Abstract
Purpose of Review
Oral contraceptive pill induced hypertension (OCPIH) and hypertensive disorders in pregnancy (HDP) share common risk factors and pathophysiological mechanisms, yet the bidirectional relationship between these two conditions is not well-established. We review and describe OCPIH and HDP to better understand how hormonal and metabolic imbalances affect hypertension.
Recent findings
Oral contraceptive pills continue to be a popular method of contraception, with an incidence of OCPIH ranging from 1%−8.5% among OCP users. HDP have an incidence of 5–10% of all pregnancies in the United States and have been shown to be a powerful predictor of lifetime adverse cardiovascular outcomes, including future hypertension. OCPIH and HDP share common risk factors such as age, BMI, past personal and family history of hypertension, as well as pathogenic mechanisms, including alterations in hormonal metabolism and the renin angiotensin aldosterone system; imbalance of vasodilator – vasoconstrictor compounds; and changes in the cardiovascular system.
Summary
Future research should address additional potential mechanisms that underlie hypertension in these two conditions where endocrine changes, either physiological (pregnancy) or iatrogenic (use of OCP), play a role. This may lead to novel, targeted treatment options to improve hypertension management and overall cardiovascular risk profile management in this subset of young female patients.
Keywords: hormone treatment, preeclampsia, gestational hypertension, drug induced hypertension
Introduction
The combined oral contraceptive pill (OCP) continues to be a popular method of contraception, with up to 80.5% of women in the United States claiming to have “ever used” the pill during their lifetimes as a contraceptive method [1]. The pill also gained popularity due its benefits beyond contraception, such as reducing symptoms of dysmenorrhea, menstrual flow regulation and control of premenstrual syndrome, and decreasing the signs of hyper-androgenism [2, 3]. One of the major adverse effects of OCP use is OCP-induced hypertension (OCPIH), which occurs in 1%−8.5% of OCP users [4–6].
Hypertensive disorders in pregnancy (HDP) have an incidence of 5– 10% of all pregnancies in the United States and are responsible for about 7% of pregnancy related deaths [7–10], as well as maternal complication-related infant deaths [11]. The most severe form of HDP, preeclampsia (PE), has an incidence of 3% among all pregnancies in the U.S. [12] and has been shown to be a powerful predictor of lifetime adverse cardiovascular outcomes [13, 14, 15 **, 10].
OCPIH and HDP share common risk factors and pathophysiological mechanisms, yet the bidirectional relationship between these two entities are not well-established. In this review, we will i) highlight the relationship between OCPs and HDP by reviewing clinical studies; ii) outline common risk factors and pathogenic mechanisms which may shed light on the potential predictive value of OCPIH in HDP; and iii) discuss treatment options that target the underlying pathophysiological mechanisms, thus potentially improving the management of OCPIH/HDP/PE. We further discuss the gaps in knowledge that need to be addressed in future research.
Oral contraceptive pill induced hypertension: review of evidence
When the OCP was discovered by Gregory Pincus, he foresaw the ovulation inhibition effects of19-nor steroids and the development of a relative pregnancy state [16]. It is therefore not surprising that there are many similarities between the effects of OCPs and pregnancy, both in terms of endocrine changes and complications such as hypertension. Research consistently has demonstrated that OCP use can cause an increase in blood pressure [17–22]. OCPs in susceptible individuals have been shown to increase systolic blood pressure by an average of 4mm Hg, diastolic blood pressure by 1 mm Hg [18], as well as increasing the mean arterial pressure [23]. Susceptible individuals displaying more exaggerated responses and malignant hypertension associated with OCP use have been reported [24–27]
OCPs are not recommended for patients with hypertension, including those with satisfactory blood pressure control. Similarly, they are not recommended for women with a history of hypertension during pregnancy [28, 29]. Different formulations of OCPs are available: monophasic preparations that have a fixed dose of estrogen and progesterone throughout the cycle; biphasic preparations, which have two different doses for one or both hormones that may vary during the cycle; and triphasic preparations in which hormone levels change during the course of three weeks [30–32]. OCPIH has been reported irrespective of the type of phasic preparation and doses of either estrogen or progesterone in OCPs.
Many studies of OCPIH were done using earlier preparations with high estrogen doses [5, 33]. However, more recent studies with low dose estrogen preparations also have shown a risk of developing high blood pressure [34 **, 35, 36], especially relative to the duration of use [34 **, 24, 37] and depending on the type of ‘phasic-combination.’ A meta-analysis demonstrated that the use of OCPs increases the risk of hypertension every 5 years by 13% [34 **]. The analysis based on the type of OCP showed that monophasic pill users demonstrated a hypertension relative risk (RR) of 2.3 (95% CI=1.7 to 3.0), biphasic pill users a RR of 1.7 (95% CI=1.2 to 2.4), and triphasic pill users a RR of 1.9 (95% CI=0.9 to 4.1) when compared to never users [35]. A study involving 1060 OCP users and 480 non users demonstrated the same effect, with higher blood pressure recordings for monophasic pill users compared to triphasic pill users [36]. This suggests that the hormonal balance between estrogen and progesterone, rather than the effect of a single hormone, influences the development of OCPIH.
There is conflicting evidence as to which dose of each particular hormone is responsible for the development of hypertension, whether from estrogen [24, 38] or progesterone [38, 35, 24, 39]. However, most studies agree that low doses have a lesser risk than the high dose estrogens [5, 35]. The risk of developing hypertension due to OCPs decreases with their discontinuation [39, 33, 35, 40], although some amount of risk persists, with a multivariate RR of 1.8 for current users and 1.2 for past users [35].
OCP use/OCPIH and HDP: a bidirectional correlation?
A majority of previous studies have demonstrated a positive correlation between having a previous history of HDP/ toxemia and OCPIH [24, 41, 42, 25, 43], with few studies reporting no correlation [18]. We found a paucity of data linking OCPs with future HDP, and no studies demonstrating an association between having a prior history of OCPIH and subsequent HDP. The following discussion presents current evidence, which is further summarized in Table 1.
Table 1.
Summary of Evidence for the Association between HDP, OCP Use and OCPIH
| Association | Supporting Studies | Studies not in Support |
|---|---|---|
| Khaw et al. [24] | ||
| Spellacy et al. [41] | ||
| HDP and subsequent OCPIH | Tsai et al. [25] | Fisch et al. [18] |
| Pritchard et al. [43] | ||
| Mason et al. [42] | ||
| OCP use and HDP | Thadhani et al. [37] | Croft et al. [44] |
| OCPIH and subsequent HDP | - | - |
HDP – hypertensive disorders of pregnancy, OCP – oral contraceptive pill, OCPIH – oral contraceptive pill induced hypertension.
Several studies have described the relationship between HDP and subsequent OCPIH. The development of diastolic hypertension while on OCPs was observed to be higher in those who had histories of HDP [41]. Similarly, those with prior histories of elevated blood pressures associated with OCPs, preeclampsia, or any history of hypertension without an identifiable cause/reason, were found to be at a higher risk of developing OCPIH [25]. These findings are consistent with that of Pritchard et al. who found that among 180 women following their first pregnancies complicated by HDP (including 26 with eclampsia), 5% developed OCPIH compared to 2.5% of 200 nulligravid women. This study further showed that among those who developed OCPIH, women who had HDP complicating their first pregnancies developed OCPIH within 3 months of initiation of OCPs, while none of the nulligravid women who developed OCPIH developed it within 3 months of starting OCPs, demonstrating an early onset in those who have had HDP. However, of those 180 women, some went on to develop HDP in subsequent pregnancies without developing OCPIH, suggesting a multifactorial pathophysiology [43].
Research regarding the development of HDP/preeclampsia in those with a history of OCPIH, however, is scarce. There are some studies showing an association between OCP use, without clear evidence of OCPIH, and subsequent HDP. Thadhani et al. demonstrated in a study of 3973 nulliparous women that users of OCPs of <2 years prior to pregnancy had a multivariate RR of 1.3 (95% CI, 0.8 –2.4) for preeclampsia. Subgroup analysis limited to nonsmokers demonstrated that OCP users for >8 years had a RR of 4.1 (95% CI, 1.9–8.7) for preeclampsia compared to never and past users of OCPs [37].
Contrary evidence is also available, such as the study of myocardial ischemia by Croft et al., which revealed that the risk of having later hypertension or toxemia in pregnancy was not altered by prior OCP use [44]. At present, additional studies are needed to elucidate the role of OCPIH as a potential risk factor for HDP, in general, and preeclampsia, in particular. New machine learning approaches may allow for further characterization of this association using large data sets.
OCPIH and HDP: Common Risk Factors
Both OCPIH and HDP share common risk factors, such as preexisting metabolic derangements (e.g. insulin resistance) and genetic predispositions (positive family history)[24, 45 *, 46, 47]. A summary of all the similarities between OCP use, OCPIH and preeclampsia are listed in Table 2. In the case of OCPIH, these preexisting conditions can be further aggravated by the inherent effects of OCPs, including alterations in lipid and carbohydrate metabolism, insulin resistance, hypertension and altered hemostasis [36, 37, 47, 48]. As normalization of metabolic derangements due to OCPs such as hypertension, dyslipidemia and insulin resistance takes time, adverse effects which stem from these metabolic derangements can be carried into pregnancy in recent OCP users [37, 42, 33, 49]. The net result may be an unfavorable cardiometabolic milieu that may increase the risk for HDP.
Table 2.
Summary Table of Shared Mechanisms and Clinical Similarities among OCP use, OPIH, and HDP
| OCP use | OCPIH | HDP | |
|---|---|---|---|
| Preeclampsia/Eclampsia | |||
| Risk factors | Not Applicable. | Age, BMI, past history of hypertension and family history of hypertension [18, 24]. | History of preeclampsia, chronic hypertension, pre-gestational diabetes mellitus, multiple gestations, pre-pregnancy BMI, APLS/ SLE, nulliparity, family history of preeclampsia [45 *, 46, 51, 50]. |
| Biological/Pathogenic mechanisms | The use of OCPs can lead to alterations in lipid and carbohydrate metabolism, insulin resistance, hypertension [36, 37, 47, 48]. | OCPIH reversible by Angiotensin converting enzyme inhibitors, but not by blocking sympathetic activity [76]. | Associated with dyslipidemia [138, 139]. |
| OCP use overrides and alters physiological estrogen/ progesterone balance [52]. | Imbalance of estrogen and progesterone: Compared to progesterone, higher estrogen levels in early pregnancy in those with preeclampsia [56–58]. | ||
| OCPs increase cortisol regardless of ACTH level. [71–73]. | Lower cortisol/cortisone ratio with a failure to demonstrate a downward trend with progression of gestation, as seen in normal pregnancy [64, 65, 67–70]. | ||
| Increase in glutathione peroxidase levels [16]. | Increase in glutathione peroxidase, while reduced levels in normal pregnancy [74]. | ||
| Increased levels of renin, angiotensinogen, angiotensin II, and aldosterone [21, 82–84, 87–89]. | Increased sensitivity to vasoconstrictor, Angiotensin II [97]. | ||
| Increased plasma renin activity [21]. | Lower plasma renin activity than normotensive pregnancy and those with chronic hypertension who subsequently develop superimposed preeclampsia [51, 91–93]. | ||
| Proteinuria [20, 95]. | Proteinuria [96]. | ||
| Renal vasoconstriction reversible by angiotensin converting enzyme inhibitors [89, 95]. | Increased AT1AA [98]. | ||
| Higher angiotensin II/angiotensin (1–7) ratio [21]. | Decrease in vasodilator, angiotensin 1–7 [107, 77]. | ||
| Inhibition of endometrial VEGF [115, 116]. | Neutralization of VEGF by high levels of sFlt-1 is seen in preeclamptic pregnancies [109–111]. | ||
| Animal studies demonstrate increases in CRP and uric acid levels [135]. | Increases in uric acid levels [136], CRP levels [137]. | ||
| Treatment | Not Applicable. | Discontinue OCPs [35, 39, 88, 146 *]. | Delivery [144 **]. |
| Long-term Outcomes | Contraindicated in those with increased cardiovascular risk including chronic hypertensives, and those with a history of HDP [28]. | Some continue to have HTN even with OCP discontinuation [33, 49]. | Increase the risk of OCPIH [24, 41, 42, 25]. |
| Increased risk of future cardiovascular diseases such as chronic hypertension, coronary artery disease and stroke [13, 15 **, 51, 153, 154]. | |||
| Increases risk of cerebral hemorrhage and coronary artery disease [146 *, 149–151]. | |||
OCP= oral contraceptive pill, OCPIH= oral contraceptive pill induced hypertension, HDP= hypertensive disorders of pregnancy, BMI= body mass index, APLS= antiphospholipid syndrome, SLE= systemic lupus erythematosus, AT1AA= angiotensin II type-1 receptor autoantibodies, AT1R= angiotensin II type 1 receptor, AT2R= angiotensin II type 2 receptor, VEGF= vascular endothelial growth factor, sFlt-1= soluble fms-like tyrosine kinase, CRP= C-reactive protein, HTN=hypertension
Similar to HDP, OCPIH is predisposed to by age, BMI, past personal and family history of hypertension [18, 24, 45 *, 46, 50, 51]. Family history is an important risk factor for both OCPIH and HDP, further supporting the role of genetic susceptibility.
Pathogenesis
Hormonal and Metabolic Alterations
The endocrinological effects of OCPs are that the exogenous estrogens and progesterone suppress endogenous hormone production enough to inhibit ovulation [52]. Changes in the metabolism of hormones, such as estrogens, progesterone, as well as cortisol, have been implicated in the development of HDP. Studies of hormonal levels in preeclampsia, however, have yielded contradictory evidence. While some studies have reported no change in estrogen levels [53, 54], others have reported reduced estrogen levels [55] or an increase in progesterone [53].
Furthermore, one study demonstrated that preeclamptic women have increased serum estradiol and increased urinary estradiol excretion of approximately 50% in early pregnancy compared to normal pregnancies [56]. This may be due to reduced hepatic blood flow and/or reduced estrogen metabolism [56–58]. Moreover, reduction in estrogen was disproportionate to that of progesterone [59], making it noteworthy that there is an imbalance in hormonal levels in preeclampsia vs. normotensive pregnancies [60].
Experiments have revealed that estrogen action also occurs through a G-protein coupled pathway found in trophoblastic cells, and that the expression of G-protein coupled estrogen receptor 1 is reduced in preeclamptic pregnancies relative to normotensive pregnancies [61]. Of note, this pathway is different from the traditionally known estrogen receptor-a (ERa) and estrogen receptor-b (ERb) pathways, which are operative in estrogen-responsive tissues [62]. This finding, along with reduced estradiol, suggests the role of endocrine dysregulation- albeit poorly studied - as one of the underlying mechanisms in the pathogenesis of preeclampsia [59, 61, 63].
In normal pregnancy, estrogens upregulate levels of the HSD11B2 enzyme, which converts active cortisol to cortisone, resulting in a decreased cortisol/cortisone ratio [64]. With progression of pregnancy, this ratio exhibits a downwards trend [65]. Preeclampsia is characterized by estrogen deficiency in placental tissue [59, 66], which in turn may down regulate the placental HSD11B2 enzyme, leading to increased placental cortisol [67–70]. In HDP and PE, the plasma cortisol/ cortisone ratio fails to demonstrate a physiological downward trend, further supporting abnormalities in cortisol metabolism [65].
OCP use has been shown to increase cortisol levels [71–73] regardless of the ACTH level [71]. This shift has been attributed to the estrogen mediated increase in corticosteroid-binding globulin production. The inhibition of HSD11B2 by hormones and its relationship to hypertension warrants further study.
Additional metabolic stress similarities of OCPs and pregnancy may result in increased need for antioxidant vitamins, such as vitamins C and E, and a higher production of lipid peroxides. Glutathione peroxidase activity is elevated in OCP users compared to non-users, as well as in preeclamptic compared to normotensive pregnancies [16, 74], further highlighting the metabolic stress similarities between OCP use and preeclampsia.
Renin – Angiotensin - Aldosterone System
Alterations in the renin-angiotensin-aldosterone system (RAAS) have been implicated in the pathogenesis of both OCPIH [75, 76] and HDP [77]. During an analysis of the sexual dimorphism of blood pressure, Bachman et al. highlighted the effects of estrogen on regulation of renin-angiotensin system related genes [78]. Several studies have demonstrated that the M235T allele of the angiotensinogen (AGT) gene results in an increased angiotensin concentration when treated with ethinyl-estradiol, and the same allele is also associated with preeclampsia and its severity [79–81].
OCP users have an increased angiotensinogen, plasma renin substrate [82], regardless of the renin concentration [83–85]. Despite the apparent compensatory suppression of renal renin release in individuals taking OCPs [84, 86], this decrease has not been shown to be capable of normalizing the RAAS abnormalities [87]. In addition, increased levels of angiotensinogen, angiotensin II, and aldosterone are also seen in OCP users [21, 84, 87–89, 9]. Moreover, exogenous estrogens have been found to be better stimulants of angiotensinogen than endogenous estrogens [90]. However, these changes were not found to affect electrolyte homeostasis, as urinary sodium and potassium excretion continued to remain the same [88].
Plasma renin activity is higher in OCP users compared to normotensive women during the follicular phase, but its activity in both groups is similar in the luteal phase [21]. While plasma renin activity was shown to be increased in women with OCPIH compared to women with essential hypertension, this study did not distinguish differences in plasma renin activity according to phase of the menstrual cycle [20]. An increased plasma renin substrate and activity is also seen in normotensive pregnancy, with higher levels compared to OCPs users [84]. Similarly, in HDP/preeclampsia, both de novo and superimposed on chronic hypertension, plasma renin activity is comparatively lower than in normotensive pregnancies [51, 91–93].
One of the possible mechanisms for hypertension that occurs in OCP users is reduced renal blood flow [94, 87]. Treatment with enalapril was shown to reduce the increase in plasma renin concentration, renin activity and angiotensin II that is due to ethinyl-estradiol [89]. The study conducted by Ahmed additionally revealed that non diabetic OCP users showed a greater renal vasodilatory response to captopril than non-diabetic nonusers, and that the vasodilatory effect was further apparent in diabetic OCP users [95]. This study also reported the development of macro-albuminuria in OCP users, while weight, HbA1c, smoking status and serum creatinine were not predictive of macro-albuminuria [95]. Albuminuria and increased renal vascular resistance in OCP users were also demonstrated by Ribstein et al. [20]. Ahmed et al. have speculated that the use of OCPs may trigger kidney injury in predisposed individuals [95]. Similarly, proteinuria is a well-known sign of kidney injury [96], as is angiotensin II mediated renal vasoconstriction in preeclampsia [97].
The Role of Angiotensin II Receptor type 1 Auto-antibodies
The identification of Angiotensin II type 1 receptor autoantibodies (AT1AA) led to the discovery of a new mechanistic pathway in the pathogenesis of preeclampsia [98]. AT1AA are produced due to placental ischemia [99, 77] and have been shown to reduce the invasiveness of trophoblast cells and to increase expression of the plasminogen activator inhibitor-1 (PAI-1) gene [100]. Mice injected with AT1AA containing sera had increased levels of soluble fms-like tyrosine kinase (sFlt)-1 [101], hypertension, proteinuria and placental abnormalities [102], and increased reactive oxygen species [103]. Studies have demonstrated that blocking the AT1 receptor in a rat model of preeclampsia improved preeclamptic symptoms such as hypertension, impaired angiogenesis, as well as preeclampsia associated vasoconstriction [104, 105]. AT1AA, furthermore, have been shown to enhance both renal angiotensin II vascular receptor sensitivity and vasoconstriction [97].
Despite the association of AT1AA with preeclampsia pathogenesis, the clinical significance of AT1AA remains unclear. AT1AA are present in normotensive pregnancies and may not always be present in preeclamptic pregnancies [99]. Even after delivery, alterations in the RAAS and levels of sFlt-1 and AT1AA remain comparatively elevated in those who had preeclamptic pregnancies compared to those with normotensive pregnancies. This may relate to the increased long term cardiovascular risks in these women [77] and their relatively early onset [10]. However, the role of AT1AA in OCPIH has not yet been studied. Future research addressing their role(s) in OCPIH can provide additional data regarding comparative findings of RAAS dysregulation between these two entities.
The Imbalance of Vasodilator – Vasoconstrictor Compounds
The RAAS has been shown to exert its effects primarily through angiotensin II, which is predominantly a vasoconstrictor, and angiotensin 1–7, which is a vasodilator. Despite the fact that estradiol has been shown to increase vasodilatory angiotensin 1–7 [17, 106], OCP users have higher angiotensin II/angiotensin (1–7) ratios, indicating that OCPs overriding effect is on the vasoconstrictor arm of the RAAS [21]. Studies similarly have shown that the hypertension seen in HDP/preeclampsia is due to the imbalance between angiotensin II and angiotensin 1–7 [107, 77].
An imbalance of pro-inflammatory (TNF-alpha, IL-6) and anti-inflammatory (IL-4, IL-10) cytokines [108], favoring the former, and resulting in systemic inflammation, has been implicated in HDP/PE. Hypertension in HDP/PE seems to be mediated by several pathways, including neutralization of angiogenic factors such as placental growth factor (PlGF) and vascular endothelial growth factor (VEGF) by sFlt-1 [109–111]; increased levels of the vasoconstrictor, endothelin-1 [91, 104]; and reduced vasodilatory nitric oxide (NO) [112], all of which have been demonstrated to contribute to vascular damage and systemic vasoconstriction in preeclampsia.
A study on the effect of OCPs on endothelin-1 did not show any correlation. However, this study did not find significant changes in blood pressure between OCP users and non-users [113]. The use of OCPs has been shown to result in reduced vasodilatory NO levels [114]. OCPs are also associated with inhibition of proangiogenic VEGF of endometrial origin [115, 116].
The hormonal effects on angiogenic balance and blood pressure have been demonstrated in preeclampsia. Treatment with the synthetic progesterone, 17-alpha-hydroxyprogesterone caproate, has been demonstrated to improve preeclampsia like symptoms in rat models [117, 118], thus raising the possibility that previously described elevations in progesterone in preeclampsia are compensatory, but likely insufficient. A study of rats with IL-6 induced hypertension, when supplemented with 17-alpha-hydroxyprogesterone caproate, demonstrated a reduction in AT1AA activity, reduced hypertension, and increased NO production [119]. These relatively higher progesterone levels could be a counterregulatory mechanism to reduce blood pressure in PE. Further studies of hormonal balance and specific vasoactive compounds may facilitate insight into the pathogenesis of preeclampsia.
Effects on Hemostasis
Alterations in hemostasis involving the procoagulant, anticoagulant, and fibrinolytic pathways due to OCPs and their relation to thrombogenicity have been well studied [120, 121]. The risk due to OCP seems to differ according to the hormonal combination in each preparation [121]. This risk of thrombosis is further enhanced in those with hereditary thrombophilia [122]. OCPs induce increases in pro-coagulant fibrinogen, prothrombin and factors VII, VIII and X, and result in changes in anti-coagulants, such as decreased antithrombin and tissue factor pathway inhibitor (TFPI), and increased fibrinolytic pathway factors such as tissue plasminogen activator and plasminogen. [123–129]. Pregnancy itself is a pro-coagulant condition with increases in factors VII, VIII, IX, X, XII, as well as a decrease in protein S [130, 131]. Compared to normal pregnancy, further hemostatic derangements are seen in preeclampsia [132, 133]. An increased risk of preeclampsia is also seen in those with thrombophilia [134]. The pro-coagulant state in preeclampsia is evident, with increases in fibrinogen and tissue factor, and decreases in antithrombin and the TFPI to tissue factor ratio [132]. The influence of hormonal imbalance on the development of hemostatic derangements may shed light on the pathogenicity of HDP/preeclampsia.
Other Cardiovascular Changes
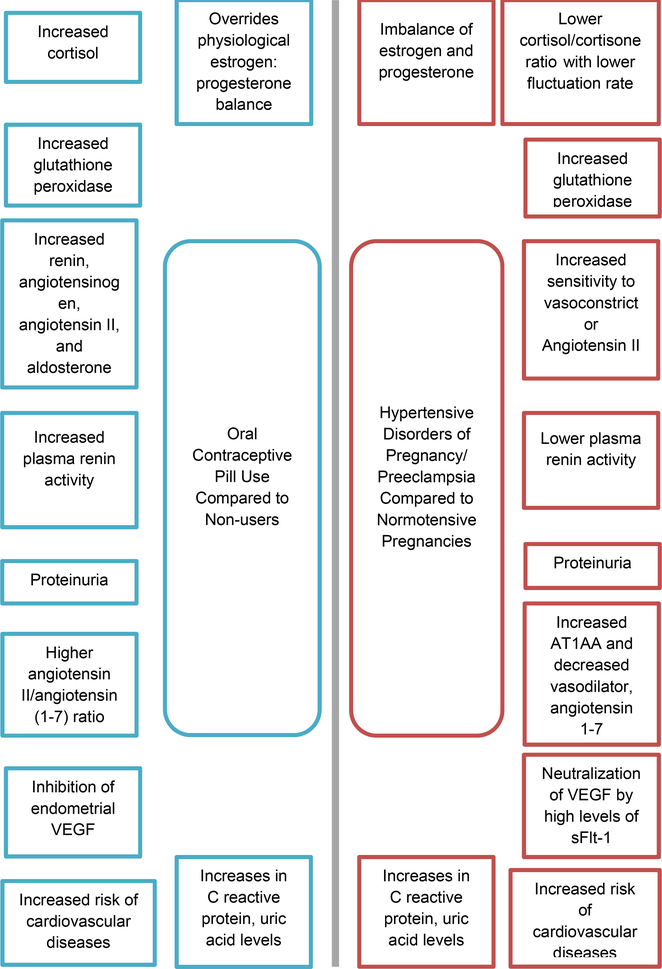
Studies have demonstrated that OCP treatment and NO deficiency display synergistic effects in increasing atherosclerotic cardiovascular (CV) risk. This phenomenon was studied in vivo in rats that were deficient in NO: when treated with combined oral contraceptives (COCs), they demonstrated increased levels of total cholesterol and low density lipoprotein cholesterol compared to the control groups, which included either COC untreated NO deficient rats and both COC-treated and untreated rats with a normal NO pathway. Furthermore, COC treatment significantly increased serum C-reactive protein and uric acid levels in rats compared to untreated controls [135]. Similarly, HDP have been associated with elevated uric acid levels [136], increased CRP [137], and dyslipidemia [138, 139]. Elevated uric acid levels have been associated with the development of cardiac hypertrophy [140] and cardiovascular disease mortality [141, 142 *], and have been identified as being associated with future cardiovascular disease [143]. Comparisons between the physiological effects of OCP use and HDP/preeclampsia are illustrated in figure 1.
Figure 1.
Comparison between Physiological Effects of OCP use (blue/on left) and HDP/Preeclampsia (red/on right). *
*Contains studies done in both humans and animal models
AT1AA= angiotensin II type-1 receptor autoantibodies, VEGF= vascular endothelial growth factor, sFlt-1= soluble fms-like tyrosine kinase-1.
Similarities in Treatment and Long-Term Outcomes
Treatment for HDP/preeclampsia mainly focuses on delivery, thereby reducing the underlying biochemical stressor [144 **]. Use of aspirin has been recommended for select patients for the prevention of HDP/preeclampsia [145]. The treatment for OCPIH similarly is to discontinue OCPs. Varying time frames [35, 39, 88] have been observed for the reversal of the increased blood pressure in OCPIH patients, and improvements in both systolic and diastolic blood pressures have been noted [146 *]. However, similar to some preeclamptic pregnancies [147], there was a susceptible group of individuals with OCPIH whose blood pressures remained elevated [33] and progressed to chronic hypertension, possibly prematurely triggered due to OCPs [148].
In multiple studies [44, 149, 150] and a meta- analysis [151] of OCP use, OCPIH [146 *, 152] and HDP [51, 153–155, 15 **, 13] have been associated with long term adverse cardiovascular effects, such as stroke and coronary artery disease. The increased risk of acute myocardial infarction (AMI) with OCP use was further increased in those with a past history of HDP compared to non-OCP users who had normotensive pregnancies [150]. This finding suggests that the adverse cardiovascular effects of OCPIH and HDP may be synergistic. Thus, OCP use is contraindicated in those with chronic hypertension, HDP, or those at risk of future adverse cardiovascular outcomes [29], and OCPs are less frequently used in women with increased cardiovascular risks [150].
Although alterations in the metabolism of lipids and carbohydrates, and increases in blood pressure and insulin resistance have been shown to reverse with cessation of OCP use [37, 17, 18], the durations of time taken for such reversals are not clear. It is therefore plausible that women who develop alterations in metabolism can carry those effects into pregnancy, or that women who have a tendency to develop such alterations in metabolism with OCP use are predisposed to adverse pregnancy outcomes such as HDP [37, 148, 33]. Although blood pressure returns to normal in both OCPIH and HDP after eliminating the stressor, the adverse cardiovascular effects triggered by them seem to persist, increasing the risk for future CVD [153–155, 15 **, 49, 150].
Conclusion and Future Direction
There are striking similarities in the risk factors, pathogenesis and outcomes of HDP and OCPIH. Most importantly, endocrine changes, either iatrogenic (OCP) or physiologic (pregnancy), result in hemodynamic and vascular changes, which, in susceptible individuals, lead to hypertension. The resultant hypertensive disorders, OPIH and HDP, respectively, share common underlying mechanisms and clinical features. Better understanding of the predictive value of OCPIH for HDP and other pregnancy complications is needed, as are the implications of both conditions for long term cardiovascular health in women. Finally, the presented data suggest that understanding the pathogenesis of HDP/preeclampsia would benefit from further studies of endocrine abnormalities that may offer new opportunities for targeted therapies. New approaches, such as machine learning techniques, are possible means by which to evaluate these mechanisms.
Acknowledgments
Declarations:
This review was supported by the National Institutes of Health under the award number: R01HL 136348 (V.D.G). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: None
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
The papers of particular interest, published recently, have been and highlight as:
• Of importance
•• Of major importance
- 1.CDC. Key Statistics from the National Survey of Family Growth - C Listing. https://www.cdc.gov/nchs/nsfg/key_statistics/c_2015-2017.htm.
- 2.Carey MS, Allen RH. Non-contraceptive uses and benefits of combined oral contraception. The Obstetrician & Gynaecologist. 2012;14(4):223–8. doi: 10.1111/j.1744-4667.2012.00126.x. [DOI] [Google Scholar]
- 3.Schindler AE. Non-contraceptive benefits of oral hormonal contraceptives. Int J Endocrinol Metab. 2013;11(1):41–7. doi: 10.5812/ijem.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs N, Düsterberg B, Weber-Diehl F, Mühe B. The effect on blood pressure of a monophasic oral contraceptive containing ethinylestradiol and gestodene. Contraception. 1995;51(6):335–9. doi: 10.1016/0010-7824(95)00097-t. [DOI] [PubMed] [Google Scholar]
- 5.Woods JW. Oral contraceptives and hypertension. Hypertension. 1988;11(3 Pt 2):Ii11–5. doi: 10.1161/01.hyp.11.3_pt_2.ii11. [DOI] [PubMed] [Google Scholar]
- 6.Perol S, Hugon-Rodin J, Plu-Bureau G. Hypertension and contraception. Presse Med. 2019;48(11 Pt 1):1269–83. doi: 10.1016/j.lpm.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related Mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130(2):366–73. doi: 10.1097/aog.0000000000002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen EE, Davis NL, Goodman D, Cox S, Mayes N, Johnston E et al. Vital Signs: Pregnancy-Related Deaths, United States, 2011–2015, and Strategies for Prevention, 13 States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423–9. doi: 10.15585/mmwr.mm6818e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. Pregnancy Mortality Surveillance System. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm. Accessed 01/23/2020.
- 10.Garovic VD, White WM, Vaughan L, Saiki M, Parashuram S, Garcia-Valencia O et al. Incidence and Long-Term Outcomes of Hypertensive Disorders of Pregnancy. J Am Coll Cardiol. 2020;75(18):2323–34. doi: 10.1016/j.jacc.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Easter SR, Bateman BT, Sweeney VH, Manganaro K, Lassey SC, Gagne JJ et al. A comorbidity-based screening tool to predict severe maternal morbidity at the time of delivery. Am J Obstet Gynecol. 2019;221(3):271.e1–.e10. doi: 10.1016/j.ajog.2019.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Garovic VD, August P. Preeclampsia and the future risk of hypertension: the pregnant evidence. Curr Hypertens Rep. 2013;15(2):114–21. doi: 10.1007/s11906-013-0329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Bmj. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Ciavatti M, Renaud S. Oxidative status and oral contraceptive. Its relevance to platelet abnormalities and cardiovascular risk. Free Radic Biol Med. 1991;10(5):325–38. doi: 10.1016/0891-5849(91)90039-6. [DOI] [PubMed] [Google Scholar]
- 17.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53(3):688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 18.Fisch IR, Frank J. Oral Contraceptives and Blood Pressure. JAMA. 1977;237(23):2499–503. doi: 10.1001/jama.1977.03270500051024. [DOI] [PubMed] [Google Scholar]
- 19.Hickson SS, Miles KL, McDonnell BJ, Yasmin, Cockcroft JR, Wilkinson IB et al. Use of the oral contraceptive pill is associated with increased large artery stiffness in young women: the ENIGMA study. J Hypertens. 2011;29(6):1155–9. doi: 10.1097/HJH.0b013e328346a5af. [DOI] [PubMed] [Google Scholar]
- 20.Ribstein J, Halimi JM, du Cailar G, Mimran A. Renal characteristics and effect of angiotensin suppression in oral contraceptive users. Hypertension (Dallas, Tex : 1979). 1999;33(1):90–5. doi: 10.1161/01.hyp.33.1.90. [DOI] [PubMed] [Google Scholar]
- 21.Davis GC, Gibson KJ, Casley D, Brown MA. Angiotensin II/Angiotensin (1–7) ratio and 24-h blood pressure throughout the menstrual cycle and in women using oral contraceptives. J Hypertens. 2017;35(6):1178–86. doi: 10.1097/HJH.0000000000001310. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro CCM, Shimo AKK, Lopes MHBdM, Lamas JLT. Effects of different hormonal contraceptives in women’s blood pressure values. Rev Bras Enferm. 2018;71(suppl 3):1453–9. doi: 10.1590/0034-7167-2017-0317. [DOI] [PubMed] [Google Scholar]
- 23.Harvey RE, Hart EC, Charkoudian N, Curry TB, Carter JR, Fu Q et al. Oral Contraceptive Use, Muscle Sympathetic Nerve Activity, and Systemic Hemodynamics in Young Women. Hypertension (Dallas, Tex : 1979). 2015;66(3):590–7. doi: 10.1161/HYPERTENSIONAHA.115.05179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khaw K-T, Peart WS. Blood pressure and contraceptive use. British Medical Journal (Clinical research ed). 1982;285(6339):403–7. doi: 10.1136/bmj.285.6339.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai CC, Williamson HO, Kirkland BH, Braun JO, Lam CF. Low-dose oral contraception and blood pressure in women with a past history of elevated blood pressure. American journal of obstetrics and gynecology. 1985;151(1):28–32. doi: 10.1016/0002-9378(85)90418-1. [DOI] [PubMed] [Google Scholar]
- 26.Dunn FG, Jones JV, Fife R. Malignant hypertension associated with use of oral contraceptives. Br Heart J. 1975;37(3):336–8. doi: 10.1136/hrt.37.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mir D, Ardabilygazir A, Afshariyamchlou S, Sachmechi I. Malignant Hypertension in Association with Low Estrogen Dose Oral Contraceptives: Case Report and Review of Literature. Cureus. 2018;10(7):e2978–e. doi: 10.7759/cureus.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medical eligibility criteria for contraceptive use. 4th Edition ed. World Health Oraganization; 2009. [PubMed] [Google Scholar]
- 29.Classifications for Combined Hormonal Contraceptives. https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/appendixd.html. Accessed 01/23/2020.
- 30.Van Vliet H, Grimes D, Helmerhorst F, Schulz K. Biphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2001(4):Cd002032. doi: 10.1002/14651858.Cd002032. [DOI] [PubMed] [Google Scholar]
- 31.Van Vliet HA, Grimes DA, Helmerhorst FM, Schulz KF. Biphasic versus triphasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006(3):Cd003283. doi: 10.1002/14651858.CD003283.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Vliet HA, Grimes DA, Lopez LM, Schulz KF, Helmerhorst FM. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2011;2011(11):Cd003553. doi: 10.1002/14651858.CD003553.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weir RJ. Effect on blood pressure or changing from high to low dose steroid preparations in women with oral contraceptive induced hypertension. Scott Med J. 1982;27(3):212–5. doi: 10.1177/003693308202700303. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Yao J, Wang W, Zhang D. Association between duration of oral contraceptive use and risk of hypertension: A meta-analysis. J Clin Hypertens (Greenwich). 2017;19(10):1032–41. doi: 10.1111/jch.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chasan-Taber L, Willett WC, Manson JE, Spiegelman D, Hunter DJ, Curhan G et al. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation. 1996;94(3):483–9. doi: 10.1161/01.cir.94.3.483. [DOI] [PubMed] [Google Scholar]
- 36.Godsland IF, Crook D, Devenport M, Wynn V. Relationships between blood pressure, oral contraceptive use and metabolic risk markers for cardiovascular disease. Contraception. 1995;52(3):143–9. doi: 10.1016/0010-7824(95)00153-2. [DOI] [PubMed] [Google Scholar]
- 37.Thadhani R, Stampfer MJ, Chasan-Taber L, Willett WC, Curhan GC. A prospective study of pregravid oral contraceptive use and risk of hypertensive disorders of pregnancy. Contraception. 1999;60(3):145–50. doi: 10.1016/s0010-7824(99)00079-7. [DOI] [PubMed] [Google Scholar]
- 38.Glisic M, Shahzad S, Tsoli S, Chadni M, Asllanaj E, Rojas LZ et al. Association between progestin-only contraceptive use and cardiometabolic outcomes: A systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25(10):1042–52. doi: 10.1177/2047487318774847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weir RJ, Briggs E, Mack A, Naismith L, Taylor L, Wilson E. Blood Pressure in Women Taking Oral Contraceptives. Br Med J. 1974;1(5907):533–5. doi: 10.1136/bmj.1.5907.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beral V, Hermon C, Kay C, Hannaford P, Darby S, Reeves G. Mortality associated with oral contraceptive use: 25 year follow up of cohort of 46 000 women from Royal College of General Practitioners’ oral contraception study. BMJ. 1999;318(7176):96–100. doi: 10.1136/bmj.318.7176.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spellacy WN, Birk SA. The effect of intrauterine devices, oral contraceptives, estrogens, and progestogens on blood pressure. Am J Obstet Gynecol. 1972;112(7):912–9. doi: 10.1016/0002-9378(72)90811-3. [DOI] [PubMed] [Google Scholar]
- 42.Mason B, Oakley N, Wynn V. Studies of carbohydrate and lipid metabolism in women developing hypertension on oral contraceptives. Br Med J. 1973;3(5875):317–20. doi: 10.1136/bmj.3.5875.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pritchard JA, Pritchard SA. Blood pressure response to estrogen-progestin oral contraceptive after pregnancy-induced hypertension. Am J Obstet Gynecol. 1977;129(7):733–9. doi: 10.1016/0002-9378(77)90390-8. [DOI] [PubMed] [Google Scholar]
- 44.Croft P, Hannaford PC. Risk factors for acute myocardial infarction in women: evidence from the Royal College of General Practitioners’ oral contraception study. Bmj. 1989;298(6667):165–8. doi: 10.1136/bmj.298.6667.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. Bmj. 2016;353:i1753. doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cincotta RB, Brennecke SP. Family history of pre-eclampsia as a predictor for pre-eclampsia in primigravidas. Int J Gynaecol Obstet. 1998;60(1):23–7. doi: 10.1016/s0020-7292(97)00241-5. [DOI] [PubMed] [Google Scholar]
- 47.Crook D, Godsland I. Safety evaluation of modern oral contraceptives. Effects on lipoprotein and carbohydrate metabolism. Contraception. 1998;57(3):189–201. doi: 10.1016/s0010-7824(98)00018-3. [DOI] [PubMed] [Google Scholar]
- 48.Godsland IF, Crook D, Simpson R, Proudler T, Felton C, Lees B et al. The effects of different formulations of oral contraceptive agents on lipid and carbohydrate metabolism. N Engl J Med. 1990;323(20):1375–81. doi: 10.1056/nejm199011153232003. [DOI] [PubMed] [Google Scholar]
- 49.Mulatero P, Morra di Cella S, Veglio F. Hypertension, genotype and oral contraceptives. Pharmacogenomics. 2002;3(1):57–63. doi: 10.1517/14622416.3.1.57. [DOI] [PubMed] [Google Scholar]
- 50.Eskenazi B, Fenster L, Sidney S. A multivariate analysis of risk factors for preeclampsia. JAMA. 1991;266(2):237–41. [PubMed] [Google Scholar]
- 51.Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res. 2019;124(7):1094–112. doi: 10.1161/circresaha.118.313276. [DOI] [PubMed] [Google Scholar]
- 52.Gaspard UJ, Romus MA, Gillain D, Duvivier J, Demey-Ponsart E, Franchimont P. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception. 1983;27(6):577–90. doi: 10.1016/0010-7824(83)90023-9. [DOI] [PubMed] [Google Scholar]
- 53.Tamimi R, Lagiou P, Vatten LJ, Mucci L, Trichopoulos D, Hellerstein S et al. Pregnancy hormones, pre-eclampsia, and implications for breast cancer risk in the offspring. Cancer Epidemiol Biomarkers Prev. 2003;12(7):647–50. [PubMed] [Google Scholar]
- 54.Pecks U, Rath W, Kleine-Eggebrecht N, Maass N, Voigt F, Goecke TW et al. Maternal Serum Lipid, Estradiol, and Progesterone Levels in Pregnancy, and the Impact of Placental and Hepatic Pathologies. Geburtshilfe Frauenheilkd. 2016;76(7):799–808. doi: 10.1055/s-0042-107078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hertig A, Liere P, Chabbert-Buffet N, Fort J, Pianos A, Eychenne B et al. Steroid profiling in preeclamptic women: Evidence for aromatase deficiency. American Journal of Obstetrics and Gynecology. 2010;203(5):477.e1–.e9. doi: 10.1016/j.ajog.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 56.Cantonwine DE, McElrath TF, Trabert B, Xu X, Sampson J, Roberts JM et al. Estrogen metabolism pathways in preeclampsia and normal pregnancy. Steroids. 2019;144:8–14. doi: 10.1016/j.steroids.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosing U, Carlström K. Serum Levels of Unconjugated and Total Oestrogens and Dehydroepiandrosterone, Progesterone and Urinary Oestriol Excretion in Pre-Eclampsia. Gynecol Obstet Invest. 1984;18(4):199–205. doi: 10.1159/000299081. [DOI] [PubMed] [Google Scholar]
- 58.Oosterhof H, Voorhoeve PG, Aarnoudse JG. Enhancement of hepatic artery resistance to blood flow in preeclampsia in presence or absence of HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets). Am J Obstet Gynecol. 1994;171(2):526–30. doi: 10.1016/0002-9378(94)90293-3. [DOI] [PubMed] [Google Scholar]
- 59.Wan J, Hu Z, Zeng K, Yin Y, Zhao M, Chen M et al. The reduction in circulating levels of estrogen and progesterone in women with preeclampsia. Pregnancy Hypertens. 2018;11:18–25. doi: 10.1016/j.preghy.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Berkane N, Liere P, Lefevre G, Alfaidy N, Nahed RA, Vincent J et al. Abnormal steroidogenesis and aromatase activity in preeclampsia. Placenta. 2018;69:40–9. doi: 10.1016/j.placenta.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Tong C, Feng X, Chen J, Qi X, Zhou L, Shi S et al. G protein-coupled receptor 30 regulates trophoblast invasion and its deficiency is associated with preeclampsia. J Hypertens. 2016;34(4):710–8. doi: 10.1097/HJH.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 62.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715–26. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyer MR, Prossnitz ER, Barton M. The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function. Vascul Pharmacol. 2011;55(1–3):17–25. doi: 10.1016/j.vph.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995;16(5):608–48. doi: 10.1210/edrv-16-5-608. [DOI] [PubMed] [Google Scholar]
- 65.Kosicka K, Siemiątkowska A, Krzyścin M, Bręborowicz GH, Resztak M, Majchrzak-Celińska A et al. Glucocorticoid Metabolism in Hypertensive Disorders of Pregnancy: Analysis of Plasma and Urinary Cortisol and Cortisone. PLoS One. 2015;10(12):e0144343–e. doi: 10.1371/journal.pone.0144343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin YY, Jeong JS, Park MN, Lee JE, An SM, Cho WS et al. Regulation of steroid hormones in the placenta and serum of women with preeclampsia. Mol Med Rep. 2018;17(2):2681–8. doi: 10.3892/mmr.2017.8165. [DOI] [PubMed] [Google Scholar]
- 67.Berkane N, Liere P, Oudinet J-P, Hertig A, Lefèvre G, Pluchino N et al. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr Rev. 2017;38(2):123–44. doi: 10.1210/er.2016-1065. [DOI] [PubMed] [Google Scholar]
- 68.He P, Chen Z, Sun Q, Li Y, Gu H, Ni X. Reduced expression of 11β-hydroxysteroid dehydrogenase type 2 in preeclamptic placentas is associated with decreased PPARγ but increased PPARα expression. Endocrinology. 2014;155(1):299–309. doi: 10.1210/en.2013-1350. [DOI] [PubMed] [Google Scholar]
- 69.Quinkler M, Stewart PM. Hypertension and the cortisol-cortisone shuttle. J Clin Endocrinol Metab. 2003;88(6):2384–92. doi: 10.1210/jc.2003-030138. [DOI] [PubMed] [Google Scholar]
- 70.Aufdenblatten M, Baumann M, Raio L, Dick B, Frey BM, Schneider H et al. Prematurity Is Related to High Placental Cortisol in Preeclampsia. Pediatric Research. 2009;65(2):198–202. doi: 10.1203/PDR.0b013e31818d6c24. [DOI] [PubMed] [Google Scholar]
- 71.Carr BR, Parker CR Jr., Madden JD, MacDonald PC, Porter JC. Plasma levels of adrenocorticotropin and cortisol in women receiving oral contraceptive steroid treatment. J Clin Endocrinol Metab. 1979;49(3):346–9. doi: 10.1210/jcem-49-3-346. [DOI] [PubMed] [Google Scholar]
- 72.Roche DJO, King AC, Cohoon AJ, Lovallo WR. Hormonal contraceptive use diminishes salivary cortisol response to psychosocial stress and naltrexone in healthy women. Pharmacol Biochem Behav. 2013;109:84–90. doi: 10.1016/j.pbb.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Cauter E, Golstein J, Vanhaelst L, Leclercq R. Effects of oral contraceptive therapy on the circadian patterns of cortisol and thyrotropin (TSH). European journal of clinical investigation. 1975;5(2):115–21. doi: 10.1111/j.1365-2362.1975.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 74.Taravati A, Tohidi F. Comprehensive analysis of oxidative stress markers and antioxidants status in preeclampsia. Taiwan J Obstet Gynecol. 2018;57(6):779–90. doi: 10.1016/j.tjog.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Olatunji LA, Seok Y-M, Igunnu A, Kang S-H, Kim I-K. Combined oral contraceptive-induced hypertension is accompanied by endothelial dysfunction and upregulated intrarenal angiotensin II type 1 receptor gene expression. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(11):1147–57. doi: 10.1007/s00210-016-1272-0. [DOI] [PubMed] [Google Scholar]
- 76.Olatunji LA, Soladoye AO. Oral contraceptive-induced high blood pressure is prevented by renin-angiotensin suppression in female rats but not by sympathetic nervous system blockade. Indian J Exp Biol. 2008;46(11):749–54. [PubMed] [Google Scholar]
- 77.J Spaan J, A Brown M. Renin-angiotensin system in pre-eclampsia: everything old is new again. Obstet Med. 2012;5(4):147–53. doi: 10.1258/om.2012.120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bachmann J, Feldmer M, Ganten U, Stock G, Ganten D. Sexual dimorphism of blood pressure: possible role of the renin-angiotensin system. J Steroid Biochem Mol Biol. 1991;40(4–6):511–5. doi: 10.1016/0960-0760(91)90270-f. [DOI] [PubMed] [Google Scholar]
- 79.Ward K, Hata A, Jeunemaitre X, Helin C, Nelson L, Namikawa C et al. A molecular variant of angiotensinogen associated with preeclampsia. Nat Genet. 1993;4(1):59–61. doi: 10.1038/ng0593-59. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H, Li Y-X, Peng W-J, Li Z-W, Zhang C-H, Di H-H et al. The Gene Variants of Maternal/Fetal Renin-Angiotensin System in Preeclampsia: A Hybrid Case-Parent/Mother-Control Study. Sci Rep. 2017;7(1):5087-. doi: 10.1038/s41598-017-05411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zitouni H, Ben Ali Gannoum M, Raguema N, Maleh W, Zouari I, Faleh RE et al. Contribution of angiotensinogen M235T and T174M gene variants and haplotypes to preeclampsia and its severity in (North African) Tunisians. J Renin Angiotensin Aldosterone Syst. 2018;19(1):1470320317753924-. doi: 10.1177/1470320317753924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goldhaber SZ, Hennekens CH, Spark RF, Evans DA, Rosner B, Taylor JO et al. Plasma renin substrate, renin activity, and aldosterone levels in a sample of oral contraceptive users from a community survey. Am Heart J. 1984;107(1):119–22. doi: 10.1016/0002-8703(84)90144-3. [DOI] [PubMed] [Google Scholar]
- 83.Low J, Oparil S. Oral contraceptive pill hypertension. J Reprod Med. 1975;15(5):201–8. [PubMed] [Google Scholar]
- 84.Derkx FH, Steunkel C, Schalekamp MP, Visser W, Huisveld IH, Schalekamp MA. Immunoreactive renin, prorenin, and enzymatically active renin in plasma during pregnancy and in women taking oral contraceptives. J Clin Endocrinol Metab. 1986;63(4):1008–15. doi: 10.1210/jcem-63-4-1008. [DOI] [PubMed] [Google Scholar]
- 85.Newton MA, Sealey JE, Ledingham JGG, Laragh JH. High blood pressure and oral contraceptives: Changes in plasma renin and renin substrate and in aldosterone excretion. American Journal of Obstetrics and Gynecology. 1968;101(8):1037–45. doi: 10.1016/0002-9378(68)90345-1. [DOI] [PubMed] [Google Scholar]
- 86.Laragh JH, Baer L, Brunner HR, Buhler FR, Sealey JE, Vaughan ED Jr. Renin, angiotensin and aldosterone system in pathogenesis and management of hypertensive vascular disease. Am J Med. 1972;52(5):633–52. doi: 10.1016/0002-9343(72)90054-x. [DOI] [PubMed] [Google Scholar]
- 87.Kang AK, Duncan JA, Cattran DC, Floras JS, Lai V, Scholey JW et al. Effect of oral contraceptives on the renin angiotensin system and renal function. American journal of physiology Regulatory, integrative and comparative physiology. 2001;280(3):R807–R13. doi: 10.1152/ajpregu.2001.280.3.R807. [DOI] [PubMed] [Google Scholar]
- 88.McAreavey D, Cumming AM, Boddy K, Brown JJ, Fraser R, Leckie BJ et al. The renin-angiotensin system and total body sodium and potassium in hypertensive women taking oestrogen-progestagen oral contraceptives. Clin Endocrinol (Oxf). 1983;18(2):111–8. doi: 10.1111/j.1365-2265.1983.tb03192.x. [DOI] [PubMed] [Google Scholar]
- 89.Byrne KB, Geraghty DP, Stewart BJ, Burcher E. Effect of contraceptive steroid and enalapril treatment of systolic blood pressure and plasma renin-angiotensin in the rat. Clin Exp Hypertens. 1994;16(5):627–57. doi: 10.3109/10641969409067966. [DOI] [PubMed] [Google Scholar]
- 90.Gordon MS, Chin WW, Shupnik MA. Regulation of angiotensinogen gene expression by estrogen. J Hypertens. 1992;10(4):361–6. doi: 10.1097/00004872-199204000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Saleh L, Verdonk K, Visser W, van den Meiracker AH, Danser AHJ. The emerging role of endothelin-1 in the pathogenesis of pre-eclampsia. Ther Adv Cardiovasc Dis. 2016;10(5):282–93. doi: 10.1177/1753944715624853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verdonk K, Saleh L, Lankhorst S, Smilde JEI, van Ingen MM, Garrelds IM et al. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension (Dallas, Tex : 1979). 2015;65(6):1316–23. doi: 10.1161/HYPERTENSIONAHA.115.05267. [DOI] [PubMed] [Google Scholar]
- 93.Malha L, Sison CP, Helseth G, Sealey JE, August P. Renin-Angiotensin-Aldosterone Profiles in Pregnant Women With Chronic Hypertension. Hypertension. 2018;72(2):417–24. doi:doi: 10.1161/HYPERTENSIONAHA.118.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hollenberg NK, Williams GH, Burger B, Chenitz W, Hoosmand I, Adams DF. Renal blood flow and its response to angiotensin II. An interaction between oral contraceptive agents, sodium intake, and the renin-angiotensin system in healthy young women. Circulation research. 1976;38(1):35–40. doi: 10.1161/01.res.38.1.35. [DOI] [PubMed] [Google Scholar]
- 95.Ahmed SB, Hovind P, Parving H-H, Rossing P, Price DA, Laffel LM et al. Oral Contraceptives, Angiotensin-Dependent Renal Vasoconstriction, and Risk of Diabetic Nephropathy. Diabetes Care. 2005;28(8):1988–94. doi: 10.2337/diacare.28.8.1988. [DOI] [PubMed] [Google Scholar]
- 96.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstetrics and gynecology. 2013;122(5):1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 97.Cunningham MW Jr., Williams JM, Amaral L, Usry N, Wallukat G, Dechend R et al. Agonistic Autoantibodies to the Angiotensin II Type 1 Receptor Enhance Angiotensin II-Induced Renal Vascular Sensitivity and Reduce Renal Function During Pregnancy. Hypertension. 2016;68(5):1308–13. doi: 10.1161/hypertensionaha.116.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–52. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walther T, Wallukat G, Jank A, Bartel S, Schultheiss H-P, Faber R et al. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension (Dallas, Tex : 1979). 2005;46(6):1275–9. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
- 100.Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig. 2003;10(2):82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 101.Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G et al. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens. 2010;23(8):911–6. doi: 10.1038/ajh.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14(8):855–62. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dechend R, Viedt C, Müller DN, Ugele B, Brandes RP, Wallukat G et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107(12):1632–9. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 104.Campbell N, LaMarca B, Cunningham MW Jr. The Role of Agonistic Autoantibodies to the Angiotensin II Type 1 Receptor (AT1-AA) in Pathophysiology of Preeclampsia. Curr Pharm Biotechnol. 2018;19(10):781–5. doi: 10.2174/1389201019666180925121254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murphy SR, Cockrell K. Regulation of soluble fms-like tyrosine kinase-1 production in response to placental ischemia/hypoxia: role of angiotensin II. Physiol Rep. 2015;3(2). doi: 10.14814/phy2.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brosnihan KB, Li P, Ganten D, Ferrario CM. Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. Am J Physiol. 1997;273(6):R1908–15. doi: 10.1152/ajpregu.1997.273.6.R1908. [DOI] [PubMed] [Google Scholar]
- 107.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) in normal and preeclamptic pregnancy. Endocrine. 2002;18(3):239–45. doi: 10.1385/ENDO:18:3:239. [DOI] [PubMed] [Google Scholar]
- 108.Aggarwal R, Jain AK, Mittal P, Kohli M, Jawanjal P, Rath G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J Clin Lab Anal. 2019;33(4):e22834–e. doi: 10.1002/jcla.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parchem JG, Kanasaki K, Kanasaki M, Sugimoto H, Xie L, Hamano Y et al. Loss of placental growth factor ameliorates maternal hypertension and preeclampsia in mice. J Clin Invest. 2018;128(11):5008–17. doi: 10.1172/jci99026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McGinnis R, Steinthorsdottir V, Williams NO, Thorleifsson G, Shooter S, Hjartardottir S et al. Variants in the fetal genome near FLT1 are associated with risk of preeclampsia. Nat Genet. 2017;49(8):1255–60. doi: 10.1038/ng.3895. [DOI] [PubMed] [Google Scholar]
- 111.Maynard SE, Min J-Y, Merchan J, Lim K-H, Li J, Mondal S et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsubara K, Higaki T, Matsubara Y, Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int J Mol Sci. 2015;16(3):4600–14. doi: 10.3390/ijms16034600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wiegratz I, Kutschera E, Lee JH, Moore C, Mellinger U, Winkler UH et al. Effect of four oral contraceptives on thyroid hormones, adrenal and blood pressure parameters. Contraception. 2003;67(5):361–6. doi: 10.1016/s0010-7824(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 114.Fallah S, Nouroozi V, Seifi M, Samadikuchaksaraei A, Aghdashi EM. Influence of oral contraceptive pills on homocysteine and nitric oxide levels: as risk factors for cardiovascular disease. J Clin Lab Anal. 2012;26(2):120–3. doi: 10.1002/jcla.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maia H Jr., Casoy J, Pimentel K, Correia T, Athayde C, Cruz T et al. Effect of oral contraceptives on vascular endothelial growth factor, Cox-2 and aromatase expression in the endometrium of uteri affected by myomas and associated pathologies. Contraception. 2008;78(6):479–85. doi: 10.1016/j.contraception.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 116.Macpherson AM, Archer DF, Leslie S, Charnock-Jones DS, Makkink WK, Smith SK. The effect of etonogestrel on VEGF, oestrogen and progesterone receptor immunoreactivity and endothelial cell number in human endometrium. Hum Reprod. 1999;14(12):3080–7. doi: 10.1093/humrep/14.12.3080. [DOI] [PubMed] [Google Scholar]
- 117.Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin JN Jr., LaMarca B. 17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension (Dallas, Tex : 1979). 2015;65(1):225–31. doi: 10.1161/HYPERTENSIONAHA.114.04484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Amaral LM, Cottrell JN, Comley KM, Cunningham MW, Witcher A, Vaka VR et al. 17-Hydroxyprogesterone caproate improves hypertension and renal endothelin-1 in response to sFlt-1 induced hypertension in pregnant rats. Pregnancy Hypertens. 2020;22:151–5. doi: 10.1016/j.preghy.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Amaral LM, Kiprono L, Cornelius DC, Shoemaker C, Wallace K, Moseley J et al. Progesterone supplementation attenuates hypertension and the autoantibody to the angiotensin II type I receptor in response to elevated interleukin-6 during pregnancy. American journal of obstetrics and gynecology. 2014;211(2):158.e1–.e1586. doi: 10.1016/j.ajog.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dragoman MV, Tepper NK, Fu R, Curtis KM, Chou R, Gaffield ME. A systematic review and meta-analysis of venous thrombosis risk among users of combined oral contraception. Int J Gynaecol Obstet. 2018;141(3):287–94. doi: 10.1002/ijgo.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stegeman BH, de Bastos M, Rosendaal FR, van Hylckama Vlieg A, Helmerhorst FM, Stijnen T et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. Bmj. 2013;347:f5298. doi: 10.1136/bmj.f5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Vlijmen EF, Wiewel-Verschueren S, Monster TB, Meijer K. Combined oral contraceptives, thrombophilia and the risk of venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2016;14(7):1393–403. doi: 10.1111/jth.13349. [DOI] [PubMed] [Google Scholar]
- 123.Kemmeren JM, Algra A, Meijers JC, Bouma BN, Grobbee DE. Effects of second and third generation oral contraceptives and their respective progestagens on the coagulation system in the absence or presence of the factor V Leiden mutation. Thromb Haemost. 2002;87(2):199–205. [PubMed] [Google Scholar]
- 124.Sidelmann JJ, Kluft C, Krug AH, Winkler U, Jespersen J, Gram JB. Fibrin clot structure - pro-fibrinolytic effect of oral contraceptives in apparently healthy women. Thromb Haemost. 2017;117(4):700–5. doi: 10.1160/th16-10-0748. [DOI] [PubMed] [Google Scholar]
- 125.Middeldorp S, Meijers JC, van den Ende AE, van Enk A, Bouma BN, Tans G et al. Effects on coagulation of levonorgestrel- and desogestrel-containing low dose oral contraceptives: a cross-over study. Thromb Haemost. 2000;84(1):4–8. [PubMed] [Google Scholar]
- 126.Sandset PM. Mechanisms of hormonal therapy related thrombosis. Thromb Res. 2013;131 Suppl 1:S4–7. doi: 10.1016/s0049-3848(13)70009-4. [DOI] [PubMed] [Google Scholar]
- 127.Harris GM, Stendt CL, Vollenhoven BJ, Gan TE, Tipping PG. Decreased plasma tissue factor pathway inhibitor in women taking combined oral contraceptives. Am J Hematol. 1999;60(3):175–80. doi:. [DOI] [PubMed] [Google Scholar]
- 128.Meijers JC, Middeldorp S, Tekelenburg W, van den Ende AE, Tans G, Prins MH et al. Increased fibrinolytic activity during use of oral contraceptives is counteracted by an enhanced factor XI-independent down regulation of fibrinolysis: a randomized cross-over study of two low-dose oral contraceptives. Thromb Haemost. 2000;84(1):9–14. [PubMed] [Google Scholar]
- 129.Gialeraki A, Valsami S, Pittaras T, Panayiotakopoulos G, Politou M. Oral Contraceptives and HRT Risk of Thrombosis. Clin Appl Thromb Hemost. 2018;24(2):217–25. doi: 10.1177/1076029616683802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bremme KA. Haemostatic changes in pregnancy. Best Practice & Research Clinical Haematology. 2003;16(2):153–68. doi: 10.1016/S1521-6926(03)00021-5. [DOI] [PubMed] [Google Scholar]
- 131.Katz D, Beilin Y. Disorders of coagulation in pregnancy. BJA: British Journal of Anaesthesia. 2015;115(suppl_2):ii75–ii88. doi: 10.1093/bja/aev374. [DOI] [PubMed] [Google Scholar]
- 132.Ismail SK, Higgins JR. Hemostasis in pre-eclampsia. Semin Thromb Hemost. 2011;37(2):111–7. doi: 10.1055/s-0030-1270336. [DOI] [PubMed] [Google Scholar]
- 133.Han L, Liu X, Li H, Zou J, Yang Z, Han J et al. Blood coagulation parameters and platelet indices: changes in normal and preeclamptic pregnancies and predictive values for preeclampsia. PLoS One. 2014;9(12):e114488–e. doi: 10.1371/journal.pone.0114488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Robertson L, Wu O, Langhorne P, Twaddle S, Clark P, Lowe GD et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 2006;132(2):171–96. doi: 10.1111/j.1365-2141.2005.05847.x. [DOI] [PubMed] [Google Scholar]
- 135.Olatunji LA, Olaniyi KS, Usman TO, Abolarinwa BA, Achile CJ, Kim I-K. Combined oral contraceptive and nitric oxide synthesis inhibition synergistically causes cardiac hypertrophy and exacerbates insulin resistance in female rats. Environ Toxicol Pharmacol. 2017;52:54–61. doi: 10.1016/j.etap.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 136.Pasyar S, Wilson LM, Pudwell J, Peng YP, Smith GN. Investigating the diagnostic capacity of uric acid in the occurrence of preeclampsia. Pregnancy Hypertens. 2019;19:106–11. doi: 10.1016/j.preghy.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 137.Correa N, Arbildi P, Rosano S, López V, Rodríguez-Camejo C, Colistro V et al. Predictive value of blood measurement of Complement System proteins and metabolic components for early detection of obstetric complications linked to poor placental function. Placenta. 2020;101:45–8. doi: 10.1016/j.placenta.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 138.Jin WY, Lin SL, Hou RL, Chen XY, Han T, Jin Y et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth. 2016;16:60. doi: 10.1186/s12884-016-0852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Serrano NC, Guio-Mahecha E, Quintero-Lesmes DC, Becerra-Bayona S, Paez MC, Beltran M et al. Lipid profile, plasma apolipoproteins, and pre-eclampsia risk in the GenPE case-control study. Atherosclerosis. 2018;276:189–94. doi: 10.1016/j.atherosclerosis.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 140.Cuspidi C, Facchetti R, Bombelli M, Sala C, Tadic M, Grassi G et al. Uric Acid and New Onset Left Ventricular Hypertrophy: Findings From the PAMELA Population. Am J Hypertens. 2017;30(3):279–85. doi: 10.1093/ajh/hpw159. [DOI] [PubMed] [Google Scholar]
- 141.Zhang W, Iso H, Murakami Y, Miura K, Nagai M, Sugiyama D et al. Serum Uric Acid and Mortality Form Cardiovascular Disease: EPOCH-JAPAN Study. J Atheroscler Thromb. 2016;23(6):692–703. doi: 10.5551/jat.31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rahimi-Sakak F, Maroofi M, Rahmani J, Bellissimo N, Hekmatdoost A. Serum uric acid and risk of cardiovascular mortality: a systematic review and dose-response meta-analysis of cohort studies of over a million participants. BMC Cardiovasc Disord. 2019;19(1):218. doi: 10.1186/s12872-019-1215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fonseca FA, Izar MC. High-Sensitivity C-Reactive Protein and Cardiovascular Disease Across Countries and Ethnicities. Clinics (Sao Paulo). 2016;71(4):235–42. doi: 10.6061/clinics/2016(04)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstetrics & Gynecology. 2020;135(6):e237–e60. doi: 10.1097/aog.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 145.ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstet Gynecol. 2018;132(1):e44–e52. doi: 10.1097/aog.0000000000002708. [DOI] [PubMed] [Google Scholar]
- 146.Lubianca JN, Moreira LB, Gus M, Fuchs FD. Stopping oral contraceptives: an effective blood pressure-lowering intervention in women with hypertension. J Hum Hypertens. 2005;19(6):451–5. doi: 10.1038/sj.jhh.1001841. [DOI] [PubMed] [Google Scholar]
- 147.Amougou SN, Mbita SMM, Danwe D, Tebeu PM. Factor associated with progression to chronic arterial hypertension in women with preeclampsia in Yaoundé, Cameroon. Pan Afr Med J. 2019;33:200. doi: 10.11604/pamj.2019.33.200.16857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mulatero P, Rabbia F, di Cella SM, Schiavone D, Plazzotta C, Pascoe L et al. Angiotensin-converting enzyme and angiotensinogen gene polymorphisms are non-randomly distributed in oral contraceptive-induced hypertension. J Hypertens. 2001;19(4):713–9. doi: 10.1097/00004872-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 149.Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1996;348(9026):498–505. [PubMed] [Google Scholar]
- 150.Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997;349(9060):1202–9. [PubMed] [Google Scholar]
- 151.Khader YS, Rice J, John L, Abueita O. Oral contraceptives use and the risk of myocardial infarction: a meta-analysis. Contraception. 2003;68(1):11–7. doi: 10.1016/s0010-7824(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 152.Prentice RL. On the ability of blood pressure effects to explain the relation between oral contraceptives and cardiovascular disease. Am J Epidemiol. 1988;127(2):213–9. doi: 10.1093/oxfordjournals.aje.a114797. [DOI] [PubMed] [Google Scholar]
- 153.Newstead J, von Dadelszen P, Magee LA. Preeclampsia and future cardiovascular risk. Expert Rev Cardiovasc Ther. 2007;5(2):283–94. doi: 10.1586/14779072.5.2.283. [DOI] [PubMed] [Google Scholar]
- 154.Brouwers L, van der Meiden-van Roest AJ, Savelkoul C, Vogelvang TE, Lely AT, Franx A et al. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG. 2018;125(13):1642–54. doi: 10.1111/1471-0528.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Berends AL, de Groot CJM, Sijbrands EJ, Sie MPS, Benneheij SH, Pal R et al. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension (Dallas, Tex : 1979). 2008;51(4):1034–41. doi: 10.1161/HYPERTENSIONAHA.107.101873. [DOI] [PubMed] [Google Scholar]