], 0.59, 0.93; P = 0.008; I2 = 80%). Studies with historical controls (RR = 0.28 [0.16, 0.49]; P < 0.001; I2 = 62%) or with an otherwise matched cohort (RR = 0.68 [0.53, 0.87]; P = 0.002; I2 = 42%) reported significant survival improvement. In contrast, RRs of death in studies with concurrent controls (RR = 1.10 (0.77, 1.56; P = 0.60; I2 = 85%; Table 2, Fig. 2) and randomized trials (RR = 1.18 (0.57, 2.44; P = 0.66; I2 = 0; Table 2, Fig. 2) showed no significant improvement in survival.
Table 2.
Risk of Survival and Surrogate Clinical Endpoints.
| Ref | Risk of death (RR [95%CI]) | Risk of ICU admission (RR [95%CI]) | Risk of invasive mechanical ventilation (RR [95%CI]) | Risk of secondary infection (RR [95%CI]) |
|---|---|---|---|---|
| Observational (historical control) | 0.28 [0.16, 0.49] | NA | 0.74 [0.40, 1.37] | 2.18 [0.68, 6.98] |
| Observational (matched) | 0.68 [0.53, 0.87] | 2.25 [1.17, 4.33] | 0.77 [0.33, 1.80] | 1.12 [0.77, 1.62] |
| Observational (concurrent control) | 1.10 [0.77, 1.56] | 1.91 [0.47, 7.69] | NA | 1.27 [0.85, 1.89] |
| Randomized controlled trials | 1.18 [0.57, 2.44] | 0.80 [0.49, 1.33] | 0.92 [0.21, 4.10] | 0.26 [0.03, 2.28] |
| Total events | 0.74 [0.59, 0.93] | 1.40 [0.64, 3.06] | 0.83 [0.57, 1.22] | 1.30 [0.97, 1.74] |
RR relative risk, NA not available.
Fig. 2. The effect of tocilizumab on survival.
Risk of death.
Surrogate clinical endpoints
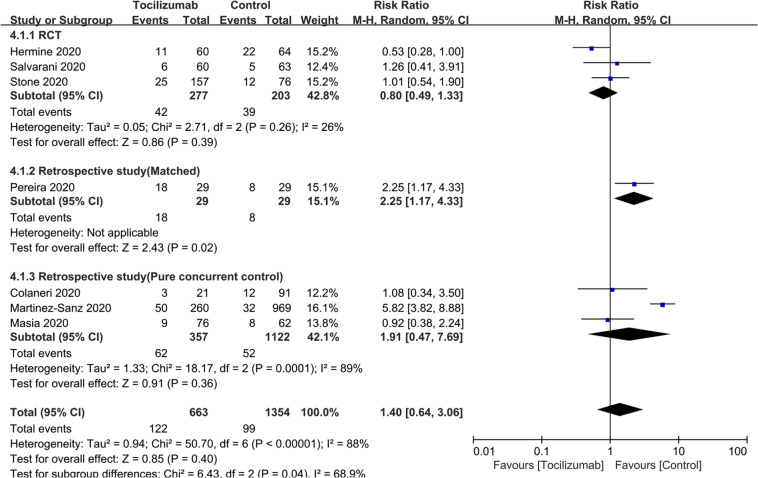
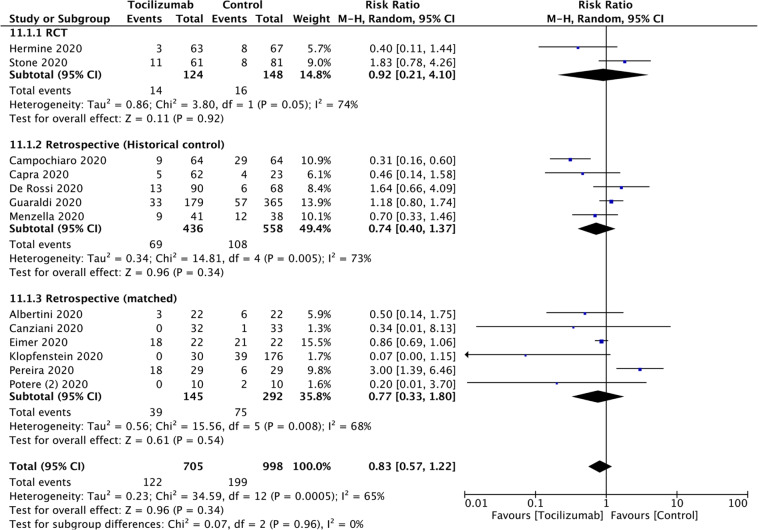
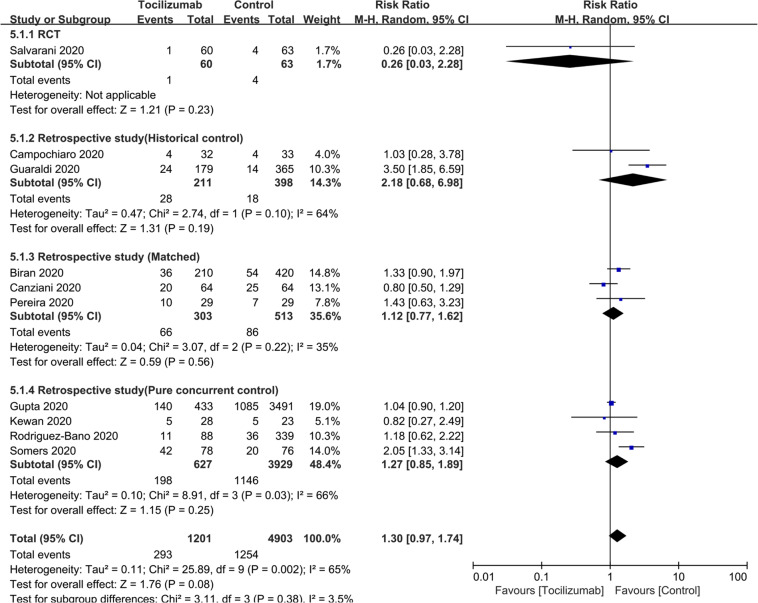
To test the efficacy of tocilizumab on rate of ICU admission we included seven studies [25–27, 31, 41, 42, 45] of 2017 subjects. RR = 1.40 (0.64, 3.06; P = 0.4; I2 = 88%). RR for RCTs and for studies with concurrent controls were RR = 0.80 (0.49, 1.33; P = 0.39; I2 = 26%) and RR = 1.91 (0.47, 7.69; P = 0.36; I2 = 89%; Table 2; Fig. 3). (There was only one study with a matched cohort and no study with a historical control). To test the efficacy of tocilizumab on rate of invasive mechanical ventilation we included 13 studies [1, 2, 25, 27, 28, 30, 32, 33, 35, 40, 43, 45, 47, 56] of 1703 subjects. RR = 0.83 (0.57, 1.22; P = 0.34; I2 = 65%). RRs of studies with historical controls (RR = 0.74 [0.40, 1.37]; P = 0.34; I2 = 73%), those with a matched cohort (RR = 0.77 [0.33, 1.80]; P = 0.54; I2 = 68%) and RCTs (RR = 0.92 [0.21, 4.10]; P = 0.92; I2 = 74) are indicated. (There were no studies with concurrent controls; Table 2; Fig. 4). To test the effect tocilizumab on rate of secondary infections we included 10 studies [1, 2, 26, 29, 35, 37, 38, 45, 51, 58] of 5495 subjects. RR = 1.30 (0.97, 1.74; P = 0.08; I2 = 65%). RR for studies with a historical controls (RR = 2.18 [0.68, 6.98]; P = 0.19; I2 = 64%), those with a matched cohort (RR = 1.12 [0.77, 1.62]; P = 0.56; I2 = 35%) and those with concurrent controls (RR = 1.27 [0.85, 1.89]; P = 0.25; I2 = 66%) were indicated in Fig. 5. (There was only one RCT). To test the impact of tocilizumab on hospitalization interval we included seven studies [30, 32, 38, 40, 42, 51, 52] of 1041 subjects. Mean difference in subjects discharged from hospital was 2 days (<1, 4 days; P = 0.006; I2 = 0; Supplement Fig. 1).
Fig. 3. The impact of tocilizumab on ICU admission.
Risk of ICU admission.
Fig. 4. The association of tocilizumab and invasive mechanical ventilation.
Risk of invasive mechanical ventilation.
Fig. 5. The correlation between tocilizumab and secondary infection.
Risk of secondary infection.
Publication bias
Potential for publication bias is shown in Fig. 6. We found potential publication bias in studies of death in subjects receiving or not receiving tocilizumab with some studies falling outside the 95% CI of the funnel plot. There was publication bias in studies included in the meta-analysis.
Fig. 6. Funnel plot.
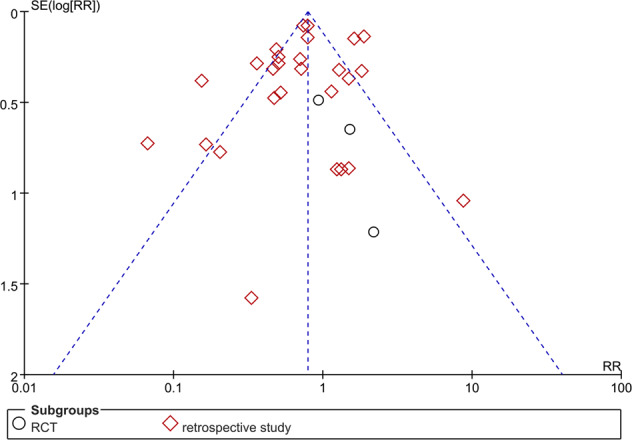
Risk of publication bias.
Discussion
Increased concentrations of inflammatory cytokines (IL-6, granulocyte-macrophage colony stimulating factor (GM-CSF) and tumor necrosis factor-a (TNF-a) are reported in persons with COVID-19 [60, 61]. IL-6 is produced by diverse immune cells and implicated in development of acute respiratory distress syndrome (ARDS) and CRS [62, 63]. Some data suggest increased IL-6 concentrations correlate with risk of death [61, 64]. Several meta-analyses claim tocilizumab is safe and effective in COVID-19 [17, 21, 23, 24]. Most studies we include gave tocilizumab to subjects with evidence of inflammation including a CRP concentration >100 mg/L, a ferritin concentration >900 ng/ml and/or a D-dimer concentration >1500 ug/L [1].
Evaluating all 32 studies we found tocilizumab reduced risk of death but not several surrogate endpoints, including ICU admission, invasive mechanical ventilation, and secondary infections. Hospitalization interval was significantly increased. However, in our analysis of RCTs and studies with a concurrent control cohort we could not confirm a decreased risk of death. This conclusion differs from most prior meta-analyses [17–24] which failed to analyze outcomes by study-design (Table 3). A recent meta-analysis concluded an association between tocilizumab and lower mortality by low certainty evidence from cohort studies [22]. Our data contradict this assumption. Two recent analyses which included only studies with a comparator cohort support our conclusion [65, 66]. Also, Mao and colleagues reported use of tocilizumab did not decrease risk of death possibly because of an increased risk of secondary bacterial infections [67].
Table 3.
Previous meta-analyses.
| Ref | N studies | N subjects | Studies included | RR or OR of death (95% CI) |
|---|---|---|---|---|
| [19] | 13 | 766 |
2 historical controls 1 matched control 4 concurrent controls 6 no control |
RR = 0.56 [0.34, 0.92] |
| [17] | 23 | 6279 |
4 historical controls 5 matched controls 14 concurrent controls |
RD = −0.06 [−0.12, −0.01] |
| [18] | 16 | 3641 |
4 historical controls 3 matched controls 9 concurrent controls |
OR = 0.57 [0.36–0.92] |
| [20] | 7 | 592 |
1 historical control 3 matched controls 3 concurrent controls |
RR = 0.62, [0.31,1.22] |
| [21] | 10 | 1358 |
3 historical controls 1 matched control 6 concurrent controls |
RR = 0.27 [0.12, 0.59] |
| [22] | 23 | 11346 |
3 historical controls 6 matched controls 8 concurrent controls 1 no control 5 RCTs |
RR for cohort studies = 0.58 [0.51–0.66] RR for RCTs = 1.09 [0.80,1.49] |
| [23] | 10 | 1675 |
3 historical controls 2 matched controls 5 concurrent controls |
OR = 0.47 [0.36, 0.60] |
| [24] | 19 | 2285 |
4 historical controls 4 matched controls 5 concurrent controls 6 no controls |
OR = 0.44 [0.36, 0.55] |
RR relative risk, OR odds ratios, RD risk differences, RCTs randomized controlled trials.
There are important limitations to our study. Firstly, subjects were heterogeneous in COVID-19 severity although most had severe to critical COVID-19. Secondly, many studies were observational and lacked an appropriate control cohort. We tried to overcome potential biases in these studies by analyzing outcomes by study-type.
In conclusion, tocilizumab decreased risk of death but not rates of surrogate endpoints including ICU admission, invasive mechanical ventilation, secondary infections and did significantly alter interval of hospitalization. A reduced risk of death was not confirmed in our meta-analysis of randomized trials or studies with a concurrent control cohort. These data question safety and efficacy of tocilizumab in persons with COVID-19.
Supplementary information
Acknowledgements
YL supported in part, by Sun Yat-sen University Cancer Center Start-Up Funding (No. 201603), and the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2017ZT07S096). RPG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Center funding scheme. We thank Prof. Juan Li for valuable comments.
Author contributions
YL, RPG, and CXC designed study. CXC and FH searched databases and processed analysis. CXC, FH, LTY, TMW, JW, YL, and RPG drafted the typescript. YL, RPG, CXC, LTY, TMW and JW revised the final typescript. YL and RPG are responsible for the paper.
Compliance with ethical standards
Conflict of interest
RPG is a consultant to: BeiGene Ltd., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Parmaceuticals Inc. and CStone Pharmaceuticals. Advisor: Antegene Biotech LLC, Medical Director: FFF Enterprises Inc. Partner: AZACA Inc. Board of Directors: RakFond Foundation for Cancer Research Support. Scientific Advisory Board, StemRad Ltd. All other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chong-xiang Chen, Fang Hu, Jin Wei
Supplementary information
The online version contains supplementary material available at 10.1038/s41375-021-01264-8.
References
- 1.Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur j of intern med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canziani LM, Trovati S, Brunetta E, Testa A, De Santis M, Bombardieri E, et al. Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case-control survival analysis of 128 patients. J of autoimmun. 2020;114:102511. doi: 10.1016/j.jaut.2020.102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur S, Bansal Y, Kumar R, Bansal G. A panoramic review of IL-6: structure, pathophysiological roles and inhibitors. Bioorg & med chem. 2020;28:115327. doi: 10.1016/j.bmc.2020.115327. [DOI] [PubMed] [Google Scholar]
- 4.Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. The oncologist. 2018;23:943–7. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dastan F, Saffaei A, Haseli S, Marjani M, Moniri A, Abtahian Z, et al. Promising effects of tocilizumab in COVID-19: a non-controlled, prospective clinical trial. Int immunopharmacol. 2020;88(Aug):106869. doi: 10.1016/j.intimp.2020.106869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández-Ruiz M, López-Medrano F, Pérez-Jacoiste Asín MA, Maestro de la Calle G, Bueno H, Caro-Teller JM, et al. Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: a single-center cohort study. J of med virol. 2021;93:831–42. doi: 10.1002/jmv.26308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fomina DS, Lysenko MA, Beloglazova IP, Mutovina ZY, Poteshkina NG, Samsonova IV, et al. Temporal clinical and laboratory response to interleukin-6 receptor blockade with tocilizumab in 89 hospitalized patients with COVID-19 pneumonia. Pathog & immun. 2020;5:327–41. doi: 10.20411/pai.v5i1.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J of med virol. 2020;92:814–8. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malekzadeh R, Abedini A, Mohsenpour B, Sharifipour E, Ghasemian R, Javad-Mousavi SA, et al. Subcutaneous tocilizumab in adults with severe and critical COVID-19: A prospective open-label uncontrolled multicenter trial. Int immunopharmacol. 2020;89:107102. doi: 10.1016/j.intimp.2020.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mastroianni A, Greco S, Apuzzo G, De Santis S, Oriolo C, Zanolini A, et al. Subcutaneous tocilizumab treatment in patients with severe COVID-19-related cytokine release syndrome: an observational cohort study. EClinicalMedicine. 2020;24:100410. doi: 10.1016/j.eclinm.2020.100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison AR, Johnson JM, Griebe KM, Jones MC, Stine JJ, Hencken LN, et al. Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving tocilizumab. Journal of autoimmunity. 2020;114:102512. doi: 10.1016/j.jaut.2020.102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel A, Shah K, Dharsandiya M, Patel K, Patel T, Patel M, et al. Safety and efficacy of tocilizumab in the treatment of severe acute respiratory syndrome coronavirus-2 pneumonia: a retrospective cohort study. Indian j of med microbiol. 2020;38:117–23. doi: 10.4103/ijmm.IJMM_20_298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrone F, Piccirillo MC, Ascierto PA, Salvarani C, Parrella R, Marata AM, et al. Tocilizumab for patients with COVID-19 pneumonia. The single-arm TOCIVID-19 prospective trial. J of transl med. 2020;18:405. doi: 10.1186/s12967-020-02573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrak RM, Skorodin NC, Van Hise NW, Fliegelman RM, Pinsky J, Didwania V, et al. Tocilizumab as a therapeutic agent for critically ill patients infected with SARS-CoV-2. Clin and transl sci. 2020. https://ascpt.onlinelibrary.wiley.com/doi/10.1111/cts.12894. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 15.Ramírez P, Gordón M, Martín-Cerezuela M, Villarreal E, Sancho E, Padrós M, et al. Acute respiratory distress syndrome due to COVID-19. Clinical and prognostic features from a medical Critical Care Unit in Valencia, Spain. Med intensiva. 2021;45:27–34. doi: 10.1016/j.medin.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc of the Natl Acad of Sci of the USA. 2020;117:10970–5. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aziz M, Haghbin H, Abu Sitta E, Nawras Y, Fatima R, Sharma S, et al. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J of med virol. 2021;93:1620–30. doi: 10.1002/jmv.26509. [DOI] [PubMed] [Google Scholar]
- 18.Boregowda U, Perisetti A, Nanjappa A, Gajendran M, Kutti Sridharan G, Goyal H. Addition of Tocilizumab to the standard of care reduces mortality in severe COVID-19: a systematic review and meta-analysis. Front in med. 2020;7:586221. doi: 10.3389/fmed.2020.586221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotak S, Khatri M, Malik M, Malik M, Hassan W, Amjad A, et al. Use of Tocilizumab in COVID-19: a systematic review and meta-analysis of current evidence. Cureus. 2020;12:e10869. doi: 10.7759/cureus.10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan SH, Lai CC, Huang HT, Chang SP, Lu LC, Hsueh PR. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int j of antimicrob agents. 2020;56:106103. doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malgie J, Schoones JW, Pijls BG. Decreased mortality in COVID-19 patients treated with Tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin infect dis. 2020;23:ciaa1445. doi: 10.1093/cid/ciaa1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tleyjeh IM, Kashour Z, Damlaj M, Riaz M, Tlayjeh H, Altannir M, et al. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin microbiol and infect. 2021;27:215–27. doi: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J, Cui W, Tian BP. Efficacy of tocilizumab treatment in severely ill COVID-19 patients. Critical care (London, England). 2020;24:524. doi: 10.1186/s13054-020-03224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao M, Lu J, Tang Y, Dai Y, Zhou J, Wu Y. Tocilizumab for treating COVID-19: a systemic review and meta-analysis of retrospective studies. Eur j of clin pharmacol. 2021;77:311–9. doi: 10.1007/s00228-020-03017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of Tocilizumab in patients hospitalized with Covid-19. The N Engl j of med. 2020;383:2333–44. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of Tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 Pneumonia: a randomized clinical trial. JAMA intern med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P. Effect of Tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA intern med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albertini L, Soletchnik M, Razurel A, Cohen J, Bidegain F, Fauvelle F, et al. Observational study on off-label use of tocilizumab in patients with severe COVID-19. Eur j of hosp pharm. 2021;28:22–27. doi: 10.1136/ejhpharm-2020-002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biran N, Ip A, Ahn J, Go RC, Wang S, Mathura S, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. The Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur j of intern med. 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms. 2020;8:695. [DOI] [PMC free article] [PubMed]
- 32.De Rossi N, Scarpazza C, Filippini C, Cordioli C, Rasia S, Mancinelli CR, et al. Early use of low dose tocilizumab in patients with COVID-19: a retrospective cohort study with a complete follow-up. EClinicalMed. 2020;25:100459. doi: 10.1016/j.eclinm.2020.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eimer J, Vesterbacka J, Svensson AK, Stojanovic B, Wagrell C, Sönnerborg A, et al. Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID-19: a retrospective cohort study. J of intern med.2021;289:434-436. [DOI] [PMC free article] [PubMed]
- 34.Galván-Román JM, Rodríguez-García SC, Roy-Vallejo E, Marcos-Jiménez A, Sánchez-Alonso S, Fernández-Díaz C, et al. IL-6 serum levels predict severity and response to Tocilizumab in COVID-19: an observational study. The J of allergy and clin immunol. 2021;147:72–80.e8. [DOI] [PMC free article] [PubMed]
- 35.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. The Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guisado-Vasco P, Valderas-Ortega S, Carralón-González MM, Roda-Santacruz A, González-Cortijo L, Sotres-Fernández G, et al. Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: a retrospective observational study (COQUIMA cohort) EClinicalMedicine. 2020;28:100591. doi: 10.1016/j.eclinm.2020.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, et al. Association between early treatment with Tocilizumab and mortality among critically ill patients with COVID-19. JAMA intern med. 2021;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kewan T, Covut F, Al-Jaghbeer MJ, Rose L, Gopalakrishna KV, Akbik B. Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMed. 2020;24:100418. doi: 10.1016/j.eclinm.2020.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimmig LM, Wu D, Gold M, Pettit NN, Pitrak D, Mueller J, et al. IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. Front Med (Lausanne). 2020;7:583897. doi: 10.3389/fmed.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klopfenstein T, Zayet S, Lohse A, Selles P, Zahra H, Kadiane-Oussou NJ, et al. Impact of tocilizumab on mortality and/or invasive mechanical ventilation requirement in a cohort of 206 COVID-19 patients. Int j of infect dis. 2020;99:491–5. doi: 10.1016/j.ijid.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martínez-Sanz J, Muriel A, Ron R, Herrera S, Pérez-Molina JA, Moreno S, et al. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicentre cohort study. Clin microbiol and infect. 2021;27:238–43. doi: 10.1016/j.cmi.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masiá M, Fernández-González M, Padilla S, Ortega P, García JA, Agulló V, et al. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: a prospective cohort study. EBioMed. 2020;60:102999. doi: 10.1016/j.ebiom.2020.102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menzella F, Fontana M, Salvarani C, Massari M, Ruggiero P, Scelfo C, et al. Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation. Critical care (London, England) 2020;24:589. doi: 10.1186/s13054-020-03306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okoh AK, Bishburg E, Grinberg S, Nagarakanti S. Tocilizumab use in COVID-19-associated pneumonia. J of med virol. 2021;93:1023–8. doi: 10.1002/jmv.26471. [DOI] [PubMed] [Google Scholar]
- 45.Pereira MR, Aversa MM, Farr MA, Miko BA, Aaron JG, Mohan S, et al. Tocilizumab for severe COVID-19 in solid organ transplant recipients: a matched cohort study. Am j of transplant. 2020;20:3198–205. doi: 10.1111/ajt.16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potere N, Di Nisio M, Cibelli D, Scurti R, Frattari A, Porreca E, et al. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: a case-control study. Ann of the rheum dis. 2021;80:1–2. doi: 10.1136/annrheumdis-2020-218243. [DOI] [PubMed] [Google Scholar]
- 47.Potere N, Di Nisio M, Rizzo G, La Vella M, Polilli E, Agostinone A, et al. Low-dose subcutaneous tocilizumab to prevent disease progression in patients with moderate COVID-19 pneumonia and hyperinflammation. Int j of infect dis. 2020;100:421–4. doi: 10.1016/j.ijid.2020.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rojas-Marte G, Khalid M, Mukhtar O, Hashmi AT, Waheed MA, Ehrlich S, et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case-controlled study. QJM. 2020;113:546–50. doi: 10.1093/qjmed/hcaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossi B, Nguyen LS, Zimmermann P, Boucenna F, Dubret L, Baucher L, et al. Effect of Tocilizumab in hospitalized patients with severe COVID-19 pneumonia: a case-control cohort study. Pharmaceuticals (Basel, Switzerland) 2020;13:317. doi: 10.3390/ph13100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossotti R, Travi G, Ughi N, Corradin M, Baiguera C, Fumagalli R, et al. Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: a comparative analysis. The J of infect. 2020;81:e11–e17. doi: 10.1016/j.jinf.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin infect dis.2020;ciaa954. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa954/5870306. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 52.Zheng KL, Xu Y, Guo YF, Diao L, Kong XY, Wan XJ, et al. Efficacy and safety of tocilizumab in COVID-19 patients. Aging. 2020;12:18878–88. doi: 10.18632/aging.103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control clin trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 54.Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet (London, England) 2001;358:870–5. doi: 10.1016/S0140-6736(01)06069-X. [DOI] [PubMed] [Google Scholar]
- 55.Fan X, Lin T, Xu K, Yin Z, Huang H, Dong W, et al. Laparoendoscopic single-site nephrectomy compared with conventional laparoscopic nephrectomy: a systematic review and meta-analysis of comparative studies. European urology. 2012;62:601–12. doi: 10.1016/j.eururo.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 56.Gokhale Y, Mehta R, Karnik N, Kulkarni U, Gokhale S. Tocilizumab improves survival in patients with persistent hypoxia in severe COVID-19 pneumonia. EClinicalMedicine. 2020;24:100467. doi: 10.1016/j.eclinm.2020.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chilimuri S, Sun H, Alemam A, Kang KS, Lao P, Mantri N, et al. Tocilizumab use in patients with moderate to severe COVID-19: a retrospective cohort study. J of clin pharm and therapeutics. 2021;46:440–6. doi: 10.1111/jcpt.13303. [DOI] [PubMed] [Google Scholar]
- 58.Rodríguez-Baño J, Pachón J, Carratalà J, Ryan P, Jarrín I, Yllescas M, et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19) Clin microbiol and infect. 2021;27:244–52. doi: 10.1016/j.cmi.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quartuccio L, Sonaglia A, McGonagle D, Fabris M, Peghin M, Pecori D, et al. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: Results from a single Italian Centre study on tocilizumab versus standard of care. J of clin virol. 2020;129:104444. doi: 10.1016/j.jcv.2020.104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395:1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA intern med. 2020;180:934–43. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lagunas-Rangel FA, Chávez-Valencia V. High IL-6/IFN-γ ratio could be associated with severe disease in COVID-19 patients. J of med virol. 2020;92:1789–90. doi: 10.1002/jmv.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin infect dis. 2020;71:1937–42. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill JA, Menon MP, Dhanireddy S, Wurfel MM, Green M, Jain R, et al. Tocilizumab in hospitalized patients with COVID-19: clinical outcomes, inflammatory marker kinetics, and safety. J of med virol. 2021;93:2270–80. doi: 10.1002/jmv.26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roumier M, Paule R, Vallée A, Rohmer J, Ballester M, Brun AL, et al. Tocilizumab for Severe Worsening COVID-19 Pneumonia: a propensity score analysis. J of clin immunol. 2021;41:303–14. doi: 10.1007/s10875-020-00911-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y, Mao Q, Zhou X. Does tocilizumab have a magical therapeutic effect on COVID-19 patients without obvious adverse reactions? Proc of the Natl Acad of Sci. of the USA. 2020;117:30896–30897. doi: 10.1073/pnas.2009961117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.