Abstract
Coronavirus disease 2019 (COVID-19), as a dangerous global pandemic, has led to high morbidity and mortality in all countries. There is a lot of evidence for the possible role of oxidative stress in COVID-19. In the present study, we aimed to measure the levels of glutathione (GSH), total antioxidant capacity (TAC) and total oxidant status (TOS) in the serum of patients with COVID-19.
A total of 96 individuals with and without COVID-19 were enrolled and divided into four groups, including hospitalised group in non–intensive care units (non-ICU) (n = 35), hospitalised group in intensive care units with endotracheal intubation (EI) (ICU with EI) (n = 19), hospitalised group in intensive care units without endotracheal intubation (ICU without EI) (n = 24) and healthy people without COVID-19 disease as our control group (n = 18). The present study revealed that the TOS level was significantly lower in the group of control (p = 0.001), and level of GSH remarkably increased in the patients' groups (p < 0.001). TAC activity in non-ICU group of patients had no significant difference in comparison with the control group. However, in hospitalised patients' groups in the ICU with and without EI this activity was significantly different from the control group (p < 0.001). Moreover, there was a significant relationship between the levels of TOS, GSH and TAC with blood oxygen saturation (SpO2), fever, duration of hospitalisation and the prognosis of this disease (p < 0.001). Area under the curve (CI, 95%) of TOS, TAC and GSH-C to predict death among patients were, respectively, 0.907 (0.841, 0.973), 0.735 (0.626, 0.843) and 0.820 (0.725, 0.914). Receiver operating characteristic curve analysis showed that TOS, TAC and GSH-C have the potential specificity and sensitivity to distinguish between alive and dead patients. We found that elevated levels of oxidative stress and reduction of antioxidant indices can aggravate disease's severity in hospitalised patients with COVID-19. Therefore, it can be suggested to apply antioxidant agents as one of the effective therapeutic strategies in these groups.
Keywords: COVID-19, glutathione (GSH), oxidative stress, total antioxidant capacity (TAC), total oxidant status (TOS)
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged in late December 2019 and was responsible for the COVID-19 pandemic [1]. COVID-19 has several unique features compared with other coronavirus infections [2]. In the most vulnerable individuals (e.g. older, obese or diabetic individuals), the virus sometimes triggers a cascade of acute biological events due to excessive levels of reactive oxygen species (ROS), which can, unfortunately, lead to ventilation of patients and even dying [3]. Critical characteristics of this pandemic are high rate of spread, lack of knowledge, lack of effective treatment and high mortality. Symptoms can vary drastically; they include fever (99%), chills, dry cough (59%), sputum production (27%), fatigue (70%), lethargy, arthralgias, myalgias (35%), headache, dyspnoea (31%), nausea, vomiting, anorexia (40%) and diarrhoea; Some carriers may be asymptomatic, whereas others can experience acute respiratory distress syndrome and death [4]. Therefore, it is required to identify complicated pathogenic mechanisms of this virus to decrease the time of hospitalisation and mortality rate. It is known that oxidative stress is associated with the severity of the disease. Oxidative stress is involved in ageing and found in certain chronic pathologies, such as diabetes mellitus, cancers, hypertension, coronary heart disease etc., and certain infections, especially by RNA viruses, belonging to coronavirus family [5]. Some authors have postulated that oxidative stress might be as an important player in the activation of the inflammasome during SARS-CoV-2 infection [6]. Over the past months, the COVID-19 crisis has seriously jeopardised the capabilities of most healthcare systems worldwide. Given to recent studies, oxidative stress is an essential factor in increasing the severity of COVID-19 in some patients, and it is associated with pulmonary dysfunction, cytokine storm and viral sepsis derived from SARS-CoV-2 infection [7]. The deleterious effects of ROS on the functions of both pulmonary cells and red blood cells can be seen as a major contributor of hypoxic respiratory failure in the most severe cases of COVID-19 [3]. Because oxidative stress seems to play an important role in the pathogenesis of respiratory syncytial virus and possibly other viral-associated lung diseases, antioxidant intervention would represent a rational approach for the treatment of lower respiratory tract infections, caused by, various respiratory viral infections [8]. The pathogenesis of many diseases can be as a result of apoptosis cascade induced by oxidative stress–induced apoptotic signalling oxidative stress, leading to ROS increases and/or antioxidant decreases, disruption of intracellular redox homeostasis and irreversible oxidative modifications of lipid, protein or DNA [9]. In general, respiratory viral infections, such as SARS-CoV-2 infection, cause cytokine production, inflammation, cell death and other pathophysiological processes, which can be linked with redox imbalance or oxidative stress [10]. All these processes are associated with the development of oxidative stress, which makes an important contribution to the pathogenesis of viral infections [11]. Strengthening immune system and reducing inflammation and oxidative stress through diet and nutrition, such as consuming sufficient protein, vitamin C, vitamin E, vitamin A, zinc, carotenoids and polyphenols play an essential role in fighting against COVID-19 [12,13]. In general, changes in redox homeostasis in infected cells are one of the key events that are linked to infection with respiratory viruses, inflammation and subsequent tissue damage [14]. It appears that oxidative stress plays a critical role in the pathogenesis of COVID-19, as perpetuating the cytokine storm cycle, blood clotting mechanism and exacerbating hypoxia [15]. It is known that oxidative stress is associated with the severity of the disease, but the status of this biomarker is unclear in patients hospitalised in the hospital with various conditions, in terms smoking and drug use, place of residence, occupation, fever, the length of hospitalisation and SpO2. Therefore, the present study was conducted to evaluate oxidative stress biomarkers' levels in hospitalised COVID-19 patients and control individuals in public hospitals of Hamadan City, in Iran, in regarding factors mentioned.
Materials and methods
Patients and informed consent
This case-control study was conducted at the public hospitals of Hamadan City, located in the west of Iran. Ninety-six COVID-19 patients were selected in four groups, including hospitalised group in non–intensive care units (non-ICU group) (n = 35), hospitalised group in intensive care units with endotracheal intubation (EI) (ICU with EI group) (n = 19), hospitalised group in intensive care units without endotracheal intubation (ICU without EI group) (n = 24) and healthy people without COVID-19 as the control group (n = 18), between May and September in 2020.
The inclusion criteria for selecting healthy people as our control group were the people who had no COVID-19 symptoms, or a history of visiting a doctor or being hospitalised due to COVID-19, and for patient groups where patients admitted in hospital due to infection with COVID-19, with a positive COVID-19 RT-PCR test, and identified based on World Health Organization interim guidelines [16]. Subjects with a history of diabetes, hypertension, cancers and autoimmune disorders were excluded from the control and case groups. Also, subjects were excluded if they had a specific regimen or took antioxidant supplements such as vitamin C, vitamin E, coenzyme Q10, N-acetylcysteine and selenium. All patients or their surrogates had completed the consent form before being involved in this investigation.
Procedure and sample collection
We used a researcher-made questionnaire that included some factors, such as age, sex, education, smoking and drug use, place of residence, occupation, fever, the length of hospitalisation and SpO2 for selected individuals in the patient and control groups. After that, venous blood samples were collected within a minimum of 24 hours after admission. The samples were centrifuged at 3000g for 10 minutes; serums were separated, liquated and stored at -20 C until analysed.
Measurement of oxidative stress markers
The serum levels of glutathione (GSH), total antioxidant capacity (TAC) and total oxidant status (TOS) were measured by using the commercially available ELISA kits as per the instructions of the company (ZellBio GmbH, Veltlinerweg 42, and 89072 Ulm, Germany).
Statistical analysis
The distribution of qualitative data was described by frequencies and percentages and compared between four groups (control group, non-ICU group, ICU without EI group and ICU with EI group) by Chi-square and Fisher exact test. The quantitative data were described as the mean ± standard deviation (SD), median and interquartile range (IQR) and their normal distribution was evaluated by Shapiro-Wilk test. In case of normal distribution, the mean of quantitative data were compared in two groups using independent t-test, and in four groups using one-way analysis of variance, otherwise Mann-Whitney and Kruskal-Wallis tests were performed. Moreover, the pairwise comparisons were carried out using Tukey post hoc test. The receiver operating characteristic (ROC) curve and the area under the curve were used to determine the feasibility of using oxidative status as a classifier to predict death among patients. All analyses were performed at 0.05 significance levels using SPSS version 23 (SPSS Inc., USA) and GraphPad Prism version 6 for Windows.
Result
Assessment of demographic characteristics of the study population
The main demographic characteristics of the study population are shown in Table 1. No significant difference in age, sex, smoking and opium use was observed between different groups (p > 0.05). There was a significant difference between these four different groups according to their education level, job position and residential area. In this regard, 73.68%, 73.68% and 63.16% of infected patients in ICU with EI were illiterate, unemployed and lived in rural areas, respectively. In accordance with the results, body temperature (fever) of patient groups was higher than that of the control group (p < 0.001), which indicated that 63.16% of patients in ICU with EI, 70.83% of patients in ICU without EI and 20.00% of patients (non-ICU) had fever. Our findings illustrated that the duration of hospitalisation of patients was variable among different groups. Over 80% of patients in ICU without EI were hospitalised for less than one week, whereas 89.47% of patients in ICU with EI were hospitalised for more than one week. Based on the results, SpO2 significantly decreased in patient groups in accordance with the severity of the disease (p < 0.001). In addition, a significant difference between case groups was observed in the outcome of the disease (p < 0.001).
Table 1.
Demographic characteristics of the study population
| Covariate | Control | Non-ICU | ICU without EI | ICU with EI | P-value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 11 (61.11) | 13 (37.14) | 16 (66.67) | 9 (47.37) | 0.117 |
| Female | 7 (38.89) | 22 (62.86) | 8 (33.33) | 10 (52.63) | |
| Age | |||||
| ≤60 | 10 (55.56) | 12 (34.29) | 11 (45.83) | 4 (21.05) | 0.141 |
| >60 | 8 (44.44) | 23 (65.71) | 13 (54.17) | 15 (78.95) | |
| Education | |||||
| Illiterate | 1 (5.56) | 13 (37.14) | 1 (4.17) | 14 (73.68) | <0.001∗ |
| Under diploma | 1 (5.56) | 13 (37.14) | 2 (8.33) | 3 (15.79) | |
| Diploma | 3 (16.67) | 5 (14.29) | 14 (58.33) | 1 (5.26) | |
| University | 13 (72.22) | 4 (11.43) | 7 (29.17) | 1 (5.26) | |
| Job | |||||
| Unemployed | 3 (16.67) | 22 (62.86) | 1 (4.17) | 14 (73.68) | <0.001 |
| Employed | 15 (83.33) | 13 (37.14) | 23 (95.83) | 5 (26.32) | |
| Residence | |||||
| City | 17 (94.44) | 29 (82.86) | 10 (41.67) | 7 (36.84) | <0.001 |
| Village | 1 (5.56) | 6 (17.14) | 14 (58.33) | 12 (63.16) | |
| Smoking | |||||
| No | 17 (100.00) | 31 (88.57) | 20 (83.33) | 17 (89.47) | 0.435∗ |
| Yes | 0 (.00) | 4 (11.43) | 4 (16.67) | 2 (10.53) | |
| Opiate | |||||
| No | 18 (100.00) | 33 (94.29) | 21 (87.50) | 18 (94.74) | 0.450∗ |
| Yes | 0 (.00) | 2 (5.71) | 3 (12.50) | 1 (5.26) | |
| Fever | |||||
| No | — | 28 (80.00) | 7 (29.17) | 7 (36.84) | <0.001 |
| Yes | — | 7 (20.00) | 17 (70.83) | 12 (63.16) | |
| Length of hospitalisation | |||||
| ≤1 week | — | 34 (100.00) | 19 (79.17) | 2 (10.53) | <0.001 |
| >1 week | — | 0 (.00) | 5 (20.83) | 17 (89.47) | |
| SpO2 | |||||
| <88 | — | 0 (.00) | 2 (8.33) | 10 (52.63) | <0.001∗ |
| 88-90 | — | 20 (57.14) | 13 (54.17) | 6 (31.58) | |
| >90 | — | 15 (42.86) | 9 (37.50) | 3 (15.79) | |
| Outcome | |||||
| Alive | — | 35 (100.00) | 18 (75.00) | 4 (21.05) | <0.001 |
| Dead | — | 0 (.00) | 6 (25.00) | 15 (78.95) | |
Without star: Chi-square test.
With star: Fisher exact test.
Assessment of TOS, GSH and TAC in serum
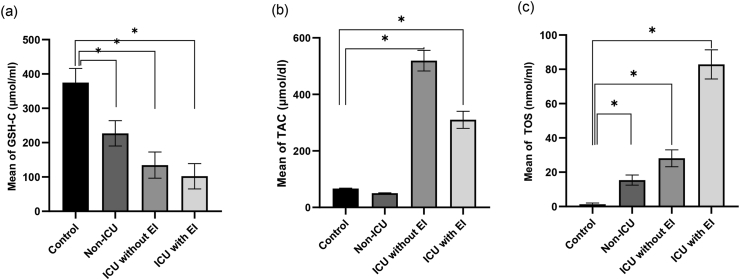
Results for oxidative stress markers, including TOS, GSH and TAC, were described by mean and 95% confidence intervals in different patient groups compared with the control group, presented in Fig. 1(a-c). In accordance with the presented results in Fig. 1(a-c), the TOS levels in different case groups were significantly higher than the control group. The mean ± SD for this biomarker was 15.40 ± 2.94 for the non-ICU group, 28.13 ± 4.87 for the ICU group without EI, 82.89 ± 8.49 for the ICU group with EI and 1.28 ± 0.75 for the control group. Moreover, in accordance with Fig. 1 a, the mean of GSH in different groups of patients was significantly lower than the control group; the mean ± SD of GSH was 227.03 ± 36.91, 134.54 ± 38.11 and 102.11 ± 36.86 in the non-ICU group, the ICU group without EI and the ICU group with EI, respectively, compared with 374.94 ± 41.15 in the control group (p < 0.001). Also, the mean ± SD of TAC in non-ICU group of patients had no significant difference with the control group (50.26 ± 9.47 and 66.72 ± 6.99), while there was a significant difference in the ICU group with and without EI (310.00 ± 131.90 and 519.26 ± 175.89) in comparison with the control group (p < 0.001). Table 2 represents the relationship between oxidative stress biomarkers and the level of other parameters in all patients. The results in this table indicate that those patients with fever, SpO2 lower than 88 percent, the length of hospitalisation higher than one week and dead patients had significantly higher mean (SD) and median (IQR) of TOS and lower mean (SD) and median (IQR) of GSH and TAC. On the other hand, the TOS/GSH and TOS/TAC ratios in both control and the patient group are shown in Table 3. In our study, the TOS/GSH ratio was 0.003 ± 0.002, 0.070 ± 0.018, 0.224 ± 0.069 and 0.907 ± 0.331 in the control group, non-ICU group, ICU without EI group and ICU with ET group, respectively, and TOS/TAC ratio was 0.019 ± 0.011, 0.323 ± 0.106, 0.061 ± 0.025 and 0.306 ± 0.129 in the control group, non-ICU group, ICU without EI group and ICU with ET group, respectively. Our study showed the TOS/GSH ratio was 0.590 ± 0.025 and 0.129 ± 0.031 in expired patients and discharged patients, respectively; and the TOS/TAC ratio was 0.318 ± 0.115 and 0.063 ± 0.015 in expired patients and discharged patients, respectively. Also, we found that 78.95% and 25% of patients in ICU with and without ET deceased, while all patients were alive in the non-ICU group.
Fig. 1.
Comparison of glutathione (GSH), total antioxidant capacity (TAC) and total oxidant status (TOS) levels between the control group (n = 18) and patient group of non–intensive care unit (non-ICU) (n = 35), intensive care unit with intubation (ICU with EI) (n = 19) and intensive care unit without intubation (ICU without EI) (n = 24); ∗p < 0.001.
Table 2.
Relationship between oxidative stress biomarkers and others demographic characteristics of the study population
| Outcome | Count | Mean | SD | Median | IQR | P-value | |
|---|---|---|---|---|---|---|---|
| TOS (nmol/mL) | |||||||
| Fever | No | 42 | 29.38 | 26.76 | 17 | 15 | 0.002 |
| Yes | 36 | 43.19 | 27.88 | 32 | 55 | ||
| SpO2 | <88 | 12 | 73.58 | 21.50 | 80.5 | 12.5 | <0.001 |
| 88-90 | 39 | 30.51 | 25.70 | 20 | 16 | ||
| ≥91 | 27 | 26.52 | 19.14 | 20 | 17 | ||
| dur_hospitalisation | =<1 week | 55 | 22.20 | 13.06 | 18 | 13 | <0.001 |
| >1 week | 23 | 70.82 | 25.10 | 78 | 17 | ||
| Outcome | Alive | 57 | 24.14 | 17.75 | 18 | 13 | <0.001 |
| Dead |
21 |
67.29 |
26.51 |
77 |
53 |
||
| GSH-C (μmol/ml) | |||||||
| Fever | No | 42 | 188.36 | 61.84 | 199.5 | 91 | 0.004 |
| Yes | 36 | 144.56 | 63.59 | 124.5 | 75 | ||
| SpO2 | <88 | 12 | 106.25 | 21.88 | 99.5 | 31.5 | 0.002 |
| 88-90 | 39 | 177.79 | 64.75 | 189 | 115 | ||
| ≥91 | 27 | 181.70 | 66.89 | 191 | 110 | ||
| dur_hospitalisation | =<1 week | 55 | 191.09 | 60.88 | 199 | 101 | <0.001 |
| >1 week | 22 | 108.36 | 34.48 | 98.5 | 34 | ||
| Outcome | Alive | 57 | 188.12 | 62.23 | 199 | 106 | <0.001 |
| Dead |
21 |
113.90 |
41.31 |
101 |
61 |
||
| TAC (μmol/dl) | |||||||
| Fever | No | 42 | 361.54 | 248.19 | 308 | 394 | |
| Yes | 36 | 165.19 | 177.89 | 55.5 | 245 | <0.001 | |
| SpO2 | <88 | 12 | 227.85 | 260.14 | 63.5 | 347 | |
| 88-90 | 39 | 243.21 | 230.49 | 67 | 345 | ||
| ≥91 | 27 | 348.58 | 161.67 | 318.5 | 160 | 0.090 | |
| dur_hospitalisation | =<1 week | 55 | 370.43 | 183.68 | 308 | 120 | |
| >1 week | 22 | 213.69 | 237.32 | 56 | 345 | 0.003 | |
| Outcome | Alive | 57 | 377.00 | 192.11 | 308 | 155 | |
| Dead | 21 | 211.44 | 232.25 | 59 | 345 | 0.002 | |
GSH, glutathione; TAC, total antioxidant capacity; TOS, total oxidant status.
Table 3.
ROS/GSH and ROS/TAC ratios in the control group and the patient groups
| Ratio | Control | Non-ICU | ICU without EI | ICU with EI | P-value |
|---|---|---|---|---|---|
| TOS/GSH | 0.003 ± 0.002 | 0.070 ± 0.018 | 0.224 ± 0.069 | 0.907 ± 0.331 | <0.001 |
| TOS/TAC | 0.019 ± 0.011 | 0.323 ± 0.106 | 0.061 ± 0.025 | 0.306 ± 0.129 | <0.001 |
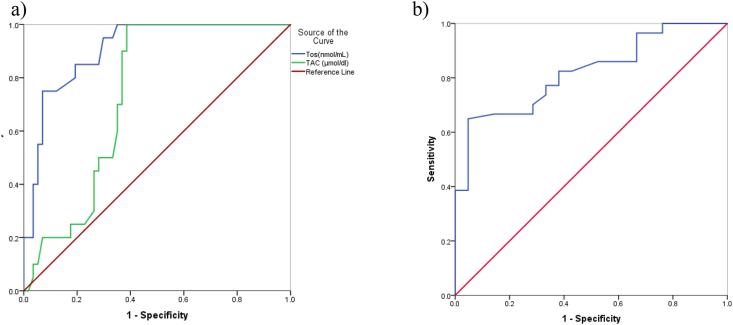
The results of the ROC analysis for discriminating the power of oxidative status to distinguish between alive and dead patients are shown in Fig. 2. This figure presents that the area under the curve (CI, 95%) for TOS, TAC and GSH-C were, respectively, 0.907 (0.841, 0.973), 0.735 (0.626, 0.843) and 0.820 (0.725, 0.914).
Fig. 2.
Receiver operating characteristic (ROC) curve analysis showed that a) TOS and TAC, b) GSH-C have the potential specificity and sensitivity to distinguish between alive and dead patients.
Discussion
In the present study, we investigated the role of oxidative stress biomarkers in different groups of patients hospitalised in all public hospitals of Hamadan city in Iran. In accordance with previous studies, an imbalance in the production of reactive species and the body's inability to detoxify these reactive species is referred to oxidative stress [17,18]. It is known that oxidative stress is triggered by a wide variety of viral infections, including HIV 1, viral hepatitis B, C and D viruses, herpes viruses, respiratory viruses, such as corona viruses [5], and is associated with severity and predictive of outcome. Although, it has been clearly understood that many of viral, bacterial and parasitic infections trigger the production of ROS and reactive nitrogen species implicated in lung tissue injury and epithelial barrier dysfunction, but understanding the molecular inflammation mechanisms of stress oxidative contributing to COVID-19 progression is a current clinical need to improve therapies in patients [19]. As mentioned in previous studies, hypoxia induced by lung injury can be due to mitochondrial dysfunction. Mitochondrial dysfunction leads to a relative decrease in oxygen and energy production and increase in ROS production. In this regard, superoxide, H2O2 and other reactive species are mainly produced by the mitochondrial respiratory chain. Hydrogen peroxide causes the expression of many genes that activate proinflammatory cytokines in macrophages, neutrophils and endothelial cells through NADPH oxidase (NOx) to produce more superoxide and H2O2 [15,20]. Also, published researches showed COVID-19 microbiota dysbiosis disturbs mitochondrial homeostasis via the production of toxic as gases hydrogen sulfide (H2S) and nitrogen oxide (NO) [20]. These processes ultimately lead to oxidative damage in COVID-19 patients. Survey data describe that oxidative damage occurs in humans and the demographic, physical or nutritional factors may be associated with it [21]. Our results demonstrated that oxidative stress profile in COVID-19 patients is closely related to patients' health level and demographic characteristics. Owing to our results, the serum level of TOS, as one of the oxidative stress biomarkers, was higher in patients with acute disease conditions, like those in ICU with and without EI, than in the control group (Fig 1a). In general, factors such as medication, environmental pollutants and dietary components highlight the importance of an optimal nutrient status to reduce inflammation and oxidative stress: thereby, they strengthen the immune system during the COVID-19 crisis [12]. Our study showed that there is a direct relationship between the serum levels of TOS in infected patients with COVID-19 and some criteria, such as fever, the length of hospitalisation more than one week, residency, the educational status and their job type, and indirect relationship with SpO2. Nucleic acid damage by the generated oxidative stress is associated with viral mutations which can potentially reduce the effectiveness of COVID-19 management, including the vaccine approach [22]. Oxidative stress via RNA virus infections can contribute to several aspects of viral disease pathogenesis, including apoptosis, loss of immune function, viral replication, inflammatory response and loss of body weight. While there is a clear correlation between oxidative stress markers and the severity of many viral diseases such as hepatitis C, for SARS-CoV-2, clinical data are limited. However, in the preclinical setting, many lines of evidence suggest that overproduction of ROS and a deprived antioxidant system play a significant role in the pathogenesis of SARS-CoV-2 infection, as well as in the progression and severity of the respiratory disease [10]. In our study, TOS levels clearly increase with the worsening of COVID-19 disease. It can be explained that the elevated level of oxidative stress has the capability to intensify the severity of COVID-19 disease, whereas antioxidant supplementation and physical exercise could reduce its severity [10]. SARS-CoV-2 has the potentials in the generation of ROS and oxidative stress [23]. High levels of ROS with deprived antioxidant mechanisms are of great importance for viruses to replicate and cause disease [24]. Although reactive species are frequently formed after viral infections and antioxidant defences, such as enzymatic and non-enzymatic components, protect against reactive species, sometimes these defences are not completely adequate [17]. The major antioxidant enzymes, directly involved in the neutralisation of ROS and reactive nitrogen species are superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase [25]. The non-enzymatic antioxidants are also divided into metabolic antioxidants and nutrient antioxidants. Metabolic antioxidants belonging to endogenous antioxidants are produced by metabolism in the body, such as lipoid acid, glutathione, L-arginine, coenzyme Q10, melatonin, uric acid, bilirubin, metal-chelating proteins, transferrin etc. [26,27]. However, nutrient antioxidants belonging to exogenous antioxidants are compounds and cannot be produced in the body and must be provided through foods or supplements, such as vitamin E, vitamin C, carotenoids, trace metals (selenium, manganese and zinc), flavonoids, omega-3 and omega-6 fatty acids etc. [18,25]. The practice of physical exercises acts as a modulator of the immune system. During and after physical exercise, pro- and anti-inflammatory cytokines are released, lymphocyte circulation increases, as well as cell recruitment. Such practice has an effect on the lower incidence, intensity of symptoms and mortality in viral infections as COVID-19 [28]. As shown in Fig. 2b, although there was a significant increase in the serum levels of TAC in ICU hospitalised patients with and without EI, no statistically significant difference of this biomarker expression was found in the control group compared with non-ICU hospitalised patients. Comparison of TAC in all four groups in this study shows that in patients with COVID-19, TAC initially decreases compared with the control group; then, it increases sharply as the disease progresses. Finally, if the disease worsens and the patient needs EI, the TAC decreases again. This decrease in TAC may lead to a reduction in patient resilience and ultimately an increase in mortality in patients with EI. Because TAC is a biochemical parameter, it can be suitable for evaluating the overall antioxidant status of cells against free radicals. Similar to our study, various investigations have indicated that when a virus enters the human body under oxidative stress conditions, the levels of antioxidant biomarkers increase to fight against oxidative compounds [13,18,29,30]. The important notion of the relationship between dietary constituents, nutrition, inflammation and oxidative stress is well-regard. So that low micronutrient status, such as of vitamin A or zinc, has been associated with increased infection risk. As previous studies have shown, decreased plasma concentrations of vitamins A, C and E vitamins, Se, Zn, Mg and Cu, and decrease activities of glutathione, glutathione peroxidase, catalase, superoxide dismutase and impairment in antioxidant system can result in a weakened immune system, and subsequently cause disease progression in patients with COVID-19 [31,32]. Moreover, it has been proved that the malnourished individuals have a rising risk of being admitted to the ICU and COVID-19-related mortality [33]. Other evidence confirmed the role of oxidative stress in COVID-19 [19,22,34,35]. GSH as a thiol compound is one of the most important small molecular weight antioxidants, produced in the cell [36]. Based on the results we obtained, GSH level as an antioxidant was significantly lower in patients with COVID-19 hospitalised in different hospital wards compared with the control group (Fig 1c). Our study also showed GSH levels in infected patients with COVID-19 have an indirect relationship with fever, duration of hospitalisation and direct relationship with SpO2. Also there was a direct relation between TOS/GSH ratio and TOS/TAC ratio and the severity of COVID-19. GSH is a peptide composed of three amino acids and a free radical scavenger, preventing damage induced by ROS in oxidative stress conditions. The thiol function of GSH gives it the role of reducing agents related to ROS. Many studies have emphasised the advantages of glutathione in the body, acting as an anti-viral factor and managing COVID-19 patients [32,[37], [38], [39], [40]]. The higher levels of GSH may improve an individual's responsiveness to viral infections [41]. In particular, GSH is known to protect host immune cells through its antioxidant mechanism, and it is also responsible for the optimal function of various cells, such as those in the immune system [[41], [42], [43]]. In accordance with several studies, COVID-19 patients with moderate and severe illness had lower levels of GSH, higher ROS levels, and greater redox status (ROS/GSH ratio) than those patients with a mild illness [36,42,44,45]. Taken together, applying antioxidants therapy can be useful as a promising approach for lowering oxidative stress and accompanying complications of viral infections. However, further experiments in vivo and clinical trials are necessary to reveal the potential effects of these therapeutic approaches on viral diseases with unknown mechanisms, like COVID-19.
Conclusion
Our studies showed a significant association between oxidative stress biomarkers and disease severity in different groups of hospitalised patients. In addition, our results illustrated that lifestyle and education level has played an important role in increasing stress levels and disease progression. In fact, there was a significant relationship between oxidative stress levels and patients' condition in terms of SpO2, fever, the median duration of hospitalisation as well as the outcome of this disease. It seems that strategies for reducing or preventing oxidative stress may help in COVID-19 management. In accordance with many studies on the role of oxidative stress in the pathophysiology of COVID-19 disease, it seems that further investigations should be conducted to determine the time of onset of antioxidants and their required dose to treat this disease.
Author contributions
All authors contributed to the work presented in the article.
Conflict of interest
All authors declared no conflict of interest regarding this article.
Funding
This research was supported by Hamadan University of Medical Sciences with the code of 9906113797 and the ethical code of IR.UMSHA.REC.1399.441.
Acknowledgements
The authors would appreciate the Deputy of Research and Technology, Hamadan University of Medical Sciences for financial support of this research.
Contributor Information
B. Karkhanei, Email: bk13472000@yahoo.com.
E. Talebi Ghane, Email: talebi.ghane@gmail.com.
F. Mehri, Email: freshteh_mehri@yahoo.com.
References
- 1.Dos Santos W.G. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed Pharmacother. 2020;129:110493. doi: 10.1016/j.biopha.2020.110493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laforge M. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valencia D.N. Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Cureus. 2020;12(3) doi: 10.7759/cureus.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ntyonga-Pono M.P. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? Pan Afr Med J. 2020;35(Suppl. 2):12. doi: 10.11604/pamj.2020.35.2.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derouiche S. Oxidative stress associated with SARS-Cov-2 (COVID-19) increases the severity of the lung disease-a systematic review. J Infect Dis Epidemiol. 2020;6:121. [Google Scholar]
- 7.Beltrán-García J. Oxidative stress and inflammation in COVID-19-associated sepsis: the potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants. 2020;9(10):936. doi: 10.3390/antiox9100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komaravelli N., Casola A. Respiratory viral infections and subversion of cellular antioxidant defenses. J Pharmacogenomic Pharmacoproteomics. 2014;5(4) doi: 10.4172/2153-0645.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Circu M.L., Aw T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48(6):749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Archiv Med Res. 2020 doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chernyak B. COVID-19 and oxidative stress. Biochemistry (Moscow) 2020;85(12):1543–1553. doi: 10.1134/S0006297920120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iddir M. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6) doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahimi B., Vesal A., Edalatifard M. Coronavirus and its effect on the respiratory system: is there any association between pneumonia and immune cells. J Fam Med Prim Care. 2020;9(9):4729–4735. doi: 10.4103/jfmpc.jfmpc_763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khomich O.A. Redox biology of respiratory viral infections. Viruses. 2018;10(8):392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cecchini R., Cecchini A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Organization W.H. World Health Organization; 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. [Google Scholar]
- 17.Camini F.C. Implications of oxidative stress on viral pathogenesis. Archiv Virol. 2017;162(4):907–917. doi: 10.1007/s00705-016-3187-y. [DOI] [PubMed] [Google Scholar]
- 18.Camini F.C. Implications of oxidative stress on viral pathogenesis. Arch Virol. 2017;162(4):907–917. doi: 10.1007/s00705-016-3187-y. [DOI] [PubMed] [Google Scholar]
- 19.Beltran-Garcia J. Oxidative stress and inflammation in COVID-19-associated sepsis: the potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants (Basel) 2020;9(10) doi: 10.3390/antiox9100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saleh J. Mitochondrion; 2020. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Block G. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156(3):274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 22.Bakadia B.M. The impact of oxidative stress damage induced by the environmental stressors on COVID-19. Life Sci. 2021;264:118653. doi: 10.1016/j.lfs.2020.118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baqi H.R. Oxidative stress and its association with COVID-19: a narrative review. Kurdistan J Appl Res. 2020:97–105. [Google Scholar]
- 24.Khomich O.A. Redox biology of respiratory viral infections. Viruses. 2018;10(8):392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 26.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 27.Willcox J.K., Ash S.L., Catignani G.L. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44(4):275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 28.da Silveira M.P. Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature. Clin Exp Med. 2020:1–14. doi: 10.1007/s10238-020-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin Gimenez V.M. Lungs as target of COVID-19 infection: protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci. 2020;254:117808. doi: 10.1016/j.lfs.2020.117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schonrich G., Raftery M.J., Samstag Y. Devilishly radical NETwork in COVID-19: oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv Biol Regul. 2020;77:100741. doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayindir M., Bayindir E.E. Synergic viral-bacterial co-infection in catalase-deficient COVID-19 patients causes suppressed innate immunity and lung damages due to detrimental elevation of hydrogen peroxide concentration. Available at SSRN 3648292. 2020 [Google Scholar]
- 32.Muhammad Y. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: a cross-sectional comparative study in Jigawa. Northwest Nigeria. 2021;9 doi: 10.1177/2050312121991246. 2050312121991246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handu D. Malnutrition care during the COVID-19 pandemic: considerations for registered dietitian nutritionists. J Acad Nutr Dietetics. 2020 doi: 10.1016/j.jand.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baqi H.R. 2020. Oxidative stress and its association with covid-19: a narrative review; pp. 97–105. [Google Scholar]
- 35.Cardinali D.P., Brown G.M., Pandi-Perumal S.R. Can melatonin Be a potential “silver bullet” in treating COVID-19 patients? Diseases. 2020;8(4) doi: 10.3390/diseases8040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sestili P., Fimognari C. Paracetamol-induced glutathione consumption: is there a link with severe COVID-19 illness? Front Pharmacol. 2020;11:579944. doi: 10.3389/fphar.2020.579944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guloyan V. Glutathione supplementation as an adjunctive therapy in COVID-19. Antioxidants (Basel) 2020;9(10) doi: 10.3390/antiox9100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horowitz R.I., Freeman P.R., Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases. Respir Med Case Rep. 2020;30:101063. doi: 10.1016/j.rmcr.2020.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khanfar A., Al Qaroot B. Could glutathione depletion be the Trojan horse of COVID-19 mortality? Eur Rev Med Pharmacol Sci. 2020;24(23):12500–12509. doi: 10.26355/eurrev_202012_24046. [DOI] [PubMed] [Google Scholar]
- 40.Silvagno F., Vernone A., Pescarmona G.P. The role of glutathione in protecting against the severe inflammatory response triggered by COVID-19. Antioxidants (Basel) 2020;9(7) doi: 10.3390/antiox9070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forman H.J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30(1–2):1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect Dis. 2020;6(7):1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 43.Jain S.K. The potential link between inherited G6PD deficiency, oxidative stress, and vitamin D deficiency and the racial inequities in mortality associated with COVID-19. Free Radic Biol Med. 2020;161:84–91. doi: 10.1016/j.freeradbiomed.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaiswal N., Bhatnagar M., Shah H. N-acetylcysteine: a potential therapeutic agent in COVID-19 infection. Med Hypotheses. 2020;144:110133. doi: 10.1016/j.mehy.2020.110133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jorge-Aarón R.-M., Rosa-Ester M.-P. N-acetylcysteine as a potential treatment for COVID-19. Future Med. 2020 doi: 10.2217/fmb-2020-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]