Abstract
Background
COVID-19 is associated with unintentional weight loss. Little is known on whether and how patients regain the lost weight. We assessed changes in weight and abdominal adiposity over a three-month follow-up after discharge in COVID-19 survivors.
Methods
In this sub-study of a large prospective observational investigation, we collected data from individuals who had been hospitalized for COVID-19 and re-evaluated at one (V1) and three (V2) months after discharge. Patient characteristics upon admission and anthropometrics, waist circumference and hunger levels assessed during follow-up were analyzed across BMI categories.
Results
One-hundred-eighty-five COVID-19 survivors (71% male, median age 62.1 [54.3; 72.1] years, 80% with overweight/obesity) were included. Median BMI did not change from admission to V1 in normal weight subjects (−0.5 [−1.2; 0.6] kg/m2, p = 0.08), but significantly decreased in subjects with overweight (−0.8 [−1.8; 0.3] kg/m2, p < 0.001) or obesity (−1.38 [−3.4; −0.3] kg/m2, p < 0.001; p < 0.05 vs. normal weight or obesity). Median BMI did not change from V1 to V2 in normal weight individuals (+0.26 [−0.34; 1.15] kg/m2, p = 0.12), but significantly increased in subjects with overweight (+0.4 [0.0; 1.0] kg/m2, p < 0.001) or obesity (+0.89 [0.0; 1.6] kg/m2, p < 0.001; p = 0.01 vs. normal weight). Waist circumference significantly increased from V1 to V2 in the whole group (p < 0.001), driven by the groups with overweight or obesity. At multivariable regression analyses, male sex, hunger at V1 and initial weight loss predicted weight gain at V2.
Conclusions
Patients with overweight or obesity hospitalized for COVID-19 exhibit rapid, wide weight fluctuations that may worsen body composition (abdominal adiposity).
ClinicalTrials.gov registration
Subject terms: Obesity, Obesity
Introduction
Unintentional weight loss and malnutrition have been recently reported to be highly prevalent in COVID-19 survivors evaluated after clinical remission [1]. Specifically, we found that more than 30% of patients, evaluated ~1 month after discharge from a hospital ward, had lost more than 5% of baseline body weight, and more than half were at risk of malnutrition [1]. Although body composition was not evaluated, it is likely that the weight loss observed in COVID-19 survivors was, at least in part, due to loss of lean body mass caused by systemic inflammation, muscle disuse and bed rest [2]. Unintentional weight loss, especially when associated with loss of fat-free mass, may negatively impact time to full recovery and patients’ health status [3, 4].
Of the numerous studies reporting the clinical consequences of unintentional weight loss due to health conditions, very few have assessed whether and how patients regain the lost weight. It has been reported that weight regain in patients with critical illness is mainly due to expansion in the fat compartment [5, 6], whereas – to the best of our knowledge - no studies are available on the patterns of weight recovery in non-critical settings. Weight recovery after weight loss is often characterized by a greater rate of fat mass vs. lean mass recovery (a phenomenon sometimes referred to as “preferential catch-up fat”) [7], with the baseline percentage of body fat being a strong predictor of fat regain [8]. This might be particularly relevant in COVID-19 patients requiring hospital admission, as the majority have overweight/obesity and increased visceral adiposity [9, 10]. In these individuals, disproportionate regain in fat mass after weight loss might further worsen body composition, functional status and cardiometabolic risk.
The aim of our study was to assess weight changes from hospital admission to three months after discharge in COVID-19 survivors across body mass index (BMI) categories, and to assess changes in abdominal adiposity, as estimated by waist circumference, in this cohort.
Subjects and methods
Study design
This was a sub-study of the COVID-BioB study, a large prospective observational investigation performed at San Raffaele University Hospital, a tertiary health-care hospital in Milan, Italy [11, 12]. The study protocol complies with the Declaration of Helsinki, was approved by the Hospital Ethics Committee (protocol no. 34/int/2020), and was registered on ClinicalTrials.gov (NCT04318366). Full description of patient management and clinical protocols were previously published [11, 12]. Signed informed consent was obtained from all patients participating in this study. We included adult (age ≥ 18 years) patients with a confirmed diagnosis of COVID-19 who had been admitted to and subsequently discharged home from a COVID-19 medical ward of San Raffaele University Hospital, and were re-evaluated one and three months after remission at the Outpatient COVID-19 Follow-Up Clinic of the same Institution from April 7, 2020, to October 6, 2020. The study size was defined by the time-window of the study. Confirmed COVID-19 was defined as positive real-time reverse-transcriptase polymerase chain reaction (RT-PCR) from a nasal and/or throat swab together with signs, symptoms, and/or radiological findings suggestive of COVID-19 pneumonia. Remission was defined as two negative RT-PCR from a nasal and/or throat swab performed 24 h apart, and no symptoms. Only patients with available anthropometrics (weight and height) recorded upon admission and at follow-up, and waist circumference recorded at both the 1-month and 3-month visits were included in the analyses. Patients admitted for other reasons and subsequently diagnosed with superimposed SARS-CoV-2 infection were excluded.
Data collection
Data were collected from medical chart review or directly by patient interview and entered in a dedicated electronic case record form (eCRF) specifically developed for the COVID-BioB study. Prior to the analysis, data were cross-checked with medical charts and verified by data managers and clinicians for accuracy. The following variables were collected for all patients: age, sex, BMI (calculated as the ratio of weight in kilograms [kg] divided by height in squared meters), PaO2/FiO2 (calculated as the ratio between the arterial partial pressure of oxygen measured on arterial blood gas analysis and the fraction of inspired oxygen), plasma glucose (mg/dL), estimated glomerular filtration rate (eGFR, as estimated by the CKD-EPI equation and expressed as ml/min/1.73 m2) and high-sensitivity C-reactive protein (CRP, mg/dL) on admission to the ED, comorbidities (including history of hypertension, diabetes mellitus, dyslipidaemia, ischemic heart disease, and active malignancy), length of stay and need of admission to the ICU. Measuring weight and height on admission was not feasible due to the workload for nurses and physicians during the peak of the pandemic and the need for contact and airborne precautions in the hospital. Therefore, weight and height on admission were self-reported by patients. Height measured at the follow-up visits was subsequently used to calculate baseline BMI for the present analysis.
The first follow-up outpatient visit was scheduled ~1 month after discharge and the second visit 3 months after discharge. Both included a complete internal medicine assessment (collection of medical history, measurement of vital signs, physical examination), and nutritional evaluation (body weight measured to the nearest 0.1 kg using a balance beam scale, height measured to the nearest 0.1 cm using a wall-mounted stadiometer, waist circumference measurements taken around the abdomen at the level of the umbilicus). Abdominal obesity was defined as a waist circumference ≥88 cm in women and ≥102 cm in men. Hunger was assessed using a visual analog scale [VAS] ranging from 0 to 100 mm [13]. Patients were considered to be weight stable if changes in body weight since the previous assessment were ≤2% [14, 15].
Statistical analysis
Descriptive statistics were obtained for all study variables. Continuous variables were expressed as medians [25th–75th percentile]. Categorical variables were summarized as counts and percentages. Fisher exact test or χ2 test and the Wilcoxon signed-rank test or the Kruskal–Wallis test were employed to determine the statistical significance of differences in proportions and medians, respectively. All statistical tests were two-sided. A p value of <0.05 was considered statistically significant. Correlations were analyzed using the Spearman rank correlation analysis. Univariable and multivariable logistic regression analyses were used to estimate the odds ratios (ORs) of weight gain with 95% confidence intervals (CIs). Demographic and clinical characteristics potentially associated with weight gain from 1 to 3 months were tested in univariable models. All variables that emerged as predictors (p < 0.05) at univariable analysis were used as covariates in the multivariable model. Missing data were not imputed. Statistical analysis was conducted using IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
Results
Study subjects
A total of 185 patients were included in the present analysis. Most patients were males (71%), median age was 62.1 [54.3; 72.1] years. Patient characteristics and laboratory results upon hospital admission are summarized in Table 1. Median BMI upon admission was 27.1 [25.5; 31.4] kg/m2. The majority (80%) of patients had overweight or obesity; the remainder had a BMI within the normal range (Table 1). Median time from hospital admission or discharge to the first follow-up outpatient visit (V1) was 43 [31; 62] and 25 [20;38] days, respectively. Median time from discharge or V1 to the second follow-up outpatient visit (V2) was 89 [79; 101] and 60 [58; 65] days, respectively.
Table 1.
Patient characteristics upon admission.
| All 185 | Missing | |
|---|---|---|
| Age, years | 62.1 (54.3; 72.1) | – |
| Male, n (%) | 132 (71.4) | – |
| Race, n (%) | ||
| White | 179 (96.8) | |
| Asian | 3 (1.6) | – |
| Black | 3 (1.6) | |
| Hypertension, n (%) | 86 (46.5) | – |
| Coronary artery disease, n (%) | 11 (5.9) | – |
| Diabetes Mellitus, n (%) | 28 (15.1) | – |
| COPD, n (%) | 9 (4.9) | – |
| CKD, n (%) | 9 (4.9) | – |
| Malignancy, n (%) | 6 (3.2) | – |
| BMI, kg/m2 | 27.7 (25.5; 31.4) | – |
| BMI category, n (%) | ||
| Underweight | 0 | – |
| Normal weight | 37 (20.0) | |
| Overweight | 81 (43.8) | |
| Obesity | 67 (36.2) | |
| PaO2/FiO2 ratio | 281.0 (238.1; 319;0) | 30 |
| CRP, mg/dl | 71.8 (28.7; 122.5) | 1 |
| Plasma glucose (mg/dL) | 108.5 (99.0; 125.0) | 7 |
| AST, U/L | 46.0 (32.0; 62.0) | 4 |
| ALT, U/L | 40.0 (25.5; 62.0) | 4 |
| eGFR (mL/min/1.73 m2) | 69.4 (41.6; 89.5) | 1 |
COPD Chronic Obstructive Pulmonary Disease, CKD Chronic Kidney Disease, BMI Body Mass Index, PaO2/FiO2 ratio ratio between the arterial partial pressure of oxygen measured on arterial blood gas analysis and the fraction of inspired oxygen, CRP C-Reactive Protein, AST aspartate aminotransferase, ALT alanine aminotransferase, eGFR estimated glomerular filtration rate, calculated using the CKD-EPI equation.
Table 2 depicts patient characteristics according to BMI category upon admission. The three groups were similar with regard to age, sex, race and comorbidities, with the exception of malignancy, which was more common in normal weight subjects; patients with obesity had a lower PaO2/FiO2 as compared to the normal weight group. No other statistically significant differences were found in laboratory results, length of stay or in the proportion of patients requiring admission to the ICU (Table 2). The timing of follow-up visits did not differ across BMI categories. Differences in BMI across groups were maintained during follow-up (p < 0.001 for all comparisons).
Table 2.
Comparison of patients with normal weight, overweight or obesity upon admission.
| Normal weight (n = 36, 19.5%) | Overweight (n = 82, 44.3%) | Obesity (n = 67, 36.2%) | P | |
|---|---|---|---|---|
| BMI, kg/m2 | 23.8 (22.1; 24.2) | 27.0 (26.2; 28.1) | 32.7 (31.1; 35.8) | 0.032 |
| Age, years | 66.6 (53.1; 75.5) | 59.8 (53.1; 69.3) | 63.6 (55.9; 74.3) | 0.415 |
| Female, n (%) | 15 (41.7) | 21 (25.6) | 17 (25.4) | 0.157 |
| Race, n (%) | 0.422 | |||
| White | 35 (34.8) | 80 (79.3) | 64 (64.8) | |
| Asian | 1 (2.8) | 0 (0) | 2 (3.0) | |
| Black | 0 (0.6) | 2 (2.4) | 1 (1.5) | |
| Hypertension, n (%) | 15 (41.7) | 33 (40.2) | 38 (56.7) | 0.109 |
| Coronary artery disease, n (%) | 1 (2.8) | 7 (4.9) | 3 (4.5) | 0.377 |
| Diabetes Mellitus, n (%) | 3 (8.3) | 11 (13.4) | 14 (20.9) | 0.200 |
| COPD, n (%) | 2 (5.6) | 4 (4.9) | 3 (4.5) | 0.971 |
| CKD, n (%) | 2 (5.6) | 2 (2.4) | 5 (7.5) | 0.342 |
| Malignancy, n (%) | 3 (8.3) | 3 (2.7) | 0 (0) | 0.038 |
| PaO2/FiO2 ratio | 307.1 (260.4; 324.8) | 290.5 (246.4; 330.0) | 269.0 (177.4; 325.8)* | 0.025 |
| CRP, mg/dl | 61.3 (20.6; 120.3) | 71.8 (32.1; 120.2) | 76.1 (28.8; 139.9) | 0.447 |
| Plasma glucose (mg/dL) | 109.0 (96.0; 72.0) | 107.5 (100.0; 121.3) | 109.0 (98.5; 126.0) | 0.770 |
| AST, U/L | 47.0 (31.0; 72.0) | 46.0 (35.0; 63.0) | 46.0 (30.0; 76.0) | 0.957 |
| ALT, U/L | 40.0 (24.0; 59.0) | 39.0 (26.0; 61.0) | 41.0 (25.0; 65.0) | 0.963 |
| eGFR (mL/min/1.73 m2) | 72.4 (38.1; 89.4) | 88.6 (41.2; 89.3) | 69.4 (49.9; 89.7) | 0.782 |
| Admission to ICU, n (%) | 1 (2.8) | 8 (8.4) | 10 (14.9) | 0.150 |
| Length of stay (days) | 10.0 (6.0; 21.8) | 13.5 (7.0; 25.3) | 15.0 (10.0; 28.0) | 0.052 |
| BMI at 1 month | 22.9 (21.4; 24.1) | 26.2 (25.2; 27.5) | 31.6 (29.7; 33.4) | <0.001 |
| WC at 1 month | 89.5 (80.3; 93.8) | 99.0 (92.8; 103.0) | 109.0 (103.0; 114.0) | <0.001 |
| BMI at 3 months | 23.3 (21.9; 24.4) | 26.7 (25.3; 27.8) | 32.4 (30.8; 34.3) | <0.001 |
| WC at 3 months | 88.5 (82.5; 97.5) | 100.0 (94.0; 104.0) | 110.0 (104.0; 116.0) | <0.001 |
| Days from admission to V1 | 44.5 (31.0; 44.5) | 44.5 (29.0; 57.0) | 42.0 (32.0; 66.0) | 0.894 |
| Days from discharge to V1 | 29.0 (20.3; 40.0) | 25.5 (29.0; 38.5) | 24.0 (20.0; 36.0) | 0.303 |
| Days from V1 to V2 | 61.5 (59.3; 70.0) | 60.0 (57.8; 63.0) | 61.0 (59.0; 65.0) | 0.116 |
*p < 0.05 vs. normal weight.
Changes in body weight and body mass index
Overall, median percent weight change from hospital admission to V1 was −3.0 [−7.2; 0.9]%. Most patients (57.8%) experienced a weight loss >2%, and 25.4% remained weight stable. The proportion of patients with a weight loss >5% was 35.1%. Median BMI did not change significantly from baseline to V1 in normal weight subjects (23.8 [22.1; 24.2] vs. 22.9 [21.4; 24.1] kg/m2, percent change −1.9 [−5.0; 2.4]%; p = 0.08), whereas it significantly decreased in subjects with overweight (27.0 [26.2; 28.1] vs. 26.2 [25.2; 28.1] kg/m2, percent change −2.8 [−6.9; 1.1]%; p < 0.001) or obesity (32.7 [31.1; 35.8] vs. 31.6 [29.7; 33.4] kg/m2, percent change −4.1 [−10.5; 0.9]%; p < 0.001). The magnitude of absolute BMI reduction from baseline to V1 was significantly greater in subjects with obesity as compared with normal weight or overweight subjects (Fig. 1A). When considering percent changes in BMI, the difference was also significant (p = 0.011). The proportion of patients who lost more than 2% of initial weight was 47, 53 and 67.2% in the normal weight, overweight and obesity group, respectively (p for trend 0.044).
Fig. 1. Changes in BMI.

BMI changes from hospital admission to V1 (A), from V1 to V2 (B) and from hospital admission to V2 (C). BMI Body Mass Index, OB Obesity, OW Overweight, NW Normal Weight.
Median percent weight change from V1 to V2 was +1.5 [0.0; 4.4]%. Overall, nearly half (47.6%) of patients gained more than 2% of V1 weight, and 43.8% remained weight stable. Median BMI did not change significantly from V1 to V2 in normal weight individuals (22.9 [21.4; 24.1] vs. 23.3 [21.9; 24.4] kg/m2, percent change +1.1 [−1.5; 4.5]%; p = 0.12), whereas it significantly increased in subjects with overweight (26.2 [25.2; 27.4] vs. 26.7 [25.3; 27.8] kg/m2, percent change +1.7 [0.0; 4.0]%; p < 0.001) or obesity (31.6 [29.7; 33.4] vs. 32.3 [30.8; 34.3] kg/m2, percent change +2.6 [0.0; 4.9]%; p < 0.001). The absolute BMI gain between the first and second follow-up visit was significantly greater in subjects with obesity as compared with normal weight subjects (Fig. 1B). When considering percent changes in BMI, this difference was borderline significant (p = 0.051). The proportion of patients who gained more than 2% of weight since V1 was 33, 48 and 55% in the normal weight, overweight and obesity group, respectively (p for trend 0.245).
Changes in BMI from hospital admission to the last follow-up visit were similar across groups (p = 0.119) (Fig. 1C). Within-group differences in BMI from admission to the last follow-up were not significant in individuals with normal weight or overweight, whereas in subjects with obesity BMI at the last follow-up was still significantly lower than baseline (p = 0.003).
Hunger
Hunger levels significantly decreased from one to three months in COVID-19 survivors with normal weight or overweight, whereas no significant change was observed in those with obesity (Fig. 2).
Fig. 2. Change in hunger from the 1-month to the 3-month follow-up visit in subjects with normal weight, overweight or obesity.
VAS visual analog scale.
Abdominal obesity
Waist circumference was higher in the obesity group as compared to the overweight and normal weight groups (109.0 [103.0; 114.0] cm vs. 99.0 [92.8; 103.0] vs. 89.5 [80.3; 93.8] cm, respectively; p < 0.001). Waist circumference significantly increased from V1 to V2 in the whole group (p < 0.001). This observation was mainly driven by changes in the overweight and obesity groups (Fig. 3). Median increase in waist circumference tended to be larger with increasing BMI (+0.5 [−1.0; 5.0], +1.0 [−0.3; 3.0] and +3.0 [0.0; 0.6] cm in subjects with normal weight, overweight and obesity, respectively; p for trend 0.079), and significantly correlated with percent weight change from V1 to V2 (Spearman’s rho 0.49, p < 0.001).
Fig. 3. Change in waist circumference from the 1-month to the 3-month follow-up visit in subjects with normal weight, overweight or obesity.
WC Waist circumference.
Abdominal obesity was found in 114 (61.6%) of patients at V1. The prevalence raised to 68.1% at three months (p = n.s.). The percentage of subjects with abdominal obesity increased with increasing BMI category, both at the first and at the second follow-up visit (Fig. 4). Of note, abdominal obesity was present in 25 and 31% of patients of normal weight at V1 and V2, respectively.
Fig. 4. Abdominal obesity.
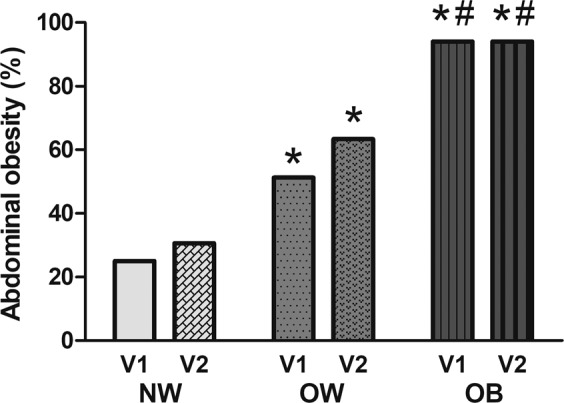
Prevalence of abdominal obesity (as defined as a waist circumference ≥88 cm in women and ≥102 cm in men) at one (V1) and three (V2) months after hospital discharge in COVID-19 survivors with normal weight (NW), overweight (OW) or obesity (OB). *p < 0.05 vs. NW; #p < .05 vs. OW.
Predictors of weight gain
At univariable logistic regression analysis including the whole cohort, male sex, baseline BMI, hunger at 1 month, abdominal obesity and percent weight loss from baseline to 1 month significantly predicted a weight gain >2% from V1 to V2 (Supplementary Table. 1). At multivariable logistic regression analysis including baseline BMI (Model 1), male sex (OR 2.58 [1.03; 6.44], p = 0.042), hunger (OR 1.02 [1.00; 1.03], p = 0.025) and weight change from hospital admission to V1 (OR 1.10 [1.04;1.17] per 1% decrease in weight from baseline, p = 0.002) were identified as independent predictors of weight gain (Supplementary Table 1). When abdominal obesity was included (Model 2), only hunger (OR 1.02 [1.00; 1.03], p = 0.032) and weight change from baseline to V1 (OR 1.09 [1.03; 1.16], p = 0.003) remained significant predictors of weight gain.
Discussion
This is the first study to assess patterns of weight change and the prevalence of abdominal obesity after hospital discharge in COVID-19 survivors, and possibly the first study to report these outcomes in a cohort of patients hospitalized for acute illness, either critical or non-critical. Weight loss was highly prevalent in COVID-19 survivors one month after hospital discharge. At three months after discharge, nearly half of patients had gained more than 2% of weight since the previous visit. Weight change patterns differed across BMI categories. Patients with obesity exhibited the largest weight fluctuations throughout the study period, whereas patients with normal weight were substantially weight stable and those with overweight exhibited an intermediate pattern. Abdominal obesity, as estimated by waist circumference, was present in most patients, both at one (62%) and three months (68%) after discharge. Consistent with the pattern of weigh regain observed, waist circumference significantly increased during follow-up, particularly in the overweight and obesity groups (Fig. 3).
It is widely recognized that hospitalization associates with inadequate nutritional intake and weight loss [16], and that loss of muscle mass is seen at an accelerated rate in catabolic illnesses, even with minimal weight loss [17]. COVID-19 has been postulated as a major cause of cachexia and sarcopenia (i.e., the reduction of muscular function in the presence of reduced muscle mass) due to marked elevation in inflammatory cytokines and immobilization [2], and is in fact associated with clinically significant weight loss [1]. Recently, a small study that included COVID-19 patients admitted to the ICU who underwent sequential computed tomography scans confirmed that a reduction in both fat and lean body mass occurs in patients without or with obesity, with the latter experiencing a greater reduction in the erector spinae muscle cross-sectional area [18]. Thus, it is likely that subjects with overweight or obesity in our cohort lost a significant amount of lean body mass. The finding that waist circumference significantly increased in subjects with overweight or obesity suggests an increase in abdominal (and possibly visceral) adiposity in these patient categories, with further worsening of body composition. This observation is in line with previous reports on ARDS survivors indicating that the gain in body weight in the year following hospital discharge was mainly due to an increase in fat mass [5, 6]. We could not find reports on weight trajectories during and after acute, non-critical illness requiring hospital admission. Studies on weight recovery in patients with anorexia nervosa show that preferential trunk or visceral fat regain may occur after severe depletion of nutritional status [19, 20]. Increasing adiposity due to disproportional visceral fat regain may have detrimental consequences on health status, especially in overweight and obesity, which are often associated with cardiometabolic abnormalities. As in a vicious cycle, cardiometabolic risk factors may favor the development and/or worsening of sarcopenia [21], which is often overlooked in patients with obesity. More than half of COVID-19 survivors in our cohort were older than 62 years. With aging, recovery of lean body mass may be challenging, thus increasing the risk of developing sarcopenia or sarcopenic obesity [22]. It should also be noticed that the prevalence of abdominal obesity was very high in our cohort, even among normal weight individuals. Abdominal adiposity as assessed by waist circumference is a strong and independent predictor of all-cause and cause-specific mortality [23], even among normal-weight subjects [24]. Our finding supports the association between visceral adiposity and risk of COVID-19 [9] or critical COVID-19 [25, 26].
Subjects with obesity experienced the greatest weight fluctuations in our study. Individuals with obesity have greater energy stores – both as fat and lean mass - as compared with normal weight subjects. These are mobilized during critical illness, possibly explaining the greater weight loss and conferring a survival advantage vs. lean individuals [27]. At multivariable regression analysis, weight loss from baseline to the 1-month visit emerged as a significant predictor of subsequent weight gain. The likelihood of gaining weight increased by 9–10% for every 1% of weight lost from baseline. Consistently, the BMI gain from one to three months was greater in subjects with obesity as compared with lean subjects, who remained relatively weight stable throughout the study period. In a systematic review and meta-regression analysis of studies in which intentional weight loss (≥5%) and subsequent weight regain (≥2%) occurred, both the amount and the rate of weight loss were significantly associated with weight regain [28]. The timing of follow-up assessments did not differ among BMI categories in our study, suggesting that the amount rather than the rate of weight loss influenced weight recovery. Other predictors of weight gain during follow-up were male sex and hunger. Male sex increased by more than twice the likelihood of weight gain in the multivariable model including baseline BMI. Male sex has been shown to associate with unfavorable relative changes in lean mass as compared with female sex over a single weight cycle [29–31]. Thus, male patients with COVID-19 may not only be at risk of more severe disease [32], but also of worsening body composition during recovery. Finally, for each unit increase in hunger assessed at 1 month, the likelihood of subsequent weight gain increased by 2%. Of note, while subjects with normal weight or overweight had returned to baseline BMI by three months after discharge and hunger levels had significantly decreased during follow-up, in individuals with obesity the BMI at three months was significantly lower as compared to baseline and hunger levels had not decreased. This is consistent with the evidence of a biological drive to regain weight after weight loss in obesity [33]. Weight loss after marked calorie restriction is associated with increased hunger and a strongly increased reward mechanism in response to food, in addition to other adaptive endocrine responses that may lead to increased food intake and weight regain [33, 34]. Recent evidence indicates that adipose tissue characteristics may also be involved [35].
Our findings should be interpreted in light of the study limitations. First, body composition was not analyzed and waist circumference was not measured at baseline, although differences in waist circumference across groups were maintained during follow-up, suggesting that similar differences were present at baseline. Further limits of our study are the use of patient-reported weight upon admission and the lack of body weight assessment at discharge, which may have led to an underestimation of baseline BMI [36, 37] and of the magnitude of weight fluctuations. The lack of anthropometrics and body composition assessment during hospital stay was due to the unprecedented workload for healthcare professionals at the beginning of the pandemic, and to the restrictions imposed by the need of isolating patients to prevent viral spread. Finally, as most patients included in the analysis were White, our findings may not be generalized to other patient populations.
In conclusion, we report, for the first time to our knowledge, weight trajectories since hospital admission and changes in waist circumference during follow-up in COVID-19 survivors. Several considerations stem from our findings. First, COVID-19 requiring hospitalization is associated with rapid and wide fluctuations in weight that may worsen body composition (increased abdominal adiposity) in subjects with overweight or obesity. Fluctuations in blood pressure, heart rate, glomerular filtration rate, sympathetic activity, blood glucose and lipids that occur during weight fluctuation could put an additional load on the cardiovascular system [38]. Nutritional management strategies [39, 40] should be implemented during hospitalization and after discharge to improve both short- and long-term outcomes, and to reduce the risk of sarcopenia and cardiometabolic alterations. Second, our findings support the association between COVID-19 and visceral adiposity previously reported [25, 26]. Very few data are available in the literature on the association of abdominal adiposity and body composition on outcomes in patients hospitalized for acute conditions, either critical or non-critical. Even less data is available on the effect of critical and non-critical acute illness on body weight and composition. Our study contributes to filling this gap, and highlights the need for addressing these points with larger, more sophisticated studies. A better knowledge of the events occurring during acute illness and in the recovery phase in people with obesity might provide a better understanding of the pathophysiology of obesity and help improve clinical care.
Supplementary information
Author contributions
Conceptualization: LDF, CC, PRQ; Data curation: LDF, RDL, EC, MF, EF, SM, GV, CC; Formal analysis: LDF, CC; Investigation: LDF, RDL, EC, MF, EF, MC, SM, GV, CC; Methodology: LDF, CC, PRQ, AG; Project administration: CC; Supervision: AG, EB, PRQ, CC; Validation: AG, EB, PRQ, CC; Writing - original draft: LDF, CC; Writing - review & editing: LDF, RDL, EC, EF, MF, MC, SM, GV, EB, AG, PRQ, CC. CC takes responsibility for the contents of the article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. CC is supported by the European Foundation for the Study of Diabetes Mentorship Programme 2019.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41366-021-00861-y.
References
- 1.Di Filippo L, De Lorenzo R, D’Amico M, Sofia V, Roveri L, Mele R, et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: a post-hoc analysis of a prospective cohort study. Clin Nutr. 2020. 10.1016/j.clnu.2020.10.043. [DOI] [PMC free article] [PubMed]
- 2.Morley JE, Kalantar-Zadeh K, Anker SD. COVID-19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle. 2020;11:863–5. doi: 10.1002/jcsm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutr Res Rev. 2009;22:93–108. doi: 10.1017/S0954422409990035. [DOI] [PubMed] [Google Scholar]
- 4.Myers J, Lata K, Chowdhury S, McAuley P, Jain N, Froelicher V. The obesity paradox and weight loss. Am J Med. 2011;124:924–30. doi: 10.1016/j.amjmed.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Chan KS, Mourtzakis M, Aronson Friedman L, Dinglas VD, Hough CL, Ely EW, et al. Evaluating muscle mass in survivors of acute respiratory distress syndrome: a 1-year multicenter longitudinal study. Crit Care Med. 2018;46:1238–46. doi: 10.1097/CCM.0000000000003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid CL, Murgatroyd PR, Wright A, Menon DK. Quantification of lean and fat tissue repletion following critical illness: a case report. Crit Care. 2008;12:R79. doi: 10.1186/cc6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosy-Westphal A, Kahlhofer J, Lagerpusch M, Skurk T, Muller MJ. Deep body composition phenotyping during weight cycling: relevance to metabolic efficiency and metabolic risk. Obes Rev. 2015;16:36–44. doi: 10.1111/obr.12254. [DOI] [PubMed] [Google Scholar]
- 8.Dulloo AG, Jacquet J, Girardier L. Autoregulation of body composition during weight recovery in human: the Minnesota Experiment revisited. Int J Obes Relat Metab Disord. 1996;20:393–405. [PubMed] [Google Scholar]
- 9.Hamer M, Gale CR, Kivimaki M, Batty GD. Overweight, obesity, and risk of hospitalization for COVID-19: a community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci USA. 2020;117:21011–3. doi: 10.1073/pnas.2011086117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marazuela M, Giustina A, Puig-Domingo M. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev Endocr Metab Disord. 2020;21:495–507. doi: 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovere Querini P, De Lorenzo R, Conte C, Brioni E, Lanzani C, Yacoub MR, et al. Post-COVID-19 follow-up clinic: depicting chronicity of a new disease. Acta Biomed. 2020;91:22–8. doi: 10.23750/abm.v91i9-S.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rovere-Querini P, Tresoldi C, Conte C, Ruggeri A, Ghezzi S, De Lorenzo R, et al. Biobanking for COVID-19 research. Panminerva Med. 2020. 10.23736/S0031-0808.20.04168-3. [DOI] [PubMed]
- 13.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 14.Look Ahead Research Group. Gregg EW, Jakicic JM, Blackburn G, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–21. doi: 10.1016/S2213-8587(16)30162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes F, Schuetz P, Bounoure L, Austin P, Ballesteros-Pomar M, Cederholm T, et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr. 2018;37:336–53. doi: 10.1016/j.clnu.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2000;904:553–7. doi: 10.1111/j.1749-6632.2000.tb06515.x. [DOI] [PubMed] [Google Scholar]
- 18.Gualtieri P, Falcone C, Romano L, Macheda S, Correale P, Arciello P, et al. Body composition findings by computed tomography in SARS-CoV-2 patients: increased risk of muscle wasting in obesity. Int J Mol Sci. 2020;21. 10.3390/ijms21134670. [DOI] [PMC free article] [PubMed]
- 19.Grinspoon S, Thomas L, Miller K, Pitts S, Herzog D, Klibanski A. Changes in regional fat redistribution and the effects of estrogen during spontaneous weight gain in women with anorexia nervosa. Am J Clin Nutr. 2001;73:865–9. doi: 10.1093/ajcn/73.5.865. [DOI] [PubMed] [Google Scholar]
- 20.Scalfi L, Polito A, Bianchi L, Marra M, Caldara A, Nicolai E, et al. Body composition changes in patients with anorexia nervosa after complete weight recovery. Eur J Clin Nutr. 2002;56:15–20. doi: 10.1038/sj.ejcn.1601290. [DOI] [PubMed] [Google Scholar]
- 21.Rubio-Ruiz ME, Guarner-Lans V, Perez-Torres I, Soto ME. Mechanisms underlying metabolic syndrome-related sarcopenia and possible therapeutic measures. Int J Mol Sci. 2019;20. 10.3390/ijms20030647. [DOI] [PMC free article] [PubMed]
- 22.Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2020;39:2368–88. doi: 10.1016/j.clnu.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177–89. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canoy D, Boekholdt SM, Wareham N, Luben R, Welch A, Bingham S, et al. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 2007;116:2933–43. doi: 10.1161/CIRCULATIONAHA.106.673756. [DOI] [PubMed] [Google Scholar]
- 25.Battisti S, Pedone C, Napoli N, Russo E, Agnoletti V, Nigra SG, et al. Computed tomography highlights increased visceral adiposity associated with critical illness in COVID-19. Diabetes Care. 2020;43:e129–e30. doi: 10.2337/dc20-1333. [DOI] [PubMed] [Google Scholar]
- 26.Foldi M, Farkas N, Kiss S, Dembrovszky F, Szakacs Z, Balasko M, et al. Visceral adiposity elevates the risk of critical condition in COVID-19: a systematic review and meta-analysis. Obesity. 2020. 10.1002/oby.23096. [DOI] [PMC free article] [PubMed]
- 27.Karampela I, Chrysanthopoulou E, Christodoulatos GS, Dalamaga M. Is there an obesity paradox in critical illness? Epidemiologic and metabolic considerations. Curr Obes Rep. 2020;9:231–44. doi: 10.1007/s13679-020-00394-x. [DOI] [PubMed] [Google Scholar]
- 28.Turicchi J, O’Driscoll R, Finlayson G, Beaulieu K, Deighton K, Stubbs RJ. Associations between the rate, amount, and composition of weight loss as predictors of spontaneous weight regain in adults achieving clinically significant weight loss: a systematic review and meta-regression. Obes Rev. 2019;20:935–46. doi: 10.1111/obr.12849. [DOI] [PubMed] [Google Scholar]
- 29.Heitmann BL, Garby L. Composition (lean and fat tissue) of weight changes in adult Danes. Am J Clin Nutr. 2002;75:840–7. doi: 10.1093/ajcn/75.5.840. [DOI] [PubMed] [Google Scholar]
- 30.Lee JS, Visser M, Tylavsky FA, Kritchevsky SB, Schwartz AV, Sahyoun N, et al. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010;65:78–83. doi: 10.1093/gerona/glp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872–8. doi: 10.1093/ajcn/82.4.872. [DOI] [PubMed] [Google Scholar]
- 32.Figliozzi S, Masci PG, Ahmadi N, Tondi L, Koutli E, Aimo A, et al. Predictors of adverse prognosis in COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020;50:e13362. doi: 10.1111/eci.13362. [DOI] [PubMed] [Google Scholar]
- 33.Vettor R, Di Vincenzo A, Maffei P, Rossato M. Regulation of energy intake and mechanisms of metabolic adaptation or maladaptation after caloric restriction. Rev Endocr Metab Disord. 2020;21:399–409. doi: 10.1007/s11154-020-09565-6. [DOI] [PubMed] [Google Scholar]
- 34.Burger KS, Stice E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage. 2011;55:233–9. doi: 10.1016/j.neuroimage.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLean PS, Higgins JA, Giles ED, Sherk VD, Jackman MR. The role for adipose tissue in weight regain after weight loss. Obes Rev. 2015;16:45–54. doi: 10.1111/obr.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flegal KM, Ogden CL, Fryar C, Afful J, Klein R, Huang DT. Comparisons of self-reported and measured height and weight, BMI, and obesity prevalence from national surveys: 1999-2016. Obesity. 2019;27:1711–9. doi: 10.1002/oby.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodge JM, Shah R, McCullough ML, Gapstur SM, Patel AV. Validation of self-reported height and weight in a large, nationwide cohort of U.S. adults. PLoS One. 2020;15:e0231229. doi: 10.1371/journal.pone.0231229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montani JP, Viecelli AK, Prevot A, Dulloo AG. Weight cycling during growth and beyond as a risk factor for later cardiovascular diseases: the ‘repeated overshoot’ theory. Int J Obes. 2006;30:S58–66. doi: 10.1038/sj.ijo.0803520. [DOI] [PubMed] [Google Scholar]
- 39.Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–8. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




