Abstract
COVID-19 pandemic has resulted in millions of deaths and a social-economic crisis. A worldwide effort was made to develop efficient vaccines for this disease. A vaccine should produce immune responses with specific and neutralizing antibodies, and without harmful effects such as the antibody-dependent enhancement that may be associated with severe acute respiratory syndrome. Vaccine design involves the selection of platforms that includes viral, viral-vector, protein, nucleic acid, or trained immunity-based strategies. Its development initiates at a pre-clinical stage, followed by clinical trials when successful. Only if clinical trials show no significant evidence of safety concerns, vaccines can be manufactured, stored, and distributed to immunize the population. So far, regulatory authorities from many countries have approved nine vaccines with phase 3 results. In the current pandemic, a paradigm for the COVID-19 vaccine development has arisen, as many challenges must be overcome. Mass-production and cold-chain storage to immunize large human populations should be feasible and fast, and a combination of different vaccines may boost logistics and immunization. In silico trials is an emerging and innovative field that can be applied to predict and simulate immune, molecular, clinical, and epidemiological outcomes of vaccines to refine, reduce, and partially replace steps in vaccine development. Vaccine-resistant variants of SARS-CoV-2 might emerge, leading to the necessity of updates. A globally fair vaccine distribution system must prevail over vaccine nationalism for the world to return to its pre-pandemic status.
Keywords: SARS-CoV-2, Coronavirus, Vaccine strategies, Vaccine safety, Emergency use
1. Vaccine development attempts for previous coronaviruses
The novel coronavirus, i.e. severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly. Coronavirus disease 2019 (COVID-19) has been the cause of millions of deaths and social and economic crises worldwide. The scientific community has been exploring all strategies to develop efficient vaccines against SARS-CoV-2, which is essential to reduce morbidity and mortality (Hodgson et al., 2020). Social distancing strategies help prevent transmission and reduce infection, but a vaccine is necessary for the population to acquire immunity against COVID-19. Attempts to develop several vaccines for β-coronaviruses, closely related to SARS-CoV-2, such as SARS-CoV and MERS-CoV, were previously performed and tested in animal models (Roper and Rehm, 2009). Most vaccines protected animals from a challenge with SARS-CoV or MERS-CoV although many of them did not induce long-term immunity. In addition, vaccination resulted in complications in some cases, including lung damage and infiltration of eosinophils in a mouse model (Bolles et al., 2011; Agrawal et al., 2016). About 40–60 % of unexposed healthy individuals from the United States (n = 20) presented SARS-CoV-2-reactive CD4 + T cells, indicating the presence of a cross-reactivity immunity for other common coronaviruses (Grifoni et al., 2020).
Efforts for vaccine development for SARS-CoV and MERS-CoV were made but failed, so the immune system managed to spontaneously suppress the infection in the population. Although their candidate vaccines have been submitted and are still in progress, there are no approved vaccines for these infectious agents so far, after 17 and 6 years of the original outbreaks, respectively (De Wit et al., 2016; Song et al., 2019). Therefore, the highest scientific standards are required for the effective development of a vaccine against COVID-19. Although previous experiments for SARS-CoV and MERS-CoV indicated potential harmful and adverse events due to increased immunity (Prompetchara et al., 2020), it is possible to learn from those vaccine attempts for other coronaviruses about how to move forward with a SARS-CoV-2 vaccine project.
2. Vaccine development for COVID-19 in a pandemic paradigm
A vaccine should produce specific and neutralizing antibodies. The goal is to expose the body to an antigen that stimulates the immune response, blocking or eliminating the virus in case of infection, without triggering COVID-19. Vaccines may cause adverse events that are harmful to the host due to unwanted immune enhancement responses, so careful and complete tests are required before the approval of a global vaccine for COVID-19 (Funk et al., 2020).
Designing a vaccine requires a selection of antigens and platforms as well as forms of administration and regimen. While only antibodies to the spike (S) protein can neutralize and prevent infection, the inclusion of the nucleocapsid (N) or a non-structural protein as antigen can probably produce a balanced humoral and T-cell immunity. The route of administration and regimen significantly depends on the vaccine strategy. However, parenteral vaccination is regarded to induce timely IgG antibodies while the respiratory route better induces resident memory T cells (TRM) and trained immunity in the lungs (Jeyanathan et al., 2020).
Scientists believe that COVID-19 severe symptoms may be better explained by the immunopathology of the Th2 response (Roncati et al., 2020). This immunopathology is based on the unregulated response of T cells, with an increased response of CD4 + T cells specific to the virus, leading to allergic inflammation and an influx of eosinophils into the lungs (Tseng et al., 2012). In vivo studies have shown that infection by SARS-CoV after vaccination failed to control viral replication, increase of clinical symptoms, and pathology characterized by distorted Th2 responses, inflammation, and eosinophilic influx (Bolles et al., 2011; Tseng et al., 2012). It was also observed that pathological development might be linked to antibodies specifically targeted to the nucleocapsid protein (Bolles et al., 2011). However, a reduction in pathology was observed in vaccination studies with the spike protein (Tseng et al., 2012).
The immunopathological effects associated with antibody-dependent enhancement (ADE) – a process by which viruses potentiate antibodies to aid infection to promote a severe inflammatory response – have been observed in viral infections, such as MERS and SARS, and have drawn attention to the possibility of ADE in COVID-19. ADE allows the infection of phagocytic antigen-presenting cells (APC), due to the binding of virus-bound antibodies to the Fc receptor on their surface (Smatti et al., 2018). Neutralizing antibodies targeting the receptor-binding domain (RBD) of MERS-CoV spike protein have been shown to mediate the entry of viruses into human cells expressing the Fc receptor in vitro (Wan et al., 2019).
Studies on small cohorts of COVID-19 patients have shown that an increased response of neutralizing IgG antibodies and a higher titer of total antibodies were associated with a severe condition of the disease (Zhang et al., 2020a,b; Zhao et al., 2020). This could be evidence of ADE in SARS-CoV-2 infection (Cao, 2020). However, animal immunization studies have shown that the spike protein RBD from SARS-CoV-2 can induce a robust response of neutralizing antibodies, without inducing ADE (Quinlan et al., 2020). Most vaccine candidates for COVID-19 under development or approved for emergency or full use aim to induce neutralizing antibodies against the viral spike protein, preventing its binding to the human angiotensin 2 converter enzyme (ACE2) receptor to block infection (Thanh Le et al., 2020). Therefore, there are doubts about the ADE relevance in COVID-19.
Developing a vaccine quickly requires a new paradigm. It is a time-consuming and expensive process (Gouglas et al., 2018). The use of state-of-the-art sequencing and reverse genetics can reduce the development time of conventional vaccines during epidemics. The urgency to develop a vaccine against SARS-CoV-2 comes at a moment of improvement in scientific understanding in areas that support vaccine development such as genomics and structural biology. Clinical trials during a pandemic show additional challenges. They are needed to confirm which populations remain at greatest risk when vaccines become available and to establish a globally fair vaccine distribution system (Lurie et al., 2020).
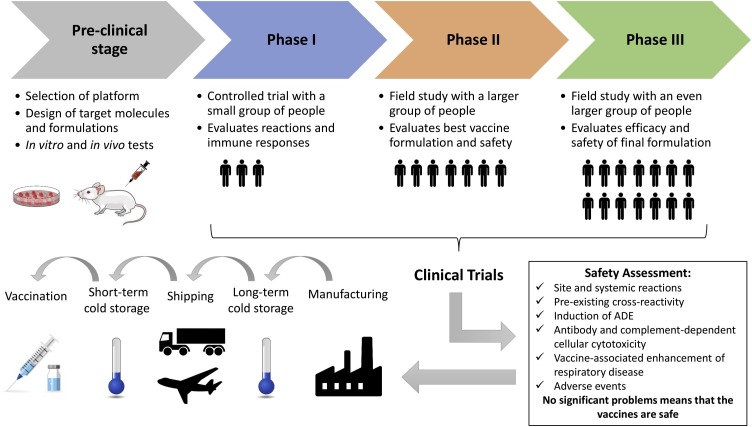
As described by Funk et al. (2020), vaccine development usually takes place in three stages. The pre-clinical stage consists of engaging research and development involving the selection of platform, design of targets and formulations, in vitro tests in cell culture, and in vivo tests in animals. If encouraging results are observed in the pre-clinical stage, the candidate vaccine proceeds to the clinical trials, which consists of testing on human volunteers in three phases: phase I, phase II, and phase III. These phases can be combined to expedite approval (e.g. phase II/III). Due to the pandemic nature of COVID-19, two phases have been carried out simultaneously.
Only if the safety and efficacy of the vaccine are achieved in human volunteers, the logistics operation stage, the third and last one, is initiated to ensure worldwide distribution in a coordinated and interconnected manner (manufacture, storage, and distribution). The vaccines that did not get satisfactory results in clinical trials do not proceed to the third stage and are discontinued. Logistically, the vaccine should be easy to administer and preferably in a single dose in the smallest possible amount. An oral or intranasal vaccine would be ideal. The vaccine should be easy to produce, and the dose mass-production necessary to immunize large human populations should be feasible and fast. Long-term storage of the vaccine at room temperature should be a goal to facilitate transportation and storage (Funk et al., 2020).
More than 160 vaccine candidates in preclinical and clinical stages are in development (Jeyanathan et al., 2020). Although produced for emergency use, they need to be safe and effective against SARS-CoV-2. Integrating knowledge on what we know so far about the virus, like infection cycle, viral recognition pathway, host immunity, and previous experiences of vaccine development attempts for other coronaviruses are important issues to consider in a pandemic scenario (Prompetchara et al., 2020). Fig. 1 shows a flowchart with major steps from the pre-clinical stage to the final goal of vaccinating the population.
Fig. 1.
Flowchart of the major steps before vaccines reach the population. Passing the pre-clinical stage, the vaccines undergo clinical trials from small to larger groups of people. If the vaccines render significant efficacy and safety, they can be manufactured in large scale, stored and distributed until vaccinations are complete.
3. In silico trials in the COVID-19 vaccine development
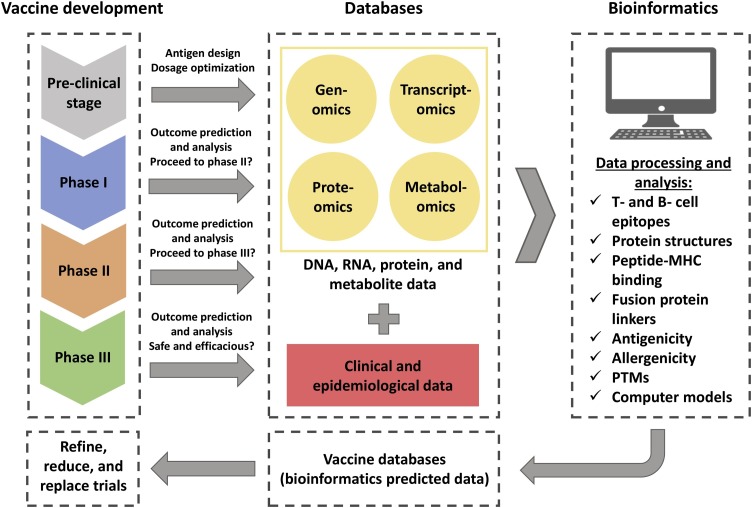
In Silico Trials (IST) technologies are indispensable in the research and development stage to predict vaccine candidates before further validation for clinical trials (Chukwudozie et al., 2021). Bioinformatics and high-throughput omics technologies enabled the progress of vaccine research into the rational design of vaccines. Databases emerged to deal with their high volume of DNA, RNA, protein, and metabolite data (He and Xiang, 2013). Several platforms have been developed to predict T- and B- cell epitopes as vaccine targets, evaluate antigenicity and allergenicity, explore antibody structures and peptide-MHC binding (data-driven and structure-based methods), simulate antigen-antibody interactions, and select immuno-adjuvants using computational pipelines (Lafuente and Reche, 2009; Chukwudozie et al., 2020). Crooke et al. (2020) used predictive algorithms to analyze all structural, non-structural, and accessory proteins from SARS-CoV-2 and identified 41 and 6 T- and B- cell epitopes, respectively.
In addition, bioinformatics can predict protein structures, post-translational modifications (PTMs), and aid in the design of linkers for fusion proteins. This way, it is possible to shorten the time spent on experimental research. Along with clinical and epidemiological data, these tools can speed up and decrease the costs of the experimental immunogenicity assessment of vaccines. Moreover, predicted data can be further stored in databases specific to vaccine design (He and Xiang, 2013; Bahrami et al., 2019). Mercurio et al. (2021) performed an in silico modeling approach to analyze structural changes in the protein S RBD favoring ACE2 binding. This method can be used to identify neutralizing antibodies or build new ones, with applications in the development of new vaccines.
Efficiency to obtain SARS-CoV-2 genome data, supported by bioinformatics platforms, was critical to design vaccines through available technologies (Chukwudozie et al., 2021). Those technologies include reverse vaccinology, which identifies novel antigens through analysis of genomic information (Ullah et al., 2020), immunoinformatics that analyzes an organism’s immunomics to make predictions of immune responses against specific molecules, and structural vaccinology that focuses on the conformational features of the viral epitope that makes them good candidate antigens (María et al., 2017).
IST can also simplify preclinical and clinical stages that can be modeled through computational approaches for the improvement of vaccine development, which is referred to as in silico clinical trials (ISCT) (Pappalardo et al., 2019). Virus–host interaction, immunization modeling, vaccination schedules, assessment of vaccine safety and efficacy commonly use agent-based modeling (ABM), a model based on the simulation of the dynamics of single entities that interact with each other following simple known rules and differential equations as computational and mathematical approaches (Pappalardo et al., 2010, 2015).
ISCT has been used effectively in preclinical studies to optimize dosage administration and schedule, maintaining a prolonged immune response that effectively protects the host (Finn and Beatty, 2016; Pappalardo et al., 2010, 2016). In clinical studies, modeling and simulation provide a reliable prediction of outcomes based on the data collected to increase confidence before investing in a new trial phase. Russo et al. (2020) applied a human immune system simulator with a SARS-CoV-2 disease model to predict the outcome of a candidate vaccine strategy. The vaccine presented a good agreement with experimental data.
Computer models can be applied in the development or regulatory assessment of vaccines to reduce, refine or partially replace pre-clinical and clinical trials. These models are useful to predict the average effect of a vaccine over a population or each individual in a population to test safety and efficacy (Pappalardo et al., 2019). A computational model indicated that COVID-19 vaccines should have an efficacy of at least 70 % to prevent epidemics and 80 % to prevent completely an epidemic without prevention measures such as social distancing (Bartsch et al., 2020). Fig. 2 illustrates the process of in silico trials applications in vaccine development.
Fig. 2.
Applications of in silico trials in vaccine development. Each vaccine development stage can use bioinformatics approaches to process and analyze viral molecular data by omics technologies and clinical and epidemiological data. The predicted data can be further deposited in vaccine databases that are used to refine, reduce, and partially replace steps in vaccine development.
In silico analysis has been an innovative and emergent area, essential and mandatory to accelerate vaccine development, especially in a pandemic scenario, and has contributed to making the COVID-19 vaccine development the fastest so far. However, in silico experiments have the disadvantages of no possible exploration of new side effects or failures never observed before (Pappalardo et al., 2019). Another important challenge is the translation of experimental data to the clinical level. Advances in technology and computational modeling are helping to overcome this translational gap (An et al., 2011). Therefore, combined use of in silico and in vivo experimentation is important as one approach compensates the limitations of the other (Carusi et al., 2012).
4. Vaccines for COVID-19: variety of strategies
There are at least eight types of vaccines against SARS-Cov-2: viral vaccines (attenuated or inactivated), viral vector-based vaccines (replicative or non-replicative), protein-based vaccines (proteins subunits or virus-like particles), and nucleic acid-based vaccines (DNA or RNA) (Callaway, 2020). They generally require two basic components: the antigen(s) and a signal of infection that activates the immune system. Non-viral or inactivated-virus vaccines can provide antigens but usually require adjuvants to induce the signal (Wang et al., 2020). Their advantages and disadvantages are summarized in Table 1 .
Table 1.
Summary of the characteristics of each vaccine platform.
| Platform | Advantages | Disadvantages |
|---|---|---|
| Live-attenuated |
|
|
| Inactivated |
|
|
| Viral-vectors |
|
|
| Protein-based |
|
|
| DNA |
|
|
| RNA |
|
|
| Trained immunity-based |
|
|
The platform, adjuvant(s), form of administration, age, and pre-existing cross-reactive immunity essentially determine the safety of a vaccine. Alum and bacterial-derived proteins as well as replicating live-attenuated virus or vectored vaccines may not be safe for respiratory administration. Possible induction of ADE and high-level secretion of proinflammatory cytokines may also raise safety concerns for the COVID-19 vaccine (Rauch et al., 2018; Jeyanathan et al., 2020).
4.1. Viral vaccines
Viral vaccines use the virus itself, in attenuated or inactivated form, with reduced virulence. This strategy can induce a rapid and strong immune response, but it can be dangerous for immunosuppressed people. Many existing vaccines are made this way, such as those against measles and polio, but require extensive safety testing. Attenuated viruses are conventionally passed through animals or human cells until they acquire mutations that make them less pathogenic. Inactivated viruses become less infective through exposure to chemicals, such as formaldehyde, or to heat. However, it requires the production of many live viruses (Callaway, 2020). The advantages consist of proven technology, triggering of strong immune responses, multivalence, and simple formulation that does not require adjuvant. The attenuated vaccines still have a proven history of good cost-benefit for large-scale manufacturing. The disadvantages are the requirement of dedicated biosecurity level installations, and in the case of attenuated viruses, there is a risk of virulence recovering (Funk et al., 2020).
4.2. Viral vector-based vaccines
Viral vector-based vaccines use a viral structure to introduce SARS-CoV-2 genes into the host. This strategy can increase immunogenicity without an adjuvant and promotes a robust cytotoxic T-cell response to eliminate virus-infected cells. In some vaccines, the vector enters the cells and manages SARS-CoV-2 protein production but cannot replicate because key genes have been disabled. In other vaccines, the vector replicates slowly, carrying SARS-CoV-2 proteins on its surface (Machhi et al., 2021). The weakened measles virus is an example of a viral vector that replicates within cells. These vaccines tend to be safe and induce a strong immune response. Existing immunity against the vector can reduce the effectiveness of the vaccine. The non-replicative viral vector, such as adenovirus, has a long history in gene therapy. An extra administration of the vaccine after an initial dose (booster shot) may be required to induce long-term immunity (Ura et al., 2014). The advantages consist of years of experience in the field of gene therapy, studying the safety and immune response, strong cellular response, and antibody production. Disadvantages include risk of chromosomal integration and oncogenesis, no possibility of used in immunocompromised individuals, pre-existence of antibodies to some vectors, potential for inflammatory adverse events, variable immunogenicity, and significant barriers for manufacturing at a large scale (Strizova et al., 2021).
4.3. Protein-based vaccines
In this type of vaccine, coronavirus proteins are injected directly into the body. Similarly, fragments of shells and proteins that mimic the virus structure can also be used. Most vaccines are based on viral protein subunits with a focus on the spike protein or, more precisely, the RBD. Similar vaccines against SARS-CoV protected monkeys from infection but have not been tested in humans. Those vaccines may require adjuvants, immunostimulant molecules, as well as multiple doses to work well. A vaccine based on virus-like particles or wrapped empty viruses that mimic SARS-CoV-2 structure is not infectious because they lack genetic material. These vaccines can trigger a strong immune response, but they can be difficult to manufacture (Park et al., 2021).
4.4. Nucleic acid-based vaccines
Here, the nucleic acid of SARS-CoV-2 is inserted into human cells, which produce copies of virus proteins that stimulate an immune response. Most of these vaccines encode the spike protein. DNA vaccines use plasmid DNA to efficiently deliver and express SARS-CoV-2 antigens to host cells. Electroporation is a delivery system that creates pores in cell membranes to increase DNA absorption. Currently, there are no DNA vaccines approved for humans. mRNA vaccines encode a SARS-CoV-2 antigen and use a delivery system, such as a liposome, to carry the mRNA molecule into host cells. The advantages of DNA and RNA-based vaccines are that they are safe and easy to develop. To produce them, only the virus genome is needed, not the complete virus. Among the disadvantages is the fact that there is no vaccine licensed with this technology so far (Delany et al., 2014; Strizova et al., 2021).
4.5. The trained immunity-based strategy
Innate immune responses are, after physical and chemical barriers, the line of defense against invading pathogens. Only when this line of defense is dominated by pathogens, the adaptive immune response (T and B cells) is activated. For a long time, it was believed that only adaptive immune system cells could generate immune memory and protect against recurrent infections. This property of the lymphocytes is the basis of vaccine effectiveness against specific infections. However, it has been shown that innate immune cells may also present adaptive characteristics after certain infections or vaccines; a property that is functionally like the construction of immune memory (Netea et al., 2020a).
This process called trained immunity is the reprogramming of the innate immune system cells by external or internal primary stimulations that lead to a higher response at a second immune challenge. It is mediated by an epigenetics reprogramming instead of the genetic recombination from the adaptive immune memory, resulting in metabolic changes and higher responsiveness with long-term effects, but generally reversible and less durable. This cellular reprogramming allows rapid accessibility of transcription factors to the promoter, potentiating regions of pro-inflammatory genes after restimulation, and facilitates gene expression. Increased metabolic activity of the cell provides rapid energy supply and metabolites needed to generate a robust immune response after restimulation (Netea et al., 2020b)
In the category of repurposed vaccines - vaccines already in use for other diseases that may protect against SARS-CoV-2 - is the vaccine based on trained immunity against Bacillus Calmette-Guerin (BCG), developed in the early 1900s as a protection against tuberculosis. Public available data suggests that countries without universal BCG vaccination were more severely affected by the COVID-19 pandemic than countries with universal and established BCG vaccination policies. This vaccine has presented protection in animals by trained immunity against infections of Candida albicans, Schistosoma mansoni, and Mycobacterium tuberculosis (Tribouley et al., 1978; Wout et al., 1992; Kaufmann et al., 2018).
5. Some of the major vaccines for COVID-19 approved for emergency and full use
In Phase 3 clinical trial, the vaccine is tested on thousands of people, who are monitored to evaluate how many will be infected, compared to those who received placebo. The volunteers do not know whether they received a placebo or vaccine. These tests can determine whether the vaccine protects against coronavirus by measuring what is known as the efficacy rate. The World Health Organization (WHO) suggested that efficacy with 50 % must be a minimum criterion for any acceptable COVID-19 vaccine, and that efficacy can be evaluated against disease development, progression of mild or moderate-to-severe disease, and/or shedding/transmission (Hodgson et al., 2020). Phase 3 trials determine vaccine safety, efficacy, and protection. Early or limited approval is based on preliminary evidence that vaccines are safe and effective. Full approval occurs when the regulatory agency reviews the complete test results and the plans to manufacture a vaccine (Funk et al., 2020). Table 2 shows information about the COVID-19 vaccines with phase 3 results approved by countries’ regulatory authorities, according to the COVID-19 Vaccine Tracker (2021) until March 27, 2021.
Table 2.
Latest information on COVID-19 vaccines with approvals and phase 3 clinical trial results.
| Developer country | Developer | Vaccine name | Strategy | Regimen | Efficacy | Storage | Countries approvals | Latest publication |
|---|---|---|---|---|---|---|---|---|
| United States and Germany | Pfizer-Biotech | BNT162b2 | mRNA | Two-dose | 95 % | −80 to -60 °C | 79 approvals (US, UK, EU, AR, AU, others) | Polack et al., 2020 |
| United States and Germany | Janssen | Ad26.COV2.S | Ad26 | One-dose | 74.4 % (US), 64.7 % (LatAm), 52.0 % (ZA) | 2 to 8 °C | 35 approvals (US, EU, CA, ZA, others) | Oliver et al., 2021 |
| United States | Moderna | mRNA-1273 | mRNA | Two-dose | 94.0 % | −25 to -15 °C | 41 approvals (US, UK, EU, IL, others) | Baden et al., 2021 |
| United Kingdom and Sweden | Oxford-AstraZeneca | AZD-1222 | ChAdOx1 | Two-dose | 70.4 % | 2 to 8 °C | 81 approvals (UK, EU, BR, IN, MA, others) | Voysey et al., 2021 |
| Russia | Gamaleya | Sputnik-V | Ad26, Ad5 | Two-dose | 91.6 % | −18 °C | 55 approvals (RU, AR, AE, GN, others) | Logunov et al., 2021 |
| China | CanSino | Ad5-nCoV (Convidecia) |
Ad5 | One-dose | NA | NA | 4 approvals (CN, HU, MX, PK) | Zhu et al., 2020 |
| China | Sinopharm | BBIBP-CorV | Inactivated | Two-dose | NA | 2 to 8 °C | 27 approvals (BH, EG, HU, IQ, PE, CS, AE, others) | Xia et al., 2021 |
| China | Sinovac | CoronaVac | Inactivated | Two-dose | 50.4 % | 2 to 8 °C | 19 approvals (BR, CL, CN, ID, TR, others) | Zhang et al., 2021 |
| India | Bharat Biotech | BBV152 (Covaxin) | Inactivated | Two-dose | NA | 2 to 8 °C | 5 approvals (IN, IR, MA, NP, ZW) | Ella et al., 2021 |
AR: Argentina; AE: United Arab Emirates; AU: Australia; BH: Bahrain; BR: Brazil; CA: Canada; CL: Chile; CN: China; EG: Egypt; EU: European Union; GN: Guinea; HU: Hungary; ID: Indonesia; IL: Israel; IN: India; IR: Iran; IQ: Iraq; LatAm: Latin American; MA: Morocco; MA: Mauritius; MX: Mexico; NP: Nepal; PE: Peru; PK: Pakistan; RU: Russia; TR: Turkey; UK: United Kingdom; CS: Republic of Serbia; ZA: South Africa; ZW: Zimbabwe; LatAm: Latin America.
NA: Not available.
It is important to consider the uncertainty of the results of vaccination that has already started. In other words, if there will be protection in the short or long term. According to Gaebler et al. (2021), levels of antibodies against SARS-CoV-2 spike protein declined over six months following infection, which has raised concerns that immunity to the virus probably declines rapidly. However, levels of memory B cells, which are specific for making antibodies against the spike protein, remained constant. Intestine sample analyzes, after 4 months of infection, revealed that half of the participants had persistent protein or RNA markers for SARS-CoV-2, potentially providing a continued source of stimulation to the immune system.
Mutations can help SARS-CoV-2 to escape the immune response of a subset of infected people. Researchers have identified thousands of mutations in SARS-CoV-2 samples, but the vast majority are unlikely to have an effect on viral biology (Greaney et al., 2021). Antibody responses were tested to spike protein samples and indicated that each sample carried different RBD versions. This spike protein sequence recognizes host cells and is a major target for antibodies. Out of thousands of RBD mutations tested, only a few reduced the antibodies' ability to bind tightly to the spike protein. At least one of these variants is easier transmitted than other forms of the virus currently in wide circulation (Wang et al., 2021). These findings suggest that vaccine-resistant variants might emerge, meaning that COVID-19 vaccines may need an update.
6. COVID-19 vaccine nationalism
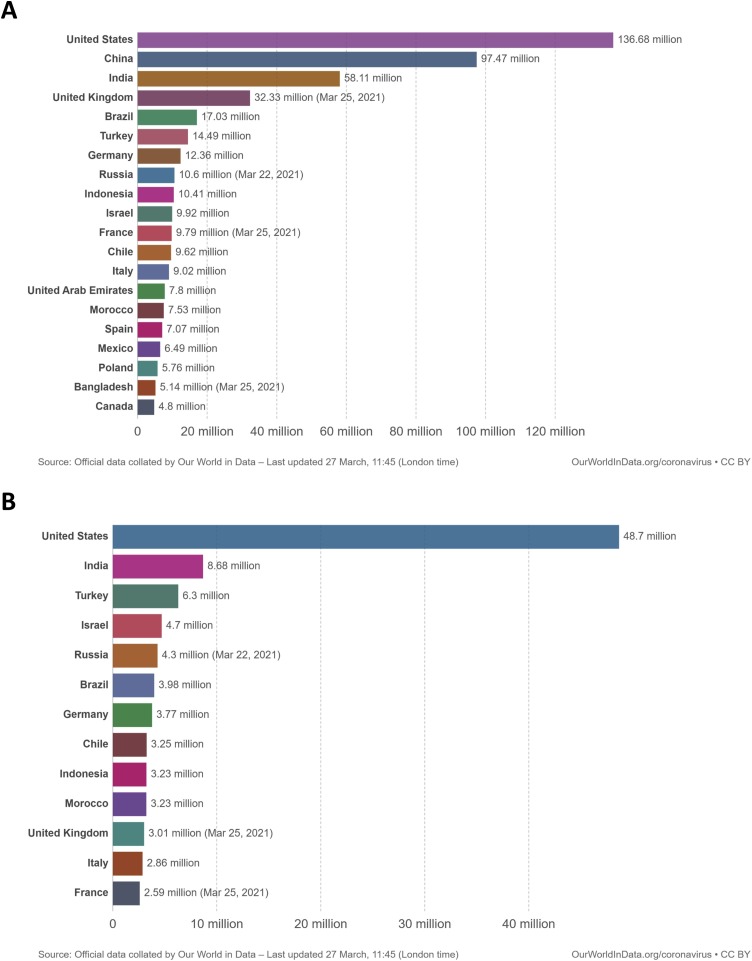
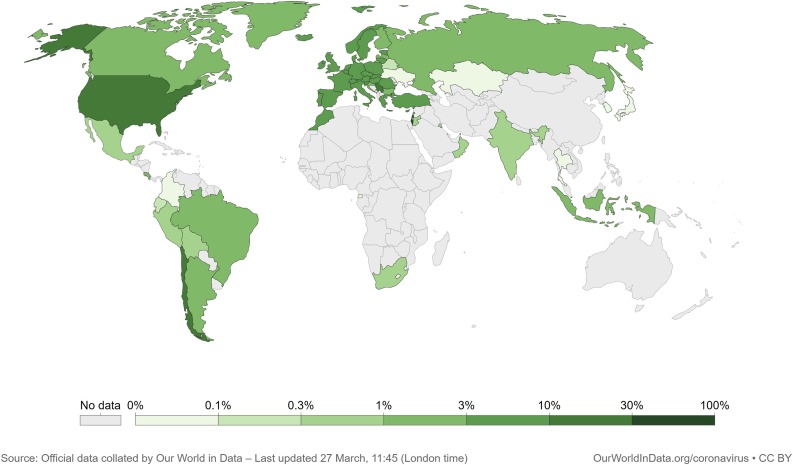
According to Ritchie et al. (2021) (Our World in Data), until March 26, 62 % of the world COVID-19 vaccine doses were administered in four countries: United States, China, India, and United Kingdom (Fig. 3 A). Only the United States accounted for 41 % of the number of people fully vaccinated in the world (Fig. 3B). Those data suggest a great inequality in the global distribution of vaccines. Fig. 4 shows that developed and developing countries lead on the share of the world population fully vaccinated, indicating that they are the most likely to first achieve herd immunity. According to the World Tourism Organization (2021), Europe shared 51 % of the world's international tourist arrivals in 2019 while North America and North-East Asia shared 10 % and 12 %, respectively. In 2020, COVID-19 was responsible for a plunge of 74 % in the number of international tourist arrivals. Therefore, vaccination needs to achieve sufficient equality between all countries for the world to return to a pre-pandemic status, especially if new mutations, capable of circumventing the immunization of current vaccines, arises in the populations of countries with a long-lasting COVID-19 epidemic.
Fig. 3.
Total number vaccine single doses administered (A) and total number of people fully vaccinated (B) for COVID-19 per country. Single doses do not represent the number of people fully vaccinated as many vaccines require two-dose regimen for complete immunization.
Fig. 4.
World map showing the share of the country population who achieved full vaccination. This map shows the percentage of the country population who received all required doses prescribed by the vaccines for complete immunization.
Despite the independent race for the vaccine, the current scenario converges towards possible cooperation to implement a strategy known as heterologous prime-boost against the coronavirus in an attempt to facilitate the logistics of immunization and to increase the immune responses in the process. Most coronavirus vaccines are administered in two doses: an initial dose followed by a booster to stimulate the immune system's memory cells and amplify the immune response. Researchers in the United Kingdom propose to test the immune responses for the combination of its Oxford-AstraZeneca and Pfizer or Sputnik V vaccines. A heterologous prime-boost combination against Ebola was approved in 2020 by European regulators. Experimental HIV vaccines also rely on this strategy (Ledford, 2021). If the production of antibodies and the safety of this strategy are experimentally proven, fighting variants of the coronavirus could be a relevant alternative that could accelerate the vaccination process and reduce the impact of any interruptions in the supply chain.
The Access to COVID-19 Tools – ACT Accelerator is an initiative of WHO with collaborators from around the world to join efforts to combat the pandemic. In an ideal scenario, the proposal was to unite the countries, aiming at the common good, focusing on the diagnosis, therapy, and vaccination for COVID-19. The vaccine pillar of ACT Accelerator is the Global Vaccine Access Instrument for COVID-19: COVAX Facility. Its purpose is to coordinate a global risk-sharing mechanism for joint acquisition, and the equitable distribution of possible vaccines of COVID-19 (WHO, 2020). However, this alliance was not established among the countries. Instead, they started an independent race for the vaccine. The WHO’s director Tedros Adhanom Ghebreyesus warned that national interests in the COVID-19 vaccine could impede global efforts and prolong the pandemic of the new coronavirus. In his words: “Sharing vaccines or sharing other tools actually helps the world to recover together. The economic recovery can be faster and the damage from COVID-19 could be less. Vaccine nationalism is not good, it will not help us. We must seize this moment to come together in national unity and global solidarity to control COVID-19. No country will be safe until we are all safe” (Shields and Burger, 2020).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Bahia State Research Support Foundation.
References
- Agrawal A.S., Tao X., Algaissi A., Garron T., Narayanan K., Peng B.H., Couch R.B., Tseng C.T.K. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccines Immunother. 2016;12:2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G., Bartels J., Vodovotz Y. In silico augmentation of the drug development pipeline: examples from the study of acute inflammation. Drug Dev. Res. 2011;72:187–200. doi: 10.1002/ddr.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami A.A., Payandeh Z., Khalili S., Zakeri A., Bandehpour M. Immunoinformatics: in silico approaches and computational design of a multi-epitope, immunogenic protein. Int. Rev. Immunol. 2019;38(6):307–322. doi: 10.1080/08830185.2019.1657426. [DOI] [PubMed] [Google Scholar]
- Bartsch S.M., O’Shea K.J., Ferguson M.C., Bottazzi M.E., Wedlock P.T., Strych U., McKinnell J.A., Siegmund S.S., Cox S.N., Hotez P.J., Lee B.Y. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am. J. Prev. Med. 2020;59(4):493–503. doi: 10.1016/j.amepre.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M., Baric R.S. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011;85:12201–12215. doi: 10.1128/jvi.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. The race for Coronavirus vaccines. Nature. 2020;580:576–577. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carusi A., Burrage K., Rodríguez B. Bridging experiments, models and simulations: an integrative approach to validation in computational cardiac electrophysiology. Am. J. Physiol. - Hear. Circ. Physiol. 2012 doi: 10.1152/ajpheart.01151.2011. [DOI] [PubMed] [Google Scholar]
- Chukwudozie O.S., Chukwuanukwu R.C., Iroanya O.O., Eze D.M., Duru V.C., Dele-Alimi T.O., Kehinde B.D., Bankole T.T., Obi P.C., Okinedo E.U. Attenuated subcomponent vaccine design targeting the SARS-CoV-2 nucleocapsid phosphoprotein RNA binding domain: in silico analysis. J. Immunol. Res. 2020;2020 doi: 10.1155/2020/2837670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukwudozie O.S., Duru V.C., Ndiribe C.C., Aborode A.T., Oyebanji V.O., Emikpe B.O. The relevance of bioinformatics applications in the discovery of vaccine candidates and potential drugs for COVID-19 treatment. Bioinform. Biol. Insights. 2021;15 doi: 10.1177/11779322211002168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Vaccine Tracker, 2021. https://covid19.trackvaccines.org/ (Accessed 27 March 2021).
- Crooke S.N., Ovsyannikova I.G., Kennedy R.B., Poland G.A. Immunoinformatic identification of B cell and T cell epitopes in the SARS-CoV-2 proteome. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-70864-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016 doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany I., Rappuoli R., De Gregorio E. Vaccines for the 21st century. EMBO Mol. Med. 2014;6(6):708–720. doi: 10.1002/emmm.201403876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ella R., Vadrevu K.M., Jogdand H., Prasad S., Reddy S., Sarangi V., Ganneru B., Sapkal G., Yadav P., Abraham P., Panda S., Gupta N., Reddy P., Verma S., Kumar Rai S., Singh C., Redkar S.V., Gillurkar C.S., Kushwaha J.S., Mohapatra S., Rao V., Guleria R., Ella K., Bhargava B. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn O.J., Beatty P.L. Cancer immunoprevention. Curr. Opin. Immunol. 2016 doi: 10.1016/j.coi.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C.D., Laferrière C., Ardakani A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front. Pharmacol. 2020 doi: 10.3389/fphar.2020.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., Cho A., Jankovic M., Schaefer-Babajew D., Oliveira T.Y., Cipolla M., Viant C., Barnes C.O., Bram Y., Breton G., Hägglöf T., Mendoza P., Hurley A., Turroja M., Gordon K., Millard K.G., Ramos V., Schmidt F., Weisblum Y., Jha D., Tankelevich M., Martinez-Delgado G., Yee J., Patel R., Dizon J., Unson-O’Brien C., Shimeliovich I., Robbiani D.F., Zhao Z., Gazumyan A., Schwartz R.E., Hatziioannou T., Bjorkman P.J., Mehandru S., Bieniasz P.D., Caskey M., Nussenzweig M.C. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021:1–10. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouglas D., Thanh Le T., Henderson K., Kaloudis A., Danielsen T., Hammersland N.C., Robinson J.M., Heaton P.M., Røttingen J.A. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob. Heal. 2018;6:e1386–e1396. doi: 10.1016/S2214-109X(18)30346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney A.J., Loes A.N., Crawford K.H., Starr T.N., Malone K.D., Chu H.Y., Bloom J.D. Comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human serum antibodies. bioRxiv 2020. 2021;12 doi: 10.1101/2020.12.31.425021. 31.425021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Smith D.M., Crotty S., Sette A., Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Jadi R.S., Marrama D., Silva A.M. De, Frazier A., Carlin A.F., Greenbaum J.A. Targets of t cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals ll article targets of t cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Xiang Z. Databases and in silico tools for vaccine design. Methods mol. Biol. (Clifton, N.J.) 2013;993:115–127. doi: 10.1007/978-1-62703-342-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson S.H., Mansatta K., Mallett G., Harris V., Emary K.R.W., Pollard A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2020 doi: 10.1016/s1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann E., Sanz J., Dunn J.L., Khan N., Mendonça L.E., Pacis A., Tzelepis F., Pernet E., Dumaine A., Grenier J.C., Mailhot-Léonard F., Ahmed E., Belle J., Besla R., Mazer B., King I.L., Nijnik A., Robbins C.S., Barreiro L.B., Divangahi M. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell. 2018;172(1-2):176–190.e19. doi: 10.1016/j.cell.2017.12.031. [DOI] [PubMed] [Google Scholar]
- Lafuente E., Reche P. Prediction of MHC-Peptide binding: a systematic and comprehensive overview. Curr. Pharm. Des. 2009;15:3209–3220. doi: 10.2174/138161209789105162. [DOI] [PubMed] [Google Scholar]
- Ledford H. Could mixing COVID vaccines boost immune response? Nature. 2021 doi: 10.1038/d41586-021-00315-5. [DOI] [PubMed] [Google Scholar]
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., Botikov A.G., Izhaeva F.M., Popova O., Ozharovskaya T.A., Esmagambetov I.B., Favorskaya I.A., Zrelkin D.I., Voronina D.V., Shcherbinin D.N., Semikhin A.S., Simakova Y.V., Tokarskaya E.A., Egorova D.A., Shmarov M.M., Nikitenko N.A., Gushchin V.A., Smolyarchuk E.A., Zyryanov S.K., Borisevich S.V., Naroditsky B.S., Gintsburg A.L. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021 doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020 doi: 10.1056/nejmp2005630. [DOI] [PubMed] [Google Scholar]
- Machhi J., Shahjin F., Das Srijanee, Patel M., Abdelmoaty M.M., Cohen J.D., Singh P.A., Baldi A., Bajwa N., Kumar R., Vora L.K., Patel T.A., Oleynikov M.D., Soni D., Yeapuri P., Mukadam I., Chakraborty R., Saksena C.G., Herskovitz J., Hasan M., Oupicky D., Das Suvarthi, Donnelly R.F., Hettie K.S., Chang L., Gendelman H.E., Kevadiya B.D. Nanocarrier vaccines for SARS-CoV-2. Adv. Drug Deliv. Rev. 2021 doi: 10.1016/j.addr.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- María R.R., Arturo C.J., Alicia J.A., Paulina M.G., Gerardo A.O. Vaccines. InTech; 2017. The impact of bioinformatics on vaccine design and development. [DOI] [Google Scholar]
- Mercurio I., Tragni V., Busto F., et al. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: from conformational changes to novel neutralizing antibodies. Cell. Mol. Life Sci. 2021;78:1501–1522. doi: 10.1007/s00018-020-03580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Domínguez-Andrés Jorge, Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., Meer J.W.Mvander, Mhlanga M.M., Mulder W.J.M., Riksen N.P., Schlitzer A., Schultze J.L., Benn C.S., Sun J.C., Xavier R.J., Latz E. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Giamarellos-Bourboulis E.J., Domínguez-Andrés J., Curtis N., van Crevel R., van de Veerdonk F.L., Bonten M. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell. 2020;181(5):969–977. doi: 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.E., Gargano J.W., Scobie H., Wallace M., Hadler S.C., Leung J., Blain A.E., McClung N., Campos-Outcalt D., Morgan R.L., Mbaeyi S., MacNeil J., Romero J.R., Keipp Talbot H., Lee G.M., Bell B.P., Dooling K. Morbidity and mortality weekly report the advisory committee on immunization practices’ interim recommendation for use of Janssen COVID-19 vaccine-united states, February 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70(9):329–332. doi: 10.15585/mmwr.mm7009e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo F., Pennisi M., Castiglione F., Motta S. Vaccine protocols optimization: in silico experiences. Biotechnol. Adv. 2010 doi: 10.1016/j.biotechadv.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Pappalardo F., Flower D., Russo G., Pennisi M., Motta S. Computational modelling approaches to vaccinology. Pharmacol. Res. 2015 doi: 10.1016/j.phrs.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Pappalardo F., Fichera E., Paparone N., Lombardo A., Pennisi M., Russo G., Leotta M., Pedretti A., De Fiore F., Motta S. A computational model to predict the immune system activation by citrus-derived vaccine adjuvants. Bioinformatics. 2016;32:2672–2680. doi: 10.1093/bioinformatics/btw293. [DOI] [PubMed] [Google Scholar]
- Pappalardo F., Russo G., Tshinanu F.M., Viceconti M. In silico clinical trials: concepts and early adoptions. Brief. Bioinformatics. 2019;20(5):1699–1708. doi: 10.1093/bib/bby043. [DOI] [PubMed] [Google Scholar]
- Park K.S., Sun X., Aikins M.E., Moon J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021;169:137–151. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pacific J. Allergy Immunol. 2020 doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- Quinlan B.D., Mou H., Zhang L., Guo Y., He W., Ojha A., Parcells M.S., Luo G., Li W., Zhong G., Choe H., Farzan M. The SARS-CoV-2 receptor-binding domain elicits a potent neutralizing response without antibody-dependent enhancement. bioRxiv. 2020 doi: 10.1101/2020.04.10.036418. [DOI] [Google Scholar]
- Rauch S., Jasny E., Schmidt K.E., Petsch B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018 doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie H., Ortiz-Ospina E., Beltekian D., Mathieu E., Hasell J., Macdonald B., Giattino C., Roser M. 2021. Coronavirus (COVID-19) Vaccinations. Our World in Data. https://ourworldindata.org/covid-vaccinations (Accessed 03 February 2021) [Google Scholar]
- Roncati L., Nasillo V., Lusenti B., Riva G. Signals of Th2 immune response from COVID-19 patients requiring intensive care. Ann. Hematol. 2020 doi: 10.1007/s00277-020-04066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper R.L., Rehm K.E. SARS vaccines: where are we? Expert Rev. Vaccines. 2009 doi: 10.1586/erv.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo G., Pennisi M., Fichera E., Motta S., Raciti G., Viceconti M., Pappalardo F. In silico trial to test COVID-19 candidate vaccines: a case study with UISS platform. BMC Bioinformatics. 2020;21:527. doi: 10.1186/s12859-020-03872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields M., Burger L. 2020. Global Recovery Could Be Faster If COVID Vaccine Made Available to All: WHO Chief. Reuters.https://www.reuters.com/article/us-health-coronavirus-who-idUSKCN25229K (Accessed 03 February 2021) [Google Scholar]
- Smatti M.K., Al Thani A.A., Yassine H.M. Viral-induced enhanced disease illness. Front. Microbiol. 2018;9:2991. doi: 10.3389/fmicb.2018.02991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., Zhu H., Zhao W., Han Y., Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019 doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizova Z., Smetanova J., Bartunkova J., Milota T. Principles and challenges in anti-COVID-19 vaccine development. Int. Arch. Allergy Immunol. 2021 doi: 10.1159/000514225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh Le T., Andreadakis Z., Kumar A., Gómez Román R., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020 doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- Tribouley J., Tribouley-Duret J., Appriou M. 1978. Effect of Bacillus callmette Guerin (BCG) on the Receptivity of Nude Mice to Schistosoma mansoni] undefined. [PubMed] [Google Scholar]
- Tseng C.-T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L., Peters C.J., Couch R.B. Correction: immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7 doi: 10.1371/annotation/2965cfae-b77d-4014-8b7b-236e01a35492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah M.A., Sarkar B., Islam S.S. Exploiting the reverse vaccinology approach to design novel subunit vaccines against Ebola virus. Immunobiology. 2020;225 doi: 10.1016/j.imbio.2020.151949. [DOI] [PubMed] [Google Scholar]
- Ura T., Okuda K., Shimada M. Developments in viral vector-based vaccines. Vaccines. 2014;2(3):624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O'Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q., He L., Chen Y., Wu J., Shi Z., Zhou Y., Du L., Li F. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 2019;94 doi: 10.1128/jvi.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Peng Y., Xu H., Cui Z., Williams R.O. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech. 2020;21:1–12. doi: 10.1208/s12249-020-01744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., Schaefer-Babajew D., Cipolla M., Gaebler C., Lieberman J.A., Oliveira T.Y., Yang Z., Abernathy M.E., Huey-Tubman K.E., Hurley A., Turroja M., West K.A., Gordon K., Millard K.G., Ramos V., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;10 doi: 10.1038/s41586-021-03324-6. 1038/s41586-021-03324-03326. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO – World Health Organization, 2020. https://www.who.int/initiatives/act-accelerator (Accessed 03 February 2021).
- World Tourism Organization . 2021. Global and Regional Tourism Performance.https://www.unwto.org/global-and-regional-tourism-performance (Accessed 03 February 2021) [Google Scholar]
- Wout J.W., Poell R., Furth R. The role of BCG/PPD-Activated macrophages in resistance against systemic candidiasis in mice. Scand. J. Immunol. 1992;36:713–720. doi: 10.1111/j.1365-3083.1992.tb03132.x. [DOI] [PubMed] [Google Scholar]
- Xia S., Zhang Y., Wang Y., Wang Hui, Yang Yunkai, Gao G.F., Tan W., Wu G., Xu M., Lou Z., Huang W., Xu W., Huang B., Wang Huijuan, Wang Wei, Zhang W., Li N., Xie Z., Ding L., You W., Zhao Y., Yang Xuqin, Liu Y., Wang Q., Huang L., Yang Yongli, Xu G., Luo B., Wang Wenling, Liu P., Guo W., Yang Xiaoming. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhou X., Zhu C., Feng F., Qiu Y., Feng J., Jia Q., Song Q., Zhu B., Wang J. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.12.20035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zeng G., Pan H., Li C., Hu Yaling, Chu K., Han W., Chen Z., Tang R., Yin W., Chen X., Hu Yuansheng, Liu X., Jiang C., Li J., Yang M., Song Y., Wang X., Gao Q., Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2020;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Ge S., Liu L., Zhang J., Xia N., Zhang Z. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. medRxiv. 2020 doi: 10.1101/2020.03.02.20030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., Wu S.P., Wang Z., Wu X.H., Xu J.J., Zhang Z., Jia S.Y., Wang B.Sen, Hu Y., Liu J.J., Zhang J., Qian X.A., Li Q., Pan H.X., Jiang H.D., Deng P., Gou J.B., Wang X.W., Wang X.H., Chen W. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]