Abstract
We reviewed the literature on cerebrospinal fluid (CSF) studies in patients who had a seizure in the setting of COVID-19 infection to evaluate for evidence of viral neuroinvasion. We performed a systematic review of Medline and Embase to identify publications that reported one or more patients with COVID-19 who had a seizure and had CSF testing preformed. The search ranged from December 1st 2019 to November 18th 2020. We identified 56 publications which described 69 unique patients who met our inclusion criteria. Of the 54 patients whose past medical history was provided, 2 (4%) had epilepsy and 1 (2%) had a prior seizure in the setting of hyperglycemia, but the remaining 51 (94%) had no history of seizures. Seizure was the initial symptom of COVID-19 for 15 (22%) patients. There were 26 (40%) patients who developed status epilepticus. SARS-CoV-2 PCR testing was performed in the CSF for 45 patients; 6 (13%) had a positive CSF SARS-CoV-2 PCR, only 1 (17%) of whom had status epilepticus. The cycle thresholds were not reported. Evaluation for CSF SARS-CoV-2 antibodies (directly or indirectly, via testing for CSF oligoclonal bands or immunoglobulins) was performed in 26 patients, only 2 (8%) of whom had evidence of intrathecal antibody synthesis. Of the 11 patients who had CSF autoimmune antibody panels tested, 1 had NMDA antibodies and 1 had Caspr-2 antibodies. Detection of SARS-CoV-2 in the CSF of patients with seizures who have COVID-19 is uncommon. Our review suggests that seizures in this patient population are not likely due to direct viral invasion of the brain.
Keywords: COVID-19, Epilepsy, Seizure, Status epilepticus, Cerebrospinal fluid
1. Introduction
Since the onset of the pandemic, there have been innumerable reports of neurologic manifestations of COVID-19, the most common of which are anosmia, ageusia, dizziness, encephalopathy, and headache [19], [20], [21]. Seizures have also been described in patients with COVID-19, but studies of patients with COVID-19 who had neurological events found that only 0.51.6% of patients had seizures [19,20]. However, given the magnitude of the pandemic and the number of people infected worldwide, this relatively rare neurologic manifestation has been reported a myriad of times.
There have been several mechanisms proposed to explain the occurrence of seizures in COVID-19 patients. While some suggest seizures in this population may be related to hypoxia or proinflammatory cytokines, it has also been hypothesized that seizures may be the result of viral neuroinvasion [22], [23], [24].
Review of cerebrospinal fluid (CSF) is one way to evaluate for viral neuroinvasion. There have been multiple reports of electroencephalography (EEG) findings in patients with COVID-19 [3]; however, the CSF findings in COVID-19 patients with seizures have not been systematically examined. Herein, we systematically review the CSF results in published case reports and case series of patients with COVID-19 who had seizures to evaluate for evidence of viral neuroinvasion.
2. Methods
As part of a larger review of published reports of patients with COVID-19 who had neurological symptoms prompting CSF testing, we identified 1,182 unique publications from December 1, 2019 and November 18, 2020 by searching Medline and Embase using the population search terms “COVID-19” or “SARS-CoV-2” and the intervention search terms “cerebrospinal fluid” or “csf” or “spinal puncture” or “spinal tap” or “lumbar puncture” or “meningitis” or “encephalitis” or “encephalomyelitis” or “seizure” or “encephalopathy” or “myelitis” or “Guillain Barre” or “polyradiculitis” or “Miller Fisher” [25]. Two neurologists (AL and KM) independently screened these references using Covidence Systematic Review Software and performed full-text review to identify documents that were written in English and provided details on at least one unique patient with COVID-19 diagnosed based on positive SARS-CoV-2 PCR or serologic testing who had a neurological symptom and CSF testing [26]. We excluded publications that described patients who had an acute neurological diagnosis that could potentially impact CSF results (such as subarachnoid hemorrhage or another intracranial infection) and added additional relevant publications based on review of references and other sources. This resulted in identification of 242 publications.
Two neurologists (EC and AL) reviewed all 242 publications to identify publications that described a patient with COVID-19 who had CSF obtained and had a seizure. This resulted in identification of 56 publications. Cases were reviewed and organized based on CSF findings. If a patient had more than one lumbar puncture, we included the results from the CSF that was acquired closest to the time of the first reported seizure. CSF results were converted to a common unit to facilitate comparison. This search was performed in accordance with PRISMA guidelines (Fig. 1 ).
Fig. 1.
Publication selection.
3. Results
Our systematic review identified 69 unique patients who had a seizure in the setting of COVID-19 and had CSF studies available for review (Supplemental Table 1) [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18],[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64]]. Patients ranged in age from 6-weeks to 96-years-old. The majority of patients had no history of seizures, and had moderate or severe COVID-19 symptoms (based on WHO guidelines [66]). Seizures were the initial symptom of COVID-19 in 15/69 (22%) patients. The majority of patients (42/69, 61%) had generalized seizures, and 26/69 (40%) patients had status epilepticus (clinical or subclinical). The time between seizure onset and lumbar puncture was specified for 15/69 (22%) patients (median of 1 day with interquartile range of -3- 4). Although the outcome for 13/69 (19%) patients remained unknown at the time of their respective article publication, the majority of patients recovered and were discharged from the hospital (43/56, 77%), but 13 (23%) died (Table 1 ).
Table 1.
Patient characteristics.
| Patient age range | 6-weeks to 96-years-old |
|---|---|
| History of seizure, n= 54 | |
| Reported history of epilepsy | 2 (4%) |
| Prior seizure not related to underlying epilepsy | 1 (2%) |
| No prior seizure history | 51 (94%) |
| COVID severity of disease, n = 44 | |
| Mild COVID symptoms | 8 (18%) |
| Moderate COVID symptoms | 18 (45%) |
| Severe COVID symptoms | 18 (45%) |
| Number of days between onset of COVID-19 symptoms and seizure n = 58 | 0–52 days [median 4 days, interquartile range (IQR) 0–10 days] |
| Seizure as the presenting symptom, n = 69 | 15 (22%) |
| Seizure semiology, n = 69 | |
| Generalized seizure | 42 (61%) |
| Focal seizure | 8 (12%) |
| Generalized and focal seizures | 2 (3%) |
| Unspecified semiology | 17 (26%) |
| EEG findings, n = 41 | |
| Normal | 5 (12%) |
| Abnormal, but without seizure or epileptiform discharges | 16 (39%) |
| Abnormal with epileptiform discharges or other patterns at high risk for seizure, but without seizure | 9 (22%) |
| Seizure | 11 (27%) |
| Status epilepticus (clinical or subclinical), n = 69 | 26 (40%) |
| Neuroimaging results, n = 65 | |
| Normal | 24 (37%) |
| Abnormal, but with no discrete lesion | 9 (14%) |
| Abnormal, with a discrete lesion | 32 (49%) |
| Outcome, n = 69 | |
| Recovered | 43 (62%) |
| Dead | 13 (19%) |
| Unknown | 13 (19%) |
3.1. CSF pleocytosis
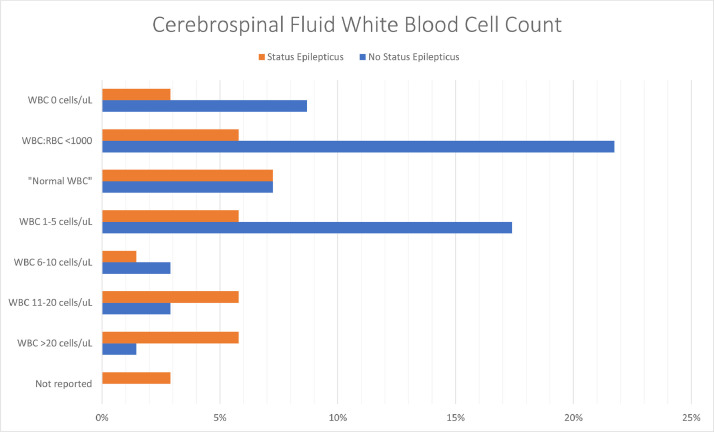
Though the CSF red blood cell count (RBC) was reported for only 19/69 (28%) patients [[2], [3], [4],13,16,18,29,31,35,41,42,45,[47], [48], [49],[53], [54], [55],67], the CSF white blood cell (WBC) count was reported for 67/69 patients (97%). The CSF WBC count ranged from 0 to 350 cells/µL; one-third of patients (22/67 patients, 33%) had pleocytosis (CSF WBC count of >5 cells/µL) [6,[8], [9], [10],[14], [15], [16],30,31,[34], [35], [36],38,42,44,48,50,53,54,56,58], but the remainder of patients had a normal CSF WBC count. Only 8/22 (36%) patients with pleocytosis also had a CSF RBC count reported, all of whom had a CSF WBC:RBC ratio >1:500 [16,30,31,35,42,48,50,53,54]. Of the 24 patients who were in status epilepticus at some point during their clinical course whose CSF WBC count was reported, 10 (42%) had pleocytosis; the CSF WBC count ranged from 0 to 76 cells/µL (Fig. 2 ) [6,8,14,15,34,35,44,54,56].
Fig. 2.
CSF white blood cell count results.
The patient with the highest CSF WBC count (350 cells/µL) was reported by Rebeiz et al; the CSF RBC count was not provided, but the CSF protein was 297 mg/dL [58]. The patient was a man in his 30s who presented with fever and encephalopathy, then developed seizures a few weeks later and was found to have diffuse cortical, basal ganglia, and corpus callosum diffusion weighted imaging (DWI) changes on his MRI, felt to be consistent with encephalitis, and ultimately progressed to brain death.
3.2. CSF Protein
The CSF protein was reported for 64/69 (93%) patients [[1], [2], [3], [4], [5], [6],[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18],[27], [28], [29], [30], [31], [32], [33], [34], [35],[37], [38], [39], [40], [41], [42],[44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55],[57], [58], [59],[61], [62], [63], [64], [65]] and ranged from 15 to 541 mg/dL. There were 27 patients (39%) with CSF protein >60 mg/dL [3,4,9,11,[13], [14], [15],17,18,27,30,34,35,37,39,40,42,43,[47], [48], [49], [50],54,57,58,63]. Half of the patients (13/26, 48%) who were in status epilepticus at some point during their clinical course had a CSF protein >60 mg/dL (Fig. 3 ) [3,4,8,11,[13], [14], [15],27,34,35,37,40,44,54,57].
Fig. 3.
CSF protein results.
The patient with the highest CSF protein (541 mg/dL) was a 33-year-old woman with generalized status epilepticus reported by Elkady et al [8]. Her CSF was also notable for a WBC count of 26 cells/µL and neuroimaging showed diffuse brain swelling with bilateral hemorrhagic thalamic and cerebellar lesions, which were attributed to acute necrotizing encephalopathy. Despite treatment with high-dose steroids, she had a cardiac arrest five days after admission and died.
3.3. CSF SARS-CoV 2 PCR
SARS-CoV-2 PCR testing was performed in the CSF for 45/69 (65%) patients [[3], [4], [5], [6], [7],[10], [11], [12], [13], [14], [15], [16],18,28,[31], [32], [33], [34], [35], [36], [37],39,41,42,45,[50], [51], [52], [53],55,56,59,60,63,64]. Of these, 6/45 (13%) had a positive CSF SARS-CoV-2 PCR, 1 (17%) of whom was in status epilepticus at some point during their clinical course [16,42,50,55,56,59,63]. The cycle threshold (the number of amplification cycles required for the target gene to exceed the threshold, which is inversely related to viral load) was not reported for any of these patients. Of the four patients with a positive CSF SARS-CoV-2 PCR whose severity of COVID-19 was noted, 2 (50%) had moderate COVID-19. EEG results were only provided for two patients with a positive CSF SARS-CoV-2 PCR; one had a normal EEG and the other had an abnormal EEG, but no epileptiform activity. The majority (4/6, 66%) of patients with a positive CSF SARS-CoV-2 PCR had abnormal neuroimaging (Table 2 ).
Table 2.
Patients with Positive SARS-CoV-2 CSF PCR.
| Author | Age/Sex | History of epilepsy | Severity of COVID-19 | Number of days between onset of illness and seizure | Status epilepticus | EEG findings | CSF RBC count (cells/µL) | CSF WBC count (cells/µL) | CSF protein (mg/dL) | SARS-CoV-2 CSF PCR | CSF SARS-CoV-2 Ab | CSF Oligoclonal Bands | CSF autoimmune antibody panel | Imaging findings | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duong et al. [28] and Huang et al [42] | 41F | N | Mild | NR | N | Abnormal, but w/o epileptiform activity | 65 | 70 | 100 | + | NR | NR | NR | Normal CT | Recovered |
| Mirzaee et al [55] | 12M | N | NR | 0 | N | NR | 100 | 0 | 21 | + | NR | NR | NR | Acute strokes in the left middle cerebral artery territory with associated microhemorrhages | Recovered |
| Moriguchi et al [56] | 24M | N | Moderate | 9 | Y | NR | NR | 12 | NR | + | NR | NR | NR | Hyperintense signal along the wall of the lateral ventricle and in the temporal lobe, hippocampal atrophy, pan-paranasal sinusitis | Unknown |
| Rifino et al [59] | 60M | N | NR | 0 | N | Normal | NR | Normal | Normal | + | NR | NR | NR | Normal CT | Recovered |
| Sattar et al [16] | 44M | N | Severe | 27 | N | NR | 1685 | 11 | 39 | + | NR | - | - | Bifrontal hyperintensities | Recovered |
| Westhoff et al [63] | 69M | N | Moderate | 10 | N | NR | NR | 1 | 110 | + | NR | NR | NR | Meningeal enhancement and white matter edema without mass effect | Recovered |
3.4. CSF SARS-CoV-2 antibody testing
SARS-CoV-2 antibody testing was reported in the CSF for 2/69 (3%) patients [2,17]; both were positive.
Song et al. evaluated CSF and serum SARS-CoV-2 antibodies in a 60-year-old man who had a seizure [17]. Though details of his clinical course were not available for review, his CSF analysis revealed a WBC count of 2 cells/µL, protein of 66 mg/dL, and positive SARS-CoV-2 antibodies. A SARS-CoV-2 epitope Luminex panel was used to compared CSF and serum SARS-CoV-2 antibodies. This demonstrated there were unique antibodies specific to different regions of the spike protein in both compartments. Though his individual CSF biomarkers were not provided, they were included in a cumulative analysis of six patients with COVID-19 who had CSF testing, and when compared to controls, the patients with COVID-19 had elevated CSF Interleukin-8 (IL-8), Interleukin-1β (IL-1β), Interleukin-12 (IL-12) and Fibroblast Growth Factor-2 (FGF-2). These biomarkers were all normal in plasma, prompting the authors to conclude patients with COVID-19 have an immunologically distinct response in the central nervous system. Clinical outcome was not reported.
Benameur et al reported a 34-year-old man with hypertension who presented with fever, shortness of breath, and cough [2]. Nasopharyngeal SARS-CoV-2 PCR was positive. His respiratory status declined and he required intubation, after which he developed encephalopathy with myoclonus on hospital day nine. EEG suggested that the myoclonus was “seizure-related.” MRI on hospital day 15 showed a non-enhancing hyperintense lesion within the splenium of the corpus callosum. CSF analysis revealed an elevated opening pressure of 48 cm H2O, no pleocytosis, and mildly increased protein. CSF SARS-CoV-2 PCR was negative, but CSF SARS-CoV-2 antibody testing was positive. No further testing was performed to distinguish intrathecal antibody synthesis from transudation of antibodies, or antibody-secreting cells, into the CSF via a damaged blood-brain barrier. Several CSF biomarkers were sent; while Interleukin-10 (IL-10) was normal, Interleukin-6 (IL-6), IL-8, Interferon Gamma Induced Protein-10 (IP-10), Tumor Necrosis Factor-α (TNF-α), and IL-1β were all elevated compared to healthy controls. Serum inflammatory markers were not sent. Clinical outcome was not reported.
3.5. Oligoclonal bands
CSF oligoclonal bands were tested in 22/69 (31%) patients [1,[4], [5], [6],[8], [9], [10], [11], [12], [13], [14], [15], [16],18,28]. One patient had oligoclonal bands specific to the CSF [10], and one patient had positive oligoclonal bands in the CSF, but it was not delineated if these were matched or unmatched in the serum [6].
Guilmot et al reported an 80-year-old patient who presented with two weeks of asthenia and weight loss, followed by paroxysmal episodes of dysarthria, a generalized tonic-clonic seizure, and neuropsychiatric symptoms [10]. He had no respiratory symptoms. MRI was unremarkable, and EEG demonstrated generalized slowing. CSF analysis was notable for a WBC count of 9 cells/µL, protein of 46 mg/dL, CSF-specific IgG oligoclonal bands, and positive anti-contactin-associated-protein 2 (Caspr2) IgG antibodies, which were also present in the serum. The authors did not mention whether an oncological workup was performed. The patient was treated with levetiracetam, methylprednisolone, and plasmapheresis, and had no additional seizures.
Dono et al reported an 81-year-old man with hypertension who presented with fever, dyspnea, and cough for seven days [6]. On day 14, he became mildly confused. EEG at that time showed no epileptiform discharges, but on day 16, his mental status worsened, and he developed myoclonic jerking; EEG was consistent with status epilepticus, and he required aggressive escalation of anti-seizure medications including a midazolam infusion. On day 23, MRI showed multiple non-enhancing hyperintense areas in the bilateral parietal cortex, left temporal cortex, and right cingulate cortex. CSF examination showed a WBC count of 26 cells/µL, normal glucose, protein of 47 mg/dL, and positive oligoclonal bands. However, it was not noted whether these bands were matched in the serum. He had a cardiac arrest and died 45 days after admission.
3.6. CSF Immunoglobulins
Of the 60 patients who had seizures in the setting of COVID-19 and did not have a positive CSF SARS-CoV-2 PCR, CSF SARS-CoV-2 antibodies or CSF-specific oligoclonal bands, 6 had CSF immunoglobulins measured, only 1 of which was elevated [2,4,7,[12], [13], [14],45,58]. Ghosh et al reported a 44-year-old woman who developed fever, myalgias, cough, and hypogeusia 10 days prior to hospital admission [9]. She became encephalopathic 7 days after symptom onset, then presented to the hospital 3 days later after she had a generalized tonic-clonic seizure. MRI brain revealed a frontoparietal hyperintense lesion with surrounding edema and a focus of hemorrhage. Her CSF analysis was notable for pleocytosis with WBC count of 20 cells/µL, normal protein, and an elevated IgG index (no value provided), with no evidence of oligoclonal bands. The authors did not conclude these findings were consistent with intrathecal antibody synthesis.
3.7. Autoimmune antibodies
In addition to the aforementioned patient reported by Guilmot et al who had Caspr2 IgG antibodies in the CSF, there were 10 other patients (11/69; 16%) whose CSF results included an autoimmune antibody panel [3,4,6,10,11,[13], [14], [15], [16],18,34]. Though the majority (9/11, 82%) of these were negative, a patient described by Monti et al had N-Methyl-D-aspartate (NMDA) antibodies in the CSF [14].
The patient was a 50-year-old man who had new psychiatric symptoms for four days then began having focal motor seizures with impaired awareness. He went on to develop refractory status epilepticus which lasted for 47 days. Initial CSF analysis was notable for WBC count of 76 cells/µL and slightly elevated protein. Repeat lumbar puncture one week later showed CSF WBC count of 25 cells/µL and normal protein. Oligoclonal bands were present, but matched in serum, and IgG index was normal. NMDA antibodies were present in the CSF, but not serum. CSF and serum IL-6 were elevated (4.58 pg/mL with normal not reported and 52 pg/mL with reported normal <10 pg/mL, respectively). CSF IL-8 was also elevated (40.1 pg/mL; normal not reported), but TNF-α and IL-1β were normal. One month later, the CSF WBC count was 16 cells/µL and the protein was 105 mg/dL, oligoclonal bands were no longer present, the IgG index was elevated (1.45 with reported normal <0.7) and NMDA antibodies were still present in the CSF, but not the serum. CSF and serum IL-6 increased further to 5.75 pg/mL and 206 pg/mL, respectively, as did CSF IL-8 (744 pg/mL). Oncologic workup was unrevealing. After a four-month hospital course, during which he was treated with plasma-exchange and two rounds of intravenous immunoglobulin, he was discharged in good condition.
3.8. Other CSF biomarkers
In addition to the aforementioned patients described by Song et al, Benameur et al, and Monti et al, CSF biomarkers were tested in the CSF of 3 or 4 other patients (Keller at al described 5 patients with COVID-19 who had neurological symptoms, only 1 of whom had seizures, and reported 4/5 had elevated CSF IL-6, but it is not clear if the patient with seizures was amongst those with elevated CSF IL-6) [2,4,7,[12], [13], [14],45].
Le Guennec et al and Delorme et al described a 69-year-old man who developed status epilepticus following five days of cough, fever, and anosmia [4,13]. CSF analysis was notable for a WBC count of 1 cell/µL, protein of 66 mg/dL, and normal glucose. IL-6 was elevated in both the CSF and serum (16 pg/mL in CSF with reported normal <2.5 pg/mL and 28.8 pg/mL in serum with reported normal <6.5 pg/mL). Tau was also markedly elevated (>2,000 pg/mL, normal range 150–450 pg/ml); the authors suggested that this could be secondary to a pre-existing neurodegenerative process but may also reflect neuronal damage in the setting of encephalitis/status epilepticus. Several additional CSF biomarkers were sent, including IL-10, which was normal; β-amyloid, which was low (570 pg/ml, normal range 650–2000 pg/ml); and Interferon-α (INF-α), which was negative. The patient improved following treatment with intravenous immunoglobulin and was successfully extubated one week after admission.
Farhadian et al reported a 78-year-old kidney transplant recipient on tacrolimus and mycophenolate mofetil who presented with 3 days of moderate COVID-19 symptoms and confusion, then had a generalized tonic-clonic seizure on the day of admission [45]. CSF analysis revealed RBC count of 350 cells/µL, WBC count of 1 cells/µL, and normal protein. IL-6, IL-8, Interleukin-17A (IL-17A), and IP-10 were elevated in both the serum and CSF when compared to control patients. IL-10 was normal in both the serum and CSF. Monocyte Chemoattractant Protein-1 (MCP-1) was uniquely elevated in the CSF. MRI brain revealed generalized atrophy and patchy subcortical white matter disease. She was treated with tocilizumab and hydroxychloroquine and was discharged after one month.
Eden et al reported a 60-year-old man with cardiac disease, diabetes mellitus, hypertension, and obesity, who developed severe COVID-19 requiring intubation [7]. He later had multiple seizures, but the semiology was not noted, and it was not mentioned if he met criteria for status epilepticus. NCHCT was normal, and EEG was remarkable only for generalized background slowing. CSF analysis revealed a normal WBC count (<3cells/µL), negative SARS-CoV-2 PCR, and normal IgG index, but elevated Neopterin (>40 nmol/L; reported normal 5.8 nmol/L) and β2-microglobulin (>2.5 mg/L; reported normal 1.8 mg/L). Serum Neopterin and β2-microglobulin were also elevated. The authors attributed these findings to indirect effects of systemic infection and immune activation on the central nervous system. Outcome was not noted.
4. Discussion
There are a number of reasons why patients with COVID-19 may develop seizures including, but not limited to: hypoxia, electrolyte derangements, systemic infections, hypo- or hyperglycemia, acute kidney injury, cytokine storming, shock, medications, or stroke [21,68]. However, it has also been hypothesized that seizures in this patient population could be the result of viral neuroinvasion [20,[68], [69], [70]]. Though neuropathological studies are the most definitive way to evaluate for viral neuroinvasion, CSF results can also demonstrate evidence of viral neuroinvasion. Thus, in this systematic review, we reviewed the CSF results for patients with COVID-19 who had a seizure.
After identifying 69 patients who had a seizure in the setting of COVID-19 and had CSF studies available for review [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18],[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65]], we found that 6/45 (13%) had a positive CSF SARS-CoV-2 PCR (though cycle threshold was not reported for any of them) [[3], [4], [5], [6], [7],[10], [11], [12], [13], [14], [15], [16],18,28,[31], [32], [33], [34], [35], [36], [37],39,41,42,45,[50], [51], [52], [53],55,56,59,60,63,64]; 2/26 (8%) demonstrated evidence of intrathecal antibody synthesis (though one had Caspr2 IgG in the CSF in addition to CSF-specific oligoclonal bands, so their bands may have been demonstrative of those antibodies rather than SARS-CoV-2 antibodies) [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]; and 2 of the remaining 24 patients who had direct or indirect testing for intrathecal antibodies had possible evidence of intrathecal antibody synthesis, but the data provided did not allow for distinction between intrathecal antibody synthesis and transudation of antibodies, or cells that secrete antibodies, to the CSF via breakdown of the blood-brain barrier [2,6]. Thus, these findings suggest that detection of SARS-CoV-2 in the CSF of patients with seizures who have COVID-19 is uncommon.
Though CSF SARS-CoV-2 PCR and antibody testing were positive in only a few cases, CSF pleocytosis and elevated protein were more common. One-third of patients had CSF pleocytosis and nearly half had hyperproteinorachia. The reported incidence of postictal pleocytosis is 4-30% and elevated CSF protein is seen relatively frequently following a seizure; some studies have reported hyperproteinorachia after seizure in up to 60% of patients [71], [72], [73]. The presence of elevated CSF protein is thought to be related to a transient disturbance of the blood-brain barrier which allows for extravasation of small molecules (such as albumin), but generally prohibits transudation of larger blood components (such as white blood cells) [71,74]. The mechanism behind postictal pleocytosis is uncertain, but it has been noted that the CSF WBC count varies based on the duration of time that elapses between the seizure and CSF collection [73]. As such, it is feasible that CSF pleocytosis and/or elevated protein in the patients included in this review may be secondary to the seizure itself, as opposed to a separate intrathecal process related to COVID-19.
CSF biomarkers were tested for a small number of patients, but all of them had at least one elevated biomarker [2,4,7,[12], [13], [14],45]. Increased serum and CSF biomarkers, in particular IL-6, have also been reported postictally in patients with epilepsy without underlying infection [74], [75], [76]. The degree of elevation has been noted to be dependent on seizure type, duration, and frequency [74,75]. Patients with generalized tonic-clonic seizures have relatively higher postictal CSF IL-6 than those who have other seizure types [74,75]. This is attributed to the widespread epileptic activity not restricted to a solitary focus during generalized tonic-clonic seizures, so there is a greater degree of leakage through the blood-brain barrier [75,76]. Animal studies examining the impact of intranasal IL-6 administration prior to induction of seizures via systemic injection of pentylenetetrazole also suggested that it is proconvulsant, and induces longer seizures with higher mortality [77]. Therefore, it is unclear whether elevated CSF biomarkers, particularly IL-6, in our patients developed due to 1) systemic infection and inflammation which then lowered the seizure threshold; 2) the seizure itself; or 3) both.
Of the 11 patients whose CSF was evaluated for autoimmune antibodies, 2 (18%) were positive [3,4,6,10,11,[13], [14], [15], [16],18,34]. A multitude of neural-specific autoantibodies in both the serum and CSF have been linked to seizures and status epilepticus [78]. The most common antibodies are NMDA antibodies and antibodies against the voltage-gated potassium channel (VGKC) complex, such as leucine-rich, glioma inactivated 1 (LGI1) and Caspr2 [78]. Accordingly, one of the patients we identified who had COVID-19 and positive CSF autoimmune antibodies had NMDA antibodies and the second had Caspr2 antibodies [10,14]. NMDA encephalitis is paraneoplastic in up to 50% of cases (dependent upon age and gender); the most common underlying tumor is an ovarian teratoma. In contrast, limbic encephalitis due to LGI1 antibodies is rarely paraneoplastic, and Caspr2 encephalitis is associated with a tumor in only 30% of cases (most typically a thymoma) [78]. In patients with autoimmune encephalitis secondary to an underlying tumor, autoimmunity is attributed to ectopic tumor expression of neuronal autoantigens. In non-paraneoplastic cases of autoimmune encephalitis, it has been suggested that a preceding infection may trigger autoimmunity, as these patients frequently present with prodromal flu-like symptoms [79]. Infection with Herpes Simplex encephalitis has also been associated with autoimmune encephalitis [80]. However, the immunological mechanism that explains the relationship between infections and autoimmune encephalitis is poorly understood [79]. Of the two patients we identified who had COVID-19 and autoimmune antibodies in the CSF, one had a 3-week history of asthenia and weight loss before onset of seizure and neuropsychiatric symptoms, but the second did not have any systemic symptoms [10]. It is unclear if their autoimmune antibodies were related to COVID-19 or if the infection was merely coincidental, and while neither was noted to have cancer, underlying undiagnosed tumor cannot be ruled out; additionally, their HLA haplotypes were not reported, but some HLA haplotypes have been associated with autoimmune encephalitis, suggesting the potential for genetic susceptibility [79].
Our findings are limited both by publication bias and our search methodology. There are likely additional patients who had seizures in the setting of COVID-19 and had CSF results that were not included herein because 1) the seizure was not clinically recognized or was non-convulsive or 2) there was no published report of the clinical details of their case during our search period so they were not captured by our search. It is also important to note that while CSF is one technique to evaluation for viral neuroinvasion, neuropathological studies can provide more definitive data. In addition, though our results suggest that the majority of patients included in this review did not have CSF evidence of viral neuroinvasion, lack of access to detailed clinical information for each patient precluded us from systematically evaluating for other causes of seizure such as hypoxia, metabolic derangements, acute kidney injury, cytokine storming, or medications. Lastly, very few authors specified the timing of CSF collection in relation to seizure onset. Because of this, it is important to note that CSF could be falsely negative if there was a delay between seizure onset and lumbar puncture.
5. Conclusion
Detection of SARS-CoV-2 in the CSF of patients with seizures who have COVID-19 is uncommon, and even when there is evidence of SARS-CoV-2 in the CSF, the significance of this finding is unknown. Neuropathological studies are needed to further investigate the impact of viral neuroinvasion on development of seizures in patients with COVID-19.
Declaration of Competing Interest
Drs Carroll, Melmed, Frontera, Placatonakis, Galetta, Balcer, and Lewis report no conflicts of interest.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors have any conflicts of interest to disclose.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.seizure.2021.05.003.
Appendix. Supplementary materials
References
- 1.Abdi S, Ghorbani A, Fatehi F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J Neurol Sci. 2020;416 doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benameur K, Agarwal A, Auld SC, et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis. 2020;26(9):2016–2021. doi: 10.3201/eid2609.202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll E, Neumann H, Aguero-Rosenfeld ME, et al. Post-COVID-19 inflammatory syndrome manifesting as refractory status epilepticus. Epilepsia. 2020;61(10):e135–e139. doi: 10.1111/epi.16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delorme C, Paccoud O, Kas A, et al. Covid-19-related encephalopathy: a case series with brain FDG-PET/CT findings. Eur J Neurol. 2020 doi: 10.1111/ene.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djellaoui A, Seddik L, Cleret De Langavant L, Cattan S, Bachoud-Lévi AC, Hosseini H. Posterior reversible encephalopathy syndrome associated with SARS-CoV-2 infection. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323923. [DOI] [PubMed] [Google Scholar]
- 6.Dono F, Carrarini C, Russo M, et al. New-onset refractory status epilepticus (NORSE) in post SARS-CoV-2 autoimmune encephalitis: a case report. Neurol Sci. 2020:1–4. doi: 10.1007/s10072-020-04846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edén A, Kanberg N, Gostner J, et al. CSF biomarkers in patients with COVID-19 and neurological symptoms: a case series. Neurology. 2020 doi: 10.1212/WNL.0000000000010977. [DOI] [PubMed] [Google Scholar]
- 8.Elkady A, Rabinstein AA. Acute necrotizing encephalopathy and myocarditis in a young patient with COVID-19. 2020;7(5):e801.
- 9.Ghosh R, Dubey S, Finsterer J, Chatterjee S, Ray BK. SARS-CoV-2-associated acute hemorrhagic, necrotizing encephalitis (AHNE) presenting with cognitive impairment in a 44-year-old woman without comorbidities: a case Report. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.925641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilmot A, Maldonado Slootjes S, Sellimi A, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. 2020:1–7. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseini AA, Shetty AK, Sprigg N, Auer DP, Constantinescu CS. Delirium as a presenting feature in COVID-19: Neuroinvasive infection or autoimmune encephalopathy? Brain Behav Immun. 2020;88:68–70. doi: 10.1016/j.bbi.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller E, Brandi G, Winklhofer S, et al. Large and small cerebral vessel involvement in severe COVID-19: detailed clinical workup of a case series. Stroke. 2020 doi: 10.1161/STROKEAHA.120.031224. Strokeaha120031224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Guennec L, Devianne J, Jalin L, et al. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020 doi: 10.1111/epi.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monti G, Giovannini G, Marudi A, et al. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure. 2020;81:18–20. doi: 10.1016/j.seizure.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilotto A, Masciocchi S, Volonghi I, et al. Clinical presentation and outcomes of SARS-CoV-2 related encephalitis: the ENCOVID multicentre study. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sattar SBA, Haider MA, Zia Z, Niazi M, Iqbal QZ. Clinical, radiological, and molecular findings of acute encephalitis in a COVID-19 patient: a rare case report. Cureus. 2020;12(9):e10650. doi: 10.7759/cureus.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song E, Chow RD, Jiang R, et al. Immunologically distinct responses occur in the CNS of COVID-19 patients. bioRxiv : the preprint server for biology.2020.
- 18.Zambreanu L, Lightbody S, Bhandari M, et al. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J Neurol Neurosurg Psychiatry. 2020;91(11):1229–1230. doi: 10.1136/jnnp-2020-323839. [DOI] [PubMed] [Google Scholar]
- 19.Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized COVID-19 patients in New York City. Neurology. 2020 doi: 10.1212/WNL.0000000000010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu L, Xiong W, Liu D, et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61(6):e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desforges M, Le Coupanec A, Brison E, Meessen-Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75–96. doi: 10.1007/978-81-322-1777-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koralnik IJ, Tyler KL. COVID-19: a global threat to the nervous system. Ann Neurol. 2020;88(1):1–11. doi: 10.1002/ana.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis A, Frontera JA, Placantonakis D, Galetta S, Balcer L, Melmed K. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J Neurol Sci. 2020 doi: 10.1016/j.jns.2021.117316. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Covidence systematic review software. In. Vol Veritas Health Innovation. Melbourne, Australia.
- 27.Abdulsalam MA, Abdulsalam AJ, Shehab D. Generalized status epilepticus as a possible manifestation of COVID-19. Acta Neurol Scand. 2020;142(4):297–298. doi: 10.1111/ane.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afshar H, Yassin Z, Kalantari S, et al. Evolution and resolution of brain involvement associated with SARS- CoV2 infection: A close Clinical - Paraclinical follow up study of a case. Multiple Scl Rel] Disord. 2020;43 doi: 10.1016/j.msard.2020.102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anand P, Al-Faraj A, Sader E, et al. Seizure as the presenting symptom of COVID-19: a retrospective case series. Epilepsy Behav. 2020;112 doi: 10.1016/j.yebeh.2020.107335. (no pagination)(107335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand P, Lau KHV, Chung DY, et al. Posterior reversible encephalopathy syndrome in patients with coronavirus disease 2019: two cases and a review of the literature. Journal of Stroke and Cerebrovascular Diseases : the Official Journal of National Stroke Association. 2020;29(11) doi: 10.1016/j.jstrokecerebrovasdis.2020.105212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayatollahi P, Tarazi A, Wennberg R. Possible autoimmune encephalitis with claustrum sign in case of Acute SARS-CoV-2 Infection. Can J Neurol Sciences Le Journal Canadien des Sciences Neurologiques. 2020:1–3. doi: 10.1017/cjn.2020.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balloy G, Leclair-Visonneau L, Péréon Y, et al. Non-lesional status epilepticus in a patient with coronavirus disease 2019. Clin Neurophysiol. 2020;131(8):2059–2061. doi: 10.1016/j.clinph.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellon M, Schweblin C, Lambeng N, et al. Cerebrospinal fluid features in SARS-CoV-2 RT-PCR positive patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1165. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernard-Valnet R, Pizzarotti B, Anichini A, et al. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol. 2020 doi: 10.1111/ene.14298. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canham LJW, Staniaszek LE, Mortimer AM, Nouri LF, Kane NM. Electroencephalographic (EEG) features of encephalopathy in the setting of Covid-19: a case series. Clin Neurophysiol Pract. 2020;5:199–205. doi: 10.1016/j.cnp.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casez O, Willaume G, Grand S, et al. SARS-CoV-2 related encephalitis: MRI pattern of the olfactory tract involvement. Neurology. 2020 doi: 10.1212/WNL.0000000000011150. [DOI] [PubMed] [Google Scholar]
- 37.Conte G, Avignone S, Carbonara M, et al. COVID-19-associated PRES-like encephalopathy with perivascular gadolinium enhancement. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dharsandiya M, Shah K, Patel K, Patel T, Patel A, Patel A. SARS-CoV-2 viral sepsis with meningoencephalitis. Indian J Med Microbiol. 2020;38(2):219–221. doi: 10.4103/ijmm.IJMM_20_291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dixon L, Varley J, Gontsarova A, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol (R) Neuroimmunol Neuroinflamm. 2020;7(5):e789. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doo FX, Kassim G, Lefton DR, Patterson S, Pham H, Belani P. Rare presentations of COVID-19: PRES-like leukoencephalopathy and carotid thrombosis. Clin Imaging. 2021;69:94–101. doi: 10.1016/j.clinimag.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dugue R, Cay-Martínez KC, Thakur KT, et al. Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94(24):1100–1102. doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav Immun. 2020;87:33. doi: 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elgamasy S, Kamel MG, Ghozy S, Khalil A, Morra ME, Islam SMS. First case of focal epilepsy associated with SARS-coronavirus-2. J Med Virol. 2020 doi: 10.1002/jmv.26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emami A, Fadakar N, Akbari A, et al. Seizure in patients with COVID-19. Neurol Sci. 2020;41(11):3057–3061. doi: 10.1007/s10072-020-04731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farhadian S, Glick LR, Vogels CBF, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. Res Square. 2020 doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.García-Howard M, Herranz-Aguirre M, Moreno-Galarraga L, et al. Case report: benign infantile seizures temporally associated with COVID-19. Front Pediatr. 2020;8:507. doi: 10.3389/fped.2020.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haddad S, Tayyar R, Risch L, et al. Encephalopathy and seizure activity in a COVID-19 well controlled HIV patient. IDCases. 2020;21:e00814. doi: 10.1016/j.idcr.2020.e00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hafizi F, Kherani S, Shams M. Meningoencephalitis from SARS-CoV-2 infection. IDCases. 2020;21:e00919. doi: 10.1016/j.idcr.2020.e00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haider A, Siddiqa A, Ali N, Dhallu M. COVID-19 and the brain: acute encephalitis as a clinical manifestation. Cureus. 2020;12(10):e10784. doi: 10.7759/cureus.10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karimi N SRA, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3) [Google Scholar]
- 52.Kihira S, Delman BN, Belani P, et al. Imaging features of acute encephalopathy in patients with COVID-19: a case series. AJNR Am J Neuroradiol. 2020;41(10):1804–1808. doi: 10.3174/ajnr.A6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyons S, O'Kelly B, Woods S, et al. Seizure with CSF lymphocytosis as a presenting feature of COVID-19 in an otherwise healthy young man. Seizure. 2020;80:113–114. doi: 10.1016/j.seizure.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McAbee GN, Brosgol Y, Pavlakis S, Agha R, Gaffoor M. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr Neurol. 2020;109:94. doi: 10.1016/j.pediatrneurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mirzaee SMM, Gonçalves FG, Mohammadifard M, Tavakoli SM, Vossough A. Focal cerebral arteriopathy in a pediatric patient with COVID-19. Radiology. 2020;297(2):e274–e275. doi: 10.1148/radiol.2020202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rebeiz T, Lim-Hing K, Khazanehdari S, Rebeiz K. Behavioral changes without respiratory symptoms as a presenting Sign of COVID-19 encephalitis. Cureus. 2020;12(9):e10469. doi: 10.7759/cureus.10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rifino N, Censori B, Agazzi E, et al. Neurologic manifestations in 1760 COVID-19 patients admitted to Papa Giovanni XXIII Hospital, Bergamo, Italy. J Neurol. 2020:1–8. doi: 10.1007/s00415-020-10251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santos De Lima F, Issa N, Seibert K, et al. Epileptiform activity and seizures in patients with COVID-19. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-324337. [DOI] [PubMed] [Google Scholar]
- 61.Shahbaznejad L, Navaeifar MR, Abbaskhanian A, Hosseinzadeh F, Rahimzadeh G, Rezai MS. Clinical characteristics of 10 children with a pediatric inflammatory multisystem syndrome associated with COVID-19 in Iran. BMC Pediatr. 2020;20(1):513. doi: 10.1186/s12887-020-02415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sripadma P, Rai A, Wadhwa C. Postpartum atypical posterior reversible encephalopathy syndrome in a COVID-19 patient - an obstetric emergency. J Stroke Cerebrovasc Dis. 2020;29(12) doi: 10.1016/j.jstrokecerebrovasdis.2020.105357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Westhoff TH, Seibert FS, Bauer F, et al. Allograft infiltration and meningoencephalitis by SARS-CoV-2 in a pancreas-kidney transplant recipient. Am J Transplant. 2020 doi: 10.1111/ajt.16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zanin L, Saraceno G, Panciani PP, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 2020;162(7):1491–1494. doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bigaut K, Mallaret M, Baloglu S, et al. Guillain-Barre syndrome related to SARS-CoV-2 infection. Neurol(R) Neuroimmunol Neuroinflamm. 2020;7(5):e785. doi: 10.1212/NXI.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.HQ TWHO. Clinical Management of COVID-19: Interim Guidance. https://www.who.int/publications/i/item/clinical-management-of-covid-19. Published 2020. Updated 27 May 2020. Accessed.
- 67.Cai Y, Kim DJ, Takahashi T, et al. Kynurenic acid underlies sex-specific immune responses to COVID-19. medRxiv : the preprint server for health sciences. 2020.
- 68.Vohora D, Jain S, Tripathi M, Potschka H. COVID-19 and seizures: Is there a link? Epilepsia. 2020;61(9):1840–1853. doi: 10.1111/epi.16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen X, Laurent S, Onur OA, et al. A systematic review of neurological symptoms and complications of COVID-19. J Neurol. 2020 doi: 10.1007/s00415-020-10067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scramstad C, Jackson AC. Cerebrospinal fluid pleocytosis in critical care patients with seizures. Can J Neurol Sci Le Journal Canadien des Sciences Neurologiques. 2017;44(4):343–349. doi: 10.1017/cjn.2016.442. [DOI] [PubMed] [Google Scholar]
- 72.Chatzikonstantinou A, Ebert AD, Hennerici MG. Cerebrospinal fluid findings after epileptic seizures. Epileptic Disord. 2015;17(4):453–459. doi: 10.1684/epd.2015.0779. [DOI] [PubMed] [Google Scholar]
- 73.Devinsky O, Nadi S, Theodore WH, Porter RJ. Cerebrospinal fluid pleocytosis following simple, complex partial, and generalized tonic-clonic seizures. Ann Neurol. 1988;23(4):402–403. doi: 10.1002/ana.410230418. [DOI] [PubMed] [Google Scholar]
- 74.Zisimopoulou V, Mamali M, Katsavos S, Siatouni A, Tavernarakis A, Gatzonis S. Cerebrospinal fluid analysis after unprovoked first seizure. Funct Neurol. 2016;31(2):101–107. doi: 10.11138/FNeur/2016.31.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alapirtti T, Lehtimaki K, Nieminen R, et al. The production of IL-6 in acute epileptic seizure: a video-EEG study. J Neuroimmunol. 2018;316:50–55. doi: 10.1016/j.jneuroim.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 76.de Vries EE, van den Munckhof B, Braun KP, van Royen-Kerkhof A, de Jager W, Jansen FE. Inflammatory mediators in human epilepsy: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2016;63:177–190. doi: 10.1016/j.neubiorev.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 77.Kalueff AV, Lehtimaki KA, Ylinen A, Honkaniemi J, Peltola J. Intranasal administration of human IL-6 increases the severity of chemically induced seizures in rats. Neurosci Lett. 2004;365(2):106–110. doi: 10.1016/j.neulet.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 78.Gaspard N. Autoimmune Epilepsy. Continuum (Minneap Minn) 2016;22(1 Epilepsy):227–245. doi: 10.1212/CON.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 79.Joubert B, Dalmau J. The role of infections in autoimmune encephalitides. Rev Neurol (Paris) 2019;175(7-8):420–426. doi: 10.1016/j.neurol.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 80.Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.