Abstract
Background
Neonatal sepsis is a major cause of morbidity and mortality. It is the third leading cause of neonatal mortality globally constituting 13% of overall neonatal mortality. Despite the high burden of neonatal sepsis, high‐quality evidence in diagnosis and treatment is scarce. Possibly due to the diagnostic challenges of sepsis and the relative immunosuppression of the newborn, many neonates receive antibiotics for suspected sepsis. Antibiotics have become the most used therapeutics in neonatal intensive care units. The last Cochrane Review was updated in 2004. Given the clinical importance, an updated systematic review assessing the effects of different antibiotic regimens for early‐onset neonatal sepsis is needed.
Objectives
To assess the beneficial and harmful effects of different antibiotic regimens for early‐onset neonatal sepsis.
Search methods
We searched the following electronic databases: CENTRAL (2020, Issue 8); Ovid MEDLINE; Embase Ovid; CINAHL; LILACS; Science Citation Index EXPANDED and Conference Proceedings Citation Index – Science on 12 March 2021. We searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi‐RCTs.
Selection criteria
We included RCTs comparing different antibiotic regimens for early‐onset neonatal sepsis. We included participants from birth to 72 hours of life at randomisation.
Data collection and analysis
Three review authors independently assessed studies for inclusion, extracted data, and assessed risk of bias. We used the GRADE approach to assess the certainty of evidence. Our primary outcome was all‐cause mortality, and our secondary outcomes were: serious adverse events, respiratory support, circulatory support, nephrotoxicity, neurological developmental impairment, necrotising enterocolitis, and ototoxicity. Our primary time point of interest was at maximum follow‐up.
Main results
We included five RCTs (865 participants). All trials were at high risk of bias. The certainty of the evidence according to GRADE was very low. The included trials assessed five different comparisons of antibiotics.
We did not conduct any meta‐analyses due to lack of relevant data.
Of the five included trials one trial compared ampicillin plus gentamicin with benzylpenicillin plus gentamicin; one trial compared piperacillin plus tazobactam with amikacin; one trial compared ticarcillin plus clavulanic acid with piperacillin plus gentamicin; one trial compared piperacillin with ampicillin plus amikacin; and one trial compared ceftazidime with benzylpenicillin plus gentamicin.
None of the five comparisons found any evidence of a difference when assessing all‐cause mortality, serious adverse events, circulatory support, nephrotoxicity, neurological developmental impairment, or necrotising enterocolitis; however, none of the trials were near an information size that could contribute significantly to the evidence of the comparative benefits and risks of any particular antibiotic regimen.
None of the trials assessed respiratory support or ototoxicity.
The benefits and harms of different antibiotic regimens remain unclear due to the lack of well‐powered trials and the high risk of systematic errors.
Authors' conclusions
Current evidence is insufficient to support any antibiotic regimen being superior to another. Large RCTs assessing different antibiotic regimens in early‐onset neonatal sepsis with low risk of bias are warranted.
Plain language summary
Antibiotic regimens for early‐onset neonatal sepsis
Review question
We reviewed available evidence on different antibiotic regimens for newborns (from birth to 72 hours of life), with early‐onset sepsis (as defined by trialists).
Background
Sepsis in newborns is a severe and potential lethal condition, caused by the body's response to an infection. Neonatal sepsis is the third leading cause of neonatal death globally. Despite the high burden of sepsis in newborns, high‐quality evidence in diagnosis and treatment is scarce. This Cochrane Review was originally published in 2004. To identify the most appropriate antibiotic policies for neonatal sepsis, there is a need to base these policies on an updated well‐conducted review. Given the clinical importance, such a review assessing the effects of different antibiotic regimens for early‐onset neonatal sepsis is needed.
Study characteristics
The evidence is current to August 2020. We included five trials randomising 865 participants. The included trials compared five different antibiotic regimens.
Key results
We included five trials: one trial compared ampicillin plus gentamicin with benzylpenicillin plus gentamicin; one trial compared piperacillin plus tazobactam with amikacin; one trial compared ticarcillin plus clavulanic acid with piperacillin plus gentamicin; one trial compared piperacillin with ampicillin plus amikacin; and one trial compared ceftazidime with benzylpenicillin plus gentamicin.
None of the five comparisons showed any difference when assessing death from all causes, serious adverse events (i.e. major complications), respiratory support, circulatory support, nephrotoxicity (toxicity in the kidneys), neurological developmental impairment (disabilities in the functioning of the brain that affect a child's behaviour, memory, or ability to learn), necrotising enterocolitis (tissues in the gut become inflamed and start to die), or ototoxicity (toxic to the ear). Current evidence cannot confirm or reject one antibiotic regimen being superior to another.
Quality of the evidence
The evidence behind our conclusions is very‐low quality. The five trials had high risk of bias (i.e. the trials were conducted in a way that may have skewed results to the positive side). In addition, the five trials included few participants, making the results of this review imprecise.
Summary of findings
Background
Description of the condition
Definition
Sepsis is defined as life‐threatening organ dysfunction caused by a dysregulated host response to infection (Singer 2016). There are internationally agreed diagnostic criteria for sepsis in both adults and children (Singer 2016; Wynn 2014), but currently there is no international consensus on specific criteria for neonatal sepsis (Wynn 2014; Wynn 2016). The most used neonatal sepsis criteria used in clinical trials are based on a combination of clinical and laboratory parameters (see Table 6) (Morris 2016; Wynn 2014).
1. Commonly used clinical and laboratory criteria of sepsis.
| Clinical criteria | Laboratory criteria |
|
|
WBC: white blood cell.
Sepsis that occurs before 28 days after birth is termed neonatal sepsis (Bakhuizen 2014; Camacho‐Gonzalez 2013). Depending on the time of onset, neonatal sepsis is referred to as either early‐ or late‐onset sepsis. The most commonly accepted distinction between these two subgroups is before and after 72 hours of age, although other definitions also exist such as 48 hours and seven days of age (Bakhuizen 2014; Bizzarro 2008; Camacho‐Gonzalez 2013; Manan 2016; NICE 2012; Shah 2014; Shane 2013; Shane 2014; Tripathi 2012; Zaidi 2009; Zea‐Vera 2015). This distinction is based on the different aetiologies and pathophysiology of pathogens typically seen before and after 72 hours (Camacho‐Gonzalez 2013; Shah 2014; Shane 2013).
It is generally accepted that the infection in early‐onset sepsis usually is vertically acquired from the mother (either because the mother is infected, or simply colonised with commonly occurring vaginal or gut bacteria), and that the infection in late‐onset sepsis is usually horizontally acquired (e.g. from the community or a nosocomial (hospital‐acquired) infection) (Park 2013; Shane 2013; Stoll 2002; Weston 2011; Zea‐Vera 2015). As some of these clinical manifestations can be non‐specific, it can be difficult to clinically distinguish between sepsis and severe infections, such as meningitis, osteomyelitis, and necrotising enterocolitis (Camacho‐Gonzalez 2013; Zea‐Vera 2015).
Epidemiology
The incidence of neonatal sepsis is estimated to be between 1 per 1000 and 12 per 1000 live births in high‐income countries (Bakhuizen 2014; Stoll 2011). The incidence in low‐ and middle‐income countries (LMICs) is higher. Reported incidences are estimated to be 7.1 per 1000 to 38 per 1000 live births in Asia, 6.5 per 1000 to 23 per 1000 live births in Africa, and 3.5 per 1000 to 8.9 per 1000 live births in South America and the Caribbean (Karunasekera 1999; Lim 1995; Moreno 1994; Robillard 1993; Tallur 2000; WHO 1999).
Early‐onset sepsis is reported to be less frequent than late‐onset sepsis. Studies from the USA and Australia suggest that early‐onset sepsis ranges from 1.5 per 1000 to 3.5 per 1000 live births, while late‐onset sepsis constitutes up to 6 per 1000 live births (Isaacs 1999; Schuchat 2000; Vergnano 2005). However, as there is no consensus on criteria for neonatal sepsis and no agreement on the cut‐off between early‐ and late‐onset sepsis (48 hours, 72 hours, or 7 days) (see 'Definition' section above), it is difficult to estimate the exact incidence of neonatal sepsis (Bakhuizen 2014). The incidence of early‐onset sepsis is higher for neonates with very low birthweight (less than 1500 g) than for term neonates, with an incidence of 4 per 1000 for low birthweight versus 0.4 per 1000 for term neonates (Bedford Russell 2015). The incidence of early‐onset sepsis is around 1 per 1000 live births in high‐income countries (Stoll 2011; Vergnano 2011), but increases with decreasing gestational age and birthweight up to approximately 11 per 1000 live births in neonates weighing 401 g to 1500 g (Stoll 2011).
Neonatal sepsis is a major cause of morbidity and mortality. Neonatal sepsis is the third leading cause of neonatal mortality globally, constituting 13% of overall neonatal mortality, only surpassed by intrapartum‐related complications (23%) and preterm birth complications (35%) (Lawn 2005; Liu 2012). In high‐income countries, the mortality rate in neonatal sepsis ranges from 5% to 20% and results in major disability or death in 39% of all cases despite initiation of conventional treatment. Mortality rates higher than 70% can be observed in some LMICs (Bakhuizen 2014; Kabwe 2016; Weston 2011; Wynn 2014).
Sepsis in the neonatal period can result in several complications, such as multiple organ failure, cerebral haemorrhage, periventricular leukomalacia, meningitis, and respiratory distress syndrome (Sharma 2007; Stoll 2010). In survivors, sepsis is associated with serious long‐term morbidity, such as cerebral palsy, cognitive and psychomotor delay, auditory and visual impairment, and bronchopulmonary dysplasia (Bakhuizen 2014; Benjamin 2006; Klinger 2010; Schlapbach 2011; Stoll 2004). Most of these associations are based on observational cohort studies and, therefore, do not distinguish between causality and association. It remains uncertain whether it is possible to prevent these subsequent sequela by treating neonatal sepsis with an appropriate empirical antibiotic regimen (Bakhuizen 2014).
Aetiology
In high‐income countries, the most common aetiological agents responsible for early‐onset sepsis are group B Streptococcus (38% to 58% of cases) and Escherichia coli (18% to 29% of cases), and together they constitute 62% to 72% of all cases of early‐onset sepsis (Bizzarro 2005; Bizzarro 2008; Stoll 2011; Vergnano 2011; Weston 2011). One study from the USA showed that most (73%) infants with group B Streptococcus isolates were term, and most (81%) with E coli were preterm infants (Stoll 2011). Other agents prevalent in early‐onset sepsis are Listeria monocytogenes, other streptococci species than group B Streptococcus (Streptococcus pyogenes, viridans group streptococci, Streptococcus pneumoniae), enterococci, staphylococci, Bacillus species, and Haemophilus influenzae (Stoll 2011; Vergnano 2011). Studies from the USA and Australia have shown a reduced incidence of early‐onset neonatal sepsis after the implementation of antenatal screening for group B Streptococcus and intrapartum antibiotic prophylaxis offered to colonised women who are group B Streptococcus positive (Isaacs 1999; Shane 2014; Stoll 2011). This preventive effect of intrapartum antibiotic prophylaxis is not seen in late‐onset sepsis (Ohlsson 2014).
The distribution of pathogens is quite different in LMICs with pathogens such as Klebsiella species and Staphylococcus aureus being the most prevalent causes of neonatal sepsis while group B Streptococcus infection is rare (Breurec 2016; Vergnano 2005; Zaidi 2005). Estimations suggest that Gram‐negative rod‐shaped bacteria (most commonly Klebsiella species) constitute approximately 60% of positive blood cultures in LMICs (Zaidi 2005).
Several risk factors are associated with an increased risk of developing early‐onset sepsis (Manan 2016). Commonly recognised risk factors are maternal intrapartum fever, urinary tract infection, prolonged labour, preterm rupture of the membrane (PROM), prolonged PROM of greater than 18 hours, meconium aspiration, multiple gestation, and chorioamnionitis (Naher 2011; Shah 2014). Prematurity (defined as neonates born before the 37th gestational week) and low birthweight are major risk factors, as one multicentre observational study showed that neonatal sepsis was most common in preterm (82%) and low birthweight neonates (81%) (Stoll 2011). This might be influenced by the fact that the risk factor intrauterine infection (e.g. chorioamnionitis and amnionitis) is a major contributor to spontaneous preterm delivery (Goldenberg 2000).
Furthermore, neonates are immunocompromised as several components of the immune system are not fully developed at birth (Camacho‐Gonzalez 2013; Kumar 2016). Preterm neonates are especially immunocompromised due to even more immature innate and adaptive immune systems (Kan 2016; Rogosch 2012; Walker 2011; Ygberg 2012; Zemlin 2007).
Description of the intervention
Treatment of neonatal sepsis is aimed at:
treating the underlying infectious cause of sepsis (i.e. the bacterial infection), which in turn depends on the presumed aetiology (Deutschman 2014; Singer 2016); and
correcting the associated organic dysfunction via, for example, respiratory support, maintenance of central and peripheral perfusion (often requiring intravenous fluids and inotropes), and correction of metabolic, temperature, and glucose derangements (Seale 2015; WHO 2013).
Antibiotics are antimicrobial drugs that are used to either kill or inhibit the growth of the bacteria and, accordingly, they are paramount in treatment of sepsis (Waksman 1947). Early initiation of antibiotic therapy in neonates with suspected sepsis reduces both mortality and morbidity (Bakhuizen 2014). According to guidelines, the treatment should be given as soon as possible and always within one hour of the decision to treat (NICE 2012; WHO 2013).
The choice of the empirical antibiotic used is based on several factors, such as age at onset, likely pathogens, and antibiotic susceptibility patterns with a special focus on group B Streptococcus, E coli, other Gram‐negative organisms, and Listeria monocytogenes (Manan 2016; NICE 2014). Most neonates who receive antibiotics have negative blood cultures (Klingenberg 2018); therefore, trials that assess empirical antibiotics need to consider, in design and analysis, the issue that neonates with true sepsis will be pooled with non‐infected neonates due to the lack of specific early diagnostic criteria. With the current diagnostic tools, the inclusion of non‐infected neonates will be inevitable, when assessing empirical antibiotics. This may potentially cause type II errors as the event rate of clinically import outcomes would be lower in a study population including healthy neonates.
Most guidelines recommend a beta‐lactam antibiotic (most commonly benzylpenicillin or ampicillin) together with an aminoglycoside (most commonly gentamicin) for empirical treatment of all cases of early‐onset neonatal sepsis (Cortese 2016; Manan 2016; NICE 2014; Vergnano 2005; WHO 2013). Beta‐lactam antibiotics are divided into four classes: penicillins, cephalosporins, monobactams, and carbapenems (Golan 2011; Katzung 2009).
Ampicillin is also frequently combined with a third‐generation cephalosporin drug (most commonly cefotaxime) (Cantey 2015; Clark 2006a; Stoll 2011; Tzialla 2015; Vergnano 2011). Other regimens, such as cephalosporins (as monotherapy) are also used (NICE 2012). However, most national and international guidelines recommend the use of a penicillin combined with an aminoglycoside, as the use of cephalosporins are thought to cause a higher incidence of drug resistance (Cortese 2016; Manan 2016; NICE 2012; NICE 2014; WHO 2013).
The duration of treatment is adjusted according to the type of pathogen, treatment response, and the possibility of the antibiotic to penetrate to the site of infection in case of, for example, meningitis (inflammation of the protective membranes covering the brain and spinal cord), encephalitis (infection of the brain), osteomyelitis (infection in a bone), or endocarditis (inflammation of the inner layer of the heart). When the pathogen is identified by cultures, the antibiotic therapy might be changed according to the antibiotic susceptibility of the pathogen. However, causative bacteria are identified only in about one‐third of the patients with presumed sepsis (Dellinger 2013; Gaieski 2010; Kumar 2006). One study found that the empirical antibiotic regimen was changed in 44% of the cases when the pathogen and susceptibility was identified, and the most frequently added antibiotics were vancomycin, cefotaxime, and penicillin (Stoll 2011). It is recommended to stop the antibiotic treatment when there are no signs and symptoms of infection and no pathogen identified (Camacho‐Gonzalez 2013; Cortese 2016).
Antibiotic susceptibility
Antibiotic resistance is a growing problem which increases the morbidity, mortality, and healthcare costs associated with infections globally (Cohen 1992; Foster 2006; Huynh 2016; Vergnano 2005). Studies indicate that bacterial resistance to antibiotics results primarily from the selective pressure exerted by the use and overuse of antibiotics (Foster 2006; Kunin 1990; McGowan 1994; Murray 1994; Sáez‐Llorens 2000). Studies that compare antibiotic susceptibility over time in the same unit show increased resistance to the most‐used antibiotics (Vergnano 2005). The spread of antibiotic‐resistant organisms within hospitals is a recognised problem, although neonates admitted from the community may also carry antibiotic‐resistant pathogens (Bhutta 1996).
The pathogens that cause neonatal infections and their antibiotic susceptibility patterns change over time and differ between countries, cities, and hospitals (Breurec 2016; Isaacs 2003; May 2005; Stoll 2003; Stoll 2005; Vergnano 2011). Furthermore, the definition and epidemiology of neonatal sepsis differ between countries, which may make the comparison of antibiotic susceptibility between countries difficult (Vergnano 2005). When comparing the epidemiology of neonatal sepsis in LMICs with high‐income countries, some important differences emerge in the pattern of aetiological pathogens and their antibiotic resistance (Khatua 1986; Tallur 2000; Tessin 1990; Vesikari 1985).
For example, data from the UK showed that 95% of the identified pathogens were susceptible to the most used empirical antibiotic regimens of penicillin and gentamicin (Vergnano 2011). One multicentre observational study from the USA showed that all group B Streptococcus isolates tested were sensitive to penicillin, ampicillin, and vancomycin, while 46% were resistant to erythromycin and 20% to clindamycin. With regards to E coli, 78% were ampicillin‐resistant, 4% were gentamicin‐resistant, and 3% were resistant to third‐generation cephalosporins (Stoll 2011).
Two multicentre studies from the USA showed an increased proportion of E coli strains resistant to ampicillin, especially among preterm neonates (Baltimore 2001; Hyde 2002). The emergence of intrapartum ampicillin exposure is thought to be a significant independent risk factor for ampicillin resistance (Bizzarro 2008). Some neonatal units have changed antibiotic policies to include a third‐generation cephalosporin in exchange for ampicillin due to a growing resistance of Gram‐negative bacteria to ampicillin and gentamicin (Camacho‐Gonzalez 2013; Meyer 2010; Sáez‐Llorens 2000; Vergnano 2005). However, several reports have also shown an emerging reduced susceptibility to third‐generation cephalosporin (Meyer 2010; Musoke 2000; Rahman 2002).
In LMICs, estimations suggest that up to 70% of pathogens isolated from neonatal sepsis may not be covered by the recommended empirical antibiotic regimen of ampicillin and gentamicin (Zaidi 2005). Some studies in LMICs have shown almost universal resistance (92% to 100% resistant) among the most common pathogens (Gram‐negative rods) to first‐line (often ampicillin and gentamicin) and second‐line antibiotics, such as third‐generation cephalosporins (Kabwe 2016; WHO 2013; Zaidi 2005). In addition, some LMICs face widespread dissemination of resistant bacterial strains, including extended‐spectrum‐lactamase‐producing bacteria and methicillin‐resistant Staphylococcus aureus (MRSA) (Cotton 2000; Gonzalez‐Vertiz 2001; Shenoy 2007; Zaidi 2005).
Adverse events
Use of ampicillin has been associated in some studies with adverse events, such as rashes, diarrhoea, nausea, and nephrotoxicity (Golan 2011; Katzung 2009; Mrvos 2013). Contrary to these findings, one systematic review of randomised controlled trials (RCTs) only found an significant increased incidence of candidiasis with no significant increase in rashes, diarrhoea, nausea, or nephrotoxicity (Gillies 2015). Nephrotoxicity has been estimated to be rare (0.03%) (Mrvos 2013).
Aminoglycosides (e.g. gentamicin) have been shown to be toxic (nephrotoxicity and ototoxicity) in adults, whereas its toxicity in neonates remains unclear (Huth 2011; Jackson 1971; Mattie 1989; McGlone 2008; Mingeot‐Leclercq 1999; Musiime 2015; Schultze 1971; Selimoglu 2007; Wargo 2014). Regarding ototoxicity in neonates, trials have presented conflicting results showing ototoxicity in 0% to 26% of neonates exposed to aminoglycoside (Agarwal 2002; Finitzo‐Hieber 1979; Itsarayoungyuen 1982; Lundergan 1999; Mercado 2004; Rastogi 2002). One meta‐analysis showed that 3% of neonates had hearing loss after treatment with gentamicin (Musiime 2015).
With regards to nephrotoxicity in neonates, the literature shows a large discrepancy between the degree of nephrotoxicity seen in neonates after gentamicin exposure (Kent 2014; Martinková 2010; McWilliam 2017). Studies span from showing no nephrotoxicity to showing the development of nephrotoxicity in 33% of the cases after aminoglycoside exposure (Martinková 2010; McWilliam 2017; Rhone 2014). In comparison, it is estimated that almost 25% of all adults who received aminoglycoside therapy develop nephrotoxicity (Lopez‐Novoa 2011; Wargo 2014).
How the intervention might work
Antibiotics are antimicrobial drugs that treat and prevent bacterial infections by either killing or inhibiting the growth of the bacteria (Waksman 1947). They can be classified based on:
their mechanism of action (bactericidal or bacteriostatic);
bacterial spectrum (broad or narrow); and
chemical structure (e.g. penicillins, macrolides, quinolones, tetracyclines, or aminoglycosides) (Bérdy 2005).
A combination of different antibiotics might have several advantages. The rational of combination therapy is to broaden the spectrum of antibiotic coverage when used empirically to increase the chance of covering the alleged causative bacteria. Theoretically, combination therapy might also suppress the development of subpopulations of micro‐organisms resistant to antibiotic (Allan 1985; Milatovic 1987; Tamma 2012).
However, it is theoretically possible that the optimal empirical antibiotic treatment should not be chosen solely based on the presumed pathogen and cultures. Antibiotics might have different effects in the human body compared to the pattern they show in vitro (e.g. cell cultures).
Why it is important to do this review
The previous version of this review (Mtitimila 2004), included two small trials and showed no evidence of a difference in effect between the compared antibiotic regimens on mortality, treatment failure, and bacteriological resistance (Mtitimila 2004). The two trials compared ticarcillin plus clavulanic acid versus piperacillin plus gentamicin (Miall‐Allen 1988), and ceftazidime versus combination therapy (benzylpenicillin and gentamicin) (Snelling 1983).
Despite the high burden of neonatal sepsis, high‐quality evidence in diagnosis and treatment is scarce (Zea‐Vera 2015). In adults, appropriate empirical antibiotic treatment reduces mortality rates by up to 50% associated with sepsis (Ibrahim 2000; Leibovici 1998; Paul 2010). Accordingly, it is currently recommended that the antibiotic empirical treatment should be broad to ensure coverage of any likely pathogen, which typically results in a composite antibiotic therapy (Cawcutt 2014; Dellinger 2013). Due to the diagnostic challenges of sepsis and the relative immunosuppression of the newborn, many neonates receive antibiotics for suspected sepsis. In fact, antibiotics have become the most used therapeutics in neonatal intensive care units (Clark 2006b), and observational studies in high‐income countries suggest that 83% to 94% of newborns treated with antibiotics for suspected sepsis have negative blood cultures (Klingenberg 2018). This presumed inappropriate use of antibiotics seems to contribute to the development and spread of resistant pathogens in neonatal intensive care units and seems to be associated with adverse events (e.g. invasive candidiasis, increased antimicrobial resistance, necrotising enterocolitis) (Clark 2006a; Cordero 2003; Cotten 2006; Cotten 2009; Foster 2006; Kuppala 2011). Adverse events of antibiotic exposure in infants is believed to be minimised through appropriate antibiotic choice and duration of treatment (Tripathi 2012).
Finally, the overuse of antibiotics has an important impact on health economic budgets. The cost of antimicrobials in children's hospitals in the USA has amounted to USD 192.9 million annually, corresponding to 17.1% of the total pharmacy budget (USD 1.13 billion) (Ross 2015). One Cochrane Review showed that the implementation of antibiotic policies/antimicrobial stewardship programmes effectively reduces the use of antibiotics (Davey 2017). To create the most appropriate antibiotic policies for neonatal sepsis, there is a need to base these policies on an updated systematic review with meta‐analysis.
In conclusion, there is a need for an updated systematic review assessing the effects of different antibiotic regimens for early‐onset neonatal sepsis.
Objectives
To assess the beneficial and harmful effects of different antibiotic regimens for early‐onset neonatal sepsis.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs, quasi‐RCTs, and cluster‐RCTs regardless of publication type, publication status, publication date, and language. We excluded crossover trials.
Types of participants
We included neonates (from birth to 72 hours of life at randomisation) clinically suspected of or diagnosed with early‐onset sepsis (as defined by trialists), severe/deep‐seated infections such as meningitis, osteomyelitis, endocarditis, or necrotising enterocolitis.
We excluded trials where the suspicion of sepsis was solely based on risk factors with no clinical signs of sepsis.
Types of interventions
We accepted any type of antibiotic or combination of antibiotics such as the following:
broad‐spectrum beta‐lactam antibiotics defined as broad‐spectrum penicillins (e.g. ampicillin, amoxicillin, piperacillin, ticarcillin, carbenicillin, and mezlocillin), cephalosporins (e.g. cefazolin, cephalexin, cefuroxime, cefotetan, cefoxitin, ceftriaxone, cefotaxime, ceftazidime, cefepime, cefazolin, ceftobiprole, ceftolozane, and cefoperazone), carbapenems (e.g. imipenem, meropenem, doripenem, and ertapenem), and monobactams (e.g. aztreonam);
narrow‐spectrum antibiotics including narrow‐spectrum penicillins (e.g. oxacillin, cloxacillin, dicloxacillin, nafcillin, methicillin, and penicillin G);
beta‐lactam antibiotics with beta‐lactamase inhibitors (e.g. avibactam, clavulanic acid, sulbactam, and tazobactam);
combinations of beta‐lactam with aminoglycoside (e.g. gentamicin);
combinations of beta‐lactam with glycopeptide (e.g. vancomycin and teicoplanin);
combinations of glycopeptide with aminoglycoside.
We planned to assess the following comparisons:
aminoglycoside added to any type of antibiotic versus antibiotic (same antibiotic as in the experimental group);
broad‐spectrum antibiotic and aminoglycoside versus narrower‐spectrum antibiotic (defined in the above description, e.g. penicillins) and aminoglycoside (same aminoglycoside as in the experimental group);
any other used antibiotic regimen (not included in the above‐mentioned comparisons) versus any other used antibiotic regimen (not included in the above‐mentioned comparisons).
Types of outcome measures
Primary outcomes
All‐cause mortality.
Secondary outcomes
-
Proportion of participants with one or more serious adverse event. We defined a serious adverse event as any untoward medical occurrence that resulted in death; was life‐threatening; jeopardised the participant; was persistent; or led to significant disability, hospitalisation, or prolonged hospitalisation (ICH‐GCP 2015). As we expected the reporting of serious adverse events in many trials to be very heterogeneous and not strictly according to the recommendations regarding good clinical practice from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH‐GCP) (ICH‐GCP 2015), we included the event as a serious adverse event if the trial authors either:
used the term 'serious adverse event' but not referred to ICH‐GCP; or
reported the proportion of participants with an event we considered fulfil the ICH‐GCP definition. If studies reported several such events, we chose the highest proportion reported in each trial to avoid double‐counting.
Respiratory support, defined as the need for respiratory support, such as non‐invasive ventilation (e.g. continuous positive airway pressure (CPAP)) or invasive ventilation (e.g. respirator).
Circulatory support, defined as the need for circulatory support such as fluid bolus or vasoactive medication (e.g. inotropic agents or vasopressors).
Nephrotoxicity (as defined by the trial author(s)).
Presence of moderate‐to‐severe neurological developmental and sensory impairment (defined as a functional abnormality in the function of the brain, spinal cord, muscles, nerves, eyes or ears; or as any significant lag in a child's physical or motor, cognitive, behavioural, emotional, or social development, in comparison with other children of the same age and sex within similar environments. If formal evaluation tools were used to assess neurodevelopmental impairment, we used a threshold of –2 standard deviations (SDs) of the normal. Furthermore, severe brain injury per se is included, such as intraventricular haemorrhage grade 3 and 4 (Papile 1978; Volpe 2008), and periventricular leukomalacia.
Necrotising enterocolitis during or after treatment, defined by Bell's criteria 2 (Bell 1978).
Ototoxicity as defined by trial author(s).
We assessed all dichotomised outcomes as proportions.
We used the trial results reported at maximum follow‐up (our primary time point of interest).
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialised register; neonatal.cochrane.org/resources-review-authors). We searched for errata or retractions 12 March 2021 from included studies published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed), and we found none.
Electronic searches
We conducted a comprehensive literature search including: the Cochrane Central Register of Controlled Trials (CENTRAL 2021, Issue 3) in the Cochrane Library; Ovid MEDLINE (1946 to 12 March 2021); Embase via Ovid (1974 to 12 March 2021); CINAHL (EBSCOhost; 12 March 2021); LILACS (Bireme; 1982 to 12 March 2021) and Science Citation Index EXPANDED and Conference Proceedings Citation Index – Science (1990 to 12 March 2021). We have included the search strategies for each database in Appendix 1.
We searched ZETOC for abstracts of scientific conferences or symposia (zetoc.jisc.ac.uk/).
We searched clinical trial registries for ongoing or recently completed trials. We searched the World Health Organization's International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en/), and the U.S. National Library of Medicine's ClinicalTrials.gov (clinicaltrials.gov), via Cochrane CENTRAL. Additionally, we searched the ISRCTN Registry for any unique trials not identified through the Cochrane CENTRAL search (www.isrctn.com/).
We applied no language restrictions. If we identified any papers in a language not known by the review author group, we sought translation assistance and acknowledged the translators in the Acknowledgements section of the review.
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review to identify additional relevant articles.
We searched clinical trial registers of Europe and the USA, websites of pharmaceutical companies, the US Food and Drug Administration (FDA), and the European Medicines Agency (EMA) websites, to identify unpublished trials.
Data collection and analysis
Selection of studies
Three review authors working in pairs (SKK, CN, and SS) independently screened titles and abstracts. We retrieved all relevant full‐text study reports/publications. Two review authors (SKK and SS) independently screened the full text and identified trials for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, we consulted a third review author (JCJ). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and a Characteristics of excluded studies table.
Where studies had multiple publications, we collated the reports of the same study so that each study, rather than each report, was the unit of interest for the review, and such studies had a single identifier with multiple references.
Data extraction and management
We used validated data collection forms for trial characteristics and outcome data. Three review authors working in pairs (SKK, CN, and SS) extracted trial characteristics from included trials. We extracted the following trials characteristics:
methods: trial design, total duration of the trial, number of trial centres and location, trial setting, withdrawals, and date of the trial;
participants: number of participants in each intervention group, mean age, age range, gender, diagnostic criteria, inclusion criteria, and exclusion criteria;
interventions: intervention (including dosage, route of administration, and length of empirical treatment) and comparison;
outcomes: primary and secondary outcomes specified and collected, and time points reported;
notes: funding for trial and notable conflicts of interest of trial authors.
Three review authors (SKK, CN, and SS) independently extracted outcome data from included trials. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by discussion, or by involving a third review author (JCJ). One review author (SKK) transferred data into Review Manager 5 (Review Manager 2020). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (SS) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (SKK and SS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane 'Risk of bias' tool (Higgins 2020), for the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved any disagreements through discussion or by consulting a third review author (JCJ). See Appendix 2 for a more detailed description of risk of bias for each domain.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Korang 2021), and planned to report any deviations from it in the Differences between protocol and review section of the review.
Measures of treatment effect
Dichotomous outcomes
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials and the neonatal unit (or subunit) for cluster‐RCTs. For cluster‐RCTs, we undertook analyses at the level of the individual while accounting for the clustering in the data using the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020).
Dealing with missing data
We did not impute missing values for any outcomes in our primary analysis.
We contacted trial investigators and sponsors to verify key trial characteristics and obtain missing numerical outcome data where possible (e.g. when we identified a study as an abstract only).
Assessment of heterogeneity
We planned to visually inspect forest plots to assess for signs of heterogeneity and explore possible heterogeneity in our prespecified subgroup analyses. We also planned to inspect trial characteristics across trials to identify clinical heterogeneity. We planned to assess the presence of statistical heterogeneity using the Chi² test (threshold P < 0.10) and measure the quantities of heterogeneity using the I²statistic (Higgins 2002; Higgins 2003). If we detected moderate or high heterogeneity (I² statistic of 50% or greater), we planned to explore the possible causes (i.e. differences in study design, participants, interventions, or completeness of outcome assessments). Ultimately, we decided that a meta‐analysis should be avoided (Higgins 2002; Higgins 2003).
Assessment of reporting biases
We planned to use a funnel plot to assess publication bias if 10 or more trials met the inclusion criteria. We planned to visually inspect funnel plots to assess the risk of bias. We tested asymmetry using the Harbord test (Harbord 2006).
Data synthesis
Meta‐analysis
We planned to undertake this meta‐analysis according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We used Review Manager 5 to analyse data (Review Manager 2020).
We planned to assess our intervention effects using fixed‐effect meta‐analyses (Demets 1987), in accordance with the policies of Cochrane Neonatal. We had one primary outcome and, therefore, we considered a P value of 0.05 or less as the threshold for statistical significance (Jakobsen 2014). We planned to use the eight‐step procedure to assess if the threshold for significance was crossed (Jakobsen 2014). Where data were only available from one trial, we planned to use Fisher's exact test for dichotomous data (Fisher 1922).
Where a trial reported multiple trial arms, we planned to only include the relevant trial arms. If two comparisons were combined in the same meta‐analysis, we would halve the control group to avoid double‐counting.
Trial sequential analysis
Traditional meta‐analysis runs the risk of random errors due to sparse data and repetitive testing of accumulating data when updating reviews. Therefore, we planned to perform trial sequential analysis (TSA) on the outcomes to calculate the required information size and the cumulative Z‐curve's breach of relevant trial sequential monitoring boundaries (Brok 2008; Brok 2009; Thorlund 2009; Thorlund 2010; Thorlund 2011; TSA 2017; Wetterslev 2008; Wetterslev 2009). We wished to control the risks of type I errors and type II errors. A more detailed description of TSA can be found at www.ctu.dk/tsa/. We planned to assess our TSA intervention effects with both a random‐effects model (DerSimonian 1986), and a fixed‐effect model (Demets 1987). We planned to use the more conservative point estimate of the two (Jakobsen 2014). The more conservative point estimate would be the estimate closest to zero effect. If the two estimates were similar, we used the estimate with the widest CI.
For dichotomous outcomes, we planned to estimate the required information size based on the observed, unweighted proportion of neonates with an outcome in the control group (the cumulative proportion of participants with an event in the control groups relative to all participants in the control groups), a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and diversity as suggested by the trials in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses for our primary outcome.
High risk of bias trials compared to low risk of bias trials.
Gestational age: term (37 weeks or greater) compared to preterm.
Trials from high‐income countries compared to trials from LMICs, as defined by the World Bank (World Bank 2019).
Early‐onset sepsis defined as onset within 48 hours, within 72 hours, within one week, or as defined by the trial authors.
Clinically suspected sepsis compared to culture‐supported suspicion of severe bacterial infection.
We planned to use the formal test for subgroup interactions in Review Manager 5 (Review Manager 2020).
Sensitivity analysis
To assess the potential impact of the missing data, we planned to perform the following two sensitivity analyses on the primary outcome.
'Best‐worst‐case' scenario: we planned to assume that all participants lost to follow‐up in the experimental group had survived; and all those participants with missing outcomes in the control group had not survived.
'Worst‐best‐case' scenario: we planned to assume that all participants lost to follow‐up in the experimental group had not survived and that all those participants lost to follow‐up in the control group had survived.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence of the following (clinically relevant) outcomes: our primary outcome (all‐cause mortality), and five secondary outcomes (serious adverse event, circulatory support, nephrotoxicity, neurological developmental impairment, and necrotising enterocolitis).
Two review authors (SKK and SS) independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create five 'Summary of findings' tables to report the certainty of the evidence for the following five comparisons of antibiotic regimens.
Ampicillin plus gentamicin compared with penicillin plus gentamicin.
Piperacillin plus tazobactam compared with amikacin.
Ticarcillin plus clavulanic acid compared with piperacillin plus gentamicin.
Piperacillin compared with ampicillin plus amikacin.
Ceftazidime compared with benzylpenicillin plus gentamicin.
The GRADE approach results in an assessment of the certainty of a body of evidence as one of four grades.
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
We assessed all studies according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020), and the protocol for this review (Korang 2021). Characteristics of each study can be found in the Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
Our initial search identified 3356 references. We deemed 56 studies relevant and obtained full texts for further evaluation (see Figure 1). Of these, we included five completed trials (Hammerberg 1989; Metsvaht 2010; Miall‐Allen 1988; Snelling 1983: Tewari 2014). We identified no ongoing trials relevant for the review.
1.
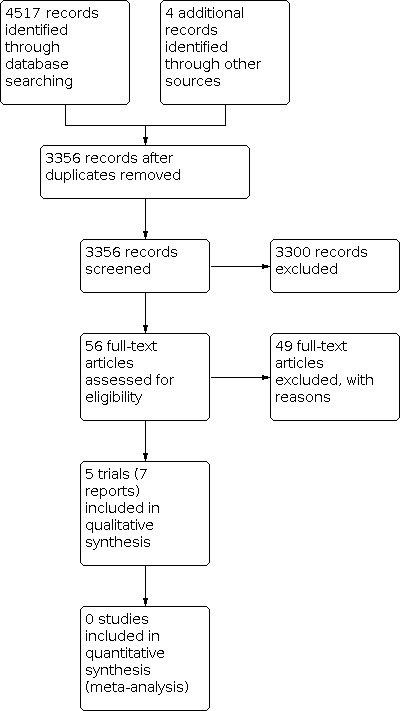
Study flow diagram.
Included studies
Five trials met our inclusion criteria (Hammerberg 1989; Metsvaht 2010; Miall‐Allen 1988; Snelling 1983; Tewari 2014). For detailed descriptions, see the Characteristics of included studies table. Two additional papers were included as secondary publications (Metsvaht 2011; Parm 2010), to Metsvaht 2010. Four were single centre trials (Hammerberg 1989; Miall‐Allen 1988; Snelling 1983; Tewari 2014), and one trial was a cluster‐RCT conducted at two centres (Metsvaht 2010).
Participants
The five included trials randomised 865 participants. The mean proportion of girls was 43% among the trials that reported the participant's gender.
Interventions
The five trials compared different antibiotic regimens.
Metsvaht 2010 compared ampicillin plus gentamicin with benzylpenicillin plus gentamicin.
Tewari 2014 compared piperacillin plus tazobactam with amikacin.
Miall‐Allen 1988 compared ticarcillin plus clavulanic acid with piperacillin plus gentamicin.
Hammerberg 1989 compared piperacillin with ampicillin plus amikacin.
Snelling 1983 compared ceftazidime with benzylpenicillin plus gentamicin.
Co‐interventions
Participants in all five included trials received standard care in addition to the allocated antibiotic regimen.
Outcomes
All five included trials reported all‐cause mortality. Five trials reported serious adverse events. None of the trials reported serious adverse events according to the ICH‐GCP, neither did they report serious adverse events as a composite outcome. Therefore, we reported the proportion of participants with an event we considered fulfilled the ICH‐GCP definition (e.g. need for vasoactive drugs or death). As there were several such events, we chose the highest proportion reported in each trial to avoid double‐counting. One trial reported circulatory support (Metsvaht 2010), nephrotoxicity (Hammerberg 1989), necrotising enterocolitis (Metsvaht 2010), and neurological developmental impairment (Metsvaht 2010). None of the trials reported respiratory support and ototoxicity.
Antibiotic resistance in included trials
One trial (from Estonia) reported three cases of resistance (to ampicillin) out of the six participants with positive cultures in the ampicillin plus gentamicin group, and five cases of resistance (to both penicillin and gentamicin) out of the eight participants with positive cultures in the penicillin plus gentamicin group (Metsvaht 2010).
One trial (from the USA) reported a single case of resistance (towards piperacillin) out of the 12 participants with positive cultures in the piperacillin group, but no resistance was reported among the 15 participants with positive cultures in the ampicillin plus amikacin group (Hammerberg 1989).
One trial (from Iran) reported one case of resistance out of the three participants with positive cultures in the piperacillin plus tazobactam group, but there was no resistance among the two participants with positive cultures in the amikacin group (Tewari 2014).
One trial (from the USA) comparing ceftazidime with benzylpenicillin plus gentamicin reported that none of the six participants with positive cultures grew any resistant isolates to the allocated antibiotics (Snelling 1983).
One trial (from the USA) reported two cases of resistance to ticarcillin out of the five participants with positive cultures in the ticarcillin plus clavulanic acid group, but no cases of resistance out of the seven participants with positive cultures in the piperacillin plus gentamicin group. (Miall‐Allen 1988).
Excluded studies
We assessed 49 trials as relevant on review of the abstract, but later excluded them upon review of the full publication.
We excluded 23 trials due to being a mix of early‐onset and late‐onset neonatal sepsis (Adelman 1987a; Adelman 1987b; Baqui 2013; Begue 1998; De Louvois 1992; Faix 1988; Fogel 1983; Gokalp 1991; Haffejee 1984; Hall 1988; Lee 2005; Marks 1978; Mir 2017; Molyneux 2017; Odio 1987; Taheri 2011; Tessin 1988; Tessin 1989; Tshefu 2015a; Tshefu 2015b; Umana 1990; Wiese 1988; Zaidi 2013).
In eight trials, both groups received the same antibiotics (Auriti 2005; Chowdhary 2006; Gathwala 2010; Hansen 1980; Langhendries 1993; McCracken 1976; Mulubwa 2020; Rohatgi 2017).
Three trials included only late‐onset neonatal sepsis (Ceriani 2014; Lutsar 2020; Millar 1992).
One trial included adults (Bassetti 1991).
Three trials were not randomised (Ebrahim 1969; Odio 1995; Oral 1998).
Eleven trials did not include neonates with early‐onset sepsis (Alinejad 2018; Aronoff 1984; Chartrand 1984; Collins 1998; Deville 2003; Feigin 1976; Jantausch 2003; Kaplan 2003; Lonnerholm 1982; Viganó 1995; Wells 1984).
When the participant age was unclear or separate data were not available for early‐onset sepsis, we contacted the trial authors. However, we obtained no additional information on these trials.
Risk of bias in included studies
We assessed all the included trials at overall high risk of bias (Figure 2). We contacted the authors for clarification, as some data were missing and several bias domains were unclear.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two trials did not describe how allocation sequence generation was performed resulting in unclear risk of bias (Miall‐Allen 1988; Snelling 1983). Three trials used a computer generated sequence, flipped a coin, or used an online randomisation service resulting in low risk of bias (Hammerberg 1989; Metsvaht 2010; Tewari 2014).
One trial used serially numbered opaque sealed envelopes to conceal allocation and was at low risk of bias (Tewari 2014). Three trials did not describe allocation concealment and were at unclear risk of bias (Hammerberg 1989; Miall‐Allen 1988; Snelling 1983). One trial was a cluster‐RCT resulting in assessment of high risk of bias (Metsvaht 2010).
Blinding
Two trials did not blind participants, treatment providers, or outcome assessors resulting in high risk of bias. Two trials did not describe blind participants, treatment providers, or outcome assessors resulting in unclear risk of bias. One trial did blind treatment providers and participants, but did not describe the blinding of outcome assessors resulting in 'low' and 'unclear' risk of bias respectively.
Incomplete outcome data
All five included trials used either intention‐to‐treat analysis or had no/few dropouts resulting in low risk of attrition bias.
Selective reporting
All five included trials reported mortality resulting in low risk of reporting bias.
Other potential sources of bias
We observed no other biases.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings 1. Ampicillin plus gentamicin compared with penicillin plus gentamicin for early‐onset neonatal sepsis.
| Ampicillin + gentamicin compared with penicillin + gentamicin for early‐onset neonatal sepsis | ||||||
|
Patient or population: neonates with early‐onset sepsis Settings: neonatal intensive care unit in Estonia Intervention: ampicillin + gentamicin Comparison: penicillin + gentamicin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Penicillin + gentamicin | Ampicillin + gentamicin | |||||
|
All‐cause mortality maximum follow‐up |
163 per 1000 | 91 per 1000 (49 to 173) |
RR 0.56 (0.30 to 1.06) |
283 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 3898 (RR 0.80, α 0.05, β 0.20) |
|
Serious adverse events maximum follow‐up |
461 per 1000 | 428 per 1000 (332 to 558) |
RR 0.93 (0.72 to 1.21) |
283 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 992 (RR 0.80, α 0.05, β 0.20) The serious adverse events were need for vasoactive drugs. |
|
Circulatory support maximum follow‐up |
461 per 1000 | 428 per 1000 (332 to 558) |
RR 0.93 (0.72 to 1.21) |
283 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 992 (RR 0.80, α 0.05, β 0,20) |
|
Neurological developmental impairment maximum follow‐up |
113 per 1000 | 92 per 1000 (45 to 183) |
RR 0.81 (0.40 to 1.61) |
283 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 5592 (RR 0.80, α 0.05, β 0.20) Participants with intraventricular haemorrhage type III to IV. |
|
Necrotising enterocolitis maximum follow‐up |
57 per 1000 | 70 per 1000 (28 to 173) |
RR 1.24 (0.50 to 3.05) |
283 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 11822 (RR 0.80, α 0.05, β 0.20) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OIS: optimal information size; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels because of very serious risk of bias, very serious imprecision of results, and serious risk of indirectness.
Summary of findings 2. Piperacillin plus tazobactam compared with amikacin for early‐onset neonatal sepsis.
| Piperacillin + tazobactam compared with amikacin for early‐onset neonatal sepsis | ||||||
|
Patient or population: neonates with early‐onset sepsis Settings: neonatal intensive care unit in India Intervention: piperacillin + tazobactam Comparison: amikacin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Amikacin | Piperacillin + tazobactam | |||||
|
All‐cause mortality maximum follow‐up |
34 per 1000 | 11 per 1000 (0 to 262) |
RR 0.32 (0.01 to 7.61) |
59 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 20142 (RR 0.80, α 0.05, β 0.20) |
|
Serious adverse events maximum follow‐up |
69 per 1000 | 67 per 1000 (10 to 442) |
RR 0.97 (0.15 to 6.41) |
59 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 9602 (RR 0.80, α 0.05, β 0.20) The serious adverse events were treatment failures. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OIS: optimal information size; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels because of very serious risk of bias, very serious imprecision of results, and serious risk of indirectness.
Summary of findings 3. Ticarcillin plus clavulanic acid compared with piperacillin plus gentamicin for early‐onset neonatal sepsis.
| Ticarcillin + clavulanic acid compared with piperacillin + gentamicin for early‐onset neonatal sepsis | ||||||
|
Patient or population: neonates with early‐onset sepsis Settings: neonatal intensive care unit in England Intervention: ticarcillin + clavulanic acid Comparison: piperacillin + gentamicin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Piperacillin + gentamicin | Ticarcillin + clavulanic acid | |||||
|
All‐cause mortality maximum follow‐up |
125 per 1000 | 94 per 1000 (24 to 363) |
RR 0.75 (0.19 to 2.90) |
72 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 5014 (RR 0.80, α 0.05, β 0.20) |
|
Serious adverse events maximum follow‐up |
125 per 1000 | 94 per 1000 (24 to 363) |
RR 0.75 (0.19 to 2.90) |
72 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 5014 (RR 0.80, α 0.05, β 0.20) The serious adverse events were deaths. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OIS: optimal information size; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels because of very serious risk of bias, very serious imprecision of results, and serious risk of indirectness.
Summary of findings 4. Piperacillin compared with ampicillin plus amikacin for early‐onset neonatal sepsis.
| Piperacillin compared with ampicillin + amikacin for early‐onset neonatal sepsis | ||||||
|
Patient or population: neonates with early‐onset sepsis Settings: NICU in Canada Intervention: piperacillin Comparison: ampicillin + amikacin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Ampicillin + amikacin | Piperacillin | |||||
|
All‐cause mortality maximum follow‐up |
138 per 1000 | 85 per 1000 (48 to 152) |
RR 0.62 (0.35 to 1.10) |
396 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 4518 (RR 0.80, α 0.05, β 0.20) |
|
Serious adverse events maximum follow‐up |
138 per 1000 | 85 per 1000 (48 to 152) |
RR 0.62 (0.35 to 1.10) |
396 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 4518 (RR 0.80, α 0.05, β 0.20) The serious adverse events were deaths. |
|
Nephrotoxicity maximum follow‐up |
229 per 1000 | 250 per 1000 (186 to 353) |
RR 1.14 (0.80 to 1.61) |
396 (1) | ⊕⊝⊝⊝ Very lowa | OIS: 4518 (RR 0.80, α 0.05, β 0.20) There might have been a lower number of participants in this outcome as only participants who received antibiotics for > 1 day were included. The exact number was unclear. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OIS: optimal information size; RR: risk ratio. | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels because of very serious risk of bias, very serious imprecision of results, and serious risk of indirectness.
Summary of findings 5. Ceftazidime compared with benzylpenicillin plus gentamicin for early‐onset neonatal sepsis.
| Ceftazidime compared with benzylpenicillin + gentamicin for early‐onset neonatal sepsis | ||||
|
Patient or population: neonates with early‐onset sepsis Settings: neonatal intensive care unit in the UK Intervention: ceftazidime Comparison: penicillin + gentamicin | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
All‐cause mortality maximum follow‐up |
Not estimable | 55 (1) | ⊕⊝⊝⊝ Very lowa | There were no deaths in either group. |
|
Serious adverse events maximum follow‐up |
Not estimable | 55 (1) | ⊕⊝⊝⊝ Very lowa | There were no serious adverse events in either group. |
| CI: confidence interval; OIS: optimal information size; RR: risk ratio. | ||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded three levels because of very serious risk of bias, very serious imprecision of results, and serious risk of indirectness.
Five trials met the inclusion criteria (Hammerberg 1989; Metsvaht 2010; Miall‐Allen 1988; Snelling 1983; Tewari 2014). We were able to assess in part all‐cause mortality as our primary outcome and the secondary outcomes serious adverse events, circulatory support, neurological developmental impairment, nephrotoxicity, and necrotising enterocolitis. However, the five trials assessed comparisons with different antibiotic regimens. Hence, we performed no meta‐analyses, TSAs, or subgroup analyses. We estimated the optimal information size for all outcomes and the optimal information size was not reached for any of the comparisons (Table 1; Table 2; Table 3; Table 4; Table 5).
Ampicillin plus gentamicin compared with benzylpenicillin plus gentamicin
We found one trial comparing ampicillin plus gentamicin with benzylpenicillin plus gentamicin (Table 1).
Primary outcome
All‐cause mortality
One trial randomising 283 participants comparing ampicillin plus gentamicin with benzylpenicillin plus gentamicin showed no evidence of a difference in all‐cause mortality (RR 0.56, 95% CI 0.30 to 1.06; very low‐certainty evidence; Analysis 1.1) (Metsvaht 2010).
1.1. Analysis.

Comparison 1: Ampicillin plus gentamicin compared with benzylpenicillin plus gentamicin, Outcome 1: All‐cause mortality
Secondary outcomes
Serious adverse events
One trial randomising 283 participants comparing ampicillin plus gentamicin with benzylpenicillin plus gentamicin showed no evidence of a difference in serious adverse events (RR 0.93, 95% CI 0.72 to 1.21; very low‐certainty evidence; Analysis 1.2) (Metsvaht 2010).
1.2. Analysis.

Comparison 1: Ampicillin plus gentamicin compared with benzylpenicillin plus gentamicin, Outcome 2: Serious adverse events
Respiratory support
The trial did not report respiratory support.
Circulatory support
One trial randomising 283 participants comparing ampicillin plus gentamicin with benzylpenicillin plus gentamicin showed no evidence of a difference in circulatory support (RR 0.93, 95% CI 0.72 to 1.21; very low‐certainty evidence; Analysis 1.3) (Metsvaht 2010).
1.3. Analysis.

Comparison 1: Ampicillin plus gentamicin compared with benzylpenicillin plus gentamicin, Outcome 3: Circulatory support
Nephrotoxicity
The trial did not report nephrotoxicity.
Neurological developmental impairment
One trial randomising 283 participants comparing ampicillin plus gentamicin with benzylpenicillin plus gentamicin showed no evidence of a difference in neurological developmental impairment (RR 0.81, 95% CI 0.40 to 1.61; very low‐certainty evidence; Analysis 1.4) (Metsvaht 2010).
1.4. Analysis.

Comparison 1: Ampicillin plus gentamicin compared with benzylpenicillin plus gentamicin, Outcome 4: Neurological developmental impairment
Necrotising enterocolitis
One trial randomising 283 participants comparing ampicillin plus gentamicin with benzylpenicillin plus gentamicin showed no evidence of a difference in necrotising enterocolitis (RR 1.24, 95% CI 0.50 to 3.05; very low‐certainty evidence; Analysis 1.5) (Metsvaht 2010).
1.5. Analysis.

Comparison 1: Ampicillin plus gentamicin compared with benzylpenicillin plus gentamicin, Outcome 5: Necrotising enterocolitis
Ototoxicity
The trial did not report ototoxicity.
Piperacillin plus tazobactam compared with amikacin
We found one trial comparing piperacillin plus tazobactam with amikacin (Table 2).
Primary outcome
All‐cause mortality
One trial randomising 59 participants comparing piperacillin plus tazobactam with amikacin showed no evidence of a difference in all‐cause mortality (RR 0.32, 95% CI 0.01 to 7.61; very low‐certainty evidence; Analysis 2.1) (Tewari 2014).
2.1. Analysis.

Comparison 2: Piperacillin plus tazobactum compared with amikacin, Outcome 1: All‐cause mortality
Secondary outcomes
Serious adverse events
One trial randomising 59 participants comparing piperacillin plus tazobactam with amikacin showed no evidence of a difference in serious adverse events (RR 0.97, 95% CI 0.15 to 6.41; very low‐certainty evidence; Analysis 2.2) (Tewari 2014).
2.2. Analysis.

Comparison 2: Piperacillin plus tazobactum compared with amikacin, Outcome 2: Serious adverse events
Respiratory support
The trial did not report respiratory support.
Circulatory support
The trial did not report circulatory support.
Nephrotoxicity
The trial did not report nephrotoxicity.
Neurological developmental impairment
The trial did not report neurological developmental impairment.
Necrotising enterocolitis
The trial did not report necrotising enterocolitis.
Ototoxicity
The trial did not report ototoxicity.
Ticarcillin plus clavulanic acid compared with piperacillin plus gentamicin
We found one trials comparing ticarcillin plus clavulanic acid compared with piperacillin (Table 3).
Primary outcome
All‐cause mortality
One trial randomising 72 participants comparing ticarcillin plus clavulanic acid with piperacillin plus gentamicin showed no evidence of a difference in all‐course mortality (RR 0.75, 95% CI 0.19 to 2.90; very low‐certainty evidence; Analysis 3.1) (Miall‐Allen 1988).
3.1. Analysis.

Comparison 3: Ticarcillin plus clavulanic acid compared with piperacillin plus gentamicin, Outcome 1: All‐cause mortality
Secondary outcomes
Serious adverse events
One trial randomising 72 participants comparing ticarcillin plus clavulanic acid with piperacillin plus gentamicin showed no evidence of a difference in serious adverse events (RR 0.75, 95% CI 0.19 to 2.90; very low‐certainty evidence; Analysis 3.2) (Miall‐Allen 1988).
3.2. Analysis.

Comparison 3: Ticarcillin plus clavulanic acid compared with piperacillin plus gentamicin, Outcome 2: Serious adverse events
Respiratory support
The trial did not report respiratory support.
Circulatory support
The trial did not report circulatory support.
Nephrotoxicity
The trial did not report nephrotoxicity.
Neurological developmental impairment
The trial did not report neurological developmental impairment.
Necrotising enterocolitis
The trial did not report necrotising enterocolitis.
Ototoxicity
The trial did not report ototoxicity.
Piperacillin compared with ampicillin plus amikacin
We found one trial comparing piperacillin with ampicillin plus amikacin (Table 4).
Primary outcome
All‐cause mortality
One trial randomising 396 participants comparing piperacillin with ampicillin plus amikacin showed no evidence of a difference in all‐course mortality (RR 0.62, 95% CI 0.35 to 1.10; very low‐certainty evidence; Analysis 4.1) (Hammerberg 1989).
4.1. Analysis.

Comparison 4: Piperacillin compared with ampicillin plus amikacin, Outcome 1: All‐cause mortality
Secondary outcomes
Serious adverse events
One trial randomising 396 participants comparing piperacillin with ampicillin plus amikacin showed no evidence of a difference in serious adverse events (RR 0.62, 95% CI 0.35 to 1.10; very low‐certainty evidence; Analysis 4.2) (Hammerberg 1989).
4.2. Analysis.

Comparison 4: Piperacillin compared with ampicillin plus amikacin, Outcome 2: Serious adverse events
Respiratory support
The trial did not report respiratory support.
Circulatory support
The trial did not report circulatory support.
Nephrotoxicity
One trial randomising 396 participants comparing piperacillin with ampicillin plus amikacin showed no evidence of a difference in nephrotoxicity (RR 1.14, 95% CI 0.80 to 1.63; very low‐certainty evidence; Analysis 4.3) (Hammerberg 1989).
4.3. Analysis.

Comparison 4: Piperacillin compared with ampicillin plus amikacin, Outcome 3: Nephrotoxicity
Neurological developmental impairment
The trial did not report neurological developmental impairment.
Necrotising enterocolitis
The trial did not report necrotising enterocolitis.
Ototoxicity
The trial did not report ototoxicity.
Ceftazidime compared with benzylpenicillin plus gentamicin
We found one trial comparing ceftazidime compared with benzylpenicillin plus gentamicin (Table 5).
Primary outcome
All‐cause mortality
One trial randomising 55 participants comparing ceftazidime with benzylpenicillin plus gentamicin reported no deaths (Analysis 5.1) (Snelling 1983).
5.1. Analysis.

Comparison 5: Ceftazidime compared with benzylpenicillin plus gentamicin, Outcome 1: All‐cause mortality
Secondary outcomes
Serious adverse events
One trial randomising 55 participants comparing ceftazidime with benzylpenicillin plus gentamicin reported no serious adverse events (Analysis 5.2) (Snelling 1983).
5.2. Analysis.

Comparison 5: Ceftazidime compared with benzylpenicillin plus gentamicin, Outcome 2: Serious adverse events
Respiratory support
The trial did not report respiratory support.
Circulatory support
The trial did not report circulatory support.
Nephrotoxicity
The trial did not report nephrotoxicity.
Neurological developmental impairment
The trial did not report neurological developmental impairment.
Necrotising enterocolitis
The trial did not report necrotising enterocolitis.
Ototoxicity
The trial did not report ototoxicity.
Discussion
Summary of main results
Evidence from five RCTs including 865 participants contributed data to our prespecified outcomes. We found insufficient information to assess the relative effects of any of the antibiotics compared. Furthermore, these trials had high risk of bias. In summary, we graded the level of evidence as very‐low certainty.
We conducted no meta‐analyses due to a lack of relevant data. The optimal information size was not reached for any of the comparisons (Table 1; Table 2; Table 3; Table 4; Table 5).
When assessing all‐cause mortality, one trial randomising 283 participants found no evidence of a difference when comparing ampicillin plus gentamicin with benzylpenicillin plus gentamicin (RR 0.56, 95% CI 0.30 to 1.06; very low‐certainty evidence) (Metsvaht 2010); one trial randomising 59 participants found no evidence of a difference when comparing piperacillin plus tazobactam with amikacin (RR 0.32, 95% CI 0.01 to 7.61; very low‐certainty evidence) (Tewari 2014); one trial randomising 72 participants found no evidence of a difference when comparing ticarcillin plus clavulanic acid with piperacillin plus gentamicin (RR 0.75, 95% CI 0.19 to 2.90; very low‐certainty evidence) (Miall‐Allen 1988); one trial randomising 396 participants found no evidence of a difference when comparing piperacillin with ampicillin plus amikacin (RR 0.62, 95% CI 0.35 to 1.10; very low‐certainty evidence) (Hammerberg 1989); and one trial randomising 55 participants comparing ceftazidime with benzylpenicillin plus gentamicin reported no deaths (Snelling 1983).
When assessing serious adverse events, one trial randomising 283 participants found no evidence of a difference when comparing ampicillin plus gentamicin with benzylpenicillin plus gentamicin (RR 0.93, 95% CI 0.72 to 1.21; very low‐certainty evidence) (Metsvaht 2010); one trial randomising 59 participants found no evidence of a difference when comparing piperacillin plus tazobactam with amikacin (RR 0.97, 95% CI 0.15 to 6.41; very low‐certainty evidence) (Tewari 2014); one trial randomising 72 participants found no evidence of a difference when comparing ticarcillin plus clavulanic acid with piperacillin plus gentamicin (RR 0.75, 95% CI 0.19 to 2.90; very low‐certainty evidence) (Miall‐Allen 1988); one trial randomising 396 participants found no evidence of a difference when comparing piperacillin with ampicillin plus amikacin (RR 0.62, 95% CI 0.35 to 1.10; very low‐certainty evidence) (Hammerberg 1989); and one trial randomising 55 participants comparing ceftazidime or benzylpenicillin plus gentamicin reported no serious adverse events (Snelling 1983).
None of the trials reported respiratory support.
When assessing circulatory support, one trial randomising 283 participants found no evidence of a difference when comparing ampicillin plus gentamicin with benzylpenicillin plus gentamicin (RR 0.93, 95% CI 0.72 to 1.21; very low‐certainty evidence) (Metsvaht 2010).
When assessing nephrotoxicity, one trial randomising 396 participants found no evidence of a difference when comparing piperacillin with ampicillin plus amikacin (RR 1.14, 95% CI 0.80 to 1.63; very low‐certainty evidence) (Hammerberg 1989).
When assessing neurological developmental impairment, one trial randomising 283 participants found no evidence of a difference when comparing ampicillin plus gentamicin with benzylpenicillin plus gentamicin (RR 0.81, 95% CI 0.40 to 1.61; very low‐certainty evidence) (Metsvaht 2010).
When assessing necrotising enterocolitis, one trial randomising 283 participants found no evidence of a difference when comparing ampicillin plus gentamicin with benzylpenicillin plus gentamicin (RR 1.24, 95% CI 0.50 to 3.05; very low‐certainty evidence) (Metsvaht 2010).
None of the trials reported ototoxicity.
The benefits and harms of different antibiotic regimens remain unclear owing to the lack of well‐powered trials and the high risks of bias.
Overall completeness and applicability of evidence
We were unable to perform any meta‐analyses due to lack of relevant data and the identified trials were underpowered. Therefore, it was not possible to conclude whether one antibiotic regimen was superior to another in neonates with early‐onset sepsis. More and larger RCTs with low risk of bias are needed.
Quality of the evidence
Heterogeneity
As no meta‐analysis was performed, we did not assess heterogeneity.
Risk of systematic error ('bias')
We found no trials and no outcome results at low risk of bias.
It was not possible to assess publication bias, as we included only five studies.
Risk of random error ('play of chance')
It was not possible to perform TSA, as we performed no meta‐analyses.
GRADE
We assessed the certainty of the evidence for each outcome using the GRADE approach. The GRADE assessment generally showed that evidence was of very‐low certainty. The reasons for the GRADE assessment are given in the footnotes of the tables (Table 1; Table 2; Table 3; Table 4; and Table 5).
Potential biases in the review process
The main limitation of this review was the low number of randomised participants and hence paucity of evidence for the use of different antibiotic regimens. Another limitation was that some trials did not distinguish between early‐onset and late‐onset neonatal sepsis, which resulted in exclusion of a large number of potentially relevant trials. Most included trials were from before 1990.
We used the broadest possible definition of early‐onset sepsis as there is no internationally agreed‐upon consensus definition of neonatal sepsis. This could potentially have caused the inclusion of trials with very a heterogeneous population. The consequence was that some trials included participants with suspected early‐onset sepsis may have included participants that did not have sepsis. We decided to use a broad definition to potentially include more trials and obtain more power. However, despite this broad approach, we only found five trials.
If we had found trials with different sepsis definitions, we would have explored the statistical and clinical heterogeneity (according to our protocol (Korang 2021)), and considered whether meta‐analysis could be justified.
As indicated in our Background section, there might be substantial differences between the pathogens across countries. The optimal antibiotic regimen might, therefore, vary according to country and local risks of antibiotic resistance. We did not include enough trials to confirm or reject that this was the case. Despite the anticipated differences between the antibiotic resistance at different sites, there could still be important differences between antibiotic regimens on clinical outcomes that would lead to generalised recommendations (Paul 2010). Furthermore, adverse events of the antibiotics are presumably similar across different populations.
For future updates, we will systematically assess the clinical heterogeneity (Barbateskovic 2021).
Agreements and disagreements with other studies or reviews
The additional trials included in this review update did not change the overall conclusions and recommendations of the former review (Mtitimila 2004).
Authors' conclusions
Implications for practice.
Current evidence does not allow confirmation or rejection of one antibiotic regimen being superior to another.
Implications for research.
The primary focus should be to develop an international consensus definition of neonatal sepsis (McGovern 2020; Wynn 2014; Wynn 2016). Then high‐quality randomised controlled trials are needed to assess the effects of different antibiotic regimens for sepsis in newborn infants. Such trials should:
randomise a sufficient number of participants to demonstrate reliable results;
assess all‐cause mortality and serious adverse events;
be conducted with low risk of bias;
adhere to consensus definitions of suspected and diagnosed early‐onset neonatal when such emerge;
measure antibiotic resistance among the culture‐positive participants;
assess differences between sites, countries, and regions included.
What's new
| Date | Event | Description |
|---|---|---|
| 12 March 2021 | Amended | Prior to updating, the authors rewrote the protocol. The protocol and subsequent review will update the previously published review of "Antibiotic regimens for suspected early neonatal sepsis" (Mtitimila 2004). |
History
Protocol first published: Issue 12, 2020
Acknowledgements
We would like to thank Cochrane Neonatal: Colleen Ovelman, Managing Editor; Jane Cracknell, Assistant Managing Editor; Roger Soll, Co‐coordinating editor; and Bill McGuire, Co‐coordinating Editor, who provided editorial and administrative support.
Carol Friesen, Cochrane Neonatal Information Specialist, and Sarah Klingenberg Cochrane Hepato‐Biliary Information Specialist designed the literature searches.
Jeffrey Horbar and James Hagadorn peer reviewed and offered feedback for this review.
The Methods section of this review was based on a standard template used by Cochrane Neonatal.
We acknowledge the work of Dr Edward Mtitimila and Dr Richard Cooke on the previously published version of the protocol (Mtitimila 2003), and the review (Mtitimila 2004).
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 3) in the Cochrane Library
#1 MeSH descriptor: [Infant] explode all trees
#2 (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
#3 #1 or #2
#4 MeSH descriptor: [Neonatal Sepsis] explode all trees
#5 (sepsis NEAR/3 (neonat* or neo nat*))
#6 (sepsis NEAR/3 (newborn* or new born* or newly born*))
#7 (septic* NEAR/3 (neonat* or neo nat*))
#8 (septic* NEAR/3 (newborn* or new born* or newly born*))
#9 (infect* NEAR/3 (neonat* or neo nat*))
#10 (infect* NEAR/3 (newborn* or new born* or newly born*))
#11 (bacter* NEAR/3 (neonat* or neo nat*))
#12 (bacter* NEAR/3 (newborn* or new born* or newly born*))
#13 (gram NEAR/2 negative)
#14 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13
#15 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees
#16 (antibiot* OR antimicrob* OR lactam* OR aminoglycoside* OR glycoprotein OR penicillin OR oxacillin OR cloxacillin OR dicloxacillin OR nafcillin OR methicillin OR ampicillin OR amoxicillin OR piperacillin OR ticarcillin OR carbenicillin OR mezlocillin OR cephalosporins OR cefazolin OR cephalexin OR cefuroxime OR cefotetan OR cefoxitin OR ceftriaxone OR cefotaxime OR ceftazidime OR cefepime OR cefazolin OR ceftobiprole OR cefoperazone OR carbapenems OR imipenem OR meropenem OR doripenem OR ertapenem OR monobactams OR aztreonam)
#17 #15 OR #16
#18 #3 and #14 and #17
MEDLINE Ovid (1946 to March 2021)
1. exp Infant/
2. (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
3. 1 or 2
4. exp Neonatal Sepsis/
5. (sepsis adj3 (neonat$ or neo nat$)).ti,ab.
6. (sepsis adj3 (newborn$ or new born$ or newly born$)).ti,ab.
7. (septic$ adj3 (neonat$ or neo nat$)).ti,ab.
8. (septic$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
9. (infect$ adj3 (neonat$ or neo nat$)).ti,ab.
10. (infect$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
11. (bacter$ adj3 (neonat$ or neo nat$)).ti,ab.
12. (bacter$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
13. (gram adj2 negative).ti,ab.
14. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13
15. exp Anti‐Bacterial Agents/
16. (antibiot* or antimicrob* or lactam* or aminoglycoside* or glycoprotein or penicillin or oxacillin or cloxacillin or dicloxacillin or nafcillin or methicillin or ampicillin or amoxicillin or piperacillin or ticarcillin or carbenicillin or mezlocillin or cephalosporins or cefazolin or cephalexin or cefuroxime or cefotetan or cefoxitin or ceftriaxone or cefotaxime or ceftazidime or cefepime or cefazolin or ceftobiprole or cefoperazone or carbapenems or imipenem or meropenem or doripenem or ertapenem or monobactams or aztreonam).ti,ab.
17. 15 or 16
18. 3 and 14 and 17
19. (randomized controlled trial or controlled clinical trial).pt. or clinical trials as topic.sh. or trial.ti.
20. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms]
21. 18 and (19 or 20)
Embase Ovid (1974 to March 2021)
1. exp infant/
2. (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
3. 1 or 2
4. exp newborn sepsis/
5. (sepsis adj3 (neonat$ or neo nat$)).ti,ab.
6. (sepsis adj3 (newborn$ or new born$ or newly born$)).ti,ab.
7. (septic$ adj3 (neonat$ or neo nat$)).ti,ab.
8. (septic$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
9. (infect$ adj3 (neonat$ or neo nat$)).ti,ab.
10. (infect$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
11. (bacter$ adj3 (neonat$ or neo nat$)).ti,ab.
12. (bacter$ adj3 (newborn$ or new born$ or newly born$)).ti,ab.
13. (gram adj2 negative).ti,ab.
14. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13
15. exp antiinfective agent/
16. (antibiot* or antimicrob* or lactam* or aminoglycoside* or glycoprotein or penicillin or oxacillin or cloxacillin or dicloxacillin or nafcillin or methicillin or ampicillin or amoxicillin or piperacillin or ticarcillin or carbenicillin or mezlocillin or cephalosporins or cefazolin or cephalexin or cefuroxime or cefotetan or cefoxitin or ceftriaxone or cefotaxime or ceftazidime or cefepime or cefazolin or ceftobiprole or cefoperazone or carbapenems or imipenem or meropenem or doripenem or ertapenem or monobactams or aztreonam).ti,ab.
17. 15 or 16
18. 3 and 14 and 17
19. Randomized controlled trial/ or Controlled clinical study/ or trial.ti.
20. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word]
21. 18 and (19 or 20)
CINAHL (EBSCOhost; March 2021)
S14 S10 AND S13
S13 S11 OR S12
S12 TX ( random* or blind* or placebo* or meta‐analys* ) OR TI trial
S11 PT randomized controlled trial OR PT controlled clinical trial
S10 S3 AND S6 AND S9
S9 S7 OR S8
S8 TI ( (antibiot* or antimicrob* or lactam* or aminoglycoside* or glycoprotein or penicillin or oxacillin or cloxacillin or dicloxacillin or nafcillin or methicillin or ampicillin or amoxicillin or piperacillin or ticarcillin or carbenicillin or mezlocillin or cephalosporins or cefazolin or cephalexin or cefuroxime or cefotetan or cefoxitin or ceftriaxone or cefotaxime or ceftazidime or cefepime or cefazolin or ceftobiprole or cefoperazone or carbapenems or imipenem or meropenem or doripenem or ertapenem or monobactams or aztreonam)
S7 MH antibiotics
S6 S4 OR S5
S5 TI ( (((sepsis or septic* or infect* or bacter*) N3 (neonat* or neo nat* or newborn* or new born* or newly born*)) or (gram N2 negative)) ) OR AB ( (((sepsis or septic* or infect* or bacter*) N3 (neonat* or neo nat* or newborn* or new born* or newly born*)) or (gram N2 negative)) )
S4 MH Neonatal Sepsis
S3 S1 OR S2
S2 TX (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
S1 MH infant
LILACS (Bireme; 1982 to March 2021)
(infan$ or newborn or neonat$ or premature or preterm or very low birth weight or low birth weight or VLBW or LBW) and (((sepsis or septic$ or infect$ or bacter$) and (neonat$ or neo nat$ or newborn$ or new born$ or newly born$)) or (gram near negative)) and (antibiot$ OR antimicrob$ OR lactam$ OR aminoglycoside$ OR glycoprotein OR penicillin OR oxacillin OR cloxacillin OR dicloxacillin OR nafcillin OR methicillin OR ampicillin OR amoxicillin OR piperacillin OR ticarcillin OR carbenicillin OR mezlocillin OR cephalosporins OR cefazolin OR cephalexin OR cefuroxime OR cefotetan OR cefoxitin OR ceftriaxone OR cefotaxime OR ceftazidime OR cefepime OR cefazolin OR ceftobiprole OR cefoperazone OR carbapenems OR imipenem OR meropenem OR doripenem OR ertapenem OR monobactams OR aztreonam) [Words] and (random$ or blind$ or placebo$ or meta‐analys$) [Words]
Science Citation Index EXPANDED (1900 to August 2020) and Conference Proceedings Citation Index – Science (1990 to March 2021) (Web of Science)
#5 #4 AND #3 AND #2 AND #1
#4 TI=(random* or blind* or placebo* or meta‐analys* or trial*) OR TS=(random* or blind* or placebo* or meta‐analys*)
#3 TS=(antibiot* OR antimicrob* OR lactam* OR aminoglycoside* OR glycoprotein OR penicillin OR oxacillin OR cloxacillin OR dicloxacillin OR nafcillin OR methicillin OR ampicillin OR amoxicillin OR piperacillin OR ticarcillin OR carbenicillin OR mezlocillin OR cephalosporins OR cefazolin OR cephalexin OR cefuroxime OR cefotetan OR cefoxitin OR ceftriaxone OR cefotaxime OR ceftazidime OR cefepime OR cefazolin OR ceftobiprole OR cefoperazone OR carbapenems OR imipenem OR meropenem OR doripenem OR ertapenem OR monobactams OR aztreonam)
#2 TS=(((sepsis or septic* or infect* or bacter*) and (neonat* or neo nat* or newborn* or new born* or newly born*)) or (gram near negative))
#1 TS=(infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. 'Risk of bias' tool
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low, high, or unclear risk for participants; and
low, high, or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we reincluded missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Were reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it was clear that all the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified outcomes of interest and were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could have put it at high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could have put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Ampicillin plus gentamicin compared with benzylpenicillin plus gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.06] |
| 1.2 Serious adverse events | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.72, 1.21] |
| 1.3 Circulatory support | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.72, 1.21] |
| 1.4 Neurological developmental impairment | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.40, 1.61] |
| 1.5 Necrotising enterocolitis | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.50, 3.05] |
Comparison 2. Piperacillin plus tazobactum compared with amikacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.61] |
| 2.2 Serious adverse events | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.15, 6.41] |
Comparison 3. Ticarcillin plus clavulanic acid compared with piperacillin plus gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause mortality | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 2.90] |
| 3.2 Serious adverse events | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 2.90] |
Comparison 4. Piperacillin compared with ampicillin plus amikacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 All‐cause mortality | 1 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.35, 1.10] |
| 4.2 Serious adverse events | 1 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.35, 1.10] |
| 4.3 Nephrotoxicity | 1 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.80, 1.63] |
Comparison 5. Ceftazidime compared with benzylpenicillin plus gentamicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 All‐cause mortality | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.2 Serious adverse events | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hammerberg 1989.
| Study characteristics | ||
| Methods | Randomised controlled trial Duration: at the discretion of the attending neonatologist. Maximum duration 10 days Date: NA Location: NICU in Canada |
|
| Participants | 396 infants suspected of early‐onset sepsis Inclusion criteria: had combination of risk factors or clinical signs (or both) compatible with sepsis; aged < 7 days of life Gender (boy/girl): NA Age: median gestational age 31.5 weeks. 97% were < 72 hours at randomisation. Exclusion criteria: previously received antibiotics, had underlying congenital conditions incompatible with life or were known to be septic. |
|
| Interventions |
Intervention 1: piperacillin 50 mg/kg and placebo (5% dextrose in water) every 12 hours Intervention 2: ampicillin 50 mg/kg and amikacin 7.5 mg/kg every 12 hours Co‐interventions: not described |
|
| Outcomes |
Primary outcome
Secondary outcomes
Follow‐up
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer‐generated randomised sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as being blinded and used placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | Reported mortality. |
| Other bias | Low risk | No other bias observed. |
Metsvaht 2010.
| Study characteristics | ||
| Methods | Cluster randomised trial Duration: NA Date: 2 August 2006 to 30 November 2007 Location: 2 tertiary NICUs in Estonia |
|
| Participants | 283 neonates admitted within 72 hours of life, needing early empiric antibiotic treatment for early‐onset neonatal sepsis or risk factors of infection according to the CDC criteria (e.g. maternal chorioamnionitis or maternal risk factors of infection or preterm labour in < 35 weeks of gestation, or a combination of these). Gender (boy/girl): 163/120 Age: median gestational age 31 weeks. < 72 hours at randomisation Exclusion criteria: prior administration of a different antibiotic regimen for > 24 hours or presence of suspected or confirmed meningitis, NEC, peritonitis, severe sepsis, or septic shock with isolation of micro‐organisms resistant to the study regimen in maternal urinary tract or birth canal or other situations that required different antibacterial treatment |
|
| Interventions |
Intervention 1: gentamicin (4–5 mg/kg 24–48 hourly, based on gestational age and postnatal age) + ampicillin (25 mg/kg 8–12 hourly, based on gestational age and postnatal age) Intervention 2: gentamicin (4–5 mg/kg 24–48 hourly, based on gestational age and postnatal age) + penicillin G (25 000 IU/kg 8–12 hourly, based on gestational age and postnatal age) Co‐interventions: not described |
|
| Outcomes |
Primary outcome
Secondary outcomes
Follow‐up
|
|
| Notes | The study was supported by Estonian Science Foundation Grant No 6984; Estonian Target Financing No 2726, and ESPID Small Grant Award. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomised trial. Was assigned randomly by flipping a coin. |
| Allocation concealment (selection bias) | High risk | Whole unit was treated the same. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment was not performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | Reported mortality and serious adverse events. |
| Other bias | Low risk | No other biases were identified. |
Miall‐Allen 1988.
| Study characteristics | ||
| Methods | Randomised controlled trial Duration: maximum 10 days, but until 48 hours if participants were asymptomatic and afebrile Date: NA Location: Hammersmith Hospital, London, UK |
|
| Participants | 72 neonates with suspected infection up to 48 hours of age Gender (boys/girls): 39/33 Age: < 48 hours at randomisation Inclusion criteria: < 48 hours after birth with confirmed sepsis, signs highly suggestive of sepsis, or who were at particular high risk of developing sepsis Exclusion criteria: not described |
|
| Interventions |
Intervention 1: ticarcillin + clavulanic acid 80 mg/kg 12 hourly or 8 hourly if > 2 kg (n = 32) Intervention 2: piperacillin 100 mg/kg 12 hourly + gentamicin 2.5 mg/kg 12 hourly (n = 40) |
|
| Outcomes |
Follow‐up
|
|
| Notes | It was not possible to contact the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only described as randomised. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | Reported mortality and serious adverse events. |
| Other bias | Low risk | No other biases identified. |
Snelling 1983.
| Study characteristics | ||
| Methods | Randomised controlled trial Duration: 7–10 days, but 48 hours if participants were asymptomatic and had negative blood cultures Date: NA Location: Liverpool Maternity Hospital, Liverpool, UK |
|
| Participants | 55 neonates with suspected serious infection within 48 hours of birth Gender (boys/girls): NA Age: < 48 hours at randomisation Inclusion criteria: < 48 hours after birth with confirmed sepsis, signs highly suggestive of sepsis or who were at particular high risk of developing sepsis Exclusion criteria: not described |
|
| Interventions |
Intervention 1: ceftazidime 50 mg/kg 12 hourly (n = 31) Intervention 2: gentamicin 3 mg/kg + benzylpenicillin 15 mg/kg 12 hourly (n = 24) |
|
| Outcomes |
Follow‐up
|
|
| Notes | Not possible to contact the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of intervention and outcome measurements not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of intervention and outcome measurements not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | Reported mortality and serious adverse events. |
| Other bias | Low risk | No other biases were identified. |
Tewari 2014.
| Study characteristics | ||
| Methods | Randomised controlled trial Duration: ≥ 48 hours Date: 1 May 2009 to 30 April 2011 Location: Neonatal Unit, Department of Pediatrics, Kerala Institute of Medical Sciences, Trivandrum, India |
|
| Participants | 59 neonates with suspected early‐onset neonatal sepsis Gender (boys/girls): NA Mean age: 1 day Diagnostic criteria: risk factors were maternal fever (> 37.8 °C) between onset of labour to delivery, prolonged rupture of membranes > 18 hours, spontaneous preterm (< 37 weeks) onset of labour, preterm (< 37 weeks) premature rupture of membranes, maternal sepsis, urinary infection or diarrhoea within 7 days to date of delivery, and features of clinical chorioamnionitis. Enrolled newborns were stratified within 1 hour of birth as asymptomatic or symptomatic based on presence of respiratory distress, apnoea, vomiting, abdominal distention, hypotension, hypoperfusion, hypoglycaemia, or hyperglycaemia. Exclusion criteria: babies with life‐threatening congenital anomalies, surgical illnesses, and indicated preterm birth for a maternal cause not associated with risk of early‐onset sepsis |
|
| Interventions |
Intervention 1: piperacillin + tazobactam 100 mg/kg IV infusion 12 hourly in 5% dextrose over 30 minutes Intervention 2: amikacin in 5% dextrose by IV infusion over 30 minutes with dose adjusted for the postmenstrual age in weeks and postnatal age in days Co‐interventions: routine and supportive care was provided using similar methods to participants in both groups as per unit guidelines. |
|
| Outcomes |
Primary outcome
Secondary outcome
Follow‐up
|
|
| Notes | Authors contacted by email: docvvt_13@hotmail.com Data for symptomatic participants were obtained from trialist. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using an online randomisation service. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment done using serially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | Reported mortality and serious adverse events. |
| Other bias | Low risk | No other biases identified. |
CDC: Centers for Disease Control; IQR: interquartile range; IV: intravenous; IVH: intraventricular haemorrhage; LOS: length of stay; n: number of participants; NA: not applicable; NEC: necrotising enterocolitis; NICU: neonatal intensive care unit; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adelman 1987a | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Adelman 1987b | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Alinejad 2018 | Participants did not have early‐onset neonatal sepsis. |
| Aronoff 1984 | Did not include neonates with sepsis. |
| Auriti 2005 | Both groups received amoxicillin. |
| Baqui 2013 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Bassetti 1991 | Participants were adults. |
| Begue 1998 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Ceriani 2014 | Included only late‐onset neonatal sepsis. |
| Chartrand 1984 | Did not include neonates with sepsis. |
| Chowdhary 2006 | Both groups received the same antibiotics. |
| Collins 1998 | Participants did not have early‐onset neonatal sepsis. |
| De Louvois 1992 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Deville 2003 | Did not have sepsis. |
| Ebrahim 1969 | Not a randomised controlled trial. |
| Faix 1988 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Feigin 1976 | Participants did not have early‐onset neonatal sepsis. |
| Fogel 1983 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Gathwala 2010 | Both groups received the same antibiotics. |
| Gokalp 1991 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Haffejee 1984 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Hall 1988 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Hansen 1980 | Both groups received ampicillin and gentamicin. |
| Jantausch 2003 | Did not have early‐onset sepsis. |
| Kaplan 2003 | Did not have early‐onset sepsis. |
| Langhendries 1993 | Both groups received the same antibiotic. |
| Lee 2005 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Lonnerholm 1982 | Participants were not suspected of having sepsis, a severe infection or deep‐seated infection. |
| Lutsar 2020 | Only included participants with late‐onset neonatal sepsis. |
| Marks 1978 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| McCracken 1976 | Both groups received the same antibiotic. |
| Millar 1992 | Only included participants with late‐onset neonatal sepsis. |
| Mir 2017 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Molyneux 2017 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Mulubwa 2020 | Both groups received the same antibiotic. |
| Odio 1987 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Odio 1995 | Not a randomised controlled trial. |
| Oral 1998 | Not a randomised controlled trial. |
| Rohatgi 2017 | Both groups received the same antibiotics |
| Taheri 2011 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Tessin 1988 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Tessin 1989 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Tshefu 2015a | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Tshefu 2015b | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Umana 1990 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Viganó 1995 | Did not have early‐onset sepsis. |
| Wells 1984 | Did not have early‐onset sepsis. |
| Wiese 1988 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
| Zaidi 2013 | Included both early‐onset and late‐onset neonatal sepsis. Unable to provide separate data for early onset. |
Differences between protocol and review
We decided to describe the antibiotic resistance occurring within the included trials towards the allocated antibiotic regimens narratively. We did this to further strengthen the review as recommended by Leibovici and colleagues (Leibovici 2016).
We decided to include a subgroup assessing the different inclusion criteria for sepsis.
Contributions of authors
SKK: conceived, designed, and drafted the review. He extracted, analysed, and interpreted the data.
SS: extracted data, and commented on and revised the review.
CN: extracted data, and commented on and revised the review.
MG: provided general advice and revised the review.
AG: provided general advice and revised the review.
GG: provided general advice and revised the review.
ULT: provided general advice and revised the review.
JCJ: conceived, designed, provided general advice and revised the review. He analysed and interpreted the data.
All authors agreed on the final review version.
Sources of support
Internal sources
No sources of support provided
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
The project received no funding.
SKK: none.
SS: none.
CN: none.
MG: none.
AG: none.
GG: none.
ULT: none.
JCJ: none.
New
References
References to studies included in this review
Hammerberg 1989 {published data only}
- Hammerberg O, Kurnitzki C, Watts J, Rosenbloom D. Randomized trial using piperacillin versus ampicillin and amikacin for treatment of premature neonates with risk factors for sepsis. European Journal of Clinical Microbiology & Infectious Diseases 1989;8(3):241-4. [DOI: 10.1007/BF01965268] [PMID: ] [DOI] [PubMed] [Google Scholar]
Metsvaht 2010 {published data only}
- Metsvaht T, Ilmoja ML, Parm U, Maipuu L, Merila M, Lutsar I. Comparison of ampicillin plus gentamicin vs penicillin plus gentamicin in empiric treatment of neonates at risk of early onset sepsis. Acta Paediatrica 2010;99(5):665-72. [DOI: 10.1111/j.1651-2227.2010.01687.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Metsvaht T, Ilmoja ML, Parm U, Merila M, Maipuu L, Muursepp P, et al. Ampicillin versus penicillin in the empiric therapy of extremely low-birthweight neonates at risk of early onset sepsis. Pediatrics International 2011;53(6):873-80. [DOI: 10.1111/j.1442-200X.2011.03468.x] [PMID: 21895866] [DOI] [PubMed] [Google Scholar]
- Parm U, Metsvaht T, Sepp E, Ilmoja ML, Pisarev H, Pauskar M, et al. Impact of empiric antibiotic regimen on bowel colonization in neonates with suspected early onset sepsis. European Journal of Clinical Microbiology & Infectious Diseases 2010;29(7):807-16. [DOI: 10.1007/s10096-010-0931-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Miall‐Allen 1988 {published data only}
- Miall-Allen VM, Whitelaw AG, Darrell JH. Ticarcillin plus clavulanic acid (Timentin) compared with standard antibiotic regimes in the treatment of early and late neonatal infections. British Journal of Clinical Practice 1988;42(7):273-9. [PMID: ] [PubMed] [Google Scholar]
Snelling 1983 {published data only}
- Snelling S, Hart CA, Cooke RW. Ceftazidime or gentamicin plus benzylpenicillin in neonates less than forty-eight hours old. Journal of Antimicrobial Chemotherapy 1983;12(Suppl A):353-6. [DOI: 10.1093/jac/12.suppl_a.353] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tewari 2014 {published data only}
- Tewari VV, Jain N. Monotherapy with amikacin or piperacillin-tazobactum empirically in neonates at risk for early-onset sepsis: a randomized controlled trial. Journal of Tropical Pediatrics 2014;60(4):297-302. [DOI: 10.1093/tropej/fmu017] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adelman 1987a {published data only}
- Adelman RD, Wirth F, Rubio T. A controlled study of the nephrotoxicity of mezlocillin and gentamicin plus ampicillin in the neonate. Journal of Pediatrics 1987;111(6 Pt 1):888-93. [DOI: 10.1016/s0022-3476(87)80212-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Adelman 1987b {published data only}
- Adelman RD, Wirth F, Rubio T. A controlled study of the nephrotoxicity of mezlocillin and gentamicin plus ampicillin in the neonate. American Journal of Diseases of Children 1987;141(11):1175-8. [DOI: 10.1001/archpedi.1987.04460110045019] [PMID: ] [DOI] [PubMed] [Google Scholar]
Alinejad 2018 {published data only}
- Alinejad S, Yousefichaijan P, Rezagholizamenjany M, Rafie Y, Kahbazi M, Arjmand A. Nephrotoxic effect of gentamicin and amikacin in neonates with Infection. Nephro-Urology Monthly 2018;10(2):e58580. [DOI: 10.5812/numonthly.58580] [DOI] [Google Scholar]
Aronoff 1984 {published data only}
- Aronoff SC, Reed MD, O'Brien CA, Blumer JL. Comparison of the efficacy and safety of ceftriaxone to ampicillin/chloramphenicol in the treatment of childhood meningitis. Journal of Antimicrobial Chemotherapy 1984;13(2):143-51. [DOI: 10.1093/jac/13.2.143] [PMID: ] [DOI] [PubMed] [Google Scholar]
Auriti 2005 {published data only}
- Auriti C, Rava L, Di Ciommo V, Ronchetti MP, Orzalesi M. Short antibiotic prophylaxis for bacterial infections in a neonatal intensive care unit: a randomized controlled trial. Journal of Hospital Infection 2005;59(4):292-8. [DOI: 10.1016/j.jhin.2004.09.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Baqui 2013 {published data only}
- Baqui AH, Saha SK, Ahmed A, Shahidullah M, Quasem I, Roth DE, et al. Safety and efficacy of simplified antibiotic regimens for outpatient treatment of serious infection in neonates and young infants 0-59 days of age in Bangladesh: design of a randomized controlled trial. Pediatric Infectious Disease Journal 2013;32(9 Suppl):S12-S8. [DOI: 10.1097/INF.0b013e31829ff790] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassetti 1991 {published data only}
- Bassetti D, Cruciani M, Solbiati M, Rubini F, Gandola L, Valenti G, et al. Comparative efficacy of ceftriaxone versus ceftazidime in the treatment of nosocomial lower respiratory tract infections. Chemotherapy 1991;37(5):371-5. [DOI: 10.1159/000238881] [PMID: ] [DOI] [PubMed] [Google Scholar]
Begue 1998 {published data only}
- Begue P, Astruc J, Francois P, Floret D. Comparison of ceftriaxone and cefotaxime in severe pediatric bacterial infections: a multicentrique study [Evaluation de la ceftriaxone et du céfotaxime dans l'infection bactérienne sévère en pédiatrie: étude multicentrique]. Medecine et Maladies Infectieuses 1998;28(4):300-6. [DOI: 10.1016/S0399-077X(98)80054-1] [DOI] [Google Scholar]
Ceriani 2014 {published data only}
- Ceriani Cernadas JM, Fernandez Jonusas S, Marquez M, Garsd A, Mariani G. Clinical outcome of neonates with nosocomial suspected sepsis treated with cefazolin or vancomycin: a non-inferiority, randomized, controlled trial. Archivos Argentinos de Pediatria 2014;112(4):308-14. [DOI: 10.5546/aap.2014.308] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chartrand 1984 {published data only}
- Chartrand SA, Marks MI, Scribner RK, Johnston JT, Frederick DF. Moxalactam therapy of Haemophilus influenzae type b meningitis in children. Journal of Pediatrics 1984;104(3):454-9. [DOI: 10.1016/s0022-3476(84)81116-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chowdhary 2006 {published data only}
- Chowdhary G, Dutta S, Narang A. Randomized controlled trial of 7-day vs. 14-day antibiotics for neonatal sepsis. Journal of Tropical Pediatrics 2006;52(6):427-32. [DOI: 10.1093/tropej/fml054] [PMID: ] [DOI] [PubMed] [Google Scholar]
Collins 1998 {published data only}
- Collins MD, Dajani AS, Kim KS, King DR, Kaplan SL, Azimi PH, et al. Comparison of ampicillin/sulbactam plus aminoglycoside vs. ampicillin plus clindamycin plus aminoglycoside in the treatment of intraabdominal infections in children. The Multicenter Group. Pediatric Infectious Disease Journal 1998;17(3 Suppl):S15-18; discussion S20-21. [DOI: 10.1097/00006454-199803001-00005] [PMID: ] [DOI] [PubMed] [Google Scholar]
De Louvois 1992 {published data only}
- De Louvois J, Dagan R, Tessin I. A comparison of ceftazidime and aminoglycoside based regimens as empirical treatment in 1316 cases of suspected sepsis in the newborn. European Journal of Pediatrics 1992;151(12):876-84. [DOI: 10.1007/BF01954122] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deville 2003 {published data only}
- Deville JG, Adler S, Azimi PH, Jantausch BA, Morfin MR, Beltran S, et al. Linezolid versus vancomycin in the treatment of known or suspected resistant Gram-positive infections in neonates. Pediatric Infectious Disease Journal 2003;22(9 Suppl):S158-63. [DOI: 10.1097/01.inf.0000086955.93702.c7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ebrahim 1969 {published data only}
- Ebrahim GJ. Sepsis of the new-born (a therapeutic trial with gentamicin). East African Medical Journal 1969;46(1):30-3. [PMID: ] [PubMed] [Google Scholar]
Faix 1988 {published data only}
- Faix RG, Polley TZ, Grasela TH. A randomized, controlled trial of parenteral clindamycin in neonatal necrotizing enterocolitis. Journal of Pediatrics 1988;112(2):271-7. [DOI: 10.1016/s0022-3476(88)80069-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Feigin 1976 {published data only}
- Feigin RD, Stechenberg BW, Chang MJ, Dunkle LM, Wong ML, Palkes H, et al. Prospective evaluation of treatment of Hemophilus influenzae meningitis. Journal of Pediatrics 1976;88(4 Pt 1):542-8. [DOI: 10.1016/s0022-3476(76)80002-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fogel 1983 {published data only}
- Fogel D, Farfel L, Miskin A, Mogilner BM. Comparison between the combination of azlocillin-gentamicin and ampicillin-gentamicin in the treatment of a nursery population. Israel Journal of Medical Sciences 1983;19(11):1009-15. [PMID: ] [PubMed] [Google Scholar]
Gathwala 2010 {published data only}
- Gathwala G, Sindwani A, Singh J, Choudhry O, Chaudhary U. Ten days vs. 14 days antibiotic therapy in culture-proven neonatal sepsis. Journal of Tropical Pediatrics 2010;56(6):433-5. [DOI: 10.1093/tropej/fmq012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gokalp 1991 {published data only}
- Gokalp AS, Oguz A, Gultekin A, Icagasioglu D. Neonatal sepsis in Turkey: the comparison between penicillin plus aminoglycoside and ampicillin plus third-generation cephalosporin chemotherapies. Materia Medica Polona. Polish Journal of Medicine and Pharmacy 1991;23(4):226-8. [DOI: 10.1093/tropej/36.4.200] [PMID: ] [DOI] [PubMed] [Google Scholar]
Haffejee 1984 {published data only}
- Haffejee IE. A therapeutic trial of cefotaxime versus penicillin-gentamicin for severe infections in children. Journal of Antimicrobial Chemotherapy 1984;14(Suppl B):147-52. [DOI: 10.1093/jac/14.suppl_b.147] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hall 1988 {published data only}
- Hall MA, Ducker DA, Lowes JA, McMichael J, Clarke P, Rowe D, et al. A randomised prospective comparison of cefotaxime versus netilmicin/penicillin for treatment of suspected neonatal sepsis. Drugs 1988;35(Suppl 2):169-77. [DOI: 10.2165/00003495-198800352-00036] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hansen 1980 {published data only}
- Hansen TN, Ritter DA, Speer ME, Kenny JD, Rudolph AJ. A randomized, controlled study of oral gentamicin in the treatment of neonatal necrotizing enterocolitis. Journal of Pediatrics 1980;97(5):836-39. [DOI: 10.1016/s0022-3476(80)80283-6] [PMID: 7000998] [DOI] [PubMed] [Google Scholar]
Jantausch 2003 {published data only}
- Jantausch BA, Deville J, Adler S, Morfin MR, Lopez P, Edge-Padbury B, et al. Linezolid for the treatment of children with bacteremia or nosocomial pneumonia caused by resistant Gram-positive bacterial pathogens. Pediatric Infectious Disease Journal 2003;22(9 Suppl):S164-71. [DOI: 10.1097/01.inf.0000086956.45566.55] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaplan 2003 {published data only}
- Kaplan SL, Deville JG, Yogev R, Morfin MR, Wu E, Adler S, et al, Linezolid Pediatric Study Group. Linezolid versus vancomycin for treatment of resistant Gram-positive infections in children. Pediatric Infectious Disease Journal 2003;22(8):677-86. [DOI: 10.1097/01.inf.0000078160.29072.42] [PMID: ] [DOI] [PubMed] [Google Scholar]
Langhendries 1993 {published data only}
- Langhendries JP, Battisti O, Bertrand JM, François A, Darimont J, Tulkens PM, et al. Once daily administration of amikacin. Adaptation to neonatology for infants less than 3 days of postnatal age [Administration en dose unique journalière de l'amikacine. Adaptation à la néonatologie pour des enfants traités avant le 3ème jour d'âge postnatal]. Médecine et Maladies Infectieuses 1993;23:44-54. [DOI: 10.1016/S0399-077X(05)80984-9] [DOI] [Google Scholar]
Lee 2005 {published data only}
- Lee SJ, Park EA. Efficacy and safety of amoxicillin-sulbactam and ampicillin-sulbactam in full term neonates. Journal of the Korean Society of Neonatology 2005;12(1):17-24. [Google Scholar]
Lonnerholm 1982 {published data only}
- Lonnerholm G, Bengtsson S, Ewald U. Oral pivampicillin and amoxycillin in newborn infants. Scandinavian Journal of Infectious Diseases 1982;14(2):127-30. [DOI: 10.3109/inf.1982.14.issue-2.10] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lutsar 2020 {published data only}
- Lutsar I, Chazallon C, Trafojer U, Vincent MC, Cinzia A, Chiara B, et al, NeoMero Consortium. Meropenem vs standard of care for treatment of neonatal late onset sepsis (NeoMero1): a randomised controlled trial. Plos One 2020;15(3):e0229380. [DOI: 10.1371/journal.pone.0229380] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marks 1978 {published data only}
- Marks S, Marks MI, Dupont C, Hammerberg S. Evaluation of three antibiotic programs in newborn infants. Canadian Medical Association Journal 1978;118(6):659-62. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
McCracken 1976 {published data only}
- McCracken GH Jr, Mize SG. A controlled study of intrathecal antibiotic therapy in Gram-negative enteric meningitis of infancy. Report of the neonatal meningitis cooperative study group. Journal of Pediatrics 1976;89(1):66-72. [DOI: 10.1016/s0022-3476(76)80929-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Millar 1992 {published data only}
- Millar MR, MacKay P, Levene M, Langdale V, Martin C. Enterobacteriaceae and neonatal necrotising enterocolitis. Archives of Disease in Childhood 1992;67(1 Spec No):53-6. [DOI: 10.1136/adc.67.1_spec_no.53] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mir 2017 {published data only}
- Mir F, Nisar I, Tikmani SS, Baloch B, Shakoor S, Jehan F, et al. Simplified antibiotic regimens for treatment of clinical severe infection in the outpatient setting when referral is not possible for young infants in Pakistan (Simplified Antibiotic Therapy Trial [SATT]): a randomised, open-label, equivalence trial. Lancet Global Health 2017;5(2):e177-85. [DOI: 10.1016/S2214-109X(16)30335-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molyneux 2017 {published data only}
- Molyneux EM, Dube Q, Banda FM, Chiume M, Singini I, Mallewa M, et al. The treatment of possible severe infection in infants: an open randomized safety trial of parenteral benzylpenicillin and gentamicin versus ceftriaxone in Infants <60 days of age in Malawi. Pediatric Infectious Disease Journal 2017;36(12):e328-33. [DOI: 10.1097/INF.0000000000001576] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulubwa 2020 {published data only}
- Mulubwa M, Griesel HA, Mugabo P, Dippenaar R, Wyk L. Assessment of vancomycin pharmacokinetics and dose regimen optimisation in preterm neonates. Drugs in Research & Development 2020;20(2):105-13. [DOI: 10.1007/s40268-020-00302-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Odio 1987 {published data only}
- Odio CM, Umana MA, Saenz A, Salas JL, McCracken GH Jr. Comparative efficacy of ceftazidime vs carbenicillin and amikacin for treatment of neonatal septicemia. Pediatric Infectious Disease Journal 1987;6(4):371-7. [DOI: 10.1097/00006454-198704000-00006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Odio 1995 {published data only}
- Odio CM. Cefotaxime for treatment of neonatal sepsis and meningitis. Diagnostic Microbiology and Infectious Disease 1995;22(1-2):111-7. [DOI: 10.1016/0732-8893(95)00093-p] [PMID: ] [DOI] [PubMed] [Google Scholar]
Oral 1998 {published data only}
- Oral R, Akisü, M, Kültürsay N, Vardar F, Tansuğ N. Neonatal klebsiella pneumonia sepsis and imipenem/cilastatin. Indian Journal of Pediatrics 1998;65(1):121-9. [DOI: 10.1007/BF02849703] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rohatgi 2017 {published data only}
- Rohatgi S, Dewan P, Faridi MM, Kumar A, Malhotra RK, Batra P. Seven versus 10 days antibiotic therapy for culture-proven neonatal sepsis: a randomised controlled trial. Journal of Paediatrics and Child Health 2017;53(6):556-62. [DOI: 10.1111/jpc.13518] [PMID: 28398692] [DOI] [PubMed] [Google Scholar]
Taheri 2011 {published data only}
- Taheri PA, Eslamieh H, Salamati P. Is ceftizoxime an appropriate surrogate for amikacin in neonatal sepsis treatment? A randomized clinical trial. Acta Medica Iranica 2011;49(8):499-503. [PMID: ] [PubMed] [Google Scholar]
Tessin 1988 {published data only}
- Tessin I, Thiringer K, Trollfors B, Brorson JE. Comparison of serum concentrations of ceftazidime and tobramycin in newborn infants. European Journal of Pediatrics 1988;147(4):405-7. [DOI: 10.1007/BF00496420] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tessin 1989 {published data only}
- Tessin I, Trollfors B, Thiringer K, Thorn Z, Larsson P. Concentrations of ceftazidime, tobramycin and ampicillin in the cerebrospinal fluid of newborn infants. European Journal of Pediatrics 1989;148(7):679-81. [DOI: 10.1007/BF00441533] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tshefu 2015a {published data only}
- Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P, et al. Simplified antibiotic regimens compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with clinical signs of possible serious bacterial infection when referral is not possible: a randomised, open-label, equivalence trial. Lancet 2015;385(9979):1767-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tshefu 2015b {published data only}
- Tshefu A, Lokangaka A, Ngaima S, Engmann C, Esamai F, Gisore P, et al. Oral amoxicillin compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with fast breathing when referral is not possible: a randomised, open-label, equivalence trial. Lancet 2015;385(9979):1758-66. [10.1016/S0140-6736(14)62285-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Umana 1990 {published data only}
- Umana MA, Odio CM, Castro E, Salas JL, McCracken GH Jr. Evaluation of aztreonam and ampicillin vs amikacin and ampicillin for treatment of neonatal bacterial infections. Pediatrics Infectious Disease Journal 1990;9(3):175-80. [DOI: 10.1097/00006454-199003000-00006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Viganó 1995 {published data only}
- Viganò A, Principi N. A randomised comparison of isepamicin and amikacin in the treatment of bacterial infections in paediatric patients. Journal of Chemotherapy 1995;7(Suppl 2):95-101. [PMID: ] [PubMed] [Google Scholar]
Wells 1984 {published data only}
- Wells TG, Trang JM, Brown AL, Marmer BC, Jacobs RF. Cefotaxime therapy of bacterial meningitis in children. Journal of Antimicrobial Chemotherapy 1984;14(Suppl B):181-9. [DOI: 10.1093/jac/14.suppl_b.181] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wiese 1988 {published data only}
- Wiese G. Treatment of neonatal sepsis with ceftriaxone/gentamicin and with azslocillin/gentamicin: a clinical comparison of efficacy and tolerability. Chemotherapy 1988;34(2):158-63. [DOI: 10.1159/000238564] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zaidi 2013 {published data only}
- Zaidi AK, Tikmani SS, Sultana S, Baloch B, Kazi M, Rehman H, et al. Simplified antibiotic regimens for the management of clinically diagnosed severe infections in newborns and young infants in first-level facilities in Karachi, Pakistan: study design for an outpatient randomized controlled equivalence trial. Pediatric Infectious Disease Journal 2013;32(Suppl 1):S19-25. [DOI: 10.1097/INF.0b013e31829ff7aa] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Agarwal 2002
- Agarwal G, Rastogi A, Pyati S, Wilks A, Pildes RS. Comparison of once-daily versus twice-daily gentamicin dosing regimens in infants > or = 2500 g. Journal of Perinatology 2002;22(4):268-74. [DOI: 10.1038/sj.jp.7210704] [PMID: ] [DOI] [PubMed] [Google Scholar]
Allan 1985
- Allan JD, Moellering RC Jr. Management of infections caused by Gram-negative bacilli: the role of antimicrobial combinations. Review of Infectious Diseases 1985;7(Suppl 4):S559-71. [DOI: 10.1093/clinids/7.supplement_4.s559] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bakhuizen 2014
- Bakhuizen SE, Haan TR, Teune MJ, Wassenaer-Leemhuis AG, Heyden JL, Ham DP, et al. Meta-analysis shows that infants who have suffered neonatal sepsis face an increased risk of mortality and severe complications. Acta Paediatrica 2014;103(12):1211-8. [DOI: 10.1111/apa.12764] [PMID: ] [DOI] [PubMed] [Google Scholar]
Baltimore 2001
- Baltimore RS, Huie SM, Meek JI, Schuchat A, O'Brien KL. Early-onset neonatal sepsis in the era of group B streptococcal prevention. Pediatrics 2001;108(5):1094-8. [DOI: 10.1542/peds.108.5.1094] [PMID: ] [DOI] [PubMed] [Google Scholar]
Barbateskovic 2021
Bedford Russell 2015
- Bedford Russell AR, Kumar R. Early onset neonatal sepsis: diagnostic dilemmas and practical management. Archives of Disease in Childhood. Fetal and Neonatal Edition 2015;100(4):F350-4. [DOI: 10.1136/archdischild-2014-306193] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1-7. [DOI: 10.1097/00000658-197801000-00001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2006
- Benjamin DK Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al, National Institute of Child Health and Human Development Neonatal Research Network. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006;117(1):84-92. [DOI: 10.1542/peds.2004-2292] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bérdy 2005
- Bérdy J. Bioactive microbial metabolites. Journal of Antibiotics 2005;58(1):1-26. [DOI: 10.1038/ja.2005.1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhutta 1996
- Bhutta ZA. Enterobacter sepsis in the newborn – a growing problem in Karachi. Journal of Hospital Infection 1996;34(3):211-6. [DOI: 10.1016/s0195-6701(96)90068-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bizzarro 2005
- Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG. Seventy-five years of neonatal sepsis at Yale: 1928-2003. Pediatrics 2005;116(3):595-602. [DOI: 10.1542/peds.2005-0552] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bizzarro 2008
- Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics 2008;121(4):689-96. [DOI: 10.1542/peds.2007-2171] [PMID: ] [DOI] [PubMed] [Google Scholar]
Breurec 2016
- Breurec S, Bouchiat C, Sire JM, Moquet O, Bercion R, Cisse MF, et al. High third-generation cephalosporin resistant Enterobacteriaceae prevalence rate among neonatal infections in Dakar, Senegal. BMC Infectious Diseases 2016;16(1):587. [DOI: 10.1186/s12879-016-1935-y] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. Journal of Clinical Epidemiology 2008;61(8):763-9. [DOI: 10.1016/j.jclinepi.2007.10.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive. Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. International Journal of Epidemiology 2009;38(1):287-98. [DOI: 10.1093/ije/dyn188] [PMID: ] [DOI] [PubMed] [Google Scholar]
Camacho‐Gonzalez 2013
- Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatric Clinics of North America 2013;60(2):367-89. [DOI: 10.1016/j.pcl.2012.12.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantey 2015
- Cantey J, Wozniak PS, Sánchez PJ. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatric Infectious Disease Journal 2015;34(3):267-72. [DOI: 10.1097/INF.0000000000000542] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cawcutt 2014
- Cawcutt KA, Peters SG. Severe sepsis and septic shock: clinical overview and update on management. Mayo Clinic Proceedings 2014;89(11):1572-8. [DOI: 10.1016/j.mayocp.2014.07.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Clark 2006a
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics 2006;117(1):67-74. [DOI: 10.1542/peds.2005-0179] [PMID: ] [DOI] [PubMed] [Google Scholar]
Clark 2006b
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 2006;117(6):1979-87. [DOI: 10.1542/peds.2005-1707] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cohen 1992
- Cohen ML. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science 1992;257(5073):1050-5. [DOI: 10.1126/science.257.5073.1050] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cordero 2003
- Cordero L, Ayers LW. Duration of empiric antibiotics for suspected early-onset sepsis in extremely low birth weight infants. Infection Control and Hospital Epidemiology 2003;24(9):662-6. [DOI: 10.1086/502270] [14510248] [DOI] [PubMed] [Google Scholar]
Cortese 2016
- Cortese F, Scicchitano P, Gesualdo M, Filaninno A, De Giorgi E, Schettini F, et al. Early and late infections in newborns: where do we stand? A review. Pediatrics and Neonatology 2016;57(4):265-73. [DOI: 10.1016/j.pedneo.2015.09.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cotten 2006
- Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK Jr, National Institute for Child Health and Human Development Neonatal Research Network. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics 2006;118(2):717-22. [DOI: 10.1542/peds.2005-2677] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cotten 2009
- Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ, et al, NICHD Neonatal Research Network. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009;123(1):58-66. [DOI: 10.1542/peds.2007-3423] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotton 2000
- Cotton MF, Wasserman E, Pieper CH, Theron DC, Tubbergh D, Campbell G, et al. Invasive disease due to extended spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal unit: the possible role of cockroaches. Journal of Hospital Infection 2000;44(1):13-7. [DOI: 10.1053/jhin.1999.0650] [PMID: ] [DOI] [PubMed] [Google Scholar]
Davey 2017
- Davey P, Marwick CA, Scott CL, Charani E, McNeil K, Brown E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD003543. [DOI: 10.1002/14651858.CD003543.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dellinger 2013
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Medicine 2013;39(2):165-228. [DOI: 10.1007/s00134-012-2769-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demets 1987
- Demets DL. Methods for combining randomized trials: strength and limitations. Statistics in Medicine 1987;6(3):341-50. [DOI: 10.1002/sim.4780060325] [PMID: ] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI: 10.1016/0197-2456(86)90046-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deutschman 2014
- Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity 2014;40(4):463-75. [DOI: 10.1016/j.immuni.2014.04.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Finitzo‐Hieber 1979
- Finitzo-Hieber T, McCracken GH Jr, Roeser RJ, Allen DA, Chrane DF, Morrow J. Ototoxicity in neonates treated with gentamicin and kanamycin: results of a four-year controlled follow-up study. Pediatrics 1979;63(3):443-50. [PMID: ] [PubMed] [Google Scholar]
Fisher 1922
- Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society 1922;85(1):87-94. [Google Scholar]
Foster 2006
- Foster KR, Grundmann H. Do we need to put society first? The potential for tragedy in antimicrobial resistance. PLoS Medicine 2006;3(2):e29. [DOI: 10.1371/journal.pmed.0030029] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaieski 2010
- Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Critical Care Medicine 2010;38(4):1045-53. [DOI: 10.1097/CCM.0b013e3181cc4824] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gillies 2015
- Gillies M, Ranakusuma A, Hoffmann T, Thorning S, McGuire T, Glasziou P, et al. Common harms from amoxicillin: a systematic review and meta-analysis of randomized placebo-controlled trials for any indication. Canadian Medical Association Journal 2015;187(1):E21-31. [DOI: 10.1503/cmaj.140848] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Golan 2011
- Golan DE, Tashlian AH, Amstrong EJ, Armstrong AW. Principles of Pharmacology: the Pathophysiologic Basis of Drug Therapy. 3rd edition. Philadelphia (PA): Lippincott Williams & Wilkins, 2011. [Google Scholar]
Goldenberg 2000
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. New England Journal of Medicine 2000;342(20):1500-7. [DOI: 10.1056/NEJM200005183422007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gonzalez‐Vertiz 2001
- Gonzalez-Vertiz A, Alcantar-Curiel D, Cuauhtli M, Daza C, Gayosso C, Solache G, et al. Multiresistant extended-spectrum beta-lactamase-producing Klebsiella pneumoniae causing an outbreak of nosocomial bloodstream infection. Infection Control and Hospital Epidemiology 2001;22(11):723-5. [DOI: 10.1086/501854] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 9 December 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014. Available at gradepro.org.
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI: 10.1002/sim.2380] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI: 10.1002/sim.1186] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Huth 2011
- Huth ME, Ricci AJ, Cheng AG. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. International Journal of Otolaryngology 2011;2011:937861. [DOI: 10.1155/2011/937861] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huynh 2016
- Huynh BT, Padget M, Garin B, Delarocque-Astagneau E, Guillemot D, BIRDY study group. Bacterial neonatal sepsis and antibiotic resistance in low-income countries. Lancet 2016;387(10018):533-4. [DOI: 10.1016/S0140-6736(16)00220-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hyde 2002
- Hyde TB, Hilger TM, Reingold A, Farley MM, O'Brien KL, Schuchat A, Active Bacterial Core surveillance (ABCs) of the Emerging Infections Program Network. Trends in incidence and antimicrobial resistance of early-onset sepsis: population-based surveillance in San Francisco and Atlanta. Pediatrics 2002;110(4):690-5. [DOI: 10.1542/peds.110.4.690] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ibrahim 2000
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000;118(1):146-55. [DOI: 10.1378/chest.118.1.146] [PMID: ] [DOI] [PubMed] [Google Scholar]
ICH‐GCP 2015
- International Conference on Harmonisation-Good Clinical Practice. ICH harmonised guideline: integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2) ICH Consensus Guideline (ICH-GCP), 2015. ichgcp.net (accessed 18 April 2021).
Isaacs 1999
- Isaacs D, Royle JA. Intrapartum antibiotics and early onset neonatal sepsis caused by group B Streptococcus and by other organisms in Australia. Australasian Study Group for Neonatal Infections. Pediatric Infectious Disease Journal 1999;18(6):524-8. [DOI: 10.1097/00006454-199906000-00009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Isaacs 2003
- Isaacs D, Australasian Study Group for Neonatal Infections. A ten year, multicentre study of coagulase negative staphylococcal infections in Australasian neonatal units. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(2):F89-93. [DOI: 10.1136/fn.88.2.f89] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Itsarayoungyuen 1982
- Itsarayoungyuen S, Riff L, Schauf V, Hamilton L, Otrembiak J, Vidyasagar D. Tobramycin and gentamicin are equally safe for neonates: results of a double-blind randomized trial with quantitative assessment of renal function. Pediatric Pharmacology (New York, N.Y.) 1982;2(2):143-55. [PMID: ] [PubMed] [Google Scholar]
Jackson 1971
- Jackson GG, Arcieri G. Ototoxicity of gentamicin in man: a survey and controlled analysis of clinical experience in the United States. Journal of Infectious Diseases 1971;124(Suppl):S130-7. [DOI: 10.1093/infdis/124.supplement_1.s130] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI: 10.1186/1471-2288-14-120] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kabwe 2016
- Kabwe M, Tembo J, Chilukutu L, Chilufya M, Ngulube F, Lukwesa C, et al. Etiology, antibiotic resistance and risk factors for neonatal sepsis in a large referral center in Zambia. Pediatric Infectious Disease Journal 2016;35(7):e191-8. [DOI: 10.1097/INF.0000000000001154] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kan 2016
- Kan B, Razzaghian HR, Lavoie PM. An immunological perspective on neonatal sepsis. Trends in Molecular Medicine 2016;22(4):290-302. [DOI: 10.1016/j.molmed.2016.02.001] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karunasekera 1999
- Karunasekera KA, Pathirana D. A preliminary study on neonatal septicaemia in a tertiary referral hospital paediatric unit. Ceylon Medical Journal 1999;44(2):81-6. [PMID: ] [PubMed] [Google Scholar]
Katzung 2009
- Katzung BG, Masters SB, Trevor AJ. Basic and Clinical Pharmacology. 11th edition. London (UK): McGraw-Hill Medical, 2009. [Google Scholar]
Kent 2014
- Kent A, Turner MA, Sharland M, Heath PT. Aminoglycoside toxicity in neonates: something to worry about? Expert Review of Anti-infective Therapy 2014;12(3):319-31. [DOI: 10.1586/14787210.2014.878648] [PMID: ] [DOI] [PubMed] [Google Scholar]
Khatua 1986
- Khatua SP, Das AK, Chatterjee BD, Khatua S, Ghose B, Saha A. Neonatal septicemia. Indian Journal of Pediatrics 1986;53(4):509-14. [DOI: 10.1007/BF02749537] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klingenberg 2018
- Klingenberg C, Kornelisse RF, Buonocore G, Maier RF, Stocker M. Culture-negative early-onset neonatal sepsis – at the crossroad between efficient sepsis care and antimicrobial stewardship. Frontiers in Pediatrics 2018;6:285. [DOI: 10.3389/fped.2018.00285] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klinger 2010
- Klinger G, Levy I, Sirota L, Boyko V, Lerner-Geva L, Reichman B, Israel Neonatal Network. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics 2010;125(4):e736-40. [DOI: 10.1542/peds.2009-2017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kumar 2006
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical Care Medicine 2006;34(6):1589-96. [DOI: 10.1097/01.CCM.0000217961.75225.E9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kumar 2016
- Kumar SK, Bhat BV. Distinct mechanisms of the newborn innate immunity. Immunology Letters 2016;173:42-54. [DOI: 10.1016/j.imlet.2016.03.009] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kunin 1990
- Kunin CM, Johansen KS, Worning AM, Daschner FD. Report of a symposium on use and abuse of antibiotics worldwide. Reviews of Infectious Diseases 1990;12(1):12-9. [DOI: 10.1093/clinids/12.1.12] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kuppala 2011
- Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. Journal of Pediatrics 2011;159(5):720-5. [DOI: 10.1016/j.jpeds.2011.05.033] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawn 2005
- Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why? Lancet 2005;365(9462):891-900. [DOI: 10.1016/S0140-6736(05)71048-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leibovici 1998
- Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. Journal of Internal Medicine 1998;244(5):379-86. [DOI: 10.1046/j.1365-2796.1998.00379.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leibovici 2016
- Leibovici L, Paul M, Garner P, Sinclair DJ, Afshari A, Pace NL, et al. Addressing resistance to antibiotics in systematic reviews of antibiotic interventions. Journal of Antimicrobial Chemotherapy 2016;71(9):2367-9. [DOI: 10.1093/jac/dkw135] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lim 1995
- Lim NL, Wong YH, Boo NY, Kasim MS, Chor CY. Bacteraemic infections in a neonatal intensive care unit – a nine-month survey. Medical Journal of Malaysia 1995;50(1):59-63. [PMID: ] [PubMed] [Google Scholar]
Liu 2012
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al, Child Health Epidemiology Reference Group of WHO and UNICEF . Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379(9832):2151-61. [DOI: 10.1016/S0140-6736(12)60560-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lopez‐Novoa 2011
- Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney International 2011;79(1):33-45. [DOI: 10.1038/ki.2010.337] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lundergan 1999
- Lundergan FS, Glasscock GF, Kim EH, Cohen RS. Once-daily gentamicin dosing in newborn infants. Pediatrics 1999;103(6 Pt 1):1228-34. [DOI: 10.1542/peds.103.6.1228] [PMID: ] [DOI] [PubMed] [Google Scholar]
Manan 2016
- Manan MM, Ibrahim NA, Aziz NA, Zulkifly HH, Al-Worafi YM, Long CM. Empirical use of antibiotic therapy in the prevention of early onset sepsis in neonates: a pilot study. Archives of Medical Science 2016;12(3):603-13. [DOI: 10.5114/aoms.2015.51208] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinková 2010
- Martínková J, Pokorná P, Záhora J, Chládek J, Vobruba V, Selke-Krulichová I, et al. Tolerability and outcomes of kinetically guided therapy with gentamicin in critically ill neonates during the first week of life: an open-label, prospective study. Clinical Therapeutics 2010;32(14):2400-14. [DOI: 10.1016/j.clinthera.2011.01.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mattie 1989
- Mattie H, Craig WA, Pechère JC. Determinants of efficacy and toxicity of aminoglycosides. Journal of Antimicrobial Chemotherapy 1989;24(3):281-93. [DOI: 10.1093/jac/24.3.281] [PMID: ] [DOI] [PubMed] [Google Scholar]
May 2005
- May M, Daley AJ, Donath S, Isaacs D, Australasian Study Group for Neonatal Infections. Early onset neonatal meningitis in Australia and New Zealand, 1992-2002. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(4):F324-7. [DOI: 10.1136/adc.2004.066134] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGlone 2008
- McGlone A, Cranswick N. Evidence behind the WHO guidelines: hospital care for children: what is the evidence of safety of gentamicin use in children? Journal of Tropical Pediatrics 2008;54(5):291-3. [DOI: 10.1093/tropej/fmn059] [PMID: ] [DOI] [PubMed] [Google Scholar]
McGovern 2020
- McGovern M, Giannoni E, Kuester H, Turner MA, den Hoogen A, Bliss JM, et al. Challenges in developing a consensus definition of neonatal sepsis. Pediatric Research 2020;88(1):14-26. [DOI] [PubMed] [Google Scholar]
McGowan 1994
- McGowan JE Jr. Do intensive hospital antibiotic control programs prevent the spread of antibiotic resistance? Infection Control and Hospital Epidemiology 1994;15(7):478-83. [DOI: 10.1086/646954] [PMID: ] [DOI] [PubMed] [Google Scholar]
McWilliam 2017
- McWilliam SJ, Antoine DJ, Smyth RL, Pirmohamed M. Aminoglycoside-induced nephrotoxicity in children. Pediatric Nephrology 2017;32(11):2015-25. [DOI: 10.1007/s00467-016-3533-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mercado 2004
- Mercado MC, Brodsky NL, McGuire MK, Hurt H. Extended interval dosing of gentamicin in preterm infants. American Journal of Perinatology 2004;21(2):73-7. [DOI: 10.1055/s-2004-820515] [PMID: ] [DOI] [PubMed] [Google Scholar]
Metsvaht 2011
- Metsvaht T, Ilmoja ML, Parm U, Merila M, Maipuu L, Muursepp P, et al. Ampicillin versus penicillin in the empiric therapy of extremely low-birthweight neonates at risk of early onset sepsis. Pediatrics international 2011;53(6):873-80. [DOI: 10.1111/j.1442-200X.2011.03468.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Meyer 2010
- Meyer E, Schwab F, Schroeren-Boersch B, Gastmeier P. Dramatic increase of third-generation cephalosporin-resistant E. coli in German intensive care units: secular trends in antibiotic drug use and bacterial resistance, 2001 to 2008. Critical Care 2010;14(3):R113. [DOI: 10.1186/cc9062] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milatovic 1987
- Milatovic D, Braveny I. Development of resistance during antibiotic therapy. European Journal of Clinical Microbiology 1987;6(3):234-44. [DOI: 10.1007/BF02017607] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mingeot‐Leclercq 1999
- Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrobial Agents and Chemotherapy 1999;43(5):1003-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moreno 1994
- Moreno MT, Vargas S, Poveda R, Sáez-Llorens X. Neonatal sepsis and meningitis in a developing Latin American country. Pediatric Infectious Disease Journal 1994;13(6):516-20. [DOI: 10.1097/00006454-199406000-00010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morris 2016
- Morris JM, Roberts CL, Bowen JR, Patterson JA, Bond DM, Algert CS, et al. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet 2016;387(10017):444-52. [DOI: 10.1016/S0140-6736(15)00724-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mrvos 2013
- Mrvos R, Pummer TL, Krenzelok EP. Amoxicillin renal toxicity: how often does it occur? Pediatric Emergency Care 2013;29(5):641-3. [DOI: 10.1097/PEC.0b013e31828e9e78] [PMID: ] [DOI] [PubMed] [Google Scholar]
Murray 1994
- Murray BE. Can antibiotic resistance be controlled? New England Journal of Medicine 1994;330(17):1229-30. [DOI: 10.1056/NEJM199404283301710] [PMID: ] [DOI] [PubMed] [Google Scholar]
Musiime 2015
- Musiime GM, Seale AC, Moxon SG, Lawn JE. Risk of gentamicin toxicity in neonates treated for possible severe bacterial infection in low- and middle-income countries: systematic review. Tropical Medicine & International Health 2015;20(12):1593-606. [DOI: 10.1111/tmi.12608] [PMID: ] [DOI] [PubMed] [Google Scholar]
Musoke 2000
- Musoke RN, Revathi G. Emergence of multidrug-resistant Gram-negative organisms in a neonatal unit and the therapeutic implications. Journal of Tropical Pediatrics 2000;46(2):86-91. [DOI: 10.1093/tropej/46.2.86] [PMID: ] [DOI] [PubMed] [Google Scholar]
Naher 2011
- Naher BS, Mannan MA, Noor K, Shahiddullah M. Role of serum procalcitonin and C-reactive protein in the diagnosis of neonatal sepsis. Bangladesh Medical Research Council Bulletin 2011;37(2):40-6. [DOI: 10.3329/bmrcb.v37i2.8432] [PMID: ] [DOI] [PubMed] [Google Scholar]
NICE 2012
- National Institute for Health and Clinical Excellence. Neonatal infection (early onset): antibiotics for prevention and treatment. Clinical guideline [CG149]. Published August 2012. www.nice.org.uk/guidance/cg149 (accessed 9 December 2019).
NICE 2014
- National Institute for Health and Clinical Excellence. Antibiotics for early-onset neonatal infection. Evidence Update June 2014. A summary of selected new evidence relevant to NICE clinical guideline 149 'Antibiotics for early-onset neonatal infection: antibiotics for the prevention and treatment of early-onset neonatal infection' (2012). Evidence Update 62. www.nice.org.uk/guidance/cg149/evidence/evidence-update-pdf-188168797 (accessed 9 December 2019). [PubMed]
Ohlsson 2014
- Ohlsson A, Shah VS. Intrapartum antibiotics for known maternal Group B streptococcal colonization. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD007467. [DOI: 10.1002/14651858.CD007467.pub4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [DOI: 10.1016/s0022-3476(78)80282-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Park 2013
- Park SE. Prevention of neonatal group B streptococcal disease. Infection & Chemotherapy 2013;45(3):343-5. [DOI: 10.3947/ic.2013.45.3.343] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parm 2010
- Parm U, Metsvaht T, Sepp E, Ilmoja ML, Pisarev H, Pauskar M, et al. Impact of empiric antibiotic regimen on bowel colonization in neonates with suspected early onset sepsis. European Journal of Clinical Microbiology & Infectious Diseases 2010;29(7):807-16. [DOI: 10.1007/s10096-010-0931-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
Paul 2010
- Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrobial Agents and Chemotherapy 2010;54(11):4851-63. [DOI: 10.1128/AAC.00627-10] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rahman 2002
- Rahman S, Hameed A, Roghani M T, Ullah Z. Multidrug resistant neonatal sepsis in Peshawar, Pakistan. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;87(1):F52-4. [DOI: 10.1136/fn.87.1.f52] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rastogi 2002
- Rastogi A, Agarwal G, Pyati S, Pildes RS. Comparison of two gentamicin dosing schedules in very low birth weight infants. Pediatric Infectious Disease Journal 2002;21(3):234-40. [DOI: 10.1097/00006454-200203000-00014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2014.
Rhone 2014
- Rhone ET, Carmody JB, Swanson JR, Charlton JR. Nephrotoxic medication exposure in very low birth weight infants. Journal of Maternal-fetal & Neonatal Medicine 2014;27(14):1485-90. [DOI: 10.3109/14767058.2013.860522] [PMID: ] [DOI] [PubMed] [Google Scholar]
Robillard 1993
- Robillard PY, Nabeth P, Hulsey TC, Sergent MP, Périanin J, Janky E. Neonatal bacterial septicemia in a tropical area. Four-year experience in Guadeloupe (French West Indies). Acta Paediatrica 1993;82(8):687-9. [DOI: 10.1111/j.1651-2227.1993.tb18041.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rogosch 2012
- Rogosch T, Kerzel S, Hoss K, Hoersch G, Zemlin C, Heckmann M, et al. IgA response in preterm neonates shows little evidence of antigen-driven selection. Journal of Immunology 2012;189(11):5449-56. [DOI: 10.4049/jimmunol.1103347] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 2015
- Ross RK, Hersh AL, Kronman MP, Newland JG, Gerber JS. Cost of antimicrobial therapy across US children's hospitals. Infection Control and Hospital Epidemiology 2015;36(10):1242-4. [DOI: 10.1017/ice.2015.159] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sáez‐Llorens 2000
- Sáez-Llorens X, Castrejón de Wong MM, Castaño E, De Suman O, De Morös D, De Atencio I. Impact of an antibiotic restriction policy on hospital expenditures and bacterial susceptibilities: a lesson from a pediatric institution in a developing country. Pediatric Infectious Disease Journal 2000;19(3):200-6. [DOI: 10.1097/00006454-200003000-00005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schlapbach 2011
- Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, et al, Swiss Neonatal Network and Follow-Up Group. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 2011;128(2):e348-57. [DOI: 10.1542/peds.2010-3338] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schuchat 2000
- Schuchat A, Zywicki SS, Dinsmoor MJ, Mercer B, Romaguera J, O'Sullivan MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics 2000;105(1 Pt 1):21-6. [DOI: 10.1542/peds.105.1.21] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schultze 1971
- Schultze RG, Winters RE, Kauffman H. Possible nephrotoxicity of gentamicin. Journal of Infectious Diseases 1971;124(Suppl):S145-7. [DOI: 10.1093/infdis/124.supplement_1.s145] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.gradepro.org/app/handbook/handbook.html (accessed prior to 18 April 2021).
Seale 2015
- Seale AC, Obiero CW, Berkley JA. Rational development of guidelines for management of neonatal sepsis in developing countries. Current Opinion in Infectious Diseases 2015;28(3):225-30. [DOI: 10.1097/QCO.0000000000000163] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Selimoglu 2007
- Selimoglu E. Aminoglycoside-induced ototoxicity. Current Pharmaceutical Design 2007;13(1):119-26. [DOI: 10.2174/138161207779313731] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shah 2014
- Shah BA, Padbury JF. Neonatal sepsis: an old problem with new insights. Virulence 2014;5(1):170-8. [DOI: 10.4161/viru.26906] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shane 2013
- Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. American Journal of Perinatology 2013;30(2):131-41. [DOI: 10.1055/s-0032-1333413] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shane 2014
- Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. Journal of Infection 2014;68(Suppl 1):S24-32. [DOI: 10.1016/j.jinf.2013.09.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharma 2007
- Sharma R, Tepas JJ 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. Journal of Pediatric Surgery 2007;42(3):454-61. [DOI: 10.1016/j.jpedsurg.2006.10.038] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shenoy 2007
- Shenoy S, Hegde A, Dominic SR, Kamath S, Arvind N. An outbreak of extended spectrum beta-lactamase producing Klebsiella pneumoniae in a neonatal intensive care unit. Indian Journal of Pathology & Microbiology 2007;50(3):669-70. [PMID: ] [PubMed] [Google Scholar]
Singer 2016
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315(8):801-10. [DOI: 10.1001/jama.2016.0287] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoll 2002
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. New England Journal of Medicine 2002;347(4):240-7. [DOI: 10.1056/NEJMoa012657] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2003
- Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Seminars in Perinatology 2003;27(4):293-301. [DOI: 10.1016/s0146-0005(03)00046-6] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2004
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al, National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004;292(19):2357-65. [DOI: 10.1001/jama.292.19.2357] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2005
- Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al, National Institute of Child Health and Human Development. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of Gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatric Infectious Disease Journal 2005;24(7):635-9. [DOI: 10.1097/01.inf.0000168749.82105.64] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2010
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443-56. [DOI: 10.1542/peds.2009-2959] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoll 2011
- Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011;127(5):817-26. [DOI: 10.1542/peds.2010-2217] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tallur 2000
- Tallur SS, Kasturi AV, Nadgir SD, Krishna BV. Clinico-bacteriological study of neonatal septicemia in Hubli. Indian Journal of Pediatrics 2000;67(3):169-74. [DOI: 10.1007/BF02723654] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tamma 2012
- Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with Gram-negative bacteria. Clinical Microbiology Reviews 2012;25(3):450-70. [DOI: 10.1128/CMR.05041-11] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tessin 1990
- Tessin I, Trollfors B, Thiringer K. Incidence and etiology of neonatal septicaemia and meningitis in western Sweden 1975-1986. Acta Paediatrica Scandinavica 1990;79(11):1023-30. [DOI: 10.1111/j.1651-2227.1990.tb11378.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? International Journal of Epidemiology 2009;38(1):276-86. [DOI: 10.1093/ije/dyn179] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta-analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV-infected individuals. Clinical Epidemiology 2010;2:57-66. [DOI: 10.2147/clep.s9242] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 7 January 2015).
Tripathi 2012
- Tripathi N, Cotten CM, Smith PB. Antibiotic use and misuse in the neonatal intensive care unit. Clinics in Perinatology 2012;39(1):61-8. [DOI: 10.1016/j.clp.2011.12.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2017
- Copenhagen Trial Unit. TSA – Trial sequential analysis, 2017. ctu.dk/tsa/ (accessed 1 May 2021).
Tzialla 2015
- Tzialla C, Borghesi A, Serra G, Stronati M, Corsello G. Antimicrobial therapy in neonatal intensive care unit. Italian Journal of Pediatrics 2015;41:27. [DOI: 10.1186/s13052-015-0117-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vergnano 2005
- Vergnano S, Sharland M, Kazembe P, Mwansambo C, Heath PT. Neonatal sepsis: an international perspective. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90(3):F220-4. [DOI: 10.1136/adc.2002.022863] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vergnano 2011
- Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, Watts T, et al. Neonatal infections in England: the NeonIN surveillance network. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(1):F9-14. [DOI: 10.1136/adc.2009.178798] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vesikari 1985
- Vesikari T, Janas M, Grönroos P, Tuppurainen N, Renlund M, Kero P, et al. Neonatal septicaemia. Archives of Disease in Childhood 1985;60(6):542-6. [DOI: 10.1136/adc.60.6.542] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Volpe 2008
- Volpe JJ. Intracranial hemorrhage: germinal matrix intraventricular hemorrhage. In: Neurology of the Newborn. 5th edition. Philadelphia (PA): Saunders Elsevier, 2008:517-88. [Google Scholar]
Waksman 1947
- Waksman SA. What is an antibiotic or an antibiotic substance? Mycologia 1947;39(5):565-9. [DOI: 10.1056/NEJM194712042372302] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walker 2011
- Walker JC, Smolders MA, Gemen EF, Antonius TA, Leuvenink J, Vries E. Development of lymphocyte subpopulations in preterm infants. Scandinavian Journal of Immunology 2011;73(1):53-8. [DOI: 10.1111/j.1365-3083.2010.02473.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wargo 2014
- Wargo KA, Edwards JD. Aminoglycoside-induced nephrotoxicity. Journal of Pharmacy Practice 2014;27(6):573-7. [DOI: 10.1177/0897190014546836] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weston 2011
- Weston EJ, Pondo T, Lewis MM, Martell-Cleary P, Morin C, Jewell B, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005-2008. Pediatric Infectious Disease Journal 2011;30(11):937-41. [DOI: 10.1097/INF.0b013e318223bad2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [DOI: 10.1016/j.jclinepi.2007.03.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Medical Research Methodology 2009;9:86. [DOI: 10.1186/1471-2288-9-86] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization Young Infants Study Group. Clinical prediction of serious bacterial infections in young infants in developing countries. Pediatric Infectious Disease Journal 1999;18(10 Suppl):S23-31. [DOI: 10.1097/00006454-199910001-00005] [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Pocket book of hospital care for children. Guidelines for the management of common childhood illnesses. Second edition. apps.who.int/iris/bitstream/10665/81170/1/9789241548373_eng.pdf?ua=1 (accessed prior to 24 May 2017).
World Bank 2019
- World Bank. World Bank country and lending groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed 9 December 2019).
Wynn 2014
- Wynn JL, Wong HR, Shanley TP, Bizzarro MJ, Saiman L, Polin RA. Time for a neonatal-specific consensus definition for sepsis. Pediatric Critical Care Medicine 2014;15(6):523-8. [DOI: 10.1097/PCC.0000000000000157] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wynn 2016
- Wynn JL. Defining neonatal sepsis. Current Opinion in Pediatrics 2016;28(2):135-40. [DOI: 10.1097/MOP.0000000000000315] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ygberg 2012
- Ygberg S, Nilsson A. The developing immune system – from foetus to toddler. Acta Paediatrica 2012;101(2):120-7. [DOI: 10.1111/j.1651-2227.2011.02494.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zaidi 2005
- Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet 2005;365(9465):1175-88. [DOI: 10.1016/S0140-6736(05)71881-X] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zaidi 2009
- Zaidi AK, Thaver D, Ali SA, Khan TA. Pathogens associated with sepsis in newborns and young infants in developing countries. Pediatric Infectious Disease Journal 2009;28(1 Suppl):S10-8. [DOI: 10.1097/INF.0b013e3181958769] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zea‐Vera 2015
- Zea-Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. Journal of Tropical Pediatrics 2015;61(1):1-13. [DOI: 10.1093/tropej/fmu079] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zemlin 2007
- Zemlin M, Hoersch G, Zemlin C, Pohl-Schickinger A, Hummel M, Berek C, et al. The postnatal maturation of the immunoglobulin heavy chain IgG repertoire in human preterm neonates is slower than in term neonates. Journal of Immunology 2007;178(2):1180-8. [DOI: 10.4049/jimmunol.178.2.1180] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Korang 2021
- Korang SK, Safi S, Gupta M, Greisen G, Lausten-Thomsen U, Jakobsen JC. Antibiotic regimens for early-onset neonatal sepsis. Cochrane Database of Systematic Reviews 2021;(1). [DOI: 10.1002/14651858.CD013837] [DOI] [PMC free article] [PubMed]
Mtitimila 2003
- Mtitimila EI, Cooke RW. Antibiotic regimens for suspected early neonatal sepsis. Cochrane Database of Systematic Reviews 2003, Issue 4. Art. No: CD004495. [DOI: 10.1002/14651858.CD004495] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mtitimila 2004
- Mtitimila EI, Cooke RW. Antibiotic regimens for suspected early neonatal sepsis. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No: CD004495. [DOI: 10.1002/14651858.CD004495.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


