THE RATIONALE FOR BARRETT’S ESOPHAGUS SCREENING
There are approximately 18,440 new cases of esophageal cancer and 16,170 deaths from esophageal cancer annually.1 Esophageal cancer has 2 histologic subtypes, adenocarcinoma and squamous cell carcinoma, and accounts for 1% of all cancers diagnosed in the United States.1 Over the past 3 to 4 decades, there has been an exponential increase in the incidence of esophageal adenocarcinoma (EAC), which now constitutes most cases of esophageal cancer diagnosed in the West.2,3 There was a 463% increase in the incidence of EAC in Americans in 2000 to 2004 as compared with 1975 to 1979.4 There is a marked male predominance for EAC with a male-to-female ratio of 9:1.5,6 Known risk factors for EAC include Barrett’s esophagus (BE), gastroesophageal reflux disease (GERD), male sex, white race, central obesity, and tobacco use.7–9
Despite advancements in its diagnosis and earlier recognition, EAC is associated with a poor 5-year survival rate of 10% to 20%.10,11 The mortal threat of this disease results from 2 key mechanisms. The first is the propensity for metastasis at the earliest of stages owing to tumor access to lymphatic drainage in the esophageal submucosa. The second reason is the paucity of symptoms until advanced stages are reached. This combination almost always ensures a poor prognosis unless screening and surveillance are implemented from a preneoplastic state, that is, BE.
BE is the sole known precursor to the development of EAC and is characterized by metaplastic changes whereby the normal squamous epithelium of the distal esophagus is replaced by specialized columnar epithelium with intestinal metaplasia (IM).7,12 BE is thought to progress to EAC in a stepwise manner, transitioning from nondysplastic BE (NDBE) to low-grade dysplasia (LGD) to high-grade dysplasia (HGD) to EAC. The risk of EAC in patients with BE is 30- to 125-fold greater than that in the general population.13,14 The prevalence of BE in the general population is approximately 1% to 2% but may be as high as 5% to 15% in those with chronic GERD.15–17
Screening for BE currently includes patients with risk factors for the disease undergoing an endoscopic evaluation with esophagoduodenoscopy (EGD) or transnasal endoscopy (TNE). Esophageal biopsies are performed if the endoscopic appearance of the distal esophagus is consistent with BE with the presence of at least 1 cm of columnar-appearing metaplasia in the tubular esophagus. Given that the progression from BE to EAC is through a metaplasia-dysplasia-carcinoma sequence as described above, screening for BE is performed with the rationale that if patients can be identified at the time of metaplasia alone, they can be placed on endoscopic surveillance to enable the detection of dysplasia (low grade or high grade) or carcinoma (at an earlier stage).18,19
Over the past decade, randomized controlled trials and several prospective and retrospective cohort studies have shown that endoscopic eradication therapy (consisting of endoscopic resection of visible lesions followed by endoscopic ablation) can reduce progression to EAC by eliminating dysplasia and IM.20–23 More recently, chemoprevention trials suggest that patients with NDBE may have prolonged time to all-cause mortality, EAC, and HGD when treated with high-dose proton pump inhibitors (PPIs) and/or aspirin.24,25 If early-stage EAC (T1a EAC) is diagnosed during surveillance, it can be successfully treated endoscopically with excellent long-term survival (in distinction to potentially poor outcomes with surgery and chemoradiation in the treatment of more advanced EAC diagnosed after the onset of symptoms).26,27
Patients with EAC and prior history of known BE (presumably undergoing surveillance) are more likely to be detected with earlier-stage disease and receive endoscopic or surgical treatment compared with those with EAC and no prior known history of BE (not diagnosed in a surveillance program). A metaanalysis demonstrated that regular surveillance was associated with lower EAC-related and all-cause mortality (relative risk 0.60, hazard ratio 0.75).28 In addition, this study showed that surveillance-detected EAC is diagnosed at earlier stages than EAC diagnosed outside of surveillance. Moreover, because there are no specific signs or symptoms associated with BE, it can only be detected via screening those with risk factors. The presence of alarm symptoms, such as dysphagia, odynophagia, and unintentional weight loss, often signal complications, such as a stricture or locally advanced EAC.
RISK FACTORS FOR BARRETT’S ESOPHAGUS AND ESOPHAGEAL ADENOCARCINOMA
Given that the metaplastic mucosa that occurs with BE is thought to occur uniformly in the presence of chronic GERD, it only stands to reason that GERD is often considered to be the primary risk factor for BE. Chronic GERD is strongly associated with BE with a summary odds ratio (OR) of 2.90 (95% confidence interval [CI], 1.86–4.54).29 GERD symptoms increase the odds of long-segment BE by almost 5-fold (OR 4.92; 95% CI, 2.01–12.0) but may not have a significant association with short-segment BE (OR 1.15; 95% CI, 0.76–1.73) as shown in a systematic review and metaanalysis.29 In patients with recurrent symptoms of GERD, the OR of EAC was 7.7 (95% CI, 5.3–11.4) compared with those without GERD symptoms.30 The more frequent, prolonged, and severe the symptoms of GERD, the greater the risk of BE and EAC.30–32 However, given that the overall prevalence of EAC is low and that 50% of the population experiences GERD symptoms on a monthly basis and 20% on a weekly basis, routine endoscopic screening of patients with GERD symptoms and no other risk factors is not supported.31 The prevalence of BE in patients with GERD alone is estimated to be 3%.33 In the setting of GERD plus another risk factor, the prevalence of BE increases to 12.2%.33
Age greater than 50 years is another strong risk factor for BE.33–36 The prevalence of BE in the Western population older than the age of 50 years is 6.1%.33 In individuals older than the age of 65 years, the prevalence of BE is approximately 16.7%.37 The association of age and BE appears to be less prominent in the non-Western population, where the prevalence of BE in those older than the age of 50 years is about 1%.38 Unless multiple other risk factors are present, screening is not recommended in those younger than 50 years even when GERD symptoms are present; for example, a man aged 35 years with GERD symptoms has a risk of EAC of 1 in 100,000.39
A substantial portion of first-degree relatives of individuals with BE have either short-segment or long-segment BE, suggesting that there may be a familial susceptibility to developing BE and EAC in a subset of the population.40 After adjusting for other risk factors, family history was independently associated with BE or EAC with an OR of 12.23 (95% CI, 3.34–44.76).41 There may be a greater than 2-fold risk of BE in first-degree relatives compared with those without a family history of BE and EAC.42 Although 1 study suggested that close to 30% of first-degree relatives of patients with BE with HGD or EAC may have BE, the overall pooled prevalence of BE in this population is estimated at 23.4%.33,43 It is estimated that 7% of cases of BE and EAC may be familial: these should be suspected in patients with younger age of onset of reflux symptoms and familial diagnosis of EAC or BE.44 In contrast, in another study, there was no statistical difference in the risk of BE in a family member of a patient with BE with reflux symptoms compared with controls with reflux. However, these family members were more likely to experience reflux symptoms compared with controls theoretically increasing their risk for erosive esophagitis (EE) or BE compared with the general population.45
Central obesity is independently associated with BE and EAC.46,47 BE may be present in 2% to 6% of patients with obesity undergoing EGD48,49 with a pooled prevalence of 1.9%.33 For every 1-kg/m2 increase in the body mass index (BMI), the risk of EAC and BE increases by 16% and 12%, respectively.46 Waist circumference is a better predictor of BE risk compared with BMI.50
Men are significantly more likely to have BE compared with women.37,51,52 The prevalence of BE in men approaches 6.8%.33 The incidence of EAC in women with GERD is extremely low and comparable to the risk of breast cancer in men.39 The length of the BE segment was also found to be greater in men compared with women in 1 study.53 Women are also less likely to have prevalent HGD or EAC compared with men (OR 0.52; 95% CI, 0.31–0.88).53 Given the very low incidence of EAC in women, routine screening for BE is not recommended for this group unless multiple other risk factors are present.
White race is also a risk factor for BE.52,54 Whites account for greater than 90% of all cases of BE.55 The white-to-African American ratio for EAC is about 3:1.56 The prevalence of BE in whites is approximately 6.1% and significantly higher compared with Hispanics (1.7%) and African Americans (1.6%).54
Tobacco use increases the risk of BE and EAC by about 2-fold.57 There is a strong dose association between pack-years of cigarettes consumed and BE/EAC risk.57 Compared with current smokers, those who quit smoking had lower risk of EAC after adjusting for pack-years.57
Most patients with BE have a hiatal hernia.58 More than 95% of patients with BE have a hernia that is 2 cm in size or longer.58 The hernia length and hiatal opening width in patients with BE were 3.95 cm and 3.52 cm, respectively, and both were significantly greater compared with controls with or without esophagitis.58
The risk factors shown to be associated with BE are summarized in Table 1. Multiple studies have demonstrated that the greater the number of risk factors present, the higher the chances of BE being present.33,34 For each risk factor, the prevalence of BE increases by 1.2%.33 When 3 or more risk factors were present, the odds of EE or BE were 3.7 times higher compared with those with 2 risk factors or less.34 Moreover, when 5 or more risk factors were present, the odds of EE or BE were 5.7 times higher.34
Table 1.
Risk factors associated with Barrett’s esophagus
| Risk Factor | Odds Ratio (95% CI) |
|---|---|
| Chronic gastroesophageal reflux disease | 2.90 (1.86–4.54)29 |
| Age >50 y | 1.60 (1.1–2.4)59 |
| Family history of Barrett’s esophagus or esophageal adenocarcinoma | 12.23 (3.34–44.76)41 |
| Central obesity | 1.98 (1.52–2.57)60 |
| Male sex | Male/female sex ratio: 1.96/1 (1.77, 2.17/1)61 |
| White race | 3.95 (3.07–5.08)62 |
| Tobacco use | 1.42 (1.15–1.76)63 |
| Hiatal hernia | 3.94 (3.02–5.13)64 |
Various risk scores and models exist that predict the risk of BE in an individual. The Michigan Barrett’s Esophagus pREdiction Tool (M-BERET) is a model that incorporates GERD, age, abdominal obesity, and cigarette use and has been shown to more accurately classify the presence of BE compared with a model based on GERD alone (area under the curve [AUC] 0.72 vs 0.61, respectively).65 The M-BERET has been externally validated in 4 independent datasets and maintains reasonable accuracy in discriminating patients with BE from population-based controls.66 In addition, a risk prediction model for BE that combined a multibiomarker score (based on serum levels of interleukin [IL] 12p70, IL-6, IL-8, IL-10, and leptin) with demographic and clinical features had greater accuracy than with using GERD alone.67 Although these risk scores and models may help identify high-risk patients and improve population screening, they are not routinely used in clinical practice given that the thresholds at which confirmatory testing is to be performed are not yet defined. These thresholds are likely dependent on the costs and performance characteristics of screening and confirmatory tests.
SELECTING CANDIDATES FOR BARRETT’S ESOPHAGUS SCREENING
National societies have independently published guidelines to select candidates for BE screening (Box 1).19,38,68–70 The identification of screening criteria for these guidelines relies for the most part on demographic and clinical characteristics found commonly in patients with BE. In general, all societies do not recommend screening in the general population.19,38,68–70 Instead, they recommend that BE screening should be considered in high-risk individuals. Moreover, before considering screening, the projected lifespan and comorbidities of the patient should be considered. Patients should be counseled that screening may detect BE, which could be followed by surveillance and/or dysplasia that could require endoscopic therapy. If BE is not found on the initial endoscopy, subsequent evaluation is not recommended given the low (<2%) yield of BE on repeat evaluation.19 However, if the initial EGD shows moderate or severe esophagitis (Los Angeles Classification B, C, or D), high-dose PPI should be prescribed and repeat endoscopy should be performed after 8 to 12 weeks of treatment to evaluate for the development of BE (which can be present in 12%–15% of cases).19
Box 1. Summary of guidelines for Barrett’s esophagus screening from gastroenterology and endoscopy societies.
Screening for BE in the general population is not recommended.
Screening for BE should be considered in those with chronic reflux and multiple risk factors, including age greater than 50 years, male sex, white race, central obesity, smoking use, first-degree relative with BE or esophageal adenocarcinoma, and presence of hiatal hernia.
Screening for BE should generally not be performed in women with chronic GERD or men younger than 50 years with chronic GERD but may be considered in these individuals if multiple other risk factors are also present.
Data from Recommendations based on BE screening guidelines from ACG (2016), ASGE (2019), AGA (2016), ACP (2012), BSG (2014), and ESGE (2020).
GUIDELINES FROM NATIONAL SOCIETIES
The American College of Gastroenterology recommends that screening for BE may be considered in men with chronic (>5 years) and/or frequent (weekly or more) symptoms of GERD and two or more of the following risk factors for BE or EAC: age greater than 50 years, white race, presence of central obesity (waist circumference >102 cm or waist-hip ratio >0.9), current or past history of smoking, and a confirmed family history of BE or EAC in a first-degree relative.19 Screening for BE in women is not recommended given the substantially lower risk of EAC in women with chronic GERD symptoms compared with men. However, screening in women may be considered in select cases when multiple risk factors are present.19
The American Gastroenterological Association (AGA) suggests screening for BE in patients with multiple risk factors for EAC, including age 50 years or older, male sex, white race, chronic GERD, hiatal hernia, elevated BMI, and intraabdominal distribution of body fat.68
The American Society of Gastrointestinal Endoscopy recommends a screening strategy that identifies individuals at risk for EAC.38 At-risk populations include those with a family history of BE and EAC (high risk) or patients with GERD and at least 1 other risk factor for EAC (moderate risk).38 These other risk factors include age greater than 50 years, obesity/central adiposity, history of smoking, or male gender.38
The American College of Physicians suggests upper endoscopy be performed in men older than 50 years with chronic GERD symptoms (greater than 5 years) and additional risk factors, such as nocturnal reflux symptoms, hiatal hernia, elevated BMI, tobacco use, and intraabdominal distribution of fat.69
The British Society of Gastroenterology recommends endoscopic screening for BE in patients with chronic GERD symptoms and multiple risk factors (at least three of the following: age 50 years or older, white race, male sex, and obesity).70 However, they suggest that the threshold of multiple risk factors should be lowered in the presence of family history, including at least 1 first-degree relative with BE or EAC.70
The European Society of Gastrointestinal Endoscopy suggests that endoscopic screening for BE should only be considered in high-risk individuals: that is, in those with long-standing GERD symptoms (ie, >5 years) and multiple risk factors (age ≥50 years, white race, male sex, obesity, or first-degree relative with BE or EAC).71
CHALLENGES WITH BARRETT’S ESOPHAGUS SCREENING
There are several challenges to screening for BE. The main goal of screening is earlier detection of dysplasia or EAC to enable improved outcomes. EAC is a cancer with a low incidence at the population level, which makes it difficult to detect. The global incidence rate of EAC worldwide is 0.7 per 100,000 person-years.7 In the United States, 17,650 new cases of esophageal cancer were diagnosed in 2019 with EAC accounting for about 60% of the cases.72,73 Despite recommendations for BE screening and surveillance, most cases (93%) of EAC are prevalent because they continue to be diagnosed after the onset of alarm symptoms, which are more indicative of advanced disease (Fig. 1).73 In contrast, the low rate of progression from BE to EAC (annual risk of 0.12%–0.5%) questions the cost-effectiveness and impact of surveillance for incident neoplasia.15,74 Furthermore, data suggest it is likely due to this low absolute rate of progression that most patients with BE die of non-EAC related causes, such as ischemic heart disease.75 Because of this low rate of progression, evidence to show that BE screening and surveillance are effective in reducing EAC-related mortality is challenging to generate. Although surveillance may be associated with earlier-stage EAC and may provide a small survival benefit, potential confounding biases (length and lead time) could account for this observation.28,76 A large prospective randomized study to assess the effectiveness of surveillance in BE to reduce EAC-related mortality is ongoing.77
Fig. 1.
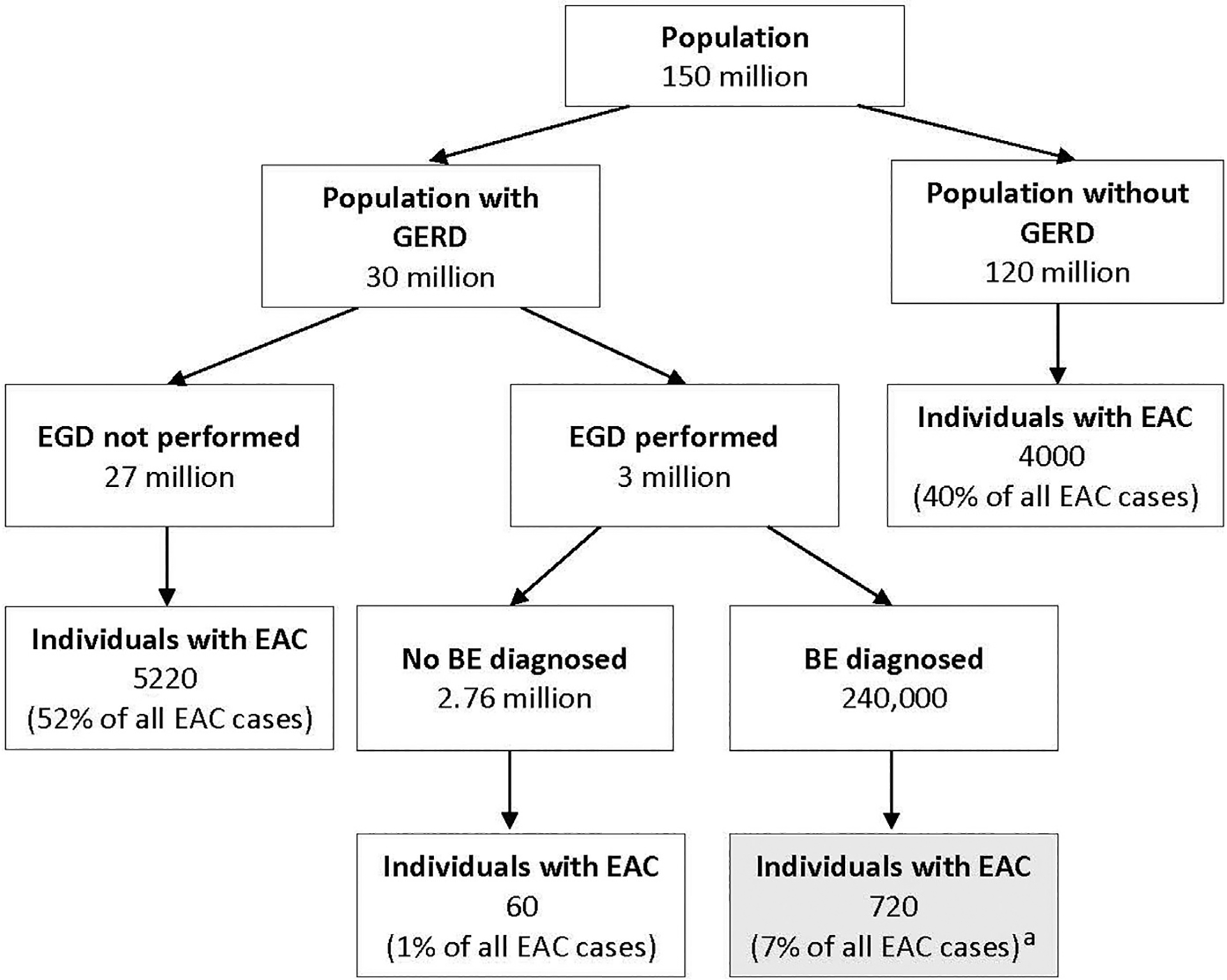
The current approach of endoscopic screening for BE identifies only a minority of patients with EAC. a Only 7% of all EAC cases are diagnosed via current screening approaches. (Adapted from Vaughan TL., Fitzgerald RC. Precision prevention of oesophageal adenocarcinoma. Nat Rev Gastroenterol Hepatol 2015:243–8; with permission.)
An area of controversy in BE is related to its diagnosis and whether the presence of an irregular Z line dictates obtaining a biopsy and instituting lifelong surveillance if IM is seen. In a study of 102 patients with an irregular Z line followed for a median of 70 months, only 2 patients developed LGD as the most advanced pathologic condition, although 8.8% of patients were subsequently diagnosed with short-segment BE.78 In another study of 86 patients with IM of the gastroesophageal junction followed for a median of 8 years, progression to LGD occurred in 6 (7%), and similarly, no patient progressed to HGD or cancer.79 Although there is some trepidation on the part of societies to definitively define a Barrett’s segment by an absolute length, the summary of this data is that, although arbitrary, BE should continue to be defined as a salmon-colored area of mucosa proximal to the gastroesophageal junction ≥1 cm with IM, although it is possible cases of HGD or cancer may develop from smaller segments. Such cases may include cancers of the gastroesophageal junction. Unfortunately, there is no recognized prelesion at this point that merits identification and surveillance.
One of the emerging trends in guidelines is reconsideration of the importance of chronic heartburn as a criterion. Although most current guidelines recommend that BE screening should be performed in patients with chronic GERD symptoms, only 1 society guideline presently (AGA) does not place undue emphasis on heartburn in its criteria. The minimization of heartburn as a key criterion comes from studies that show neither adequate sensitivity nor specificity of this symptom in identifying patients with BE or EAC. In fact, 40% to 50% of patients with BE or EAC do not report chronic GERD symptoms, such as heartburn or regurgitation, and therefore, do not benefit from enrollment in a screening followed by surveillance program (see Fig. 1).30 In a prospective study applying several guidelines for Barrett’s screening to a large patient population, GERD symptoms alone identified patients with BE with an AUC of only 0.579 and performed less well than other guideline criteria.80 Although long-standing GERD is certainly associated with an increased risk of BE, patients with BE often have fewer symptoms of GERD because of impaired esophageal sensitivity.81,82 As discussed earlier, there are numerous other risk factors for BE besides chronic GERD.
One of the greatest challenges in EAC is shifting the vast number of patients with prevalent to incident EAC. In other words, most patients with EAC present de novo, without prior identification of BE and enrollment in surveillance program. Several studies have demonstrated that at best 15% of EACs are found during surveillance.83–85 This dilemma rests in part on the above discussion documenting the poor performance of heartburn as an indicator of BE. Furthermore, there is also some evidence to suggest that there may be 2 phenotypes of EAC, one arising in a background of grossly visible BE and/or histologically identifiable IM and another form without.86 The latter form may be associated with poorer outcomes. The true precursor to this latter form of EAC is not yet known, and hence, this may also reduce the pool of EAC that can be prevented by screening for BE for lack of identification of a clear endoscopic Barrett’s segment.86 Although this concept is emerging, it potentially confounds the approach of identifying patients with endoscopic evidence of BE.
Another aspect of improving BE and EAC screening is adherence to established guidelines for identifying patients at risk for BE and EAC who require endoscopy. Only about 10% of US patients with chronic reflux symptoms undergo endoscopic screening.87,88 The cause of this low screening rate is multifactorial with potential explanations including lack of knowledge about BE by primary care physicians, the patient not bringing heartburn symptoms to the attention of the caregiver because of their mild nature or effective empiric treatment with medical therapy, or hesitancy with physician ordering or patients proceeding with endoscopy.88,89 Nevertheless, this leads to most BE cases in the community remaining undiagnosed, which leaves most BE cases (the likely source of most EACs) outside of surveillance programs (see Fig. 1).79
The absence of a widely applicable minimally or noninvasive tool for BE screening is also a major challenge to implementing BE screening. Sedated endoscopy (EGD) is not ideal because it requires the procedure to be performed in an endoscopy suite and carries with it small but significant risks from sedation and the procedure itself.90 Endoscopic screening is also associated with both direct (staff, monitoring, recovery) and indirect costs (lost productivity because of patient and family members needing time off work) with 1 study showing median 30-day costs from endoscopic screening to be $2000.90 Although unsedated transnasal endoscopy (uTNE) is safe, well tolerated, accurate, less expensive, and cost-effective, its utilization remains limited likely because of a host of patient- and provider-related limitations.91–94 To overcome this limitation, nonendoscopic and imaging-based techniques are being developed, including capsule sponge/balloon cytology sampling devices combined with biomarkers (such as TFF3 and methylated DNA markers), esophageal capsule endoscopy, and tethered capsule endomicroscopy.94–98 Although these technologies are promising and continue to be investigated, standard endoscopy remains the mainstay for screening of BE at this time.
Another future direction for improving endoscopic screening in BE might be using physician extenders to perform endoscopy or uTNE. The evidence that using physician extenders to perform endoscopy will lead to cost savings is variable. In a multi-center UK modeling study, physicians were more cost-effective measured in quality-adjusted life-years (QALYs) than nurses in performing endoscopy.99 Nevertheless, the ability to expand the work force capable of doing endoscopy has merit. In 2016, it was estimated that there are more than 450 nurses performing endoscopy in Europe.100 One concern is whether the quality of physician extenders performing endoscopy will be adequate. However, multiple studies have demonstrated similar accuracy to physicians.101,102 Studies have also shown success in training physician extenders to perform uTNE.
Screening with sedated EGD and uTNE has been shown to be cost-effective for patients with chronic GERD and other risk factors.103–105 Screening is associated with gains in QALYs in patients with chronic reflux at costs comparable to other preventive measures.106,107 Screening patients with chronic GERD with EGD costs $22,200 per QALY gained, which falls within willingness to pay threshold.108 Regardless, the cost-effectiveness of BE screening is dynamic and likely to change with development of new screening modalities, improvements in risk stratification of patients, and decreases in procedural costs. Some investigators have proposed the routine use of EGD for BE screening at the time of screening colonoscopy at age 50. Although this strategy may result in a relatively high yield of BE, the cost-effectiveness of such a strategy in the general population remains unproven, but estimates place this strategy at close to 5 times the cost of using guideline criteria.109
SUMMARY
EAC incidence is steadily increasing over the past few decades and continues to have a poor 5-year survival of less than 20%. BE is the only known precursor to EAC. The risk of EAC in patients with BE is significantly higher compared with the general population. Endoscopic screening and surveillance are performed to identify patients earlier in the metaplasia-dysplasia-carcinoma sequence from BE to EAC followed by endoscopic therapy to prevent and/or treat EAC. Given that BE does not have any specific presenting signs or symptoms, it can only be detected via screening. Therefore, current guidelines suggest that BE screening should be considered in individuals who have multiple risk factors, including chronic GERD, age greater than 50 years, male sex, white race, central obesity, smoking use, and family history of BE or EAC. Importantly, it should be recognized that screening only patients with chronic GERD is likely not sufficient to identify all those at risk for BE and EAC. A major limitation remains the absence of a minimally invasive screening tool, which would enable the detection of a larger pool of BE patients at risk for developing EAC. New nonendoscopic biomarker-based and imaging-based techniques for BE screening may be on the horizon. Additional progress on dysplasia detection is also critical to make adequate progress in EAC prevention.
KEY POINTS.
Only 7% of the approximately 10,000 esophageal adenocarcinomas diagnosed annually in the United States are detected through current screening and surveillance practices. This likely reflects the low proportion (33%) of prevalent Barrett’s esophagus (BE) in the community that is currently in the surveillance pool.
Screening for BE should be considered in those with multiple risk factors, including age greater than 50 years, male sex, chronic gastroesophageal reflux disease, white race, central obesity, smoking use, first-degree relative with BE or esophageal adenocarcinoma, and presence of hiatal hernia. Screening is not recommended in the general population.
The greater the number of risk factors present, the higher the prevalence of BE.
If initial evaluation is negative for BE, repeat evaluation for BE detection is not indicated given low yield, unless new clinical symptoms develop.
CLINICS CARE POINTS.
Screening for Barrett’s esophagus should be considered in those with multiple risk factors, including age greater than 50 years, male sex, chronic gastroesophageal reflux disease (>5 years and/or frequent symptoms), white race, central obesity, smoking, and first-degree relative with Barrett’s esophagus or esophageal adenocarcinoma. Screening of the general population is not recommended at this time.
If initial evaluation for Barrett’s esophagus is negative, repeat screening is not recommended at this time.
Screening needs to be coupled with improved techniques and technology for dysplasia detection to make real progress in improving EAC outcomes.
Funding:
Funded in part by NCI R01 grant CA241164 (to P.G. Iyer).
Conflicts:
A.K. Kamboj: None. D.A. Katzka: Education: Takeda. P.G. Iyer: Research funding from Exact Sciences, Pentax Medical, Medtronic, Nine Point Medical; Consulting: Medtronic, Symple Surgical.
REFERENCES
- 1.American Cancer Society. Key statistics for esophageal cancer. Available at: https://www.cancer.org/cancer/esophagus-cancer/about/key-statistics.html. Accessed February 10, 2020.
- 2.Edgren G, Adami HO, Vainio EW, et al. A global assessment of the oesophageal adenocarcinoma epidemic. Gut 2013;62(10):1406–14. [DOI] [PubMed] [Google Scholar]
- 3.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. Br J Cancer 2009;101(5):855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nassri A, Zhu H, Muftah M, et al. Epidemiology and survival of esophageal cancer patients in an American Cohort. Cureus 2018;10(4):e2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie SH, Lagergren J. The male predominance in esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2016;14(3):338–47. [DOI] [PubMed] [Google Scholar]
- 6.Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin 2013;63(4):232–48. [DOI] [PubMed] [Google Scholar]
- 7.Coleman HG, Xie SH, Lagergren J. The epidemiology of esophageal adenocarcinoma. Gastroenterology 2018;154(2):390–405. [DOI] [PubMed] [Google Scholar]
- 8.Turati F, Tramacere I, La Vecchia C, et al. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17. [DOI] [PubMed] [Google Scholar]
- 9.Tramacere I, La Vecchia C, Negri E. Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology 2011;22(3):344–9. [DOI] [PubMed] [Google Scholar]
- 10.Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J Gastroenterol Hepatol 2016;31(6):1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavin AT, Francisci S, Foschi R, et al. Oesophageal cancer survival in Europe: a EUROCARE-4 study. Cancer Epidemiol 2012;36(6):505–12. [DOI] [PubMed] [Google Scholar]
- 12.Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med 2014;371(9):836–45. [DOI] [PubMed] [Google Scholar]
- 13.Runge TM, Abrams JA, Shaheen NJ. Epidemiology of Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am 2015;44:203–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron AJ, Ott BJ, Payne WS. The incidence of adenocarcinoma in columnar-lined (Barrett’s) esophagus. N Engl J Med 1985;313(14):857–9. [DOI] [PubMed] [Google Scholar]
- 15.Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet 2009;373(9666):850–61. [DOI] [PubMed] [Google Scholar]
- 16.Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005;61(2):226–31. [DOI] [PubMed] [Google Scholar]
- 17.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology 2005;129(6): 1825–31. [DOI] [PubMed] [Google Scholar]
- 18.Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA 2013;310:627–36. [DOI] [PubMed] [Google Scholar]
- 19.Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016;111(1):30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009;360(22):2277–88. [DOI] [PubMed] [Google Scholar]
- 21.Wolf WA, Pasricha S, Cotton C, et al. Incidence of esophageal adenocarcinoma and causes of mortality after radiofrequency ablation of Barrett’s esophagus. Gastroenterology 2015;149(7):1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phoa KN, Pouw RE, Van Vilsteren FGI, et al. Remission of Barrett’s esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology 2013;145(1):96–104. [DOI] [PubMed] [Google Scholar]
- 23.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57(9):1200–6. [DOI] [PubMed] [Google Scholar]
- 24.Jankowski JAZ, de Caestecker J, Love SB, et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet 2018;392(10145):400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Husain NS, El-Serag HB. Chemoprevention of Barrett’s oesophagus: a step closer with PPIs and aspirin. Nat Rev Clin Oncol 2018;15(12):728–30. [DOI] [PubMed] [Google Scholar]
- 26.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137(3):815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zehetner J, Demeester SR, Hagen JA, et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg 2011;141(1):39–47. [DOI] [PubMed] [Google Scholar]
- 28.Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology 2018;154(8):2068–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor JB, Rubenstein JH. Meta-analyses of the effect of symptoms of gastroesophageal reflux on the risk of Barrett’s esophagus. Am J Gastroenterol 2010;105:1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagergren J, Bergström R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340(11):825–31. [DOI] [PubMed] [Google Scholar]
- 31.Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. JAMA 2002;287:1972–81. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman DA, Oehlke M, Helfand M. Risk factors for Barrett’s esophagus in community-based practice. GORGE consortium. Gastroenterology Outcomes Research Group in Endoscopy. Am J Gastroenterol 1997;92(8):1293–7. [PubMed] [Google Scholar]
- 33.Qumseya BJ, Bukannan A, Gendy S, et al. Systematic review and meta-analysis of prevalence and risk factors for Barrett’s esophagus. Gastrointest Endosc 2019;90(5):707–17. [DOI] [PubMed] [Google Scholar]
- 34.Crews NR, Johnson ML, Schleck CD, et al. Prevalence and predictors of gastroesophageal reflux complications in community subjects. Dig Dis Sci 2016;61(11):3221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003;125(6):1670–7. [DOI] [PubMed] [Google Scholar]
- 36.Gerson LB, Banerjee S. Screening for Barrett’s esophagus in asymptomatic women. Gastrointest Endosc 2009;70(5):867–73. [DOI] [PubMed] [Google Scholar]
- 37.Ward EM, Wolfsen HC, Achem SR, et al. Barrett’s esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am J Gastroenterol 2006;101(1):12–7. [DOI] [PubMed] [Google Scholar]
- 38.Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc 2019;90(3):335–59. [DOI] [PubMed] [Google Scholar]
- 39.Rubenstein JH, Scheiman JM, Sadeghi S, et al. Esophageal adenocarcinoma incidence in individuals with gastroesophageal reflux: synthesis and estimates from population studies. Am J Gastroenterol 2011;106(2):254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chak A, Faulx A, Kinnard M, et al. Identification of Barrett’s esophagus in relatives by endoscopic screening. Am J Gastroenterol 2004;99(11):2107–14. [DOI] [PubMed] [Google Scholar]
- 41.Chak A, Lee T, Kinnard MF, et al. Familial aggregation of Barrett’s oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut 2002;51(3):323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero Y Familial association in Barrett esophagus. Gastroenterol Hepatol (N Y) 2007;3(5):346–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Juhasz A, Mittal SK, Lee TH, et al. Prevalence of Barrett esophagus in first-degree relatives of patients with esophageal adenocarcinoma. J Clin Gastroenterol 2011;45(10):867–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verbeek RE, Spittuler LF, Peute A, et al. Familial clustering of Barrett’s esophagus and esophageal adenocarcinoma in a European cohort. Clin Gastroenterol Hepatol 2014;12(10):1656–63. [DOI] [PubMed] [Google Scholar]
- 45.Romero Y, Cameron AJ, Schaid DJ, et al. Barrett’s esophagus: prevalence in symptomatic relatives. Am J Gastroenterol 2002;97(5):1127–32. [DOI] [PubMed] [Google Scholar]
- 46.Thrift AP, Shaheen NJ, Gammon MD, et al. Obesity and risk of esophageal adenocarcinoma and Barrett’s esophagus: a Mendelian randomization study. J Natl Cancer Inst 2014;106(11):dju252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamat P, Wen S, Morris J, et al. Exploring the association between elevated body mass index and Barrett’s esophagus: a systematic review and meta-analysis. Ann Thorac Surg 2009;87(2):655–62. [DOI] [PubMed] [Google Scholar]
- 48.D’Silva M, Bhasker AG, Kantharia NS, et al. High-percentage pathological findings in obese patients suggest that esophago-gastro-duodenoscopy should be made mandatory prior to bariatric surgery. Obes Surg 2018;28(9):2753–9. [DOI] [PubMed] [Google Scholar]
- 49.Csendes A, Burgos AM, Smok G, et al. Endoscopic and histologic findings of the foregut in 426 patients with morbid obesity. Obes Surg 2007;17(1):28–34. [DOI] [PubMed] [Google Scholar]
- 50.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology 2007;133(1):34–41. [DOI] [PubMed] [Google Scholar]
- 51.Gerson LB, Edson R, Lavori PW, et al. Use of a simple symptom questionnaire to predict Barrett’s esophagus in patients with symptoms of gastroesophageal reflux. Am J Gastroenterol 2001;96(7):2005–12. [DOI] [PubMed] [Google Scholar]
- 52.Ford AC, Forman D, Reynolds PD, et al. Ethnicity, gender, and socioeconomic status as risk factors for esophagitis and Barrett’s esophagus. Am J Epidemiol 2005;162(5):454–60. [DOI] [PubMed] [Google Scholar]
- 53.Falk GW, Thota PN, Richter JE, et al. Barrett’s esophagus in women: demographic features and progression to high-grade dysplasia and cancer. Clin Gastroenterol Hepatol 2005;3(11):1089–94. [DOI] [PubMed] [Google Scholar]
- 54.Abrams JA, Fields S, Lightdale CJ, et al. Racial and ethnic disparities in the prevalence of Barrett’s esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol 2008;6(1):30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma P, Weston AP, Morales T, et al. Relative risk of dysplasia for patients with intestinal metaplasia in the distal oesophagus and in the gastric cardia. Gut 2000;46(1):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991;265(10):1287–9. [PubMed] [Google Scholar]
- 57.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the International BEACON Consortium. J Natl Cancer Inst 2010;102(17):1344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cameron AJ. Barrett’s esophagus: prevalence and size of hiatal hernia. Am J Gastroenterol 1999;94(8):2054–9. [DOI] [PubMed] [Google Scholar]
- 59.Edelstein ZR, Bronner MP, Rosen SN, et al. Risk factors for Barrett’s esophagus among patients with gastroesophageal reflux disease: a community clinic-based case-control study. Am J Gastroenterol 2009;104(4):834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11(11):1399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett’s esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol 2005;162(11):1050–61. [DOI] [PubMed] [Google Scholar]
- 62.Alkaddour A, Palacio C, Vega KJ. Risk of histologic Barrett’s esophagus between African Americans and non-Hispanic whites: a meta-analysis. United European Gastroenterol J 2018;6(1):22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andrici J, Cox MR, Eslick GD. Cigarette smoking and the risk of Barrett’s esophagus: a systematic review and meta-analysis. J Gastroenterol Hepatol 2013;28(8):1258–73. [DOI] [PubMed] [Google Scholar]
- 64.Andrici J, Tio M, Cox MR, et al. Hiatal hernia and the risk of Barrett’s esophagus. J Gastroenterol Hepatol 2013;28(3):415–31. [DOI] [PubMed] [Google Scholar]
- 65.Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barrett’s esophagus among men. Am J Gastroenterol 2013;108(3):353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thrift AP, Vaughan TL, Anderson LA, et al. External validation of the Michigan Barrett’s Esophagus Prediction Tool. Clin Gastroenterol Hepatol 2017;15(7):1124–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thrift AP, Garcia JM, El-Serag HB. A multibiomarker risk score helps predict risk for Barrett’s esophagus. Clin Gastroenterol Hepatol 2014;12(8):1267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.American Gastroenterological Association Spechler SJ, Sharma P, et al. American gastroenterological association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140(3):1084–91. [DOI] [PubMed] [Google Scholar]
- 69.Shaheen NJ, Weinberg DS, Denberg TD, et al. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med 2012;157(11):808–17. [DOI] [PubMed] [Google Scholar]
- 70.Fitzgerald RC, Di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014;63(1):7–42. [DOI] [PubMed] [Google Scholar]
- 71.Săftoiu A, Hassan C, Areia M, et al. Role of gastrointestinal endoscopy in the screening of digestive tract cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2020;52(4):293–304. [DOI] [PubMed] [Google Scholar]
- 72.National Cancer Institute. Surveillance, Epidemiology and ERP. Cancer stat facts: esophageal cancer Available at: https://seer.cancer.gov/statfacts/html/esoph.html. Accessed February 10, 2020.
- 73.Vaughan TL, Fitzgerald RC. Precision prevention of oesophageal adenocarcinoma. Nat Rev Gastroenterol Hepatol 2015;12:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med 2011;365(15):1375–83. [DOI] [PubMed] [Google Scholar]
- 75.Solaymani-Dodaran M, Card TR, West J. Cause-specific mortality of people with Barrett’s esophagus compared with the general population: a population-based cohort study. Gastroenterology 2013;144(7):1375–83. [DOI] [PubMed] [Google Scholar]
- 76.Tramontano AC, Sheehan DF, Yeh JM, et al. The impact of a prior diagnosis of Barrett’s esophagus on esophageal adenocarcinoma survival. Am J Gastroenterol 2017;112(8):1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Old O, Moayyedi P, Love S, et al. Barrett’s Oesophagus Surveillance versus endoscopy at need Study (BOSS): protocol and analysis plan for a multicentre randomized controlled trial. J Med Screen 2015;22(3):158–64. [DOI] [PubMed] [Google Scholar]
- 78.Itskoviz D, Levi Z, Boltin D, et al. Risk of neoplastic progression among patients with an irregular Z line on long-term follow-up. Dig Dis Sci 2018;63(6):1513–7. [DOI] [PubMed] [Google Scholar]
- 79.Jung KW, Talley NJ, Romero Y, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. Am J Gastroenterol 2011;106(8):1447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubenstein JH, McConnell D, Waljee AK, et al. Validation and comparison of tools for selecting individuals to screen for Barrett’s esophagus and early neoplasia. Gastroenterology 2020;158(8):2082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brandt MG, Darling GE, Miller L. Symptoms, acid exposure and motility in patients with Barrett’s esophagus. Can J Surg 2004;47(1):47–51. [PMC free article] [PubMed] [Google Scholar]
- 82.Byrne PJ, Mulligan ED, O’Riordan J, et al. Impaired visceral sensitivity to acid reflux in patients with Barrett’s esophagus. The role of esophageal motility. Dis Esophagus 2003;16(3):199–203. [DOI] [PubMed] [Google Scholar]
- 83.Dulai GS, Gornbein J, Kahn KL, et al. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology 2002;122(1):26–33. [DOI] [PubMed] [Google Scholar]
- 84.Verbeek RE, Leenders M, Ten Kate FJW, et al. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am J Gastroenterol 2014;109(8):1215–22. [DOI] [PubMed] [Google Scholar]
- 85.Cooper GS, Kou TD, Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. Am J Gastroenterol 2009;104(6):1356–62. [DOI] [PubMed] [Google Scholar]
- 86.Sawas T, Killcoyne S, Iyer PG, et al. Identification of prognostic phenotypes of esophageal adenocarcinoma in 2 independent cohorts. Gastroenterology 2018;155(6):1720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143(5):1179–87.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Menezes A, Tierney A, Yang YX, et al. Adherence to the 2011 American Gastroenterological Association medical position statement for the diagnosis and management of Barrett’s esophagus. Dis Esophagus 2015;28(6):538–46. [DOI] [PubMed] [Google Scholar]
- 89.Pohl H, Robertson D, Welch HG. Repeated upper endoscopy in the Medicare population: a retrospective analysis. Ann Intern Med 2014;160(3):154–60. [DOI] [PubMed] [Google Scholar]
- 90.Sami SS, Ragunath K, Iyer PG. Screening for Barrett’s esophagus and esophageal adenocarcinoma: rationale, recent progress, challenges, and future directions. Clin Gastroenterol Hepatol 2015;13(4):623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sami SS, Dunagan KT, Johnson ML, et al. A randomized comparative effectiveness trial of novel endoscopic techniques and approaches for Barrett’s esophagus screening in the community. Am J Gastroenterol 2015;110(1):148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sami SS, Iyer PG, Pophali P, et al. Acceptability, accuracy, and safety of disposable transnasal capsule endoscopy for Barrett’s esophagus screening. Clin Gastroenterol Hepatol 2019;17(4):638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blevins CH, Iyer PG. Putting it through the nose: the ins and outs of transnasal endoscopy. Am J Gastroenterol 2016;111:1371–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Honing J, Kievit W, Bookelaar J, et al. Endosheath ultrathin transnasal endoscopy is a cost-effective method for screening for Barrett’s esophagus in patients with GERD symptoms. Gastrointest Endosc 2019;89(4):712–22. [DOI] [PubMed] [Google Scholar]
- 95.Iyer PG, Taylor WR, Johnson ML, et al. Highly discriminant methylated DNA markers for the non-endoscopic detection of Barrett’s esophagus. Am J Gastroenterol 2018;113(8):1156–66. [DOI] [PubMed] [Google Scholar]
- 96.Kadri PSR, Lao-Sirieix I, O’Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ 2010;341(7773):595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018;10(424). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gora MJ, Sauk JS, Carruth RW, et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat Med 2013;19(2):238–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richardson G, Bloor K, Williams J, et al. Cost effectiveness of nurse delivered endoscopy: findings from randomised multi-institution nurse endoscopy trial (MllNuET). BMJ 2009;338(7693):b270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pfeifer UG, Schilling D. Non-physician endoscopy: how far can we go? Visc Med 2016;32:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meaden C, Joshi M, Hollis S, et al. A randomized controlled trial comparing the accuracy of general diagnostic upper gastrointestinal endoscopy performed by nurse or medical endoscopists. Endoscopy 2006;38(6):553–60. [DOI] [PubMed] [Google Scholar]
- 102.Stephens M, Hourigan LF, Appleyard M, et al. Non-physician endoscopists: a systematic review. World J Gastroenterol 2015;21(16):5056–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inadomi JM, Saxena N. Screening and surveillance for Barrett’s esophagus: is it cost-effective? Dig Dis Sci 2018;63:2094–104. [DOI] [PubMed] [Google Scholar]
- 104.Rubenstein JH, Inadomi JM, Brill JV, et al. Cost utility of screening for Barrett’s esophagus with esophageal capsule endoscopy versus conventional upper endoscopy. Clin Gastroenterol Hepatol 2007;5(3):312–8. [DOI] [PubMed] [Google Scholar]
- 105.Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003;138(3):176–86. [DOI] [PubMed] [Google Scholar]
- 106.Nietert PJ, Silverstein MD, Mokhashi MS, et al. Cost-effectiveness of screening a population with chronic gastroesophageal reflux. Gastrointest Endosc 2003;57(3):311–8. [DOI] [PubMed] [Google Scholar]
- 107.Gerson LB, Groeneveld PW, Triadafilopoulos G. Cost-effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2004;2(10):868–79. [DOI] [PubMed] [Google Scholar]
- 108.Benaglia T, Sharples LD, Fitzgerald RC, et al. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett’s esophagus. Gastroenterology 2013;144(1):62–73. [DOI] [PubMed] [Google Scholar]
- 109.Gupta N, Bansal A, Wani SB, et al. Endoscopy for upper GI cancer screening in the general population: a cost-utility analysis. Gastrointest Endosc 2011;74(3):610–24. [DOI] [PubMed] [Google Scholar]


