Abstract
Infections that stem from bacterial biofilms are difficult to eradicate. Within a biofilm state, bacteria are upwards of 1000-fold more resistant to conventional antibiotics, necessitating the development of alternative approaches to treat biofilm-based infections. One such approach is the development of small molecule adjuvants that can inhibit/disrupt bacterial biofilms. When such molecules are paired with conventional antibiotics, these dual treatments present a combination approach to eradicate biofilm-based infections. Previously, we have demonstrated that small molecules containing either a 2-amino pyrimidine (2-AP) or a 2-aminoimidazole (2-AI) heterocycle are potent anti-biofilm agents. Herein, we now report a scaffold hopping strategy to generate new aryl 2-AP analogs that inhibit biofilm formation by methicillin-resistant Staphylococcus aureus (MRSA). These molecules also suppress colistin resistance in colistin resistant Klebsiella pneumoniae, lowering the minimum inhibitory concentration (MIC) by 32-fold.
Infections that stem from bacterial biofilms are difficult to eradicate.
Introduction
Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and the Enterobacter species are opportunistic bacteria dubbed the ESKAPE pathogens and are notorious for their impact on human health.1 Multi-drug resistant isolates of these pathogens pose some of the most serious health threats, as a result of a diminishing number of treatment options.1 Of the ESKAPE pathogens, methicillin resistant S. aureus (MRSA) remains one of the highest in terms of infections and mortality, and is responsible for approximately 80 000 severe infections, which result in over 10 000 deaths annually in the United States alone.2
Outside of acquired resistance mechanisms, one way in which many bacteria evade the action of antibiotics is by adopting a biofilm phenotype. Biofilms are surface-associated communities of microorganisms surrounded by an extracellular matrix made up of biomolecules called the extracellular polymeric substance (EPS). As a result of several factors, bacterial cells within a biofilm exhibit up to 1000-fold increased tolerance to antibiotics than their free-floating (planktonic) counterparts. Such factors include protection imparted by the biofilm matrix itself, higher cell density, and varying metabolic rates of cells within the biofilm.3–6 Biofilms are typically present in chronic, difficult/impossible to treat infections including lung infections of cystic fibrosis (CF) patients, infections of indwelling medical devices, as well as chronic and diabetic wounds. For this reason, inhibiting biofilm formation and/or dispersing preformed biofilms is one approach to aid in combatting these recalcitrant infections.
Although the development of small molecules with anti-biofilm activity is an attractive approach to fighting chronic bacterial infections, there are still relatively few chemical scaffolds that have been developed that are effective at modulating the bacterial biofilm life cycle.7 Our group, among others, has disclosed a handful of structures with such activities.8 Many of our earlier reported scaffolds focused on functionalized 2-aminoimidazoles (2-AIs), seen in compound 1 (Fig. 1), to harness structural similarities to marine alkaloid natural products that display anti-biofilm activity against marine biofilms (commonly referred to as microfouling). Analogues of 1 were shown to inhibit the formation of Escherichia coli and P. aeruginosa biofilms.9 Later studies demonstrated that derivatized 2-aminopyrimidines (2-AP), as seen in compounds 2 and 3 (Fig. 1) inhibited the formation of MRSA biofilms10,11 In an initial study, compound 2 was the most active derivative, displaying an IC50 of 72 μM (where the IC50 is defined as the concentration at which a compound inhibits 50% of biofilm formation compared to untreated bacteria).10 Recently, we posited that the meridianins, a family of natural products that contain a 2-AP subunit, possessed anti-biofilm activity. To test this hypothesis, we synthesized a family of meridianin D derivatives and discovered that compound 3 (Fig. 1)11 inhibited the formation of MRSA biofilms and surprisingly suppressed colistin resistance in colistin resistant Gram-negative bacteria. Given the activity of these 2-AP analogs, we hypothesized that a scaffold hopping approach centered around replacing the 2-AI of 1 with a 2-AP would deliver compounds with anti-biofilm activity and/or the ability to suppress colistin resistance. By replacing the 2-AI with the 2-AP we remove a hydrogen bond donating group and increase the size of the ring. Additionally, we change the placement and identity of the aromatic rings attached to the 2-AP which proved effective on compound 3. In order to probe the SAR of this proposed class of aryl 2-AP analogs, we synthesized a library of compounds utilizing six distinct orientations: ortho, meta, and para substituted phenyl core with either C-4 or C-5 attachment to the pyrimidine head group (4, Fig. 1).
Fig. 1. Previous biofilm life cycle modulating molecules and new scaffold design.
Results and discussion
Synthesis
The synthetic approach to access C-4 linked 2-AP derivatives is outlined in Scheme 1A. Each acetophenone (5a–c) was reacted with DMF/dimethylformamide-dimethylacetal (DMF-DMA) to generate their corresponding vinylogous amide, which underwent cyclization with guanidine hydrochloride to afford the C-4 substituted 2-AP nitro intermediates 6a–c. Reduction of the nitro group followed by coupling with the appropriate derivatized benzoyl chloride in the presence of tri-basic potassium phosphate allowed access to the aryl 2-AP analogs. After purification, each 2-AP analog (8a–h) was then converted to the corresponding HCl salt for biological testing. C-5 linked derivatives were synthesized using the route depicted in Scheme 1B. Each boronic acid (10a–c) was cross-coupled with 2-amino-5-bromopyrimidine to afford 2-AP derivatives 11a–c. Reduction, coupling, and conversion to the HCl salt as above generated target analogs 13a–e.
Scheme 1. Reagents and conditions for general synthesis: A: (a) DMF-DMA, 110 °C 4 h; (b) guanidine hydrochloride, 2-methoxyethanol, reflux, 16 h; (c) H2, Pd/carbon, rt, 16 h; (d) ArCOCl, K3PO4, dry THF, 0 °C to rt, 10 h; (e) HCl/MeOH; B: (a) PdCl2(PPH3)2, Na2CO3, dry THF, reflux 20 h; (b) H2, Pd/carbon, rt, 16 h; (c) ArCOCl, K3PO4, dry THF, 0 °C to rt, 10 h; (d) HCl/MeOH.
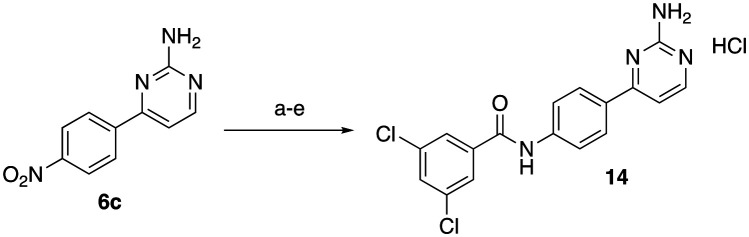
While this synthetic route was used for the majority of analogues, initially we attempted to mask the reactivity of the exocyclic pyrimidine amine using Boc protection. Although this ultimately proved unnecessary, five analogues (compounds 14, and 15a–d) were synthesized via this lengthier route that involved protection and deprotection steps. To synthesize the C-4 linked 2-AP with a 3,5-dichlorobenzoyl tail (14, Scheme 2), intermediate 6c was di-Boc protected using Boc-anhydride and DMAP. Following Boc-protection, reduction, acylation, and purification proceeded smoothly. The intermediate 2-AP was then deprotected using TFA in DCM and converted to its corresponding HCl salt for testing.
Scheme 2. Reagents and condition for synthesis of compound 14: (a) Boc-anhydride, DMAP, DCM, 18 h; (b) H2, Pd/carbon, rt, 16 h; (c) 3,5-dichlorobenzoyl chloride, K3PO4, dry THF, 0 °C to rt, 10 h; (d) TFA, DCM (e)HCl/MeOH.
The four remaining compounds were accessed using the synthetic approach summarized in Scheme 3. 2-Amino-5-bromopyrimidine (9) was Boc protected using Boc anhydride and pyridine. Following Boc protection, a Suzuki reaction was carried out as before with either 2- or 3-nitroboronic acid. With 2-nitro boronic acid, we noted that both Boc groups remained intact after cross-coupling, while reaction with 3-nitroboronic acid resulted in cleavage of one of the Boc groups. Following reduction of the nitro-groups to their corresponding anilines, acylation was carried out as before. Finally, each 2-AP analog was deprotected using TFA in DCM, that, following salt exchange, delivered compounds 15a–d (Scheme 3).
Scheme 3. Reagents and condition for synthesis of compounds 15a–d: (a) Boc-anhydride, pyridine 18 h (b) boronic acid, PdCl2(PPh3)2, Na2CO3, dry THF, reflux 20 h; (c) H2, Pd/carbon, rt, 16 h; (d) corresponding benzoyl chloride, K3PO4, dry THF, 0 °C to rt, 10 h; (e) TFA, DCM (f) HCl/MeOH.
Biological evaluation
All compounds were first evaluated for their ability to inhibit MRSA biofilms. Initially, each compound was tested at 200 μM against MRSA (strain ATCC 43300) and inhibition was measured using a crystal violet assay as previously reported.12 Compounds that exhibited greater than 50% inhibition at 200 μM were then subjected to a dose response assay to determine their IC50 values (Table 1).
IC50's of aryl 2-APs against MRSA 43300 biofilms.
| Compound | 8a | 8b | 8c | 8d | 8e | 8f |
| IC50 (μM) | >200 | >200 | >200 | >50 | 26.4 ± 4.9 | >200 |
| Compound | 8g | 8h | 13a | 13b | 13c | 13d |
| IC50 (μM) | 17.4 ± 6.4 | >50 | >200 | >200 | >200 | 43 ± 8.3 |
| Compound | 13e | 14 | 15a | 15b | 15c | 15d |
| IC50 (μM) | >100 | 41 ± 12 | >200 | >100 | >200 | 69 ± 16 |
From the 18-compound pilot library, only five analogues had IC50's less than 100 μM (Table 1). Two of these analogues contained the 3,5-dichloro tail while the other three compounds possessed the 3,5-dibromo tail. The highest activity was observed when the 2-AP was substituted at C-4 (versus the C-5) and the amide linker was positioned meta to the 2-AP (8e and 8g). These two compounds displayed IC50's of 26.4 ± 4.9 μM (8e) and 17.4 ± 6.4 μM (8g). Compounds 14, 13d and 15d all displayed moderate activity. Analog 14 (IC50 = 41 ± 12 μM) possessed a 3,5-dichloro tail placed at the para position of the phenyl core and substituted at C-4 of the 2-AP. Compound 13d (IC50 = 43 ± 8.3 μM) had a 3,5-dibromo tail placed at the meta position of the phenyl core and substituted at C-5 of the 2-AP. Finally, 15d (IC50 = 69 ± 16 μM) had the 3,5-dibromo tail placed at the ortho position of the phenyl core and substituted at C-5 of the 2-AP.
We next tested the two most active compounds, 8e and 8g, for their anti-biofilm activity against additional MRSA strains, as well as a methicillin susceptible S. aureus strain. Both compounds had comparable IC50's across each of the four strains tested (Table 2) demonstrating that these compounds are active against multiple MRSA strains. Following this, we sought to determine whether these compounds were specifically inhibiting biofilm formation, and not simply inhibiting planktonic growth under the conditions of the biofilm assay. To that end, we constructed time kill curves using strain 43 300 as our test bacterium and compared growth in the absence and presence of compounds 8e and 8g at their IC50 concentration (Fig. S1†). Growth curves in the absence and presence of either compound were similar, establishing that these compounds are not toxic to planktonic bacteria and could potentially demonstrate a lower frequency of resistance.
IC50's of lead aryl 2-AP analogues against panel of S. aureus strains biofilms.
| Compound | MRSA 43300 (μM) | MRSA BAA-44 (μM) | MRSA 33591 (μM) | S. aureus 6538 (μM) |
|---|---|---|---|---|
| 8e | 26.4 ± 4.9 | 29.5 ± 2.1 | 26.6 ± 4.1 | 24.9 ± 4.2 |
| 8g | 17.4 ± 6.4 | 14.1 ± 2.2 | 20.2 ± 3.0 | 15.6 ± 6.4 |
Next, we screened our compounds for their ability to disperse preformed biofilms. None of the 18 compounds demonstrated any dispersion activity against MRSA ATCC 43300 at a concentration of 100 μM.
Due to the activity of the meta 3,5-dichloro (8e) and the meta 3,5-dibromo (8g) analogues, we elected to construct and screen a hybrid analogue containing one chloro and one bromo substituent. To access this derivative, we acylated compound 7b with 3-bromo-5-chlorobenzoic acid (16) under standard EDC coupling conditions. Purification and conversion to the corresponding HCl salt as previously described yielded compound 17 (Scheme 4). With this compound in hand, we first assessed the MIC of the compound and determined it to be >200 μM. We then evaluated its biofilm inhibition properties and determined that its IC50 was 27.6 ± 9.6 μM, which is within the error of both 8e and 8g.
Scheme 4. Reagents and condition for synthesis of compound 17: (a) EDC, DMAP, DCM, 18 h (b) HCl/MeOH.
Finally, having previously shown that analogs of the 2-aminopyrimidine-containing marine sponge natural product meridianin D potentiated colistin in Gram-negative bacteria, we probed whether these compounds also potentiated colistin. To that end, we first determined the minimum inhibitory concentration (MIC) of each compound against A. baumannii (AB 4106) and K. pneumoniae (KP B9), both of which have chromosomally encoded colistin resistance. All 18 compounds registered an MIC of ≥200 μM. We then screened each compound at 60 μM in combination with colistin to determine the effect on colistin activity. The MIC of colistin was 1024 and 512 μg mL−1 against AB 4106 and KP B9, respectively, as previously reported.11,13
None of the compounds lowered the MIC of colistin against AB 4106 below 64 μg mL−1 at 60 μM. However, one compound from this pilot library, compound 8g, did lower the MIC of colistin from 512 to 16 μg mL−1 against KP B9, a 32-fold reduction. Interestingly, compound 8g was also the most active MRSA biofilm inhibitor out of the 18-compound library. With antibiotic-adjuvants, we look for adjuvants that can lower the MIC of an antibiotic to its breakpoint against a bacterial strain. The colistin breakpoint in AB 4106 and KP B9 is 2 μg mL−1; therefore, further diversification of this scaffold may allow for a new class of molecules that can potentiate colistin activity.
To determine if 8g exhibits synergy with colistin, we performed checkerboard assays with compound 8g and colistin in KP B9, and observed a fractional inhibitory concentration index (FICI) of ≤0.09 (Table S3†). We also performed the checkerboard assay in two additional strains of K. pneumoniae; A5, an additional highly colistin resistant clinical isolate, and F2210219mcr-1, an engineered strain harboring a plasmid containing the mcr-1 gene.14 In both strains, compound 8g exhibited synergy with colistin, returning FICI values of ≤0.31 and ≤0.19 for A5 and F2210219mcr-1 respectively (Table S3†).
Resistance to colistin typically involves modification of the lipid A anchor of the lipopolysaccharide (LPS) of Gram-negative bacteria. To begin probing the mechanism of action for this decrease in colistin resistance we analyzed lipid A extracted from KP B9 grown in the absence and presence of 8gvia mass spectrometry. No change in the lipid A substitution pattern was noted between the two samples, indicating that these compounds potentiate colistin through a mechanism not dependent upon reversing lipid A modification. Further investigation into the mechanism of action is ongoing.
Finally, we tested the hemolytic activity of compounds 8g and 8e by performing a hemolysis assay using defibrinated sheep's blood challenged with either compound. Triton-X (1%) used as the 100% lysis marker, and phosphate buffered saline as the 0% lysis marker. Compounds 8e and 8g only lysed 5% and 3.4% of cells respectively when dosed at 200 μM, a concentration higher than the IC50 of either compound suggesting that there would be little to no lysis of eukaryotic cells when dosed at a lower concentration.
Conclusions
In conclusion, we have employed a scaffold hopping strategy to develop compounds with anti-biofilm activity against MRSA. While the lead compound 8g shows comparable inhibitory activity with previously reported compounds, none of the disclosed compounds were able to disperse preformed biofilms. The lead compound 8g also suppressed resistance to the polymyxin antibiotic colistin in K. pneumoniae, encouraging future structural derivatizations that could increase this activity. We determined that the specific connectivity between the 2-AP head, the phenyl core, and the aryl tail has a significant effect on activity, and studies to diversify this scaffold further are underway.
The authors would like to thank the National Institutes of Health (AI136904 and DE022350) for support.
Conflicts of interest
Dr. C. Melander is a co-founder of Agile Sciences, a biotechnology company seeking to commercialize antibiotic adjuvants.
Supplementary Material
Electronic supplementary information (ESI) available: Synthetic methods, compound characterization, and assays methods. See DOI: 10.1039/d0md00238k
Notes and references
- Rice L. B. J. Infect. Dis. 2008;197(8):1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- CDC, 2013
- Davies D. Nat. Rev. Drug Discovery. 2003;2(2):114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- Stewart P. S. Costerton J. W. Lancet. 2001;358(9276):135–138. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- Donlan R. M. Costerton J. W. Clin. Microbiol. Rev. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T. B. Givskov M. Int. J. Med. Microbiol. 2006;296(2–3):149–161. doi: 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Richards J. J. Melander C. ChemBioChem. 2009;10(14):2287–2294. doi: 10.1002/cbic.200900317. [DOI] [PubMed] [Google Scholar]
- Yan Z. Huang M. Melander C. Kjellerup B. V. J. Appl. Microbiol. 2020;128(5):1279–1288. doi: 10.1111/jam.14491. [DOI] [PubMed] [Google Scholar]
- Bunders C. A. Richards J. J. Melander C. Bioorg. Med. Chem. Lett. 2010;20(12):3797–3800. doi: 10.1016/j.bmcl.2010.04.042. [DOI] [PubMed] [Google Scholar]
- Lindsey E. A. Worthington R. J. Alcaraz C. Melander C. Org. Biomol. Chem. 2012;10(13):2552–2561. doi: 10.1039/C2OB06871K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins W. M. Barker W. T. Baker J. T. Hahn N. A. Melander R. J. Melander C. ACS Med. Chem. Lett. 2018;9(7):702–707. doi: 10.1021/acsmedchemlett.8b00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole G. A. Kolter R. Mol. Microbiol. 1998;30(2):295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- Nemeth A. M. Basak A. K. Weig A. W. Marrujo S. A. Barker W. T. Jania L. A. Hendricks T. A. Sullivan A. E. O'Connor P. M. Melander R. J. Koller B. H. Melander C. ChemMedChem. 2020;15(2):210–218. doi: 10.1002/cmdc.201900560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Y. Chandler C. E. Leung L. M. McElheny C. L. Mettus R. T. Shanks R. M. Liu J. H. Goodlett D. R. Ernst R. K. Doi Y. Antimicrob. Agents Chemother. 2017;61(6):e00580-17. doi: 10.1128/AAC.00580-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.