Abstract
Background
Helminthiasis is an infestation of the human body with parasitic worms. It is estimated to affect 44 million pregnancies, globally, each year. Intestinal helminthiasis (hookworm infestation) is associated with blood loss and decreased supply of nutrients for erythropoiesis, resulting in iron‐deficiency anaemia. Over 50% of the pregnant women in low‐ and middle‐income countries (LMIC) suffer from iron‐deficiency anaemia. Though iron‐deficiency anaemia is multifactorial, hookworm infestation is a major contributory cause in women of reproductive age in endemic areas. Antihelminthics are highly efficacious, but evidence of their beneficial effect and safety when given during pregnancy has not been established. This is an update of a Cochrane Review last published in 2015.
Objectives
To determine the effects of mass deworming with antihelminthics for soil‐transmitted helminths (STH) during the second or third trimester of pregnancy on maternal and pregnancy outcomes.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP) (8 March 2021) and reference lists of retrieved studies.
Selection criteria
We included all prospective randomised controlled trials evaluating the effect of administration of antihelminthics versus placebo or no treatment during the second or third trimester of pregnancy; both individual‐randomised and cluster‐randomised trials were eligible. We excluded quasi‐randomised trials and studies that were only available as abstracts with insufficient information.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data, checked accuracy and assessed the certainty of the evidence using the GRADE approach.
Main results
We included a total of six trials (24 reports) that randomised 7873 pregnant women. All of the included trials were conducted in antenatal clinics within hospitals in LMICs (Uganda, Nigeria, Peru, India, Sierra Leone and Tanzania). Among primary outcomes, five trials reported maternal anaemia, one trial reported preterm birth and three trials reported perinatal mortality. Among secondary outcomes, included trials reported maternal worm prevalence, low birthweight (LBW) and birthweight. None of the included studies reported maternal anthropometric measures or infant survival at six months. Overall, we judged the included trials to be generally at low risk of bias for most domains, while the certainty of evidence ranged from low to moderate.
Analysis suggests that administration of a single dose of antihelminthics in the second trimester of pregnancy may reduce maternal anaemia by 15% (average risk ratio (RR) 0.85, 95% confidence interval (CI) 0.72 to 1.00; I²= 86%; 5 trials, 5745 participants; low‐certainty evidence). We are uncertain of the effect of antihelminthics during pregnancy on preterm birth (RR 0.84, 95% CI 0.38 to 1.86; 1 trial, 1042 participants; low‐certainty evidence) or perinatal mortality (RR 1.01, 95% CI 0.67 to 1.52; 3 trials, 3356 participants; low‐certainty evidence).
We are uncertain of the effect of antihelminthics during pregnancy on hookworm (average RR 0.31, 95% CI 0.05 to 1.93; Tau² = 1.76, I² = 99%; 2 trials, 2488 participants; low‐certainty evidence). Among other secondary outcomes, findings suggest that administration of antihelminthics during pregnancy may reduce the prevalence of trichuris (average RR 0.68, 95% CI 0.48 to 0.98; I²=75%; 2 trials, 2488 participants; low‐certainty evidence) and ascaris (RR 0.24, 95% CI 0.19 to 0.29; I²= 0%; 2 trials, 2488 participants; moderate‐certainty evidence). Antihelminthics during pregnancy probably make little or no difference to LBW (RR 0.89, 95% CI 0.69 to 1.16; 3 trials, 2960 participants; moderate‐certainty evidence) and birthweight (mean difference 0.00 kg, 95% CI ‐0.03 kg to 0.04 kg; 3 trials, 2960 participants; moderate‐certainty evidence).
Authors' conclusions
The evidence suggests that administration of a single dose of antihelminthics in the second trimester of pregnancy may reduce maternal anaemia and worm prevalence when used in settings with high prevalence of maternal helminthiasis. Further data is needed to establish the benefit of antihelminthic treatment on other maternal and pregnancy outcomes.
Future research should focus on evaluating the effect of these antihelminthics among various subgroups in order to assess whether the effect varies. Future studies could also assess the effectiveness of co‐interventions and health education along with antihelminthics for maternal and pregnancy outcomes.
Plain language summary
Effect of drugs to treat intestinal worms from contaminated soil in pregnant women
What is the issue?
Parasitic worm infections from contaminated soil include hookworm, roundworm, and whipworm. These intestinal worms (helminths) feed on blood and can contribute to iron‐deficiency anaemia in women of reproductive age. Parasitic worms also release substances that stop blood from clotting, so cause further bleeding. Affected women often experience anorexia, vomiting and diarrhoea, which reduces the supply of essential nutrients for producing blood cells. As a result, the health of pregnant women and their unborn babies can be affected.
Antihelminthics are drugs that force parasitic worms out of the body, either by stunning or killing them, without causing damage to the host. Antihelminthics are highly effective against these worms, but evidence of their beneficial effect and safety when given during pregnancy is limited.
Why is this important?
Women in low‐ and middle‐income countries (LMICs) are especially likely to have worms that can lead to anaemia, since they may be pregnant or lactating for as much as half of their reproductive lives. Women who are anaemic during pregnancy are more likely to have ill health, give birth prematurely, and have low birthweight (LBW) babies with low iron reserves. A lack of iron can reduce the babies' mental abilities and development as well as their physical growth.
What evidence did we find?
We searched for evidence in March 2021 and identified six randomised controlled studies (in 24 reports) that included 7873 pregnant women. All of the included studies were conducted in antenatal clinics within hospitals in LMICs (Uganda, Nigeria, Peru, India, Sierra Leone and Tanzania). All except one of the included trials gave iron supplementation to the women participating in the studies, as well as the antihelminthic drugs.
Evidence from five trials (5745 women) suggests that deworming using a single dose of antihelminthics in the second trimester of pregnancy may reduce maternal anaemia (low‐certainty evidence). We are uncertain about the effects on preterm birth (1042 women in 1 study) or perinatal mortality (3356 women in 3 studies), with low‐certainty evidence for both outcomes. Antihelminthics probably make little or no difference to LBW babies (3301 women in 4 studies) or birthweight (3301 women in 4 studies), with moderate‐certainty evidence for both outcomes. The number of women with worms was reduced (2488 women in 2 studies; low‐certainty evidence).
What does this mean?
Antihelminthic drugs given during the second trimester of pregnancy may reduce maternal anaemia and the number of women with worms, but with no impact on other maternal or pregnancy outcomes. Further research is needed among particular groups of women and on the effectiveness of additional interventions with antihelminthics, including health education.
Summary of findings
Summary of findings 1. Antihelminthics versus control for soil‐transmitted helminths during pregnancy.
| Antihelminthics versus control for soil‐transmitted helminths during pregnancy | ||||||
|
Patient or population: pregnant women in second trimester of pregnancy
Settings: antenatal clinics (Sierra Leone, Peru, Uganda, Nigeria, Tanzania, India)
Intervention: antihelminthics Comparison: placebo/control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antihelminthics | |||||
| Maternal anaemia in third trimester (< 11 g/dL) | Study population | RR 0.85 (0.72 to 1.00) | 5745 (5 studies) | ⊕⊕⊝⊝ lowa,b | ||
| 447 per 1000 | 383 per 1000 | |||||
| Preterm birth (birth before 37 weeks of gestation) | Study population | RR 0.84 (0.38 to 1.86) | 1042 (1 study) | ⊕⊕⊝⊝ lowc | ||
| 25 per 1000 | 21 per 1000 | |||||
| Perinatal mortality | Study population | RR 1.01 (0.67 to 1.52) | 3356 (3 studies) | ⊕⊕⊝⊝ lowd,e | ||
| 28 per 1000 | 30 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded by one level for serious limitations in study design (high risk of attrition bias in Torlesse 2001 and high risk from lack of blinding in Urassa 2011). bDowngraded by one level for serious limitations relating to inconsistency (I2 = 86%). cDowngraded by two levels serious imprecision due to a small number of events and wide CI. dDowngraded by one level for serious indirectness (the studies were not powered to capture mortality). eDowngraded by one level for serious imprecision – wide CI crossing the line of no effect.
Background
Description of the condition
Helminthiasis is an infestation of the human body with parasitic worms. There are about 20 major helminth infections of humans, and all have some public health significance, but among the commonest of all human infections are those from soil‐transmitted helminths (STH) (Keiser 2019). Altogether, these STH infect over one billion people and account for about three million disability‐adjusted life years (Hay 2017; Hotez 2014). An estimated 438.9 million people were infected with hookworm in 2010, 819.0 million with roundworm (Ascaris lumbricoides), and 464.6 million with whipworm (Trichuris trichiura). Overall, STH contributed to a total of 4.98 million years lived with disability (YLDs) (Pullan 2014). Of these YLDs, 65% were attributable to hookworm, 22% to roundworm and the remaining 13% to whipworm. In terms of geographical distribution, around 67% of STH infections occurred in Asia and contributed to 68% of the YLDs (Pullan 2014).
Intestinal helminths contribute to anaemia as they feed on blood and cause further haemorrhage by releasing anticoagulant compounds, thereby leading to iron‐deficiency anaemia. They also contribute by affecting the supply of nutrients necessary for erythropoiesis (Hotez 1983; Torlesse 2000). Although iron‐deficiency anaemia is multifactorial, hookworm infection is an important contributory factor in endemic areas, especially among women of reproductive age. It is the leading cause of pathological blood loss in tropical and subtropical regions, posing a serious threat to the health of mothers and foetuses (Bundy 1995; Pawlowski 1991). Women in low‐ and middle‐income countries (LMIC) may be pregnant or lactating for as much as half of their reproductive lives (WHO 1994); estimates indicate that over 50% of the pregnant women in LMIC have iron‐deficiency anaemia (ACC/SCN 2000; WHO 1997). T trichura also causes intestinal blood loss, although much less so than hookworms on a per‐worm basis (Bundy 1989). A lumbricoides interferes with the utilisation of vitamin A, which is required for hematopoiesis. All three intestinal helminths may reduce the intake and absorption of iron and other hematopoietic nutrients by causing anorexia, vomiting and diarrhoea (WHO 2003). Anaemia during pregnancy is associated with premature delivery, low birthweight (LBW), maternal ill health, and maternal death (Seshadri 1997). Favourable pregnancy outcomes occur 30% to 45% less often in anaemic mothers, and their infants have less than one half of normal iron reserves (Rahman 2016). Iron deficiency adversely affects cognitive performance and development as well as the physical growth of these infants (WHO 2001).
Description of the intervention
Antihelminthic treatment is regarded as the most effective means of controlling mortality and morbidity due to intestinal helminth infections (WHO 1994). Antihelminthics such as levamisole, mebendazole, albendazole and pyrantel are highly efficacious and have minimal side‐effects, but data about their use in pregnancy are limited. In 1994, the World Health Organization (WHO) convened an informal consultation on hookworm infection and anaemia in girls and women, which promoted the use of antihelminthics in pregnancy after the first trimester in areas where these infections are endemic (prevalence > 20% to 30%) and where anaemia is prevalent, but it also recommended evaluation of the long‐term safety, particularly in terms of birth outcomes (WHO 1994). With regards to mass deworming during pregnancy, data about deworming drug use in pregnancy are scarce (WHO 1994; WHO 2018). Adverse events associated with deworming in girls and women themselves have rarely been published, and usually only within the context of specific research studies (Keiser 2008). A cross‐sectional retrospective study in Sri Lanka in 1995, assessing the effect of mebendazole during pregnancy on birth outcome, found beneficial effects of the therapy on birth outcome, with significantly lower rates of stillbirths, perinatal deaths and very low birthweight babies in the mebendazole group than in the control group. A slightly higher rate of congenital defects was found in women who had taken the drug in the first trimester of pregnancy, but the difference was non‐significant (de Silva 1999). Another non‐randomised effectiveness study, also conducted in Sri Lanka, involved iron folate supplementation along with a single dose of mebendazole in the second trimester of pregnancy (Atukorala 1994). Comparison of compliants versus non‐compliants of the therapy showed an improvement in the iron status of pregnant women in the iron folate mebendazole group. An Indian community‐based pre‐post experimental study demonstrated a decrease in the prevalence of anaemia and increased mean haemoglobin in both second and third trimester in the group receiving education focusing on anaemia, plus iron supplementation and 100 mg mebendazole taken twice daily for three days (Abel 2000). Similar results were found in a non‐randomised community‐based study in Nepal, which showed an increase in haemoglobin levels and a lower proportion of anaemia in the third trimester in women receiving albendazole in the second trimester (Christian 2004).
The WHO recommends mass deworming for STH depending on the prevalence of worm infection (WHO 2017). Deworming, using single‐dose albendazole (400 mg) or mebendazole (500 mg), is recommended as a public health intervention to reduce the worm burden of hookworm and T trichiura infection for pregnant women after the first trimester, living in areas where both:
the baseline prevalence of hookworm or T trichiura infection is 20% or higher among pregnant women, and
anaemia is a severe public health problem, with a prevalence of 40% or higher among pregnant women.
Antihelminthics are not recommended in the first trimester of pregnancy.
How the intervention might work
Measures to prevent and treat helminth infections aim to alleviate suffering, reduce poverty, and support equal opportunities for men and women (WHO 2006). Since many of the antihelminthic drugs are broad spectrum, treatment can result in targeting several diseases simultaneously. Preventive chemotherapy (either alone or in combination) is used as a public heath tool for preventing morbidity due to infection, usually with more than one helminth at a time.
Anthelmintics are selectively toxic to the parasite and not the host. Some work by inhibiting metabolic processes that are vital to the parasite but absent or not vital in the host. Other anthelmintics are poorly absorbed through the gut, which means the parasite is exposed to much higher concentrations of the anthelmintic than the host. This results in starvation or paralysis of the parasite, followed by subsequent expulsion or digestion (Drugs.com 2021).
Why it is important to do this review
Mass deworming with antihelminthics is generally accepted as an effective measure to prevent and treat STH along with the water, sanitation and hygiene (WASH) measures. However, very little is currently known about the effects of antihelminthics on birth outcome (WHO 2006; WHO 2018). Furthermore, critical appraisal of existing studies suggests that these fail to account for various factors that could modify the effectiveness of deworming, including nutritional status, type of infection, worm burden and concomitant interventions (Barry 2013; Turner 2015). Hence, the aim of this review is to identify the effects of administering antihelminthics during pregnancy on maternal and pregnancy outcomes. This is an update of a review last published in 2015.
Objectives
To determine the effects of mass deworming with antihelminthics for soil‐transmitted helminths (STH) during the second or third trimester of pregnancy on maternal and pregnancy outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) that assessed the effects of administration of antihelminthics during the second or third trimester of pregnancy, irrespective of language or publication status. Both individual‐randomised and cluster‐randomised trials were eligible. We excluded quasi‐randomised trials and studies that were available as abstracts only with insufficient information.
Types of participants
We included pregnant women in the second or third trimester. We excluded studies that enrolled HIV‐infected women only.
Types of interventions
The comparison of interest was mass deworming with antihelminthics versus placebo or no treatment. In case of co‐interventions other than antihelminthics, both groups should have received the same co‐intervention.
Types of outcome measures
Primary outcomes
Maternal anaemia in the third trimester of pregnancy (haemoglobin less than 11 g/dL)
Preterm birth (birth before 37 weeks of gestation)
Perinatal mortality (includes foetal death after 28 weeks of gestation and infant death that occurs at less than seven days of life)
Secondary outcomes
Maternal worm burden/prevalence (measured as faecal egg counts, in eggs per gram of faeces or as reported by the study authors)
Maternal anthropometric measures (weight, height and body mass index (BMI))
Low birthweight (less than 2500 g)
Birthweight
Infant survival at six months
Search methods for identification of studies
The following search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (8 March 2021).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase and CINAHL (Cumulative Index to Nursing and Allied Health Literature); the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service, please follow this link to: pregnancy.cochrane.org/pregnancy-and-childbirth-groups-trials-register.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of CENTRAL;
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL EBSCO;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
To maintain the register, two people screen the search results and review the full text of all relevant trial reports identified through the searching activities described. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (8 March 2021) using the search methods detailed in Appendix 1.
Searching other resources
We searched reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Salam 2015.
For this update, we used the following methods to assess the reports identified as a result of the updated search.
The following methods section is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. We entered the data into Review Manager software and checked for accuracy (Review Manager 2020).
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described, for each outcome or class of outcomes, the completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where a study reported sufficient information, or the trial authors could supply this, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as‐treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (see: Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
We used the mean difference (MD) as trials measured outcomes in the same way. In future updates, if appropriate, we will use the standardised mean difference (SMD) to combine trials that measured the same outcome but use different methods.
Unit of analysis issues
Cluster‐randomised trials
One of the included trials was a cluster‐randomised trial (Urassa 2011). In this study the trial authors used appropriate methods for cluster adjustment, so the estimates that we used in the meta‐analysis had already been adjusted for the effects of clustering.
In future updates, we will adjust the sample sizes or standard errors using the methods described in the Handbook using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population (Higgins 2011). We did not use ICC from other sources, but if we use ICCs from other sources in future updates we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. We considered it reasonable to combine the results from both the cluster‐randomised trial and the other RCTs as there was little heterogeneity between the study designs and we considered interaction between the effect of intervention and the choice of randomisation unit to be unlikely.
Cross‐over trials
Cross‐over designs are not a valid study design for Pregnancy and Childbirth reviews, and so were not eligible for inclusion.
Studies with multiple arms
In instances where the included studies had more than two arms that were relevant to the review, we combined the groups to create a single pair‐wise comparison. For Elliott 2005, we merged the two relevant intervention arms ('albendazole (400 mg) and placebo' and 'albendazole and praziquantel') and compared it with the placebo group to create a single pair‐wise comparison. For https://revman.cochrane.org/#/995704122310113533/htmlView/7.24#STD‐Torlesse‐2001, we included four arms in two separate pair‐wise comparisons (Torlesse 2001 (1): albendazole and daily iron folate versus daily iron folate and calcium vitamin D tablets as albendazole control; Torlesse 2001 (2): albendazole and calciferol tablets as iron folate control versus calcium vitamin D tablets as albendazole control and calciferol tablets as iron folate control). In order to reduce heterogeneity, we kept the Torlesse 2001 study arms separate since one arm is albendazole with iron/folate while one is other is albendazole without iron/folate.
Dealing with missing data
We noted the levels of attrition for the included studies. If future updates include more eligible studies, we will use sensitivity analysis to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect.
For all outcomes, we carried out analyses on an intention‐to‐treat basis as far as possible, i.e. we attempted to include in the analyses all participants randomised to each group. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero or there was a low P value (less than 0.10) from the Chi² test for heterogeneity. If we identified substantial heterogeneity (I² above 30%), we planned to explore it by prespecified subgroup analysis.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis in future updates, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (Review Manager 2020). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and we judged the trials’ populations and methods to be sufficiently similar.
If there was sufficient clinical heterogeneity to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary for outcomes if we considered that an average treatment effect across trials was clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects and discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, we presented the results as the average treatment effect with 95% confidence intervals and gave the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We planned to investigate any identified substantial heterogeneity using subgroup analyses and sensitivity analyses.
We had specified the following subgroup analyses for our primary outcome (maternal anaemia).
Differences in type of antihelminthic (albendazole, mebendazole, praziquantel, others)
Baseline worm burden at the individual level (across four levels of none, light, moderate and heavy, using the WHO cutoffs for each helminth) (Montresor 1998)
Co‐interventions other than antihelminthics (concomitant iron/folic acid supplementation versus no supplementation)
We could not conduct the planned subgroup analysis since there were too few studies in each subgroup to make any meaningful conclusions.
In future updates, we will attempt to conduct the subgroup analysis and assess subgroup differences by interaction tests available within Review Manager 2020. We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test's I² value.
Sensitivity analysis
We planned to carry out sensitivity analysis to explore the effect of risk of bias, assessed by concealment of allocation, high attrition rates, or both. We planned to exclude high risk of bias studies from the analysis to assess whether this made any difference to the overall result. However, there were too few studies included in any meta‐analysis to carry out meaningful sensitivity analysis in this update.
Summary of findings and assessment of the certainty of the evidence
We used the GRADEpro Guideline Development Tool to import data from Review Manager 2020 and create a summary of findings table for the main comparison (antihelminthics versus control). We produced a summary of the intervention effect and a measure of certainty for the following outcomes, using the approach outlined in the GRADE handbook.
Maternal anaemia in third trimester (< 11 g/dL)
Preterm birth (birth before 37 weeks of gestation)
Perinatal mortality
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
See: Figure 1.
1.
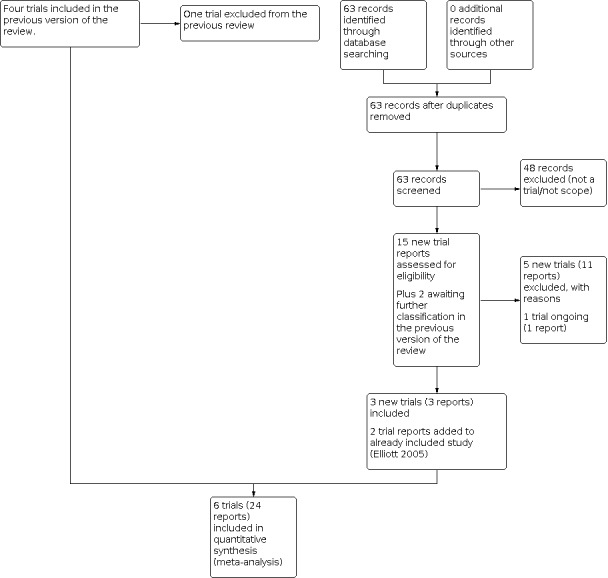
Study flow diagram.
For this update, we assessed 15 new trial reports and reassessed two that were awaiting classification in the previous version of the review (Friedman 2007; Urassa 2011). We included three new trials (Akpan 2018; Deepti 2015; Urassa 2011), and added two new reports to one previously included trial (Elliott 2005). We excluded five new trials (Asbjornsdottir 2018; Friedman 2007; Ivan 2015; Mofid 2017; NCT04171388 (first received 2019 Nov 20)a) (eleven reports). We also excluded one trial previously included in the review (Ndyomugyenyi 2008), since it did not fulfil the definition of mass deworming. One study is ongoing (NCT04391998 (first received 2020 May 18)a.
Included studies
Design
All of the included studies were RCTs. One of the trials was a cluster‐RCT (Urassa 2011), while all of the other included trials were individually randomised.
Sample sizes
The trials included a total of 7873 pregnant women. Sample size in the included trials ranged from a minimum of 184 pregnant women in the study by Torlesse 2001 to a maximum of 3080 pregnant women in the Urassa 2011 trial. The number of participants in the Akpan 2018 study was 560; there were 2507 women in the study by Elliott 2005 ; 1042 in Larocque 2006; and 500 in the trial by Deepti 2015.
Setting
All of the included trials were conducted in antenatal clinics within hospitals. All the trials were conducted in low‐ and middle‐income countries: Uganda (Elliott 2005); Nigeria (Akpan 2018); Peru (Larocque 2006); India (Deepti 2015); Sierra Leone (Torlesse 2001); and Tanzania (Urassa 2011).
Participants
The majority of the trials enrolled pregnant women in their second trimester. Torlesse 2001 and Urassa 2011 enrolled women in their first trimester but delivered the intervention in the second/third trimester. Deepti 2015 enrolled women in their second and third trimester. Mean age of the participants ranged from a minimum of 22 years to a maximum of 29 years. One trial did not specify the mean age of the pregnant women (Deepti 2015).
Interventions and comparisons
The intervention included administration of albendazole or mebendazole. Three trials provided albendazole (Elliott 2005; Torlesse 2001; Urassa 2011); two trials provided mebendazole (Akpan 2018; Larocque 2006); while one trial provided both albendazole and mebendazole (Deepti 2015). One trial also provided praziquantel for schistosomiasis (Elliott 2005). With the exception of the study by Deepti 2015, all of the trials provided iron/folic acid supplementation along with the antihelminthic drugs.
Outcomes
Among primary outcomes, five trials reported maternal anaemia, one trial reported preterm birth and three trials reported perinatal mortality.
Among secondary outcomes, included trials reported maternal worm prevalence, low birthweight and birthweight. None of the included studies reported any of the maternal anthropometric measures (including weight, height and body mass index) or infant survival at six months.
Deepti 2015 did not report outcomes specific to the study arms, so we were unable to include data from this trial in the meta‐analyses. Please refer to the Characteristics of included studies table for more details.
Trial dates
Trials took place between 1995 and 2015. Specific start and end dates for all trials were as follows: January 2015 to December 2015 (Akpan 2018); April 2003 to November 2005 (Elliott 2005); April 2003 to July 2004 (Larocque 2006); August 2011 to February 2012 (Deepti 2015); December 1995 to June 1996 (Torlesse 2001); March 2001 to February 2003 (Urassa 2011).
Funding sources
One trial did not receive any financial support (Akpan 2018); another did not specify funding sources (Deepti 2015). The remaining trials received funding from a Wellcome Trust Fellowship (Elliott 2005); the Canadian Institutes of Health Research (CIHR) (Larocque 2006); a research grant from the Institute of Biomedical and Life Sciences, University of Glasgow, UK (Torlesse 2001); and the Swedish agency for research and co‐operation with developing countries (Sida‐SAREC) (Urassa 2011).
Conflicts of interest
The authors of two trials declared that there were no competing interests (Akpan 2018; Elliott 2005). The remaining trials did not mention conflicts of interest.
Excluded studies
We excluded eleven trials as they did not satisfy the inclusion criteria of the review (Asbjornsdottir 2018; Basra 2013; Bhutta 2007; Friedman 2007; Ivan 2015; Mofid 2017; NCT04171388 (first received 2019 Nov 20)a; Ndyomugyenyi 2008; Nery 2013; Tehalia 2011; Villar 1998). Asbjornsdottir 2018 was a mass population drug administration study and included men, women and children; Basra 2013 assessed the efficacy of mefloquine; Bhutta 2007 did not have an appropriate comparison group; Friedman 2007 assessed deworming for schistosomiasis; Ivan 2015 only included HIV‐infected pregnant women; NCT04171388 (first received 2019 Nov 20)a was withdrawn due to Covid‐19 with no participants being enrolled; Mofid 2017 focused on deworming among postpartum women; Ndyomugyenyi 2008 included women infected with any STH and compared them to a reference group without any STH; Nery 2013 did not target pregnant women; while two studies were only available as abstracts with insufficient information (Tehalia 2011; Villar 1998).
More details are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
All the included trials in this review were randomised controlled trials. Figure 2 and Figure 3 provide a graphical summary of the risk of bias assessments for the included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Five trials were at low risk of bias for random sequence generation, since they used appropriate randomisation methods (Akpan 2018; Elliott 2005; Larocque 2006; Deepti 2015; Torlesse 2001). We judged the Urassa 2011 trial to be at unclear risk, since the trial authors did not specify the process of randomisation.
Allocation was adequately concealed in four trials (Akpan 2018; Elliott 2005; Larocque 2006; Deepti 2015). Two trials provided no information on allocation concealment (Torlesse 2001; Urassa 2011).
Blinding
We considered all six trials to have adequate measures for blinding of participants and personnel, so assessed them to be at low risk (Akpan 2018; Elliott 2005; Larocque 2006; Deepti 2015; Torlesse 2001; Urassa 2011).
Five trials also had adequate measures for blinding of outcome assessors, so we considered these to be at low risk of bias (Akpan 2018; Elliott 2005; Larocque 2006; Deepti 2015; Torlesse 2001). We judged the trial by Urassa 2011 to be at high risk for blinding of outcome assessors.
Incomplete outcome data
Five trials were at low risk of attrition bias (Akpan 2018; Elliott 2005; Larocque 2006; Deepti 2015; Urassa 2011). These trials provided reasons for attrition and exclusions at each level, along with the distribution across the study arms. Torlesse 2001 was at high risk for attrition (with a rate of 29%).
Selective reporting
We judged two trials to be at low risk for selective reporting, since these two trials provided trial registration details and their results sections reported all the outcomes prespecified in the protocol (Elliott 2005; Larocque 2006). We judged three trials to be at unclear risk for selective reporting because we could not find any trial registration details or published protocols (Akpan 2018Torlesse 2001; Urassa 2011). However, these trials reported all of the outcomes specified in their methodology sections in their results sections. We judged the trial by Deepti 2015 to be at high risk for selective reporting, since we could not find any details on trial registration or a published protocol and the trial did not report outcomes specific to the study groups.
Other potential sources of bias
We judged all of the trials to be at low risk for other biases because we did not identify any other concerns. The cluster‐randomised trial used appropriate methods for cluster adjustment, so we judged this to be at low risk of bias.
Effects of interventions
See: Table 1
See: Table 1.
Comparison: Antihelminthics versus control
Primary outcomes
Maternal anaemia in third trimester
Five trials reported maternal anaemia (Akpan 2018; Elliott 2005; Larocque 2006; Torlesse 2001; Urassa 2011). Administration of a single dose of antihelminthics in the second trimester of pregnancy may reduce maternal anaemia in the third trimester by 15% (average RR 0.85, 95% CI 0.72 to 1.00; Tau² = 0.03, I² = 86%; 5745 participants, 5 trials; low‐certainty evidence; Analysis 1.1). However, heterogeneity was high for this outcome so these results should be viewed with caution; this could not be explored through sensitivity or subgroup analysis due to the limited number of studies included.
1.1. Analysis.

Comparison 1: Antihelminthics versus control, Outcome 1: Maternal anaemia in third trimester (< 11 g/dL)
Preterm birth
One trial reported preterm birth (Larocque 2006). We are uncertain of the effect of antihelminthics during pregnancy on preterm birth (RR 0.84, 95% CI 0.38 to 1.86; 1042 participants, 1 trial; low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Antihelminthics versus control, Outcome 2: Preterm birth (birth before 37 weeks of gestation)
Perinatal mortality
Three trials reported perinatal mortality (Akpan 2018; Elliott 2005; Larocque 2006). We are uncertain of the effect of antihelminthics during pregnancy on perinatal mortality (RR 1.01, 95% CI 0.67 to 1.52; I² = 0%; 3356 participants, 3 trials; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Antihelminthics versus control, Outcome 3: Perinatal mortality
Secondary outcomes
Maternal worm prevalence
Two trials reported on the prevalence of hookworm, trichuris and ascaris (Elliott 2005; Larocque 2006). Administration of antihelminthics during pregnancy may reduce the prevalence of trichuris (average RR 0.68, 95% CI 0.48 to 0.98; Tau² = 0.05, I²= 75%; 2488 participants, 2 trials; low‐certainty evidence; Analysis 1.4.1); and ascaris (average RR 0.24, 95% CI 0.19 to 0.29; Tau² = 0.00, I²= 0%; 2488 participants, 2 trials; moderate‐certainty evidence; Analysis 1.4.2). We are uncertain of the effect of antihelminthics during pregnancy on hookworm (average RR 0.31, 95% CI: 0.05 to 1.93; Tau² = 1.76, I²=99%; 2488 participants, 2 trials; low‐certainty evidence; Analysis 1,4.3). Substantial statistical heterogeneity is present in the meta‐analyses for hookworm and trichuris, so these results should be interpreted with caution. The reason for this heterogeneity could be the different drugs used in the trials. In the Elliott 2005 trial, the drug used was albendazole, while in the Larocque 2006 trial it was mebendazole. It is known that albendazole is more effective against hookworms whereas mebendazole is more effective against ascaris.
Maternal anthropometric measures
None of the included trials reported this outcome.
Low birthweight
Three trials reported low birthweight as an outcome (Akpan 2018; Elliott 2005; Larocque 2006). A single dose of antihelminthics in the second trimester of pregnancy probably makes little or no difference to low birthweight (RR 0.89, 95% CI 0.69 to 1.16; I²= 0%; 2960 participants, 3 trials; moderate‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Antihelminthics versus control, Outcome 5: Low birthweight
Birthweight
Three trials reported birthweight (Akpan 2018; Elliott 2005; Larocque 2006). A single dose of antihelminthics in the second trimester of pregnancy probably makes little or no difference on to birthweight (mean difference 0.00 kg, 95% CI ‐0.03 kg to 0.04 kg; I²= 0%; 2960 participants, 3 trials; moderate‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Antihelminthics versus control, Outcome 6: Birthweight
Infant survival at six months
None of the included trials reported this outcome.
Discussion
Summary of main results
This review summaries findings from six trials (24 reports) including 7873 pregnant women. Among primary outcomes, five trials reported maternal anaemia, one trial reported preterm birth and three trials reported perinatal mortality. Findings suggest that administration of a single dose of antihelminthics in the second trimester of pregnancy may reduce maternal anaemia by 15%. However, we are uncertain of the effect of antihelminthics during pregnancy on preterm birth and perinatal mortality. Among secondary outcomes, the included trials reported maternal worm prevalence, low birthweight (LBW) and birthweight. None of the included trials reported any other secondary outcomes (including maternal anthropometric measures and infant survival at six months). Findings suggest that administration of antihelminthics during pregnancy may reduce the prevalence of trichuris and ascaris and probably makes little or no difference to LBW and birthweight.
Overall completeness and applicability of evidence
We found six randomised controlled trials evaluating the impact of antihelminthic treatment in the second trimester of pregnancy. All trials were conducted in low‐ and middle‐income countries (LMIC) in which a single dose of antihelminthic in the second trimester of pregnancy was compared against the control group. Iron supplementation was given as a co‐intervention in all except one of the included trials (Deepti 2015). The review findings showed some positive impact of antihelminthic treatment in the second trimester of pregnancy on maternal anaemia and worm prevalence, while there was no impact on any of the other maternal or pregnancy outcomes. However, these findings should be interpreted with caution due to high heterogeneity; we could not explore the reasons through sensitivity or subgroup analysis due to the limited number of included studies.
We could not evaluate the impact on maternal anthropometric measures and infant survival at six months of age due to the non‐availability of data from the included trials. We could not conduct subgroup analyses according to the different type of antihelminthics, co‐interventions other than antihelminthics and baseline worm burden due to there being too little data in each subgroup for any meaningful analysis. The findings of the review are generalisable to LMIC settings.
Quality of the evidence
We judged the included trials to be at low risk of bias overall for most risk of bias domains, with a few exceptions: we judged Urassa 2011 to be at high risk for blinding; Deepti 2015 to be at high risk for selective reporting; and Torlesse 2001 to be at high risk for incomplete outcome data. The overall GRADE ratings of the certainty of evidence ranged from low to moderate. We downgraded outcomes due to study limitations, imprecision, indirectness and inconsistency. We graded the outcome 'maternal anaemia' as low‐certainty evidence. We downgraded this outcome by two levels; firstly due to a high risk of attrition bias in Torlesse 2001 and a high risk of blinding in Urassa 2011; and secondly due to inconsistency (I² = 86%). We downgraded preterm birth by two levels for very serious imprecision due to a small number of events and a wide 95% confidence interval. We downgraded perinatal mortality by one level due to serious indirectness, since the studies were not powered to capture mortality, and by one level for serious imprecision because the wide 95% confidence intervals crossed the line of no effect.
Potential biases in the review process
We minimised biases in the review process. There was a systematic evaluation at all stages, including literature search screening, full‐text eligibility and data extraction. Two review authors did this independently and resolved discrepancies by discussion among all the review authors. All of the outcomes were prespecified in the protocol.
Agreements and disagreements with other studies or reviews
There has been limited data pertaining to deworming among pregnant women and hence there is a consequent gap in the evidence related to the health impacts. A recent individual participant data analysis (IPD) suggested that mass deworming during pregnancy is associated with reducing anaemia, with no evidence of impact on any other maternal or pregnancy outcomes (Salam 2019). These findings are in concordance with the findings of our review. Furthermore, the IPD analysis also suggested that findings were limited by the availability of data in relation to subgroups and effect modification. In our review, we could not perform the planned subgroup analyses due to limited data availability.
Our review findings are also in concordance with a review assessing the effect of antihelminthics among women of reproductive age and adolescent girls. That review's findings suggested that the intervention probably reduces the prevalence of soil‐transmitted helminths (STH) but may have little or no effect on anaemia and iron‐deficiency in adolescent girls and non‐pregnant women in comparison to no intervention or placebo (Ghogomu 2018). These results were also limited by sparse data and the moderate‐ to very low‐certainty of evidence available.
A recent IPD assessing the effect of mass deworming among children suggested that there might be small effects on weight but not height or haemoglobin among children with moderate or heavy intensity infections (using WHO cut‐offs) (Welch 2019). The analysis concluded that the effects of deworming are uncertain in children with heavy intensity infections. A recent systematic review and network meta‐analysis evaluating the effects of mass deworming for STH on growth, educational achievement, cognition, school attendance, quality of life, and adverse effects in children in endemic helminth areas suggested that mass deworming for STH, with or without deworming for schistosomiasis, had little effect (Welch 2017).
Authors' conclusions
Implications for practice.
Our review findings suggest that administration of antihelminthics during pregnancy may reduce maternal anaemia and worm prevalence. However, we did not find any effect on any other maternal or pregnancy outcomes, including preterm birth, perinatal mortality, LBW and birthweight.
Implications for research.
Existing data on the use of antihelminthics among pregnant women is scarce. We could not conduct planned subgroup analysis based on type of antihelminthics, baseline worm burden and co‐interventions since there were too few studies in each subgroup for any meaningful conclusions. Future research should focus on evaluating the effect of these antihelminthics among various subgroups to assess whether the effect varies. Future studies should also assess the effectiveness of co‐interventions, mainly WASH interventions and health education, along with antihelminthics for maternal and pregnancy outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 8 March 2021 | New citation required and conclusions have changed | Previously, the review concluded that there was no effect of administration of a single dose of antihelminthics in the second trimester of pregnancy on maternal anaemia, low birthweight, preterm birth or perinatal mortality. The updated analysis suggests that administration of a single dose of antihelminthics in the second trimester of pregnancy may reduce maternal anaemia in the third trimester by 15%. However, there was no effect on preterm birth and perinatal mortality. |
| 8 March 2021 | New search has been performed | Search updated. We included three new trials (Akpan 2018; Deepti 2015; Urassa 2011). One study previously included was excluded in this update (Ndyomugyenyi 2008). This review now includes a total of six trials (24 reports). |
History
Protocol first published: Issue 4, 2005 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 29 June 2015 | Amended | Analysis 1.1 corrected to display totals. |
| 31 January 2015 | New citation required but conclusions have not changed | No change in conclusions. |
| 31 January 2015 | New search has been performed | Search updated and one study added. |
| 12 November 2008 | Amended | Converted to new review format. |
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser (2020 version). The authors are grateful to the following peer reviewer for their time and comments: David Lissauer, University of Liverpool.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
ICTRP
Each line was searched separately (with no restrictions)
helminth(s) AND pregnancy
helminth(s) AND pregnant
deworming AND pregnancy
deworming AND pregnant
worm(s) AND pregnancy
worm(s) AND pregnant
antihelminthic AND pregnancy
antihelminthic AND pregnant
ClinicalTrials.gov
Advanced search
pregnancy | Interventional Studies | Worms
pregnancy | Interventional Studies | anthelmintics
pregnancy | deworming
Data and analyses
Comparison 1. Antihelminthics versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Maternal anaemia in third trimester (< 11 g/dL) | 5 | 5745 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.00] |
| 1.2 Preterm birth (birth before 37 weeks of gestation) | 1 | 1042 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.86] |
| 1.3 Perinatal mortality | 3 | 3356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.52] |
| 1.4 Maternal worm prevalence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Trichuris trichiura | 2 | 2488 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.48, 0.98] |
| 1.4.2 Ascaris lumbricoides | 2 | 2488 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.19, 0.29] |
| 1.4.3 Hookworm | 2 | 2488 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.05, 1.93] |
| 1.5 Low birthweight | 3 | 2960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.16] |
| 1.6 Birthweight | 3 | 2960 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.03, 0.04] |
1.4. Analysis.

Comparison 1: Antihelminthics versus control, Outcome 4: Maternal worm prevalence
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akpan 2018.
| Study characteristics | ||
| Methods |
Design: randomised controlled trial Unit of randomisation: individual |
|
| Participants |
Location/Setting: University of Calabar Teaching Hospital Antenatal Clinic, a tertiary health facility in Calabar Metropolis, the capital of Cross River State which is located in South‐South Zone of Nigeria Sample size: 560 healthy pregnant women in their second trimester Dropouts/withdrawals: 39 Mean age: 29.3 years (± 4.4 years) Inclusion criteria: 1) consenting women; 2) gestational age from 14 weeks to 28 weeks by last menstrual period or ultrasound, and 3) singleton pregnancy Exclusion criteria: 1) history of vaginal bleeding in current pregnancy; 2) medical, surgical or obstetric complication; 3) diagnosed or suspected multiple pregnancies; 4) women with haemoglobinopathy; 5) women with moderate to severe anaemia; and 6) allergy to mebendazole or sulphadoxine |
|
| Interventions |
Intervention: (n = 300) A single 500 mg oral dose of mebendazole plus a daily iron supplement, (60 mg elemental iron) and folic acid Control: (n = 260) A single dose placebo plus a daily iron supplement (60 mg elemental iron) and folic acid Intervention was administered after the first trimester of pregnancy. |
|
| Outcomes |
Outcomes: maternal and perinatal outcomes including prevalence of peripartum anaemia (PCV < 33%), mode of delivery postpartum haemorrhage, and other maternal morbidity like puerperal pyrexia; perinatal outcome included the proportion of low birthweight, birth asphyxia, congenital abnormally and perinatal mortality Timing of outcome assessment: delivery and immediate postpartum period |
|
| Notes |
Study start date: 1 January 2015 Study end date: 31 December 2015 Funding source: this research did not receive any financial support. Conflicts of interest: the authors have declared that no competing interests exist. Comment: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Computer‐generated random numbers were used for sampling." Comment: adequately done |
| Allocation concealment (selection bias) | Low risk |
Quote: "Single tablet (500 mg) of mebendazole was wrapped in a paper and labelled with a number for identification from the placebo which was also wrapped with same paper to blind the patients and the dispenser." Comment: adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "Single tablet (500 mg) of mebendazole was wrapped in a paper and labelled with a number for identification from the placebo which was also wrapped with same paper to blind the patients and the dispenser." Comment: adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Single tablet (500 mg) of mebendazole was wrapped in a paper and labelled with a number for identification from the placebo which was also wrapped with same paper to blind the patients and the dispenser." Comment: adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 39/360 (10.83%) loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial registration not reported. Outcome specified in the methods section have been reported in the results section. |
| Other bias | Low risk | Comment: no other biases identified. |
Deepti 2015.
| Study characteristics | ||
| Methods |
Design: randomised controlled trial Unit of randomisation: individual |
|
| Participants |
Location/Setting: Sree Balaji Medical College And Hospital, Chennai, India Sample size: 500 pregnant women Dropouts/Withdrawals: no dropouts Mean age: not specified Inclusion criteria: women were eligible if they were healthy on recruitment day, a resident in the study area, planning to deliver at the hospital, willing to know their HIV status, prepared to participate in the study, and in their second or third trimester (based on last menstrual period and midwife's assessment) Exclusion criteria: haemoglobin level < 8 g/dL, clinically apparent severe liver disease, history of diarrhoea with blood in stool, abnormal pregnancy, previous adverse reaction to anthelminthics, or enrolment during a previous pregnancy. |
|
| Interventions |
Intervention: (number of participants in each group was not specified) A: Albendazole (400 mg) and placebo B: Mebendazole (100 mg BD for 3 days) and placebo C: Albendazole and mebendazole Control: placebo and placebo |
|
| Outcomes |
Outcomes: anaemia, birthweight, low birthweight TIming of outcome assessment: at the time of delivery |
|
| Notes |
Study start date: August 2011 Study end date: February 2012 Funding source: not specified Conflict of interest: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The randomisation sequence was prepared with blocks of 100 by the trial statistician with use of Stata, version 7 (Stata)." Comment: adequately done |
| Allocation concealment (selection bias) | Low risk |
Quote: "Researchers in SBMCH who were not otherwise involved in the study prepared opaque, sealed envelopes numbered with the randomisation code that contained albendazole tablets (GlaxoSmithKline) or matching placebo and 12 capsules of Mebendazole 100mg or matching placebo." Comment: adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "Staff and participants were blinded to the treatment allocation" Comment: adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Staff and participants were blinded to the treatment allocation" Comment: adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there was no loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Comment: trial registration not specified. Outcomes are not reported according to the intervention group assignment. |
| Other bias | Low risk | Comment: no other biases identified |
Elliott 2005.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled trial Unit of randomisation: individual |
|
| Participants |
Location/Setting: Entebbe Hospital, Uganda Sample size: 2507 pregnant women Dropouts/Withdrawals: 159 Mean age: 23.5 years Inclusion criteria: mothers in the second trimester of pregnancy, residing in the study area, planning to deliver in hospital and willing to know their HIV status were eligible for inclusion in the study. Exclusion criteria: mothers with Hb < 8 g/dL were excluded and treated for hookworm and anaemia. Other exclusion criteria were abnormal pregnancy or history of adverse reaction to antihelminthic drugs. |
|
| Interventions |
Intervention: A: albendazole (400 mg) and placebo (n = 629) B: praziquantel (40 mg/kg) and placebo (n = 628) C: albendazole and praziquantel (n = 628) Control: placebo/placebo (n = 630) All women received a month’s supply of daily ferrous sulphate (200 mg; 60 mg elemental iron) and folic acid (0.25 mg) at each antenatal visit and intermittent presumptive sulphadoxine pyrimethamine treatment for malaria twice after the first trimester. For analysis, we have merged the data for groups A and C as intervention group and compared it with the placebo/placebo group. |
|
| Outcomes |
Outcomes: immune responses in mothers and infants, infant response to immunisation, infant eczema, maternal anaemia, birthweight, perinatal mortality, congenital anomalies, infant motor and neurocognitive function. Timings of outcome assessment: at delivery, infant survival at one week and infant assessment at 1 and 5 years of age. |
|
| Notes |
Study start date: April 2003 Study end date: November 2005 Funding source: Wellcome Trust Fellowship (064693 to first author); albendazole and matching placebo were provided by GlaxoSmithKline. Conflict of interest: All authors declared no conflict of interest. Comment: as a preliminary study, 103 were randomised to treatment with single‐dose albendazole (400 mg) or placebo. Study enrolment was then stopped due to new guidelines by the World Health Organization which recommended inclusion of treatment of women with schistosomiasis. The protocol was revised and then a total of 2507 participants were assigned to receive albendazole (400 mg) and placebo, praziquantel (40 mg/kg) and placebo, albendazole and praziquantel, or placebo and placebo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The randomisation sequence was prepared with blocks of 100 by the trial statistician". Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk |
Quote: "Researchers in Entebbe who were not otherwise involved in the study prepared opaque, sealed envelopes numbered with the randomisation code". Comment: adequately done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "double blind"; "contained albendazole tablets or matching placebo and praziquantel (300 mg; Medochemie) or matching placebo" "Staff and participants were blinded to the treatment allocation". Comment: adequately done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "All other staff and participants remain blinded to treatment allocation as follow up continues". Comment: adequately done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: Placebo/placebo: 37/630: 5.8% Albendazole/placebo: 44/629: 6.9% Placebo/praziquantel: 41/628: 6.5% Albendazole/praziquantel: 37/628: 5.8% |
| Selective reporting (reporting bias) | Low risk | Comment: trial registration reported (ISRCTN32849447). Authors reported all the outcomes mentioned in the protocol. |
| Other bias | Low risk | Comment: study enrolment was stopped after 104 women due to new guidelines by the World Health Organization which recommended inclusion of treatment of women with schistosomiasis. |
Larocque 2006.
| Study characteristics | ||
| Methods |
Design: randomised, double‐blind, placebo‐controlled trial Unit of randomisation: individual |
|
| Participants |
Location/Setting: 12 health centres in the Iquitos region of Peru Sample size: 1042 second trimester pregnant women Dropouts/Withdrawals: 36 Mean age: 25.3 years Inclusion criteria: pregnant women in second trimester (>= 18 weeks; < 26 weeks) between 18 and 44 years of age (gestational age was assessed by using a combination of fundal height and the first day of last menstrual period); not having received anthelminthic treatment for 6 months prior to recruitment; residing in rural or peri‐urban areas (defined as having no running water or flushing toilet facility at home) and giving consent. Exclusion criteria: any participants having severe anaemia (Hb < 7 g/dL) or a medical condition requiring follow‐up were excluded. |
|
| Interventions |
Intervevention: (n = 522) Intervention group received a single dose of mebendazole (500 mg) plus a daily iron supplement (60 mg elemental iron, ferrous sulphate). Control: (n = 520) Control group received a single dose placebo plus a daily iron supplement (60 mg elemental iron, ferrous sulphate). |
|
| Outcomes |
Outcomes: mean infant birthweight, low birthweight, maternal anaemia in third trimester Timing of outcome assessment: at delivery |
|
| Notes |
Study start date: April 2003 Study end date: July 2004 Funding source: funding was provided by the Canadian Institutes of Health Research (CIHR) (grant no. MCT 53575). Conflict of interest: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "computer‐generated randomly ordered blocks of 4, 6 and 8 were used to randomly allocate women to each intervention group". Comment: adequately done. |
| Allocation concealment (selection bias) | Low risk |
Quote: "2 researchers not otherwise involved in the trial prepared sealed envelopes containing the intervention assignment". Comment: adequately done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "double‐blind"; ''the local project director, field workers, obstetrics, laboratory technologists and pregnant women were all blind to the group assignment"; and "placebo tablets were similar in appearance, smell and taste to the mebendazole tablets". Comment: adequately done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: ''the local project director, field workers, obstetrics, laboratory technologists and pregnant women were all blind to the group assignment". Comment: adequately done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Comment: 36/1042 (3.4%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: trial registry specified (ISRCTN08446014). Outcomes mentioned in the methods section were presented in the results. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Torlesse 2001.
| Study characteristics | ||
| Methods |
Design: randomised controlled trial Unit of randomisation: individual |
|
| Participants |
Location/Setting: 3 antenatal clinics in peri‐urban Freetown and 6 in rural areas in Port Loko District, Sierra Leone Sample size: 184 pregnant women Dropouts/Withdrawals: 53 Mean age: 25.1 (SD 5.5) years Inclusion criteria: women with a Hb >= 8 g/dL and gestational age < 14 weeks at baseline were eligible for the study. Exclusion criteria: any women with Hb < 8 g/dL at any stage of the study was treated immediately with appropriate therapy and withdrawn from the study in accordance with World Health Organization ethical guidelines. |
|
| Interventions |
Intervention: Group A (FeA): daily iron folate supplements (Fe) 36 mg and single‐dose albendazole (A) 2 × 200 mg Group B (FeC): daily iron folate supplements (Fe) 36 mg and placebo control in place of albendazole (C) (tablets containing calcium with vitamin D) Group C: (CA): placebo control in place of iron folate (C) (calciferol tablets (1.25 mg)) and single‐dose albendazole (A) 2 × 200 mg Control: Placebo controls for iron folate supplement and Albendazole Intervention was administered after the first trimester of pregnancy. |
|
| Outcomes |
Outcomes: maternal anaemia, iron deficiency and anaemia, cure rate, egg reduction rate
Anaemia in pregnancy is defined as Hb < 11 g/dL. Timing of outcome assessment: Third trimester, within one week of delivery, and one month post partum. |
|
| Notes |
Study start date: December 1995 Study end date: June 1996 Funding source: the study was funded by a research grant from the Institute of Biomedical and Life Sciences, University of Glasgow, UK. Conflict of interest: not specified In this review, we included following comparisons. Torlesse 2001 (1): albendazole and daily iron folate versus daily iron folate and calcium vitamin D tablets as albendazole control (Group A versus Group B) Torlesse 2001 (2): albendazole and calciferol tablets as iron folate control versus calcium vitamin D tablets as albendazole control and calciferol tablets as iron folate control (Group C versus Control). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Intervention groups were allocated using random number tables to generate the random‐number sequence". Comment: adequately done. |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "intervention groups were allocated using random number tables to generate the random‐number sequence". Comment: not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "Albendazole and control calcium with vitamin D tablets used were similar in colour, shape and size"; "calciferol tablets which were used as control for iron folate supplements were similar to the iron supplements in shape and size but were different in colour". Comment: adequately done. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Albendazole and control calcium with vitamin D tablets used were similar in colour, shape and size"; "calciferol tablets which were used as control for iron folate supplements were similar to the iron supplements in shape and size but were different in colour". Comment: adequately done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 53/184 (28.8%) loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Study protocol was not available and trial registration details not specified, but outcomes mentioned in the methods section were presented in the results. |
| Other bias | Low risk | Comment: no other biases identified. |
Urassa 2011.
| Study characteristics | ||
| Methods |
Design: cluster‐randomised controlled trial Unit of randomisation: cluster (health institutions) |
|
| Participants |
Location/Setting: 16 health institutions in Rufiji district, Tanzania Sample size: 3080 pregnant women Dropouts/Withdrawals: 651 Mean age: median age 22 years Inclusion criteria: pregnant women booking for antenatal care for the first time after first trimester but before 24 weeks of pregnancy Exclusion criteria: women with haemoglobin < 60 g/l were excluded from the study. |
|
| Interventions |
Intervention: (n = 1475) Study arm received albendazole Control: (n = 1605) Control arm received placebo All women also received routine daily iron folate supplements (36 mg iron and 5 mg folate), and sulphadoxine pyrimethamine to prevent malaria. |
|
| Outcomes |
Outcomes: prevalence of anaemia at term and 4 months postpartum, haemoglobin Timing of outcome assessment: at booking, at term and at 4 months postpartum |
|
| Notes |
Study start date: March 2001 Study end date: February 2003 Funding source: Swedish agency for research and co‐operation with developing countries (Sida‐SAREC) Conflict of interest: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "out of the 16 eligible health institutions eight were allocated to the study and control arms" Comment: randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "out of the 16 eligible health institutions eight were allocated to the study and control arms" Comment: allocation not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "The placebo was made up of starch with the same size and colour as the true albendazole and it was supplied by the same company that supplied albendazole tablets." "The study was single blinded i.e. neither the health worker nor the participants were aware of the intervention allocation" Comment: adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "The study was single blinded i.e. neither the health worker nor the participants were aware of the intervention allocation. Only the principal investigator who made the randomisation and was supplying the drugs knew which heath institutions received albendazole and which one received placebo" Comment: not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 651/3080 (21.1%) loss to follow‐up. The study reported no difference between the women remaining in the study arm versus those lost to follow up and attrition was below 25%. |
| Selective reporting (reporting bias) | Unclear risk | Comment: trial registration details not specified, but outcomes specified in the methodology section were reported in the results section. |
| Other bias | Low risk | Comment: no other biases identified. The study used appropriate methods for cluster adjustment of the sample size and estimates reported are cluster‐adjusted. |
Hb: haemoglobin LBW: low birthweight PCV: packed cell volume SD: standard deviation VLBW: very low birthweight
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asbjornsdottir 2018 | 380,000 participants including men, women and children with mass population drug administration of albendazole ‐ so participants and study design not eligible. End date 2013. |
| Basra 2013 | This study focused only on the efficacy of mefloquine and did not report any of the primary or secondary outcomes of the review. |
| Bhutta 2007 | The study compared different regimens of antihelminthic treatment (single dose versus 3 days mebendazole). There was no appropriate control group. |
| Friedman 2007 | This study focused on deworming for Schistosomiasis not STH. |
| Ivan 2015 | This study focused on HIV‐infected women. |
| Mofid 2017 | This study focused on deworming for postpartum women. |
| NCT04171388 (first received 2019 Nov 20)a | Withdrawn due to Covid‐19, no participants enrolled. |
| Ndyomugyenyi 2008 | The study randomised women infected with any STH to three treatment arms and compared them to a reference group without any STH. |
| Nery 2013 | This study assessed the antihelminthic efficacy of a single dose of albendazole in communities and did not specifically target pregnant women. |
| Tehalia 2011 | Published abstract with insufficient information available. |
| Villar 1998 | Published abstract with insufficient information available. |
STH: soil‐transmitted helminths
Characteristics of ongoing studies [ordered by study ID]
NCT04391998 (first received 2020 May 18)a.
| Study name | Parasitic Infection in Anemic Pregnant Women |
| Methods | Randomized, parallel open label |
| Participants | 200 women, second or third trimester pregnancy with an Hb of below 10.5 mg /dL. |
| Interventions | Group A: iron + antiparasitic treatment as follows:
Group B: received iron only |
| Outcomes | Hb above 11.0 mg/dL |
| Starting date | Estimated end date: September 2021 |
| Contact information | Ahmed Maged, MD 01005227404 prof.ahmedmaged@gmail.com |
| Notes |
Differences between protocol and review
We added an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
Previously, the review only had one primary outcome (maternal anaemia). In this update we have three primary outcomes (maternal anaemia, preterm birth and perinatal mortality). Among secondary outcomes, we have added three new outcomes, including maternal worm burden, maternal anthropometric measures and birthweight.
Previosuly, we planned to carry out the following subgroup analyses for our primary outcome (maternal anaemia).
Differences in type, dosage, duration and frequency of antihelminthics
Differences in baseline infant mortality
Co‐interventions other than antihelminthics
Prevalence of malaria
However, for this update we had planned the following subgroup analyses for all the primary outcomes.
Differences in type of antihelminthics (albendazole, mebendazole, praziquantel, others)
Baseline worm burden (across four levels of none, light, moderate and heavy, using the WHO cut‐offs for each helminth)
Co‐interventions other than antihelminthics (concomitant iron/folic acid supplementation versus no supplementation)
However, we could not conduct the planned subgroup analysis since there were too few studies in each subgroup to make any meaningful conclusions.
Contributions of authors
The protocol for the review was written by Batool Azra Haider (BAH) under the guidance of Professor Zulfiqar A Bhutta (ZAB). For this update, Rehana A Salam (RAS) and Jai K Das (JKD) participated in all steps of manuscript preparation, including screening, data extraction, analysis and manuscript writing. ZAB provided support and guidance for the review.
Sources of support
Internal sources
The Aga Khan University, Pakistan
External sources
No sources of support provided
Declarations of interest
Rehana A Salam: no conflict of interest
Jai K Das: no conflict of interest
Zulfiqar A Bhutta: no conflict of interest
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Akpan 2018 {published data only}
- Akpan UB, Asibong U, Okpara HC, Monjok E, Etuk S. Antenatal deworming and materno-perinatal outcomes in Calabar, Nigeria. Open Access Macedonian Journal of Medical Sciences 2018;6(5):901-7. [CENTRAL: CN-01666522] [EMBASE: 624817663] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deepti 2015 {published data only}
- Deepti SS, Nandini L. Effects of deworming during pregnancy on maternal and perinatal outcomes: A randomized controlled trial. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2015;6(1):1521-6. [CENTRAL: CN-01047596] [EMBASE: 601626821] [Google Scholar]
Elliott 2005 {published data only}
- Elliott AM, Kizza M, Quigley MA, Ndibazza J, Nampijja M, Muhangi L, et al. The impact of helminths on the response to immunization and on the incidence of infection and disease in childhood in Uganda: design of a randomized, double-blind, placebo-controlled, factorial trial of deworming interventions delivered in pregnancy and early childhood. Clinical Trials 2007;4:42-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AM, Mpairwe H, Quigley MA, Nampijja M, Muhangi L, Oweka-Onyee J, et al. Helminth infection during pregnancy and development of infantile eczema. Journal of the American Medical Association 2005;294(16):2032-4. [DOI] [PubMed] [Google Scholar]
- Elliott AM, Namujju PB, Mawa PA, Quigley MA, Nampijja M, Nkurunziza PM, et al. A randomised controlled trial of the effects of albendazole in pregnancy on maternal responses to mycobacterial antigens and infant responses to bacille calmette-guerin (BCG) immunisation. BMC Infectious Diseases 2005;5:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard JD, Muhangi L, Sewankambo M, Ndibazza J, Elliott AM, Webb EL. Assessing the external validity of a randomized controlled trial of anthelminthics in mothers and their children in Entebbe, Uganda. Trials 2014;15(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpairwe H, Webb EL, Muhangi L, Ndibazza J, Akishule D, Nampijja M, et al. Antihelminthic treatment during pregnancy is associated with increased risk of infantile eczema: randomised-controlled trial results. Pediatric Allergy and Immunology 2011;22(3):305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namara B, Nash S, Lule SA, Akurut H, Mpairwe H, Akello F, et al. Effects of treating helminths during pregnancy and early childhood on risk of allergy-related outcomes: follow-up of a randomized controlled trial. Pediatric Allergy and Immunology 2017;28(8):784-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nampijja M, Apule B, Lule S, Akurut H, Muhangi L, Webb EL, et al. Effects of maternal worm infections and anthelminthic treatment during pregnancy on infant motor and neurocognitive functioning. Journal of the International Neuropsychological Society 2012;18(6):1019-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S, Mentzer AJ, Lule SA, Kizito D, Smits G, Klis FR, et al. The impact of prenatal exposure to parasitic infections and to anthelminthic treatment on antibody responses to routine immunisations given in infancy: secondary analysis of a randomised controlled trial. PLoS Neglected Tropical Diseases 2017;11(2):e0005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndibazza J, Mpairwe H, Webb EL, Mawa PA, Nampijja M, Muhangi L, et al. Impact of anthelminthic treatment in pregnancy and childhood on immunisations, infections and eczema in childhood: a randomised controlled trial. PLOS One 2012;7(12):e50325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndibazza J, Muhangi L, Akishule D, Kiggundu M, Ameke C, Oweka J, et al. Effects of deworming during pregnancy on maternal and perinatal outcomes in Entebbe, Uganda: a randomized controlled trial. Clinical Infectious Diseases 2010;50(4):531-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweyongyere R, Mawa PA, Emojong NO, Mpairwe H, Jones FM, Duong T, et al. Effect of praziquantel treatment of Schistosoma mansoni during pregnancy on intensity of infection and antibody responses to schistosome antigens: results of a randomised, placebo-controlled trial. BMC Infectious Diseases 2009;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweyongyere R, Mawa PA, Kihembo M, Jones FM, Webb EL, Cose S, et al. Effect of praziquantel treatment of Schistosoma mansoni during pregnancy on immune responses to schistosome antigens among the offspring: results of a randomised, placebo-controlled trial. BMC Infectious Diseases 2011;11:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweyongyere R, Mawa PA, Ngom-Wegi S, Ndibazza J, Duong T, Vennervald BJ, et al. Effect of praziquantel treatment during pregnancy on cytokine responses to schistosome antigens: results of a randomized, placebo-controlled trial. Journal of Infectious Diseases 2008;198(12):1870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweyongyere R, Naniima P, Mawa PA, Jones FM, Webb EL, Cose S, et al. Effect of maternal schistosoma mansoni infection and praziquantel treatment during pregnancy on schistosoma mansoni infection and immune responsiveness among offspring at age five years. PLOS Neglected Tropical Diseases 2013;7(10):e2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb EL, Kyosiimire-Lugemwa J, Kizito D, Nkurunziza P, Lule S, Muhangi L, et al. The effect of anthelmintic treatment during pregnancy on HIV plasma viral load: results from a randomized, double-blind, placebo-controlled trial in Uganda. Journal of Acquired Immune Deficiency Syndromes 2012;60(3):307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb EL, Mawa PA, Ndibazza J, Kizito D, Namatovu A, Kyosiimire-Lugemwa J, et al. Effect of single-dose anthelmintic treatment during pregnancy on an infant's response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double-blind, placebo-controlled trial. Lancet 2011;377(9759):52-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larocque 2006 {published data only}
- Gyorkos TW, Gilbert NL, Larocque R, Casapia M. Trichuris and hookworm infections associated with anaemia during pregnancy [Infections au second trimestre par Trichuris et l'ankylostome, associees a l'anemie au troisieme trimestre dans une population de femmes enceintes peruviennes]. Tropical Medicine and International Health 2011;16(4):531-7. [DOI] [PubMed] [Google Scholar]
- Gyorkos TW, Larocque R, Casapia M, Gotuzzo E. Lack of risk of adverse birth outcomes after deworming in pregnant women. Pediatric Infectious Disease Journal 2006;25(9):791-4. [DOI] [PubMed] [Google Scholar]
- Larocque R, Casapia M, Gotuzzo E, MacLean JD, Soto JC, Rahme E, et al. A double-blind randomized controlled trial of antenatal mebendazole to reduce low birthweight in a hookworm-endemic area of Peru. Tropical Medicine and International Health 2006;11(10):1485–95. [DOI] [PubMed] [Google Scholar]
Torlesse 2001 {published data only}
- Torlesse H, Hodges M. Albendazole therapy and reduced decline in haemoglobin concentrations during pregnancy (Sierra Leone). Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95:195-201. [DOI] [PubMed] [Google Scholar]
- Torlesse H, Hodges M. Antihelminthic treatment and haemoglobin concentrations during pregnancy. Lancet 2000;356:1083. [DOI] [PubMed] [Google Scholar]
Urassa 2011 {published data only}
- Urassa DP, Nystrom L, Carlsted A. Effectiveness of routine antihelminthic treatment on anaemia in pregnancy in Rufiji District, Tanzania: a cluster randomised controlled trial. East African Journal of Public Health 2011;8(3):176-84. [PubMed] [Google Scholar]
References to studies excluded from this review
Asbjornsdottir 2018 {published data only}
- Asbjornsdottir KH, Ajjampur SSR, Anderson RM, Bailey R, Gardiner I, Halliday KE, et al. Assessing the feasibility of interrupting the transmission of soil-transmitted helminths through mass drug administration: the DeWorm3 cluster randomized trial protocol [Assessing the feasibility of interrupting the transmission of soil-transmitted helminths through mass drug administration: the DeWorm3 cluster randomized trial protocol]. PLoS Neglected Tropical Diseases January 2018;12(1):e1-16. [CENTRAL: CN-01623002] [EMBASE: 620498567] [Google Scholar]
- NCT03014167. Field Studies on the Feasibility of Interrupting the Transmission of Soil-transmitted Helminths (STH) [Field Studies on the Feasibility of Interrupting the Transmission of Soil-transmitted Helminths (STH)]. Https://clinicaltrials.gov/show/nct03014167 (first received 2016 Dec 30). [CENTRAL: CN-01592491]
- The DeWorm3 Trials Team. Baseline patterns of infection in regions of Benin, Malawi and India seeking to interrupt transmission of soil transmitted helminths (STH) in the DeWorm3 trial. PLoS Neglected Tropical Diseases 2020;14(11):e0008771 https://doi.org/10.1371/journal.pntd.0008771. [CENTRAL: CN-02202287] [EMBASE: 633328479] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Basra 2013 {published data only}
- Basra A, Mombo-Ngoma G, Melser MC, Diop DA, Wurbel H, Mackanga JR, et al. Efficacy of mefloquine intermittent preventive treatment in pregnancy against Schistosoma haematobium infection in Gabon: a nested randomized controlled assessor-blinded clinical trial. Clinical Infectious Diseases 2013;56(6):e68-75. [DOI] [PubMed] [Google Scholar]
Bhutta 2007 {published data only}
- Bhutta Z, Klemm R, Shahid F, Rizvi A, Rah JH, Christian P. Treatment response to iron and folic acid alone is the same as with multivitamins and/or anthelminthics in severely anemic 6- to 24-month-old children. Journal of Nutrition Aug 2009;139(8):1568-74. [DOI] [PubMed] [Google Scholar]
- Bhutta ZA. Severe anaemia treatment trial, Pakistan. clinicaltrials.gov/show/NCT00116493 (first accessed 2007).
- Christian P, Shahid F, Rizvi A, Klemm RD, Bhutta ZA. Treatment response to standard of care for severe anemia in pregnant women and effect of multivitamins and enhanced anthelminthics. American Journal of Clinical Nutrition 2009;89(3):853-61. [DOI] [PubMed] [Google Scholar]
Friedman 2007 {published data only}
- Abioye AI, McDonald EA, Park S, Joshi A, Kurtis JD, Wu H, et al. Maternal, placental and cord blood cytokines and the risk of adverse birth outcomes among pregnant women infected with Schistosoma japonicum in the Philippines. PLoS Neglected Tropical Diseases 2019;13(6):e0007371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00486863. S. japonicum and pregnancy outcomes: a randomized, double blind, placebo controlled trial (RCT). clinicaltrials.gov/ct2/show/NCT00486863 (first accessed 15 June 2007).
- Olveda RM, Acosta LP, Tallo V, Baltazar PI, Lesiguez JL, Estanislao GG, et al. A randomized double blind placebo controlled trial assessing the safety and efficacy of praziquantel for the treatment of schistosomiasis during human pregnancy. In: Pediatric Academic Societies Annual Meeting; 2016 April 30-May 3; Baltimore (MD). 2016:2715.1.
- Olveda RM, Acosta LP, Tallo V, Baltazar PI, Lesiguez JL, Estanislao GG, et al. Efficacy and safety of praziquantel for the treatment of human schistosomiasis during pregnancy: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Infectious Diseases 2016 Feb;16(2):199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ivan 2015 {published data only}
- Ivan E, Crowther NJ, Mutimura E, Rucogoza A, Janssen S, Njunwa KK, et al. Effect of deworming on disease progression markers in HIV-1-infected pregnant women on antiretroviral therapy: a longitudinal observational study from Rwanda. Clinical Infectious Diseases 2015;60(1):135-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mofid 2017 {published data only}
- Mofid LS, Casapía M, Aguilar E, Silva H, Montresor A, Rahme E, et al. A double-blind randomized controlled trial of maternal postpartum deworming to improve infant weight gain in the Peruvian Amazon. PLoS Neglected Tropical Diseases 2017;11(1):e0005098. [CENTRAL: CN-01401425] [EMBASE: 614416205] [PMID: ] [DOI] [PMC free article] [PubMed]
- Mofid LS, Casapía M, Montresor A, Rahme E, Fraser WD, Marquis GS, et al. Maternal deworming research study (MADRES) protocol: a double-blind, placebo-controlled randomised trial to determine the effectiveness of deworming in the immediate postpartum period. BMJ Open 2015;5(6):e008560. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04171388 (first received 2019 Nov 20)a {published data only}
- NCT04171388. Enhancing Nutrition and Antenatal Infection Treatment for Maternal and Child Health in Ethiopia [Enhancing Nutrition and Antenatal Infection Treatment for Maternal and Child Health in Amhara Region, Ethiopia]. Https://clinicaltrials.gov/show/NCT04171388 (first received 2019 Nov 20). [CENTRAL: CN-02010018]
Ndyomugyenyi 2008 {published data only}
- Ndyomugyenyi R, Kabatereine N, Olsen A, Magnussen P. Efficacy of ivermectin and albendazole alone and in combination for treatment of soil-transmitted helminths in pregnancy and adverse events: a randomized open label controlled intervention trial in Masindi district, western Uganda. American Journal of Tropical Medicine and Hygiene 2008;79(6):856-63. [PubMed] [Google Scholar]
Nery 2013 {published data only}
- Nery SV, Gomes S, Traub R, McCarthy J, Llewellyn S, Andrews R, et al. Assessment of the efficacy of albendazole for the treatment of soil transmitted helminths in East Timor. American Journal of Tropical Medicine and Hygiene 2013;89(5 Suppl 1):229. [Google Scholar]
Tehalia 2011 {published data only}
- Tehalia MK. Impact of deworming on anaemia in pregnancy. In: 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5-9; Hyderabad, Andhra Pradesh, India. 2011:63.
Villar 1998 {published data only}
- Villar MA, Dala FE, Cardona VA. Nematode infections in pregnancy: the pyrantel experience. American Journal of Obstetrics and Gynaecology 1998;178(1 Pt 2):S214. [Google Scholar]
References to ongoing studies
NCT04391998 (first received 2020 May 18)a {published data only}
- NCT04391998. Parasitic Infection in Anemic Pregnant Women [Parasitic Infection in Anemic Pregnant Women. Prevalence and Effects in Rural Area in Egypt]. Https://clinicaltrials.gov/show/NCT04391998 (first received 2020 May 18). [CENTRAL: CN-02124718]
Additional references
Abel 2000
- Abel R, Rajaratnam J, Kalaimani A, Kirubakaran S. Can iron status be improved in each of the three trimesters? A community-based study. European Journal of Clinical Nutrition 2000;54:490-3. [DOI] [PubMed] [Google Scholar]
ACC/SCN 2000
- United Nations Administrative Committee on Coordination, Subcommittee on Nutrition. Fourth Report on the World Nutrition Situation. Geneva: World Health Organization, 2000. [Google Scholar]
Atukorala 1994
- Atukorala TM, Silva LD, Dechering WH, Dassenaeike TS, Perera RS. Evaluation of effectiveness of iron-folate supplementation and anthelminthic therapy against anaemia in pregnancy - a study in the plantation sector of Sri Lanka. American Journal of Clinical Nutrition 1994;60:286-92. [DOI] [PubMed] [Google Scholar]
Barry 2013
- Barry MA, Simon GG, Mistry N, Hotez PJ. Global trends in neglected tropical disease control and elimination: impact on child health. Archives of Disease in Childhood 2013;98(8):635-41. [DOI] [PubMed] [Google Scholar]
Bundy 1989
- Bundy DA, Cooper ES. Trichuris and trichuriasis in humans. Advances in Parasitology 1989;28:108-73. [DOI] [PubMed] [Google Scholar]
Bundy 1995
- Bundy DA, Chan MS, Savioli L. Hookworm infection in pregnancy. Transactions of the Royal Society of Tropical Hygiene and Medicine 1995;89:521-2. [DOI] [PubMed] [Google Scholar]
Christian 2004
- Christian P, Khatry SK, West KP Jr. Antenatal anthelmintic treatment, birthweight, and infant survival in rural Nepal. Lancet 2004;364:981-3. [DOI] [PubMed] [Google Scholar]
de Silva 1999
- Silva NR, Sirisena JL, Gunasekera DP, Ismail MM, Silva HJ. Effect of mebendazole therapy during pregnancy on the birth outcome. Lancet 1999;353:1145-9. [DOI] [PubMed] [Google Scholar]
Drugs.com 2021
- Drugscom. Anthelmintics. www.drugs.com/drug-class/anthelmintics.html (accessed 25th January 2021).
Ghogomu 2018
- Ghogomu ET, Suresh S, Rayco-Solon P, Hossain A, McGowan J, Peña-Rosas JP, et al. Deworming in non-pregnant adolescent girls and adult women: a systematic review and meta-analysis. Systematic Reviews 2018;7(1):239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hay 2017
- Hay SI, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1260-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hotez 1983
- Hotez PJ, Cerami A. Secretion of a proteolytic anticoagulant by ancylostoma duodenale hookworms. Journal of Experimental Medicine 1983;157:1594-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hotez 2014
- Hotez PJ, Alvarado M, Basáñez MG, Bolliger I, Bourne R, Boussinesq M, et al. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Neglected Tropical Diseases 2014;8(7):e2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keiser 2008
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. Journal of the American Medical Association 2008;299(16):1937-48. [DOI] [PubMed] [Google Scholar]
Keiser 2019
- Keiser J, Utzinger J. Community-wide soil-transmitted helminth treatment is equity-effective. Lancet 2019;393(10185):2011-2. [DOI] [PubMed] [Google Scholar]
Montresor 1998
- Montresor A, Crompton DW, Hall A, Bundy DA, Savioli L, World Health Organization. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level: a guide for managers of control programmes. World Health Organization 1998.
Pawlowski 1991
- Pawlowski ZS, Schad GA, Stott GJ. Hookworm Infection And Anaemia: Approaches To Prevention And Control. Geneva: World Health Organization, 1991. [Google Scholar]
Pullan 2014
- Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites & Vectors 2014;7(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2016
- Rahman MM, Abe SK, Rahman MS, Kanda M, Narita S, Bilano V, Ota E, Gilmour S, Shibuya K. Maternal anemia and risk of adverse birth and health outcomes in low-and middle-income countries: systematic review and meta-analysis. The American Journal of Clinical Nutrition 2016 Feb 1;102(2):495-504. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Salam 2019
- Salam RA, Cousens S, Welch V, Gaffey M, Middleton P, Makrides M, et al. Mass deworming for soil‐transmitted helminths and schistosomiasis among pregnant women: a systematic review and individual participant data meta-analysis. Campbell Systematic Reviews 2019;15(3):e1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seshadri 1997
- Seshadri S. Nutritional anaemia in South Asia. In: Gillespie S, editors(s). Malnutrition in South Asia. Kathmandu: United Nations Children's Fund, 1997:75-124. [Google Scholar]
Torlesse 2000
- Torlesse H, Hodges M. Anthelminthic treatment and haemoglobin concentrations during pregnancy. Lancet 2000;356:1083. [DOI] [PubMed] [Google Scholar]
Turner 2015
- Turner HC, Truscott JE, Hollingsworth TD, Bettis AA, Brooker SJ, Anderson RM. Cost and cost-effectiveness of soil-transmitted helminth treatment programmes: systematic review and research needs. Parasites & Vectors 2015;8(1):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welch 2017
- Welch VA, Ghogomu E, Hossain A, Awasthi S, Bhutta ZA, Cumberbatch C, et al. Mass deworming to improve developmental health and wellbeing of children in low-income and middle-income countries: a systematic review and network meta-analysis. Lancet Global Health 2017;5(1):e40-50. [DOI] [PubMed] [Google Scholar]
Welch 2019
- Welch VA, Hossain A, Ghogomu E, Riddle A, Cousens S, Gaffey M, et al. Deworming children for soil-transmitted helminths in low and middle-income countries: systematic review and individual participant data network meta-analysis. Journal of Development Effectiveness 2019;11(3):288-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1994
- World Health Organization. WHO Report of the Informal Consultation on Hookworm Infection and Anaemia in Girls and Women. (WHO/CTD/SIP/96.1). Geneva: World Health Organization, 1994. [Google Scholar]
WHO 1997
- World Health Organization. The World Health Report 1997: Conquering Suffering, Enriching Humanity. Geneva: World Health Organization, 1997. [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. Iron Deficiency Anaemia Assessment, Prevention and Control: a Guide for Program Managers. Geneva: World Health Organization, 2001. [Google Scholar]
WHO 2003
- World Health Organization. Controlling Disease due to Helminth Infections. Geneva: World Health Organization, 2003. [Google Scholar]
WHO 2006
- World Health Organization. Preventive Chemotherapy in Human Helminthiasis. Coordinated use of Anthelminthic Drugs in Control Interventions — Guidelines for Health Professionals and Programme Managers. Geneva: World Health Organization, 2006. [Google Scholar]
WHO 2017
- World Health Organization. Guideline: Preventive Chemotherapy To Control Soil-transmitted Helminth Infections In At-risk Population Groups. Geneva: World Health Organization, 2017. [PubMed] [Google Scholar]
WHO 2018
- World Health Organization. Reaching girls and women of reproductive age with deworming. Report of the Advisory Group on deworming in girls and women of reproductive age. Rockefeller Foundation Bellagio Center, Bellagio, Italy 28–30 June 2017. www.who.int/publications/i/item/WHO-CDS-NTD-PCT-2018.01 2018.
References to other published versions of this review
Haider 2009
- Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil transmitted helminths during pregnancy. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD005547. [DOI: 10.1002/14651858.CD005547.pub2] [DOI] [PubMed] [Google Scholar]
Salam 2015
- Salam RA, Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil-transmitted helminths during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD005547. [DOI: 10.1002/14651858.CD005547.pub3] [DOI] [PubMed] [Google Scholar]


