Abstract
BACKGROUND
Population-based estimates of the risk of breast cancer associated with germline pathogenic variants in cancer-predisposition genes are critically needed for risk assessment and management in women with inherited pathogenic variants.
METHODS
In a population-based case–control study, we performed sequencing using a custom multigene amplicon-based panel to identify germline pathogenic variants in 28 cancer-predisposition genes among 32,247 women with breast cancer (case patients) and 32,544 unaffected women (controls) from population-based studies in the Cancer Risk Estimates Related to Susceptibility (CARRIERS) consortium. Associations between pathogenic variants in each gene and the risk of breast cancer were assessed.
RESULTS
Pathogenic variants in 12 established breast cancer–predisposition genes were detected in 5.03% of case patients and in 1.63% of controls. Pathogenic variants in BRCA1 and BRCA2 were associated with a high risk of breast cancer, with odds ratios of 7.62 (95% confidence interval [CI], 5.33 to 11.27) and 5.23 (95% CI, 4.09 to 6.77), respectively. Pathogenic variants in PALB2 were associated with a moderate risk (odds ratio, 3.83; 95% CI, 2.68 to 5.63). Pathogenic variants in BARD1, RAD51C, and RAD51D were associated with increased risks of estrogen receptor–negative breast cancer and triple-negative breast cancer, whereas pathogenic variants in ATM, CDH1, and CHEK2 were associated with an increased risk of estrogen receptor–positive breast cancer. Pathogenic variants in 16 candidate breast cancer–predisposition genes, including the c.657_661del5 founder pathogenic variant in NBN, were not associated with an increased risk of breast cancer.
CONCLUSIONS
This study provides estimates of the prevalence and risk of breast cancer associated with pathogenic variants in known breast cancer–predisposition genes in the U.S. population. These estimates can inform cancer testing and screening and improve clinical management strategies for women in the general population with inherited pathogenic variants in these genes. (Funded by the National Institutes of Health and the Breast Cancer Research Foundation.)
Germline Pathogenic Variants in cancer-predisposition genes included in hereditary cancer multigene testing panels have been associated with an increased risk of breast cancer.1–4 Identification of pathogenic variants in predisposition genes has provided benefit through improving access to risk-reducing prophylactic surgery and targeted therapies among carriers of pathogenic variants in BRCA1 and BRCA2 and access to enhanced mammography and magnetic resonance imaging (MRI)– based screening among carriers of pathogenic variants in several established breast cancer–predisposition genes.5,6 The aggregate prevalence of pathogenic variants in these genes has been estimated at 7 to 10% among women with breast cancer.1,7–10 However, these prevalences and associated risks of breast cancer are based on high-risk populations enriched with women who had a family history of breast and ovarian cancers, received a breast cancer diagnosis at a young age, had estrogen receptor (ER)–negative tumors, or had founder mutations. Only studies of limited size have evaluated pathogenic variants in multigene panels in women with breast cancer unselected for family history or age at diagnosis.11,12 Thus, current risk estimates of breast cancer with respect to predisposition genes have uncertain application to the general population.13
Genetic testing recommendations have been developed to provide guidance on the selection of women for multigene panel testing. The U.S. Preventive Services Task Force has suggested that the selection of unaffected women for testing be based on risk stratification.14 The National Comprehensive Cancer Network has also suggested that risk stratification be used in the selection of unaffected and affected women for testing.15 In contrast, the American Society of Breast Surgeons has recommended that germline genetic testing for hereditary cancer be performed in all women with breast cancer. Separately, population-based screening for BRCA1 and BRCA2 in all women older than 30 years of age has been proposed.16 However, large-scale population-based studies that provide estimates of the prevalence of pathogenic variants in predisposition genes in the general population are lacking.
Cancer Risk Estimates Related to Susceptibility (CARRIERS) is a United States–based consortium consisting of population-based and family-based studies of breast cancer. Here, we used the population-based studies in the CARRIERS consortium to estimate the prevalence and risk of breast cancer associated with pathogenic variants in breast cancer–predisposition genes in the U.S. general population.
METHODS
STUDY POPULATION
The CARRIERS consortium includes 17 studies — 7 nested case–control studies in prospective cohorts, 2 case–cohort studies in prospective cohorts, 3 case–control studies, and 5 case–control o rcase–cohort studies enriched with women with early-onset disease or a family history of breast cancer (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). The characteristics of the 39,553 women with breast cancer (case patients) and 35,867 study-matched unaffected women (controls) are provided in Table S2. Population-based estimates were derived from 32,247 case patients and 32,544 controls from the 12 studies in the CARRIERS consortium that were not enriched with patients with a family history or early onset of disease (the Black Women’s Health Study, the Cancer Prevention Study II, the Cancer Prevention Study 3, the California Teachers’ Study, the Mayo Clinic Breast 3 Cancer Study, the Multiethnic Cohort Study, the Mayo Mammography Health Study, the Nurses’ Health Study, the Nurses’ Health Study II, the Women’s Circle of Health Study, the Women’s Health Initiative, and the Wisconsin Women’s Health Study). All participants provided informed consent for research. The CARRIERS study was approved by the institutional review board at the Mayo Clinic.
DNA SEQUENCING AND BIOINFORMATICS ANALYSIS
Germline DNA samples were subjected to dual bar-coded QIAseq (Qiagen) multiplex amplicon-based analysis of 746 target regions in 37 cancer-predisposition genes.17 Libraries from 768 samples 6 were pooled and sequenced in each lane of a HiSeq 4000 system (Illumina). Genetic variants were identified with the use of the Genome Analysis Toolkit (GATK) Haplotype Caller tool and Var-Dict variant caller tool. High-quality sequence data (read depth of >20 times) were obtained for 99.3% of the target regions. Twenty-eight cancer predisposition genes including 12 established breast cancer–predisposition genes (ATM, BARD1, BRCA1, BRCA2, CDH1, CHEK2, NF1, PALB2, PTEN, RAD51C, RAD51D, and TP53) and 16 candidate predisposition genes were evaluated (Table S3).18–30 Loss-of-function variants and variants identified as “pathogenic” or “likely pathogenic” in the ClinVar database were classified as pathogenic variants (see the Supplementary Appendix).17 Pathogenic variants in NF1 and TP53 were restricted to those with an alternate allele fraction (calculated as the number of alternate allele reads divided by the total number of reads at a specific genomic position) between 0.3 and 0.7 in an effort to exclude potential clonal hematopoiesis variants.31
STATISTICAL ANALYSIS
Prevalences of pathogenic variants and variants of uncertain significance in each gene were tabulated for the case patients and controls in the population-based CARRIERS analysis, and 95% confidence intervals were estimated with the use of the Wilson score method without continuity correction. A generalized additive model for pathogenic-variant status and a smoothing spline function for age32 were used to estimate the relationship between the prevalence of a pathogenic variant and age. Associations between pathogenic variants in each gene and the risk of breast cancer were assessed by means of logistic regression, with adjustment for study, age, first-degree family history of breast cancer, and race or ethnic group. Sensitivity analyses were conducted to assess the effect of each of the 12 studies with the use of a leave-one-study-out cross-validation approach. Comparisons between unaffected controls and women with ER-positive cancer, women with ER-negative cancer, and women with triple-negative breast cancer (ER-negative, progesterone receptor [PR]– negative, and human epidermal growth factor receptor type 2 [HER2]–negative) were conducted with the use of binomial logistic-regression models. All analyses were performed with R software (version 3.5.2), and all tests were two-sided. Lifetime absolute risk of breast cancer to age 85 years was estimated for pathogenic-variant carriers by combining age-specific odds ratio estimates with age-specific breast cancer incidence rates from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (Supplementary Appendix).
RESULTS
PARTICIPANT CHARACTERISTICS
The distributions of age at diagnosis for 39,553 case patients and age at the time of selection into the study for 35,867 controls from the 17 studies in the CARRIERS consortium are shown in Figure S1. In the 12 population-based studies, the mean age at the time of breast cancer diagnosis among 32,247 case patients was 62.1 years, and the mean age at the time of enrollment among 32,544 controls was 61.2 years, ages that are similar to those derived from the SEER 18 registries (Table 1 and Table S4). A family history of breast cancer was reported in 20.4% of case patients and 14.3% of controls (Table 1). Among the case patients with available data on tumor biomarkers, 82.9% had ER-positive breast cancer and 11.3% had triple-negative breast cancer (Table 1), prevalences that are consistent with those derived from the SEER 18 registries. Data on HER2 status were available for only 41.1% of tumors.
Table 1.
Demographic and Clinical Characteristics of the Participants in the Population-Based CARRIERS Analysis.*
| Variable | Case Patients (N = 32,247) | Controls (N = 32,544) |
|---|---|---|
| Demographic characteristic | ||
| Age† | ||
| Mean — yr | 62.07±11.44 | 61.22±11.82 |
| Range — yr | 21.00–94.00 | 21.80–94.30 |
| Distribution — no./total no. (%) | ||
| ≤40 yr | 1,099/31,708 (3.5) | 1,599/32,542 (4.9) |
| 41–50 yr | 4,197/31,708 (13.2) | 4,443/32,542 (13.7) |
| 51–60 yr | 7,999/31,708 (25.2) | 8,580/32,542 (26.4) |
| 61–70 yr | 10,357/31,708 (32.7) | 10,095/32,542 (31.0) |
| >70 yr | 8,056/31,708 (25.4) | 7,825/32,542 (24.0) |
| Race or ethnic group — no./total no. (%)‡ | ||
| Asian | 1,282/32,068 (4.0) | 1,269/32,498 (3.9) |
| Non-Hispanic Black | 3,946/32,068 (12.3) | 4,954/32,498 (15.2) |
| Hispanic | 1,019/32,068 (3.2) | 998/32,498 (3.1) |
| Non-Hispanic White | 25,287/32,068 (78.9) | 24,770/32,498 (76.2) |
| Other | 534/32,068 (1.7) | 507/32,498 (1.6) |
| Participants in included study — no./total no. (%) | ||
| BWHS | 1,437/32,247 (4.5) | 2,896/32,544 (8.9) |
| CPSII | 4,037/32,247 (12.5) | 3,935/32,544 (12.1) |
| CPS3 | 1,537/32,247 (4.8) | 1,729/32,544 (5.3) |
| CTS | 2,226/32,247 (6.9) | 2,134/32,544 (6.6) |
| MCBCS | 4,517/32,247 (14.0) | 3,249/32,544 (10.0) |
| MEC | 3,641/32,247 (11.3) | 3,689/32,544 (11.3) |
| MMHS | 291/32,247 (0.9) | 1,257/32,544 (3.9) |
| NHS | 2,088/32,247 (6.5) | 2,420/32,544 (7.4) |
| NHSII | 935/32,247 (2.9) | 1,391/32,544 (4.3) |
| WCHS | 2,215/32,247 (6.9) | 1,705/32,544 (5.2) |
| WHI | 4,994/32,247 (15.5) | 4,535/32,544 (13.9) |
| WWHS | 4,329/32,247 (13.4) | 3,604/32,544 (11.1) |
| Family history of breast cancer — no./total no. (%)§ | ||
| No | 24,873/31,234 (79.6) | 27,016/31,527 (85.7) |
| Yes | 6,361/31,234 (20.4) | 4,511/31,527 (14.3) |
| Family history of ovarian cancer — no./total no. (%)§ | ||
| No | 27,494/28,534 (96.4) | 28,002/28,949 (96.7) |
| Yes | 1,040/28,534 (3.6) | 947/28,949 (3.3) |
| Clinical characteristic | ||
| Tumor behavior — no./total no. (%) | ||
| In situ | 4,446/31,221 (14.2) | NA |
| Invasive | 26,775/31,221 (85.8) | NA |
| Estrogen-receptor status — no./total no. (%) | ||
| Negative | 3,805/22,233 (17.1) | NA |
| Positive | 18,428/22,233 (82.9) | NA |
| Progesterone-receptor status — no./total no. (%) | ||
| Negative | 6,186/21,643 (28.6) | NA |
| Positive | 15,457/21,643 (71.4) | NA |
| HER2 status — no./total no. (%) | ||
| Negative | 11,077/13,252 (83.6) | NA |
| Positive | 2,175/13,252 (16.4) | NA |
| Triple-negative breast cancer — no./total no. (%) | ||
| No | 11,452/12,915 (88.7) | NA |
| Yes | 1,463/12,915 (11.3) | NA |
Plus-minus values are means ±SD. Percentages may not total 100 because of rounding. The population-based Cancer Risk Estimates Related to Susceptibility (CARRIERS) analysis included data from the Black Women’s Health Study (BWHS), the Cancer Prevention Study II (CPSII), the Cancer Prevention Study 3 (CPS3), the California Teachers’ Study (CTS), the Mayo Clinic Breast Cancer Study (MCBCS), the Multiethnic Cohort Study (MEC), the Mayo Mammography Health Study (MMHS), the Nurses’ Health Study (NHS), the Nurses’ Health Study II (NHSII), the Women’s Circle of Health Study (WCHS), the Women’s Health Initiative (WHI), and the Wisconsin Women’s Health Study (WWHS). NA denotes not applicable and HER2 human epidermal growth factor receptor 2.
Age at the time of breast cancer diagnosis was used for case patients, and age at the time of selection into the study was used for controls. Data on age were missing for 539 case patients and for 2 controls.
Race or ethnic group was reported by the participant.
Family history was restricted to first-degree relatives.
PREVALENCE OF PATHOGENIC VARIANTS IN PREDISPOSITION GENES
In the overall CARRIERS analysis that included data from all 17 studies, the prevalence of pathogenic variants in 12 established breast cancer– predisposition genes (ATM, BARD1, BRCA1, BRCA2, CDH1, CHEK2, NF1, PALB2, PTEN, RAD51C, RAD51D, and TP53) was 5.67% (95% confidence interval [CI], 5.44 to 5.90) among case patients and 1.73% (95% CI, 1.60 to 1.87) among controls (Tables S5 and S6). However, in the population-based CARRIERS analysis, the prevalence was 5.03% (95% CI, 4.79 to 5.27) among cases patients and 1.63% (95% CI, 1.50 to 1.78) among controls (Table 2). The prevalence of pathogenic variants was similar among non-Hispanic White, non-Hispanic Black, and Hispanic case patients and controls (Table S7). A lower overall prevalence of pathogenic variants was detected among Asian American case patients (1.64%; 95% CI, 1.07 to 2.49) (Table S7). Among the case patients, the highest prevalence of pathogenic variants was observed for BRCA2 (1.29%; 95% CI, 1.18 to 1.42), CHEK2 (1.08%; 95% CI, 0.98 to 1.20), and BRCA1 (0.85%; 95% CI, 0.76 to 0.96) (Table 2). BRCA1 carriers had a relatively young mean (±SD) age at diagnosis (50.9±13.3 years among those with ER-positive status and 50.3±12.4 among those with ER-negative status), whereas BRCA2 carriers had a slightly older mean age at diagnosis (55.4±12.8 years among those with ER-positive status and 58.6±12.2 among those with ER-negative status) (Table S8). The prevalence of variants of uncertain significance in the 12 established breast cancer genes was 18.9% (95% CI, 18.5 to 19.4) among case patients and 18.5% (95% CI, 18.1 to 19.0) among controls (Table S9).
Table 2.
Associations between Pathogenic Variants in Established Breast Cancer–Predisposition Genes and Risk of Breast Cancer.*
| Breast Cancer–Predisposition Gene1,2,7 | Case Patients (N = 32,247) | Controls (N = 32,544) | Odds Ratio (95% CI)† | P Value |
|---|---|---|---|---|
| no. with pathogenic variant (%) | ||||
| ATM | 253 (0.78) | 134 (0.41) | 1.82 (1.46–2.27) | <0.001 |
| BARD1 | 49 (0.15) | 35 (0.11) | 1.37 (0.87–2.16) | 0.18 |
| BRCA1 | 275 (0.85) | 37 (0.11) | 7.62 (5.33–11.27) | <0.001 |
| BRCA2 | 417 (1.29) | 78 (0.24) | 5.23 (4.09–6.77) | <0.001 |
| CDH1 | 17 (0.05) | 6 (0.02) | 2.50 (1.01–7.07) | 0.06 |
| CHEK2 | 349 (1.08) | 138 (0.42) | 2.47 (2.02–3.05) | <0.001 |
| NF1‡ | 19 (0.06) | 11 (0.03) | 1.93 (0.91–4.31) | 0.09 |
| PALB2 | 148 (0.46) | 38 (0.12) | 3.83 (2.68–5.63) | <0.001 |
| PTEN | 8 (0.02) | 3 (0.01) | NA | NA |
| RAD51C | 41 (0.13) | 35 (0.11) | 1.20 (0.75–1.93) | 0.44 |
| RAD51D | 26 (0.08) | 14 (0.04) | 1.72 (0.88–3.51) | 0.12 |
| TP53‡ | 19 (0.06) | 2 (0.01) | NA | NA |
| Total | 1621 (5.03) | 531 (1.63) | — | — |
The studies in the CARRIERS consortium that were included in this population-based analysis were BWHS, CPSII, CPS3, CTS, MCBCS, MEC, MMHS, NHS, NHSII, WCHS, WHI, and WWHS. NA denotes not applicable (too few events [<5] to calculate a stable odds ratio).
Odds ratio estimates for any breast cancer were adjusted for study, age, family history of breast cancer, and race or ethnic group.
Pathogenic variants in NF1 and TP53 were restricted to those with an alternate allele fraction (calculated as the number of alternate allele reads divided by the total number of reads at a specific genomic position) between 0.3 and 0.7.
PATHOGENIC VARIANTS IN PREDISPOSITION GENES AND BREAST CANCER RISK
Case–control association analyses, with adjustment for study, age, family history of breast cancer, and race or ethnic group, were performed with data from all 17 studies in the CARRIERS consortium and with data from the 12 population-based studies in the CARRIERS consortium (Table 2 and Table S5 and S6). In the population-based studies, BRCA1 and BRCA2 were associated with a high risk of breast cancer, with odds ratios of 7.62 (95% CI, 5.33 to 11.27) and 5.23 (95% CI, 4.09 to 6.77), respectively (Table 2 and Table S10). Pathogenic variants in PALB2 and CHEK2 were associated with a moderate risk, with odds ratios of 3.83 (95% CI, 2.68 to 5.63) and 2.47 (95% CI, 2.02 to 3.05), respectively. The common CHEK2 pathogenic variants p.Ile157Thr and p.Ser428Phe had limited clinical importance (i.e., odds ratio, <1.5), with odds ratios of 1.30 (95% CI, 1.06 to 1.59; P=0.01) and 1.26 (95% CI, 0.76 to 2.12; P=0.37), respectively, and were excluded from the analyses. Pathogenic variants in ATM and NF1 were associated with an increased risk of breast cancer, with odds ratios of 1.82 (95% CI, 1.46 to 2.27) and 1.93 (95% CI, 0.91 to 4.31), respectively. Pathogenic variants in BARD1, RAD51C, and RAD51D were associated with a moderate risk of ER-negative breast cancer and triple-negative breast cancer but not ER-positive breast cancer, whereas pathogenic variants in ATM, CDH1, and CHEK2 were associated only with ER-positive breast cancer (Table 3). Limited numbers of women with pathogenic variants in PTEN and TP53 did not allow us to assess associations with breast cancer (Tables 2 and 3). Sensitivity analyses verified that individual studies did not influence associations with breast cancer risk (Fig. S2).
Table 3.
Associations between Pathogenic Variants in Established Breast Cancer–Predisposition Genes and Risk of Breast Cancer According to Estrogen-Receptor and Triple-Negative Breast Cancer Status of Tumors.*
| Breast Cancer–Predisposition Gene | ER-Positive Breast Cancer (N = 18,428) | ER-Negative Breast Cancer (N = 3805) | Triple-Negative Breast Cancer (N = 1463) | |||
|---|---|---|---|---|---|---|
| Participants with Pathogenic Variant | Odds Ratio (95% CI)† | Participants with Pathogenic Variant | Odds Ratio (95% CI)† | Participants with Pathogenic Variant | Odds Ratio (95% CI)† | |
| no. (%) | no. (%) | no. (%) | ||||
| ATM | 151 (0.82) | 1.96 (1.52–2.53) | 19 (0.50) | 1.04 (0.59–1.72) | 5 (0.34) | 0.50 (0.12–1.36) |
| BARDI | 20 (0.11) | 0.91 (0.49–1.64) | 11 (0.29) | 2.52 (1.18–5.00) | 6 (0.41) | 3.18 (1.16–7.42) |
| BRCA1 | 73 (0.40) | 3.39 (2.17–5.45) | 114 (3.00) | 26.33 (17.28–41.52) | 65 (4.44) | 42.88 (26.56–71.25) |
| BRCA2 | 201 (1.09) | 4.66 (3.52–6.23) | 82 (2.16) | 8.89 (6.36–12.47) | 30 (2.05) | 9.70 (5.97–15.47) |
| CDH1 | 13 (0.07) | 3.37 (1.24–10.72) | 3 (0.08) | NA | 1 (0.07) | NA |
| CHEK2 | 205 (1.11) | 2.60 (2.05–3.31) | 20 (0.53) | 1.40 (0.83–2.25) | 8 (0.55) | 1.63 (0.72–3.20) |
| NF1‡ | 10 (0.05) | 1.63 (0.65–4.03) | 2 (0.05) | NA | 1 (0.07) | NA |
| PALB2 | 64 (0.35) | 3.13 (2.02–4.96) | 42 (1.10) | 9.22 (5.63–15.25) | 24 (1.64) | 13.03 (7.08–23.75) |
| PTEN | 3 (0.02) | NA | 0 | NA | 0 | NA |
| RAD51C | 16 (0.09) | 0.83 (0.44–1.54) | 9 (0.24) | 2.19 (0.97–4.49) | 4 (0.27) | NA |
| RAD51D | 13 (0.07) | 1.61 (0.71–3.70) | 7 (0.18) | 3.93 (1.40–10.29) | 1 (0.07) | NA |
| TP53‡ | 9 (0.05) | NA | 2 (0.05) | NA | 2 (0.14) | NA |
The studies in the CARRIERS consortium that were included in this population-based analysis were BWHS, CPSII, CPS3, CTS, MCBCS, MEC, MMHS, NHS, NHSII, WCHS, WHI, and WWHS. ER denotes estrogen-receptor.
Odds ratio estimates for any breast cancer were adjusted for study, age, family history of breast cancer, and race or ethnic group.
Pathogenic variants in NF1 and TP53 were restricted to those with an alternate allele fraction between 0.3 and 0.7.
None of the 16 candidate predisposition genes, including the mismatch repair genes (MLH1, MSH2, and MSH6), were significantly associated with increased risk of breast cancer in analyses involving the participants overall (Table S10) or the participants stratified according to ER status (Table S11) (P>0.05 for all). An increased risk of breast cancer was not observed among the participants with any pathogenic variant in NBN (odds ratio, 1.05; 95% CI, 0.71 to 1.56) or among those with the NBN pathogenic variant c.657_661del5 (odds ratio, 0.93; 95% CI, 0.52 to 1.68), which was previously associated with breast cancer, or those with c.657_661del5 who were homozygous for the GG allele of the c.553 (Table S10).33,34
To investigate the influence of a family history of breast cancer on associations with breast cancer risk in the general population, analyses were conducted separately for the pathogenic-variant carriers with (20.4%) or without (79.6%) a first-degree relative with breast cancer. Among the participants with a family history, pathogenic variants in BRCA1, BRCA2, CDH1, and PALB2 were associated with a high risk of breast cancer (odds ratio, >4) and pathogenic variants in ATM and RAD51D were associated with a moderate risk (odds ratio, >2) (Table S12). The influence of age at breast cancer diagnosis on associations was also evaluated.35 With respect to the pathogenic variants in the 12 established predisposition genes, the associations with breast cancer risk were unchanged among the participants who received a breast cancer diagnosis at an age of 50 years or younger, except among those with pathogenic variants in BRCA1 or BRCA2, who were at an increased risk (Table S13). Among the participants who received a diagnosis of breast cancer at an age of more than 50 years, the associations with breast cancer observed for pathogenic variants in most genes were similar to those among the participants overall (Table S14). To assess the influence of older age at enrollment, associations with breast cancer were evaluated in the studies in which women younger than 45 years of age were eligible for enrollment; the results did not differ from those of the population-based CARRIERS analysis (Table S15).
LIFETIME ABSOLUTE RISK OF BREAST CANCER
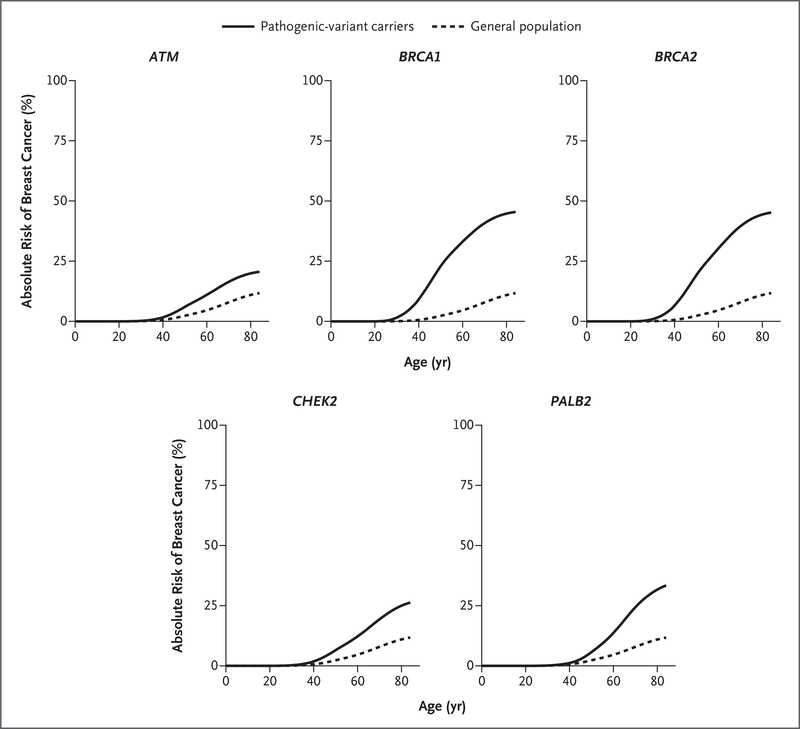
The prevalences of pathogenic variants in the commonly mutated genes ATM, BRCA1, BRCA2, CHEK2, and PALB2 were assessed among the case patients according to age at diagnosis and among the controls according to age at the time of selection into the study (Fig. S3). The prevalence of pathogenic variants in BRCA1 and BRCA2 among case patients decreased rapidly after age 40 years, whereas a constant and limited decline in the prevalence of pathogenic variants in ATM, CHEK2, and PALB2 was observed among the case patients 40 to 85 years of age (Fig. S3). Pathogenic variants in ATM, BRCA1, BRCA2, CHEK2, and PALB2 were associated with lifetime absolute risk of breast cancer of greater than 20% by age 85 years among non-Hispanic Whites; pathogenic variants in BRCA1 or BRCA2 yielded a lifetime risk of approximately 50%, and in PALB2, a lifetime risk of approximately 32% (Fig. 1).
Figure 1. Population-Based Lifetime Absolute Risk of Breast Cancer Development According to Age and the Commonly Mutated Genes ATM, BRCA1, BRCA2, CHEK2, and PALB2.
The Cancer Risk Estimates Related to Susceptibility (CARRIERS) consortium studies that were included in the analysis of the absolute risk of breast cancer among pathogenic-variant carriers were the Cancer Prevention Study II, the Cancer Prevention Study 3, the California Teachers’ Study, the Mayo Clinic Breast Cancer Study, the Multiethnic Cohort Study, the Mayo Mammography Health Study, the Nurses’ Health Study, the Nurses’ Health Study II, the Women’s Circle of Health Study, the Women’s Health Initiative, and the Wisconsin Women’s Health Study. The analysis in the general population was performed with the use of age-specific breast cancer incidence data (restricted to non-Hispanic Whites) from the Surveillance, Epidemiology, and End Results 21 registries.
DISCUSSION
Here, we report the prevalence of pathogenic variants in breast cancer–predisposition genes among 32,247 women with breast cancer and 32,544 study-matched unaffected women from U.S. population-based studies in the CARRIERS consortium and provide estimates of breast cancer risk with respect to these pathogenic variants in the general population. On the basis of the American Cancer Society estimate of 276,000 new diagnoses of breast cancer in the United States in 2020, the population-based CARRIERS analysis suggests that at least 13,800 (approximately 5%) will occur in women with germline pathogenic variants in predisposition genes. However, many of these women are not known to have underlying genetic susceptibility to breast cancer.
Currently, there are differing recommendations for the selection of patients with breast cancer for clinical genetic testing, with considerable controversy regarding which patients to test and which genes to include in the testing process.15,36–38 The National Comprehensive Cancer Network and the National Institute for Health and Care Excellence provide criteria for the selection of women with breast cancer, ovarian cancer, or both for testing on the basis of personal and family history of these and other cancers.15,36–38 In contrast, the American Society of Breast Surgeons has suggested offering testing to all patients with breast cancer, which increases the number of pathogenic-variant carriers by 30%.37 Among the patients with breast cancer in the population-based CARRIERS analysis, 5.03% had pathogenic variants in the 12 established, clinically actionable predisposition genes, with 0.85% and 1.29% having pathogenic variants in BRCA1 and BRCA2, respectively. These refined estimates of the prevalences of pathogenic variants among women with breast cancer in the overall population, as opposed to selected high-risk patients, may inform ongoing discussions regarding testing in patients with breast cancer. The risks of breast cancer associated with pathogenic variants in the genes evaluated in the population-based CARRIERS analysis also provide important information for risk assessment and counseling of women with breast cancer who do not meet high-risk selection criteria.
The population-based CARRIERS analysis also showed that certain subgroups of patients with breast cancer are at substantially increased risk of having pathogenic variants in high-penetrance, clinically actionable genes. For instance, 7 pathogenic variants in BRCA1, BRCA2, and PALB2 were observed in 8.13% of the patients with triple-negative breast cancer, as compared with 1.84% of the patients with ER-positive breast cancer. In addition, approximately 3.3% of women with ER-positive breast cancer without a family history had pathogenic variants in actionable breast cancer genes, with pathogenic variants in ATM, CHEK2, and BRCA2 accounting for the majority. Furthermore, pathogenic variants in BARD1, RAD51C, and RAD51D showed weak associations with breast cancer risk among the participants overall but were associated with a moderate risk of ER-negative breast cancer (odds ratio, >2). These findings were consistent with previously reported associations with ER-negative and triple-negative breast cancer among women who qualified for clinical genetic testing3,39 and among non-Hispanic Black women with breast cancer, a relatively high proportion of whom have ER-negative disease.4 Thus, risk stratification of women with breast cancer in the general population based on features such as tumor markers is an important method for identifying women at the highest risk of having a mutation, especially in underserved, minority populations.
There is also increasing discussion regarding screening for pathogenic variants in BRCA1 and BRCA2 in the unaffected population.40 Such testing for the Ashkenazi Jewish founder mutations in BRCA1 and BRCA2 is currently availablein Israel.41 However, beyond founder mutations, estimates of the prevalence of pathogenic variants in BRCA1 and BRCA2 or other breast cancer–pre-disposition genes are not well established in the general population. Here, we provide prevalence estimates for the 12 predisposition genes, showing that pathogenic variants in CHEK2 and ATM are the most common, and we note that pathogenic variants in BRCA1 and BRCA2 were found in 0.35% of the participants (or, 1 in 280). These estimates may inform the debate about population-based testing.
Most commercial genetic testing for hereditary cancer is based on multigene panels. However, many genes included on commercially available panels were not associated with an increased risk of breast cancer in the population-based CARRIERS analysis. Furthermore, several genes previously associated with an increased risk of breast cancer, including NBN, BRIP1, and RECQL, showed no associations in this population-based study. In particular, the finding that the NBN c.657_661del5 Slavic founder mutation was not associated with an increased risk of breast cancer suggests that the recommendation by management guidelines15,36-38 to increase screening among women with NBN pathogenic variants may need to be reevaluated. Among the established breast cancer–predisposition genes, ATM yielded an odds ratio of 1.82 (95% CI, 1.46 to 2.27) among all the women in the population-based CARRIERS analysis, an odds ratio of 1.72 (95% CI, 1.37 to 2.16) among women with no family history of breast cancer, and an odds ratio of 1.68 (95% CI, 1.31 to 2.17) among those who received a diagnosis of breast cancer at an age of more than 50 years. These findings suggest that carriers of pathogenic variants in ATM in the general population may have a substantially lower risk than what is often communicated to carriers of pathogenic variants who are identified through clinical testing (i.e., a risk that is said to be 2.5 times as high as that among noncarriers).1,42 However, the estimated lifetime risk of breast cancer by age 85 years among carriers of pathogenic variants in ATM was still over the 20% threshold used clinically for enhanced screening. Pathogenic variants in PALB2 were associated with a moderate risk of breast cancer in the population-based CARRIERS analysis (odds ratio, 3.83; 95% CI, 2.68 to 5.63), a finding that is similar to that in a study of two PALB2 founder mutations in a prospective cohort study in Poland (odds ratio, 4.39; 95% CI, 2.30 to 8.37).43 However, PALB2 was identified as a high-risk gene (odds ratio, 8.04; 95% CI, 5.33 to 12.29) among case patients with a family history of breast cancer in the population-based CARRIERS analysis (Table S12), a finding consistent with the results of a study of 524 families with PALB2 mutations (relative risk, 7.18; 95% CI, 5.82 to 8.85).44 These findings confirmed the effect of family history on breast cancer risk and identified family history as a critical factor for risk stratification of patients.
This study has some limitations. Enrollment was restricted to women 50 years of age or older in certain population-based studies in the CARRIERS consortium, which had the potential to influence the generalizability of the aggregate estimates of the prevalence of pathogenic variants in BRCA1 and BRCA2 to younger women; however, sensitivity analyses that excluded the Women’s Health Initiative and the Cancer Prevention Study II (studies that involved women at an older age at enrollment) did not substantially influence the findings. In addition, the statistical model for penetrance estimation was based on the assumptions that the underlying population-based SEER rates, prevalence of pathogenic variants, and age-specific estimates of odds ratios reflect those in the general population. Future studies are needed to evaluate the calibration of the probability estimates. Another potential limitation is that the sequencing was conducted in a research laboratory rather than a commercial genetic-testing facility. However, the custom QIAseq panel was shown to have high sensitivity and specificity for pathogenic variants in predisposition genes.17 Furthermore, because all samples were sequenced in a single center and variants were called through a single pipeline, issues with bioinformatics and batch effects were minimized. In addition, it was not possible to study the effects of individual pathogenic variants on breast cancer risk because of the rarity of the variants.
Overall, the results of the population-based CARRIERS analysis showed that pathogenic variants in the predisposition genes ATM, BRCA1, BRCA2, CHEK2, and PALB2 were associated with increased risks of breast cancer and that pathogenic variants in BARD1, RAD51C, and RAD51D were associated with increased risks of ER-negative breast cancer in the general population. To 3 date, the management recommendations for women with pathogenic variants in these genes have been based on risk estimates from studies involving women at high risk. We anticipate that the estimates from the population-based CARRIERS analysis will inform cancer screening and other risk-management strategies for women with pathogenic variants in cancer-predisposition genes in the general population.
Supplementary Material
Acknowledgments
Supported in part by the National Institutes of Health (NIH) (grants R01CA192393, R01CA225662, and R35CA253187), the NIH Specialized Program of Research Excellence (SPORE) in Breast Cancer (grant P50CA116201), and the Breast Cancer Research Foundation.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank Samantha J. McDonough, M.S., and Jin Jen, M.D., Ph.D., from Mayo Clinic Medical Genome Facility for technical support.
Contributor Information
Chunling Hu, Mayo Clinic, Rochester, MN
Steven N. Hart, Mayo Clinic, Rochester, MN
Rohan Gnanaolivu, Mayo Clinic, Rochester, MN
Hongyan Huang, Harvard University T.H. Chan School of Public Health, Boston
Kun Y. Lee, Mayo Clinic, Rochester, MN
Jie Na, Mayo Clinic, Rochester, MN
Chi Gao, Harvard University T.H. Chan School of Public Health, Boston
Jenna Lilyquist, Mayo Clinic, Rochester, MN
Siddhartha Yadav, Mayo Clinic, Rochester, MN
Nicholas J. Boddicker, Mayo Clinic, Rochester, MN
Raed Samara, Qiagen, Hilden, Germany
Josh Klebba, Qiagen, Hilden, Germany
Christine B. Ambrosone, Roswell Park Comprehensive Cancer Center, Buffalo, New York
Hoda Anton-Culver, University of California, Irvine, California
Paul Auer, University of Wisconsin-Milwaukee Joseph J. Zilber School of Public Health, Milwaukee
Elisa V. Bandera, Cancer Prevention and Control Program, Rutgers Cancer Institute of New Jersey, State University of New Jersey, New Brunswick
Leslie Bernstein, Beckman Research Institute of City of Hope, Duarte, California
Kimberly A. Bertrand, Slone Epidemiology Center at Boston University, Boston
Elizabeth S. Burnside, University of Wisconsin-Madison, Madison
Brian D. Carter, Behavioral and Epidemiology Research Group, American Cancer Society, Atlanta
Heather Eliassen, Brigham and Women's Hospital, Boston
Susan M. Gapstur, Behavioral and Epidemiology Research Group, American Cancer Society, Atlanta
Mia Gaudet, Behavioral and Epidemiology Research Group, American Cancer Society, Atlanta
Christopher Haiman, Keck School of Medicine, University of Southern California, Los Angeles, California
James M. Hodge, Behavioral and Epidemiology Research Group, American Cancer Society, Atlanta
David J. Hunter, Harvard University T.H. Chan School of Public Health, Boston University of Oxford, Oxford, United Kingdom.
Eric J. Jacobs, Behavioral and Epidemiology Research Group, American Cancer Society, Atlanta
Esther M. John, Stanford University School of Medicine, Stanford, California
Charles Kooperberg, Fred Hutchinson Cancer Research Center, Seattle
Allison W. Kurian, Stanford University School of Medicine, Stanford, California
Loic Le Marchand, Epidemiology Program, University of Hawaii Cancer Center, Honolulu
Sara Lindstroem, Department of Epidemiology, University of Washington, Seattle
Tricia Lindstrom, Mayo Clinic, Rochester, MN
Huiyan Ma, Beckman Research Institute of City of Hope, Duarte, California
Susan Neuhausen, Beckman Research Institute of City of Hope, Duarte, California
Polly A. Newcomb, Fred Hutchinson Cancer Research Center, Seattle
Katie M. O’Brien, National Institute of Environmental Health Sciences, Durham, NC
Janet E. Olson, Mayo Clinic, Rochester, MN
Irene M. Ong, University of Wisconsin-Madison, Madison
Tuya Pal, Vanderbilt University, Nashville
Julie R. Palmer, Slone Epidemiology Center at Boston University, Boston
Alpa V. Patel, Behavioral and Epidemiology Research Group, American Cancer Society, Atlanta
Sonya Reid, Vanderbilt University, Nashville
Lynn Rosenberg, Slone Epidemiology Center at Boston University, Boston
Dale P. Sandler, National Institute of Environmental Health Sciences, Durham, NC
Christopher Scott, Mayo Clinic, Rochester, MN
Rulla Tamimi, Weill Cornell Medicine, New York
Jack A. Taylor, National Institute of Environmental Health Sciences, Durham, NC
Amy Trentham-Dietz, University of Wisconsin-Madison, Madison
Celine M. Vachon, Mayo Clinic, Rochester, MN
Clarice Weinberg, National Institute of Environmental Health Sciences, Durham, NC
Song Yao, Roswell Park Comprehensive Cancer Center, Buffalo, New York
Argyrios Ziogas, University of California, Irvine, California
Jeffrey N. Weitzel, Beckman Research Institute of City of Hope, Duarte, California
David E. Goldgar, University of Utah, Salt Lake City
Susan M. Domchek, Department of Medicine and the Basser Center for BRCA, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania, Philadelphia
Katherine L. Nathanson, Department of Medicine and the Basser Center for BRCA, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania, Philadelphia
Peter Kraft, Harvard University T.H. Chan School of Public Health, Boston
Eric C. Polley, Mayo Clinic, Rochester, MN
Fergus J. Couch, Prof., Mayo Clinic, Rochester, MN
REFERENCES
- 1.Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol 2017;3:1190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Easton DF, Pharoah PDP, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl Med 2015;372:2243–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimelis H, LaDuca H, Hu C, et al. Triple-negative breast cancer risk genes identified by multigene hereditary cancer panel testing. J Natl Cancer Inst 2018;110: 855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer JR, Polley EC, Hu C, et al. Contribution of germline predisposition gene mutations to breast cancer risk in African American women. J Natl Cancer Inst 2020. May 19 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domchek SM, Friebel TM, Singr CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010;304:?967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tung NM, Garber JE. BRCA1/2 testing: therapeutic implications for breast cancer management. Br J Cancer 2018; 119:141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buys SS, Sandbach JF, Gammon A, et al. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer 2017; 123:1721–30. [DOI] [PubMed] [Google Scholar]
- 8.Hauke J, Horvath J, Groß E, et al. Gene panel testing of 5589 BRCA1/2-negative index patients with breast cancer in a routine diagnostic setting: results of the German Consortium for Hereditary Breast and Ovarian Cancer. Cancer Med 2018;7: 1349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurian AW, Hughes E, Handorf EA, et al. Breast and ovarian cancer penetrance estimates derived from germline multiple-gene sequencing results in women. JCO Precis Oncol 2017;1:1–12. [DOI] [PubMed] [Google Scholar]
- 10.Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer 2015;121:25–33. [DOI] [PubMed] [Google Scholar]
- 11.Momozawa Y, Iwasaki Y, Parsons MT, et al. Germline pathogenic variants of 11 breast cancer genes in 7,051 Japanese patients and 11,241 controls. Nat Commun 2018:9:4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol 2016;34:1460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terry MB, Liao Y, Whittemore AS, et al. 10-Year performance of four models of breast cancer risk: a validation study. Lancet Oncol 2019;20:504–17. [DOI] [PubMed] [Google Scholar]
- 14.US Preventive Services task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA 2019;322:652–65.. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology — genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 1. 2020. (https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf). [DOI] [PubMed]
- 16.King M-C, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA 2014;312:1091–2. [DOI] [PubMed] [Google Scholar]
- 17.Hu C, Hart SN, Polley EC, et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 2018;319?2401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogdanova N, Feshchenko S, Schürmann P, et al. Nijmegen Breakage Syndrome mutations and risk of breast cancer. Int J Cancer 2008;122:802–6. [DOI] [PubMed] [Google Scholar]
- 19.Borg A, Sandberg T, Nilsson K, et al. High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J Natl Cancer Inst 2000;92 1260–6. [DOI] [PubMed] [Google Scholar]
- 20.Damiola F, Pertesi M, Oliver J, et al. Rare key functional domain missense substitutions in MRE11A, RAD50, and NBN contribute to breast cancer susceptibility: results from a Breast Cancer Family Registry case-control mutation-screening study. Breast Cancer Res 2014;16(3):R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg M, Bell K, Aronson M, et al. Association between the Lynch syndrome gene MSH2 and breast cancer susceptibility in a Canadian familial cancer registry. J Med Genet 2017;54:742–6. [DOI] [PubMed] [Google Scholar]
- 22.Harkness EF, Barrow E, Newton K, et al. Lynch syndrome caused by MLH1 mutations is associated with an increased risk of breast cancer: a cohort study. J Med Genet 2015;52:553–6. [DOI] [PubMed] [Google Scholar]
- 23.Kiiski JI, Pelttari LM, Khan S, et al. Exome sequencing identifies FANCM as a susceptibility gene for triple-negative breast cancer. Proc Natl Acad Sci U S A 2014;111:15172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park DJ, Lesueur F, Nguyen-Dumont T, et al. Rare mutations in XRCC2 increase the risk of breast cancer. Am J Hum Genet 2012;90:734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park DJ, Tao K, Le Calvez-Kelm F, et al. Rare mutations in RINT1 predispose carriers to breast and Lynch syndrome-spectrum cancers. Cancer Discov 2014;4:804–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts ME, Jackson SA, Susswein LR, et al. MSH6 and PMS2 germ-line pathogenic variants implicated in Lynch syndrome are associated with breast cancer. Genet Med 2018;20:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seal S, Thompson D, Renwick A, et al. Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet 2006;38:1239–41. [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Wang Y, Xia Y, et al. Mutations in RECQL gene are associated with predisposition to breast cancer. PLoS Genet 2015;11(5):e1005228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson ER, Doyle MA, Ryland GL, et al. Exome sequencing identifies rare deleterious mutations in DNA repair genes FANCC and BLM as potential breast cancer susceptibility alleles. PLoS Genet 2012;8(9):e1002894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vijai J, Topka S, Villano D, et al. A recurrent ERCC3 truncating mutation confers moderate risk for breast cancer. Cancer Discov 2016;6:1267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weitzel JN, Chao EC, Nehoray B, et al. Somatic TP53 variants frequently confound germ-line testing results. Genet Med 2018;20:809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood SN. Generalized additive models: an introduction with R. New York: Chapman & Hall/CRC Texts in Statistical Science, 2017. [Google Scholar]
- 33.Steffen J, Nowakowska D, Niwinska A, et al. Germline mutations 657del5 of the NBS1 gene contribute significantly to the incidence of breast cancer in Central Poland. Int J Cancer 2006;119:472–5. [DOI] [PubMed] [Google Scholar]
- 34.Rusak B, Kluzniak W, Wokolorczyk D, et al. Allelic modification of breast cancer risk in women with an NBN mutation. Breast Cancer Res Treat 2019;178:427–31. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell KN, Wubbenhorst B, D’Andrea K, et al. Prevalence of mutations in a panel of breast cancer susceptibility genes in BRCA1/2-negative patients with early-onset breast cancer. Genet Med 2015;17:630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manahan ER, Kuerer HM, Sebastian M, et al. Consensus guidelines on genetic testing for hereditary breast cancer from the American Society of Breast Surgeons. Ann Surg Oncol 2019;26:3025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yadav S, Hu C, Hart SN, et al. Evaluation of germline genetic testing criteria in a hospital-based series of women with breast cancer. J Clin Oncol 2020;38:1409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Institute for Health and Care Excellence. Familial breast cancer:classification, care and managing breast cancer and related risks in people with a family history of breast cancer. 2019. (www.nice.org.uk/guidance/CG164). [PubMed]
- 39.Couch FJ, Hart SN, Sharma P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol 2015;33:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manchanda R, Patel S, Gordeev VS, et al. Cost-effectiveness of population-based BRCA1, BRCA2, RAD51C, RAD51D, BRIP1, PALB2 mutation testing in unselected general population women. J Natl Cancer Inst 2018;110:714–25. [DOI] [PubMed] [Google Scholar]
- 41.Manchanda R, Lieberman S, Gaba F, Lahad A, Levy-Lahad E. Population screening for inherited predisposition to breast and ovarian cancer. Annu Rev Genomics Hum Genet 2020;21:373–412. [DOI] [PubMed] [Google Scholar]
- 42.LaDuca H, Polley EC, Yussuf A, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med 2020;22:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cybulski C, Kluzniak W, Huzarski T, et al. Clinical outcomes in women with breast cancer and a PALB2 mutation: a prospective cohort analysis. Lancet Oncol 2015;16:638–44. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Leslie G, Doroszuk A, et al. Cancer risks associated with germline PALB2 pathogenic variants: an international study of 524 families. J Clin Oncol 2020;38:674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.