Abstract
Coronavirus (SARS-CoV-2) is spreading rapidly in the world and is still taking a heavy toll. Studies show that cytokine storms and imbalances in T-helper (Th)1/Th2 play a significant role in most acute cases of the disease. A number of medications have been suggested to treat or control the disease but have been discontinued due to their side effects. Melatonin, as an intrinsic molecule, possesses pharmacological anti-inflammatory and antioxidant properties that decreases in concentration with age; as a result, older people are more prone to various diseases. In this study, patients who were hospitalized with a diagnosis of coronavirus disease 2019 (COVID-19) were given a melatonin adjuvant (9 mg daily, orally) for fourteen days. In order to measure markers of Th1 and Th2 inflammatory cytokines (such as interleukin (IL)-2, IL-4, and interferon (IFN)-γ) as well as the expression of Th1 and Th2 regulatory genes (signal transducer and activator of transcription (STAT)4, STAT6, GATA binding protein 3 (GATA3), and T-box expressed in T cell (T-bet)), blood samples were taken from patients at the beginning and end of the treatment. Adjuvant therapy with melatonin controlled and reduced inflammatory cytokines in patients with COVID-19. Melatonin also controlled and modulated the dysregulated genes that regulate the humoral and cellular immune systems mediated by Th1 and Th2. In this study, it was shown for the first time that melatonin can be used as a medicinal adjuvant with anti-inflammatory mechanism to reduce and control inflammatory cytokines by regulating the expression of Th1 and Th2 regulatory genes in patients with COVID-19.
Keywords: Melatonin, COVID-19, Humoral immunity, Cellular immunity, T helper, Adjunctive therapy
1. Introduction
In late 2019, a viral illness was reported in Wuhan City, Hubei Province, China, indicating a disease with symptoms similar to but different from the common cold, which spread rapidly worldwide and caused a global pandemic (Akbariqomi et al., 2020). The new virus, which belongs to the coronavirus family and the beta (β)-coronavirus subfamily, was named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) by the International Committee for the Taxonomy of Viruses (ICTV). The World Health Organization (WHO) has officially named the disease SARS-CoV-2 “coronavirus disease 2019” (COVID-19) (Ahn et al., 2020; Nikpouraghdam et al., 2020). Many studies have been conducted on the pathophysiology and pathogenesis mechanisms of COVID-19 to identify possible pathways as well as treatment. It has been shown that in moderate and severe cases of the disease, cytokine storm plays an important role in COVID- 19 due to an excessive increase in inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-2, interferon (IFN)-γ, and imbalance in Th1 and Th2 responses (Bouadma et al., 2020; Gadotti et al., 2020). Furthermore, preliminary clinical studies on COVID-19 show that people are more susceptible to the disease as well as at risk of death as they become older (Gadotti et al., 2020). Many organizations and companies worldwide are finding and testing vaccines and effective medicines for COVID-19 with or without an acceptable level of side effects. Although some pharmaceutical companies in a number of countries have found an effective vaccine for COVID-19, there is still a long way until it is available to all people in the world. Nevertheless, the SARS-CoV-2 virus is spreading at an alarming rate regardless of these issues, and the lack of a comprehensive treatment strategy to date remains a major challenge to control it in most countries (Acuña-Castroviejo et al., 2020; Farnoosh et al., 2020). Therefore, the need for a safe medicine with no side effects to control or at least reduce the effects of COVID-19 is currently strongly felt.
Melatonin (5-methoxy-N-acetyltryptamine) is a small neuroendocrine molecule that is naturally secreted from the pineal gland in response to the circadian rhythm. Melatonin has many properties and plays many roles in the body. In addition to regulating sleep and wakefulness and cardiovascular effects, it is a highly potent anti-inflammatory and antioxidant agent. It also protects the mitochondria against free radicals. Melatonin is not a viricidal agent but due to its anti-inflammatory and antioxidant properties it can counteract acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) (Acuña-Castroviejo et al., 2020; Bahrampour Juybari et al., 2020; Reiter et al., 2020; Shneider et al., 2020). It is surprising that the level of endogenous melatonin decreases with age, which is in keeping with the findings of clinical studies of patients with COVID-19, according to which the risk of contracting the disease and death is higher in the elderly than in the youth (Reiter et al., 2020; Shneider et al., 2020).
However, little information is available on the properties of melatonin as an adjunct, its dosage, and its effect in COVID-19. This study examined the profile of changes in inflammatory cytokines as well as the expression of Th1 and Th2 regulatory genes following the use of melatonin as adjuvant therapy in patients with COVID-19 to better understand the possible mechanisms in regulating immune responses using melatonin.
2. Materials and methods
2.1. Study design
This study was conducted on a cohort of patients diagnosed with COVID-19 who were admitted to Baqiyatallah Hospital in Tehran, Iran during April to June 2020. Patients were included in the study only after receiving the approval by the ethics scientific committee (ethics number IR.BMSU.REC.1399.165) and the consent form approval. Due to the dropouts, a total of 40 male and female patients in the age range of 30–65 (20 in the intervention and 20 in the control group) completed the scheduled treatment and were analyzed.
Melatonin (9 mg daily) was given orally to patients for 14 days along with standard treatment. Control patients, on the other hand, received only standard treatment. The standard treatment for COVID-19 at the discretion of the treating physician and according to the Iranian National Treatment Protocol for COVID-19 included antiviral agents, antibiotics, and glucocorticoids. Blood samples from intervention and control patients were collected at the beginning of the study and after 14 days to measure inflammatory cytokines as well as the expression of target genes.
2.1.1. Inclusion criteria
Diagnosis of COVID-19 was performed by polymerase chain reaction (PCR) as well as clinical chest tomography (CT scan) which showed infection with COVID-19. Eligible patients for inclusion in the study were those with mild to moderate COVID-19, men and non-pregnant women aged 18 years or older, who willingly consented to participate in the study.
2.1.2. Exclusion criteria
Pregnancy, neurological diseases, other viral diseases such as hepatitis and human immunodeficiency virus (HIV), organ transplant, and allergy to melatonin were the factors leading to the exclusion of patients from the study.
2.2. Evaluation of cytokines
Patients' blood was collected and aliquoted at the beginning and end of the intervention to obtain plasma and was stored at −80 °C until cytokines measurement. Cytokines were measured with conventional enzyme-linked immunosorbent assay (ELISA) kits for IL-2, IL-4 and IFN-γ (Karmania Pars Gene, Iran) according to the manufacturer's instructions at a wavelength of 450 nm. The sensitivity of the kit for IL-2 was 4 pg/ml and its accuracy was intra assay <3%, inter assay <10%. Moreover, the sensitivity of the kit for IL-4 was 4 pg/ml and its accuracy was intra assay <3%, inter assay <8%. Finally, the sensitivity of the kit for IFN-γ was 3 pg/ml and its accuracy was intra assay <3%, inter assay <10%.
2.3. Evaluation of expression of target genes
Target genes were measured with quantitative polymerase chain reaction (qPCR) from patients' blood samples. To this end, full RNA was isolated using the FavorPrep Blood/Cultured Cell Total RNA Mini Kit (Favorgen Biotech Inc., Taiwan). The purity and the amount obtained were evaluated using a nanodrop device (NanoDrop, 2000; Thermo Fisher Scientific, USA). As a next step, the PrimeScript 1st strand cDNA Synthesis Kit (Takara Bio Inc., Japan) was used to make cDNA. All steps were followed according to the manufacturer's instructions. The SYBER Green I method was used to measure the expression of target genes, for which a 2X Real-Time PCR Master Mix kit (BioFACT, South Korea) was used. Primers pertaining to each gene designed for qPCR (Oligo 7, Molecular Biology Insight, Inc., USA) are shown in Table 1 . The actin beta (ACTB) gene was used as an internal control for each sample. PCR reaction included enzyme activation and initial denaturation at 95 °C for 10 min, followed by 40 denaturation cycles at 95 °C for 20 s, annealing at 60 °C for 30 s and 72 °C for 30 s. All samples were launched in duplicate. Finally, the raw qPCR data were quantified using the ΔΔCt formula and expressed as fold changes.
Table 1.
Primer sequences for identifying the expression of target (STAT4, STAT6, GATA3, and T-bet) and reference (ACTB) genes in real-time PCR.
| Gene | Forward primer | Reverse primer | Product length (bp) |
|---|---|---|---|
| STAT4 | 5′-CCCAGAGACTCGAACACTGA-3′ | 5′-CCTTACAAGAGGCAGCCAGA-3′ | 203 |
| STAT6 | 5′-GGCAGGTTCTCTATCGCTCA-3′ | 5′-GGTCCTCCTCACTCCTCAGA-3′ | 151 |
| GATA3 | 5′-GGCGCCGTCTTGATACTT T-3′ | 5′-CTGGGTAGCGAAGAGCAGAG-3′ | 188 |
| T-bet | 5′-ATGATTGTGCTCCAGTCCC-3′ | 5′-CCTCTGGCTCTCCGTCGTTC-3′ | 76 |
| ACTB | 5′-AACTTGTGTTGCATGCCTGA-3′ | 5′-ACCGTTGCCAATCTAAGTGC-3′ | 176 |
Abbreviations: STAT4, signal transducer and activator of transcription 4; STAT6, signal transducer and activator of transcription 6; GATA3, GATA binding protein 3; T-bet, T-box expressed in T cell; ACTB, actin beta.
2.4. Statistical analysis
Data obtained from the measurement of inflammatory cytokines and expression of target genes were analyzed using the GraphPad Prism software. Normal distribution of variables was assessed by the Kolmogorov-Smirnov test. Continuous variables were expressed as median (min, max) percent of baseline and compared using the t-test or Mann-Whitney U test. A confidence level of less than 0.05 was considered significant.
3. Results
3.1. Clinical characteristics of hospitalized patients with COVID-19
The median (interquartile range (IQR)) age in the melatonin and control groups was 53 (40–60) and 52 (38.5–56.5) years, respectively, and gender ratio (male/female) in the melatonin and control groups were 1.4 and 1.5 respectively. There was no significant difference in terms of clinical characteristics, and underlying diseases between the two groups at baseline. Hypertension, diabetes, rheumatic disease, cardiovascular diseases, and cancer were common underlying diseases between the two groups. Furthermore, during follow-up, patients treated with melatonin had significantly shorter recovery period than control patients (11 [IQR, 7–20] days vs. 21 [IQR, 14–36] days, P = 0.001; respectively). It is noteworthy that no patients died in either groups. Likewise, no adverse conditions were observed in patients receiving melatonin during the treatment period.
3.2. Effect of melatonin on plasma levels of Th1 profile of IL-2 and IFN-γ
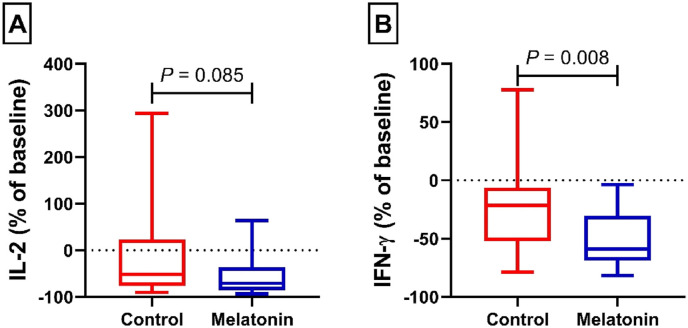
Fig. 1A shows the plasma level of IL-2 in the control and intervention groups. No significant difference was found following 14 days of treatment in the control and intervention groups (51.30 [IQR, 23.18–76.00]% vs. 70.69 [IQR, 36.37–85.08]%, P = 0.085; respectively). Fig. 1 B illustrates the plasma level of IFN-γ in the control and intervention groups. Patients receiving melatonin compared with control patients showed a significant improvement of IFN-γ (21.57 [IQR, 6.29–52.01]% vs. 58.79 [IQR, 30.29–68.58]%, P = 0.008) at the end of treatment.
Fig. 1.
Plasma level of IL-2 (A) and IFN-γ (B) from patients with mild and moderate COVID-19 infection in the control and melatonin cohorts. Data presented as the median (min, max) percent of baseline.
3.3. Effect of melatonin on plasma levels of Th2 profile of IL-4
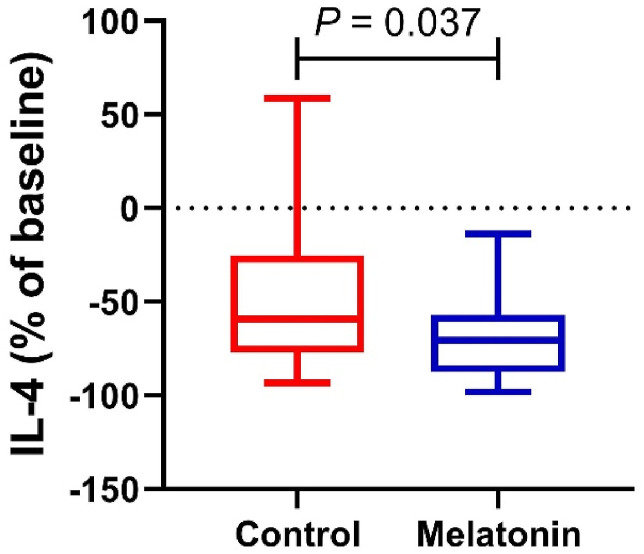
Fig. 2 shows the plasma level of IL-4 in the control and intervention groups. Plasma levels of IL-4 in the control and intervention groups reached 59.19 [IQR, 25.40–77.20]% vs. 70.80 [IQR, 57.06–87.58]%, respectively, indicating a significant difference between the intervention group with melatonin after 14 days of treatment and the control group (P = 0.037).
Fig. 2.
Plasma level of IL-4 from patients with mild and moderate COVID-19 infection in the control and melatonin cohorts. Data presented as the median (min, max) percent of baseline.
3.4. Effect of melatonin on the expression of Th1 regulatory genes
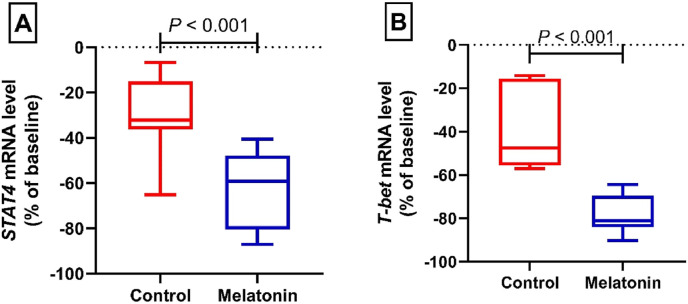
Fig. 3A shows the relative expression of the signal transducer and activator of transcription (STAT)4 gene in the control and intervention groups. After 14 days of treatment, the relative expression of STAT4 gene changed to 32.16 [IQR, 15. 20–36.30]% vs. 59.20 [IQR, 47.82–80.47]% in the control and intervention groups, respectively. According to the results of data analysis, there is a significant difference between the intervention group with melatonin after 14 days of treatment and the control group (P < 0.001). Fig. 3 B illustrates the relative expression of the T-box expressed in T cell (T-bet) gene in the control and intervention groups. After 14 days of treatment, the relative expression of T-bet gene reached 47.58 [IQR, 15.58–55.63]% vs. 81.12 [IQR, 69.44–84.05]% in the control and intervention groups, respectively. Statistical analysis indicated a significant difference between the intervention group and the control group (P < 0.001).
Fig. 3.
Relative mRNA level of STAT4 (A) and T-bet (B) from patients with mild and moderate COVID-19 infection in the control and melatonin cohorts. Data presented as the median (min, max) percent of baseline.
3.5. Effect of melatonin on the expression of Th2 regulatory genes
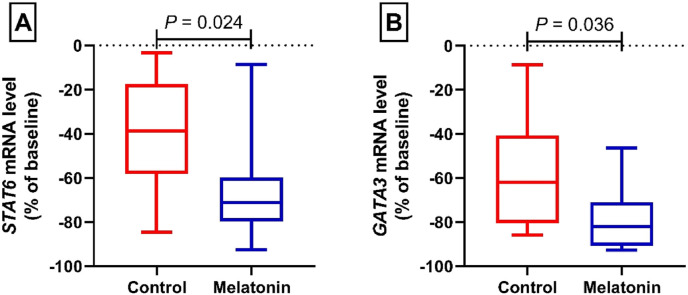
Fig. 4A shows the relative expression of the STAT6 gene in the control and intervention groups. Following 14 days of treatment, the relative expression of STAT6 gene reached 38.63 [IQR, 17.39–58.21]% vs. 71.17 [IQR, 59.84–79.73]% in the control and intervention groups, respectively. These changes showed a statistically significant difference between the intervention group with melatonin after 14 days of treatment and the control group (P = 0.024). Fig. 4 B depicts the relative expression of the GATA binding protein 3 (GATA3) gene in the control and intervention groups. At the end of treatment, the relative expression of GATA3 gene changed to 61.84 [IQR, 40.63–80.52]% vs. 81.92 [IQR, 71.03–90.69]% in the control and intervention groups, respectively. In statistical terms, these changes were indications of a significant difference between the intervention group with melatonin after 14 days of treatment and the control group (P = 0.036).
Fig. 4.
Relative mRNA level of STAT6 (A) and GATA3 (B) from patients with mild and moderate COVID-19 infection in the control and melatonin cohorts. Data presented as the median (min, max) percent of baseline.
4. Discussion
In this study, the cytokine profile of Th1 containing cytokines IL-2 and IFN-γ as well as the profile of Th2 containing cytokine IL-4 were measured in patients with COVID-19 following the melatonin adjuvant therapy. The results of this study showed that increased levels of cytokines were present in the cohorts who did not receive melatonin, indicating that the immune system was involved with a viral agent. Glucocorticoids could downregulate blood levels of inflammatory cytokines (Coutinho and Chapman, 2011; Ingawale and Mandlik, 2020). It should be note that no difference was found in the use of glucocorticoids between the control and melatonin groups. Therefore, a significant effect of melatonin on inflammatory cytokines in the blood from patients with mild to moderate COVID-19 appears to be unaffected by the use of glucocorticoids. IFN-γ and IL-2 are secreted from activated lymphocytes containing cluster of differentiation (CD)4, CD8 T, and natural killer (NK) cells and play an important role in the development and differentiation of naive CD4+ T cells into Th1, which are involved in cellular immunity (Burke and Young, 2019). On the other hand, IL-4 is mainly secreted by Th2 cells and is involved in activating B cells as well as suppressing Th1 cells (Vaz de Paula et al., 2020). Although many studies have demonstrated that Th1 and Th2 responses have an inhibitory effect on each other (Dittmer et al., 2001; Nair et al., 2002; So et al., 2000), the highly antagonistic relationship between IL-4 and IFN-γ is hardly in line with the observation that these two cytokines are often secreted simultaneously during the immune response. The observed pattern in relation to Th1 and Th2 responses and secretion of cytokine profiles of these two cells is more common in chronic diseases (Noble and Kemeny, 1995).
As regards COVID-19, studies on the cytokine profiles of IL-2, IFN-γ, and IL-4 show that these cytokines increase in these patients to a great extent (Akbari et al., 2020; Gadotti et al., 2020). Moreover, Th2 responses are more prevalent in COVID-19 patients (Gadotti et al., 2020). The results of these studies are in line with the results in the present study, in which patients showed an increased cytokine profile of IL-2, IFN-γ, and IL-4. Melatonin treatment in patients with COVID-19 after 14 days led to a significant reduction in the cytokine profile of IFN-γ and IL-4 compared to patients who used standard medications. However, we did not note a significant reduction on IL-2 following melatonin treatment in the present study. Studies in humans and animal models have shown that melatonin has anti-inflammatory properties in the ALI model or in other diseases and reduces the production of IFN-γ and IL-4 (Huang et al., 2010; Pontes et al., 2007; Rodríguez et al., 2007). Lower respiratory tract damage is associated with excessive levels of reactive oxygen species (ROS) in patients with COVID-19, which promote a cascade of biological events that drive pathological host responses and common symptoms in COVID-19. ROS induce tissue damage, immune response imbalance, and pro-inflammatory cytokine production which contribute to COVID-19 disease severity (Laforge et al., 2020). Melatonin participates in many important physiological functions, including anti-inflammation and acting as an antioxidant with a broad spectrum (Huang et al., 2010). It also suggests that free radical scavengers could be beneficial for the most vulnerable patients in COVID-19 (Laforge et al., 2020). Study in animal shows that melatonin ameliorates respiratory syncytial virus (RSV)-induced oxidative injury, in part, due to its ability in antioxidant effects and scavenging free radicals. Furthermore, its correct the aberrant immune responses due to immunomodulatory effects (Huang et al., 2010).
In this study, the expression of Th1 and Th2 regulatory genes in patients with COVID-19 was examined to better understand the anti-inflammatory mechanisms of melatonin. As the results of this study revealed, melatonin decreased the expression of STAT4 and T-bet genes, which play an important role in regulating Th1 cells, as well as the expression of STAT6 and GATA3 genes, which also have a significant role in regulating Th2 cells (Zhang et al., 2014). To date, very few studies have been carried out on the effects of COVID-19 on the expression of Th1 and Th2 regulatory genes. It was shown in a study that T-bet gene expression significantly increased in patients with COVID-19 (Kaneko et al., 2020). Another study of patients with COVID-19 as well as acute malaria indicated the expression of T-bet gene in both CD8+ and CD4+ cells had a significant increase in patients with COVID-19 compared to patients with acute malaria (Herrmann et al., 2020). The results of these studies are congruent with the results of the present study in relation to increased expression of T-bet gene in patients with COVID-19. Further, in one study, GATA3 gene expression increased following the use of SARS-CoV-2 spike proteins in NK cells (Bortolotti et al., 2020), which confirms the findings of the present study regarding increased GATA3 gene expression in patients with COVID-19. To date, no study has been reported on the expression of STAT4 and STAT6 genes in COVID-19. However, considering that T-bet and GATA3 genes require the activation of STAT4 and STAT6 in order to be expressed (Zhang et al., 2014), it can be extrapolated that the expression of these genes also increases in COVID-19. Similarly, in the existence of IFN-γ, naive CD4 T cells differentiate to Th1 in a course that is reliant on the T-bet and STAT4 activities. Th1 cells produce large amounts of IFN-γ and play a critical role in protective immunity against intracellular pathogens through the activation of macrophages. IL-4 promotes Th2 differentiation through the STAT6 and GATA3 activation. Th2 cells produce IL-4, IL-5, IL-13 and IL-25, which are important for the orchestration of humoral immune responses clearing extracellular pathogens and parasites through the induction of immunoglobulin class switching to immunoglobulin G1 and immunoglobulin E, respectively (Leung et al., 2010; Zhang et al., 2014). The results of this study indicate the alignment of cytokine profile and gene expression in COVID-19. Melatonin treatment reduced the expression of genes regulating the Th1 and Th2 pathways after 14 days in patients with COVID-19. Little research has been done on the effect of melatonin on the expression of Th1 and Th2 regulatory genes. Melatonin has been shown to reduce GATA3 gene expression in human breast cancer cells MCF-7 (Alonso-González et al., 2018), which supports the results of the present study.
This study has some limitations and strengths that are noteworthy. As a limitation, our study was limited to patients who had gone to the hospital at the beginning of the outbreak. Moreover, patients with severe symptoms were not examined in this study due to some restrictions. The pathology of these patients might be different compared to individuals with mild and moderate symptoms, which might have affected the results of the study. The second concern pertains to the dose of melatonin used, in the sense that it was difficult to use higher doses due to the special conditions of the disease. Further, in this study we used whole peripheral blood mononuclear cell for gene expression analysis and this study did not distinct particular subset of CD4 or CD8 T cells or NK cells, yet the results of this study could pave the way for further investigations. It is also suggested that in order to determine the role of the immune system and the effects of melatonin in the pathogenesis of COVID-19, other pathways such as the regulatory T cells as well as the inflammasome activation and oxidative stress pathway and the expression of genes in the relevant pathways should be examined.
5. Conclusion
In this study, the Th1 and Th2 responses during COVID-19 as well as following the use of melatonin were examined for the first time. The results of this study showed that melatonin, as an anti-inflammatory medicinal adjuvant, attenuates and controls inflammatory cytokines from Th1 and Th2 by regulating the expression of Th1 and Th2 regulatory genes in patients with COVID-19.
CRediT authorship contribution statement
Abdolkarim Hosseini: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Hadi Esmaeili Gouvarchin Ghaleh: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. Hossein Aghamollaei: Project administration, Resources, Writing – original draft, Writing – review & editing. Mahdi Fasihi Ramandi: Resources, Software, Writing – original draft, Writing – review & editing. Gholamhossein Alishiri: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. Alireza Shahriary: Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing. Kazem Hassanpour: Methodology, Project administration, Writing – original draft, Writing – review & editing. Mahdi Tat: Resources, Software, Writing – original draft, Writing – review & editing. Gholamreza Farnoosh: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
None to declare.
Acknowledgments
The authors would like to thank the Clinical Research Development Unit of Baqiyatallah Hospital, Tehran, Iran.
References
- Acuña-Castroviejo D., Escames G., Figueira J.C., de la Oliva P., Borobia A.M., Acuña-Fernández C. Clinical trial to test the efficacy of melatonin in COVID-19. J. Pineal Res. 2020;69 doi: 10.1111/jpi.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn D.G., Shin H.J., Kim M.H., Lee S., Kim H.S., Myoung J., Kim B.T., Kim S.J. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J. Microbiol. Biotechnol. 2020;30:313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari H., Tabrizi R., Lankarani K.B., Aria H., Vakili S., Asadian F., Noroozi S., Keshavarz P., Faramarz S. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Life Sci. 2020;258:118167. doi: 10.1016/j.lfs.2020.118167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbariqomi M., Hosseini M.S., Rashidiani J., Sedighian H., Biganeh H., Heidari R., Moghaddam M.M., Farnoosh G., Kooshki H. Clinical characteristics and outcome of hospitalized COVID-19 patients with diabetes: a single-center, retrospective study in Iran. Diabetes Res. Clin. Pract. 2020;169:108467. doi: 10.1016/j.diabres.2020.108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-González C., Menéndez-Menéndez J., González-González A., González A., Cos S., Martínez-Campa C. Melatonin enhances the apoptotic effects and modulates the changes in gene expression induced by docetaxel in MCF-7 human breast cancer cells. Int. J. Oncol. 2018;52:560–570. doi: 10.3892/ijo.2017.4213. [DOI] [PubMed] [Google Scholar]
- Bahrampour Juybari K., Pourhanifeh M.H., Hosseinzadeh A., Hemati K., Mehrzadi S. Melatonin potentials against viral infections including COVID-19: current evidence and new findings. Virus Res. 2020;287:198108. doi: 10.1016/j.virusres.2020.198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotti D., Gentili V., Rizzo S., Rotola A., Rizzo R. SARS-CoV-2 spike 1 protein controls natural killer cell activation via the HLA-E/NKG2A pathway. Cells 9. 2020 doi: 10.3390/cells9091975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouadma L., Wiedemann A., Patrier J., Surénaud M., Wicky P.H., Foucat E., Diehl J.L., Hejblum B.P., Sinnah F., de Montmollin E., Lacabaratz C., Thiébaut R., Timsit J.F., Lévy Y. Immune alterations in a patient with SARS-CoV-2-related acute respiratory distress syndrome. J. Clin. Immunol. 2020;40:1082–1092. doi: 10.1007/s10875-020-00839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J.D., Young H.A. IFN-γ: a cytokine at the right time, is in the right place. Semin. Immunol. 2019;43:101280. doi: 10.1016/j.smim.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A.E., Chapman K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer U., Peterson K.E., Messer R., Stromnes I.M., Race B., Hasenkrug K.J. Role of interleukin-4 (IL-4), IL-12, and gamma interferon in primary and vaccine-primed immune responses to Friend retrovirus infection. J. Virol. 2001;75:654–660. doi: 10.1128/JVI.75.2.654-660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnoosh G., Ghanei M., Khorramdelazad H., Alishiri G., Jalali Farahani A., Shahriary A., Hosseini Zijoud S.R. Are Iranian sulfur mustard gas-exposed survivors more vulnerable to SARS-CoV-2? Some similarity in their pathogenesis. Disaster Med. Public Health Prep. 2020:1–7. doi: 10.1017/dmp.2020.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadotti A.C., de Castro Deus M., Telles J.P., Wind R., Goes M., Garcia Charello Ossoski R., de Padua A.M., de Noronha L., Moreno-Amaral A., Baena C.P., Tuon F.F. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289:198171. doi: 10.1016/j.virusres.2020.198171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Schulte S., Wildner N.H., Wittner M., Brehm T.T., Ramharter M., Woost R., Lohse A.W., Jacobs T., Schulze Zur Wiesch J. Analysis of Co-inhibitory receptor expression in COVID-19 infection compared to acute plasmodium falciparum malaria: LAG-3 and TIM-3 correlate with T cell activation and course of disease. Front. Immunol. 2020;11:1870. doi: 10.3389/fimmu.2020.01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.H., Cao X.J., Liu W., Shi X.Y., Wei W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J. Pineal Res. 2010;48:109–116. doi: 10.1111/j.1600-079X.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- Ingawale D.K., Mandlik S.K. New insights into the novel anti-inflammatory mode of action of glucocorticoids. Immunopharmacol. Immunotoxicol. 2020;42:59–73. doi: 10.1080/08923973.2020.1728765. [DOI] [PubMed] [Google Scholar]
- Kaneko N., Kuo H.H., Boucau J., Farmer J.R., Allard-Chamard H., Mahajan V.S., Piechocka-Trocha A., Lefteri K., Osborn M., Bals J., Bartsch Y.C., Bonheur N., Caradonna T.M., Chevalier J., Chowdhury F., Diefenbach T.J., Einkauf K., Fallon J., Feldman J., Finn K.K., Garcia-Broncano P., Hartana C.A., Hauser B.M., Jiang C., Kaplonek P., Karpell M., Koscher E.C., Lian X., Liu H., Liu J., Ly N.L., Michell A.R., Rassadkina Y., Seiger K., Sessa L., Shin S., Singh N., Sun W., Sun X., Ticheli H.J., Waring M.T., Zhu A.L., Alter G., Li J.Z., Lingwood D., Schmidt A.G., Lichterfeld M., Walker B.D., Yu X.G., Padera R.F., Jr., Pillai S. Loss of bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell. 2020;183:143–157. doi: 10.1016/j.cell.2020.08.025. e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S., Liu X., Fang L., Chen X., Guo T., Zhang J. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell. Mol. Immunol. 2010;7:182–189. doi: 10.1038/cmi.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair M.P., Kandaswami C., Mahajan S., Chadha K.C., Chawda R., Nair H., Kumar N., Nair R.E., Schwartz S.A. The flavonoid, quercetin, differentially regulates Th-1 (IFNgamma) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim. Biophys. Acta. 2002;1593:29–36. doi: 10.1016/s0167-4889(02)00328-2. [DOI] [PubMed] [Google Scholar]
- Nikpouraghdam M., Jalali Farahani A., Alishiri G., Heydari S., Ebrahimnia M., Samadinia H., Sepandi M., Jafari N.J., Izadi M., Qazvini A., Dorostkar R., Tat M., Shahriary A., Farnoosh G., Hosseini Zijoud S.R., Taghdir M., Alimohamadi Y., Abbaszadeh S., Gouvarchin Ghaleh H.E., Bagheri M. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in Iran: a single center study. J. Clin. Virol. 2020;127:104378. doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A., Kemeny D.M. Interleukin-4 enhances interferon-gamma synthesis but inhibits development of interferon-gamma-producing cells. Immunology. 1995;85:357–363. [PMC free article] [PubMed] [Google Scholar]
- Pontes G.N., Cardoso E.C., Carneiro-Sampaio M.M., Markus R.P. Pineal melatonin and the innate immune response: the TNF-alpha increase after cesarean section suppresses nocturnal melatonin production. J. Pineal Res. 2007;43:365–371. doi: 10.1111/j.1600-079X.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- Reiter R.J., Sharma R., Ma Q., Dominquez-Rodriguez A., Marik P.E., Abreu-Gonzalez P. Melatonin inhibits COVID-19-induced cytokine storm by reversing aerobic glycolysis in immune cells: a mechanistic analysis. Med Drug Discov. 2020;6:100044. doi: 10.1016/j.medidd.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez M.I., Escames G., López L.C., López A., García J.A., Ortiz F., Acuña-Castroviejo D. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J. Pineal Res. 2007;42:272–279. doi: 10.1111/j.1600-079X.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Shneider A., Kudriavtsev A., Vakhrusheva A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020;39:153–162. doi: 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- So E.Y., Park H.H., Lee C.E. IFN-gamma and IFN-alpha posttranscriptionally down-regulate the IL-4-induced IL-4 receptor gene expression. J. Immunol. 2000;165:5472–5479. doi: 10.4049/jimmunol.165.10.5472. [DOI] [PubMed] [Google Scholar]
- Vaz de Paula C.B., de Azevedo M.L.V., Nagashima S., Martins A.P.C., Malaquias M.A.S., Miggiolaro A., da Silva Motta Júnior J., Avelino G., do Carmo L.A.P., Carstens L.B., de Noronha L. IL-4/IL-13 remodeling pathway of COVID-19 lung injury. Sci. Rep. 2020;10:18689. doi: 10.1038/s41598-020-75659-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang Y., Gu W., Sun B. TH1/TH2 cell differentiation and molecular signals. Adv. Exp. Med. Biol. 2014;841:15–44. doi: 10.1007/978-94-017-9487-9_2. [DOI] [PubMed] [Google Scholar]