ABSTRACT
Our aim was to evaluate the efficacy and toxicity of interstitial multicatheter high dose rate brachytherapy (imHDR-BRT) as accelerated partial breast irradiation (APBI) after second breast-conserving surgery (BCS) in patients with ipsilateral breast tumor recurrence (IBTR). Between January 2010 and December 2019, 20 patients with IBTR who refused salvage mastectomy (sMT) were treated with second BCS and post-operative imHDR-BRT as APBI. All patients had undergone primary BCS followed by adjuvant external beam radiotherapy. Median imHDR-BRT dose was 32 Gy delivered in twice-daily fractions of 4 Gy. Five-year IBTR-free survival, distant metastasis-free survival (DMFS), overall survival (OS) as well as toxicity and cosmesis were evaluated in the present retrospective analysis. Median age at recurrence and median time from the first diagnosis to IBTR was 65.1 years and 12.2 years, respectively. After a median follow-up of 69.9 months, two patients developed a second local recurrence resulting in 5-year IBTR free-survival of 86.8%. Five-year DMFS and 5-year OS were 84.6% and 92.3%, respectively. Grade 1–2 fibrosis was noted in 60% of the patients with no grade 3 or higher toxicity. Two (10%) cases of asymptomatic fat necrosis were documented. Cosmetic outcome was classified as excellent in 6 (37.5%), good in 6 (37.5%), fair in 3 (18.75%) and poor in 1 (6.25%) patient, respectively. We conclude that imHDR-BRT as APBI re-irradiation is effective and safe for IBTR and should be considered in appropriately selected patients.
Keywords: breast cancer, local recurrence, salvage mastectomy, interstitial multicatheter brachytherapy
INTRODUCTION
Adjuvant external beam radiation therapy (EBRT) can decrease the incidence of breast cancer (BCA) recurrence and cancer-specific mortality in patients treated with breast-conserving surgery (BCS) [1]. The management of ipsilateral breast tumor recurrence (IBTR), however is posing a clinical challenge and is mainly determined by previously applied therapeutic modalities. Ipsilateral breast tumor recurrence after adjuvant EBRT accounts for 9% after up to 15 years [2] and represents 80% of all locoregional recurrences [3, 4]. These manifestations are associated with a higher risk of concurrent or subsequent systemic progression [5], thus necessitating comprehensive treatment. Although comparative phase III evidence for salvage mastectomy (sMT) versus re-BCS is lacking, sMT is broadly recommended for IBTR with a second local relapse in the form of a chest wall recurrence occurring in 3–32% of cases [6]. Repeat BCS alone cannot be considered as an option, since it is associated with increased cancer-specific and overall mortality [7]. However, a recent systematic review showed at least non-inferior oncological outcomes in comparison with sMT when repeat BCS is combined with repeat irradiation [8]. Re-irradiation can be offered either as partial breast EBRT or as partial breast interstitial brachytherapy due to the risk of excessive toxicity after repeat EBRT [9]. Although, the role of interstitial multicatheter accelerated partial breast irradiation (APBI) after initial BCS has been well established through prospective trials [10,11], its role as a re-irradiation modality after second BCS has not been extensively studied. In this retrospective analysis, we evaluate the clinical outcome of interstitial multicatheter high dose rate brachytherapy (imHDR-BRT) as APBI in a group of patients presenting with IBTR following EBRT.
MATERIALS AND METHODS
Between January 2010 and December 2019, a total of 20 patients were treated with computed tomography (CT)-guided imHDR-BRT for IBTR. All patients had undergone primary BCS with axillary lymph node dissection or sentinel lymph node biopsy followed by adjuvant EBRT with or without regional nodal irradiation according to axillary lymph node status. Endocrine therapy and adjuvant systemic treatment were administered according to hormone receptor status and national guidelines at the time of diagnosis. During follow-up (FU), all patients developed a histologically proven IBTR and were subsequently staged for regional and distant metastases. After exclusion of metastatic disease, all cases were discussed in an interdisciplinary gynecological tumorboard. Mastectomy was offered to all patients as the gold standard. For those patients refusing sMT, repeat BCS with subsequent imHDR-BRT was offered as an alternative provided that: (i) the tumor measured <3 cm pre-operatively; (ii) the last day of EBRT dated back preferably >5 years; (iii) a negative resection margin was warranted; and (iv) the cosmetic result could be deemed acceptable in accordance with oncosurgical standards.
Patient and tumor characteristics for the primary and recurrent tumor are shown in Table 1. Among the 19 (95%) patients with known primary and recurrent tumor histology, 14 (73.7%) were of the same and 5 (26.3%) of different histological subtype, thus considered as new primaries. Tumor lesions were located in the left breast in 11 (55%) patients and in the right breast in 9 (45%) patients. Tumor location for both primary and recurrent tumor according to quadrant was known in 16 (80%) patients. Among them, 10 (62.5%) recurrent lesions were located in the same quadrant as the primary and 6 (37.5%) in a different quadrant. Regarding operation technique, in all cases except one the dissected cavity was closed. No operation included major oncoplastic surgery with the need for mobilization of more than one breast quadrant. At first tumor diagnosis, axillary dissection was performed in 16 (80%) patients and sentinel node biopsy in 4 (20%). Of note, 14 (70%) patients were operated before 2004, which explains the high rate of axillary dissections at first diagnosis as sentinel node biopsy started emerging in the early 2000s and became the standard procedure for clinical node-negative axilla during the end of the first half of the 2010s following the publication of randomized trials [12, 13] and the publication of recommendations from major surgical and oncological associations [14, 15].
Table 1.
Tumor and treatment characteristics
| Characteristic | Primary tumor | Secondary/recurrent tumor | |||
|---|---|---|---|---|---|
| Patients (n = 20) | % | Patients (n = 20) | % | ||
| Tumor location (quadrant) | upper outer | 10 | 50 | 9 | 45 |
| upper inner | 2 | 10 | 3 | 15 | |
| lower outer | 3 | 20 | 3 | 15 | |
| lower inner | 1 | 5 | 2 | 10 | |
| unknown | 4 | 20 | 3 | 15 | |
| Type of BCS | Lumpectomy | 5 | 25 | 3 | 15 |
| Segmentectomy | 12 | 60 | 16 | 80 | |
| Quadrantectomy | 3 | 15 | 1 | 5 | |
| Histology | NST | 13 | 65 | 14 | 70 |
| Lobular | 2 | 10 | 1 | 5 | |
| DCIS | 3 | 15 | 5 | 25 | |
| unknown | 1 | 5 | 0 | 0 | |
| pT stage | yT0 | 1 | 5 | 0 | 0 |
| Tis | 3 | 15 | 5 | 25 | |
| T1 | 14 | 70 | 11 | 55 | |
| T2 | 2 | 10 | 4 | 20 | |
| Surgical LN assessment | Axillary dissection | 16 | 80 | 2 | 10 |
| Sentinel node biopsy | 4 | 20 | 1 | 5 | |
| cN0 | 17 | 85 | |||
| pN stage | Nx | 1 | 5 | ||
| N0 | 17 | 85 | 3 | 15 | |
| N1 | 2 | 10 | 0 | 0 | |
| Grading | G1 | 2 | 10 | 2 | 10 |
| G2 | 9 | 45 | 12 | 60 | |
| G3 | 7 | 35 | 6 | 30 | |
| unknown | 2 | 10 | |||
| ER status | positive | 10 | 50 | 18 | 90 |
| negative | 2 | 10 | 1 | 5 | |
| unknown | 8 | 40 | 1 | 5 | |
| PR status | positive | 10 | 50 | 16 | 80 |
| negative | 2 | 10 | 2 | 10 | |
| unknown | 8 | 40 | 2 | 10 | |
| Her2neu status | positive | 1 | 5 | 2 | 10 |
| negative | 9 | 45 | 17 | 85 | |
| unknown | 10 | 55 | 1 | 5 | |
| Resection margin | Rx | 7 | 35 | 2 | 10 |
| R0 | 13 | 65 | 18 | 90 | |
| ≥2 mm | n. a. | n. a. | 15 | 83.3 | |
| 1 mm | n. a. | n. a. | 2 | 11.1 | |
| ≤1 mm | n. a. | n. a. | 1 | 5.6 | |
| Endocrine therapy | yes | 10 | 50 | 11 | 55 |
| no | 4 | 20 | 2 | 10 | |
| unknown | 6 | 30 | 7 | 35 | |
| Chemotherapy | yes | 10 | 50 | 1 | 5 |
| no | 10 | 50 | 19 | 95 | |
Abbreviations: BCS, breast-conserving surgery; NST, non-special type; DCIS, ductal carcinoma in situ; LN, lymph node; ER, estrogen; PR, progesterone; n.a., not available
Treatment technique
Interstitial multicatheter HDR-BRT was carried out within 6 weeks after repeat BCS. For this purpose, brachytherapy catheters were implanted free-hand (Fig. 1) under CT guidance with the patients being under general anesthesia. Round-tip plastic catheters of 6F diameter and 200 mm length (OncoSmart ProGuide Round Needle, Elekta, Sweden) were implanted using a rigid tungsten alloy obturator of the same length and diameter (OncoSmart ProGuide Obturator, Elekta, Sweden). This allowed for maintenance of catheter integrity and stability during insertion. After removal of the obturator, positional control was obtained by generating CT images with the catheter in situ. Thus, the direction and position of the implanted catheters were estimated by interactive CT scanning. The number and geometrical alignment of the catheters were dependent on the size and location of the tumor bed. After catheter placement, a spiral CT scan (slice thickness of 1.5 mm) without contrast agent was acquired for three-dimensional (3D) treatment planning. For the purpose of tumor bed localization, pre-operative imaging, including any available mammograms, ultrasound or magnetic resonance imaging (MRI), surgical reports, surgical clips and the position of the skin scar were taken into consideration. The clinical target volume (CTV) was defined according to the recommendations of the GEC-ESTRO Breast Cancer Working Group [16, 17]. The safety margin was calculated by taking into account the size of the free resection margin in the respective direction. The total size of the safety margin was 20 mm as the sum of the surgical and added safety margins. The CTV was limited to chest wall/pectoral muscles and 5 mm below the skin surface. No additional margin was added for the planning target volume (PTV). Dose constraints were used in line with the ESTRO-ACROP guideline [18]. For 3D dose optimization, performed using Oncentra Brachytherapy (Elekta, Sweden), active source dwell positions were selected along the catheters to ensure placement inside the PTV (Fig. 2). The dose distribution was normalized relative to the calculated mean dose value on the PTV surface, with the reference dose specified as the 100% value. The dose was delivered over consecutive days with an interfractional interval of at least 6 h. Catheters stayed in place throughout the fractionation schedule and were removed immediately after the last treatment fraction. All irradiations were performed using an Iridium-192 (192Ir) HDR-afterloading system (Elekta, Sweden) with an apparent initial source activity of ~370 GBq. This analysis was approved by the local research ethics board.
Fig. 1.
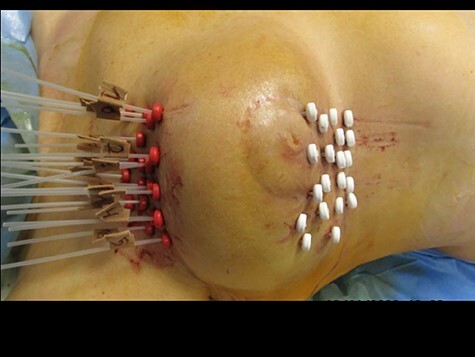
Interstitial multicatheter HDR-BRT implant for a centrally located right-sided breast cancer recurrence after second breast-conserving surgery. Implantation of 20 catheters. The white buttons are fixed in the catheters and fixate them from the one side of the breast, whereas the externally overlaid red radiopaque buttons fixate the catheters from the other side.
Fig. 2.
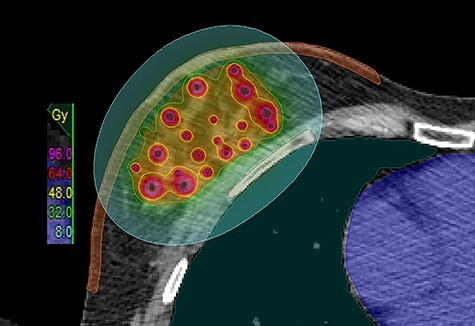
Axial view of an imHDR-APBI treatment plan with overlaid dose distribution. The isodose lines color code convention is: pink = 96.0 Gy; red = 64 Gy; yellow = 48 Gy; green = 32; turquoise = 8 Gy.
Statistics
Patient data were retrieved retrospectively from institutional databases in accordance with institutional ethical policies. For patients who received their initial whole-breast BRT other than at our department, all treatment-related documents were collected. Overall survival (OS) was calculated from the end of BRT until the last date of FU or death. Locoregional control (LC) was calculated from the end of BRT until the diagnosis of recurrent disease. Ipsilateral breast tumor recurrence was defined as any relapse within the ipsilateral breast tissue, while regional recurrence was considered as occurrence of regional lymph node metastases (axilla, supraclavicular fossa or internal mammary chain). The Kaplan–Meier method was used to estimate 5-year IBTR-free survival, 5-year distant metastasis-free survival (DMFS) and 5-year OS. Patients alive at FU were censored. The likelihood of events was thereafter compared using the log-rank test, setting a two-sided P value of ≤0.05 as statistically significant. All statistical calculations were performed and evaluated using the statistical software Winstat® (R.Fitch Software, Bad Kronzingen, Germany). Acute toxicity was defined as any side effect occurring within 3 months following BRT, while later occurrences were classified as late adverse events. Toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Cosmetic outcome was determined according to the 4-point Harvard breast cosmesis scale [19] and classified by two independent observers (G.C. and N.T.). Post-treatment FU including toxicity and cosmetic outcome assessment was performed initially at 3 months, 6 monthly thereafter in the first 2 years and annually thereafter.
RESULTS
Median age at recurrence was 65.1 years (range 47.9–81.5). Median time from the first diagnosis to IBTR was 12.2 years (range 2.5–25.6). As for radiation treatment, all patients at first diagnosis received adjuvant EBRT of a median 55.8 Gy (range 45–66.6 Gy). At recurrence, all patients received imHDR-BRT, which was performed under general anesthesia. Median number of catheters implanted was 11.5 (range 6–20), whereas median total physical dose was 32 Gy (range 20–32 Gy), with a median single fraction dose of 4 Gy (range 2.5–4 Gy), applied in a median of eight fractions (range 8–11). The median PTV was 69.2 cm3 (range 35.6–217.6 cm3). No complication was documented during BRT.
Oncological outcomes
After a median FU of 69.6 months (range 10.4–140.3 months), two (10%) patients experienced a second IBTR (both local recurrences) 13.4 and 27.7 months after imHDR-BRT, respectively. The 5-year IBTR-free survival was 86.8%. Of these two patients, one received sMT and remains free of disease, whereas the second one developed distant metastasis, being currently free from progression 3 years after chemotherapy. Both patients with second IBTR had an in-breast but not local recurrence at first IBTR. Both had an R0 resection with a >3 mm tumor-free margin at second BCS. No regional recurrence was observed. Two (10%) patients developed distant metastasis 30.6 and 44.2 months after imHDR-BRT, for a 5-year DMFS of 84.6%. Five-year OS was 92.3% (Fig. 3).
Fig. 3.
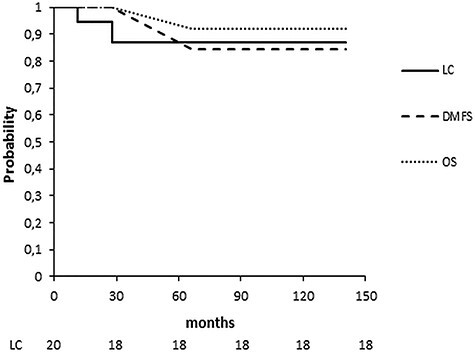
Kaplan–Meier survival curves of LC, DMFS and OS.
Toxicity and cosmetic result
Grade I–II fibrosis was noted in 60% of the patients and was manifested in most of the cases within the first 2 years after imHDR-BRT. Telangiectasia and hyperpigmentation were documented in 15% and 10% of the patients, respectively. There was one (5%) case of breast retraction and two (10%) cases of asymptomatic fat necrosis (Fig. 4). The two latter cases were recorded 2 and 7 years after the imHDR-BRT. There was no grade III–IV toxicity. Among the 16 patients (80%) available for cosmetic assessment, the result was scored as excellent for 6 (37.5%), good for 6 (37.5%), fair for 3 (18.75%) and poor for 1 (6.25%). Figure 5a and b depicts the excellent cosmetic outcome of a patient before and 3 years after imHDR-BRT. Patient’s self-evaluation did not differ from that of the physician, except for one patient who evaluated her cosmetic outcome as fair instead of good (physician’s evaluation).
Fig. 4.
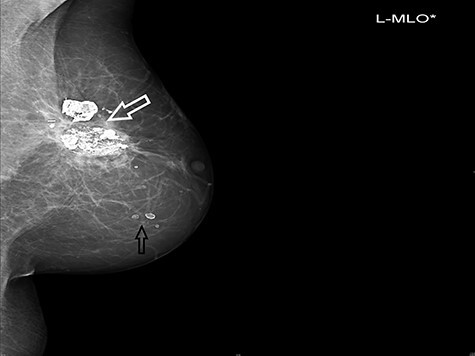
Mammogram of the left breast 25 months after imHDR-BRT. Mediolateral oblique view showing calcified fat necrosis seen in the left upper outer quadrant (white arrow) and micro- and macrocalcifications (black arrow).
Fig. 5.
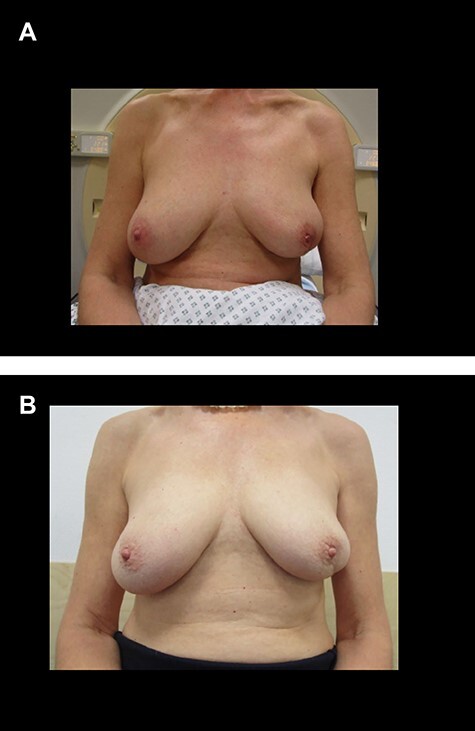
(a) Pre-imHDR-BRT image of a patient with a left breast cancer (the tumor was pre-operatively located in the lower outer quadrant). (b) Image of the same patient showing an excellent cosmetic result 3 years after imHDR-BRT.
DISCUSSION
Treatment of locally recurrent BCA remains an interdisciplinary challenge. So far, sMT has been recommended as the standard therapy in cases of IBTR, yielding an IBTR rate after 5 years of ~10–18% in the recent literature [20–23]. However, patients undergoing MT may suffer from impaired body self-image and reduced self-esteem, followed by emotional and physical distress negatively impacting quality of life and sexual performance [24]. Hence, breast preservation should always be considered and offered after careful evaluation and appropriate patient selection [25]. However, second BCS alone is associated with a significantly higher rate of second IBTR compared with sMT [26, 27], which is in turn associated with poor prognosis [7, 28]. Combination of repeat breast conservation with a second course of radiotherapy as APBI is reducing the increased risk of second IBTR compared with second BCS alone, yielding similar oncological outcomes to sMT [8].
The largest experience with repeat radiotherapy by means of APBI exists for imHDR-BRT [23, 29–31]. The outcomes of these studies are summarized in Table 2. Interstitial multicatheter HDR-BRT after second BCS results in an excellent 5-year LC rate of >85% and a 5-year OS of > 80%. In the largest series to date, Hannoun-Levi et al. [29] treated 217 patients overall, of which 102 (47%), 88 (40.6%) and 27 (12.4%) were treated with HDR, pulse dose rate (PDR) and low dose rate (LDR) BRT, respectively. Median time to IBTR was 10.1 years (range 1.1–35.3). Most of the recurrent tumors were pT1, with positive hormone receptor status and negative Her2neu status. Interstitial multicatheter HDR-BRT was delivered in 5–10 fractions of 3.6–4.4 Gy. After a median FU of 47 months, 5-year second IBTR-free survival was 94%, with a 5-year DMFS and OS of 88.9% and 88.7%, respectively. In univariate analysis, age at the time of IBTR (≤55 versus >55 years, P = 0.035), histological grade (I–II versus III; P = 0.0003) and hormone receptor status (positive versus negative, P = 0.001) were prognostic factors for second IBTR, whereas in multivariate analysis only histological grade remained significant for second IBTR (P = 0.008). Most of the patients experienced grade I–II toxicity, predominantly (sub-) cutaneous fibrosis, and were evaluated as having good to excellent cosmesis (85%). In comparison, in our analysis, 5-year second IBTR-free survival, 5-year DMFS and OS were 86.6, 84.6 and 92.3, respectively, with similar toxicity and cosmesis outcomes. Low-dose-rate BRT as well as PDR-BRT have also been used for re-irradiation after second BCS [32–36]. The results and toxicity do not differ significantly from those of HDR-BRT, with 5-year freedom from second IBTR in the range of 77–93%.
Table 2.
Studies of HDR-BRT as re-irradiation after repeat breast-conserving surgery
| Study | Patient number | Fraction × dose in Gy | Median FU (months) | 5-year FFLR (%) | 5-year DMFS (%) | 5-year OS (%) | Excellent and good cosmesis (%) |
|---|---|---|---|---|---|---|---|
| Hannoun-Levi et al. (28) | 102 | med. 8 × 4 (5–10 × 3.6–4.4) | 47 | 94 | 88.9 | 88.7 | 85 |
| Forster et al. (26) | 11 | 9 × 3.8 or 8 × 4 | 65 | 100 | 100 | 100 | N.R. |
| Smanyko et al. (19) | 39 | 5 × 4.4 | 59 | 94 | 76 | 81 | 70 |
| Cozzi et al. (27) | 40 | 8 × 4 or 10 × 3.4 | 61.5 | 96.6 | 94.8 | 85.3 | 57.5 |
| This study | 20 | 8 × 2.5–4 (med. 4 Gy) | 69.9 | 86.6 | 84.6 | 92.3 | 75 |
Abbreviations: FU, follow up; FFLR, freedom from local failure; DMFS, distant metastases-free survival; OS, overall survival; N.R., not reported; med., median.
Although there is reluctance in undertaking a second course of EBRT due to older studies showing inferior oncological and cosmesis outcomes [37], the recently published RTOG 1014 trial [38] showed that a second course of EBRT as APBI is feasible. In this prospective phase II study, 58 patients after second BCS were re-irradiated with 3D conformal EBRT up to a total reference dose of 45 Gy delivered in twice-daily fractions of 1.5 Gy. All tumors were <3 cm in size, node negative and estrogen receptor status positive in 75% of the patients, resembling, in fact, our own cohort. After a median FU of 5.5 years (range 0.1–7.2 years), there were four total second in-breast recurrences reported, representing a 5-year cumulative incidence of 5.2%. Toxicity was somewhat higher than that reported with HDR-BRT, with grade 3 events occurring in 4 (7.0%) patients. Janssen et al. [39] also evaluated the role of repeat EBRT after second BCS. In their study, 42 patients received partial breast repeat EBRT of 45 Gy of 1.8 Gy per fraction. After a median FU of 35 months, the IBTR rate was 14.5%. Of note, the study also included patients with T3–T4 tumors, which showed a significant inferior result for OS compared with T1–T2 tumors. No grade 3 toxicity was observed.
As toxicity after repeat BCS and re-irradiation seems to be acceptable with preponderantly grade 1–2 and low rates of grade 3 adverse events, identifying risk factors for second IBTR is crucial in order to properly select the patients that would benefit from this approach. A shorter interval between initial treatment and first local recurrence is negatively correlated with freedom from second IBTR, as shown by Hannoun-Levi et al. and Kauer-Dorner et al. [32, 36]. In the era of molecular subtyping of BCA, the discriminative analysis of the results by Anderson et al. [40] regarding the patterns of LR after initial imHDR-BRT as APBI according to molecular subtype may further contribute to appropriate patient selection. In this analysis, Luminal A/B subtypes showed a 5-year second IBTR-free survival of 3.5–5.2%, whereas this rate was at least doubled in the range of 11.3–13.3% for triple-negative and Her2neu-positive tumors.
In our study the median time from the first diagnosis to first IBTR was 12.2 years (range 2.5–25.6). This finding is in accordance with that reported in the literature for early stage low-risk breast cancer, in which median times vary between 9.4 and 13.3 years (range 1.1–35.4) [23, 29–31, 33]. The long latency time until the first IBTR in that patient cohort, with recurrences/new primaries occurring even 35.4 years after first BCS, implies that it may be advisable to extend FU even beyond 10 years [41].
The limitations of our analysis are the relatively low patient number, the absence of evaluation of the cosmetic status before the second BCS, which can lead to an overestimation of the side effects caused by salvage therapy, as well as its retrospective nature. At this point, it might be practically impossible to conduct a prospective randomized trial comparing second breast conservation with BCS-imHDR-BRT versus sMT because of the patients’ reluctance in accepting randomization. Despite these limitations, our study corroborates the current literature data, confirming the efficacy and safety of imHDR-BRT for the treatment of IBTR after second breast conservation.
CONCLUSION
Interstitial multicatheter HDR-BRT as APBI re-irradiation is effective and safe for IBTR and should be considered in appropriately selected patients. A significant number of those patients are deprived of re-irradiation due to fear of higher grade adverse events and are unnecessarily exposed to symptomatic local progression and eventually systemic treatments. An early multidisciplinary management approach for IBTR considering a second course of BCS and re-irradiation can ensure a substantial benefit for this group of patients.
Conflict of interest
None declared.
Contributor Information
Georgios Chatzikonstantinou, Department of Radiotherapy and Oncology, University Hospital, Goethe University Frankfurt am Main, Frankfurt, Germany.
Iosif Strouthos, German Oncology Center, Limassol, Cyprus.
Christian Scherf, Department of Radiotherapy and Oncology, University Hospital, Goethe University Frankfurt am Main, Frankfurt, Germany.
Janett Köhn, Department of Radiotherapy and Oncology, University Hospital, Goethe University Frankfurt am Main, Frankfurt, Germany.
Christine Solbach, Department of Gynecology and Obstetrics, University Hospital, Goethe University Frankfurt am Main, Frankfurt, Germany.
Claus Rödel, Department of Radiotherapy and Oncology, University Hospital, Goethe University Frankfurt am Main, Frankfurt, Germany.
Nikolaos Tselis, Department of Radiotherapy and Oncology, University Hospital, Goethe University Frankfurt am Main, Frankfurt, Germany.
References
- 1. Darby S, McGale P, Correa Cet al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jobsen J, Job P, Riemersma Set al. Pattern of ipsilateral breast tumor recurrence after breast-conserving therapy. Int J Radiat Oncol Biol Phys 2014;89:1006–14. [DOI] [PubMed] [Google Scholar]
- 3. Sjöström M, Lundstedt D, Hartman Let al. Response to radiotherapy after breast-conserving surgery in different breast cancer subtypes in the Swedish breast cancer group 91 radiotherapy randomized clinical trial. J Clin Oncol 2017;35:3222–9. [DOI] [PubMed] [Google Scholar]
- 4. Wahl AO, Rademaker A, Kiel KDet al. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys 2008;70:477–84. [DOI] [PubMed] [Google Scholar]
- 5. Tienhoven G, Voogd A, Peterse Jet al. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). Eur J Cancer 1999;35:32–8. [DOI] [PubMed] [Google Scholar]
- 6. Alpert TE, Kuerer HM, Arthur DWet al. Ipsilateral breast tumor recurrence after breast conservation therapy: outcomes of salvage mastectomy vs. salvage breast-conserving surgery and prognostic factors for salvage breast preservation. Int J Radiat Oncol Biol Phys 2005;63:845–51. [DOI] [PubMed] [Google Scholar]
- 7. Su Y, Guo R, Xue Jet al. Increased mortality with repeat lumpectomy alone after ipsilateral breast tumor recurrence. Oncologist 2019;24:e818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walstra CJEF, Schipper Ret al. Repeat breast-conserving therapy for ipsilateral breast cancer recurrence: a systematic review. Eur J Surg Oncol 2019;45:1317–27. [DOI] [PubMed] [Google Scholar]
- 9. Sedlmayer F, Zehentmayr F, Fastner G. Partial breast re-irradiation for local recurrence of breast carcinoma: benefit and long term side effects. Breast 2013;22:S141–6. [DOI] [PubMed] [Google Scholar]
- 10. Strnad V, Ott OJ, Hildebrandt Get al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet 2016;387:229–38. [DOI] [PubMed] [Google Scholar]
- 11. Polgár C, Major T, Fodor Jet al. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother Oncol 2010;94:274–9. [DOI] [PubMed] [Google Scholar]
- 12. Veronesi U, Paganelli G, Viale Get al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546–53. [DOI] [PubMed] [Google Scholar]
- 13. Tafra L, Lannin DR, Swanson MSet al. Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg 2001;233:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuehn T, Vogl FD, Helms Get al. Sentinel-node biopsy for axillary staging in breast cancer: results from a large prospective German multi-institutional trial. Eur J Surg Oncol 2004;30:252–9. [DOI] [PubMed] [Google Scholar]
- 15. Lyman GH, Giuliano AE, Somerfield MRet al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 2005;23:7703–20. [DOI] [PubMed] [Google Scholar]
- 16. Strnad V, Hannoun-Levi J, Guinot Jet al. Recommendations from GEC ESTRO breast cancer working group (I): target definition and target delineation for accelerated or boost partial breast irradiation using multicatheter interstitial brachytherapy after breast conserving closed cavity surgery. Radiother Oncol 2015;115:342–8. [DOI] [PubMed] [Google Scholar]
- 17. Major T, Gutiérrez C, Guix Bet al. Recommendations from GEC ESTRO breast cancer working group (II): target definition and target delineation for accelerated or boost partial breast irradiation using multicatheter interstitial brachytherapy after breast conserving open cavity surgery. Radiother Oncol 2016;118:199–204. [DOI] [PubMed] [Google Scholar]
- 18. Strnad V, Major T, Polgar Cet al. ESTRO-ACROP guideline: interstitial multi-catheter breast brachytherapy as accelerated partial breast irradiation alone or as boost - GEC-ESTRO breast cancer working group practical recommendations. Radiother Oncol 2018;128:411–20. [DOI] [PubMed] [Google Scholar]
- 19. Harris JR, Levene MB, Svensson Get al. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys 1979;5:257–61. [DOI] [PubMed] [Google Scholar]
- 20. Botteri E, Rotmensz N, Sangalli Cet al. Unavoidable mastectomy for ipsilateral breast tumour recurrence after conservative surgery: patient outcome. Ann Oncol 2009;20:1008–12. [DOI] [PubMed] [Google Scholar]
- 21. Lindford AJ, Meretoja TJ, Smitten KAJ, Jahkola TA. Skin-sparing mastectomy and immediate breast reconstruction in the management of locally recurrent breast cancer. Ann Surg Oncol 2010;17:1669–74. [DOI] [PubMed] [Google Scholar]
- 22. Tanabe M, Iwase T, Okumura Yet al. Local recurrence risk after previous salvage mastectomy. Eur J Surg Oncol 2016;42:980–5. [DOI] [PubMed] [Google Scholar]
- 23. Smanykó V, Mészáros N, Újhelyi Met al. Second breast-conserving surgery and interstitial brachytherapy vs. salvage mastectomy for the treatment of local recurrences: 5-year results. Brachytherapy 2019;18:411–9. [DOI] [PubMed] [Google Scholar]
- 24. Zehra S, Doyle F, Barry Met al. Health-related quality of life following breast reconstruction compared to total mastectomy and breast-conserving surgery among breast cancer survivors: a systematic review and meta-analysis. Breast Cancer 2020;27:534–66. [DOI] [PubMed] [Google Scholar]
- 25. Harms W, Geretschläger A, Cescato Cet al. Current treatment of isolated locoregional breast cancer recurrences. Breast Care 2015;10:265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gentilini O, Botteri E, Veronesi Pet al. Repeating conservative surgery after ipsilateral breast tumor reappearance: criteria for selecting the best candidates. Ann Surg Oncol 2012;19:3771–6. [DOI] [PubMed] [Google Scholar]
- 27. Vila J, Garcia-Etienne CA, Vavassori Aet al. Conservative surgery for ipsilateral breast tumor recurrence. J Surg Oncol 2014;110:62–7. [DOI] [PubMed] [Google Scholar]
- 28. Wapnir IL, Gelber S, Anderson SJet al. Poor prognosis after second locoregional recurrences in the CALOR trial. Ann Surg Oncol 2017;24:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hannoun-Levi J, Resch A, Gal Jet al. Accelerated partial breast irradiation with interstitial brachytherapy as second conservative treatment for ipsilateral breast tumour recurrence: multicentric study of the GEC-ESTRO breast cancer working group. Radiother Oncol 2013;108:226–31. [DOI] [PubMed] [Google Scholar]
- 30. Forster T, Akbaba S, Schmitt Det al. Second breast conserving therapy after ipsilateral breast tumor recurrence—a 10-year experience of re-irradiation. J Contemp Brachytherapy 2019;11:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cozzi S, Jamal DN, Slocker Aet al. Second breast-conserving therapy with interstitial brachytherapy (APBI) as a salvage treatment in ipsilateral breast tumor recurrence: a retrospective study of 40 patients. J Contemp Brachytherapy 2019;11:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hannoun-Levi J, Houvenaeghel G, Ellis Set al. Partial breast irradiation as second conservative treatment for local breast cancer recurrence. Int J Radiat Oncol Biol Phys 2004;60:1385–92. [DOI] [PubMed] [Google Scholar]
- 33. Chadha M, Feldman S, Boolbol Set al. The feasibility of a second lumpectomy and breast brachytherapy for localized cancer in a breast previously treated with lumpectomy and radiation therapy for breast cancer. Brachytherapy 2008;7:22–8. [DOI] [PubMed] [Google Scholar]
- 34. Houvenaeghel G, Boher JM, Michel Vet al. Survival after breast cancer local recurrence according to therapeutic strategies. Eur J Surg Oncol 2017;43:1409–14. [DOI] [PubMed] [Google Scholar]
- 35. Resch A, Fellner C, Mock Uet al. Locally recurrent breast cancer: pulse dose rate brachytherapy for repeat irradiation following lumpectomy—a second chance to preserve the breast. Radiology 2002;225:713–8. [DOI] [PubMed] [Google Scholar]
- 36. Kauer-Dorner D, Pötter R, Resch Aet al. Partial breast irradiation for locally recurrent breast cancer within a second breast conserving treatment: alternative to mastectomy? Results from a prospective trial. Radiother Oncol 2012;102:96–101. [DOI] [PubMed] [Google Scholar]
- 37. Deutsch M. Repeat high-dose external beam irradiation for in-breast tumor recurrence after previous lumpectomy and whole breast irradiation. Int J Radiat Oncol Biol Phys 2002;53:687–91. [DOI] [PubMed] [Google Scholar]
- 38. Arthur DW, Winter KA, Kuerer HMet al. Effectiveness of breast-conserving surgery and 3-dimensional conformal partial breast reirradiation for recurrence of breast cancer in the ipsilateral breast: the NRG oncology/RTOG 1014 phase 2 clinical trial. JAMA Oncol 2019;6:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Janssen S, Rades D, Meyer Aet al. Local recurrence of breast cancer: conventionally fractionated partial external beam re-irradiation with curative intention. Strahlenther Onkol 2018;194:806–14. [DOI] [PubMed] [Google Scholar]
- 40. Anderson BM, Kamrava M, Wang Pet al. Locoregional recurrence by molecular subtype after multicatheter interstitial accelerated partial breast irradiation: results from the pooled registry of multicatheter interstitial sites research group. Brachytherapy 2016;15:788795. [DOI] [PubMed] [Google Scholar]
- 41. www.ago-online.de/fileadmin/ago-online/downloads/_leitlinien/kommission_mamma/ 2020/PDF_EN/2020E 16_Breast Cancer Follow-up.pdf. (11 June 2020, date last acessed).


