Abstract
This study aimed to evaluate the long-term efficacy of proton beam therapy (PBT) for unresectable benign meningiomas at the University of Tsukuba, Japan. From 1986–1998, 10 patients were treated at the Particle Radiation Medical Science Center (PRMSC) with a relative biological effectiveness (RBE) value of 1.0 using an accelerator built for physics experiments. The total dose was compensated with an X-ray in three patients. Following that, from 2002–2017, 17 patients were treated with a RBE value of 1.1 at the Proton Medical Research Center (PMRC) which was built for medical use. At the PRMSC, the total dose ranged from 50.4–66 Gy (median: 54 Gy). During the follow-up, which lasted between 3.8 and 31.6 years (median: 25.1 years), the 5-, 10-, 15-, 20- and 30-year local control rates were 100%, and the 5-, 10-, 15-, 20- and 30-year survival rates were 90, 80, 70, 70 and 36%, respectively. One patient died of brainstem radiation necrosis 5.1 years after PBT. At PMRC, the total dose ranged from 45.0–61.2 GyE, with a median of 50.4 GyE. During the follow-up, which lasted between 3 and 17 years with a median of 10.5 years, the 5-, 10- and 15-year local control rates were 94.1%, and the 5-, 10- and 15-year survival rates were 100, 100 and 88.9%, respectively. Neither malignant transformation nor secondary malignancy was observed, indicating that fractionated PBT may be effective and safely control benign unresectable meningioma even for the lifelong period of time.
Keywords: benign meningioma, long-term control, proton beam, radiotherapy
INTRODUCTION
Meningioma is the most common nonglial primary intracranial tumor, accounting for approximately 30% of all primary central nervous system tumors [1–3]. Its incidence increases with age, with a peak at 40 years of age and a female-to-male ratio of approximately 2:1 [4,5]. Regarding the location of tumors, 90% are within the supratentorial compartment; the sagittal sinus, the falx, the convexity and the sphenoid ridge are the most common sites, in descending order [4,5].
Most meningiomas are benign; however, up to 15% are atypical and 2% are anaplastic according to the World Health Organization (WHO) histological criteria [6]. Surgical removal is the mainstay treatment [7,8]; however, it is sometimes difficult to achieve complete removal because of its complex shape or difficult location, especially given that it may involve vital structures such as the brain stem, cranial nerves or major vessels [7,8,9]. Cranial nerve deficits have been reported in 22–91% of patients who underwent surgical removal for petroclival meningiomas [10,11,12]. For the preservation of neurological functions in these difficult cases, intentional subtotal resection may be performed; however, it has been reported that the local recurrence rates are approximately 10, 20 and 25% at 5, 10 and 15 years after complete resection, and approximately 50, 60 and 70% at 5, 10 and 15 years after subtotal resection, respectively [13,14].
Post-operative radiotherapy has been reported to improve local control and overall survival rates in patients who undergo incomplete resection [15,16,17]. However, the long-term effects are not yet clear. It has been reported that proton beam therapy (PBT) can provide an excellent dose localization for relatively large or irregularly-shaped tumors with a higher rate of preservation of healthy brain tissue compared to other photon therapies [18,19]. Here, we analyzed the clinical data of patients with unresectable benign meningiomas treated with PBT at our institute to verify its long-term efficacy.
Treatment methods and patients
Proton beam therapy
From 1986–1998, 10 patients with benign meningiomas were treated at the Particle Radiation Medical Science Center (PRMSC) where a booster synchrotron for physics research was used to generate 250 MeV proton beams [20]. From 2002–2017, 17 patients were treated at the Proton Medical Research Center (PMRC) using proton beams of 155–250 MeV generated by a synchrotron dedicated to medical use [21].
At PRMSC, the accelerated proton beams were taken to the treatment rooms on demand; each was equipped with a horizontal or vertical beamline. The machine availability for PBT at this institute was 3–3.5 hours per day, 27–30 weeks, with clustered periods of 9–10 weeks [20]. Based on the limited machine availability for PBT, we had to change the fraction size from 2.5–4.0 Gy. When the total dose was insufficient conventional X-ray therapy was used for compensation. For treatment planning, computed tomographic (CT) images were obtained at 5 mm intervals in the treatment position. The contours of the target volume, including tumor attachment (corresponding to clinical target volume [CTV]), were manually outlined on serial CT sections displayed on a monitor, and the planned target volume (PTV) was made by overwriting on the same images by adding 2–3 mm margins to cover set-up errors. The proton beams were spread out and shaped with a ridge filter, double-scattering, multi-leaf collimators and a custom-made bolus covering the target volume. The patient’s irradiation position was adjusted using fluoroscopy before every treatment.
At PMRC, the beams were delivered using a rotating gantry through one to three ports with coplanar angles [21]. Treatment planning for PBT was performed using CT images at 3 mm intervals in the treatment position. Similarly, the proton beams were spread out and shaped with a ridge filter, double-scattering, multi-leaf collimators and a custom-made bolus covering the target volume. The proton beam was generally delivered from two directions, and all the fields were treated daily. The gross target volume (GTV) was defined as the area of contrast enhancement plus the tumor attachment on magnetic resonance imaging, and the CTV was made by adding 5 mm margins to the tumor attachment to cover the area where tumor cells are to be infiltrated. In patients who received surgical resection, the CTV did not enclose the entire area of the initial tumor, but only the residual or recurrent site and the attachment. Finally, the PTV was obtained by adding 3 mm margins to the CTV to cover the set-up error. Dose prescription to PTV was determined based on the following dose constraint on the organ at risk at our institute; Dmax < 6 GyE at the lens, Dmax < 44 GyE at the retina, Dmax < 50 GyE at the optic nerve, the optic chiasm and the whole brain stem (up to 60 GyE when the irradiated volume is smaller in the pons).
Patients
The present clinical studies at the PRMSC and the PMRC were conducted according to the principles of the Helsinki Declaration [22] and were approved by the Ethics Committee of the University of Tsukuba. All patients provided written informed consent.
Reviewing the medical records, we identified 27 patients with benign meningiomas treated with PBT between 1986 and 2017 at the PRMSC and the PMRC. It was confirmed that none of them had previously received any kind of cranial radiotherapy, including stereotactic radiosurgery (SRS). Their characteristics are detailed in Table 1. In this study, we excluded pathologically diagnosed WHO grade II and III meningiomas mainly because they were few and partly because we focused on the evaluation of long-term tumor control and the occurrence of malignant transformation or secondary malignancy after PBT.
Table 1.
Patient characteristics
| the PRMSC (n = 10) | the PMRC (n = 17) | |||
|---|---|---|---|---|
| Gender | ||||
| Male | 2 | 6 | ||
| Female | 8 | 11 | ||
| Age (years) | ||||
| Median | 54 | 53 | ||
| Range | 31–74 | 8–78 | ||
| Tumor maximum diameter (mm) | ||||
| Median | 38 | 50 | ||
| Range | 15–100 | 20–95 | ||
| Anatomical site (number of patients) | ||||
| Falx/Parasagittal | 2 | 2 | ||
| Cavernous sinus | 2 | |||
| Parasellar | 1 | 1 | ||
| tuberculum sellae | 1 | |||
| Optic nerve sheath | 3 | |||
| Olfactory groove | 1 | |||
| Sphenoidal ridge | 1 | 3 | ||
| Cerebellopontine angle | 1 | 1 | ||
| Middle cranial fossa | 1 | 3 | ||
| Tentorial | 3 | |||
| Petroclival | 1 | 1 | ||
| Surgery (number of patients) | ||||
| None | 3 | 7 | ||
| Biopsy | 3 | 1 | ||
| Removal | 5 | 9 | ||
| Simpson grade* | ||||
| I | 1 | |||
| II | 2 | |||
| III | 1 | |||
| IV | 1 | 9 | ||
| Interval between surgery (removal) and PBT (months) | ||||
| Median | 18.2 | 21.6 | ||
| Range | 0.4–251.9 | 3.1–127.1 | ||
| Histology WHO grade I (number of patients) | ||||
| Meningothelial | 4 | 5 | ||
| Fibrous | 2 | 1 | ||
| Transitional | 3 | |||
| Not documented | 2 | 1 | ||
| * Simpson Grade Meningioma Removal | ||||
| Grade | Tumor Resection | |||
| I | Macroscopically complete removal of dura, bone | |||
| II | Macroscopically complete removal, dural coagulation | |||
| III | Complete tumor resection, dura not coagulated | |||
| IV | Partial removal |
At the PRMSC, the male-to-female ratio was 2:8, and the ages ranged from 31–74 years with a median of 54 years. Of 10 patients, five had recurrent cases after subtotal or total resection in whom a median interval between surgery and PBT ranged from 0.4–251.9 months with a median of 18.2 months, three had biopsy alone and two did not undergo surgical intervention. Consequently, eight patients had a histological diagnosis of meningioma of WHO grade I, and two cases were diagnosed as benign based on clinical observation and imaging by neurosurgeons and radiation oncologists. Seven patients were treated with PBT alone, and three patients were treated with PBT and conventional X-ray therapy for dose compensation. The tumor maximum diameter ranged from 15–100 mm, with a median of 38 mm. The anatomical sites were as follows: falx/parasagittal area in two, parasellar in one, tuberculum sellae in one, optic nerve sheath in three, sphenoidal ridge in one, cerebellopontine angle in one, middle cranial fossa in one and petroclival in one. One patient had two lesions at different sites. The total dose ranged from 50.4–66 Gy and a median of 54 Gy was delivered to the target. The doses per fraction ranged from 1.8–3.96 Gy, with a median of 2.27 Gy because the treatment schedule was based on the accelerator machine availability. For the same reason, the total dose was compensated by linear accelerator (LINAC) with a dose ranging from 10.8–18.0 Gy in three patients. The relative biological effectiveness value (RBE) for 60Cobalt in the institute was determined as 1.0 based on biological experiments [23]. The treatment details are shown in Table 2.
Table 2.
PBT details
| the PRMSC (n = 10) | the PMRC (n = 17) | ||
|---|---|---|---|
| CTV (cc) | |||
| Median | not measurable | 46.3 | |
| Range | not measurable | 5.8–295.5 | |
| Proton beam dose | |||
| Median | 51.2 Gy (RBE = 1) | 50.4 GyE (RBE = 1.1) | |
| Range | 43–60 Gy (RBE = 1) | 45–61.2 GyE (RBE = 1.1) | |
| Fraction number | |||
| Median | 21 | 28 | |
| Range | 14–29 | 25–34 | |
| Treatment duration (Days) | |||
| Median | 39 | 44 | |
| Range | 24–57 | 30–53 | |
| Combined case using LINAC* (number of patients) | |||
| 10.8 Gy (6 fractions) | 1 | - | |
| 12.0 Gy (6 fractions) | 1 | - | |
| 18.0 Gy (10 fractions) | 1 | - | |
| Total dose (PBT + LINAC) | |||
| Median | 54 Gy (RBE = 1) | 50.4 GyE (RBE = 1.1) | |
| Range | 50.4–66 Gy (RBE = 1) | 45.0–61.2 GyE (RBE = 1.1) | |
*LINAC, Linear Accelerator.
At the PMRC, all 17 patients were treated with PBT alone. The male-to-female ratio was 6:11, and the age ranged from 8–78 years, with a median age of 53 years. Of the 17 patients, nine had recurrent cases after subtotal resection in whom a median interval between surgery and PBT ranged from 3.1–127.1 months with a median of 21.6 months, one had a biopsy and seven did not undergo a surgical procedure. Thus, 10 patients had a histological diagnosis of meningioma WHO grade I, and seven were diagnosed as benign from clinical observation and imaging by both neurosurgeons and radiation oncologists. The tumor maximum diameter ranged from 20–95 mm with a median of 50 mm. The anatomical sites were as follows: falx/parasagittal area in two, cavernous sinus in two, parasellar in one, olfactory groove in one, sphenoidal ridge in three, cerebellopontine angle in one, middle cranial fossa in three, tentorial in three and petroclival in one. The CTV ranged from 5.8–295.5 cc, with a median of 46.3 cc. The administered doses to the target ranged from 45.0– 61.2 GyE, with a median of 50.4 GyE. The doses per fraction ranged from 1.8–2.0 GyE, with a median of 1.8 GyE. The RBE for 10 MV X-rays in the institute was determined to be 1.1 based on our biological experiments [24].
Evaluation and statistical analyses
The patients were followed-up through hospital visits, mail or telephone calls to either the patients or their referring physicians. SPSS II for Windows (IBM, Chicago IL) was used for the statistical analysis. The overall survival and local control rates were calculated using the Kaplan–Meier method. The acute reactions were scored according to the Common Terminology Criteria for Adverse Events version 4.0 [25]. The late toxicities were scored according to the late effects of normal tissues-subjective, objective, management and analytic scoring system [26].
Results
Old facility (PRMSC)
During the follow-up lasting between 3.8 and 31.6 years, with a median of 25.1 years, four patients were alive and five had died of diseases unrelated to treatment at our old facility. In August 2020, four patients had been followed-up for over 20 years, and three for over 30 years. The pre-existing symptoms improved in four patients, were stable in five patients and had deteriorated in one patient. One asymptomatic patient also showed no change. The clinical responses of the patients are summarized in Table 3.
Table 3.
Clinical responses of patients
| the PRMSC (n = 10) | PMRC (n = 17) | ||||
|---|---|---|---|---|---|
| Clinical symptoms (number of patient) | Improved | Unchanged | Worsened | Improved | Unchanged |
| Visual disturbance | 2 | 2 | 6 | ||
| Narrowing of visual field | 2 | 3 | 1 | 2 | |
| Trigeminal neuralgia | 2 | 2 | 2 | ||
| Exophthalmus | 2 | 1 | 1 | ||
| Epilepsy | 1 | 3 | |||
| Hemiparesis | 1 | 3 | |||
| Headache | 2 | 1 | |||
| Double vision | 1 | 2 | |||
| Asymptomatic | 1 | 2 | |||
| Hoarseness | 1 | 1 | |||
| Hyposmia | 2 | ||||
| Sensory disturbance | 1 | ||||
| Hearing loss | 1 | ||||
| Orbital pain | 1 | ||||
| Tinnitus | 1 | ||||
| Parotid swelling | 1 | ||||
| Ocular motility disorder | 1 | ||||
| Fourth cranial nerve palsy | 1 | ||||
| Sixth cranial nerve palsy | 1 | ||||
One patient died of pneumonia 5.1 years after the occurrence of brainstem radiation necrosis that was observed 1.3 years after PBT. The death of the remaining five patients was not associated with PBT; one died of colon cancer at 29.7 years after PBT, one died of suffocation at 27.8 years, one died of femoral fracture at 21.8 years, one died of pneumonia at 13.8 years and one died of acute myocardial infarction at 3.8 years. Consequently, the 5-, 10-, 15-, 20- and 30-year local control rates were 100%, and the 5-, 10-, 15-, 20- and 30-year survival rates were 90, 80, 70, 70 and 36%, respectively (Figs 1 and 2).
Fig. 1.
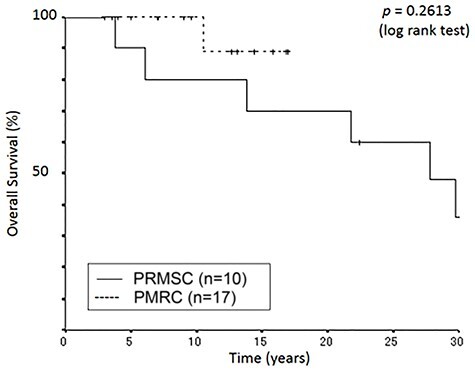
Kaplan–Meier curves of overall survival as a function of time (years).
Fig. 2.
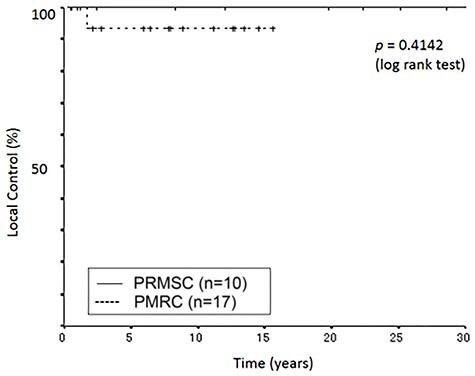
Kaplan–Meier curves of local control rate as a function of time (years).
Concerning treatment-related toxicities, 10 types of grade I or II acute adverse effects were observed at the PRMSC as shown in Table 4; however, no patient needed to discontinue the treatment. As for late toxicity greater than grade III, one patient suffered from brainstem radiation necrosis. This patient was a 68-year-old woman who had a right petroclival meningioma with a maximum diameter of 52 mm significantly compressing the brainstem. PBT was performed with 58 Gy (RBE = 1.0) in 29 fractions. Although the tumor was controlled, brainstem radiation necrosis developed 1.3 years after PBT. The patient suffered from the gradual progression of consciousness and cranial nerve disorders and died of pneumonia 5.1 years after PBT.
Table 4.
Acute and late treatment-related toxicity
| Side effect | Acute toxicity | Late toxicity | |||
|---|---|---|---|---|---|
| (number of patients) | the PRMSC (n = 10) | the PMRC (n = 17) | the PRMSC (n = 10) | the PMRC (n = 17) | |
| Low grade (CTCAE I—II) | |||||
| Radiation dermatitis | 1 | 7 | |||
| Conjunctivitis | 3 | ||||
| Vomiting | 2 | 1 | |||
| Alopecia | 2 | 2 | |||
| Middle ear inflammation | 2 | ||||
| Headache | 1 | 1 | |||
| White blood cell decreased | 1 | ||||
| Dizziness | 1 | ||||
| Eye pain | 1 | ||||
| Facial pain | 1 | ||||
| Anorexia | 1 | ||||
| Mucositis oral | 1 | ||||
| Gastritis | 1 | ||||
| High grade (CTCAE III) | |||||
| Radionecrosis | 1 | ||||
Table 5.
Summary of results on main published studies on the IMRT and PBT for benign meningiomas
| Authors, year [ref.] | Modality | Number of patient | Median dose | Median fraction number | Median volume (cc) | Local control rate (%) | Overall survival rate (%) | Median follow-up (years) |
| (Gy or GyE) | ||||||||
| Pirzkall et al. 2003 [17] | IMRT | 20 | 55.8–58.2 Gy | 32 | TV 108 | NA | NA | 3 |
| Milker-Zabel et al. 2007 [28] | IMRT | 51 | 57.6 Gy | 32 | TV 81.4 | 96.3 (5 years) | 97 (5 years) | 4.4 |
| Wenkel et al. 2000 [15] | combined PBT | 46 | 59 GyE | PBT 25 Ph 6 | CTV 76.5 | 100 (5 years) 88 (10 years) | 93 (5 years) 77 (10 years) | 4.4 |
| Weber et al. 2004 [31] | PBT | 11 | 56 GyE | 28 | PTV 107.7 | 91.7 (3 years) | 92.7 (3 years) | 2.8 |
| Noël et al. 2005 [32] | combined PBT | 51 | 60.6 GyE | PBT 15 Ph 17 | GTV 17 | 98 (4 years) | 100 (4 years) | 2.1 |
| Weber et al. 2012 [33] | PBT | 23 | 56 GyE | 28 | GTV 21.5 | 100 (5 years) | NA | 5.2*** |
| Murray et al. 2017 [34] | PBT | 61 | 54 GyE | NA | GTV 21.4 | 95.7 (5 years) | 92.1 (5 years) | 4.7 |
| EL Shafie et al. 2018 [35] | PBT | 102 | 54 GyE | 27 | CTV 31.5 | PFS 96.6 (5 years) | 96.2 (5 years) | 4.8 |
| Our study (PRMSC, 1986–1998) | PBT* | 10 | 54 Gy | 21 | ** | 100 (5 years) 100 (10 years) | 90 (5 years) 80 (10 years) | 25.1 |
| Our study (PMRC, 2002–2017) | PBT | 17 | 50.4 GyE | 28 | CTV 46.3 | 93.3 (5 years) 93.3 (10 years) | 100 (5 years) 100 (10 years) | 10.5 |
IMRT, intensive modulated radiotherapy; PBT, proton beam therapy; GyE, proton Gy × 1.1 RBE; RBE, relative biologic effectiveness; Ph, photons; TV, target volume; CTV, clinical target volume; PTV, planning target volume; GTV, gross tumor volume; PFS, progression-free survival; NA, not available.
*series includes three cases by combined PBT, ** not measurable, *** mean.
New facility (PMRC)
During the follow-up lasting between 3 and 17 years at the PMRC, with a median of 10.5 years, 16 patients were alive and one died of a disease unrelated to the treatment. Nine patients were followed-up for more than 10 years, including four for more than 15 years, at the time of analysis in August 2020 August 2020. The pre-existing symptoms improved in five patients, and they were stable in 12 patients. None of the patients showed deterioration, this included two patients who were asymptomatic before PBT. Their clinical responses are shown in Table 3. One died of pneumonia 10.5 years after PBT. Consequently, the 5-, 10- and 15-year local control rates were all 94.1%. The 5-, 10- and 15-year survival rates were 100, 100 and 88.9%, respectively (Figs 1 and 2).
Concerning the treatment-related toxicities, seven types of acute adverse effects were observed, as shown in Table 4; however, no patient needed to discontinue the treatment. At the time of analysis in August 2020, no late toxicity was observed in this cohort.
One patient was not locally controlled by PBT. A 60-year-old woman underwent partial resection of the right sphenoid wing meningioma spreading from the right middle fossa to the pterygopalatine fossa. Three years later, she underwent a second partial resection due to local recurrence followed by PBT of 50.4 GyE in 28 fractions using two oblique beams overlapping on the PVT which was set-up using the same method described above. At the time of PBT, the size of the CTV was 122.5 cc including the infiltrated portion at the base of the skull. However, the tumor regrew mostly in the pterygopalatine fossa. Thereafter, tumor resection was performed three times, and stereotactic radiotherapy with a Cyberknife (28 Gy/7 Fr) was performed once. The pathological diagnosis of the tumor removed by these four surgeries was WHO grade I transitional meningioma, and malignant changes were not observed. In the last surgery, a radical resection of the tumor in the right cavernous sinus including the carotid artery was performed, which unfortunately resulted in the ipsilateral massive cerebral infarction. The patient was hospitalized and the tumor was under observation.
Representative cases
Case 1
A 50-year-old woman with decreased visual acuity and right exophthalmos due to a tumor extending from the right sphenoid wing to the orbit was treated at the PRMSC (Fig. 3a). The planning of PBT is shown in Fig. 3b. After a combination treatment of 48 Gy (RBE = 1) in 24 fractions of PBT using two orthogonal beams and 18 Gy in 10 fractions of LINAC using the two similar orthogonal X-ray beams, the tumor gradually decreased in both size and Gadolinium enhancement effect on MRI (Fig. 3c). Although the patient’s right-side blindness was unchanged, exophthalmos gradually improved and the left visual acuity and visual field were maintained during the 22.4 years of follow-up. No adverse effects were observed.
Fig. 3.
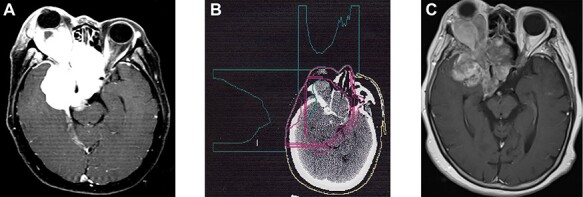
Case 1 (The clinical course is written in the text.) (a): Contrast enhanced MRI (axial view). Before PBT (b): Treatment plan (c): Contrast enhanced MRI (axial view). 22.4 years after PBT.
Case 2
A 51-year-old woman with a tentorial meningioma underwent partial tumor resection. She had reduced right visual acuity and sub-optimal ocular movement due to the regrowth of the tumor 10.6 years after the surgery (Fig. 4a and b). At the time of PBT at the PMRC, the size of the CTV was 148.6 cc. Initially, 50.4 GyE in 28 fractions was delivered to the PTV, then the irradiation field was focused on the attachment part and irradiated up to 61.2 GyE in 34 fractions using one port. Only the initial planning of PBT is shown in Fig. 4c and d. The tumor gradually shrank and her visual field defect improved (Fig. 4e and f). The patient has been stable for 12.7 years after PBT.
Fig. 4.
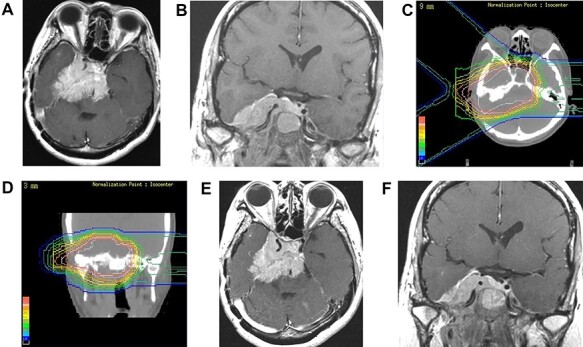
Case 2 (The clinical course is written in the text.) (a, b): Contrast enhanced MRI (axial and coronal views). Before PBT (c, d): Treatment plan (e, f): Contrast enhanced MRI (axial and coronal views). 3.5 years after PBT.
Case 3
A 53-year-old man with a tentorial meningioma spreading from the left middle fossa to the pterygopalatine fossa underwent partial intracranial tumor resection. However, he developed left ocular movement disorder and left trigeminal neuralgia 1.9 years after surgery (Fig. 5a and b). At the time of PBT at the PMRC, the size of the CTV was 158.6 cc. He was treated with PBT of 50.4 GyE in 28 fractions using two oblique beams overlapping on the PTV. After PBT (Fig. 5c and d), the intracranial tumor gradually shrank and the trigeminal neuralgia improved remarkably (Fig. 5e and f) and the tumor has been controlled for 7 years.
Fig. 5.
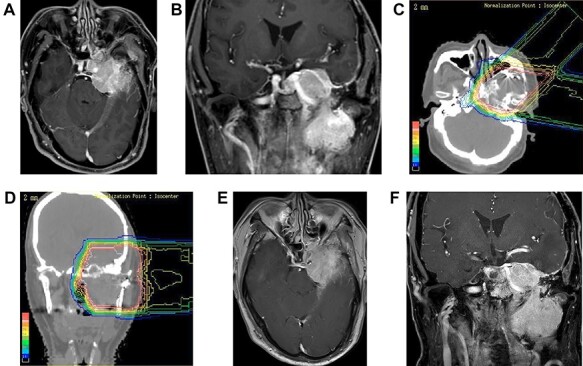
Case 3 (The clinical course is written in the text.) (a, b): Contrast enhanced MRI (axial and coronal views). Before PBT (c, d): Treatment plan (e, f): Contrast enhanced MRI (axial and coronal views). 7.0 years after PBT.
Discussion
Although surgery is the gold standard therapy for meningioma, radical resection may not be possible because of the technical difficulty and high risk of morbidity and mortality [8–12]. Condra et al. reported that radiotherapy after subtotal resection improved cause-specific survival and quality of life [13]. Later, Rogers et al. reviewed the results of external-beam radiation therapy (EBRT) either as an adjuvant or a primary therapy for meningiomas [27]. They mention that 5- to 10-year progression-free survival (PFS) rates have ranged from 80–100% with fractionated EBRT and from 75–100% with SRS. Although the results were comparable, fractionated EBRT appeared to carry a smaller risk of side effects compared with SRS [27]. Among various kinds of modalities of EBRT, PBT is advantageous for treating large or complex-shaped tumors, especially for those adjacent to critical regions [19]. Intensity modulated radiotherapy (MRI) also gives excellent dose distribution with the avoidance of surrounding healthy organs [28,29]; however, Kosaki et al. reported that PBT was superior to IMRT in reducing the dose to the brainstem in patients with complex-shaped meningiomas located at the base of the skull [30].
Studies on IMRT and PBT for benign meningiomas from the literature are summarized in Table 5. Although the number of patients included and the follow-up periods vary, the treatment outcomes in these reports are similarly favorable. The descriptions on clinical observations including appearance of toxicities are as follows: In IMRT, Pirzkall et al. showed that pre-existing neurological symptoms improved in 12 patients after a median follow-up of 3 years; the pre-existing pituitary dysfunction worsened in one patient and pre-operative low vision worsened in one patient [17]. Milker-Zabel et al. mentioned that the worsening of pre-existing neurological symptoms was seen in 4.3%, and two patients developed new clinical symptoms such as worsening of hearing or trigeminal dysesthesia [28].
As for PBT, Wenkel et al. reported that one patient died of brainstem necrosis 22 months after treatment, and eight patients suffered from late treatment-related toxicities of grade 3 or 4, including four patients with ophthalmologic toxicities. From these experiences, the optic apparatus constraints were determined to be 54 GyE [15]. Also, Weber et al. reported that two patients suffered from late visual toxicities [31]. Noël et al. reported that one patient presented with complete hypophysis insufficiency after receiving a maximal dose of 60.6 GyE, and one patient experienced severe hearing loss after receiving a maximal dose of 59.4 GyE in the internal ear and cochlea [32]. Weber et al. reported that the cumulative 5-year grade 3 late toxicity-free survival rate was 84.5% [33]. Murray et al. reported that only one patient experienced acute grade 3 brain edema, and the 5-year grade 3 late toxicity-free survival rate was 89.1% [34]. Finally, El Shafie et al. reported that two patients had late side effects of grade 3 radio-necrosis, and one patient had late side effects of grade 3 asthenia secondary to hypopituitarism [35].
The treatment outcome of our study is almost comparable to other reports; however, the follow-up period of a median of 25.1 years at the PRMSC was significantly longer than others. Although the pre-existing symptoms improved in four patients and remained stable in five patients. One female patient with a large petroclival meningioma developed brainstem radiation necrosis 1.3 years after PBT of 58 Gy in 29 fractions with an RBE value of 1.0, as previously mentioned. Retrospectively, the RBE might be higher in human brain tissue. In addition, it might have been even higher than expected because the distal end of the peak was located at the boundary between the tumor and brainstem [36]. At present, we treat patients using an RBE of 1.1, and a maximum dose of 60 GyE in 30 fractions and 54 GyE in 27 fractions at the surface and the center of the brainstem, respectively.
The induction of malignant transformation or secondary malignancy after radiotherapy for ‘benign’ meningiomas is of great concern. Pollock et al. reported that malignant transformation occurred in seven out of 316 patients with meningiomas (2.2%) after single-fraction SRS with a median follow-up of 9 years. They insist that the risk of secondary tumors or malignant transformation after SRS is very low [37]. However, Ichimura et al. warned recently that post-operative radiotherapy using a gamma-knife or LINAC induces malignant transformation during the recurrence of meningiomas at the base of the skull. They reported that the rate of malignant transformation in the patients with recurrence who received both radiotherapy and surgery was 57.1%, which was higher than that for surgery alone (18.2%) [38]. Regarding the induction of radiation-related secondary malignancies, Schneider et al. showed that the use of spot-scanned protons could reduce the incidence secondary cancers by as much as 50% [39]. In addition, Dennis et al. reported that IMRT has a 2-fold higher risk of secondary intracranial tumors compared to proton therapy, and the benefit of proton therapy over IMRT may be more substantial in patients with tumors close to critical structures [40]. These findings are in agreement with our results; there was no therapy-related malignancy or malignant transformation at the PRMSC and the PMRC with significantly longer follow-ups. These results may indicate that PBT may be a suitable modality for patients with unresectable large meningiomas with predictable long-term survival.
In our study at the PMRC, one female patient was not locally controlled by PBT, and she underwent multiple surgeries as mentioned previously. Commins et al. reported that routine histological examination may fail to identify the subset of WHO grade I tumors that behave aggressively. They also mentioned that an understanding of the genetic changes that underlie tumor progression will help in predicting the behavior of meningiomas [6]. Therefore, reliable biomarkers at the genome level have been sought because of incongruence between the clinical course and WHO grades. Mirian et al. reported that TERT-alt is an important biomarker for significantly higher risks of recurrence and death from meningiomas [41]. Further investigation is required.
Regarding the limitations of our study, the patients were reviewed retrospectively, and the number of cases was limited. It has been proposed that large prospective randomized trials are still needed to assess the clinical advantages of PBT in comparison with SRT, SRS or IMRT for surgically unresectable meningiomas [19,33]. However, Maclean et al. stated that randomized studies have proved challenging to carry out, and research strategies similar to those undertaken for other rare tumors should be adopted [42]. As for ‘resectability’ of meningiomas, it is determined mainly based on the technical difficulties of surgery; however, it may depend not only on the technical standard of each neurosurgeon or institute, but also on the patient’s background or wishes. In our study, nine patients did not undergo any surgical procedure, including biopsy; however, some of them were considered to be technically resectable and pathological diagnosis could have been obtained. Among these, two refused surgery for religious reasons and one had hypertrophic cardiomyopathy. In another six patients, many of them selected PBT as an alternative at the presentation of treatment options.
In conclusion, it was indicated that fractionated PBT may be effective for benign unresectable meningioma even for the lifelong period of time. Particularly, 50.4 GyE/28 fractions may be sufficient and safe.
ACKNOWLEDGEMENTS
The authors thank all staffs of the Proton Medical Research Center for their excellent support.
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (18 K19465).
Contributor Information
Hiroshige Sato, Biomedical Sciences, Graduate School of Comprehensive Human Sciences, University of Tsukuba, 1-1-1 Tennohdai, Tsukuba, Ibaraki 305-8575, Japan.
Masashi Mizumoto, Department of Radiation Oncology, Proton Beam Therapy Center, University of Tsukuba Hospital, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576, Japan.
Toshiyuki Okumura, Department of Radiation Oncology, Proton Beam Therapy Center, University of Tsukuba Hospital, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576, Japan.
Hideyuki Sakurai, Department of Radiation Oncology, Proton Beam Therapy Center, University of Tsukuba Hospital, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576, Japan.
Noriaki Sakamoto, Department of Diagnostic Pathology, Faculty of Medicine, University of Tsukuba, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576, Japan.
Hiroyoshi Akutsu, Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576, Japan.
Eiichi Ishikawa, Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576, Japan.
Koji Tsuboi, Tumor Therapy Center, Tsukuba Central Hospital, 1589-3 Kashiwada, Ushiku, Ibaraki 300-1211, Japan.
Funding
Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (18 K19465).
CONFLICT OF INTEREST
The authors report no conflicts of interest.
CONFERENCE PRESENTATION
The academic general meeting of the Japan Neurosurgical Society on 12 October 2018.
Clinical Trial Registration Number: H29–278: Organized No. of T-CReDO (Tsukuba Clinical Research & Development Organization).
References
- 1. Ostrom QT, Cioffi G, Gittleman Het al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol 2019;21:v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuratsu J, Kochi M, Ushio Y. Incidence and clinical features of asymptomatic meningiomas. J Neurosurg 2000;92:766–70. [DOI] [PubMed] [Google Scholar]
- 3. Claus EB, Bondy ML, Schildkraut JMet al. Epidemiology of intracranial meningioma. Neurosurgery 2005;57:1088–95. [DOI] [PubMed] [Google Scholar]
- 4. Rockhill J, Mrugala M, Chamberlain MC. Intracranial meningiomas: an overview of diagnosis and treatment. Neurosurg Focus 2007;23(4):E1. [DOI] [PubMed] [Google Scholar]
- 5. Longstreth WT Jr, Dennis LK, McGuire VMet al. Epidemiology of intracranial meningioma. Cancer 1993;72:639–48. [DOI] [PubMed] [Google Scholar]
- 6. Commins DL, Atkinson RD, Burnett ME. Review of meningioma histopathology. Neurosurg Focus 2007;23(4):E3. [DOI] [PubMed] [Google Scholar]
- 7. Apra C, Peyre M, Kalamarides M. Current treatment options for meningioma. Expert Rev Neurother 2018;18:241–9. [DOI] [PubMed] [Google Scholar]
- 8. Euskirchen P, Peyre M. Management of meningioma. Presse Med 2018;47:245–52. [DOI] [PubMed] [Google Scholar]
- 9. Sekhar LN, Swamy NK, Jaiswal Vet al. Surgical excision of meningiomas involving the clivus: preoperative and intraoperative features as predictors of postoperative functional deterioration. J Neurosurg 1994;81:860–8. [DOI] [PubMed] [Google Scholar]
- 10. Mayberg MR, Symon L. Meningiomas of the clivus and apical petrous bone. Report of 35 cases. J Neurosurg 1986;65:160–7. [DOI] [PubMed] [Google Scholar]
- 11. Samii M, Tatagiba M. Experience with 36 surgical cases of petroclival meningiomas. Acta Neurochir 1992;118:27–32. [DOI] [PubMed] [Google Scholar]
- 12. Couldwell WT, Fukushima T, Giannotta SLet al. Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg 1996;84:20–8. [DOI] [PubMed] [Google Scholar]
- 13. Condra KS, Buatti JM, Rhoton ALet al. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys 1997;39:427–36. [DOI] [PubMed] [Google Scholar]
- 14. Mirimanoff RO, Dosoretz DE, Linggood RMet al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 1985;62:18–24. [DOI] [PubMed] [Google Scholar]
- 15. Wenkel E, Thornton AF, Finkelstein Det al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys 2000;48:1363–70. [DOI] [PubMed] [Google Scholar]
- 16. Vernimmen FJ, Harris JK, Wilson JAet al. Stereotactic proton beam therapy of skull base meningiomas. Int J Radiat Oncol Biol Phys 2001;49:99–105. [DOI] [PubMed] [Google Scholar]
- 17. Pirzkall A, Debus J, Haering Pet al. Intensity modulated radiotherapy (IMRT) for recurrent, residual, or untreated skull-base meningiomas: preliminary clinical experience. Int J Radiat Oncol Biol Phys 2003;55:362–72. [DOI] [PubMed] [Google Scholar]
- 18. Palm A, Johansson KA. A review of the impact of photon and proton external beam radiotherapy treatment modalities on the dose distribution in field and out-of-field: implications for the long-term morbidity of cancer survivors. Acta Oncol 2007;46:462–73. [DOI] [PubMed] [Google Scholar]
- 19. Lesueur P, Calugaru V, Nauraye Cet al. Proton therapy for treatment of intracranial benign tumors in adults: a systematic review. Cancer Treat Rev 2019;72:56–64. [DOI] [PubMed] [Google Scholar]
- 20. Tsujii H, Tsuji H, Inada Tet al. Clinical results of fractionated proton therapy. Int J Radiat Oncol Biol Phys 1993;25:49–60. [DOI] [PubMed] [Google Scholar]
- 21. Kagei K, Tokuuye K, Sugahara Set al. Initial experience of proton beam therapy at the new facility of the University of Tsukuba. Nihon Igaku Hoshasen Gakkai Zasshi 2004;64:225–30. [PubMed] [Google Scholar]
- 22. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 23. Ando K, Koike S, Kawachi Ket al. Relative biological effectiveness of the therapeutic proton beams at NIRS and Tsukuba University. Nihon Igaku Hoshasen Gakkai Zasshi 1985;45:531–5. [PubMed] [Google Scholar]
- 24. Gerelchuluun A, Hong Z, Sun Let al. Induction of in situ DNA double-strand breaks and apoptosis by 200 MeV protons and 10 MV X-rays in human tumour cell lines. Int J Radiat Biol 2011;87:57–70. [DOI] [PubMed] [Google Scholar]
- 25. U.S. Department of Health and Human Services NIH, National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (7 November 2020, date last accessed).
- 26. Davidson SE, Burns M, Routledge Jet al. Short report: a morbidity scoring system for Clinical Oncology practice: questionnaires produced from the LENT SOMA scoring system. Clin Oncol. 2002;14:68–9. [DOI] [PubMed] [Google Scholar]
- 27. Rogers L, Mehta M. Role of radiation therapy in treating intracranial meningiomas. Neurosurg Focus 2007;23(4):E4. [DOI] [PubMed] [Google Scholar]
- 28. Milker-Zabel S, Zabel-du Bois A, Huber Pet al. Intensity-modulated radiotherapy for complex-shaped meningioma of the skull base: long-term experience of a single institution. Int J Radiat Oncol Biol Phys 2007;68:858–63. [DOI] [PubMed] [Google Scholar]
- 29. Maclean J, Fersht N, Bremner Fet al. Meningioma causing visual impairment: outcomes and toxicity after intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2013;85:e179–86. [DOI] [PubMed] [Google Scholar]
- 30. Kosaki K, Ecker S, Habermehl Det al. Comparison of intensity modulated radiotherapy (IMRT) with intensity modulated particle therapy (IMPT) using fixed beams or an ion gantry for the treatment of patients with skull base meningiomas. Radiat Oncol 2012;7:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weber DC, Lomax AJ, Rutz HPet al. Spot-scanning proton radiation therapy for recurrent, residual or untreated intracranial meningiomas. Radiother Oncol 2004;71:251–8. [DOI] [PubMed] [Google Scholar]
- 32. Noël G, Bollet MA, Calugaru Vet al. Functional outcome of patients with benign meningioma treated by 3D conformal irradiation with a combination of photons and protons. Int J Radiat Oncol Biol Phys 2005;62:1412–22. [DOI] [PubMed] [Google Scholar]
- 33. Weber DC, Schneider R, Goitein Get al. Spot scanning-based proton therapy for intracranial meningioma: long-term results from the Paul Scherrer Institute. Int J Radiat Oncol Biol Phys 2012;83:865–71. [DOI] [PubMed] [Google Scholar]
- 34. Murray FR, Snider JW, Bolsi Aet al. Long-term clinical outcomes of pencil beam scanning proton therapy for benign and non-benign intracranial meningiomas. Int J Radiat Oncol Biol Phys 2017;99:1190–8. [DOI] [PubMed] [Google Scholar]
- 35. El Shafie RA, Czech M, Kessel KAet al. Clinical outcome after particle therapy for meningiomas of the skull base: toxicity and local control in patients treated with active rasterscanning. Radiat Oncol 2018;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsumoto Y, Matsuura T, Wada Met al. Enhanced radiobiological effects at the distal end of a clinical proton beam: in vitro study. J Radiat Res 2014;55:816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pollock BE, Link MJ, Stafford SLet al. The risk of radiation-induced tumors or malignant transformation after single-fraction intracranial radiosurgery: results based on a 25-year experience. Int J Radiat Oncol Biol Phys 2017;97:919–23. [DOI] [PubMed] [Google Scholar]
- 38. Ichimura S, Kawase T. Effects of surgery and radiotherapy on recurrent skull base meningiomas: clinical and biological analyses. J Neurol Surg B Skull Base 2019;80:474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schneider U, Lomax A, Besserer Jet al. The impact of dose escalation on secondary cancer risk after radiotherapy of prostate cancer. Int J Radiat Oncol Biol Phys 2007;68:892–7. [DOI] [PubMed] [Google Scholar]
- 40. Dennis ER, Bussiere MR, Niemierko Aet al. A comparison of critical structure dose and toxicity risks in patients with low grade gliomas treated with IMRT versus proton radiation therapy. Technol Cancer Res Treat 2013;12:1–9. [DOI] [PubMed] [Google Scholar]
- 41. Mirian C, Duun-Henriksen AK, Juratli Tet al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: An individual patient data meta-analysis. J Neurol Neurosurg Psychiatry 2020;91:378–87. [DOI] [PubMed] [Google Scholar]
- 42. Maclean J, Fersht N, Short S. Controversies in radiotherapy for meningioma. Clin Oncol (R Coll Radiol) 2014;26:51–64. [DOI] [PubMed] [Google Scholar]


