Abstract
Objective.
Determine if chikungunya virus (CHIKV) persists in synovial fluid post-infection, potentially serving as a causative mechanism of persistent arthritis.
Methods.
This is a cross-sectional study of 38 Colombian participants with clinical CHIKV infection during the 2014–2015 epidemic who reported chronic arthritis and ten location-matched CHIKV- and arthritis-negative controls. Prior CHIKV infection status was serologically confirmed and the presence of synovial fluid CHIKV, viral RNA, and viral proteins was measured by viral culture, quantitative reverse transcription PCR (qRT-PCR), and mass spectrometry. Biomarkers were assessed by multiplex analysis.
Results.
Serologically confirmed CHIKV arthritis patients (33/38, 87%) were predominantly female (82%) and Afro-Colombian (55%) or White-Colombian (33%) with (Median 22-months (IQR 21–23) post-CHIKV infection) moderate disease activity (Disease Activity Score-28 of 4.52 ± 0.77). Initial symptoms of CHIKV infection included joint pain (97%), swelling (97%), stiffness (91%), and fever (91%). The most commonly affected joints were knees (87%), elbows (76%), wrists (75%), fingers (56%), and toes (56%). Synovial fluid analysis of all CHIKV arthritis participants was CHIKV qRT-PCR negative, showed no viral proteins by mass spectrometry, and was culture negative. Case versus control plasma cytokine/chemokine concentrations did not differ significantly.
Conclusions.
This is one of the largest observational studies involving synovial fluid analysis of chikungunya arthritis patients. Synovial fluid analysis revealed no detectable CHIKV. This finding suggests that CHIKV may cause arthritis through induction of potential host autoimmunity suggesting a role for immunomodulating medications in the treatment of CHIKV arthritis or that low-level viral persistence exists in synovial tissue only that is undetectable in synovial fluid.
Chikungunya virus (CHIKV) infected patients present with fever, headache, muscle pain, rash, and joint pain. Following resolution of acute CHIKV infection, chronic arthritis may develop, often lasting months to years (1,2). CHIKV had previously been restricted to Africa, Asia, Europe, and the Indian and Pacific Ocean regions (3), but, in 2013, CHIKV was first described in the Americas and has now infected over 1.5 million people in this region (1). Outbreaks of the Asian strain of CHIKV leave approximately one-third of patients with persistent joint pain at 18-months post-infection (4,5).
After transmission by an Aedes aegypti or Aedes albopictus mosquito bite, CHIKV undergoes local replication and then dissemination to lymphoid tissue (6). While viremia lasts only 5–12 days (7,8), a study in non-human primates demonstrated that CHIKV persists in lymphoid organs, liver, joint, muscle, and macrophages for up to 3 months and that CHIKV RNA remains present in spleen, liver, and muscle for extended periods (9). In another study, CHIKV RNA was detected in the synovial tissue of a patient 18-months post-infection (10). Similar findings of inflammatory macrophage infiltrates (11,12) and synovial viral RNA persistence (13) have been seen in polyarthritis caused by another alphavirus, Ross River virus. These findings have led to the hypothesis that CHIKV might persist in the joint in cases of chronic arthritis.
CHIKV is known to evade neutralizing antibodies by residing within apoptotic blebs (14). While in the blebs, CHIKV can infect neighboring cells in vitro (15). Furthermore, in vitro CHIKV inhibits RNA kinase needed to make anti-viral mRNA transcripts, thereby freezing host anti-viral protein synthesis (16). IL-6, MCP1, and IFNα have been detected during the acute phase of infection, and elevated analytes, including IL-6, MCP1, IP-10, IL-1Rα, Eotaxin, IL17, and GMCSF, have further been associated with disease severity, chronic arthralgia, and/or viral load. (17–19)
There is currently no standard treatment for CHIKV arthritis (20). However, several small studies have demonstrated clinical benefit from treatment with antivirals such as ribavirin (21) and immunosuppressant medications such as methotrexate (22–24), hydroxychloroquine (22), etanercept (23), adalimumab (23), and sulfasalazine (24). Further characterization of the CHIKV disease pathophysiology is needed to provide a rationale for large-scale randomized therapeutic clinical trials to evaluate the effectiveness of these potential therapeutics. If persistent CHIKV infection is responsible for ongoing arthritis, immunocompromising disease modifying agents may be improper and potentially dangerous treatments. Alternatively, if CHIKV does not persist in the joint, then evaluation of immunomodulating arthritis agents could be useful. The objective of the Study of Chikungunya Arthritis Mechanisms in the Americas (CAMA) was to determine if there was evidence of CHIKV in the synovial fluid of patients with chikungunya arthritis in order to understand disease pathogenesis and, perhaps, guide chikungunya arthritis therapy.
Patients and Methods
Setting.
Patients were recruited from the Atlántico and Bolívar Departments, Colombia. In September 2014, the first locally acquired CHIKV case was reported the Bolívar department. During the height of the epidemic from 2014–2015, many suspected CHIKV cases were reported in the Departments of Atlántico (2,480 cases) and Bolivar (5,997 cases).
Inclusion criteria.
Included participants were adults ≥18 years old and Spanish-speaking. Chronic chikungunya arthritis was defined as clinical or laboratory confirmed diagnosis of CHIKV infection, with persistent arthritis or arthralgias. Arthritis and arthralgias included knee pain and swelling for at least three months after diagnosis of CHIKV infection as well as current joint pain at the time of follow-up. As per the Colombian Institute of Health, a clinically confirmed case of CHIKV infection is defined as a patient presenting with fever greater than 38°C, severe joint pain or arthritis, and the acute onset of erythema multiforme with symptoms not explained by other medical conditions. In addition, these individuals must reside or have visited a municipality where evidence of CHIKV transmission is present or have traveled within 30 kilometers of confirmed viral circulation. No patients were excluded for prior arthritis; history of prior arthritis status was included in the analysis. All suspected CHIKV cases were laboratory confirmed for the purposes of this study. Healthy controls were defined as participants from the same region, who reported no history of prior CHIKV infection and did not present with arthritis.
Exclusion criteria.
Subjects were excluded if they reported a known bleeding disorder or were receiving anticoagulant medications. The study also excluded children, adults unable to give consent, prisoners, and pregnant women.
Recruitment.
In 2014–2015, as part of a CHIKV surveillance study across the Atlántico and Bolívar Departments, 907 patients with clinically (n = 424) or laboratory (n =483) confirmed CHIKV infection were referred by their primary care providers from clinics located in Baranquilla, Atlántico; Sabanalarga, Atlántico; and San Juan Nepomuceno, Bolívar. From these patients, 65 patients were randomly selected for eligibility screening of which 38 patients were eligible for study participation in the chronic arthritis group with current knee joint pain. Patients were not eligible if they did not have persistent knee pain after CHIKV infection. Ten healthy controls were also recruited from volunteers at the clinic in Baranquilla, Atlántico. Synovial fluid was not collected from healthy controls as it would not have been clinically indicated.
Ethics statement.
The study protocol was approved by the George Washington University Institutional Review Board (Protocol # 041612), the Universidad El Bosque (UB 387–2015), and the United States Army Research Institute of Infectious Disease Human Research Protections Office (FY15–32). Research on human subjects was conducted in compliance with Department of Defense, Federal, and State statutes and regulations relating to the protection of human subjects, and adheres to principles identified in the Belmont Report (1979). All data and human subjects research were gathered and conducted for this publication under an IRB approved protocol. All participants were adults and provided written informed consent during an in-person interview. The study was also registered in the ClinicalTrials.gov registry (NCT02463968).
Primary outcome.
We hypothesized that persistent active viral replication is responsible for chronic arthritis and joint pain. Therefore, the primary outcome of the current study protocol was the identification of the presence of CHIKV in the synovial fluid. Attempts to find evidence of CHIKV in synovial fluid included viral culture, quantitative reverse transcription polymerase chain reaction (qRT-PCR) for CHIKV RNA, and mass spectrometry analysis for viral proteins.
Secondary outcomes.
As part of this study, we evaluated clinical outcomes, such as the effect on daily living and arthritis severity as measured by the Disease Activity Score (DAS-28) (25), a validated rheumatoid arthritis (RA) assessment tool that is a composite score of the number of tender joints, swollen joints, global disease activity during the most recent week measured from 0–100, and the C-reactive protein (CRP). This clinical outcomes questionnaire was administered to all the participants in a face-to-face interview. Laboratory studies in these patients included plasma C-reactive protein (CRP), serum rheumatoid factor IgM antibody, rheumatoid factor IgG antibody, anti-cyclic citrullinated peptide (anti-CCP) antibody, and selected cytokines and chemokines.
Sample Collection.
Following informed consent and administration of the questionnaire concerning the participant’s demographics and symptom history, blood was obtained by venipuncture. An orthopedic surgeon performed an arthrocentesis for primary evaluation of the swollen knee joint with needle lavage where 0–20 ml of saline was injected at clinician discretion. Samples collected in this manner are referred to as “synovial fluid” within this manuscript. Blood samples were collected into 8 ml Sodium Citrate Cell Preparation Tubes (CPT) (BD Cat No. 362761) and synovial fluid was transferred to CPT in an effort to isolate cells from the fluid.
Sample Preparation.
The samples were centrifuged at room temperature (18–25ºC) in a horizontal rotor for 20 minutes at 1500 relative centrifugal force (RCF). Plasma was removed from the blood collection tubes and frozen to −80ºC until analysis. Synovial fluid samples were similarly centrifuged and frozen for subsequent analysis. There was no visible cell pellet after centrifugation of the synovial fluid, therefore supernatant and any present cells were stored as one specimen at −80ºC.
Data management.
All patients were assigned a unique patient identification number, which was used in the database and for patient sample labeling. All patient data was void of personal identifiers and was stored in REDCap database at George Washington University.
Anti-CHIKV IgG and IgM.
IgG and IgM levels in plasma were assayed using the InBios CHIKjj Detect™ ELISA assays (CHKG-R and CHKM-R). These assays provide a qualitative evaluation of the presence or absence of anti-CHIKV IgG and IgM and provide controls to calculate an Immune Status Ratio (ISR). Plasma was diluted 1:100 in kit dilution buffer and tested in duplicate, as per manufacturer’s instructions.
RNA isolation and qRT-PCR.
RNA isolation was attempted from 140 µl of plasma or synovial fluid using the QIAGEN QIAamp Viral RNA Mini Kit (Qiagen, Cat no. 52904). Control samples contained spiked RNA, which was isolated in parallel to ensure recovery and detection by qRT-PCR. Samples were run on the ABI 7500 HT using the conditions listed below.
Both plasma and synovial fluid samples were evaluated using the RNA UltraSense One-Step Quantitative RT-PCR System. A standard curve was run in parallel with samples, with duplicate evaluation of samples ranging from 1 x 10^7 to 1 x 10^2 copies/ml. The assay limit of detection (LOD) is approximately 80 copies/ml. Forward primer: 5´-GGGCTATTCTCTAAACCGTTGGT-3´. Reverse primer: 5´-CTCCCGGCCTATTATCCCAAT-3´. Probe: 5’-FAM-TCTGTGTATTACGCGGATAA-3’ MGBNFQ. These primer and probe sequences (located in the peptidase C9 domain of the nonstructural polyprotein) were designed against CHIKV of Asian lineage, as the strains currently circulating in South America are of the Asian lineage. The cycling program was as follows: 15 min at 50°C hold; 2 min at 95°C hold; 95°C for 15 sec and 60°C for 30 sec for 40 cycles.
To confirm the results of the first assay, a second qRT-PCR assay was used to re-test the synovial fluid samples using different primers that were specifically designed against isolates emerging from Colombia (Genbank KX496989.1 and KT192707.1) and also target sequences in the peptidase C9 region of the nonstructural polyprotein. Samples were tested using Power SYBR One Step RNA to Ct (ABI Cat No. 4389986). The assay LOD is approximately 100 copies/ml. Forward primer: 5´-GGCAGTGGTCCCAGATAATTCAAG. Reverse primer: 5´-GCTGTCTAGATCCACCCCATACATG-3´. The cycling program was as follows: 48˚C for 30 min; 95˚C for 10 min; 95˚C for 15 sec, 60˚C for 1 min for 40 cycles.
Culture of Synovial Fluid.
Vero cells were cultured in 12 well plates to 90% confluency. The culture media consisted of Roswell Park Memorial Institute medium (RPMI) 1640, 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid). Media was removed and 500 µL of synovial fluid was added to each well and incubated for one hour. Where more than 500 µL of synovial fluid was available, multiple cultures were established. As a positive control, CHIKV (strain 15661) was added to two wells each at approximately 10 pfu/well and 1 pfu/well, with the aim of confirming detection of low levels of viremia in the samples. After one hour, 2.5 mL of complete media was added to each well, and the cells were incubated for four days (passage 1). On the fourth day, the supernatant was transferred to fresh Vero cells (90% confluency) in a 6 well plate (passage 2). An additional 3 mL of media was added and the cells were cultured an additional 3 days. On the third day, a 140 µL aliquot of supernatant was collected for analysis. Then 3 mL of the supernatant was transferred again to fresh Vero cells (90% confluency) in a 6 well plate (passage 3). An additional 3 ml of media was added and the cells were cultured an additional 3 days. On the final day, a 140 µl aliquot of supernatant was collected for analysis. Remaining supernatant was then removed and the cells were lysed in Buffer AVL (Qiagen). Buffer AVL was also added to the supernatant samples in accordance with the manufacturer’s instructions. The samples were heated at 56°C for one hour, removed from the BSL-3, and the presence of viral nucleic acid was measured using PCR as described above.
Biomarker analysis.
Levels of IgG and IgM rheumatoid factor (RF) were measured using an Inova Diagnostics QUANTA Lite® kit in accordance with the manufacturer’s instructions, with plasma samples instead of serum samples. Anti-cyclic citrullinated peptide (anti-CCP) antibodies were measured using the Inova Diagnostics QUANTA Lite® CCP3.1 IgG/IgA ELISA (Cat no. 704550) in accordance with the manufacturer’s instructions. Plasma samples were diluted 1:100 and quantified based on the assay standard curve. Multiplex assessment of a panel of cytokines, chemokines, and other acute biomarkers was conducted using a custom Meso Scale Discovery (MSD) assay kit. Analytes included IFNα2a, CRP, IFNγ, IL1β, IL2, IL4, IL6, IL8, IL10, IL12p70, GMCSF, IL1α, IL12/23p40, IL15, IL17A, eotaxin, MIP1β, IP10, MIP1α, and MCP1. Samples were diluted in accordance with the manufacturer’s instructions for each analyte.
Mass Spectrometry analysis.
Sample Preparation:
Twenty-five µL of each synovial fluid sample were added to 200 µL Solution UT8 (8M Urea) and processed by Filter Assisted Sample Processing (FASP) per the manufacturers protocol. Briefly, proteins are bound to the FASP filter (Millipore MRCF0R030) in UT8 and alkylated in 55Mm iodoacetamide followed by digestion with 40 ng/µL Trypsin/Lys-C (Promega) overnight at 37°C. Peptides were eluted in 50 mM NaCl and subsequently desalted using C18 spin columns (Pierce 89870) per manufacturer’s instructions. Eluted peptides were dried to completion. Digests were stored at −20°C until analyzed by LC MS/MS.
LC-MS/MS Analysis and Protein Search:
Sample digests were re-suspended in 20 µL of 0.1% formic acid and mixed briefly. A Dionex 3000 RSLCnano system (Thermo Scientific) injected 2.5 µL of each digest onto a pre-column (C18 PepMap 100, 5 µm particle size, 5mm length x 0.3mm internal diameter) housed in a 10-port nano switching valve using a flow rate of 10 µL/minute. The loading solvent was 0.1 % formic acid in HPLC grade water. The pre-column eluent was directed to waste. After 5 minutes, the switching valve changed to backflush the trapped peptides from the pre-column onto an Easy-Spray analytical column (15 cm x 75 µm) packed with PepMap C18, 3 µm particle size, 100A porosity particles (Thermo Scientific, Inc.). A 2–42% B gradient elution in 95 minutes was formed using Pump-A (0.1% formic acid) and pump-B (85% acetonitrile in 0.1% formic acid) at a flow rate of 300nL/minute. The column eluent was connected to an Easy-Spray nanospray source (Thermo Scientific) with an electrospray ionization voltage of 2.2kV. An Orbitrap Elite mass spectrometer (Thermo Scientific, Inc.) with an ion transfer tube temperature of 300°C and an S-lens setting of 50% was used to focus the peptides. Low-resolution rapid CID ms/ms spectra were acquired with an AGC of 1e4 ions and a maximum injection time of 50ms. The isolation width for ms/ms CID fragmentation was set to 2 daltons. The normalized collision energy was 35% with a Q value of 0.250. The dynamic exclusion duration was 30 seconds.
Searches were performed with ProteomeDiscoverer 2.1 (Thermo Scientific) using a Human and CHIKV subset of the SwissProt_2016_10_05 database. Variable modifications used were Methyl (DE), Acetyl (K), Deamidated (NQ), and Oxidation (M) and Carbamyl (K). Cysteine carbamidomethylation was specified as a constant modification. The false discovery rate (FDR) was set at 0.1%. Mass tolerances were 10 ppm for the MS1 scan and 200 ppm for all ms/ms scans. Search results were filtered such that only high-confidence/unambiguous Peptide Spectral Matches (PSM) were used.
Statistical Analysis.
For univariate tests across diagnostic groups, chi-square or Fishers Exact test were used to compare categorical variables and analysis of variance for normally distributed continuous variables; the Kruskal-Wallis test was used for skewed continuous variables. SAS (version 9.3, Cary, NC) was used for data analysis, with p<0.05 considered significant.
Sample size and statistical power.
Given the sample size, there was sub-optimal statistical power for some comparisons of secondary outcomes. For example, when comparing CHIKV-confirmed cases with and without prior arthritis (n=33) to controls without CHIKV or arthritis (n=10) on categorical variables, using 2-tailed chi-square with alpha=0.05, power was >0.80 only for an effect size where the proportions positive were on the order of 40% vs. 1%. However, we had more robust power for detecting differences in continuous variables. For example, the power was >0.80 for detecting a difference of 39±10 versus 30±10 (Cohen’s d = 0.9) between CHIKV cases & Controls using a 2-tailed t-test.
Results
Baseline characteristics.
Prior chikungunya infection was serologically confirmed in 33/38 (87%) of the cases by IgM 1/33 (3%) and IgG ELISA 33/33 (100%). Confirmed chikungunya arthritis patients were predominantly women 27/33 (82%), Afro-Colombian 18/33 (55%) or White-Colombian 11/33 (33%) with high school or less level of education 31/33 (94%). As compared to healthy controls, the patients with CHIKV arthritis tended to be older, with less education and had at least one comorbidity (Table 1). Participants with CHIKV arthritis with a history of prior arthritis compared to those with no history of prior arthritis were comparable in terms of age, gender, ethnicity, and education level. One patient with confirmed CHIKV exposure self-reported pre-existent rheumatoid arthritis but was found to be rheumatoid factor (RF) and anti-CCP antibody negative.
Table 1.
Baseline characteristics of CAMA study participants.
| Serologically confirmed CHIKV arthritis cases |
||||
|---|---|---|---|---|
| Characteristic | No prior arthritis (n=25) | Prior history of arthritis (n= 8) | Controls (n= 10) | p-value |
| Agea | 56.0 (10.0) | 59.6 (12.2) | 31.7 (7.8) | <0.0001 |
| Body Mass Indexa | 30.0 (4.5) | 27.1 (5.8) | 24.7 (5.3) | 0.03 |
| Female Genderb | 20/25 (80%) | 7/8 (88%) | 7/10 (78%) | 0.99 |
| Afro-Colombian ethnicityb | 13/25 (52%) | 5/8 (63%) | 5/10 (50%) | 0.90 |
| White-Colombian ethnicityb | 8/25 (32%) | 3/8 (38%) | 4/10 (40%) | |
| High school or less educational levelb | 23/25 (92%) | 8/8 (100%) | 0 (0%) | <0.0001 |
| Presence of comorbiditiesb | 9/25 (36%) | 6/8 (75%) | 0 (0%) | 0.0025 |
| >3 comorbiditiesb | 0 | 1 (13%) | 0 | 0.19 |
| Comorbiditiesb | ||||
| Rheumatoid arthritis | 0 | 1 (12.5%) | 0 | 0.19 |
| Osteoarthritis | 0 | 3 (37.5%) | 0 | 0.0045 |
| Ischemic heart disease | 0 | 0 | 0 | NA |
| Chronic kidney disease | 0 | 0 | 0 | NA |
| Chronic pulmonary disease | 0 | 0 | 0 | NA |
| Diabetes | 1 (4.0%) | 1 (12.5%) | 0 | 0.39 |
| Hypertension | 7 (28.0%) | 4 (50.0%) | 0 | 0.05 |
| Depression | 1 (4.0%) | 0 | 0 | 0.99 |
mean(sd)
n(%)
Chikungunya infection related symptoms.
CHIKV arthritis patients were a median 22-months (Interquartile range 21–23) post-CHIKV infection. Initial symptoms of CHIKV infection included joint pain (97%), joint swelling (97%), joint stiffness (91%), fever (91%), and rash (88%) (Table 2); these symptoms were reported at the time of initial infection. The most commonly affected initial joints were knees (87%), elbows (76%), wrists (75%), fingers (56%), ankles (56%), and toes (56%). At follow-up, most participants reported an effect on their activities of daily living from their arthritis (82%) (Table 3) however patients were not necessarily experiencing a disease flare at the time of sample collection. Thirty-eight percent of participants reported missing school or work during their initial infection. At the time of sample collection, participants had on average 5.5 ± 5.4 tender joints and 3.0 ± 2.8 swollen joints. Patient-reported global disease activity measure (scored from 0–100 with 100 being the most active) in the last week was 93 ± 14. The disease severity was moderate as shown by an average Disease Activity Score-28 using C-reactive protein (DAS-28) of 4.52 ± 0.77. There were no significant differences between CHIKV arthritis patients with or without a prior history of arthritis with the exception of approximately one additional joint being tender and swollen in the participants with prior arthritis.
Table 2.
Chikungunya infection related symptoms at time of initial presentation
| Serologically confirmed CHIKV |
||||
|---|---|---|---|---|
| Characteristic | All CHIKV arthritis cases (n=33) | Divided by Subgroup | p-value | |
| No prior history of arthritis (n=25) | Prior history of arthritis (n= 8) | |||
| CHIKV related symptoms, n(%) | ||||
| Joint tenderness | 32/33 (97%) | 25/25 (100%) | 7/8 (88%) | 0.24 |
| Joint swelling | 32/33 (97%) | 25/25 (100%) | 7/8 (88%) | 0.24 |
| Joint stiffness | 30/33 (91%) | 22/25 (88%) | 8/8 (100%) | 0.56 |
| Fever | 30/33 (91%) | 23/25 (92%) | 7/8 (88%) | 0.99 |
| Rash | 29/33 (88%) | 22/25 (88%) | 7/8 (88%) | 0.99 |
| Commonly affected initial joints, n(%) | ||||
| Knees | 27/33 (87%) | 19/25 (83%) | 8/8 (100%) | 0.55 |
| Elbow | 25/33 (76%) | 19/25 (76%) | 6/8 (75%) | 0.99 |
| Wrist | 24/33 (75%) | 18/25 (75%) | 6/8 (75%) | 0.99 |
| Fingers | 18/33 (56%) | 14/25 (58%) | 4/8 (50%) | 0.70 |
| Ankles | 18/33 (56%) | 13/25 (57%) | 5/8 (63%) | 0.99 |
| Toes | 18/33 (56%) | 15/25 (63%) | 3/8 (38%) | 0.25 |
| Hips | 8/33 (26%) | 6/25 (26%) | 2/8 (25%) | 0.99 |
Table 3.
Chikungunya infection related effects assessed at follow-up visit median 22 months post-infection
| Serologically confirmed CHIKV |
||||
|---|---|---|---|---|
| Characteristic | All CHIKV arthritis cases (n=33) | Divided by Subgroup | p-value | |
| No prior history of arthritis (n=25) | Prior history of arthritis (n= 8) | |||
| Effect on activities of daily livinga | 27/33 (82%) | 19/25 (76%) | 8 (100%) | 0.30 |
| Missed Work/ Schoola | 12/33 (38%) | 7/25 (29%) | 5 (63%) | 0.12 |
| Tender Joint Countb | 5.5 (5.4) | 5.4 (5.5) | 6.0 (5.2) | 0.0002 c |
| Swollen Joint Countb | 3.0 (2.8) | 2.8 (2.4) | 3.9 (4.0) | <0.0001 c |
| Global disease measureb | 93.4 (14.3) | 91.3 (15.9) | 100.0 (0) | 0.14 |
| DAS-28b | 4.52 ± 0.77 | 4.44 ± 0.73 | 4.78 ± 0.87 | 0.29 |
n(%yes)
mean(sd)
Using non-parametric Kruskal-Wallis test.
Virologic and Serologic Outcomes.
All samples tested for persistent viral RNA (both plasma and synovial fluid) were negative for CHIKV by two separate qRT-PCR assays. To more rigorously evaluate if low level viremia might be present in the samples, synovial fluid samples were added to cell cultures in an attempt to expand out any replication competent virus. Cultures of synovial fluid from CHIKV arthritis patients also showed no viral growth after three passages and ten days of culture; in contrast, controls using low quantities of virus (~1 pfu/well) yielded growth and detection of virus.
Plasma markers for rheumatoid arthritis were present in only a fraction of participants with CHIKV arthritis: Rheumatoid factor (RF) IgM antibody (9%), RF IgG antibody (12%) and anti-cyclic citrullinated peptide (anti-CCP) antibody (0%). Subjects with CHIKV associated arthritis had no significant increase in rheumatoid arthritis associated markers or CRP (Figure 1). Interestingly, two of the five individuals who were enrolled in the study as having clinical CHIKV-associated arthralgia, but who were found to be seronegative for CHIKV, exhibited positive RF and/or anti-CCP antibody, suggesting they may have actually had rheumatoid arthritis or another related disease unlinked to CHIKV infection.
Figure 1.
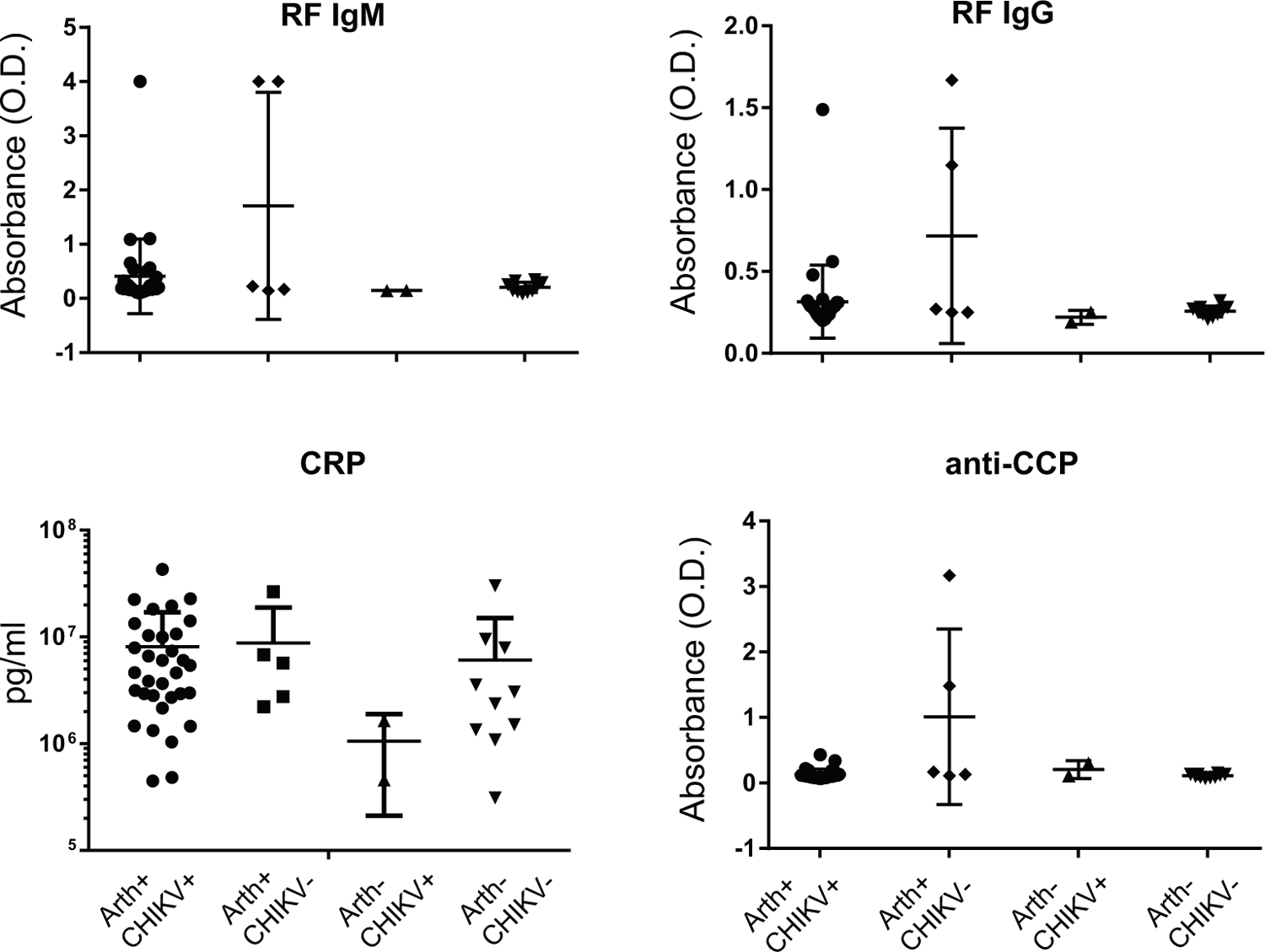
Subjects with CHIKV-associated arthritis had no significant increase in RA associated markers or CRP. Subjects were divided into four groups based on whether they presented with arthralgia or not (Arth+/−) and on whether they were IgG or IgM positive for anti-CHIKV antibody (CHIKV+/−).
Plasma cytokine and chemokine data demonstrated trends that pointed to differences between the CHIKV subjects with arthralgia and their controls, but which failed to reach statistical significance (Table 4). As observed in other publications, IL6, IL12p70, MCP-1, MIP-1β and IL8 were modestly elevated in convalescent subjects compared to controls.
Table 4.
Plasma cytokine and chemokine concentrations at median 22-months post-infection.
| Analyte | CHIKV cases n=33 in pg/ml | Controls n=10 in pg/ml | p-value |
|---|---|---|---|
| Cytokines | |||
| Interleukin-1α (IL-1α) | 1.29 (0.58–3.82) | 1.42 (0–4.93) | 0.39 |
| Interleukin-1β (IL-1β) | 0.27 (0.17–0.69) | 0.20 (0.10–0.28) | 0.10 |
| Interleukin-2 (IL-2) | 1.70 (1.25–2.89) | 2.06 (1.70–2.89) | 0.12 |
| Interleukin-4 (IL-4) | 1.08 (0.76–2.80) | 1.31 (0.61–2.51) | 0.80 |
| Interleukin-6 (IL-6) | 1.69 (1.01–3.18) | 1.32 (0.73–1.69) | 0.09 |
| Interleukin-10 (IL-10) | 1.80 (1.29–2.56) | 1.92 (1.29–2.08) | 0.85 |
| Interleukin-12 (IL-12/p40) | 111 (92.0–137) | 116 (87.4–161) | 0.55 |
| Interleukin-12 (IL-12/p70) | 1.91 (1.37–6.20) | 1.78 (1.05–6.79) | 0.81 |
| Interleukin-15 (IL-15) | 2.55 (2.01–2.97) | 2.64 (1.89–3.32) | 0.63 |
| Interleukin-17α (IL-17a) | 7.81 (6.35–9.19) | 7.2 (4.95–8.44) | 0.41 |
| Interferon-γ (IFN-γ) | 7.44 (3.92–16.1) | 6.7 (3.92–13.0) | 0.82 |
| GM_CSFa | 0.93 (0.60–1.30) | 0.80 (0.32–1.15) | 0.55 |
| Chemokines | |||
| Eotaxin | 101 (68.0–136) | 104 (65.0–134) | 0.93 |
| MIP-1αb | 23.9 (18.2–36.3) | 27.6 (23.8–37.9) | 0.22 |
| IP-10 | 162 (123–227) | 153 (133–197) | 0.67 |
| MCP-1d | 123 (88.3–140) | 106 (87.5–144) | 0.75 |
| MIP-1βe | 46.6 (33.8–56.5) | 38.13 (32.7–54.0) | 0.40 |
| Interleukin-8 (IL-8) | 7.91 (6.15–16.9) | 6.7 (5.44–11.4) | 0.12 |
Granulocyte-Monocyte CSF
Macrophage inhibitory protein
Interferon-γ induced Protein
Monocyte chemotactic protein-1
Macrophage inhibitory protein
Proteomic analysis of synovial fluid.
Mass spectrometry (MS) was used to identify proteins present in the synovial fluid of patients with arthralgia associated with CHIKV. The primary aim of the analysis was to determine if MS could detect the presence of viral proteins, as this would suggest persistence of antigen. However, MS did not identify any CHIKV viral proteins in the fluid.
Discussion.
The CAMA study is the largest observational study involving synovial fluid analysis of patients with CHIKV arthritis in the Americas to date. We hypothesized that persistent active CHIKV is responsible for chronic arthritis and joint pain and that CHIKV viral RNA would be present in the synovial fluid. However, this study did not detect viable virus after culture of synovial fluid in any of the participants who were studied a median 22 months after infection. Similarly, PCR analysis could not detect viral RNA in the plasma or in the synovial fluid. Furthermore, proteomic analysis found no evidence of viral proteins in the synovial fluid. These results have important implications for determining mechanisms of the persistent arthritis in CHIKV patients and suggest that either there is no CHIKV in synovial fluid or that CHIKV does not replicate to high enough levels for detection in the synovial fluid 2-years post-infection.
This study was inspired by the work of Hoarau et al. (10), who found CHIKV antigen in macrophages and CHIKV RNA in synovial biopsy tissue at 18-months post-CHIKV infection in a single subject. In contrast, in our analysis of 33 patients (many with relapsing and remitting disease) 22-months after acute infection, we did not identify viral RNA or proteins, suggesting that perhaps viral persistence may not be a requirement for persistent joint pain. Another consideration is that synovial tissue analysis as opposed to synovial fluid analysis may permit improved viral recovery. Furthermore, this study may suggest that the pathophysiology behind human relapsing and remitting CHIKV arthritic disease may differ from the pathophysiology of continuous erosive arthropathy seen in some patients.
In the context of animal studies, studies of non-human primates (9) and mice (26) have demonstrated viral persistence up to 44 and 100 days respectively. Given our evaluation at significantly longer times post infection (22-month) it is conceivable that any virus present was eliminated by this later end point. Furthermore, murine models have yet to reproduce the relapsing/remitting nature of human chikungunya arthritis suggesting that differing pathophysiology may be at play.
From a clinical perspective, CHIKV arthritis participants described substantial clinical disease burden. Eighty-two percent reported arthritis affecting their daily living and moderate disease severity, as measured by the Disease Activity Score-28. This is consistent with other studies following patients after CHIKV infection, which have demonstrated symptoms of persistent arthralgia that may be relapsing or unremitting, often affect multiple joints, and are associated with functional loss impairing activities of daily living and reduced quality of life (27,28).
Multiple studies have found elevated inflammatory analytes during acute CHIKV infection with IL8, MCP1, IL6, MIP1α, IL1α, and MIP1β reportedly elevated in some chronic CHIKV arthralgia cohorts (10,29,30). We measured these cytokines and chemokines as well as several relevant RA-associated biomarkers. Consistent with the literature, RA-associated factors like RF and anti-CCP were not elevated in our CHIKV-associated arthralgia cohort (2,23,31). Of interest, there were no significant differences in cytokine and chemokine levels within our cohort compared with location-matched controls at median 22-months. This might be the result of the very late stage of disease that we studied or the size of our control cohort. There is a trend favoring elevation of proinflammatory markers in our cohort (most notably IL-6), but whether a greater number of subjects would yield statistical significance, we can only speculate. It is possible that the apparent lack of a consistent pattern of inflammatory markers in patients with reported joint pain may be related to the relapsing/remitting nature of the joint pain described by CHIKV arthritis patients. Patients describe periods of relative relief and “flare” periods of worsened joint symptoms in response to physical stress or infection. Such relapse has also been described by Borgherini, et. al. from the Reunion Island outbreak (32). In our study, while all patients reported joint pain, the relative intensity varied.
Given these results, additional potential mechanisms for the persistence of arthritis symptoms in the absence of CHIKV persistence in the synovial fluid should be considered. First, it is possible that CHIKV or viral antigens persist at low levels in synovial tissue that cannot be detected in the synovial fluid. Other potential mechanisms include CHIKV-induced epigenetic modifications of host DNA resulting in persistent alterations of host gene transcription, as has been seen in other viruses such as Epstein Barr Virus (33). Alternatively, macrophages could be modified through epigenetic imprinting, much like fibroblast-like synoviocytes are in RA, leading to more aggressive cell behavior even in the absence of replicating virus (34). Molecular mimicry may also play a role in CHIKV-induced arthritis where the continued production of a CHIKV-specific antibody that cross-reacts with antigen in the synovium could account for CHIKV-associated inflammation. Finally, although unlikely, patients could have seronegative RA; or alternatively seronegative RA could reflect prior infection with CHIKV or other arthritogenic viruses.
There are several limitations to this study that need to be addressed. First, during collection, 0–20 ml saline was used to flush the joints and this could affect our ability to detect virus in the synovial fluid samples. To mitigate for this problem, we cultured 0.5 ml – 1.5 ml (as available) of the collected synovial fluid from each patient to attempt to expand any replication-competent virus in the samples. We also utilized two complementary PCR assays to detect nucleic acid as well as a proteomic approach to look for viral proteins. Proving the absence of a target is difficult and we recognize that it is possible that our approach failed to detect low-level viral antigen; however, our orthogonal approach clearly demonstrates that if viral antigen exists in the synovial fluid, it is at extremely low levels. Though it is a more invasive procedure, future studies may benefit from focusing on synovial biopsy rather than fluid. The advent of new ultrasound guided biopsy techniques may permit this approach in the future. A second limitation of this study is lack of control subjects who had CHIKV infection history without chronic arthritis, as well as a lack of age and gender-matched healthy controls. Furthermore, our healthy controls were on average younger than the CHIKV affected patients and age is known to be associated with increased inflammatory cytokine production, most notably interleukin-6 (35,36).
These study findings may have important clinical relevance for CHIKV in the Americas. Since there is no current standard of care guidance for treatment of CHIKV arthritis, some patients are currently being treated with immunosuppressant medications such as methotrexate (22–24), hydroxychloroquine (22), etanercept (23), adalimumab (23), sulfasalazine (24), fingolimod (37), abatacept and tofacitinib (38). This practice could be potentially harmful in the setting of replicating virus in the synovium as it could permit re-emergence of a systemic viral infection. To date, no such resurgence has been reported. The failure to detect viral persistence in the synovial fluid in the present study may provide some reassurance that treatment with immunosuppressant anti-rheumatic medications two years after infection is a viable option.
Acknowledgements.
This study was made possible by the support of the following institutions and individuals: Allied Research Society, Falab Laboratory, la Universidad Metropolitiano, la Universidad El Bosque, The George Washington University, La Fundación Santa Fe, El Laboratorio Fals Borda, Lorena Encinales, Walberto Mariaga, Slyvana Insignares, Cesly Arteta, Martha Castillo Jimenez, Jaime Cantillo, April Barbour, Alan Wasserman, Donna Embersit, Sofia Tang, Armando Gonzalez Brunal, Olga Lucia Barran, Margarita Arroyave-Wessel, Fabiola Pomgrico, Julio Cesar Padilla, Jose Ziade, Beda Brichacek, Michael Bukrinksy, Pierre Roques, David Boyle, and Andreas Suhrbier.
This publication was supported by The Rheumatology Research Foundation and Award Numbers UL1TR001876 and KL2TR001877 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessary represent the official views of the National Center for Advancing Translational Sciences, the National Institutes of Health, or The Rheumatology Research Foundation.
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: Disclaimer. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the United States Army.
References.
- (1).Arboviral Disease Branch. Chikungunya virus-resources for healthcare providers. Center for Disease Control. 2016. Available from https://www.cdc.gov/chikungunya/hc/resources.html. [Google Scholar]
- (2).Manimunda SP, Vijayachari P, Uppoor R, Sugunan AP, Singh SS, Rai SK, et al. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans R Soc Trop Med Hyg 2010;104(6):392–399. [DOI] [PubMed] [Google Scholar]
- (3).Powers AM. Risks to the Americas associated with the continued expansion of chikungunya virus. J Gen Virol 2015;96(Pt 1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Rahim AA, Thekkekara RJ, Bina T, Paul BJ. Disability with Persistent Pain Following an Epidemic of Chikungunya in Rural South India. J Rheumatol 2016;43(2):440–444. [DOI] [PubMed] [Google Scholar]
- (5).Lanciotti RS, Lambert AJ. Phylogenetic Analysis of Chikungunya Virus Strains Circulating in the Western Hemisphere. Am J Trop Med Hyg 2016;94(4):800–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nature Reviews Microbiology 2010;8(7):491–500. [DOI] [PubMed] [Google Scholar]
- (7).Laurent P, Le Roux K, Grivard P, Bertil G, Naze F, Picard M, et al. Development of a sensitive real-time reverse transcriptase PCR assay with an internal control to detect and quantify chikungunya virus. Clin Chem 2007;53(8):1408–1414. [DOI] [PubMed] [Google Scholar]
- (8).Parola P, de Lamballerie X, Jourdan J, Rovery C, Vaillant V, Minodier P, et al. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis 2006;12(10):1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest 2010;120(3):894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol 2010;184(10):5914–5927. [DOI] [PubMed] [Google Scholar]
- (11).Morrison TE, Whitmore AC, Shabman RS, Lidbury BA, Mahalingam S, Heise MT. Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis. J Virol 2006;80(2):737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Herrero LJ, Nelson M, Srikiatkhachorn A, Gu R, Anantapreecha S, Fingerle-Rowson G, et al. Critical role for macrophage migration inhibitory factor (MIF) in Ross River virus-induced arthritis and myositis. Proc Natl Acad Sci U S A 2011;108(29):12048–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Soden M, Vasudevan H, Roberts B, Coelen R, Hamlin G, Vasudevan S, et al. Detection of viral ribonucleic acid and histologic analysis of inflamed synovium in Ross River virus infection. Arthritis & Rheumatism 2000;43(2):365. [DOI] [PubMed] [Google Scholar]
- (14).Suhrbier A, La Linn M. Clinical and pathologic aspects of arthritis due to Ross River virus and other alphaviruses. Curr Opin Rheumatol 2004;16(4):374–379. [DOI] [PubMed] [Google Scholar]
- (15).Krejbich-Trotot P, Denizot M, Hoarau JJ, Jaffar-Bandjee MC, Das T, Gasque P. Chikungunya virus mobilizes the apoptotic machinery to invade host cell defenses. FASEB J 201;25(1):314–325. [DOI] [PubMed] [Google Scholar]
- (16).White LK, Sali T, Alvarado D, Gatti E, Pierre P, Streblow D, et al. Chikungunya virus induces IPS-1-dependent innate immune activation and protein kinase R-independent translational shutoff. J Virol 2011;85(1):606–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Teng T, Kam Y, Lee B, Hapuarachchi HC, Wimal A, Ng L, et al. A systematic meta-analysis of immune signatures in patients with acute chikungunya virus infection. J Infect Dis 2015;211(12):1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chow A, Her Z, Ong EK, Chen JM, Dimatatac F, Kwek DJ, et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis 2011;203(2):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Reddy V, Mani RS, Desai A, Ravi V. Correlation of plasma viral loads and presence of Chikungunya IgM antibodies with cytokine/chemokine levels during acute Chikungunya virus infection. J Med Virol 2014;86(8):1393–1401. [DOI] [PubMed] [Google Scholar]
- (20).Caglioti C, Lalle E, Castilletti C, Carletti F, Capobianchi MR, Bordi L. Chikungunya virus infection: an overview. New Microbiol 2013;36(3):211–227. [PubMed] [Google Scholar]
- (21).Ravichandran R, Manian M. Ribavirin therapy for Chikungunya arthritis. The Journal of Infection in Developing Countries 2008;2(02):140–142. [PubMed] [Google Scholar]
- (22).Pandya S. Methotrexate and hydroxychloroquine combination therapy in chronic chikungunya arthritis: a 16-week study. Indian Journal of Rheumatology 2008;3(3):93–97. [Google Scholar]
- (23).Bouquillard É, Combe B. A report of 21 cases of rheumatoid arthritis following Chikungunya fever. A mean follow-up of two years. Joint Bone Spine 2009;76(6):654–657. [DOI] [PubMed] [Google Scholar]
- (24).Ganu MA, Ganu A. Post-chikungunya chronic arthritis—our experience with DMARDs over two year follow up. J Assoc Physicians India 2011;59:83–86. [PubMed] [Google Scholar]
- (25).Prevoo M, Van’t Hof M, Kuper H, Van Leeuwen M, Van de Putte L, Van Riel P. Modified disease activity scores that include twenty‐eight‐joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis & Rheumatism 1995;38(1):44–48. [DOI] [PubMed] [Google Scholar]
- (26).Poo YS, Rudd PA, Gardner J, Wilson JA, Larcher T, Colle M, et al. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl Trop Dis 2014;8(12):e3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Couturier E, Guillemin F, Mura M, Leon L, Virion JM, Letort MJ, et al. Impaired quality of life after chikungunya virus infection: a 2-year follow-up study. Rheumatology (Oxford) 2012;51(7):1315–1322. [DOI] [PubMed] [Google Scholar]
- (28).Marimoutou C, Vivier E, Oliver M, Boutin JP, Simon F. Morbidity and impaired quality of life 30 months after chikungunya infection: comparative cohort of infected and uninfected French military policemen in Reunion Island. Medicine (Baltimore) 2012;91(4):212–219. [DOI] [PubMed] [Google Scholar]
- (29).Chaaithanya IK, Muruganandam N, Sundaram SG, Kawalekar O, Sugunan AP, Manimunda SP, et al. Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol 2011;24(4):265–271. [DOI] [PubMed] [Google Scholar]
- (30).Schilte C, Staikovsky F, Couderc T, Madec Y, Carpentier F, Kassab S, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis 2013;7(3):e2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Gasque P, Couderc T, Lecuit M, Roques P, Ng LF. Chikungunya virus pathogenesis and immunity. Vector-Borne and Zoonotic Diseases 2015;15(4):241–249. [DOI] [PubMed] [Google Scholar]
- (32).Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, et al. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on reunion island. Clin Infect Dis 2008. August 15;47(4):469–475. [DOI] [PubMed] [Google Scholar]
- (33).Asgari S Epigenetic modifications underlying symbiont-host interactions. Adv Genet 2014;86:253–276. [DOI] [PubMed] [Google Scholar]
- (34).Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nature Reviews Rheumatology 2013;9(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 2000;51(1):245–270. [DOI] [PubMed] [Google Scholar]
- (36).Ershler WB, Sun WH, Binkley N, Gravenstein S, Volk MJ, Kamoske G, et al. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res 1993;12(4):225–230. [PubMed] [Google Scholar]
- (37).Teo TH, Chan YH, Lee WW, Lum FM, Amrun SN, Her Z, et al. Fingolimod treatment abrogates chikungunya virus-induced arthralgia. Sci Transl Med 2017;9(375): 10.1126/scitranslmed.aal1333. [DOI] [PubMed] [Google Scholar]
- (38).Miner JJ, Cook LE, Hong JP, Smith AM, Richner JM, Shimak RM, et al. Therapy with CTLA4-Ig and an antiviral monoclonal antibody controls chikungunya virus arthritis. Sci Transl Med 2017;9(375): 10.1126/scitranslmed.aah3438. [DOI] [PMC free article] [PubMed] [Google Scholar]


