Abstract
Background
Neonatal hypoglycaemia is a common condition that can be associated with brain injury. Current practice usually includes early identification of at‐risk infants (e.g. infants of diabetic mothers; preterm, small‐ or large‐for‐gestational‐age infants), and prophylactic measures are advised. However, these measures usually involve use of formula milk or admission to the neonatal unit. Dextrose gel is non‐invasive, inexpensive and effective for treatment of neonatal hypoglycaemia. Prophylactic dextrose gel can reduce the incidence of neonatal hypoglycaemia, thus potentially reducing separation of mother and baby and supporting breastfeeding, as well as preventing brain injury.
This is an update of a previous Cochrane Review published in 2017.
Objectives
To assess the effectiveness and safety of oral dextrose gel given to newborn infants at risk of hypoglycaemia in preventing hypoglycaemia and reducing long‐term neurodevelopmental impairment.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 10) in the Cochrane Library; and Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) on 19 October 2020. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing oral dextrose gel versus placebo, no intervention, or other therapies for the prevention of neonatal hypoglycaemia.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias. We contacted investigators to obtain additional information. We used fixed‐effect meta‐analyses. We used the GRADE approach to assess the certainty of evidence.
Main results
We included two studies conducted in high‐income countries comparing oral dextrose gel versus placebo in 2548 infants at risk of neonatal hypoglycaemia. Of these, one study was included in the previous version of this review. We judged these two studies to be at low risk of bias, and that the evidence for most outcomes was of moderate certainty.
Meta‐analysis of the two studies showed that oral dextrose gel reduces the risk of hypoglycaemia (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.79 to 0.95; risk difference (RD) ‐0.06, 95% CI ‐0.10 to ‐0.02; 2548 infants; high certainty evidence). One study reported that oral dextrose gel probably reduces the risk of major neurological disability at two years' corrected age (RR 0.21, 95% CI 0.05 to 0.78; RD ‐0.05, 95% CI ‐0.09 to 0.00; 360 infants; moderate certainty evidence).
Meta‐analysis of the two studies showed that oral dextrose gel probably reduces the risk of receipt of treatment for hypoglycaemia during initial hospital stay (RR 0.89, 95% CI 0.79 to 1.00; 2548 infants; moderate certainty evidence) but makes little or no difference to the risk of receipt of intravenous treatment for hypoglycaemia (RR 1.01, 0.68 to 1.49; 2548 infants; moderate certainty evidence). Oral dextrose gel may have little or no effect on the risk of separation from the mother for treatment of hypoglycaemia (RR 1.12, 95% CI 0.81 to 1.55; two studies, 2548 infants; low certainty evidence).
There is probably little or no difference in the risk of adverse events in infants who receive oral dextrose gel compared to placebo gel (RR 1.22, 95% CI 0.64 to 2.33; two studies, 2510 infants; moderate certainty evidence), but there are no studies comparing oral dextrose with other comparators such as no treatment, standard care or other therapies.
No data were available on exclusive breastfeeding after discharge.
Authors' conclusions
Oral dextrose gel reduces the risk of neonatal hypoglycaemia in at‐risk infants and probably reduces the risk of major neurological disability at two years of age or greater without increasing the risk of adverse events compared to placebo gel. Additional large follow‐up studies at two years of age or older are required. Future research should also be undertaken in low‐ and middle‐income countries, preterm infants, using other dextrose gel preparations, and using comparators other than placebo gel. There are three studies awaiting classification and one ongoing study which may alter the conclusions of the review when published.
Plain language summary
Oral dextrose gel for prevention of low blood glucose levels in newborn babies
Review question
Is oral dextrose gel effective and safe in preventing low blood glucose levels and reducing long‐term disability in newborn babies at risk of low blood glucose levels?
Background
Low blood sugar (glucose) levels are important because they are common and are associated with brain injury in newborn babies. Up to 15 of every 100 babies will have low blood glucose levels over the first few days after they are born, and as many as half of babies who are at higher risk (those born preterm, or smaller or larger than usual, or whose mothers have diabetes).
Low blood glucose levels can cause problems with academic achievement and development during childhood. Some evidence suggests that even one episode of low blood glucose or episodes that are undetected can contribute to these problems. Therefore, it would be useful to prevent low glucose levels from occurring. Additionally, treatments for low glucose levels often include formula milk or admission to the neonatal unit, leading to the separation of mother and baby. The treatments and the separation may both impair breastfeeding.
Dextrose (sugar) gel can be rubbed on the inside of a baby's mouth, where the sugar can be absorbed and help raise blood glucose levels, thus potentially helping prevent low glucose levels.
Study characteristics
We identified two studies in high‐income countries that compared oral dextrose gel with placebo (inactive) gel for preventing low blood glucose levels in 2548 at‐risk infants. The evidence in this Cochrane Review is current to October 2020.
Key results
Two studies showed that preventative oral dextrose gel reduces the risk of low blood glucose levels in newborn infants at risk. A single study reporting two‐year outcomes in 360 infants found that oral dextrose gel given to at‐risk infants to prevent low blood glucose levels probably reduces the risk of major disability at two years of age, but additional follow‐up studies are needed.
Two studies showed that oral dextrose gel probably reduces the chance of receiving any treatment for low blood glucose during initial hospital stay but makes little or no difference to the chance of receiving intravenous treatment for low glucose, or separation from the mother for treatment of low glucose levels.
Evidence from two studies suggests that infants given oral dextrose gel are not at a higher risk of adverse events (harms) such as choking or vomiting compared with infants given placebo gel, but there was no information to assess whether oral dextrose gel is safer than no treatment or other therapies. No data were available on exclusive breastfeeding after discharge.
We assessed the risk of bias in the included studies as low, meaning it is unlikely that there is a systematic error in estimating the effect of the intervention.
Future research should be undertaken in low‐ and middle‐income countries, preterm infants, using other dextrose gel preparations and comparators other than placebo gel. There are three studies awaiting classification and one ongoing study which may alter the conclusions of the review when they are published.
Certainty of evidence
We graded the certainty of the evidence as moderate for all outcomes except risk of low blood glucose levels (assessed as high certainty) and separation from mother (assessed as low certainty).
Summary of findings
Summary of findings 1. Dextrose gel compared with placebo for prevention of hypoglycaemia in newborn infants.
| Dextrose gel compared with placebo for prevention of hypoglycaemia in newborn infants | ||||||
| Patient or population: newborn infants at risk of neonatal hypoglycaemia Setting: New Zealand and Australia Intervention: dextrose gel Comparison: placebo gel | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with dextrose gel | |||||
| Hypoglycaemia (investigator‐defined) | 433 per 1000 | 377 per 1000 (342 to 411) | RR 0.87 (0.79 to 0.95) |
2548 (2 RCTs) |
⊕⊕⊕⊕ HIGH | |
| Major neurological disability at 2 years of age or older (Defined as any of the following: legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy or developmental delay/intellectual impairment (developmental quotient or intelligence quotient lower than 2 SD below the mean)) |
60 per 1000 | 47 per 1000 (3 to 41) | RR 0.21 (0.05 to 0.78) | 360 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | Low event rates: 3/277 in dextrose gel group and 7/138 in placebo group. |
| Receipt of treatment for hypoglycaemia during initial hospital stay (investigator‐defined, any treatment ‐ oral dextrose gel, intravenous dextrose, or other drug therapy) during initial hospital stay (yes/no) |
316 per 1000 | 281 per 1000 (249 to 316) | RR 0.89 (0.79 to 1.00) | 2548 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb | |
| Receipt of intravenous treatment for hypoglycaemia (yes/no) |
37 per 1000 | 37 per 1000 (25 to 55) | RR 1.01 (0.68 to 1.49) | 2548 (2 RCTs) | ⊕⊕⊕⊝ MODERATEc | |
| Adverse events# (e.g. choking or vomiting at time of administration) | 10 per 1000 | 12 per 1000 (6 to 24) |
RR 1.22 (0.64 to 2.33) |
2510 (2 RCTs) |
⊕⊕⊕⊝ MODERATEc | Low event rates: 27/1322 in dextrose gel group and 12/1188 in placebo group. |
| Separation from mother for treatment of hypoglycaemia (infant nursed in an environment that is not in the same room as the mother, e.g. for NICU admission or the like) (yes/no)) |
50 per 1000 | 56 per 1000 (40 to 77) | RR 1.12 (0.81 to 1.55) |
2548 (2 RCTs) |
⊕⊕⊝⊝ LOWc,d |
|
| Breastfeeding (exclusive after discharge) ‐ not reported (WHO 2008 definition (yes/no)) |
‐ | ‐ | ‐ | ‐ | ‐ | No data were reported for this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); #Placebo gel was the comparator. CI: confidence interval; NICU: neonatal intensive care unit; OR: odds ratio; RR: risk ratio; SD: standard deviations | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for serious imprecision (due to low event rates) bDowngraded one level for serious imprecision (due to the CI including 1) cDowngraded one level for serious imprecision (due to the CI including possibility of both benefits or harms) dDowngraded one level for serious inconsistency (due to the high I2 value of 83%)
Background
Description of the condition
Hypoglycaemia is the most common metabolic disorder of the newborn (Hay 2009), and is potentially preventable (Chertok 2009; Singhal 1991; Singhal 1992). Neonatal hypoglycaemia can cause both brain damage (Burns 2008; Duvanel 1999; Kerstjens 2012; Koh 1988), and death (Achoki 2010; Cornblath 1965; Nadjm 2013; Willcox 2010). Some authors have reported brain abnormalities associated with neonatal hypoglycaemia on magnetic resonance imaging (MRI). Early studies reported that the most common site of damage is the occipital cortex (Alkalay 2005; Spar 1994). However, more recent studies have reported widespread MRI changes in the temporoparietal region, cerebral cortex and basal ganglia/thalamus (Burns 2008).
Up to 15% of newborn infants will have low blood glucose concentrations (Hay 2009). This rate is much higher among infants with additional risk factors: up to 50% in infants of diabetic mothers (Maayan‐Metzger 2009), and 66% in preterm infants (Lucas 1988). Those at highest risk of hypoglycaemia are infants of diabetic mothers (Agrawal 2000; Maayan‐Metzger 2009), born large for gestation (Weissmann‐Brenner 2012), small for gestation (Hawdon 1993), or preterm (Kerstjens 2012). Fifty per cent of these infants will develop at least one episode of hypoglycaemia, and 20% will have more than one episode (Harris 2012). Additional risk factors for neonatal hypoglycaemia include perinatal asphyxia (Salhab 2004), prolonged labour, hypothermia, sepsis, and maternal medications such as b‐agonists (Kurtoglu 2005), and beta‐blockers (Daskas 2013).
The definition of neonatal hypoglycaemia remains controversial, and various definitions have been proposed (Agrawal 2000; Burns 2008; Kerstjens 2012; Maayan‐Metzger 2009), or suggested as thresholds for intervention (Adamkin 2011; Cornblath 2000). A blood glucose concentration of less than 2.6 mmol/L has been widely accepted as a definition of hypoglycaemia (Harris 2014). This was heavily influenced by two studies. One described abnormal sensory evoked potentials among infants with blood glucose concentrations lower than 2.6 mmol/L (Koh 1988). The second reported a relationship between the number of days on which blood glucose measurements lower than 2.6 mmol/L were recorded in preterm infants and neurodevelopmental impairment at 18 months (Lucas 1988), and at seven to eight years (Lucas 1999). Infants who are ‘asymptomatic’ during periods of hypoglycaemia were previously considered to have a better outcome than those who exhibit signs (Hawdon 1993; Kalhan 2000; Koivisto 1972). However, Stenninger and colleagues found that longer‐term neurodevelopmental outcomes did not differ between infants who exhibited signs and those who did not (Stenninger 1998).
The effects of transient neonatal hypoglycaemia on longer‐term outcomes are not yet fully defined. However, some evidence suggests that even transient and undetected episodes may be associated with adverse outcomes (McKinlay 2017). For example, a retrospective population study demonstrated an association between a single initial blood glucose concentration of less than 2.6 mmol/L, followed by a repeat result above this, and lower academic test scores at 10 years of age (Kaiser 2015). Further, a prospective cohort study of infants at risk of neonatal hypoglycaemia, half of whom became hypoglycaemic and were treated to maintain blood glucose concentrations of 2.6 mmol/L or higher, found that clinically undetected low interstitial glucose concentrations were associated with an increased risk of executive dysfunction at four and a half years of age (McKinlay 2017). These findings suggest that even an effective treatment for neonatal hypoglycaemia may not be enough to optimise outcomes of all babies, and that prophylaxis should be considered.
Treatment of neonatal hypoglycaemia commonly requires admission to a newborn intensive care unit (NICU) or special care baby unit (SCBU), separating mothers and infants and interfering with the establishment of breastfeeding, thus incurring a high social and financial cost. The World Health Organization (WHO) states “… an approach aimed first at the prevention of hypoglycaemia, second at its reliable detection in infants at risk and third at appropriate treatment which will not be deleterious to breastfeeding is … of global importance” (WHO 1997). The American Academy of Pediatrics recommends “… early identification of the at‐risk infant and institution of prophylactic measures to prevent neonatal hypoglycaemia” (Adamkin 2011).
Widely accepted clinical monitoring and management of infants at risk of neonatal hypoglycaemia involves:
early identification of pertinent risk factors;
early feeding, skin‐to‐skin contact and ensuring that newborns are kept warm and dry;
pre‐feed blood glucose concentration measurement to determine blood glucose concentrations at the time when it is most at risk of being low; and
monitoring during the period of highest risk until blood glucose concentration is demonstrated to remain above the chosen threshold for intervention (Adamkin 2011; CPSFNC 2004; NICE 2008; UNICEF 2013; WHO 1997).
However, despite recommendations to prevent neonatal hypoglycaemia, there is little evidence of effective interventions to achieve this.
Early skin‐to‐skin contact may reduce the incidence of neonatal hypoglycaemia because it has a protective influence on thermo‐regulation (UNICEF 2013). A meta‐analysis of two randomised trials of low birthweight infants found that compared with standard of care, skin‐to‐skin contact was associated with a decreased risk of neonatal hypoglycaemia (Boundy 2016). Further, a Cochrane Review of three randomised trials of 144 healthy full‐term, low birthweight infants reported that skin‐to‐skin contact during the establishment of breastfeeding was associated with a 10 mg/dL (0.6 mmol/L) higher blood glucose concentration at 75 to 90 minutes after birth compared with standard contact. However, the evidence was graded as low certainty (Moore 2016). A retrospective pre‐ and post‐intervention study of 561 infants at risk of neonatal hypoglycaemia reported that prolonged skin‐to‐skin contact for at least 12 hours after birth during blood glucose monitoring was associated with a 4.6% reduction in admission rates to the NICU for neonatal hypoglycaemia and a 3.8% reduction in the number of infants receiving intravenous dextrose (Chiruvolu 2017). However, the small sample sizes and poor quality of some of these studies limit confidence in the findings.
Early feeding is also recommended for prevention of neonatal hypoglycaemia. In infants of diabetic mothers, early feeding (within 30 minutes of birth) was reported to decrease the incidence of subsequent neonatal hypoglycaemia, and increase mean blood glucose concentration (Chertok 2009). However, two other studies showed that the early initiation of breastfeeding within 30 minutes to one hour after birth does not affect blood glucose concentration at one and two hours in infants without risk factors for neonatal hypoglycaemia (Sweet 1999; Zhou 2017).
Antenatal expression of colostrum may potentially increase the available colostrum supply for infants soon after birth, while reducing the time taken to establish breastfeeding and decreasing the use of formula (Cox 2006). However, the Diabetes and Antenatal Milk Expressing (DAME) study that randomised women with diabetes to antenatal expression of breastmilk or standard care reported no differences between groups for mean blood glucose concentrations (Forster 2011), incidence of neonatal hypoglycaemia, admission to NICU for hypoglycaemia or time until euglycaemic status was achieved (defined as three consecutive blood glucose measurements of 2.6 mmol/L or higher) (Forster 2017). This is consistent with a post hoc analysis of a randomised controlled trial which showed that breastmilk expressed either before or after birth and given to hypoglycaemic infants did not increase glucose concentrations (Harris 2017).
Supplementation or substitution of breastfeeding with fluid or foods other than expressed breast milk may reduce the duration of breastfeeding (Blomquist 1994; Demir 2020; Smith 2016). Therefore, the commonly accepted practice is to advise exclusive breastfeeding (Eidelman 2012; UNICEF 2013). Healthy newborn infants will usually maintain their blood glucose concentration despite the small‐volume, low‐energy food source provided by colostrum. However, colostrum alone cannot be relied upon to meet the essential energy needs of infants with additional risk factors for neonatal hypoglycaemia. Thus, infants at risk of hypoglycaemia frequently receive supplemental or complementary feeding during establishment of feeding (Blomquist 1994; Harris 2013).
Powdered sugar has been used as an addition to formula in an attempt to prevent neonatal hypoglycaemia. Two randomised trials in India compared formula with formula plus added powdered sugar for prevention of subsequent hypoglycaemia in infants at risk of hypoglycaemia (small or large for gestational age) (Singhal 1991; Singhal 1992). Both studies reported a significant reduction in the incidence of subsequent hypoglycaemia among infants who received formula plus powdered sugar. In contrast, a randomised trial in Thailand of 425 infants at risk of neonatal hypoglycaemia reported no difference in the blood glucose concentrations measured at one, three and six hours after birth of infants who received 200 mg/kg prophylactic oral sucrose solution plus feeding versus infants who received feeding alone (Surachaidungtavil 2020).
The ideal intervention would be effective in preventing hypoglycaemia while reducing the need for artificial formula, improving breastfeeding rates, reducing costs, as well as potentially reducing the risk of later adverse outcomes. Oral dextrose gel is an effective first line treatment for neonatal hypoglycaemia (Harris 2013), that also improves breastfeeding (Weston 2016), reduces costs (Glasgow 2018), and appears safe (Harris 2016). This is an updated review of the previously published Cochrane Review, “Oral dextrose gel to prevent hypoglycaemia in at‐risk neonates,” which found that oral dextrose gel was reported to be effective and safe in reducing the incidence of neonatal hypoglycaemia in one randomised trial (Hegarty 2017). No longer‐term outcomes were available.
Description of the intervention
Dextrose gel is a non‐proprietary, low‐cost, simple carbohydrate in concentrated thickened aqueous solution, which can be administered by direct application to the oral mucosa ‐ buccal or sublingual. Administration via these highly vascularised, thin mucous membranes allows rapid access to the circulation. Some of the administered gel may be swallowed and absorbed from the gastrointestinal tract.
Commercially manufactured gel costs approximately USD (US dollars) 70 per 100 mL. Alternatively, gel can be prepared in hospital pharmacies (Harris 2013). Ingredients vary by pharmaceutical manufacturer but commonly include water, glucose, a gelling agent and preservative(s). Some preparations include flavourings and colourings. Suitability of the gel for use in neonates should be assessed on an individual basis. The difference in effectiveness of various formulations is unknown.
How the intervention might work
Dextrose gel administered to the oral mucosa will enter the systemic circulation via the lingual vein and the internal jugular vein. This contrasts with oral‐gastrointestinal administration, whereby the first pass effect of the portal circulation may diminish the systemic blood glucose concentration achieved. Prevention of neonatal hypoglycaemia achieved by providing additional glucose during the neonatal metabolic transition period may reduce the medical prescription of artificial formula feeds, reduce admission to the NICU for intravenous dextrose, and prevent the neurodevelopmental impairment associated with neonatal hypoglycaemia.
Why it is important to do this review
Neonatal hypoglycaemia is important because it is common and is associated with brain injury in newborn infants. Risk factors for neonatal hypoglycaemia are known, so specific groups of newborn infants are routinely targeted for screening (i.e. infants of diabetic mothers, those of high or low birth weight, preterm infants, and those with poor feeding). These infants are frequently treated prophylactically with supplemental formula milk or admission to the neonatal unit for intravenous dextrose, or both. Supplemental formula may impair establishment of breastfeeding; intravenous treatment is expensive, is not always available in resource‐poor settings, and usually requires separation of mother and infant.
Oral dextrose gel is simple to administer and inexpensive. Therefore, if it were found to be effective and safe in preventing neonatal hypoglycaemia, its use would provide many advantages, particularly in low‐resource settings. Results of this review may help to inform those preparing clinical practice guidelines, such as those currently available to guide the care of babies at risk of neonatal hypoglycaemia (Adamkin 2011; NICE 2008; UNICEF 2013).
Objectives
To assess the effectiveness and safety of oral dextrose gel given to newborn infants at risk of hypoglycaemia in preventing hypoglycaemia and reducing long‐term neurodevelopmental impairment.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs, including cluster‐randomised trials but not cross‐over trials. We included both published and unpublished studies. We included unpublished studies and studies published only as abstracts when assessment of study quality was possible and if other criteria for inclusion were fulfilled.
Types of participants
Newborn infants at risk of hypoglycaemia, including infants of diabetic mothers (all types), large for dates, small for dates, and those born preterm (< 37 weeks) or with other risk factors as determined by investigators (e.g. maternal medication such as beta‐blockers from birth to 24 hours of age), who had not yet received a diagnosis of hypoglycaemia (blood glucose concentration below normal range, investigator‐defined) and had not received treatment for hypoglycaemia.
Types of interventions
Dextrose gel, of any concentration and at any dose or number of doses, given orally compared with placebo, no treatment/standard care, or other therapies (such as antenatal expression of colostrum, early initiation of breastfeeding, supplementation or substitution of breastfeeding with formula milk), for prevention of hypoglycaemia at any gestational age and commenced within the first 24 hours following birth.
Types of outcome measures
Primary outcomes
Hypoglycaemia (investigator‐defined)
Major neurological disability at two years of age or older, defined as any of the following: legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay/intellectual impairment (developmental quotient or intelligence quotient lower than two standard deviations below the mean)
Secondary outcomes
Hypoglycaemia (any blood glucose concentration < 2.6 mmol/L) during initial hospital stay (yes/no)
Receipt of treatment for hypoglycaemia (investigator‐defined, any treatment ‐ oral dextrose gel, intravenous dextrose, or other drug therapy) during initial hospital stay (yes/no)
Receipt of intravenous treatment for hypoglycaemia (yes/no)
Receipt of oral dextrose gel treatment for hypoglycaemia (yes/no)
Receipt of any medication for hypoglycaemia, such as glucagon or corticosteroids (yes/no)
Number of episodes of hypoglycaemia (investigator‐defined) (total number per infant)
Adverse events (e.g. choking or vomiting at time of administration) (yes/no)
Separation from mother for treatment of hypoglycaemia (infant nursed in an environment that is not in the same room as the mother, e.g. for NICU admission or the like) (yes/no)
Neonatal seizures (yes/no)
Abnormal MRI of the brain in the neonatal period (yes/no)
Duration of initial hospital stay (days)
Breastfeeding (any) after discharge (yes/no)
Exclusive breastfeeding after discharge ‐ WHO 2008 definition (yes/no)
Exclusive breastfeeding at six months of age ‐ WHO 2008 definition (yes/no)
Developmental disability at two years of age or older ‐ investigator‐defined (yes/no)
Visual impairment and severity of impairment at two years of age or older
Hearing impairment and severity of impairment at two years of age or older
Cerebral palsy and severity of disorder at two years of age or older
Developmental delay/intellectual impairment and severity of impairment at two years of age or older
Executive dysfunction and severity of dysfunction at two years of age or older
Behavioural problems and severity of problems at two years of age or older
Abnormal MRI of the brain at two years of age or older
Search methods for identification of studies
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 10) in the Cochrane Library; and Ovid MEDLINE(R) and EpubAhead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 19 October 2020). We have included the search strategies for each database in Appendix 1. We did not apply language restrictions.
We searched clinical trial registries for ongoing or recently completed trials. We searched the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/) and the US National Library of Medicine’s ClinicalTrials.gov (clinicaltrials.gov) via Cochrane CENTRAL. Additionally, we searched the ISRCTN Registry (www.isrctn.com) and the ANZCTR Registry (www.anzctr.org.au) for any unique trials not found through the Cochrane CENTRAL search.
For the 2020 update, we developed a new search strategy. The previous search methods are available in Appendix 2.
Searching other resources
We searched the reference lists of included studies and approached well‐known researchers in this clinical area to identify any unpublished or ongoing research.
Data collection and analysis
We used standard methods of the Cochrane Neonatal and GRADE to assess the certainty of evidence.
Selection of studies
Two review authors (TE, GL) independently assessed the eligibility of each study for inclusion. We did not encounter any disagreements.
Data extraction and management
Two review authors (TE, GL) independently extracted data from the eligible studies. We contacted investigators of two studies included in the meta‐analysis for additional data (Harding 2020; Hegarty 2016a). We did not encounter any disagreements.
Assessment of risk of bias in included studies
Two review authors (TE, GL) independently assessed the risk of bias (low, high, or unclear) of all included studies using the Cochrane ‘Risk of bias’ tool (Higgins 2011), for these domains:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
any other bias.
We resolved any disagreements encountered by discussion or consultation with a third review author. See Appendix 3 for a more detailed description of each domain.
Measures of treatment effect
We used the numbers of events in control and intervention groups of each study to calculate risk ratios (RRs) for dichotomous data. We calculated mean differences (MDs) between treatment groups when outcomes were measured in the same way for continuous data. We reported risk differences (RDs), and when a significant effect was found, we calculated numbers needed to treat for an additional beneficial outcome (NNTB), or numbers needed to treat for an additional harmful outcome (NNTH). We reported 95% confidence intervals (CIs) for all outcomes.
Unit of analysis issues
We planned to include cluster‐randomised trials in analyses along with individually randomised trials, but we did not identify any cluster‐randomised trials for inclusion.
Dealing with missing data
We carried out analyses on an intention‐to‐treat basis for all outcomes. We contacted the original investigators to request missing data when possible.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary by assessing statistical heterogeneity using the Chi2 test and the I2 statistic, considering an I2 value of less than 25% to be none, 25% to 49% low, 50% to 74% moderate, and 75% or greater to be high heterogeneity. We took an I2 value greater than 50% and a low P value (< 0.10) from the Chi2 test for heterogeneity to indicate substantial heterogeneity (Higgins 2019). If we detected substantial heterogeneity, we planned to explore possible explanations by performing sensitivity or subgroup analyses but we were unable to do this given the small number of eligible trials. We took statistical heterogeneity into account when interpreting results, especially when we noted variation in the direction of effect.
Assessment of reporting biases
Reporting biases arise when dissemination of research findings is influenced by the nature and direction of results. Some types of reporting bias (e.g. publication bias, multiple publication bias, language bias) reduce the likelihood that all studies eligible for a review will be retrieved. If all eligible studies are not retrieved, the review may be biased. We conducted a comprehensive search for eligible studies and were alert for duplication of data. We were unable to assess publication bias by visually inspecting a funnel plot because we did not identify 10 or more trials to make such an inspection valid. Two review authors (TE, GL) examined the methods of each study for prespecified outcomes and if all prespecified outcomes were reported in the results, we considered the study carried low risk of bias. If any prespecified outcome was not reported in the results, we considered the study to carry higher risk of bias.
Data synthesis
We evaluated studies for potential clinical diversity and planned to restrict meta‐analysis to situations in which clinical consistency was apparent. We evaluated studies for bias, as above, and restricted meta‐analysis if bias would be compounded. We used a fixed‐effect model to combine data when it was reasonable to assume that studies were estimating the same underlying treatment effect. If we found evidence of clinical heterogeneity, we planned to try to explain this on the basis of different study characteristics and subgroup analyses.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses using a fixed‐effect model.
Reason for risk of hypoglycaemia (infant of diabetic mother, preterm, small, large, other).
Gestation at birth (term and post‐term versus late preterm 35 to 36 weeks versus moderately preterm 30 to 34 weeks versus extremely preterm < 30 weeks).
Actual mode of feeding (formula versus breast versus mixed).
Method of administration of gel (rubbed into buccal mucosa versus sublingual versus other).
Dose of dextrose gel per administration (≤ 200 mg/kg versus > 200 mg/kg).
Number of dextrose gel doses administered (one versus > one dose).
Time of administration of first dose of gel (≤ one hour of age versus after one hour of age versus after two hours of age).
Sensitivity analysis
We planned to conduct sensitivity analysis by examining only trials considered to have a low risk of bias across all domains as determined by the Cochrane ‘Risk of bias’ tool (Higgins 2011). We planned to report results of sensitivity analyses for primary outcomes only. However, we did not conduct the planned sensitivity analysis because included trials had a low risk of bias.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following (clinically relevant) outcomes: hypoglycaemia; major neurological disability at two years of age or older; receipt of treatment for hypoglycaemia during initial hospital stay; receipt of intravenous treatment for hypoglycaemia; adverse events; separation from the mother for treatment of hypoglycaemia; and exclusive breastfeeding after discharge.
Two review authors (TE, GL) independently assessed the certainty of evidence for each of the outcomes. We considered evidence from randomised controlled trials as high certainty but downgraded the evidence by one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the (GRADEpro GDT) Guideline Development Tool to create ‘Table 1’ to report the certainty of evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
We included two studies in this update: one new study (Harding 2020), that was identified as an ongoing study in the previous Cochrane Review (Hegarty 2017); and one study that was included in the previous review (Hegarty 2016a), that had new follow‐up data.
Results of the search
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies.
Our search, in October 2020, identified 78 records, of which 18 were duplicates. After screening the titles and abstracts of 60 records, we excluded 48 because they were not relevant, and assessed the full text of 12 records (see Figure 1). We excluded four primary and two secondary references (Bourchier 1992; Coors 2018; Retbi 2013; Van Loghum 2014). One study is ongoing (NCT04353713); and three are awaiting classification (CTRI/2017/11/010645; NCT04185766; TCTR20190805003).
1.
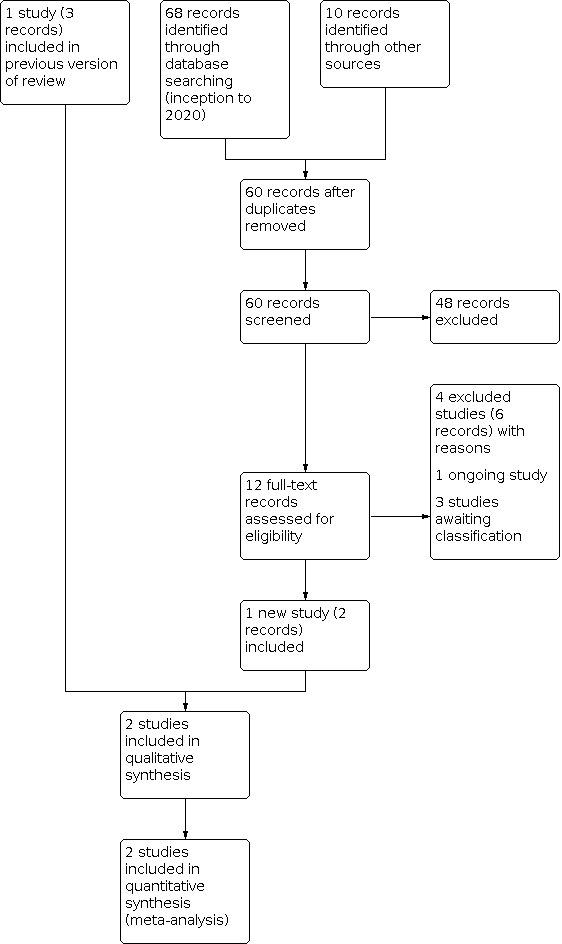
Study flow diagram
Included studies
We identified two eligible randomised, blinded, placebo‐controlled trials (Harding 2020; Hegarty 2016a), that enrolled a total of 2548 late preterm and term infants born at risk of neonatal hypoglycaemia (see Characteristics of included studies).
The study by Hegarty 2016a took place in two centres in New Zealand. They randomised 416 infants (including one infant randomised in error) in a 2:1 ratio to one of the following dose regimens of 40% dextrose gel or placebo: 0.5 mL/kg once, 1 mL/kg once, 0.5 mL/kg for four doses, and 1 mL/kg once followed by 0.5 mL/kg for three additional doses. Gel was massaged into the buccal mucosa at one hour after birth, followed by a breastfeed. Blood glucose concentration was checked using the glucose oxidase method at two hours after birth, and subsequent measurements were performed according to local hospital protocol (two to four hours pre‐feed for at least the first 12 hours). Of those randomised, 401 infants were eligible for follow‐up at two years' corrected age (13 withdrawals, one death) and a total of 360 were assessed.
The study by Harding 2020 recruited from 18 maternity hospitals in New Zealand and Australia. A total of 2149 infants born at risk of neonatal hypoglycaemia but unlikely to require neonatal unit admission for other reasons were randomised to receive a single dose of 40% dextrose gel or placebo gel massaged into the buccal mucosa at one hour after birth followed by a breastfeed. Blood glucose concentration was measured at two hours after birth and then according to hospital standard practice.
Excluded studies
We excluded four studies (six records) for the following reasons:
Three studies were commentaries which evaluated dextrose gel as a treatment for neonatal hypoglycaemia (Bourchier 1992; Retbi 2013; Van Loghum 2014).
One study (three records) was a non‐randomised study which investigated oral dextrose gel as prophylaxis against transient neonatal hypoglycaemia in at‐risk infants (Coors 2018).
See Characteristics of excluded studies for more details.
Risk of bias in included studies
Refer to Figure 2 and Figure 3 for a summary of the ‘Risk of bias’ assessment and Characteristics of included studies for more details.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Both studies reported using computer‐generated block randomisation with variable block sizes (Harding 2020; Hegarty 2016a). Allocation was concealed by a central randomisation system which assigned infants to a numbered trial pack containing identical‐looking syringes of either dextrose or placebo gel. We judged these studies to be at a low risk of selection bias.
Blinding
Both studies reported that clinicians, families and all study personnel were masked to treatment allocation (Harding 2020; Hegarty 2016a). The two‐year follow‐up of one study reported that follow‐up assessors were unaware of the infant’s treatment allocation (Hegarty 2016a). We judged these studies to be at low risk of performance and detection bias.
Incomplete outcome data
Both studies included all randomised infants in the intention‐to‐treat analysis of neonatal outcomes (Harding 2020; Hegarty 2016a). Hegarty 2016a assessed 90% of those eligible at two years' corrected age (dextrose 243/271 and placebo 117/130) and 87% of those randomised (dextrose 243/277 and placebo 117/138). Maternal and child characteristics were similar in those who were and were not assessed at two years. We judged these studies to be at low risk of attrition bias.
Selective reporting
Both studies reported data for all outcomes prespecified in the trial registration documentation (Harding 2020; Hegarty 2016a). We judged these studies to be at low risk of reporting bias.
Other potential sources of bias
Both studies reported that the demographic and prognostic characteristics of infants in each trial arm were balanced at baseline (Harding 2020; Hegarty 2016a). We did not identify any other potential sources of bias. We judged these studies to be at low risk of other bias.
Effects of interventions
See: Table 1
Primary outcomes
1.1 Hypoglycaemia (investigator‐defined)
Meta‐analysis showed that oral dextrose gel reduces the risk of neonatal hypoglycaemia (defined as a blood glucose concentration < 2.6 mmol/L) compared with placebo gel (RR 0.87, 95% CI 0.79 to 0.95; two studies, 2548 infants; high certainty evidence; Analysis 1.1). The RD was ‐0.06 (95% CI ‐0.10 to ‐0.02), and on average, 17 infants would have to receive prophylactic dextrose gel to prevent one additional case of neonatal hypoglycaemia.
1.1. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 1: Hypoglycaemia
1.2 Major neurological disability at two years or older
Additional data from one study (Hegarty 2016a) showed that oral dextrose gel probably reduces the risk of major neurological disability at two years’ corrected age compared with placebo gel (RR 0.21, 95% CI 0.05 to 0.78; one study, 360 infants; moderate certainty evidence, downgraded for serious imprecision due to low event rates; Analysis 1.2). The RD was ‐0.05 (95% CI ‐0.09 to 0.00), and on average, 20 infants would have to receive prophylactic dextrose gel to prevent one additional case of major neurological disability.
1.2. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 2: Major neurological disability at two years of age or older
Secondary outcomes
1.3 Hypoglycaemia (any blood glucose concentration less than 2.6 mmol/L) during initial hospital stay
No data were available for this outcome.
1.4 Receipt of treatment for hypoglycaemia during initial hospital stay
Meta‐analysis showed that oral dextrose gel probably reduces the risk of receipt of treatment for neonatal hypoglycaemia compared with placebo gel (RR 0.89, 95% CI 0.79 to 1.00; two studies, 2548 infants; moderate certainty evidence, downgraded for serious imprecision; Analysis 1.3).
1.3. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 3: Receipt of treatment for hypoglycaemia during initial hospital stay
1.5 Receipt of intravenous treatment for hypoglycaemia
Meta‐analysis showed that there is probably little or no difference in the risk of receipt of intravenous treatment for hypoglycaemia after oral dextrose gel compared to placebo gel (RR 1.01, 95% CI 0.68 to 1.49; two studies, 2548 infants; moderate certainty evidence, downgraded for serious imprecision; Analysis 1.4). We received additional data from the authors of one study (Harding 2020).
1.4. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 4: Receipt of intravenous treatment for hypoglycaemia
1.6 Receipt of oral dextrose gel treatment for hypoglycaemia
Meta‐analysis showed that there is probably little or no difference in receipt of oral dextrose gel for treatment of hypoglycaemia after oral dextrose gel compared to placebo gel (RR 0.90, 95% CI 0.79 to 1.01; two studies, 2548 infants; Analysis 1.5).
1.5. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 5: Receipt of oral dextrose gel treatment for hypoglycaemia
1.7 Receipt of any medication for hypoglycaemia
Additional data received from the authors of both studies showed that no infants in either the dextrose gel or placebo groups received any medication for hypoglycaemia, such as glucagon or corticosteroids.
1.8 Number of episodes of hypoglycaemia per infant
Additional data from one study (Hegarty 2016a) showed that there is probably little or no difference in the number of episodes of hypoglycaemia per infant after oral dextrose gel compared to placebo gel (MD ‐0.18, 95% CI ‐0.55 to 0.19; one study, 186 infants; Analysis 1.6).
1.6. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 6: Number of episodes of hypoglycaemia (glucose oxidase method) (total number per infant)
1.9 Adverse events
Meta‐analysis showed that there is probably little or no difference in the risk of adverse events from use of oral dextrose gel compared to placebo gel (RR 1.22, 95% CI 0.64 to 2.33; two studies, 2510 infants; moderate certainty evidence, downgraded for serious imprecision; Analysis 1.7). We received additional data from the authors of one study (Harding 2020).
1.7. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 7: Adverse effects (e.g. choking or vomiting at time of administration)
1.10 Separation from mother for treatment of hypoglycaemia
Meta‐analysis showed that oral dextrose gel may have little or no effect on the risk of separation of the infant from the mother for treatment of neonatal hypoglycaemia compared to placebo gel (RR 1.12, 95% CI 0.81 to 1.55; two studies, 2548 infants; low certainty evidence, downgraded for imprecision and substantial heterogeneity (I2 = 83%); Analysis 1.8). We were unable to perform subgroup analysis to further explore sources of heterogeneity as there were insufficient studies.
1.8. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 8: Separation from mother for treatment of hypoglycaemia (admission to NICU for hypoglycaemia)
1.11 Neonatal seizures
Only one event of neonatal seizures was reported in the dextrose gel group (1/1347; 0.1%) in (Hegarty 2016a), and one event of neonatal seizures was reported in the placebo group (1/1201; 0.1%) in (Harding 2020), (RR 0.69, 95% CI 0.08 to 5.69; two studies, 2548 infants; Analysis 1.9). Both studies reported that the seizures were unrelated to neonatal hypoglycaemia.
1.9. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 9: Neonatal seizures
1.12 Abnormal MRI of the brain in the neonatal period
No data were available for this outcome.
1.13 Duration of initial hospital stay
We received additional data from the authors of both studies which showed that oral dextrose gel makes little or no difference to the mean duration of initial hospital stay (days) (MD 0.06, 95% CI ‐0.13 to 0.24; two studies, 2537 infants; Analysis 1.10).
1.10. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 10: Duration of initial hospital stay (days)
1.14 Breastfeeding (any) after discharge
We received additional data from the authors of both studies which showed that there is little or no difference in breastfeeding (any) after discharge after oral dextrose gel compared to placebo gel (RR 1.01, 95% CI 0.98 to 1.05; two studies, 2323 infants; Analysis 1.11). Oral dextrose gel also made no difference to full or exclusive breastfeeding after discharge (RR 1.00, 95% CI 0.98 to 1.03); two studies, 2528 infants).
1.11. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 11: Breastfeeding (any) after discharge
1.15 Exclusive breastfeeding after discharge – WHO 2008 definition
No data were available for this outcome.
1.16 Exclusive breastfeeding at six months of age – WHO 2008 definition
No data were available for this outcome.
1.17 Developmental disability at two years of age or greater
One study (Hegarty 2016a) reported that oral dextrose gel may have little or no effect on the risk of any developmental disability at two years’ corrected age (RR, 0.75, 95% CI 0.49 to 1.17; one study, 359 infants; Analysis 1.12)
1.12. Analysis.

Comparison 1: Dextrose gel versus control, Outcome 12: Neurodevelopmental and disability outcomes at two years of age or greater
1.18 Visual impairment and severity at two years of age or greater
No infants were blind at two years’ corrected age (one study, 359 infants).
1.19 Hearing impairment at two years of age or greater
One infant in the placebo group was deaf at two years’ corrected age (1/116; 0.01%, RR 0.16, 95% CI 0.01 to 3.89; one study; 359 infants; Analysis 1.12).
1.20 Cerebral palsy at two years of age or greater
No infant had cerebral palsy at two years’ corrected age (one study, 359 infants).
1.21 Developmental delay/intellectual impairment and severity at two years of age or greater
Additional data from one study (Hegarty 2016a) reported that oral dextrose gel may have little to no effect on the risk of developmental delay/intellectual impairment at two years’ corrected age (RR 0.75, 95% CI 0.49 to 1.17; one study, 359 infants; Analysis 1.12).
1.22 Executive dysfunction and severity at two years of age or greater
Additional data from one study (Hegarty 2016a) reported that oral dextrose gel probably reduces the risk of executive dysfunction at two years’ corrected age compared with placebo gel (RR 0.48, 95% CI 0.23 to 0.99; one study, 357 infants; Analysis 1.12). The risk difference (RD) was ‐0.06 (95% CI ‐0.12 to 0.01), and on average, 17 infants would have to receive prophylactic dextrose gel to prevent one additional case of executive dysfunction. No severity data were available.
1.23 Behavioural problems and severity at two years of age or greater
No data were available for this outcome.
1.24 Abnormal MRI of the brain at two years of age or greater
No data were available for this outcome.
Discussion
Summary of main results
This updated review included two studies that evaluated oral dextrose gel compared with placebo gel in preventing neonatal hypoglycaemia in 2548 at‐risk infants (Harding 2020; Hegarty 2016a).
High certainty evidence from the two studies showed that oral dextrose gel reduces the risk of neonatal hypoglycaemia. On average, 17 at‐risk infants would need to be treated with oral dextrose gel to prevent one additional case of neonatal hypoglycaemia (95% CI 10 to 50). There was moderate certainty evidence from one study in 360 infants that oral dextrose gel compared with placebo gel probably reduces the risk of major neurological disability at two years of age or older (NNTB 20, 95% 11 to ∞) (see Table 1).
Oral dextrose gel probably reduces the risk of receipt of treatment for hypoglycaemia during the initial hospital stay but makes little or no difference to intravenous treatment, may have little or no effect on separation of the mother from the infant for treatment of hypoglycaemia, and probably does not result in increased adverse events compared with placebo gel.
Oral dextrose gel probably also has little or no effect on receipt of oral dextrose gel for the treatment of hypoglycaemia, the number of hypoglycaemic episodes, neonatal seizures, duration of initial hospital stay and whether an infant is breastfed after discharge. The findings from a single study (360 infants) suggest that oral dextrose gel compared with placebo gel may have little or no effect on the risks of any developmental disability, developmental delay/intellectual impairment or hearing impairment, but probably reduces executive dysfunction (NNTB 17, 95% CI 8 to 100). No infants were blind or had cerebral palsy at two years of age or greater.
Overall completeness and applicability of evidence
More data have become available on the long‐term neurodevelopmental and disability outcomes since the first version of this review, although this comes from a single small study (Hegarty 2017). No data were available for the following prespecified secondary outcomes: abnormal MRI of the brain in the neonatal period and at two years of age or greater; exclusive breastfeeding after discharge or at six months of age; and behavioural problems and severity at two years of age or greater. Both included studies compared a single preparation of oral dextrose gel with placebo in late preterm and term infants in two high‐income countries (Australia and New Zealand) and therefore, the applicability of these findings to other preparations of gel, extremely and moderately preterm infants and other healthcare settings remains unknown. There were no randomised trials that compared oral dextrose gel with no treatment, standard care or other therapies (such as antenatal expression of colostrum, early initiation of breastfeeding and the supplementation of breastfeeding with formula milk).
Quality of the evidence
We assessed both studies as low risk of bias across all domains. The certainty of evidence assessed by GRADE was moderate for most outcomes, except for the co‐primary outcome of hypoglycaemia, which was graded as high certainty, and separation of mother and infant, which was graded as low certainty (see Table 1). The most common reason for downgrading the evidence was imprecision due to wide confidence intervals and low event rates.
For the co‐primary outcome of major neurological disability at two years of age or older, data were available from only one small study with low event rates, so we graded the certainty of this evidence as moderate due to a lack of precision. Additional studies are needed to clarify the certainty of this finding.
We downgraded one outcome (separation from the mother for treatment of hypoglycaemia) to low certainty because of a lack of precision and substantial unexplained heterogeneity. We were unable to further explore this with planned subgroup analyses because there were only two studies. However, it is possible that this heterogeneity may be due to variations between hospitals in policies for admission to the NICU.
We downgraded the outcome of adverse events to moderate certainty because of imprecision due to low event rates. Thus, there is moderate certainty evidence that dextrose gel does not increase the risk of an infant choking or vomiting during administration compared to those who received placebo gel, and that the risk is low (< 1% in both groups), but there is no evidence about whether receipt of oral dextrose gel may increase the risk of these events or other harms compared with no treatment or other therapies such as formula feeding.
Potential biases in the review process
We believe that we have made every effort to minimise bias in the review process. We conducted a systematic search of the literature for randomised controlled trial evidence, not restricted by language or date of publication. When necessary, we contacted authors of primary studies to obtain additional outcome data. We adhered to the Cochrane methods of searching and performing data extraction and analysis.
Agreements and disagreements with other studies or reviews
This update agrees with the first version of this review (Hegarty 2017), which reported that oral dextrose gel compared with placebo was associated with a reduced risk of neonatal hypoglycaemia in at‐risk infants with no evidence of adverse events. The first version included a single study of 415 at‐risk infants. That study was included in this updated version and contributed data to the meta‐analysis.
Moreover, the Cochrane Review, “Oral dextrose gel for the treatment of hypoglycaemia in newborn infants,” (Weston 2016), reported that treatment of hypoglycaemia with oral dextrose gel was associated with a reduction in separation of the mother and infant and increased likelihood of full breastfeeding after discharge. Weston 2016 also found no evidence of adverse events of dextrose gel during the neonatal period or at two years' corrected age.
Authors' conclusions
Implications for practice.
The available evidence suggests that giving prophylactic oral dextrose gel to 100 at‐risk late preterm and term infants will prevent approximately six cases of neonatal hypoglycaemia and probably prevent five cases of major neurological disability at two years' corrected age, without increasing the risk of adverse events.
A cost‐utility analysis has also reported that prophylactic oral dextrose gel is likely to be cost‐effective, reducing healthcare costs while improving quality of life (Glasgow 2020). Clinicians and policy‐makers should now consider whether this evidence from two high‐quality trials in high‐income countries is sufficient to warrant introduction of prophylactic dextrose gel into clinical practice.
Implications for research.
Further research on long‐term neurodevelopmental and disability outcomes are required. An update of this review when data become available from an ongoing follow‐up study of both the included studies will clarify the certainty of this evidence. Additional data are also required on the effects of prophylactic dextrose gel on exclusive breastfeeding, and if the effects on the separation of mother from infant differ in hospitals with different NICU admission policies.
There is no evidence about the effects of prophylactic oral dextrose gel in low‐ and middle‐income countries, extremely and moderately preterm infants and using other dextrose gel preparations. However, the results of three studies awaiting classification set in India (CTRI/2017/11/010645), Thailand (TCTR20190805003) and Italy (NCT04185766), and an ongoing study in very preterm infants (NCT04353713), may help to address these knowledge gaps. Future research should also compare prophylactic oral dextrose gel with other active therapies and assess adverse events compared with no treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 19 October 2020 | New search has been performed | The literature was searched in October 2020 using a new search strategy which we ran without date limits. One new published study, one ongoing study and three studies awaiting classification were identified. |
| 19 October 2020 | New citation required but conclusions have not changed | There has been a change in authorship. One trial reported on long‐term neurodevelopmental and disability outcomes at two years of age or greater. |
History
Protocol first published: Issue 4, 2016 Review first published: Issue 7, 2017
Acknowledgements
We acknowledge the contribution of Julie Brown who was a co‐author on the protocol and first version of this Cochrane Review (Hegarty 2016b; Hegarty 2017).
We would like to thank Cochrane Neonatal: Colleen Ovelman, Managing Editor; Jane Cracknell, Assistant Managing Editor; Roger Soll, Co‐coordinating editor; and Bill McGuire, Co‐coordinating Editor, who provided editorial and administrative support. Colleen Ovelman peer reviewed the Ovid MEDLINE search strategy.
Nai Ming Lai has peer reviewed and offered feedback for this updated review.
David Osborn reviewed and was the sign‐off editor for this updated review.
Appendices
Appendix 1. 2020 Search methods
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2019). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist.
CENTRAL via CRS Web:
Terms:
MESH DESCRIPTOR Hypoglycemia EXPLODE ALL AND CENTRAL:TARGET
hypogly* AND CENTRAL:TARGET
#2 OR #1 AND CENTRAL:TARGET
MESH DESCRIPTOR Glucose EXPLODE ALL AND CENTRAL:TARGET
MESH DESCRIPTOR Sweetening Agents EXPLODE ALL AND CENTRAL:TARGET
dextrose* or glucose* or sweetening agent* AND CENTRAL:TARGET
#6 OR #5 OR #4 AND CENTRAL:TARGET
MESH DESCRIPTOR Gels EXPLODE ALL AND CENTRAL:TARGET
gel or gels AND CENTRAL:TARGET
#9 OR #8 AND CENTRAL:TARGET
MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET
infant or infants or infant's or "infant s" or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET
#12 OR #11 AND CENTRAL:TARGET
#3 AND #7 AND #10 AND #13 AND CENTRAL:TARGET
# of results: 41
MEDLINE via Ovid:
Terms:
exp Hypoglycemia/
hypogly*.mp.
1 or 2
exp Glucose/
exp Sweetening Agents/
(dextrose* or glucose* or sweetening agent*).mp.
4 or 5 or 6
exp Gels/
(gel or gels).mp.
8 or 9
exp infant, newborn/
(newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or 'infant s' or infant's or infantile or infancy or neonat*).ti,ab.
11 or 12
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/14‐21
exp animals/ not humans.sh.
22 not 23
13 and 24
randomi?ed.ti,ab.
randomly.ti,ab.
trial.ti,ab.
groups.ti,ab.
((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab.
placebo*.ti,ab.
26 or 27 or 28 or 29 or 30 or 31
12 and 32
limit 33 to yr="2018 ‐Current"
25 or 34
3 and 7 and 10 and 35
# of results: 27
ISRCTN:
Terms: ( Interventions: Dextrose* or glucose* or sweetening agent* AND Participant age range: Neonate ) "Dextrose* or glucose* or sweetening agent* AND ( Participant age range: Neonate )" Dextrose* within Participant age range: Neonate Condition: hypoglycaemia AND Interventions: Dextrose Condition: hypoglycaemia AND Interventions: Glucose
# of results: 5
ANZCTR:
Terms:
(hypoglycaemia AND dextrose AND randomised)
Description of intervention(s) / exposure: dextrose* OR glucose* OR “sweetening agent” AND hypoglycaemia
Allocation to intervention: randomised
Age group: under 18yrs
# of results: 4
Appendix 2. Previous search methods
Review authors conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12) in the Cochrane Library; MEDLINE via PubMed (1966 to 23 January 2017); Embase (1980 to 23 January 2017); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 23 January 2017) and did not apply language restrictions.
The following search strategy was used:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Review authors also searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/), the ISRCTN Registry).
Appendix 3. Risk of Bias tool
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias)
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Dextrose gel versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Hypoglycaemia | 2 | 2548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.79, 0.95] |
| 1.2 Major neurological disability at two years of age or older | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.05, 0.78] |
| 1.3 Receipt of treatment for hypoglycaemia during initial hospital stay | 2 | 2548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.79, 1.00] |
| 1.4 Receipt of intravenous treatment for hypoglycaemia | 2 | 2548 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.68, 1.49] |
| 1.5 Receipt of oral dextrose gel treatment for hypoglycaemia | 2 | 2548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.01] |
| 1.6 Number of episodes of hypoglycaemia (glucose oxidase method) (total number per infant) | 1 | 186 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.55, 0.19] |
| 1.7 Adverse effects (e.g. choking or vomiting at time of administration) | 2 | 2510 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.64, 2.33] |
| 1.8 Separation from mother for treatment of hypoglycaemia (admission to NICU for hypoglycaemia) | 2 | 2548 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.81, 1.55] |
| 1.9 Neonatal seizures | 2 | 2548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.08, 5.69] |
| 1.10 Duration of initial hospital stay (days) | 2 | 2537 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.13, 0.24] |
| 1.11 Breastfeeding (any) after discharge | 2 | 2323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.05] |
| 1.12 Neurodevelopmental and disability outcomes at two years of age or greater | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.12.1 Developmental disability at two years of age or greater | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.17] |
| 1.12.2 Hearing impairment at two years of age or greater | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.89] |
| 1.12.3 Developmental delay/intellectual impairment at two years of age or greater | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.17] |
| 1.12.4 Executive dysfunction at two years of age or greater | 1 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 0.99] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Harding 2020.
| Study characteristics | ||
| Methods | Randomised parallel controlled trial | |
| Participants | 2149 infants (16 infants randomised in error) Inclusion criteria: infants of diabetic mothers or late preterm infants (35 to 36 weeks' gestation) or small (< 2.5 kg or < 10th percentile) or large (> 4.5 kg or > 90th percentile) infants Exclusion criteria: major congenital abnormality; previous formula feed or intravenous fluids; previous diagnosis of hypoglycaemia; admitted to NICU; imminent admission to NICU Setting: multi‐centre Timing: January 2015 to May 2019 |
|
| Interventions | 40% dextrose gel massaged into buccal mucosa as a single dose (0.5 mL/kg) 1 hour after birth (n = 1070) vs Placebo gel massaged into buccal mucosa using same protocol as the intervention (n = 1063) |
|
| Outcomes |
Primary outcome Admission to NICU (defined as admission to NICU (or special care baby unit (SCBU) for hospitals using that name) for > 4 hours) ‐ no difference between oral dextrose and placebo groups Secondary outcomes • Hypoglycaemia (any blood glucose concentration < 2.6 mmol/L in first 48 hours) ‐ lower incidence in oral dextrose gel group • Admission to NICU for hypoglycaemia ‐ no difference between oral dextrose and placebo groups • Hyperglycaemia (any blood glucose concentration > 10 mmol/L) ‐ no cases • Breastfeeding at discharge from hospital (full or exclusive) ‐ no difference between oral dextrose and placebo groups • Receipt of any formula before discharge from hospital ‐ no difference between oral dextrose and placebo groups • Formula feeding at 6 weeks of age ‐ no difference between oral dextrose and placebo groups • Cost of care until primary discharge home ‐ reported separately • Maternal satisfaction (via telephone questionnaire at 6 weeks) ‐ high • Neurosensory disability at 2 years’ corrected age (any of the following: legal blindness; sensorineural deafness requiring hearing aids; cerebral palsy; Bayley Scale of Infant Development Version III cognitive, language or motor score < 1 standard deviation below the mean) ‐ follow‐up in progress |
|
| Notes | Trials registration: ACTRN12614001263684 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Babies will be assigned randomly via an internet randomisation service to the dextrose or placebo group with priority stratification for collaborating centre and risk factor" |
| Allocation concealment (selection bias) | Low risk | Quote: "Staff at the study site accessed a centralised internet based randomisations service within the first hour after birth to receive a study number which corresponded to a study treatment pack containing a single pre‐packaged syringe of 40% dextrose gel or identical appearing 2% hydroxymethylcellulose placebo gel (1:1 ratio)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote. "Families, study and site staff and investigators were all blinded to treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...study and site staff and investigators were all blinded to treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All analyses were pre‐specified and carried out using a modified intention‐to‐treat approach in which babies randomised in error (did not meet eligibility criteria at randomisation) were excluded, but all other babies were analysed in the groups which they were allocated". |
| Selective reporting (reporting bias) | Low risk | Yes all outcomes mentioned in the protocol were published |
| Other bias | Low risk | Groups were well balanced for maternal and demographic variables. We conclude that the study is not at risk of other bias. |
Hegarty 2016a.
| Study characteristics | ||
| Methods | Two centre randomised, double‐blind, placebo‐controlled trial | |
| Participants | 416 infants (one infant randomised in error) Inclusion criteria: infants of diabetic mothers or late preterm infants (35 to 36 weeks' gestation) or small‐for‐gestational age (< 2.5 kg or < 10th percentile) or large‐for‐gestational age (> 4.5 kg or > 90th percentile) infants Exclusion criteria: major congenital abnormality, previously fed by formula or received intravenous fluid, previous diagnosis of hypoglycaemia, admission to NICU or imminent admission to NICU Setting: 2 hospitals providing maternity and neonatal services (Auckland City Hospital and Waitakere Hospital) in Auckland, New Zealand Timing: Recruitment: August 2013 to November 2014 Follow‐up: August 2015 to February 2017 |
|
| Interventions | 40% dextrose gel massaged into the buccal mucosa as a single dose (0.5 mL/kg or 1 mL/kg at 1 hour) or multiple doses (additional 0.5 mL/kg 3 times pre‐feed in first 12 hours) (n = 277) vs Placebo gel massaged into the buccal mucosa using same protocol and volume as the intervention (n = 138) |
|
| Outcomes |
Primary outcome Hypoglycaemia, defined as any blood glucose concentration < 2.6 mmol/L in the first 48 hours after birth ‐ lower incidence in any dextrose group Secondary outcomes
|
|
| Notes | This trial was funded by the A+ Trust (www.adhb.govt.nz; A+5696); Auckland Medical Research Foundation (www.medicalresearch.org.nz; 1113012); Cure Kids (www.curekids.org.nz; 3537); and Lottery Health Research (http://www.communitymatters.govt.nz; 326844), and by philanthropic donations to the University of Auckland Foundation (www.auckland.ac.nz). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Australian New Zealand Clinical Trials Registry ACTRN12613000322730 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used computer‐generated blocked randomisation with variable block sizes" |
| Allocation concealment (selection bias) | Low risk | Centralised allocation. Quote: "Research staff entered demographic and entry criteria data into an online randomisation website that provided a number corresponding to a numbered trial pack" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians, families, and all study investigators were masked to treatment group allocation throughout the study and remain so for planned follow‐up |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At 24 months’ corrected age, children underwent a comprehensive assessment of neurodevelopment, growth and general health by doctors trained in all assessments who were unaware of the child’s randomisation group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Except for the one infant randomised in error, all infants had primary outcome data available and were included in the intention‐to‐treat analysis. The follow‐up rate at two years' corrected was high (87% of those randomised and 90% of those eligible for follow‐up) and maternal and child characteristics were similar between those who were and were not assessed at two years of age. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported. Trial registration was viewed |
| Other bias | Low risk | Demographic and baseline characteristics were similar for all randomisation groups. Primary risk factors for hypoglycaemia were similar across all treatment groups. The authors concluded that the study is not at risk of other bias. |
NICU: neonatal intensive care unit; SCBU: special care baby unit; vs: versus; aRR: adjusted risk ratio
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bourchier 1992 | The intervention was oral dextrose gel as a treatment for neonatal hypoglycaemia |
| Coors 2018 | Not a randomised controlled trial |
| Retbi 2013 | The intervention was oral dextrose gel as a treatment for neonatal hypoglycaemia |
| Van Loghum 2014 | The intervention was oral dextrose gel as a treatment for neonatal hypoglycaemia |
Characteristics of studies awaiting classification [ordered by study ID]
CTRI/2017/11/010645.
| Methods | Randomised parallel controlled trial |
| Participants | 611 infants Inclusion criteria: infants of diabetic mothers, > 35 weeks’ gestation and birthweight > 2 kg Exclusion criteria: major congenital malformation or requiring admission to NICU before randomisation for any of the following: < 35 weeks’ gestation, birthweight < 2 kg, respiratory distress, major lethal congenital malformation, antenatal ultrasound findings with absent/reversal of diastolic mesenteric flow, Rh negative mother with DCT positive baby and indication suggested by consultants. Setting: India |
| Interventions | 40% dextrose gel (0.5 ml/L) massaged into the buccal mucosa after birth followed by a breastfeed within 30 minutes after birth vs Breastfeed within 30 minutes after birth |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Trial registration: CTRI/2017/11/010645. Trial completed but unpublished ‐ authors contacted in August 2020 for additional information |
NCT04185766.
| Methods | Randomised parallel controlled trial |
| Participants | 172 infants Inclusion criteria: late preterm and term infants (34 to 42 weeks’ gestation), small (< 10th centile) or large‐for‐gestational age (> 90thcentile), natural birth, rooming‐in and body temperature between 36.5 to 37.5 degrees. Mother intends to breastfeed and has a BMI between 19 and 24. Exclusion criteria: major congenital malformations, blood glucose concentration < 47 mg/dL (2.6 mmol/L), NICU admission, milk intake in formula, intravenous infusion of 10% glucose solution, metabolic and respiratory acidosis (pH: 7.28 to 7.38) or mother taking medications during pregnancy. Setting: Poliambulanza Foundation Hospital Institute, Brescia, Italy. |
| Interventions | 40% Destrogel as a single dose (0.5 mL/kg or 1 mL/kg) massaged into the buccal mucosa at one hour after birth (n = 86) vs Placebo gel massaged into the buccal mucosa using the same protocol and volume as the intervention (n = 86) |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Trial registration: NCT04185766. Trial completed but unpublished ‐ authors contacted in August 2020 for additional information |
TCTR20190805003.
| Methods | Randomised double‐blind, placebo‐controlled trial |
| Participants | 600 infants Inclusion criteria: late preterm (34 to 37 weeks' gestation), small (< 10th centile) or large‐for‐gestational age (> 90th centile), infants of diabetic mothers, low birthweight (< 2500 g) or macrosomia ( > 4000 g). Exclusion criteria: received oral feed or intravenous treatment for hypoglycaemia, congenital abnormality, admission to NICU, birthweight < 1800 grams. Setting: Thailand |
| Interventions | 40% dextrose gel (0.5 ml/L) applied into the buccal mucosa within 48 hours after birth vs arboxymethyl cellulose placebo gel massaged into the buccal mucosa using the same protocol as the intervention |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Trial registration: TCTR20190805003. Trial completed but unpublished ‐ authors contacted in October 2020 for additional information |
BMI: body mass index; DCT: Direct Coombs Test; NICU: neonatal intensive care unit; Rh: rhesus; vs: versus.
Characteristics of ongoing studies [ordered by study ID]
NCT04353713.
| Study name | Gel for early hypoglycaemia prevention in preterm infants |
| Methods | Multicentre, randomised double‐blind, placebo‐controlled trial |
| Participants | 534 infants Inclusion criteria: Infants ≤ 32 weeks’ gestation Exclusion criteria: planned comfort care (palliative approach) after delivery Setting: Ireland |
| Interventions | 40% oral dextrose gel (0.5 ml/L) massaged into the buccal mucosa immediately after stabilisation or resuscitation at birth prior to in‐house transport from delivery ward to the NICU vs 2% hydroxymethylcellulose placebo gel massaged into the buccal mucosa using the same protocol and volume as the intervention |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 1 July 2020 |
| Contact information | jkelleher@coombe.ie |
| Notes | Trial registration: NCT04353713 |
NICU: neonatal intensive care unit; vs: versus
Differences between protocol and review
The original protocol was published in 2016 (Hegarty 2016b), and informed the first version of this Cochrane Review titled “Oral dextrose gel to prevent hypoglycaemia in at‐risk neonates” (Hegarty 2017). For the 2020 update, we developed a new search strategy, which we ran without date limits (Appendix 1).
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. RCTs and controlled clinical trials (CCTs) from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see how CENTRAL is created). Cochrane Neonatal has validated their searches to ensure that relevant Embase records are found while searching CENTRAL. Further, Cochrane Neonatal no longer searches for RCTs and CCTs from ClinicalTrials.gov or from The World Health Organizationís International Clinical Trials Registry Platform (ICTRP) , as records from both platforms are added to CENTRAL on a monthly basis (see how CENTRAL is created). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN (at http://www.isrctn.com formerly controlled‐trials.com), is searched separately. Starting in September 2020, Cochrane Neonatal no longer searches CINAHL for reviews, as records are identified and added to CENTRAL on a monthly basis through Cochrane's Centralised Search Service Project (see how CENTRAL is created).
The term 'adverse effects' has been changed to 'adverse events'.
Contributions of authors
2020 update
TE:
screened studies for eligibility and extracted data;
prepared the first draft and revised subsequent drafts under the supervision of Jane Harding;
contributed to subsequent drafts and approved the final version.
JEHegarty:
contributed to subsequent drafts and approved the final version.
JEHarding:
supervised the first draft and revised subsequent drafts; and
approved the final version.
CAC:
contributed to subsequent drafts and approved the final version.
JA:
contributed to subsequent drafts and approved the final version.
GL:
screened studies for eligibility and extracted data;
contributed to subsequent drafts and approved the final version.
Sources of support
Internal sources
-
Liggins Institute, University of Auckland, New Zealand
PhD Scholarship for Taygen Edwards
-
Auckland District Health Board, New Zealand
Clinical Salary for Jo Hegarty
-
Liggins Institute, University of Auckland, New Zealand
University appointments for Jane Harding, Caroline Crowther, and Jane Alsweiler
-
Aotearoa Foundation, New Zealand
Undergraduate Clinical Research Internship for Gordon Liu
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families
Declarations of interest
TE has no interest to declare. TE independently screened studies for eligibility, extracted data and performed risk of bias assessment of included studies.
JEHegarty is an author of the included studies (Hegarty 2016a; Harding 2020).
JEHarding is an author of the included studies (Hegarty 2016a; Harding 2020).
CAC is an author of the included studies (Hegarty 2016a; Harding 2020).
JA is an author of the included studies (Hegarty 2016a; Harding 2020).
GL has no interest to declare. GL independently screened studies for eligibility, extracted data and performed risk of bias assessment of included studies.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Harding 2020 {published and unpublished data}
- Harding JE, Hegarty JE, Crowther CA, Edlin R, Gamble GD, Alsweiler JM. Evaluation of oral dextrose gel for prevention of neonatal hypoglycemia (hPOD): A multicenter, double-blind randomized controlled trial. PLoS Medicine 2021;18(1):e1003411. [DOI: 10.1371/journal.pmed.1003411] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JE, Hegarty JE, Crowther CA, Edlin R, Gamble GD, Alsweiler JM. Randomised trial of neonatal hypoglycaemia prevention with oral dextrose gel (hPOD): study protocol. BMC Pediatrics 2015;15(120):1-5. [DOI: 10.1186/s12887-015-0440-6] [PMID: 26377909 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hegarty 2016a {published and unpublished data}
- Griffiths RJ, Hegarty JE, Alsweiler JM, Gamble GD, May R, McKinlay CJ, et al. Two-year outcomes after dextrose gel prophylaxis for neonatal hypoglycaemia. Archives of Disease in Childhood - Fetal and Neonatal Edition 2021;206:F278-F285. [DOI: 10.1136/archdischild-2020-320305] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty JE, Harding JE, Gamble GD, Crowther CA, Edlin R, Alsweiler JM. Preventing neonatal hypoglycaemia with oral dextrose gel: a randomised controlled trial. In: Journal of Perinatal Medicine. Vol. 43. 2015. [DOI: 10.1515/jpm-2015-2002] [DOI]
- Hegarty JE, Harding JE, Gamble GD, Crowther CA, Edlin R, Alsweiler JM. Prophylactic oral dextrose gel for newborn babies at risk of neonatal hypoglycaemia: a randomised controlled dose-finding trial (the Pre-hPOD Study). PLOS Medicine 2016;13(10):e1002155. [DOI: 10.1371/journal.pmed.1002155] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bourchier 1992 {published data only}
- Bourchier D, Weston P, Heron P. Hypostop for neonatal hypoglycaemia. New Zealand Medical Journal 1992;105(926):22. [PMID: ] [PubMed] [Google Scholar]
Coors 2018 {published data only}
- Coors S, Cousin J, Hagan J, Kaiser J. Prophylactic dextrose gel does not prevent neonatal hypoglycemia: a quasi-experimental pilot study. Journal of Pediatrics 2018;198:156-61. [DOI: 10.1016/j.jpeds.2018.02.025] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hagan J, Coors SM, Cousin J, Kaiser JR. Prophylactic dextrose gel does not prevent neonatal hypoglycemia: a pilot study. In: Pediatric Academic Societies Annual Meeting; 2017 May 06 - 09. San Francisco (CA), 2017. [DOI] [PubMed]
- NCT02523222. Prophylactic dextrose gel for newborns at high-risk for hypoglycemia. clinicaltrials.gov/show/NCT02523222 (first received 14 August 2015).
Retbi 2013 {published data only}
- Retbi J-M. Glucose gel for hypoglycaemia in the newborn. Soins Pediatrie, Puericulture 2013;275:8. [PMID: ] [PubMed] [Google Scholar]
Van Loghum 2014 {published data only}
- Van Loghum BS. Oral dextrose gel is effective in the treatment of neonatal hypoglycemia [Orale dextrose-gel is effectief in de behandeling van neonatale hypoglykemie]. Tijdschrift Voor Kindergeneeskunde 2014;82:147-8. [DOI: 10.1007/s12456-014-0029-2] [DOI] [Google Scholar]
References to studies awaiting assessment
CTRI/2017/11/010645 {published data only}
- CTRI/2017/11/010645. Prevention of low blood sugar by 40% oral dextrose gel in newborn infant of diabetic mother. who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/11/010645 (first received 27 November 2017).
NCT04185766 {published data only}
- NCT04185766. Prevention of hypoglycaemia by oral 40% destrogel. clinicaltrials.gov/show/NCT04185766 (first received 4 December 2019).
TCTR20190805003 {published data only}
- TCTR20190805003. Oral dextrose gel for management of neonates at risk for hypoglycemia. who.int/trial2.aspx?TrialID=TCTR20190805003 (first received 3 August 2019).
References to ongoing studies
NCT04353713 {published data only}
- NCT04353713. Gel for early hypoglycaemia prevention in preterm infants (GEHPPI) [Oral dextrose gel to prevent early hypoglycaemia in very preterm infants]. clinicaltrials.gov/ct2/show/NCT04353713 (first received 20 April 2020).
Additional references
Achoki 2010
- Achoki R, Opiyo N, English M. Mini-review: management of hypoglycaemia in children aged 0–59 months. Journal of Tropical Pediatrics 2010;56(4):227-4. [DOI: 10.1093/tropej/fmp109] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adamkin 2011
- Adamkin DH, Committee on Fetus and Newborn. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics 2011;127(3):575-9. [DOI: 10.1542/peds.2010-3851] [PMID: ] [DOI] [PubMed] [Google Scholar]
Agrawal 2000
- Agrawal RK, Lui K, Gupta JM. Neonatal hypoglycaemia in infants of diabetic mothers. Journal of Paediatrics and Child Health 2000;36(4):354–6. [DOI: 10.1046/j.1440-1754.2000.00512.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Alkalay 2005
- Alkalay AL, Flores-Sarnat L, Sarnat HB, Moser FG, Simmons CF. Brain imaging findings in neonatal hypoglycemia: case report and review of 23 cases. Clinical Pediatrics 2005;44(9):783–90. [DOI: 10.1177/000992280504400906] [PMID: ] [DOI] [PubMed] [Google Scholar]
Blomquist 1994
- Blomquist HK, Jonsbo F, Serenius F, Persson LA. Supplementary feeding in the maternity ward shortens the duration of breast feeding. Acta Paediatrica 1994;83(11):1122-6. [DOI: 10.1111/j.1651-2227.1994.tb18263.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Boundy 2016
- Boundy EO, Dastjerdi R, Spiegelman D, Fawzi WW, Missmer SA, Lieberman E, et al. Kangaroo mother care and neonatal outcomes: a meta-analysis. Pediatrics 2016;137(1):e20152238. [DOI: 10.1542/peds.2015-2238] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burns 2008
- Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics 2008;122(1):65-74. [DOI: 10.1542/peds.2007-2822] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chertok 2009
- Chertok IR, Raz I, Shoham I, Haddad H, Wiznitzer A. Effects of early breastfeeding on neonatal glucose levels of term infants born to women with gestational diabetes. Journal of Human Nutrition & Dietetics 2009;22(2):166-9. [DOI: 10.1111/j.1365-277X.2008.00921.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chiruvolu 2017
- Chiruvolu A, Miklis KK, Stanzo KC, Petrey B, Groves CG, McCord K, et al. Effects of skin-to-skin care on late preterm and term infants at-risk for neonatal hypoglycemia. Pediatric Quality & Safety 2017;2(4):e030. [DOI: 10.1097/pq9.0000000000000030] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornblath 1965
- Cornblath M, Reisner SH. Blood glucose in the neonate and its clinical significance. New England Journal of Medicine 1965;273(7):378-81. [DOI: 10.1056/nejm196508122730707] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cornblath 2000
- Cornblath M, Hawdon JM, Williams AF, Aynsley-Green A, Ward-Platt MP, Schwartz R, et al. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics 2000;105(5):1141-5. [DOI: 10.1542/peds.105.5.1141] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cox 2006
- Cox SG. Expressing and storing colostrum antenatally for use in the newborn period. Breastfeeding Review 2006;14(3):11-6. [PMID: ] [PubMed] [Google Scholar]
CPSFNC 2004
- Canadian Pediatric Society Fetal and Newborn Committee. Screening guidelines for newborns at risk for low blood glucose. Paediatrics & Child Health 2004;9(10):723-40. [DOI: 10.1093/pch/9.10.723] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daskas 2013
- Daskas N, Crowne E, Shield JP. Is labetalol really a culprit in neonatal hypoglycaemia? Archives of Disease in Childhood. Fetal & Neonatal Edition 2013;98(2):F185. [DOI: 10.1136/archdischild-2012-303057] [PMID: ] [DOI] [PubMed] [Google Scholar]
Demir 2020
- Demir F, Ghosh P, Liu Z. Effects of motherhood timing, breastmilk substitutes and education on the duration of breastfeeding: evidence from Egypt. World Development 2020;133(105014):1-14. [DOI: 10.1016/j.worlddev.2020.105014] [DOI] [Google Scholar]
Duvanel 1999
- Duvanel CB, Fawer CL, Cotting J, Hohlfeld P, Matthieu JM. Long-term effects of neonatal hypoglycemia on brain growth and psychomotor development in small-for-gestational-age preterm infants. Journal of Pediatrics 1999;134(4):492-8. [DOI: 10.1016/s0022-3476(99)70209-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Eidelman 2012
- Eidelman AI. Breastfeeding and the use of human milk: an analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeeding Medicine 2012;7(5):323-4. [DOI: 10.1089/bfm.2012.0067] [PMID: ] [DOI] [PubMed] [Google Scholar]
Forster 2011
- Forster DA, McEgan K, Ford R, Moorhead A, Opie G, Walker S, et al. Diabetes and antenatal milk expressing: a pilot project to inform the development of a randomised controlled trial. Midwifery 2011;27(2):209-14. [DOI: 10.1016/j.midw.2009.05.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Forster 2017
- Forster DA, Moorhead AM, Jacobs SE, Davis PG, Walker SP, McEgan KM, et al. Advising women with diabetes in pregnancy to express breastmilk in late pregnancy (Diabetes and Antenatal Milk Expressing [DAME]): a multicentre, unblinded, randomised controlled trial. Lancet 2017;389(10085):2204–13. [DOI: 10.1016/S0140-6736(17)31373-9] [DOI: 10.1016/S0140-6736(17)31373-9] [DOI] [PubMed] [Google Scholar]
Glasgow 2018
- Glasgow MJ, Harding JE, Edlin R, for the CHYLD Study Group. Cost analysis of treating neonatal hypoglycaemia with dextrose gel. Journal of Paediatrics and Child Health 2018;198(S1):21-2. [DOI: 10.1111/jpc.13882_51] [DOI] [Google Scholar]
Glasgow 2020
- Glasgow MJ, Edlin R, Harding JE. Cost-utility analysis of prophylactic dextrose gel vs. standard care for neonatal hypoglycemia in at-risk infants. Journal of Pediatrics 2020;S0022-3476(20):30827-1. [DOI: 10.1016/j.jpeds.2020.06.073] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 10 April 2017. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Harris 2012
- Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. Journal of Pediatrics 2012;161(5):787-91. [DOI: 10.1016/j.jpeds.2012.05.022] [PMID: ] [DOI] [PubMed] [Google Scholar]
Harris 2013
- Harris DL, Weston PJ, Signal M, Chase JG, Harding JE. Dextrose gel for neonatal hypoglycaemia (the Sugar Babies Study): a randomised, double-blind, placebo-controlled trial. Lancet 2013;382(9910):2077-83. [DOI: 10.1016/S0140-6736(13)61645-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Harris 2014
- Harris DL, Weston PJ, Battin MR, Harding JE. A survey of the management of neonatal hypoglycaemia within the Australian and New Zealand Neonatal Network. Journal of Paediatrics and Child Health 2014;50(10):E55-62. [DOI: 10.1111/j.1440-1754.2009.01599.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Harris 2016
- Harris D, Alsweiler JM, Ansell JM, Gamble GD, Thompson B, Wouldes TA, et al, Children with Hypoglycaemia and their Later Development (CHYLD) Study Team. Outcome at 2 years after dextrose gel treatment for neonatal hypoglycaemia: follow-up of a randomized trial. Journal of Pediatrics 2016;170:54‐9. [DOI: 10.1016/j.jpeds.2015.10.066] [PMID: 26613985] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2017
- Harris D, Gamble GD, Weston PJ, Harding JE. What happens to blood glucose concentrations after oral treatment for neonatal hypoglycemia? Journal of Pediatrics 2017;190:136-41. [DOI: 10.1016/j.jpeds.2017.06.034] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hawdon 1993
- Hawdon JM, Aynsley-Green A, Bartlett K, Ward Platt MP. The role of pancreatic insulin secretion in neonatal glucoregulation. II. Infants with disordered blood glucose homoeostasis. Archives of Disease in Childhood 1993;68(3 Spec No):280-5. [DOI: 10.1136/adc.68.3_spec_no.280] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hay 2009
- Hay WW Jr, Raju TN, Higgins RD, Kalhan SC, Devaskar SU. Knowledge gaps and research needs for understanding and treating neonatal hypoglycemia: workshop report from Eunice Kennedy Shriver National Institute of Child Health and Human Development. Journal of Pediatrics 2009;155(5):612-7. [DOI: 10.1016/j.jpeds.2009.06.044] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Kaiser 2015
Kalhan 2000
- Kalhan SC, Peter-Wohl S. Hypoglycemia: what is it for the neonate? American Journal of Perinatology 2000;17(1):11-8. [DOI: 10.1055/s-2000-7296] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kerstjens 2012
- Kerstjens JM, Bocca-Tjeertes IF, Winter AF, Reijneveld SA, Bos AF. Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics 2012;130(2):e265-72. [DOI: 10.1542/peds.2012-0079] [PMID: ] [DOI] [PubMed] [Google Scholar]
Koh 1988
- Koh TH, Aynsley-Green A, Tarbit M, Eyre JA. Neural dysfunction during hypoglycaemia. Archives of Disease in Childhood 1988;63(11):1353-8. [DOI: 10.1136/adc.63.11.1353] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koivisto 1972
- Koivisto M, Blanco-Sequeiros M, Krause U. Neonatal symptomatic and asymptomatic hypoglycaemia: a follow-up study of 151 children. Developmental Medicine and Child Neurology 1972;14(5):603-14. [DOI: 10.1111/j.1469-8749.1972.tb02642.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kurtoglu 2005
- Kurtoglu S, Akcakus M, Keskin M, Ozcan A, Hussain K. Severe hyperinsulinaemic hypoglycaemia in a baby born to a mother taking oral ritodrine therapy for preterm labour. Hormone Research 2005;64(2):61-3. [DOI: 10.1159/000087471] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lucas 1988
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ 1988;297(6659):1304-8. [DOI: 10.1159/000087471] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucas 1999
- Lucas A, Morley R. Authors' reply to Cornblath - outcome of neonatal hypoglycaemia. Complete data are needed. BMJ 1999;318(7177):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maayan‐Metzger 2009
- Maayan-Metzger A, Lubin D, Kuint J. Hypoglycemia rates in the first days of life among term infants born to diabetic mothers. Neonatology 2009;96(2):80-5. [DOI: 10.1159/000203337] [PMID: ] [DOI] [PubMed] [Google Scholar]
McKinlay 2017
- McKinlay CJ, Alsweiler JM, Anstice NS, Burakevych N, Chakraborty A, Chase JG, et al, Children With Hypoglycemia and Their Later Development (CHYLD) Study Team. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatrics 2017;171(10):972–983. [DOI: 10.1001/jamapediatrics.2017.1579] [PMID: 28783802] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2016
- Moore ER, Bergman N, Anderson GC, Medley N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD003519. [DOI: 10.1002/14651858.CD003519.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadjm 2013
- Nadjm B, Mtove G, Amos B, Hildenwall H, Najjuka A, Mtei F, et al. Blood glucose as a predictor of mortality in children admitted to the hospital with febrile illness in Tanzania. American Journal of Tropical Medicine & Hygiene 2013;89(2):232-7. [DOI: 10.4269/ajtmh.13-0016] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence (NICE) UK . Diabetes in Pregnancy; March 2008. Available at www.nice.org.uk/guidance/cg63.
Salhab 2004
- Salhab WA, Wyckoff MH, Laptook AR, Perlman JM. Initial hypoglycemia and neonatal brain injury in term infants with severe fetal acidemia. Pediatrics 2004;114(2):361-6. [DOI: 10.1542/peds.114.2.361] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Singhal 1991
- Singhal PK, Singh M, Paul VK, Malhotra AK, Deorari AK, Ghorpade MD. A controlled study of sugar-fortified milk feeding for prevention of neonatal hypoglycaemia. Indian Journal of Medical Research 1991;94:342-5. [PMID: ] [PubMed] [Google Scholar]
Singhal 1992
- Singhal PK, Singh M, Paul VK, Lamba IM, Malhotra AK, Deorari AK, et al. Prevention of hypoglycemia: a controlled evaluation of sugar fortified milk feeding in small-for-gestational age infants. Indian Pediatrics 1992;29(11):1365-9. [PMID: ] [PubMed] [Google Scholar]
Smith 2016
- Smith HA, Becker GE. Early additional food and fluids for healthy breastfed full-term infants. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD006462. [DOI: 10.1002/14651858.CD006462.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spar 1994
- Spar JA, Lewine JD, Orrison WW Jr. Neonatal hypoglycemia: CT and MR findings. American Journal of Neuroradiology 1994;15(8):1477-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Stenninger 1998
- Stenninger E, Flink R, Eriksson B, Sahlen C. Long-term neurological dysfunction and neonatal hypoglycaemia after diabetic pregnancy. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(3):F174-9. [DOI: 10.1136/fn.79.3.f174] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Surachaidungtavil 2020
- Surachaidungtavil S, Chanvorachote P, Suksumek N. A randomized control trial of oral sucrose solution for prevention of hypoglycemia in high risk infants. In Vivo 2020;34(3):1493-1497. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sweet 1999
- Sweet DG, Hadden D, Halliday HL. The effect of early feeding on the neonatal blood glucose level at 1-hour of age. Early Human Development 1999;55(1):63-6. [DOI: 10.1016/s0378-3782(99)00004-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
UNICEF 2013
- UNICEF UK - The Baby Friendly Initiative. Guidance on the development of policies and guidelines for the prevention and management of hypoglycaemia of the newborn; July 2013. Available at www.unicef.org.uk/babyfriendly/wp-content/uploads/sites/2/2010/10/hypo_policy.pdf.
Weissmann‐Brenner 2012
- Weissmann-Brenner A, Simchen MJ, Zilberberg E, Kalter A, Weisz B, Achiron R, et al. Maternal and neonatal outcomes of large for gestational age pregnancies. Acta Obstetricia et Gynecologica Scandinavica 2012;91(7):844-9. [DOI: 10.1111/j.1600-0412.2012.01412.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weston 2016
- Weston PJ, Harris D, Battin M, Brown J, Hegarty JE, Harding JE. Oral dextrose gel for the treatment of hypoglycaemia in newborn infants. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD011027. [DOI: 10.1002/14651858.CD011027.pub2] [DOI] [PubMed] [Google Scholar]
WHO 1997
- World Health Organization. Hypoglycaemia of the newborn; 1997. Available at www.who.int/maternal_child_adolescent/documents/chd_97_1/en/.
WHO 2008
- World Health Organization. Indicators for assessing infant and young child feeding practices - part I: definition. 2008. Available at http://whqlibdoc.who.int/publications/2008/9789241596664_eng.pdf?ua=1.
Willcox 2010
- Willcox ML, Forster M, Dicko MI, Graz B, Mayon-White R, Barennes H. Blood glucose and prognosis in children with presumed severe malaria: is there a threshold for 'hypoglycaemia'? Tropical Medicine & International Health 2010;15(2):232-40. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhou 2017
- Zhou Y, Bai S, Bornhorst JA, Elhassan NO, Kaiser JR. The effect of early feeding on initial glucose concentrations in term newborns. Journal of Pediatrics 2017;181:112-5. [DOI: 10.1016/j.jpeds.2016.10.032] [DOI: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hegarty 2016b
- Hegarty JE, Harding JE, Crowther CA, Brown J, Alsweiler J. Oral dextrose gel for the prevention of hypoglycaemia in newborn infants. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD012152. [DOI: 10.1002/14651858.CD012152] [DOI] [Google Scholar]
Hegarty 2017
- Hegarty JE, Harding JE, Crowther CA, Brown J, Alsweiler J. Oral dextrose gel to prevent hypoglycaemia in at-risk neonates. Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD012152. [DOI: 10.1002/14651858.CD012152.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


