Abstract
Background
Cognitive and behavioral impairment are common in children living with perinatally acquired HIV (pHIV) and children exposed to HIV in utero but uninfected (HEU).
Methods
We sought to determine the prevalence of adverse behavioral symptomatology using a Thai-translated and validated version of the SNAP-IV questionnaire and assess cognitive function utilizing the Children's Color Trails Test, Delis-Kaplan Executive Function System, and the Wechsler Intelligence Scales, in our cohort of Thai adolescents (10–20 years old) with well-controlled pHIV compared to HEU and HIV-unexposed, uninfected youth. We then evaluated the interaction between HIV status, behavioral impairment, and executive function outcomes independent of demographic variables.
Results
After controlling for demographic factors of age and household income, adolescents with pHIV had higher inattentive symptomatology and poorer neuropsychological test scores compared to uninfected controls. Significant interactions were found between inattention and executive function across multiple neurocognitive tests.
Conclusions
Behavioral impairment and poor executive functioning are present in adolescents with well-controlled pHIV compared to HIV-uninfected matched peers. The SNAP-IV questionnaire may be a useful tool to identify those with attentional impairment who may benefit from further cognitive testing in resource-limited settings.
Key words: Adolescents, behavioral health, cognition, perinatal HIV
Introduction
Children and adolescents living with perinatally acquired HIV (pHIV) or exposed to HIV in utero but uninfected (HEU) are prone to cognitive impairment and psychiatric disorders, including executive dysfunction and attention-deficit and hyperactivity disorder (ADHD) (Gadow et al., 2010; Nichols et al., 2016). Cognitive impairment and psychiatric disorders often co-occur and are linked to poor academic performance (Evans et al., 2019). Therefore, efficient tools to identify children who are most at risk for cognitive impairment are important for the millions of children living with pHIV, particularly those residing in resource-limited settings.
Complex interactions exist between demographic factors and HIV-associated co-morbidities, both of which may independently influence neurologic conditions in adolescents affected by HIV (Mellins et al., 2009). Differentiating the impact of demographic factors, psychiatric disorders, and HIV disease on cognitive functioning remains difficult. Additionally, although there are relatively limited international studies on neurodevelopment in adolescents with HIV living in low- and middle-income countries (LMIC), those that exist lack appropriate local control groups or utilize normative data from native English speakers to identify cognitive impairment in non-English-speaking populations. These studies may, therefore, inaccurately characterize the prevalence of behavioral and cognitive impairment in adolescents with well-controlled HIV infection in LMIC settings (Phillips et al., 2018).
Prior work by our group has demonstrated higher rates of interruptive and hyperactive behaviors as assessed by the Child Behavior Checklist (CBCL) in Thai and Cambodian children with pHIV compared to normative HIV-uninfected controls (Kerr et al., 2019). In particular, for Thai adolescents with pHIV, more caregiver-reported behavioral problems on the CBCL correlated with lower full-scale IQ scores, while no correlation was found between cognitive function and behavioral symptoms among HIV-uninfected youth (Puthanakit et al., 2013). In this study, we sought to expand on these findings by (1) determining the prevalence of inattention and hyperactive symptomatology using the Thai translated and validated SNAP-IV questionnaire in our cohort of Thai adolescents with well-controlled pHIV, (2) determining the prevalence of executive dysfunction in our cohort using regression-based norms extrapolated from our healthy Thai uninfected youth who served as controls, and (3) evaluating the correlation between HIV status, behavioral symptomatology, and cognitive measures of executive function.
Methods
Study participants
Two hundred and five Thai adolescents, ages 10–20 years, were included in the current study. Participants were recruited from a larger sample enrolled in the Resilience study (R01MH102151-05). The Resilience study is a longitudinal cohort study evaluating psychiatric and cognitive outcomes of adolescents living with pHIV, adolescents with HIV exposure in utero, but uninfected (HEU) and HIV-unexposed, uninfected (HUU), age and sex-matched peers at seven clinical sites in Thailand (Puthanakit et al., 2013). HUU and HEU youth were recruited from local health clinics or were siblings of our pHIV participants. HIV status was confirmed using standard immunoassay for serum HIV antibodies.
All participants with pHIV received combination antiretroviral therapy (cART) for a minimum of 12 months prior to the start of the study and had no history of AIDS-defining illnesses (CDC category C) or in utero exposure to maternal antiretroviral therapy or illicit substances. HIV viral load and CD4 count were assessed during a concurrent clinical visit. First-line cART included zidovudine, lamivudine, or nevirapine. Children who experienced side effects to these regimens were switched to efavirenz or lopinavir with ritonavir. Demographic information was collected using questionnaires provided directly to caregivers to read and complete. For caregivers who were unable to read, the study staff read the questions aloud and the caregivers' verbal responses were recorded. Questionnaire data included monthly household income, caregiver status [living with parent(s), relatives, or in an orphanage], and caregiver education (years of schooling completed).
Informed consent was obtained from caregivers in writing and verbal assent from adolescents prior to study enrollment. The study was approved by Thai national and site-specific institutional review boards.
Measures
SNAP-IV questionnaire
The SNAP-IV questionnaire (Swanson et al., 1981) is a caregiver-rated questionnaire, which evaluates inattentive and hyperactive symptomatology in children aged 6 years and older. The questionnaire is comprised of 18 questions, answered using a four-point rating scale for each question (0 = not at all, 1 = just a little, 2 = pretty much, 3 = very much). Subscale scores for inattention (nine items) and hyperactivity (nine items) are calculated by summing scores on individual questions. The SNAP-IV questionnaire has been translated from English into Thai and validated in a healthy Thai pediatric cohort (Pityaratstian et al., 2014). This validation study reported questionnaire cutoff scores for identifying Thai children with symptomatic impairment on the inattentive (cutoff score of 14) and hyperactive (cutoff score of 12) subscales (Pityaratstian et al., 2014). These subscale cutoff scores were applied in the current study to identify participants with inattentive or hyperactive behavioral impairment.
Neurocognitive measures
Neuropsychological testing was conducted by Thai psychologists or Thai nurses. Trained nurses were certified after correctly completing and scoring a minimum of 10 participants per test under supervision. Test instructions were translated and back-translated into Thai by bilingual translators (Puthanakit et al., 2013). Executive function measures included the Children's Color Trails Test (CCT) (Llorente et al., 2008) for youth <17 years, the design fluency and verbal fluency measures of the Delis-Kaplan Executive Function System (D-KEFS) (Delis et al., 2001), and the freedom from distractibility index on the Wechsler Intelligence Scale for Children, Third Edition (WISC-III) (Wechsler, 1997) for participants 17 years and under or the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) (Wechsler, 2003) for those 18 years or older. The CCT1 and 2 measure sustained attention, sequencing, psychomotor speed, and planning. In addition, CCT2 also measures task shifting and inhibition. The design fluency and verbal fluency subtests assess verbal and non-verbal fluency, inhibition, problem-solving, attention, and concept formation. Working memory and attention were assessed using the freedom from distractibility index on the WISC-III or WAIS-III.
Statistical analyses
Analyses were conducted using SPSS (Version 26.0, Armony, NY, USA, IBM Corp).
Calculating neuropsychology test Z-scores using a regression-based method
Regression-based norms were derived from the HUU group. Regression-based normalization methodology accounts for general developmental and demographic trends in the data. Using linear regression modeling, we controlled for demographic effects on neuropsychology test performance by generating continuous norms. Using the HUU normative sample, participants raw scores were transformed to regression-based normalized Z-scores (higher Z-score = better performance; lower Z-score = worse performance) for each neuropsychology test based on β weight values derived for age, sex, caregiver education, and household income from the HUU normative sample (Sithinamsuwan et al., 2014).
In addition, we examined all variables included in the final analyses for outliers, non-normative distributions, and missing variables. Few outliers (i.e. Z-score above +3 or below −3) were found after transforming neuropsychological test scores. HIV group sizes differed between analyses due to missing data on the focal outcome variables. Sample size for each analysis is provided in online Supplementary Table S1.
Determining demographic covariates of interest
We conducted correlational (for continuous data) and χ2 tests (for nominal data) to determine which demographic variables were related to outcome variables in each analysis. Variables found to differentiate an outcome variable were included as covariates. Results indicated that children's age and household income were related to SNAP-IV subscales and several, but not all neuropsychological measures. Thus, to keep the analyses parallel and to improve our ability to compare results across analyses, we included household income and age as covariates across all analyses described below.
Evaluating HIV group differences in SNAP-IV and neuropsychological test scores
To test for HIV group differences on SNAP-IV inattentive and hyperactive subscale scores and neuropsychological performance, we utilized MANCOVA. For the MANCOVA, HIV group (pHIV, HEU, and HUU) was the independent variable, age and household income were included as covariates, and SNAP-IV inattention and hyperactive subscale or neuropsychological test scores were the outcome variables.
Evaluating the interaction between SNAP-IV scores and executive function measures by HIV status
Lastly, we conducted a series of five separate multiple linear regression analyses to examine the association between inattention and hyperactivity on neuropsychology test performance. For all regression models, inattentive and hyperactive subscale scores were the independent variables, age and household income were the covariates, and CCT1, CCT2, verbal fluency, design fluency, and freedom from distractibility index were tested separately as the five outcome variables. Bonferroni adjustment was utilized to account for multiple comparisons. To examine the main effects of HIV status and the two SNAP-IV subscale scores as well as the interaction between HIV status and SNAP-IV subscale scores, we used the PROCESS macro for SPSS 26 (Hayes and Rockwood, 2017). This analysis allowed us to test whether HIV status had a significant main effect on the focal neurocognitive outcome measures and whether the expected effect of HIV status on neurocognitive outcomes was moderated by SNAP-IV inattentive or hyperactive subscale scores. We conducted five separate regression analyses as follows: (1) age and income were included as covariates; (2) HIV status, inattentive subscale scores, and hyperactive subscale scores were included as main effects, and (3) HIV status × inattentive subscale scores as well as HIV status × hyperactive subscale scores were analyzed as two separate two-way interactions. All analyses were conducted using n = 5000 bootstrap samples and bias-corrected 95% confidence intervals. Interactions were considered significant if the results demonstrated a significant (p < 0.05) change in R2 above and beyond the variance accounted for by covariates or main effects. False discovery rate of p < 0.05 was used to account for multiple comparison conducted within each regression.
Results
Two hundred and five adolescent-caregiver dyads (n = 59 pHIV, n = 67 HEU, and n = 79 HUU) completed the SNAP-IV questionnaire. Table 1 displays the demographic characteristics for the total sample and Table 2 provides clinical information specific to the pHIV subgroup. Median age was 15 years and slightly over half (56%) were female. The majority (75%) resided with at least one biologic parent who was the primary caregiver. All adolescents with pHIV were on cART for at least 3 years (ranging from 3 to 12 years) with 93% achieving viral suppression (defined as viral load <40 copies/mL) at the time the questionnaires were completed. The median age of cART initiation was 6 years (ranging from 1 to 15 years of age). The HEU and HUU groups were more likely than the pHIV group to live with their biologic parent. Additionally, Thai adolescents affected by HIV (pHIV and HEU groups) had significantly lower household income and had caregivers with fewer years of education compared to the HUU group (Table 1).
Table 1.
Demographic characteristics of HIV subgroups and total sample
| pHIV | HEU | HUU | Total sample | p value | |
|---|---|---|---|---|---|
| Age: mean (s.d.) | 15.28 (2.04) | 14.57(2.02) | 14.62 (2.23) | 14.79 (2.13) | 0.11 |
| Sex: n (%) | |||||
| Female | 35 (59%) | 37 (55%) | 43 (54%) | 115 (56.1%) | 0.84 |
| Male | 24 (41%) | 30 (45%) | 36 (46%) | 90 (43.9%) | |
| Primary caregiver: n (%) | |||||
| Biologic parent | 24 (41%) | 61 (92%) | 66 (85%) | 151 (74%) | <0.001 |
| Other relative | 20 (34%) | 4 (6%) | 12 (15%) | 36 (18%) | |
| Orphanage | 15 (25%) | 1 (2%) | 0 (0%) | 16 (8%) | |
| Caregiver education | |||||
| ⩽6 years n (%) | 28 (48%) | 32 (48%) | 14 (18%) | 74 (36%) | <0.001 |
| 7–12 years | 14 (34%) | 27 (40%) | 25 (32%) | 66 (32%) | |
| >12 years | 16 (16%) | 8 (12%) | 40 (51%) | 64 (32%) | |
| Household income (Thai baht) | |||||
| Monthly income; mean (s.d.) | 18480 (16208) | 21674 (19377) | 36843 (23685) | 26914 (21999) | <0.001 |
s.d., standard deviation, pHIV, perinatally acquired HIV; HEU, HIV exposure in utero but HIV uninfected; HUU, HIV-unexposed and uninfected.
Table 2.
Clinical characteristics of children in the pHIV group
| Variable | pHIV |
|---|---|
| CDC-category: n (%) | |
| N | 10 (17%) |
| A | 33 (57%) |
| B | 15 (26%) |
| Viral load | |
| <40 | 53 (93%) |
| Detectable (>40) | 4 (7%) |
| Current CD4 count (range) | 788 (104–1590) |
| Current CD4 % (range) | 31 (5.7–42) |
| CD4 nadir (range) | 415 (4–1108) |
| History of efavirenz exposure (%) | |
| Yes | 24 (40.6%) |
| No | 35 (59.3%) |
| On cART (%) | 59 (100%) |
| Average duration of ART exposure (range in years) | 9 (3–12) |
| Average age at ART initiation (range in years) | 6 (1–15) |
SNAP-IV by HIV subgroup
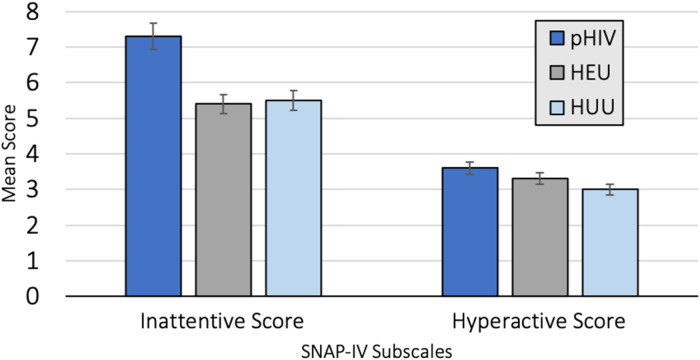
Twenty children received scores above the impairment threshold on the SNAP-IV questionnaire (9.8% of the total cohort). Of these children, 11 (5.4%) had the primary inattentive subtype, four (2%) had the primary hyperactive subtype, and five (2.4%) had both inattentive and hyperactive subtypes. Results from the MANCOVA analysis indicated that HIV group status had a significant multivariate effect on SNAP-IV scores, Wilks' λ = 0.95, F(4,384) = 2.50, p = 0.04, partial η2 = 0.03, CI 0.0004–0.05. Univariate follow-up analyses indicated that HIV group status had a significant effect on the SNAP-IV inattentive subscale but not the hyperactive subscale (see Table 3 and Fig. 1). Post hoc analyses on the inattentive subscale were conducted using Bonferroni-adjusted pairwise comparisons. Results indicated that children in the pHIV group had significantly higher inattention subscale scores compared to children in the HEU (mean difference = 2.12, p = 0.03, partial η2 = 0.04, 95% CI 0.02–0.14) and HUU (mean difference = 2.47, p = 0.008, partial η2 = 0.05, 95% CI 0.004–0.14) groups after accounting for covariates of household income and age. The HEU and HUU groups did not differ significantly on inattention subscale scores (mean difference = 0.35, p = 0.74, partial η2 = 0.001, 95% CI 0–0.03). There was no significant effect of HIV status on hyperactive subscale scores.
Table 3.
MANCOVA and ANCOVA results examining the effect of HIV group status on neurocognitive functioning
| Neuropsychology tests | pHIV | HEU | HUU | F(df) | p | η2 | η2 CI 95% |
|---|---|---|---|---|---|---|---|
| MANCOVA #1 | |||||||
| Children's Color Trails Test 1 | −0.50 (1.31) | −0.41 (1.17) | 0.03 (0.98) | 2.13(2134) | 0.12 | 0.03 | 0.0 to 0.10 |
| Children's Color Trails Test 2 | −0.62 (1.10) | −0.27 (1.01) | 0.02 (0.96 | 5.17(2134) | 0.007 | 0.07 | 0.01 to 0.16 |
| MANCOVA #2 | |||||||
| Verbal fluency | −0.38 (0.86) | −0.12 (0.85) | −0.02 (0.94) | 1.16(2175) | 0.32 | 0.01 | 0.0 to.06 |
| Design fluency | −0.64 (1.00) | −0.21 (0.99) | −0.03 (0.92) | 4.45(2175) | 0.01 | 0.05 | 0.002 to 0.12 |
| Freedom from distractibility | −0.95 (0.91) | −0.43 (0.98) | −0.04 (0.93) | 11.43(2175) | 0.00002 | 0.12 | 0.04 to 0.20 |
Mean and (standard deviations) for neurocognitive Z-scores are reported.
Fig. 1.
(a) HIV group status and SNAP-IV subscale scores with 95% confidence intervals. (b) Results from univariate analyses examining the effect of HIV group on SNAP-IV scores.
Cognitive testing by HIV subgroup
Children's Color Trail Tests 1 and 2 (CCT1 and CCT2)
Results from the first MANCOVA indicated a significant multivariate effect of HIV group status on the CCT1 and 2 [Wilks' λ = 0.95, F(4,266) = 2.79, p = 0.03, partial η2 = 0.04, CI 0.002–0.07]. Univariate follow-up results indicated that HIV status did not have a significant effect on CCT1 scores but did have a significant effect on CCT2 scores (see Table 3, MANCOVA #1). Post hoc analyses examining the effect of HIV group classification on CCT2 scores indicated that the pHIV group had significantly lower scores on the CCT2 neurocognitive test compared to the HEU (mean difference = 0.49, p = 0.03, partial η2 = 0.06, 95% CI 0.002–0.15) and HUU groups (mean difference = 0.70, p = 0.001, partial η2 = 0.12, 95% CI 0.03–0.22). The HEU and HUU groups did not differ significantly on CCT2 performance (mean difference = 0.22, p = 0.15, partial η2 = 0.02, 95% CI 0–0.09).
Freedom from distractibility, design fluency, and verbal fluency
Findings from the MANCOVA indicated a significant multivariate effect of HIV group status on the three neurocognitive test outcomes [Wilks' λ = 0.88, F(6,346) = 3.76, p = 0.001, partial η2 = 0.06, CI 0.02–0.09]. As seen in Table 3, under MANCOVA #2, there was a significant univariate effect of HIV group status on design fluency as well as freedom from distractibility scores, but no effect of HIV group on verbal fluency. Post hoc analyses were conducted to examine the effect of HIV group on the two significant outcomes. Specifically, the pHIV group scored significantly lower on the design fluency task compared to the HEU (mean difference = 0.45, p = 0.02, partial η2 = 0.05, 95% CI 0.004–0.13) and HUU (mean difference = 0.53, p = 0.003, partial η2 = 0.07, 95% CI 0.02–0.16) groups. The HEU and HUU groups did not differ significantly on verbal fluency performance (mean difference = 0.08, p = 0.60, partial η2 = 0.002, 95% CI 0.0–0.03). Participants in the pHIV group scored significantly lower on the freedom from distractibility task compared to the HEU (mean difference = 0.58, p = 0.0002, partial η2 = 0.11, 95% CI 0.03–0.21) and HUU (mean difference = 0.84, p = 0.000005, partial η2 = 0.15, 95% CI 0.07–0.25) groups. Furthermore, the HEU group scored significantly lower on the freedom from distractibility task compared to HUU group (mean difference = 0.26, p = 0.04, partial η2 = 0.03, 90% CI 0.001–0.09).
SNAP-IV subscale scores predicting neurocognitive performance
Multiple linear regression was conducted to assess the effect of participants SNAP-IV scores on neurocognitive measures of executive function, while controlling for age and household income (Table 4).
Table 4.
Results of the five multiple regression analyses
| t | p | β | F | df | p | R2adjusted | |
|---|---|---|---|---|---|---|---|
| Model 1 | |||||||
| Children's Color Trails Test 1 | 4.13 | 7131 | 0.0004 | 0.16 | |||
| Age | 2.31 | 0.02 | 0.13 | ||||
| Household income | 1.92 | 0.06 | 0.09 | ||||
| Inattention subscale | −3.27 | 0.001 | −0.10 | ||||
| Hyperactive subscale | 0.56 | 0.57 | 0.02 | ||||
| HIV status | −1.23 | 0.22 | −0.16 | ||||
| HIV status × inattention subscale | 1.15 | 0.25 | 0.04 | ||||
| HIV status × hyperactive subscale | −0.20 | 0.84 | −0.01 | ||||
| Model 2 | |||||||
| Children's Color Trails Test 2 | 3.13 | 7131 | 0.004 | 0.14 | |||
| Age | 2.90 | 0.004 | 0.16 | ||||
| Household income | 0.56 | 0.58 | 0.03 | ||||
| Inattention subscale | −2.42 | 0.02 | −0.07 | ||||
| Hyperactive subscale | 0.33 | 0.74 | 0.01 | ||||
| HIV status | −2.34 | 0.02 | −0.27 | ||||
| HIV status × inattention subscale | 0.33 | 0.74 | 0.01 | ||||
| HIV status × hyperactive subscale | 0.13 | 0.90 | 0.006 | ||||
| Model 3 | |||||||
| Verbal fluency | 2.38 | 7173 | 0.02 | 0.05 | |||
| Age | −0.44 | 0.66 | −0.02 | ||||
| Household income | 2.35 | 0.02 | 0.00 | ||||
| Inattention subscale | −1.90 | 0.06 | −0.04 | ||||
| Hyperactive subscale | 1.04 | 0.30 | 0.03 | ||||
| HIV status | −0.79 | 0.43 | −0.07 | ||||
| HIV status × inattention subscale | −0.42 | 0.67 | −0.01 | ||||
| HIV status × hyperactive subscale | 1.04 | 0.30 | 0.04 | ||||
| Model 4 | |||||||
| Design fluency | 5.37 | 7173 | 0.00001 | 0.15 | |||
| Age | 1.31 | 0.19 | 0.05 | ||||
| Household income | 1.96 | 0.05 | 0.000007 | ||||
| Inattention subscale | −3.62 | 0.0004 | −0.08 | ||||
| Hyperactive subscale | 1.71 | 0.09 | 0.04 | ||||
| HIV status | −2.02 | 0.04 | −0.19 | ||||
| HIV status × inattention subscale | 0.57 | 0.56 | 0.02 | ||||
| HIV status × hyperactive subscale | 0.74 | 0.46 | 0.03 | ||||
| Model 5 | |||||||
| Freedom from distractibility | 9.97 | 7187 | <0.00001 | 0.21 | |||
| Age | 3.06 | 0.002 | 0.11 | ||||
| Household income | 2.47 | 0.01 | 0.000007 | ||||
| Inattention subscale | −1.97 | 0.05 | −0.04 | ||||
| Hyperactive subscale | 1.28 | 0.20 | 0.03 | ||||
| HIV status | −4.82 | 0.000003 | −0.43 | ||||
| HIV status × inattention subscale | 1.34 | 0.18 | 0.04 | ||||
| HIV status × hyperactive subscale | −1.69 | 0.09 | −0.06 | ||||
Dependent variable and overall model fit statistics for each of the five models tested are shaded in grey.
Children's Color Trail 1 (CCT1)
Our results indicated that children who were younger, from households with lower incomes, and had higher inattentive subscale scores had worse CCT1 scores. Hyperactive subscale scores were not significantly associated with CCT1 scores.
Children's Color Trail 2 (CCT2)
Results indicated that younger age and higher inattentive subscale scores were significantly associated with lower scores on the CCT2. Household income and hyperactive scores were not associated with CCT2 performance.
Verbal fluency (VF)
Lower household income and higher inattentive subscale scores were predictive of poorer performance on the VF test. Participants' age and hyperactive subscale scores were not significantly related to VF scores.
Design fluency (DF)
Lower household income and higher inattention SNAP-IV subscale scores predicted significantly worse DF scores. Age and hyperactive subscale scores were not significantly related to children's performance on DF.
Freedom from distractibility (FD)
Worse performance on the freedom from distractibility task was associated with younger age, lower household income, and higher scores on the inattentive SNAP-IV subscale. Children's scores on the hyperactive subscale were not significantly associated with FD performance.
Results examining main and interactive effects of HIV status and SNAP-IV subscales on neurocognitive performance are provided in Table 4. Poorer performance on cognitive testing was predicted by higher SNAP-IV inattentive subscale scores and HIV status on select tests. These results were not related to the interaction between HIV status and SNAP-IV subscale scores. These data indicate that HIV status and higher inattentive subscale scores were significant independent predictors of worse cognitive performance on executive function, but the interaction between these two effects was not significant.
Discussion
Our study revealed that Thai adolescents with well-controlled HIV display similar rates of impairment in attention and hyperactivity compared to other cohorts of adolescents with pHIV in the USA and Kenya (12%; Gadow et al., 2010; Mellins et al., 2010; Kamau et al., 2012) and higher rates than those reported in children with pHIV residing in Uganda (6%) and Nigeria (4.9%; Mpango et al., 2017; Adefalu et al., 2018). This variability likely reflects cultural, cohort, and methodological study differences. Our findings demonstrate the relatively higher prevalence of inattention symptomatology even in adolescents with HIV who are virally suppressed on cART compared to local age- and sex-matched HIV-uninfected peers after controlling for demographic differences. Adolescents with pHIV also had significantly lower scores on the measures of executive function compared to HIV-uninfected controls across multiple neuropsychological tests. These findings are consistent with published findings by our group and mirror those from prior studies in the US-based PHACS cohort (Nichols et al., 2016). Additionally, the HEU group, which matched the pHIV group in terms of household income and caregiver education, performed similarly to the HUU group on most measures of behavioral symptomatology and executive function with the exception of the freedom from distractibility index. These data suggest that HIV may play a significant role in neurologic injury in youth living with perinatally acquired HIV despite adequate viral suppression on cART. Alternatively, though our study controlled for many sociodemographic variables, including household income and caregiver education and included age- and gender-matched controls, factors such as genetic predisposition, stigma, and parental loss may independently influence neurologic outcomes in youth living with perinatal HIV and result in delayed development compared to uninfected peers (Louw et al., 2016).
Participants with higher inattention scores on the SNAP-IV questionnaire scored poorly on the measures of executive function even after controlling for relevant demographic factors, while hyperactive subscale scores did not correlate with cognitive measures. Cognitive evaluations require the presence of trained individuals and are time consuming, which limit their feasibility in resource-limited settings. Additionally, behavioral symptomatology may be the predictors of poor societal and academic functioning (Evans et al., 2019) and problems with attention in children are often missed by parents and educators. Therefore, behavioral questionnaires may potentially be more relevant to a child's outlook than cognitive testing alone. The inattentive subscale on the SNAP-IV questionnaire is a useful tool to evaluate the behavioral health of adolescents affected by HIV and may identify those for whom further cognitive testing and intervention may be warranted. The SNAP-IV questionnaire is brief, validated across many languages and cultures and is available to researchers and clinicians at no cost. Importantly, early identification and treatment, both with medication and non-pharmacologic therapy, have been shown to improve academic performance, self-esteem, and quality of life for children living with ADHD (Arnold et al., 2015; Harpin et al., 2016). The utility of behavioral health questionnaires in adolescents affected by HIV to identify those who may benefit from cognitive testing is an area which warrants further study.
Our study has several strengths including the use of local normative cognitive data and Thai-translated and validated questionnaires as well as testing and inclusion of adolescents with well-controlled pHIV on long-term cART. Limitations of the study include the cross-sectional design, which hinders our interpretation of the directionality of associations and lack of data regarding other factors which influence cognition including genetics variables and the stability of the home and learning environment. Our results may not be generalizable to the global HIV adolescent population, as over half of adolescents living with HIV do not achieve viral suppression and many are diagnosed with AIDS-defining illnesses (Kahana et al., 2015). Additionally, the SNAP-IV questionnaire is a screening tool based on subjective caregiver responses, and therefore, may be prone to bias. Regardless, our study adds to the current literature by displaying that adolescents with pHIV without in utero exposure to recreational and antiretroviral drugs and without history of CDC-class C diagnoses on cART have significant behavioral symptomatology and worse cognitive outcomes on the measures of executive functioning compared to HIV-uninfected peers. These findings underscore the importance of improving access to behavioral health screening tools and cognitive assessments for high-risk populations of adolescents affected by HIV globally.
Financial support
This research was supported by the US National Institute of Mental Health (PP, grant number: 7K23MH119914-02; TP, grant number: R01MH102151) and by the US National Institute of Allergy and Infectious Diseases (U19 AI053741) and the US National Institute of Mental Health. Antiretroviral drugs were provided by ViiV Healthcare (AZT, 3TC), Boehringer Ingelheim (NVP), Merck (EFV), Abbott (RTV), and Roche (NFV).
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/gmh.2021.1.
click here to view supplementary material
Conflict of interest
None.
References
- Adefalu MO, Tunde-Ayinmode MF, Issa BA, Adefalu AA and Adepoju SA (2018) Psychiatric morbidity in children with HIV/AIDS at a tertiary health institution in North-central Nigeria. Journal of Tropical Pediatrics 64, 38–44. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Hodgkins P, Caci H, Kahle J and Young S (2015) Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review. PLoS ONE 10, e0116407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kaplan E and Kramer J (2001) The Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Evans SC, Cooley JL, Blossom JB, Pederson CA, Tampke EC and Fite PJ (2019) Examining ODD/ADHD symptom dimensions as predictors of social, emotional, and academic trajectories in middle childhood. Journal of Clinical Child And Adolescent Psychology 49, 912–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Chernoff M, Williams PL, Brouwers P, Morse E, Heston J, Hodge J, Di Poalo V, Deygo NS and Nachman S (2010) Co-occurring psychiatric symptoms in children perinatally infected with HIV and peer comparison sample. Journal of Developmental and Behavioral Pediatrics 31, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpin V, Mazzone L, Raynaud JP, Kahle J and Hodgkins P (2016) Long-term outcomes of ADHD: a systematic review of self-esteem and social function. Journal of Attention Disorders 20, 295–305. [DOI] [PubMed] [Google Scholar]
- Hayes AF and Rockwood NJ (2017) Regression-based statistical mediation and moderation analysis in clinical research: observations, recommendations, and implementation. Behaviour Research and Therapy 98, 39–57. [DOI] [PubMed] [Google Scholar]
- Kahana SY, Fernandez MI, Wilson PA, Bauermeister JA, Lee S, Wilson CM and Hightow-Weidman LB (2015) Rates and correlates of antiretroviral therapy use and virologic suppression among perinatally and behaviorally HIV-infected youth linked to care in the United States. Journal of Acquired Immune Deficiency Syndrome 68, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamau JW, Kuria W, Mathai M, Atwoli L and Kangethe R (2012) Psychiatric morbidity among HIV-infected children and adolescents in a resource-poor Kenyan urban community. AIDS Care 24, 836–842. [DOI] [PubMed] [Google Scholar]
- Kerr SJ, Puthanakit T, Malee KM, Thongpibul K, Ly PS, Sophonphan J, Suwanlerk T, Kosalararaksa P, Ounchanum P, Aurpibul L, Kanjanavanit S, Ngampiyaskul C, Chettra K, Robbins R, Paul R, Ananworanich J and Mellins CA (2019) Increased risk of executive function and emotional behavioral problems among virologically well-controlled perinatally HIV-infected adolescents in Thailand and Cambodia. Journal of Acquired Immune Deficiency Syndrome 82, 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente AM, Voigt RG, Williams J, Frailey JK, Satz P, D'Elia LF (2008) Children's Color Trails Test 1 & 2: test-retest reliability and factorial validity. Clinical Neuropsychology 23, 645–660. [DOI] [PubMed] [Google Scholar]
- Louw K-A, Ipser J, Phillips N and Hoare J (2016) Correlates of emotional and behavioural problems in children with perinatally acquired HIV in Cape Town, South Africa. AIDS Care 28, 842–850. [DOI] [PubMed] [Google Scholar]
- Mellins CA, Elkington KS, Bauermeister JA, Brackis-Cott E, Dolezal C, McKay M, Wiznia A, Bamji M and Abrams EJ (2009) Sexual and drug use behavior in perinatally HIV-infected youth: mental health and family influences. Journal of the American Academy of Child & Adolescent Psychiatry 48, 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins CA, Elkington KS, Leu CS, Santamaria EK, Dolezal C, Wizniz A, Bamji M, McKay MM and Abrams EJ (2010) Prevalence and change in psychiatric disorders among perinatally HIV-infected and HIV-exposed youth. AIDS Care 24, 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpango RS, Kinyanda E, Rukundo GZ, Levin J, Gadow KD and Patel V (2017) Prevalence and correlates for ADHD and relation with social and academic functioning among children and adolescents with HIV/AIDS in Uganda. BMC Psychiatry 17, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols SL, Chernoff MC, Malee KM, Sirois PA, Woods SP, Williams PL, Yildrim C, Delis D, Kammerer B and Memory and Executive Functioning Study Group (2016) Executive functioning in children and adolescents with perinatal HIV infection and perinatal HIV exposure. Journal of the Pediatric Infectious Diseases Society 5(suppl 1), S15–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips NJ, Hoare J, Stein DJ, Myer L, Zar HJ and Thomas KGF (2018) HIV-associated cognitive disorders in perinatally infected children and adolescents: a novel composite cognitive domains score. AIDS Care 30(sup1), 8–16. [DOI] [PubMed] [Google Scholar]
- Pityaratstian N, Booranasuksakul T, Juengsiragulwit D and Benyakorn S (2014) ADHD screening properties of the Thai version of Swanson, Nolan, and Pelham IV scale (SNAP-IV) and Strengths and Difficulties Questionnaire (SDQ). Journal of Psychiatric Association of Thailand 59, 97–110. [Google Scholar]
- Puthanakit T, Ananworanich J, Vonthanak S, Kosalaraksa P, Hansudewechakul R, van der Lugt J, Kerr SJ, Kanhanavanit S, Ngampiyaskul C, Wongsawat J, Luesomboon W, Vibol U, Pruksakaew K, Suwarnlerk T, Apornpong T, Ratanadilok K, Paul R, Mofenson LM, Fox L, Valcour V, Brouwers P, Ruxrungtham K and PREDICT study group (2013) Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. Pediatric Infectious Disease Journal 32, 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithinamsuwan P, Hutchings N, Ananworanich J, Wendelken L, Saengtawan P, Paul R, Chomchey N, Fletcher JL, Chalermchai T and Valcour V (2014) Practice effect and normative data of an HIV-specific neuropsychological testing battery among healthy Thais. Journal of the Medical Association of Thailand = Chotmaihet Thangphaet. 97 (Suppl 2), S222–S233. [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Nolan W and Pelham WE (1981) The SNAP rating scale for the diagnosis of attention deficit disorder. Paper presented at the meeting of the American Psychological Association; Los Angeles, CA.
- Wechsler D (1997) Manual for the Wechsler Intelligence Scale for Children, 3rd Edn., San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D (2003) Manual for the Wechsler Adult Intelligence Scale, 3rd Edn., San Antonio, TX: Psychological Corporation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/gmh.2021.1.
click here to view supplementary material