Abstract
Azithromycin, a member of the macrolide family of antibiotics, is commonly used to treat respiratory bacterial infections. Nevertheless, multiple pharmacological effects of the drug have been revealed in several investigations. Conceivably, the immunomodulatory properties of azithromycin are among its critical features, leading to its application in treating inflammatory diseases, such as asthma and chronic obstructive pulmonary disease (COPD). Additionally, azithromycin may directly inhibit viral load as well as its replication, or it could demonstrate indirect inhibitory impacts that might be associated with the expression of antiviral genes. Currently, coronavirus disease 2019 (COVID-19) is an extra urgent issue affecting the entire world, and it is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Acute respiratory distress syndrome (ARDS), which is associated with hyper inflammation due to cytokine release, is among the leading causes of death in COVID-19 patients with critical conditions. The present paper aims to review the immunomodulatory and antiviral properties of azithromycin as well as its potential clinical applications in the management of COVID-19 patients.
Keywords: Antiviral, Azithromycin, COVID-19, Immunomodulatory, SARS-CoV-2
1. Introduction
Coronaviruses (CoVs), belonging to the coronavirinae subfamily, can infect mammals and several other animals (Gorbalenya et al., 2020). While a group of CoVs (e.g., 229E, NL63, HKU1, and OC43) is recognized as low pathogenic, the groups, such as severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1), Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) demonstrated highly pathogenic capabilities. SARS-CoV-2 was discovered in December 2019 in Wuhan, China, and caused coronavirus disease in 2019 (COVID-19). The epidemic of COVID-19 was declared a pandemic in march 2020 by the World Health Organization (Li et al., 2020a; Lai et al., 2020; Organization, 2020). The symptoms of the so-called viral infection predominantly include a non-productive cough, fever, fatigue, myalgia, and dyspnea (Huang et al., 2020). Acute respiratory distress syndrome (ARDS), which is caused by dyspnea development, is the leading cause of mortality in COVID-19 (Ruan et al., 2020). The correlation between ARDS and hyper inflammation in COVID-19 is discussed in the following sections.
Macrolides are a group of antibiotics that are originated from Streptomyces erythreus. They are demonstrated to inhibit the protein synthesis in bacteria through binding to their ribosome and are known for their effects on airway infection treatment (Gaynor and Mankin, 2003). Azithromycin is a member of the macrolide family with oral administration and is structurally related to erythromycin (Peters et al., 1992).
The first step in the pathogenic mechanism of SARS-CoV-2 in the lung is entering the cells. The process occurs through the recognition of a host cell called angiotensin-converting enzyme 2 (ACE2). SARS-CoV-2 recognizes ACE2 via receptor binding domains of the viral spike, a structural protein on the envelope (Zhou et al., 2020a; Kim et al., 2020). Following the entry to the cell, activation of inflammation pathways leads to cytokine storms, that is, the over-production of pro-inflammatory cytokines, by which the severity of the disease is determined (Mehta et al., 2020). Therefore, the suppression of inflammatory pathways can be beneficial for alleviating respiratory symptoms. Anti-inflammatory properties of azithromycin in different respiratory diseases, such as chronic obstructive pulmonary disease (COPD) (Albert et al., 2011) and asthma (Gibson et al., 2017), could be generalized to COVID-19. Therefore, the present review aims to explain the potential mechanisms of azithromycin in suppressing the SARS-CoV-2-induced inflammation. Furthermore, the clinical studies in this area are discussed in the current review.
2. Immunopathogenesis of hyperinflammation in COVID-19
Excessive production of cytokines caused by hyperactivation of immune cells is a cytokine storm associated with ARDS and introduced as the foremost cause of death in COVID-19 patients (Mehta et al., 2020; Li et al., 2020b). Major pro-inflammatory cytokines involved in SARS-CoV-2-induced cytokine storm are interferon-γ (IFN-γ), interleukin (IL)-6, IL-33, IL-1β, IL-12, IL-18, transforming growth factor-β (TGF-β), and tumor necrosis factor- α (TNF-α) (Ronconi et al., 2020). The other cytokines and chemokines that are associated with cytokine storm are granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), platelet-derived growth factor subunit B (PDGFB), vascular endothelial growth factor A (VEGFA), macrophage inflammatory protein-1β (MIP1β), interferon gamma-induced protein 10 (IP10), monocyte chemoattractant protein-1 (MCP1), and MIP1α (Rothan and Byrareddy, 2020). Following the binding of SARS-CoV-2 spike protein to the ACE2 host cells, the virus enters the cell by transmembrane serine protease 2 (TMPRSS2) intervention (Hoffmann et al., 2020). The spike protein holds two subunits called S1 and S2. The S1 subunit binds to its receptor on the host cells, and the RNA genome of the virus is released into the cell following the fusion through the cytoplasmic membrane by mediating the S2 region. Cleavage of the spike protein within the S2 subunit is of excessive necessity for cell entry (Hirano and Murakami, 2020). SARS-CoV-2 induces an immune response by mediating T helper-1 (Th-1) cells, leading to the production of pro-inflammatory cytokines, such as GM-CSF and IL-6. Pattern recognition receptors (e.g., Toll-like receptors) are responsible for recognition of viral genome, nuclear Factor kappa-light-chain-enhancer of activated B (NF-ҡB) activation, and eventually immune response induction (Li et al., 2020a, 2020b; Prompetchara et al., 2020; Yi et al., 2020). GM-CSF causes considerable quantities of TNF-α and IL-6 by CD14+ CD16+ inflammatory monocytes production (Zhou et al., 2020b). However, high expression of TNF-α and IL-6 is the leading cause of cytokine storm. According to a proposed mechanism (Hirano and Murakami, 2020), ACE2, which inactivates angiotensin II (Ang II), plays a critical role in cytokine release in COVID-19. ACE2 downregulation has been shown in lung injury models, and recombinant ACE2 inhibits ARDS development (Imai et al., 2005). Downregulation of ACE2 is associated with edema, bleeding, alveolar wall thickening, and the recruiting of inflammatory cells in different models of lung injury (Imai et al., 2005; Hung et al., 2016; Lin et al., 2018; Kuba et al., 2005). Endocytosis of ACE2 with SARS-CoV contributes to the reduction of ACE2 on the surface of cells and eventually leads to the surge in serum Ang II (Kuba et al., 2005). Ang II can activate macrophages and the other immune system cells and intensify TNF-α, IL-6, and other inflammatory cytokines (Bernstein et al., 2018; Recinos et al., 2007; Yamamoto et al., 2011; Lee et al., 2002). Following the binding of Ang II to the angiotensin AT1 receptor, the activation of NF-ҡB, a disintegrin, and metalloprotease 17 (ADAM17) occur. ADAM17 causes maturation of TNF-α and epidermal growth factor receptor ligands, which leads to NF-ҡB stimulation (Eguchi et al., 2018). Additionally, the Ang II- angiotensin AT1 receptor axis causes the formation of a soluble form of IL-6 receptor α (sIL-6Rα) by mediating ADAM17 (Eguchi et al., 2018). The binding of IL-6 to sIL-6Rα contributes to signal transducer and activator of transcription 3 (STAT3) activation in non-immune cells. Furthermore, full activation of NF-ҡB requires STAT3 (Murakami et al., 2019). Eventually, the production of VEGF, MCP-1, IL-6, and IL-8 occurs by activation of STAT3 and NF-ҡB through the IL-6 amplifier (Murakami et al., 2019). On the other hand, IL-6 induces energy-dependent neutrophil extracellular traps (NETs) formation (Joshi et al., 2013). NETs are capable of promoting fibrosis (Chrysanthopoulou et al., 2014) and lung damage (Lefrançais et al., 2018) detected in lung samples of COVID-19 patients (Radermecker et al., 2020; Middleton et al., 2020). The other inducer of NET formation is myeloperoxidase (MPO), which can be complexed with DNA and is rising in COVID-19 patients (Radermecker et al., 2020). NET formation capability is a feature of activated neutrophils and increased neutrophil recruitment to the lungs, which may lead to auto-inflammation reactions (Barnes et al., 2020; Chen et al., 2020; Liu et al., 2020). Additionally, neutrophils may contribute to the stress oxidative, shown in patients with COVID-19, and leads to tissue damage (Abouhashem et al., 2020; Laforge et al., 2020). Lymphocytopenia is an imperative clinical indicator of SARS-CoV-2 severity (Tan et al., 2020) which might be caused by infection of lymphocytes and recruitment of lymphocytes to the lung tissue (Wang et al., 2020b). On the other hand, it has been indicated that ACE2 is not expressed in the lymphocytes (Hamming et al., 2004). Therefore, there must be another receptor for SARS-CoV-2 entry to the cells. It has been revealed that cluster of differentiation 147 (CD147) (also known as EMMPRIN or Basigin) is the other receptor that could interact with the spike protein of SARS-CoV-2 (Chen et al., 2020). It has also been indicated that mepolizumab, an anti-CD147 monoclonal antibody, suppresses the virus entry. Besides, CD147 can escalate the production of matrix metalloproteinases, leading to invasion and metastasis of tumor cells (Biswas et al., 1995). The immunological effects of CD147 can regulate the activation of T cells (Igakura et al., 1996). The involvement of CD147 in different inflammatory diseases, such as atherosclerosis, acute asthmatic disease, cardiac infarction, and rheumatoid arthritis, has been publicized (Schmidt et al., 2006; Gwinn et al., 2006; Schulz et al., 2011; Seizer et al., 2011; Wang et al., 2012). Additionally, CD147 promotes activation of NF-ҡB, leading to IL-1β expression (Xu et al., 2020), and inhibition of CD147 decreases reactive oxygen species (ROS) generation (Wang et al., 2020a). Moreover, CD147 is acknowledged as a co-receptor for entry into the human immunodeficiency virus 1 (HIV-1) (Pushkarsky et al., 2001) and the Plasmodium falciparum (Pushkarsky et al., 2001). Collectively, it can be cognized that a therapeutic approach may depend on the inhibition of SARS-CoV-2 entry to the host cells and the prevention of impaired hyper inflammation.
3. Azithromycin for immunomodulation
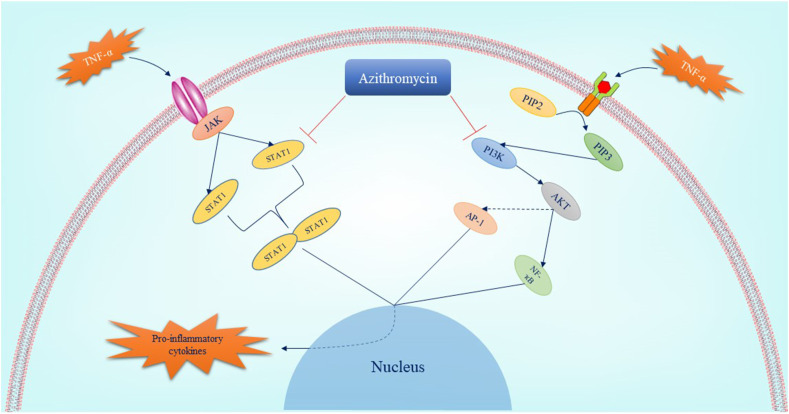
Azithromycin, a member of the macrolide family with anti-inflammatory properties, is employed to treat lower and upper respiratory tract infections (Peters et al., 1992). The anti-inflammatory effects of azithromycin have led to its use in inflammatory lung diseases, such as asthma (Gibson et al., 2017), COPD (Albert et al., 2011), and cystic fibrosis (Cigana et al., 2006). It has been shown that azithromycin is capable of inhibiting NF-ҡB during lung and other tissue inflammation (Stellari et al., 2014). NF-ҡB is one of the leading transcription factors of inflammatory cytokines, such as IL-6 (Libermann and Baltimore, 1990). Phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway is one of the upstream regulators of NF-ҡB (Kane et al., 1999), which might be inhibited by azithromycin (Wang et al., 2018; Zhao et al., 2018). The other activator of NF-ҡB is extracellular signal-regulated kinase 1/2 (ERK1/2) (Shim et al., 2011), which is also suppressed by azithromycin (Blau et al., 2007). Simultaneous inhibition of PI3K/AKT/NF-ҡB and ERK1/2/NF-ҡB by azithromycin contributes to the suppression of pro-inflammatory cytokines production (Wang et al., 2018; Blau et al., 2007). The other transcription factors involved in the inflammation induction are activator protein 1 (AP-1) and STAT proteins (Schonthaler et al., 2011). These factors are activated by molecules such as TNF-α (Desai et al., 2012; Rahman et al., 2002; Guo et al., 1998; Miscia et al., 2002). Thereby, azithromycin can inhibit the inflammation induced through STATs and AP-1 by two mechanisms: the reduction of the TNF-α levels (Blasi et al., 2010) and the direct inhibition of STATs and AP-1 activation (Haydar et al., 2019; Bosnar et al., 2011). Additionally, the accumulation of azithromycin in inflammatory cells, especially macrophages and neutrophils, have been demonstrated in different studies (Gladue et al., 1989; Wildfeuer et al., 1989, 1996). Azithromycin reduces the expression of adhesion molecules such as intercellular adhesion molecule (ICAM) (Cigana et al., 2006) and vascular cell adhesion protein (VCAM) (Bartold et al., 2013), leading to neutrophil recruitment suppression (Tsai et al., 2004). It has been revealed that the ICAM and VCAM expression is controlled by the PI3K/AKT signaling pathway (Tsoyi et al., 2010; Lin et al., 2019). Consequently, the inhibitory effects of azithromycin on ICAM and VCAM molecules may be associated with inhibition of the PI3K/AKT signaling pathway. Regarding the impact of azithromycin on the formation of NETs, it has been indicated that pre-treatment with the drug reduces the release of NETs (Bystrzycka et al., 2017). The so-called effect might be associated with lowering the MPO activity proved in several studies (Culic et al., 2005; Legssyer et al., 2006). The expression of GM-CSF, which has been indicated to regulate neutrophils activity and its high expression in lung injuries, is augmented by TNF-α through PI3K/AKT signaling pathway (Li et al., 2014). Thus, the reduction of the GM-CSF expression by azithromycin may be linked to its effect on suppressing TNF-α expression (Ivetić Tkalcević et al., 2006) and PI3K/AKT signaling pathway. On the other hand, GM-CSF stimulates PI3K/AKT signaling pathway to induce its effect (Qiu et al., 2014). The other factor involved in inflammatory respiratory diseases (e.g., asthma) is a ligand for chemokine receptor CXCR3 called IP-10 that is expressed in epithelial/T cells and required chemotaxis of Th1 cells (Cole et al., 1998). It has been indicated that azithromycin suppresses the expression of IP-10 through NF-ҡB/p65 and mitogen-activated protein kinase (MAPK)– Jun N-terminal kinases (JNKs)/ERK pathways (Kuo et al., 2019). In addition to the stated arguments, azithromycin enhances the phagocytosis of neutrophils or epithelial cells by alveolar macrophages (Hodge et al., 2006). Fig. 1 represents the role of azithromycin in controlling the immune system.
Fig. 1.
The pathways which are inhibited by azithromycin leading to hyper inflammation suppression. Azithromycin suppresses two main pathways involved in pro-inflammatory cytokines production including janus kinase (JAK)/STAT and PI3K/AKT signaling pathways. As a result, azithromycin inhibits the activation of AP-1, NF-ҡB, and STAT dimerization by mediating these pathways suppression. Reduction of the expression of pro-inflammatory cytokines such as ILs, TNF-α is the result of this inhibition. AP-1: activated protein-1; IL: interleukin; JAK: Janus kinase; NF-ҡB: Nuclear Factor kappa-light-chain-enhancer of activated B; PI3K: Phosphatidylinositol-3-kinase; STAT: Signal Transducer and Activator of Transcription; TNF-α: tumor necrosis factor-α.
4. Azithromycin for opportunistic infections in viral diseases
There is no adequate evidence concerning the effect of azithromycin on the viral load in various infections. Although it has generally been demonstrated that azithromycin holds antiviral properties, the actual mechanism is not distinctly understood. Even though Azithromycin might inhibit viral replication, the majority of studies accentuate boosting the immune system to fight against the virus rather than relying on Azithromycin. In a study by Beigelman et al. it has been observed that azithromycin reduces the inflammatory mediators after induction of viral bronchoalveolar infection in mice. Additionally, it has been indicated that azithromycin attenuates post-viral weight loss and reduces total leukocyte accumulation (Beigelman et al., 2010).
Furthermore, it has been reported that azithromycin induces the expression of antiviral genes such as IFNs and IFN-stimulated genes, including oligoadenylate synthase, melanoma differentiation-associated gene 5, a retinoic acid-inducible gene I, MxA, and viperin. Besides, it has been shown that azithromycin reduces the replication and release of rhinoviruses in bronchial epithelial cells (Gielen et al., 2010; Menzel et al., 2016). Zika virus is the next virus on which the effect of azithromycin has been studied in terms of virus replication. In the study by Bosseboeuf et al. the impact of azithromycin was investigated on Zika virus-infected Vero cells, and it was indicated that azithromycin inhibits the Zika virus replication in Vero cells (Bosseboeuf et al., 2018). Moreover, in another study, Retallack et al. have demonstrated that azithromycin reduces the proliferation of the Zika virus and its cytopathic effects (Retallack et al., 2016). Furthermore, it has been reported that pre-treatment with azithromycin has the same results against the Ebola virus (Kouznetsova et al., 2014; Du et al., 2020; Madrid et al., 2015) and Dengue (Li et al., 2019). One of the most thought-provoking studies regarding the antiviral potential of azithromycin is the study on influenza A (H1N1) pdm09 virus infection. In the study by Tran et al. it has been demonstrated that although azithromycin validates no effect on the attachment of the virus to the cell surface, it suppresses virus entry into the host cell during the early phase of infection. Moreover, the effects of this drug were shown to be independent of anti-influenza conventional medicines (Tran et al., 2019).
5. Azithromycin for management of COVID-19
Regardless of the immunomodulatory role of azithromycin, its direct antiviral effects are also an important issue. In this regard, ACE2 and TMPRSS2 are the extra attractive goals. Concerning the ACE2, its downregulation leads to inflammation induction (Shi et al., 2013). There are two types of ACE2: the first type is attached to the membrane, and the other is the soluble form (Batlle et al., 2020). Based on the in silico studies, it has been estimated that azithromycin can target the binding interaction point between ACE2 and spike protein of the SARS-CoV-2 (Braz et al., 2020). As described earlier, the downregulation of ACE2 by the virus leads to increased Ang II levels. On the other hand, it has been demonstrated that Ang II induces the PI3K/AKT signaling pathway through the angiotensin AT1 receptor and eventually contributes to the inflammatory pathway (Zhang et al., 2016; Zhao et al., 2014). Thus, it can be concluded that azithromycin could suppress Ang II-induced inflammation through inhibition of the PI3K/AKT signaling pathway. Activation of the angiotensin AT1 receptor by Ang II contributes to the upregulation of the ADAM17 expression leading to cleavage of the membrane-anchored ACE2 and changing to an active soluble form (Xu et al., 2017). It has been shown that overexpression of ACE2 enhances the entry of SARS-CoV to the host cells in a mouse model (Yang et al., 2007). It seems that the overexpression of membrane-anchored ACE2 leads to an increase of the virus entry to the cells, and the soluble form of ACE2 decreases viral entrance. It has been indicated that human recombinant soluble ACE2 inhibits SARS-CoV infection by two mechanisms: a) by neutralizing the virus without endocytosis b) by suppressing inflammation induced by the virus (Monteil et al., 2020; Xue et al., 2014). However, differences in the amino acid sequence of the ACE and ACE2 cause ACE inhibitors to have no effect on the COVID-19 treatment (Crackower et al., 2002). Concerning the TMPRSS2 as the other therapeutic target, its expression is engaged with the PI3K/AKT signaling pathway (Mishra and Dey, 2021). The expression of TMPRSS2 is upregulated by androgen receptors due to several androgen receptor elements located on the promoter of the TMPRSS2 gene, and it can be a reason for higher sensitivity of men to COVID-19 (Shen et al., 2017; Wambier et al., 2020). On the other hand, it has been demonstrated that IL-6 inhibits androgen receptors transactivation through the PI3K/AKT signaling pathway (Yang et al., 2003), and the activation of androgen receptors by androgens leads to PI3K/AKT signaling pathway activation (Sun et al., 2003). Therefore, as a target for azithromycin, although activation of this pathway may be a mechanism for TMPRSS2 expression, further research is required to approve the so-called notion. Additionally, it has been indicated that azithromycin suppresses the pathways involved in the TMPRSS2 expression (Renteria et al., 2020). On the other hand, the effect of azithromycin on influenza replication might be related to the downregulation of the TMPRSS2 (Bertram et al., 2010; Tran et al., 2019). Besides, it has been demonstrated that the endocytosis of SARS-CoV-2 is performed through a clathrin-mediated pathway (Bayati et al., 2021). From a different perspective, the PI3K/AKT signaling pathway and clathrin-mediated endocytosis are shown to be in a mutual relationship with each other. PI3K/AKT signaling pathway regulates clathrin-mediated endocytic processes, and clathrin is required for AKT activation (Bhattacharya et al., 2016; Garay et al., 2015).
Additionally, it has been represented that the activation of the PI3K/AKT signaling pathway is required for bovine ephemeral fever virus entry to the host cells by enhancing the clathrin-mediated virus endocytosis (Cheng et al., 2015). The involvement of this pathway in the entry of transmissible gastroenteritis virus, which demands clathrin for its endocytosis, has been shown in another study (Hu et al., 2018). Based on the aforementioned findings, the suppression of the PI3K/AKT signaling pathway by azithromycin may inhibit SARS-CoV-2 entry to the host cells through clathrin-dependent endocytosis.
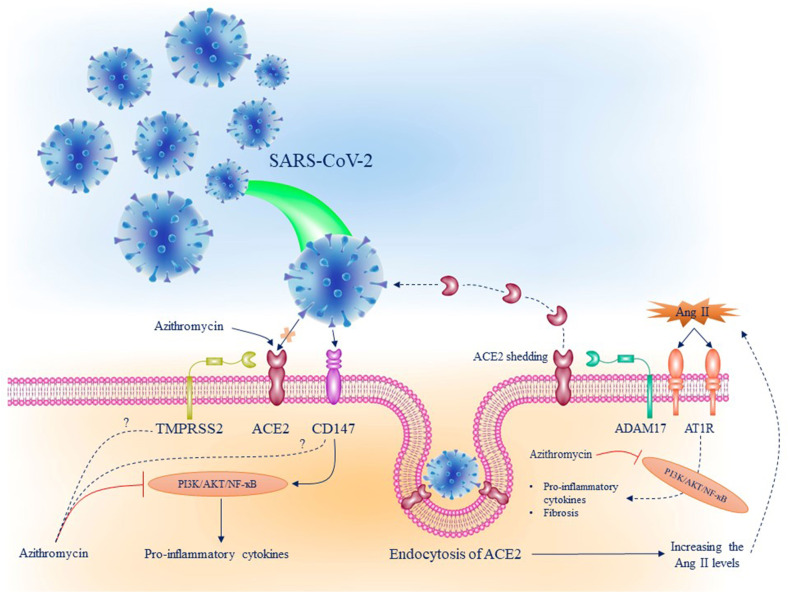
After all, the effect of azithromycin on SARS-CoV-2 replication has been evaluated in a study by Touret et al. In this study, which was accomplished in Vero cells, pre-treatment with 2.12 μM of azithromycin as EC50 inhibited the replication of the virus (Touret et al., 2020). Although the exact mechanism of this inhibition is not evidently cognized, according to a hypothesis, it may be related to the pH of the lysosome, which is required for the shedding of the viral genetic. It is proposed that an acidic environment may be required for uncoating of coronaviruses similar to the other enveloped viruses such as HIV and influenza (Greber et al., 1994). Because of the weak base feature of azithromycin, it can be declared that azithromycin upsurges the pH level and disrupts acidic conditions, which are compulsory for the uncoating process (Damle et al., 2020). The effects of azithromycin on the other SARS-CoV-2 receptor, CD147, are not fittingly scrutinized; however, there exist several hypotheses. The effect of azithromycin on reducing the MMPs' expression related to CD147 formulates the hypothesis that azithromycin may inhibit CD147, and eventually, virus entry to the host cells (Ulrich and Pillat, 2020). Inhibition of Plasmodium falciparum invasion by azithromycin in different cases makes the idea extra potent (Wilson et al., 2015). On the other hand, it has been demonstrated that CD147 induces the PI3K/AKT signaling pathway activation, contributing to NF-ҡB induction and pro-inflammatory cytokines production (Chen et al., 2009; Fang et al., 2015). In addition to inflammation, PI3K/AKT signaling pathway is involved in the fibrosis induction in different organs (Zang et al., 2019; Qiu et al., 2019). Over-activation of this pathway by SARS-CoV-2 through CD147 and angiotensin AT1 receptor may be the major cause of fibrosis in COVID-19 patients. In addition, once the expression of TMPRSS2 is regulated by PI3K/AKT signaling pathway, its overexpression by the virus may lead to an increase in the TMPRSS2 expression and eventually more virus entry to the cells. Fig. 2 represents the mechanisms of SARS-CoV-2 entry to the host cells, its pathogenicity, and possible mechanisms of azithromycin to suppress these processes. Most clinical studies on the effects of azithromycin on the COVID-19 treatment have been conducted in combination with hydroxychloroquine. The first clinical study to inspect the effects of azithromycin in the treatment of COVID-19 was done in Marseilles, France. In this study, patients were divided into three groups: hydroxychloroquine received group, hydroxychloroquine + azithromycin-treated group, and a group consisting of the patients with no hydroxychloroquine treatment as a control group. Based on the results of this study, twenty patients were treated in different groups, and a combination of azithromycin with hydroxychloroquine was shown to be more efficient for reducing viral load. All the patients who received hydroxychloroquine in combination with azithromycin had a negative SARS-CoV-2 test of nasopharyngeal polymerase chain reaction (PCR) in comparison with 57.1% of patients who received hydroxychloroquine alone, and 12.5% of patients in the control group on day 6 of post inclusion (Gautret et al., 2020a). In a pilot observational study, the effect of the combination of azithromycin and hydroxychloroquine on the viral load and clinical features of 80 patients was determined. In this study, reduced viral load was observed on days seven and eight in 83% and 93% of patients, respectively. In addition, on day 5, the results of virus culture from the patients’ respiratory system samples were negative in 97.5% of patients (Gautret et al., 2020b). According to the results of another study on 1061 patients with COVID-19, treatment with hydroxychloroquine + azithromycin is associated with a low mortality rate. In this study, patients were treated with azithromycin in a dose of 500 mg on day one and 250 mg daily for the next four days and hydroxychloroquine in a dose of 200 mg three times a day for ten days. In 91.7% of patients, satisfactory clinical outcomes were observed, and the result of the PCR test was negative in almost all patients on day 15 (Million et al., 2020). Treatment with hydroxychloroquine + azithromycin was associated with a reduction in the mortality rate, the risk of hospitalization for more than ten days, and shortness of viral shedding duration based on a retrospective analysis (Lagier et al., 2020). In another retrospective study, the same results have been confirmed, in which 2541 patients with COVID-19 with a median age of 64 years were divided into four treatment groups, including hydroxychloroquine alone, azithromycin + hydroxychloroquine, azithromycin alone, and control group. The overall mortality rate was 18.1% in the entire cohort. The mortality rates were 13.5% in the hydroxychloroquine alone group, 22.4% among those receiving azithromycin alone, 20.1% among the hydroxychloroquine + azithromycin group and 26.4% for neither drugs. Thus, it can be deduced that treatment with azithromycin is associated with a significant reduction of COVID-19-related mortality (Arshad et al., 2020). In addition to the so-called studies, Albani and et al. (Albani et al., 2020) evaluated the impact of azithromycin on hospital mortality in COVID-19 patients alone or combined with hydroxychloroquine. In this study, 1430 patients with COVID-19 were admitted to the hospital, and the outcome was available for 1376 of them. A group of 587 patients received azithromycin, and the other group with 377 patients was treated with hydroxychloroquine alone or combined with azithromycin. According to the results of this study, treatment of COVID-19 patients with azithromycin was associated with lower in-hospital mortality, and hydroxychloroquine was not associated with reduced or increased mortality. A retrospective cohort study, including 377 patients hospitalized for pneumonia caused by COVID-19 treatment with hydroxychloroquine combined with azithromycin, reduced the mortality rate compared to no treatment (Lauriola et al., 2020). Interestingly, in this study, treatment with hydroxychloroquine alone was not associated with lower mortality rate and days of in-hospital remaining, whereas in combination with azithromycin was inversely associated. Conversely, several studies discussed the adverse effects of azithromycin and hydroxychloroquine combination or the antiviral power of the drugs. In a pre-proof study, it has been indicated that the combination treatment of azithromycin and hydroxychloroquine has no evidence of clinical benefit and strong antiviral activity in severe patients (Molina et al., 2020). Besides, Furtado et al. (2020) evaluated the impact of azithromycin in addition to the standard of care treatment in patients admitted to the hospital with severe COVID-19. In this clinical trial, 214 of 397 patients received azithromycin compared with 183 patients as the control group. It was shown that in severe COVID-19, patients treated with azithromycin did not improve clinical outcomes. In response to this study, it can be referred to another study that examined the effect of azithromycin on patients with COVID-19 at the onset of early symptoms. This study included 1061 patients treated with azithromycin combined with hydroxychloroquine prior to the occurrence of COVID-19 complications demonstrated a low mortality rate and decent clinical outcome in patients (Million et al., 2020). Based on the results of other studies, QT interval prolongation, which is the widely acknowledged adverse effect of hydroxychloroquine, is increased once hydroxychloroquine is combined with azithromycin for COVID-19 treatment (Chorin et al., 2020a, 2020b; Maraj et al., 2020).
Fig. 2.
SARS-CoV-2 entry to the host cells, its pathogenicity pathways, and possible mechanisms of azithromycin to suppress these processes. SARS-CoV-2 binds to ACE2 and causes its downregulation on the cell surface. Eventually, the levels of Ang II increases leading to PI3K/AKT signaling pathway activation by mediating angiotensin AT1 receptor and inducing the expression of the pro-inflammatory cytokines such as IL-6. First, azithromycin prevents the binding of the virus spike to ACE2 because of its more affinity with the ACE2, secondly, azithromycin inhibits PI3K/AKT signaling pathway activation and suppresses Ang II-induced inflammation and fibrosis. On the other hand, the other SARS-CoV-2 receptor, CD147, activates the PI3K/AKT signaling pathway that contributes to inducing the expression of the pro-inflammatory cytokines. Thus, azithromycin suppresses CD147-dependent inflammation through this pathway. The effect of azithromycin on the expression of the TMPRSS2 and CD147 and its exact mechanisms are not understood yet. ACE2: angiotensin-converting enzyme 2; IL-6: interleukin-6; PI3K: phosphatidylinositol-3-kinase; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; TMPRSS2: transmembrane serine protease 2.
6. Conclusion
Although various studies have been conducted to gauge the effect of azithromycin on COVID-19, the majority of these studies have examined its adjuvant impact along with hydroxychloroquine. There is a lack of adequate information concerning the combination of azithromycin with the other drugs employed for COVID-19 treatment. Due to the effect of azithromycin on the suppression of the SARS-CoV-2-induced inflammation and its replication in human cells, it can be utilized in combination with other medications such as corticosteroids, antiviral agents, and antibodies. In addition, since azithromycin affects the transcription of numerous factors, its usage in the early stages of the disease might play a crucial role in its efficacy. However, it requires further investigation in order to prove the consistency of the results.
References
- Abouhashem A.S., Singh K., Azzazy H.M.E., Sen C.K. Is low alveolar type II cell SOD3 in the lungs of elderly linked to the observed severity of COVID-19? Antioxidants Redox Signal. 2020;33:59–65. doi: 10.1089/ars.2020.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani F., Fusina F., Giovannini A., Ferretti P., Granato A., Prezioso C., Divizia D., Sabaini A., Marri M., Malpetti E., Natalini G. Impact of azithromycin and/or hydroxychloroquine on hospital mortality in COVID-19. J. Clin. Med. 2020;9:2800. doi: 10.3390/jcm9092800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert R.K., Connett J., Bailey W.C., Casaburi R., Cooper J.A., Jr., Criner G.J., Curtis J.L., Dransfield M.T., Han M.K., Lazarus S.C., Make B., Marchetti N., Martinez F.J., Madinger N.E., Mcevoy C., Niewoehner D.E., Porsasz J., Price C.S., Reilly J., Scanlon P.D., Sciurba F.C., Scharf S.M., Washko G.R., Woodruff P.G., Anthonisen N.R. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K., Brar I., Alangaden G.J., Ramesh M.S., Mckinnon J.E., O'neill W., Zervos M. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., Loda M., Looney M.R., Mcallister F., Rayes R., Renaud S., Rousseau S., Salvatore S., Schwartz R.E., Spicer J.D., Yost C.C., Weber A., Zuo Y., Egeblad M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartold P.M., Du Bois A.H., Gannon S., Haynes D.R., Hirsch R.S. Antibacterial and immunomodulatory properties of azithromycin treatment implications for periodontitis. Inflammopharmacology. 2013;21:321–338. doi: 10.1007/s10787-012-0165-1. [DOI] [PubMed] [Google Scholar]
- Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. (Lond.) 2020;134:543–545. doi: 10.1042/cs20200163. [DOI] [PubMed] [Google Scholar]
- Bayati A., Kumar R., Francis V., Mcpherson P.S. SARS-CoV-2 infects cells following viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021;296:100306. doi: 10.1016/j.jbc.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigelman A., Mikols C.L., Gunsten S.P., Cannon C.L., Brody S.L., Walter M.J. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir. Res. 2010;11:90. doi: 10.1186/1465-9921-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein K.E., Khan Z., Giani J.F., Cao D.Y., Bernstein E.A., Shen X.Z. Angiotensin-converting enzyme in innate and adaptive immunity. Nat. Rev. Nephrol. 2018;14:325–336. doi: 10.1038/nrneph.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram S., Glowacka I., Blazejewska P., Soilleux E., Allen P., Danisch S., Steffen I., Choi S.Y., Park Y., Schneider H., Schughart K., Pöhlmann S. TMPRSS2 and TMPRSS4 facilitate trypsin-independent spread of influenza virus in Caco-2 cells. J. Virol. 2010;84:10016–10025. doi: 10.1128/jvi.00239-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S., Mcelhanon K.E., Gushchina L.V., Weisleder N. Role of phosphatidylinositol-4,5-bisphosphate 3-kinase signaling in vesicular trafficking. Life Sci. 2016;167:39–45. doi: 10.1016/j.lfs.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas C., Zhang Y., Decastro R., Guo H., Nakamura T., Kataoka H., Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Canc. Res. 1995;55:434–439. [PubMed] [Google Scholar]
- Blasi F., Bonardi D., Aliberti S., Tarsia P., Confalonieri M., Amir O., Carone M., Di Marco F., Centanni S., Guffanti E. Long-term azithromycin use in patients with chronic obstructive pulmonary disease and tracheostomy. Pulm. Pharmacol. Therapeut. 2010;23:200–207. doi: 10.1016/j.pupt.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Blau H., Klein K., Shalit I., Halperin D., Fabian I. Moxifloxacin but not ciprofloxacin or azithromycin selectively inhibits IL-8, IL-6, ERK1/2, JNK, and NF-kappaB activation in a cystic fibrosis epithelial cell line. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;292:L343–L352. doi: 10.1152/ajplung.00030.2006. [DOI] [PubMed] [Google Scholar]
- Bosnar M., Čužić S., Bošnjak B., Nujić K., Ergović G., Marjanović N., Pašalić I., Hrvačić B., Polančec D., Glojnarić I., Eraković Haber V. Azithromycin inhibits macrophage interleukin-1β production through inhibition of activator protein-1 in lipopolysaccharide-induced murine pulmonary neutrophilia. Int. Immunopharm. 2011;11:424–434. doi: 10.1016/j.intimp.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Bosseboeuf E., Aubry M., Nhan T., De Pina J., Rolain J., Raoult D., Musso D. Azithromycin inhibits the replication of Zika virus. J. Antivir. Antiretrovir. 2018;10:6–11. doi: 10.4172/1948-5964.1000173. [DOI] [Google Scholar]
- Braz H.L.B., Silveira J.A.M., Marinho A.D., De Moraes M.E.A., Moraes Filho M.O., Monteiro H.S.A., Jorge R.J.B. In silico study of azithromycin, chloroquine and hydroxychloroquine and their potential mechanisms of action against SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020;56:106119. doi: 10.1016/j.ijantimicag.2020.106119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrzycka W., Manda-Handzlik A., Sieczkowska S., Moskalik A., Demkow U., Ciepiela O. Azithromycin and chloramphenicol diminish neutrophil extracellular traps (NETs) release. Int. J. Mol. Sci. 2017;18:2666. doi: 10.3390/ijms18122666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Gou X., Horikawa Y., Xing J., Chen Z. Upregulation of HAb18G/CD147 in activated human umbilical vein endothelial cells enhances the angiogenesis. Canc. Lett. 2009;278:113–121. doi: 10.1016/j.canlet.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Cheng C.Y., Huang W.R., Chi P.I., Chiu H.C., Liu H.J. Cell entry of bovine ephemeral fever virus requires activation of Src-JNK-AP1 and PI3K-Akt-NF-κB pathways as well as Cox-2-mediated PGE2/EP receptor signalling to enhance clathrin-mediated virus endocytosis. Cell Microbiol. 2015;17:967–987. doi: 10.1111/cmi.12414. [DOI] [PubMed] [Google Scholar]
- Chorin E., Dai M., Shulman E., Wadhwani L., Bar-Cohen R., Barbhaiya C., Aizer A., Holmes D., Bernstein S., Spinelli M., Park D.S., Chinitz L.A., Jankelson L. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat. Med. 2020;26:808–809. doi: 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- Chorin E., Wadhwani L., Magnani S., Dai M., Shulman E., Nadeau-Routhier C., Knotts R., Bar-Cohen R., Kogan E., Barbhaiya C., Aizer A., Holmes D., Bernstein S., Spinelli M., Park D.S., Stefano C., Chinitz L.A., Jankelson L. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17:1425–1433. doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysanthopoulou A., Mitroulis I., Apostolidou E., Arelaki S., Mikroulis D., Konstantinidis T., Sivridis E., Koffa M., Giatromanolaki A., Boumpas D.T., Ritis K., Kambas K. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J. Pathol. 2014;233:294–307. doi: 10.1002/path.4359. [DOI] [PubMed] [Google Scholar]
- Cigana C., Nicolis E., Pasetto M., Assael B.M., Melotti P. Anti-inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem. Biophys. Res. Commun. 2006;350:977–982. doi: 10.1016/j.bbrc.2006.09.132. [DOI] [PubMed] [Google Scholar]
- Cole K.E., Strick C.A., Paradis T.J., Ogborne K.T., Loetscher M., Gladue R.P., Lin W., Boyd J.G., Moser B., Wood D.E., Sahagan B.G., Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J. Exp. Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-Dos-Santos A.J., Da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- Culic, O., Parnham, M., Erakovic, V., 2005. Novel therapeutic indication of azithromycin for treatment of non-infective inflamatory diseases. U.S. Patent Application No. 10/476, 377.
- Damle B., Vourvahis M., Wang E., Leaney J., Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19. Clin. Pharmacol. Ther. 2020;108:201–211. doi: 10.1002/cpt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Darland G., Bland J.S., Tripp M.L., Konda V.R. META060 attenuates TNF-α-activated inflammation, endothelial-monocyte interactions, and matrix metalloproteinase-9 expression, and inhibits NF-κB and AP-1 in THP-1 monocytes. Atherosclerosis. 2012;223:130–136. doi: 10.1016/j.atherosclerosis.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Du X., Zuo X., Meng F., Wu F., Zhao X., Li C., Cheng G., Qin F.X. Combinatorial screening of a panel of FDA-approved drugs identifies several candidates with anti-Ebola activities. Biochem. Biophys. Res. Commun. 2020;522:862–868. doi: 10.1016/j.bbrc.2019.11.065. [DOI] [PubMed] [Google Scholar]
- Eguchi S., Kawai T., Scalia R., Rizzo V. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension. 2018;71:804–810. doi: 10.1161/hypertensionaha.118.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Wang L., Zhang S., Fang Q., Hao F., Sun Y., Zhao L., Chen S., Liao H., Wang L. CD147 modulates autophagy through the PI3K/Akt/mTOR pathway in human prostate cancer PC-3 cells. Oncol Lett. 2015;9:1439–1443. doi: 10.3892/ol.2015.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado R.H.M., Berwanger O., Fonseca H.A., Correa T.D., Ferraz L.R., Lapa M.G., Zampieri F.G., Veiga V.C., Azevedo L.C.P., Rosa R.G., Lopes R.D., Avezum A., Manoel A.L.O., Piza F.M.T., Martins P.A., Lisboa T.C., Pereira A.J., Olivato G.B., Dantas V.C.S., Milan E.P., Gebara O.C.E., Amazonas R.B., Oliveira M.B., Soares R.V.P., Moia D.D.F., Piano L.P.A., Castilho K., Momesso R., Schettino G.P.P., Rizzo L.V., Neto A.S., Machado F.R., Cavalcanti A.B. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396:959–967. doi: 10.1016/s0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay C., Judge G., Lucarelli S., Bautista S., Pandey R., Singh T., Antonescu C.N. Epidermal growth factor-stimulated Akt phosphorylation requires clathrin or ErbB2 but not receptor endocytosis. Mol. Biol. Cell. 2015;26:3504–3519. doi: 10.1091/mbc.E14-09-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Sevestre J., Mailhe M., Doudier B., Aubry C., Amrane S., Seng P., Hocquart M., Eldin C., Finance J., Vieira V.E., Tissot-Dupont H.T., Honore S., Stein A., Million M., Colson P., La Scola B., Veit V., Jacquier A., Deharo J.C., Drancourt M., Fournier P.E., Rolain J.M., Brouqui P., Raoult D. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Trav. Med. Infect. Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor M., Mankin A.S. Macrolide antibiotics: binding site, mechanism of action, resistance. Curr. Top. Med. Chem. 2003;3:949–961. doi: 10.2174/1568026033452159. [DOI] [PubMed] [Google Scholar]
- Gibson P.G., Yang I.A., Upham J.W., Reynolds P.N., Hodge S., James A.L., Jenkins C., Peters M.J., Marks G.B., Baraket M., Powell H., Taylor S.L., Leong L.E.X., Rogers G.B., Simpson J.L. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659–668. doi: 10.1016/s0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010;36:646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- Gladue R.P., Bright G.M., Isaacson R.E., Newborg M.F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob. Agents Chemother. 1989;33:277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., De Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., Coronaviridae Study Group Of The International Committee On Taxonomy Of, V. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U.F., Singh I., Helenius A. Mechanisms of virus uncoating. Trends Microbiol. 1994;2:52–56. doi: 10.1016/0966-842x(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Guo D., Dunbar J.D., Yang C.H., Pfeffer L.M., Donner D.B. Induction of Jak/STAT signaling by activation of the type 1 TNF receptor. J. Immunol. 1998;160:2742–2750. [PubMed] [Google Scholar]
- Gwinn W.M., Damsker J.M., Falahati R., Okwumabua I., Kelly-Welch A., Keegan A.D., Vanpouille C., Lee J.J., Dent L.A., Leitenberg D., Bukrinsky M.I., Constant S.L. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J. Immunol. 2006;177:4870–4879. doi: 10.4049/jimmunol.177.7.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., Van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydar D., Cory T.J., Birket S.E., Murphy B.S., Pennypacker K.R., Sinai A.P., Feola D.J. Azithromycin polarizes macrophages to an M2 phenotype via inhibition of the STAT1 and NF-κB signaling pathways. J. Immunol. 2019;203:1021–1030. doi: 10.4049/jimmunol.1801228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge S., Hodge G., Brozyna S., Jersmann H., Holmes M., Reynolds P.N. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur. Respir. J. 2006;28:486–495. doi: 10.1183/09031936.06.00001506. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Zhang S., Shen Y., Yang Q. Epidermal growth factor receptor is a co-factor for transmissible gastroenteritis virus entry. Virology. 2018;521:33–43. doi: 10.1016/j.virol.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y.H., Hsieh W.Y., Hsieh J.S., Liu F.C., Tsai C.H., Lu L.C., Huang C.Y., Wu C.L., Lin C.S. Alternative roles of STAT3 and MAPK signaling pathways in the MMPs activation and progression of lung injury induced by cigarette smoke exposure in ACE2 knockout mice. Int. J. Biol. Sci. 2016;12:454–465. doi: 10.7150/ijbs.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igakura T., Kadomatsu K., Taguchi O., Muramatsu H., Kaname T., Miyauchi T., Yamamura K., Arimura K., Muramatsu T. Roles of basigin, a member of the immunoglobulin superfamily, in behavior as to an irritating odor, lymphocyte response, and blood-brain barrier. Biochem. Biophys. Res. Commun. 1996;224:33–36. doi: 10.1006/bbrc.1996.0980. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivetić Tkalcević V., Bosnjak B., Hrvacić B., Bosnar M., Marjanović N., Ferencić Z., Situm K., Culić O., Parnham M.J., Eraković V. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. Eur. J. Pharmacol. 2006;539:131–138. doi: 10.1016/j.ejphar.2006.03.074. [DOI] [PubMed] [Google Scholar]
- Joshi M.B., Lad A., Bharath Prasad A.S., Balakrishnan A., Ramachandra L., Satyamoorthy K. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 2013;587:2241–2246. doi: 10.1016/j.febslet.2013.05.053. [DOI] [PubMed] [Google Scholar]
- Kane L.P., Shapiro V.S., Stokoe D., Weiss A. Induction of NF-kappaB by the akt/PKB kinase. Curr. Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- Kim Y.I., Kim S.G., Kim S.M., Kim E.H., Park S.J., Yu K.M., Chang J.H., Kim E.J., Lee S., Casel M.A.B., Um J., Song M.S., Jeong H.W., Lai V.D., Kim Y., Chin B.S., Park J.S., Chung K.H., Foo S.S., Poo H., Mo I.P., Lee O.J., Webby R.J., Jung J.U., Choi Y.K. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27:704–709. doi: 10.1016/j.chom.2020.03.023. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouznetsova J., Sun W., Martínez-Romero C., Tawa G., Shinn P., Chen C.Z., Schimmer A., Sanderson P., Mckew J.C., Zheng W., García-Sastre A. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg. Microb. Infect. 2014;3:e84. doi: 10.1038/emi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C.H., Lee M.S., Kuo H.F., Lin Y.C., Hung C.H. Azithromycin suppresses Th1- and Th2-related chemokines IP-10/MDC in human monocytic cell line. J. Microbiol. Immunol. Infect. 2019;52:872–879. doi: 10.1016/j.jmii.2019.10.001. [DOI] [PubMed] [Google Scholar]
- Laforge M., Elbim C., Frere C., Hemadi M., Massaad C., Nuss P., Benoliel J.J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier J.C., Million M., Gautret P., Colson P., Cortaredona S., Giraud-Gatineau A., Honore S., Gaubert J.Y., Fournier P.E., Tissot-Dupont H., Chabriere E., Stein A., Deharo J.C., Fenollar F., Rolain J.M., Obadia Y., Jacquier A., La Scola B., Brouqui P., Drancourt M., Parola P., Raoult D. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Trav. Med. Infect. Dis. 2020;36:101791. doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauriola M., Pani A., Ippoliti G., Mortara A., Milighetti S., Mazen M., Perseghin G., Pastori D., Grosso P., Scaglione F. Effect of combination therapy of hydroxychloroquine and azithromycin on mortality in patients with COVID-19. Clin Transl Sci. 2020;13:1071–1076. doi: 10.1111/cts.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.B., Nagai A., Kim S.U. Cytokines, chemokines, and cytokine receptors in human microglia. J. Neurosci. Res. 2002;69:94–103. doi: 10.1002/jnr.10253. [DOI] [PubMed] [Google Scholar]
- Lefrancais E., Mallavia B., Zhuo H., Calfee C.S., Looney M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight. 2018;3 doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legssyer R., Huaux F., Lebacq J., Delos M., Marbaix E., Lebecque P., Lison D., Scholte B.J., Wallemacq P., Leal T. Azithromycin reduces spontaneous and induced inflammation in DeltaF508 cystic fibrosis mice. Respir. Res. 2006;7:134. doi: 10.1186/1465-9921-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zu S., Deng Y.Q., Li D., Parvatiyar K., Quanquin N., Shang J., Sun N., Su J., Liu Z., Wang M., Aliyari S.R., Li X.F., Wu A., Ma F., Shi Y., Nielsevn-Saines K., Jung J.U., Qin F.X., Qin C.F., Cheng G. Azithromycin protects against Zika virus infection by upregulating virus-induced type I and III interferon responses. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/aac.00394-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., Zhang Q., Wu J. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li P., Lin S.H., Zheng Y.Q., Zheng X.X. Paeonol inhibited TNFα-induced GM-CSF expression in fibroblast-like synoviocytes. Int. J. Clin. Pharm. Ther. 2014;52:986–996. doi: 10.5414/cp202127. [DOI] [PubMed] [Google Scholar]
- Libermann T.A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.I., Tsai C.H., Sun Y.L., Hsieh W.Y., Lin Y.C., Chen C.Y., Lin C.S. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int. J. Biol. Sci. 2018;14:253–265. doi: 10.7150/ijbs.23489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.T., Chen L.K., Jian D.Y., Hsu T.C., Huang W.C., Kuan T.T., Wu S.Y., Kwok C.F., Ho L.T., Juan C.C. Visfatin promotes monocyte adhesion by upregulating ICAM-1 and VCAM-1 expression in endothelial cells via activation of p38-PI3K-akt signaling and subsequent ROS production and IKK/NF-κB activation. Cell. Physiol. Biochem. 2019;52:1398–1411. doi: 10.33594/000000098. [DOI] [PubMed] [Google Scholar]
- Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., Wang L., Zhou W., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid P.B., Panchal R.G., Warren T.K., Shurtleff A.C., Endsley A.N., Green C.E., Kolokoltsov A., Davey R., Manger I.D., Gilfillan L., Bavari S., Tanga M.J. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect. Dis. 2015;1:317–326. doi: 10.1021/acsinfecdis.5b00030. [DOI] [PubMed] [Google Scholar]
- Maraj I., Hummel J.P., Taoutel R., Chamoun R., Workman V., Li C., Tran L., Delvecchio A., Howes C., Akar J.G. Incidence and determinants of QT interval prolongation in COVID-19 patients treated with hydroxychloroquine and azithromycin. J. Cardiovasc. Electrophysiol. 2020;31:1904–1907. doi: 10.1111/jce.14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., Mcauley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/s0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel M., Akbarshahi H., Bjermer L., Uller L. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci. Rep. 2016;6:28698. doi: 10.1038/srep28698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E.S., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., Rondina M.T., Egeblad M., Schiffman J.D., Yost C.C. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million M., Lagier J.C., Gautret P., Colson P., Fournier P.E., Amrane S., Hocquart M., Mailhe M., Esteves-Vieira V., Doudier B., Aubry C., Correard F., Giraud-Gatineau A., Roussel Y., Berenger C., Cassir N., Seng P., Zandotti C., Dhiver C., Ravaux I., Tomei C., Eldin C., Tissot-Dupont H., Honore S., Stein A., Jacquier A., Deharo J.C., Chabriere E., Levasseur A., Fenollar F., Rolain J.M., Obadia Y., Brouqui P., Drancourt M., La Scola B., Parola P., Raoult D. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Trav. Med. Infect. Dis. 2020;35:101738. doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miscia S., Marchisio M., Grilli A., Di Valerio V., Centurione L., Sabatino G., Garaci F., Zauli G., Bonvini E., Di Baldassarre A. Tumor necrosis factor alpha (TNF-alpha) activates Jak1/Stat3-Stat5B signaling through TNFR-1 in human B cells. Cell Growth Differ. 2002;13:13–18. [PubMed] [Google Scholar]
- Mishra D., Dey C.S. Type-2 diabetes, a co-morbidity in Covid-19: does insulin signaling matter? Biochem. Soc. Trans. 2021 doi: 10.1042/bst20201062. [DOI] [PubMed] [Google Scholar]
- Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., De Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Maladies Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkruys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado Del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Kamimura D., Hirano T. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity. 2019;50:812–831. doi: 10.1016/j.immuni.2019.03.027. [DOI] [PubMed] [Google Scholar]
- Organization W.H. 2020. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19-11 March 2020. Geneva, Switzerland. [Google Scholar]
- Peters D.H., Friedel H.A., Mctavish D. Azithromycin. A review of its antimicrobial activity, pharmacokinetic properties and clinical efficacy. Drugs. 1992;44:750–799. doi: 10.2165/00003495-199244050-00007. [DOI] [PubMed] [Google Scholar]
- Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/ap-200220-0772. [DOI] [PubMed] [Google Scholar]
- Pushkarsky T., Zybarth G., Dubrovsky L., Yurchenko V., Tang H., Guo H., Toole B., Sherry B., Bukrinsky M. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C., Xie Q., Zhang D., Chen Q., Hu J., Xu L. GM-CSF induces cyclin D1 expression and proliferation of endothelial progenitor cells via PI3K and MAPK signaling. Cell. Physiol. Biochem. 2014;33:784–795. doi: 10.1159/000358652. [DOI] [PubMed] [Google Scholar]
- Qiu T., Tian Y., Gao Y., Ma M., Li H., Liu X., Wu H., Zhang Y., Ding H., Cao M., Zhang J., Dai J., Chen J., Cai H. PTEN loss regulates alveolar epithelial cell senescence in pulmonary fibrosis depending on Akt activation. Aging (N Y) 2019;11:7492–7509. doi: 10.18632/aging.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermecker C., Detrembleur N., Guiot J., Cavalier E., Henket M., D'emal C., Vanwinge C., Cataldo D., Oury C., Delvenne P., Marichal T. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020;217 doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I., Gilmour P.S., Jimenez L.A., Macnee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol. Cell. Biochem. 2002;234–235:239–248. [PubMed] [Google Scholar]
- Recinos A., 3rd, Lejeune W.S., Sun H., Lee C.Y., Tieu B.C., Lu M., Hou T., Boldogh I., Tilton R.G., Brasier A.R. Angiotensin II induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice. Atherosclerosis. 2007;194:125–133. doi: 10.1016/j.atherosclerosis.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria A.E., Mfuna Endam L., Adam D., Filali-Mouhim A., Maniakas A., Rousseau S., Brochiero E., Gallo S., Desrosiers M. Azithromycin downregulates gene expression of IL-1β and pathways involving TMPRSS2 and TMPRSS11D required by SARS-CoV-2. Am. J. Respir. Cell Mol. Biol. 2020;63:707–709. doi: 10.1165/rcmb.2020-0285LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallack H., Di Lullo E., Arias C., Knopp K.A., Laurie M.T., Sandoval-Espinosa C., Mancia Leon W.R., Krencik R., Ullian E.M., Spatazza J., Pollen A.A., Mandel-Brehm C., Nowakowski T.J., Kriegstein A.R., Derisi J.L. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl. Acad. Sci. U. S. A. 2016;113:14408–14413. doi: 10.1073/pnas.1618029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronconi G., Tete G., Kritas S.K., Gallenga C.E., Caraffa A., Ross R., Conti P. SARS-CoV-2, which induces COVID-19, causes kawasaki-like disease in children: role of pro-inflammatory and anti-inflammatory cytokines. J. Biol. Regul. Homeost. Agents. 2020;34:767–773. doi: 10.23812/editorial-ronconi-e-59. [DOI] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Bultmann A., Ungerer M., Joghetaei N., Bulbul O., Thieme S., Chavakis T., Toole B.P., Gawaz M., Schömig A., May A.E. Extracellular matrix metalloproteinase inducer regulates matrix metalloproteinase activity in cardiovascular cells: implications in acute myocardial infarction. Circulation. 2006;113:834–841. doi: 10.1161/circulationaha.105.568162. [DOI] [PubMed] [Google Scholar]
- Schonthaler H.B., Guinea-Viniegra J., Wagner E.F. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann. Rheum. Dis. 2011;70(Suppl. 1):i109–i112. doi: 10.1136/ard.2010.140533. [DOI] [PubMed] [Google Scholar]
- Schulz C., Von Bruhl M.L., Barocke V., Cullen P., Mayer K., Okrojek R., Steinhart A., Ahmad Z., Kremmer E., Nieswandt B., Frampton J., Massberg S., Schmidt R. EMMPRIN (CD147/basigin) mediates platelet-monocyte interactions in vivo and augments monocyte recruitment to the vascular wall. J. Thromb. Haemostasis. 2011;9:1007–1019. doi: 10.1111/j.1538-7836.2011.04235.x. [DOI] [PubMed] [Google Scholar]
- Seizer P., Ochmann C., Schönberger T., Zach S., Rose M., Borst O., Klingel K., Kandolf R., Macdonald H.R., Nowak R.A., Engelhardt S., Lang F., Gawaz M., May A.E. Disrupting the EMMPRIN (CD147)-cyclophilin A interaction reduces infarct size and preserves systolic function after myocardial ischemia and reperfusion. Arterioscler. Thromb. Vasc. Biol. 2011;31:1377–1386. doi: 10.1161/atvbaha.111.225771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L.W., Mao H.J., Wu Y.L., Tanaka Y., Zhang W. TMPRSS2: a potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1–10. doi: 10.1016/j.biochi.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Zhang B., Chen X.J., Xu D.Q., Wang Y.X., Dong H.Y., Ma S.R., Sun R.H., Hui Y.P., Li Z.C. Osthole protects lipopolysaccharide-induced acute lung injury in mice by preventing down-regulation of angiotensin-converting enzyme 2. Eur. J. Pharmaceut. Sci. 2013;48:819–824. doi: 10.1016/j.ejps.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Shim Y.J., Kang B.H., Jeon H.S., Park I.S., Lee K.U., Lee I.K., Park G.H., Lee K.M., Schedin P., Min B.H. Clusterin induces matrix metalloproteinase-9 expression via ERK1/2 and PI3K/Akt/NF-κB pathways in monocytes/macrophages. J. Leukoc. Biol. 2011;90:761–769. doi: 10.1189/jlb.0311110. [DOI] [PubMed] [Google Scholar]
- Stellari F.F., Sala A., Donofrio G., Ruscitti F., Caruso P., Topini T.M., Francis K.P., Li X., Carnini C., Civelli M., Villetti G. Azithromycin inhibits nuclear factor-κB activation during lung inflammation: an in vivo imaging study. Pharmacol Res Perspect. 2014;2 doi: 10.1002/prp2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Yang L., Feldman R.I., Sun X.M., Bhalla K.N., Jove R., Nicosia S.V., Cheng J.Q. Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85alpha, androgen receptor, and Src. J. Biol. Chem. 2003;278:42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touret F., Gilles M., Barral K., Nougairede A., Van Helden J., Decroly E., De Lamballerie X., Coutard B. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 2020;10:13093. doi: 10.1038/s41598-020-70143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D.H., Sugamata R., Hirose T., Suzuki S., Noguchi Y., Sugawara A., Ito F., Yamamoto T., Kawachi S., Akagawa K.S., Ōmura S., Sunazuka T., Ito N., Mimaki M., Suzuki K. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J. Antibiot. (Tokyo) 2019;72:759–768. doi: 10.1038/s41429-019-0204-x. [DOI] [PubMed] [Google Scholar]
- Tsai W.C., Rodriguez M.L., Young K.S., Deng J.C., Thannickal V.J., Tateda K., Hershenson M.B., Standiford T.J. Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection. Am. J. Respir. Crit. Care Med. 2004;170:1331–1339. doi: 10.1164/rccm.200402-200OC. [DOI] [PubMed] [Google Scholar]
- Tsoyi K., Jang H.J., Nizamutdinova I.T., Park K., Kim Y.M., Kim H.J., Seo H.G., Lee J.H., Chang K.C. PTEN differentially regulates expressions of ICAM-1 and VCAM-1 through PI3K/Akt/GSK-3β/GATA-6 signaling pathways in TNF-α-activated human endothelial cells. Atherosclerosis. 2010;213:115–121. doi: 10.1016/j.atherosclerosis.2010.07.061. [DOI] [PubMed] [Google Scholar]
- Ulrich H., Pillat M.M. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev Rep. 2020;16:434–440. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambier C.G., Goren A., Vaño-Galván S., Ramos P.M., Ossimetha A., Nau G., Herrera S., Mccoy J. Androgen sensitivity gateway to COVID-19 disease severity. Drug Dev. Res. 2020;81:771–776. doi: 10.1002/ddr.21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.H., Yao H., Chen L.N., Jia J.F., Wang L., Dai J.Y., Zheng Z.H., Chen Z.N., Zhu P. CD147 induces angiogenesis through a vascular endothelial growth factor and hypoxia-inducible transcription factor 1α-mediated pathway in rheumatoid arthritis. Arthritis Rheum. 2012;64:1818–1827. doi: 10.1002/art.34341. [DOI] [PubMed] [Google Scholar]
- Wang J., Xie L., Wang S., Lin J., Liang J., Xu J. Azithromycin promotes alternatively activated macrophage phenotype in systematic lupus erythematosus via PI3K/Akt signaling pathway. Cell Death Dis. 2018;9:1080. doi: 10.1038/s41419-018-1097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Xu B., Fan K., Wu J., Wang T. Inflammation suppression by dexamethasone via inhibition of CD147-mediated NF-κB pathway in collagen-induced arthritis rats. Mol. Cell. Biochem. 2020;473:63–76. doi: 10.1007/s11010-020-03808-5. [DOI] [PubMed] [Google Scholar]
- Wang X., Xu W., Hu G., Xia S., Sun Z., Liu Z., Xie Y., Zhang R., Jiang S., Lu L. Retracted article: SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell. Mol. Immunol. 2020:1–3. doi: 10.1038/s41423-020-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wildfeuer A., Laufen H., Muller-Wening D., Haferkamp O. Interaction of azithromycin and human phagocytic cells. Uptake of the antibiotic and the effect on the survival of ingested bacteria in phagocytes. Arzneimittelforschung. 1989;39:755–758. [PubMed] [Google Scholar]
- Wildfeuer A., Laufen H., Zimmermann T. Uptake of azithromycin by various cells and its intracellular activity under in vivo conditions. Antimicrob. Agents Chemother. 1996;40:75–79. doi: 10.1128/aac.40.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.W., Goodman C.D., Sleebs B.E., Weiss G.E., De Jong N.W., Angrisano F., Langer C., Baum J., Crabb B.S., Gilson P.R., Mcfadden G.I., Beeson J.G. Macrolides rapidly inhibit red blood cell invasion by the human malaria parasite, Plasmodium falciparum. BMC Biol. 2015;13:52. doi: 10.1186/s12915-015-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Sriramula S., Xia H., Moreno-Walton L., Culicchia F., Domenig O., Poglitsch M., Lazartigues E. Clinical relevance and role of neuronal AT(1) receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Circ. Res. 2017;121:43–55. doi: 10.1161/circresaha.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Liu R., Huang L., Xu Y., Su M., Chen J., Geng L., Xu W., Gong S. CD147 aggravated inflammatory bowel disease by triggering NF-κB-Mediated pyroptosis. Biomed Res Int. 2020 doi: 10.1155/2020/5341247. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue T., Wei N., Xin Z., Qingyu X. Angiotensin-converting enzyme-2 overexpression attenuates inflammation in rat model of chronic obstructive pulmonary disease. Inhal. Toxicol. 2014;26:14–22. doi: 10.3109/08958378.2013.850563. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Yancey P.G., Zuo Y., Ma L.J., Kaseda R., Fogo A.B., Ichikawa I., Linton M.F., Fazio S., Kon V. Macrophage polarization by angiotensin II-type 1 receptor aggravates renal injury-acceleration of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011;31:2856–2864. doi: 10.1161/atvbaha.111.237198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Wang L., Lin H.K., Kan P.Y., Xie S., Tsai M.Y., Wang P.H., Chen Y.T., Chang C. Interleukin-6 differentially regulates androgen receptor transactivation via PI3K-Akt, STAT3, and MAPK, three distinct signal pathways in prostate cancer cells. Biochem. Biophys. Res. Commun. 2003;305:462–469. doi: 10.1016/s0006-291x(03)00792-7. [DOI] [PubMed] [Google Scholar]
- Yang X.H., Deng W., Tong Z., Liu Y.X., Zhang L.F., Zhu H., Gao H., Huang L., Liu Y.L., Ma C.M., Xu Y.F., Ding M.X., Deng H.K., Qin C. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp. Med. 2007;57:450–459. [PubMed] [Google Scholar]
- Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X.J., Li L., Du X., Yang B., Mei C.L. LncRNA TUG1 inhibits the proliferation and fibrosis of mesangial cells in diabetic nephropathy via inhibiting the PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23:7519–7525. doi: 10.26355/eurrev_201909_18867. [DOI] [PubMed] [Google Scholar]
- Zhang F., Ren X., Zhao M., Zhou B., Han Y. Angiotensin-(1-7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci. Rep. 2016;6:34621. doi: 10.1038/srep34621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Yu F.Q., Huang X.J., Xu B.Y., Li Y.L., Zhao X.Y., Guo H.F., Luan B. Azithromycin influences airway remodeling in asthma via the PI3K/Akt/MTOR/HIF-1α/VEGF pathway. J. Biol. Regul. Homeost. Agents. 2018;32:1079–1088. [PubMed] [Google Scholar]
- Zhao Y., Wang H., Li X., Cao M., Lu H., Meng Q., Pang H., Li H., Nadolny C., Dong X., Cai L. Ang II-AT1R increases cell migration through PI3K/AKT and NF-κB pathways in breast cancer. J. Cell. Physiol. 2014;229:1855–1862. doi: 10.1002/jcp.24639. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. National Science Review. 2020 doi: 10.1093/nsr/nwaa041. nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]