Abstract
Since sexual function and testosterone levels after image-guided proton therapy (IGPT) have not yet been examined in detail, we prospectively evaluated changes before and after IGPT. Among patients treated with IGPT with or without combined androgen blockade (CAB) therapy between February 2013 and September 2014, patients who agreed to participate in the study and were followed up for >3 years after IGPT were evaluated. Serum testosterone levels were regularly measured together with prostate-specific antigen (PSA) levels before and after IGPT. The Erection Hardness Score (EHS) and the sexual domain summary, function subscale and bother subscale of the sexual domain in the Expanded Prostate Cancer Index Composite (EPIC) were assessed. There were 38 low-risk, 46 intermediate-risk and 43 high- or very-high-risk patients (NCCN classification). Although serum testosterone levels in low-risk patients did not decrease after IGPT, reductions were observed in the average EHS and the sexual domain summary score of the EPIC. In intermediate-, high- and very-high-risk patients, testosterone and PSA levels both increased following the termination of CAB after IGPT, and the average EHS increased. The sexual domain summary score gradually increased, but not above minimally important differences. In intermediate-risk patients, the function subscale increased from 4.4 to 14.8 (P < 0.05) 12 months after IGPT and reached a plateau after 60 months. The results of the present study would suggest the potential of IGPT, and further prospective studies to directly compare IGPT with other modalities are warranted.
Keywords: testosterone, Expanded Prostate Cancer Index Composite, Erection Hardness Score, image-guided proton therapy, combined androgen blockade therapy, prostate cancer
INTRODUCTION
Radical prostatectomy and radiation therapy, including proton therapy (PT), are two main options for the definitive treatment of localized prostate cancer. The overall prognosis of patients is favorable after both types of treatments [1–5]; however, adverse effects differ. While urinary incontinence and impotence are the two major complications of prostatectomy, acute genitourinary disorders and late rectal bleeding are major complications of radiation therapy. Since prostate cancer patients are expected to have a long-term prognosis, quality of life (QOL) is very important after any type of treatment [6, 7], and potency is one of the important factors in QOL. Erectile function is often maintained after definitive radiation therapy, and this is an advantage of radiotherapy over surgery [8]. However, hormonal therapy is generally combined with radiation therapy in intermediate- and high-risk patients; therefore, potency is weakened due to androgen suppression. Therefore, further studies are needed to evaluate sexual function and testosterone levels after combined hormonal and radiation therapy as well as radiation therapy alone for prostate cancer.
Serum testosterone levels influence glucose metabolism, muscle strength, mental status and self-perceived vitality. Low testosterone levels have been shown to correlate with insulin resistance [9, 10]. The maintenance of muscle mass and metabolism also contributes to QOL during long-term survival. Recent studies reported a transient decline in testosterone levels shortly after intensity-modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT), which was normalized by 1–2 years post-treatment [11–14]. Testicular atrophy and variation of the male sex hormones and quality of sexual life following prostate radiotherapy have also been observed [14]. On the other hand, testosterone suppression was not observed after PT, and this may have been due to lower scatter radiation doses delivered to testicular Leydig cells [15, 16]. Yoon et al. [17] reported that secondary doses during prostate PT were ~10−4 Sv Gy–1 at 50 cm from the beam isocenter and were an order of magnitude lower than during conventional IMRT. In addition, a systematic review and meta-analysis revealed a greater reduction in testosterone level after conventional radiotherapy and IMRT/SBRT than after PT [18].
Since QOL and testosterone levels in relation to PSA levels after PT have not yet been investigated in detail in long-term follow-ups in Japanese patients, we herein prospectively evaluated changes in and correlations between sexual function and serum testosterone levels before and after image-guided PT (IGPT) with or without hormonal treatments.
MATERIALS AND METHODS
Study design, patient eligibility and treatment protocols
This was a subanalysis of a prospective study of IGPT for localized prostate cancer based on our protocol. Subjects were patients who agreed to join this study to evaluate serum testosterone levels and sexual function. The initial results of the prospective study were previously reported [19]; the study used 2.0 Gy relative biological effectiveness (RBE) daily fractions up to 74 Gy RBE for low-risk (according to the National Comprehensive Cancer Network risk classification) patients and 78 Gy RBE for intermediate-, high- and very-high-risk patients. Low-risk patients did not receive hormone therapy, whereas intermediate- and high-risk patients received neoadjuvant combined androgen blockade (CAB) therapy for 6–8 months before the start of IGPT. Adjuvant hormonal therapy for 16–18 months (a total of 24 months) was also recommended for high-risk and very-high-risk patients.
Between February 2013 and September 2014, a total of 254 patients with localized prostate cancer were prospectively treated with this protocol at the Nagoya Proton Therapy Center. IRB approval numbers were 14-02-17(14) and 14-02-18(15) for the conventional fractionation trials (the former was for hormone therapy-naïve patients and the latter for patients who had received hormone therapy at the initial visit). The 14-02-18(15) study included low-risk patients who received hormonal therapy and patients who received CAB for apparently longer or shorter times than our protocol. In October 2014, a new dose fractionation protocol using a moderate dose was initiated. In addition, due to national health insurance assessments, the serial routine measurement of testosterone levels became impossible from 2018; therefore, testosterone levels were measured up to the third year after the end of IGPT.
In the present study, only patients treated with the 14-02-17(14) protocol were included to precisely match the duration of hormonal treatment. In addition, we excluded patients who refused to undergo serial evaluations of serum testosterone levels and those who were unable to continue CAB because of liver dysfunction or heavy hot flushes. Five patients were excluded because of biochemical failure after ≥36 months based on the Phoenix definition (nadir + 2.0 ng ml–1) [20].
Treatment systems and planning
The treatment machines and systems at our institution were previously described in detail [21, 22]. The RBE value for our proton beams was 1.1 [21]. Briefly, proton treatments were delivered by PROBEAT III (Hitachi, Ltd, Tokyo, Japan) and planned with VQA (Hitachi, Ltd). A passive scattering technique using a range modulation wheel and spot scanning technique using single field optimization were used.
Our method of IGPT for prostate cancer was previously described in detail [19]. Briefly, prior to the treatment, two gold fiducial markers of 0.5 mm in diameter and 5 mm in length (VISICOIL; RadioMed, Barlett, TN, USA) were placed into the prostate [23]. Daily patient alignments were achieved by matching fiducial markers using the PIAS system (Hitachi, Ltd). The clinical target volume (CTV) was defined as only the prostate for low-risk patients, the prostate plus one-third of the seminal vesicles (SVs) for intermediate-risk patients and the prostate plus two-thirds of SVs for high-risk patients. If the dose to the rectum, bladder or intestines was markedly higher than tolerable levels, a cone-down shrunk plan was initiated from the 31st fraction. The urine volume in the bladder was controlled at 150–250 ml during the treatment using a bladder ultrasound scan (BVI 6400; Verathon Medical UK, Sandford, UK).
Follow-up evaluation and statistical analysis
Serum testosterone and prostate-specific antigen (PSA) levels were evaluated once every 3 months up to 6 months, every 6 months from 6 to 36 months, and once a year thereafter after completion of IGPT. Patient self-reported data were prospectively acquired within 1 month before starting IGPT and 1, 6, 12, 36 and 60 months after completion of IGPT. Patient self-reported data were evaluated with the Japanese version of the Expanded Prostate Cancer Index Composite (EPIC) and the Erection Hardness Score (EHS). Regarding the former, sexual function was evaluated with sexual domain and subscale scores consisting of sexual function and sexual bother in the Japanese version of the EPIC. It was adapted for Japanese patients from the original EPIC [24]. Scores range from 0 to 100, with higher values representing a more favorable health-related QOL. Minimally important differences (MIDs) in the sexual domain are 10–12 points [25]. Regarding the latter, the EHS, which is an elemental component of men’s sexual QOL, is good for evaluating erectile function and is often used to question patients because it is really simple [25, 26]. A score of 1 indicates that the penis is larger than normal, but not hard; 2 means the penis is hard, but not hard enough for penetration; 3 means the penis is hard enough for penetration, but not completely hard; and 4 indicates that the penis is completely hard and fully rigid.
Correlation coefficients for all data on serum testosterone and PSA levels measured during this study were evaluated. ‘R’ is the correlation coefficient, and ‘R2’ is the coefficient of determination. Changes in serum testosterone levels, PSA and QOL scores were analyzed with a Wilcoxon signed-rank test in each risk group using EZR [27], which is based on R and R commander (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient and treatment characteristics
Among 254 patients treated with IGPT, 127 were excluded according to the above-described criteria; therefore, the total patient number was 127. Patient and treatment characteristics are summarized in Table 1.
Table 1.
Patient and treatment characteristics
| Risk group | Low | Intermediate | High |
|---|---|---|---|
| Number | 38 | 46 | 43 |
| Age, median (range) (years) | 69 (52–77) | 68 (53–78) | 70 (54–80) |
| iPSA, median (range) (ng ml–1) | 6.2 (3.7–9.1) | 7.7 (4.4–19.7) | 14.6 (4.1–77.0) |
| T1c/T2a/T2b/T2c/T3a/T3b | 21/17/0/0/0/0 | 9/20/8/9/0/0 | 8/10/7/5/11/2 |
| Gleason score 6/7/8/9/10 | 38/0/0/0/0 | 10/36/0/0/0 | 1/7/24/11/0 |
| HbA1c (</≥ 6.5%) | 31/7 | 44/2 | 34/9 |
| Prostate volume, median (range) (ml) | 43.4 (22.3–82.1) | 28.2 (16.8–48.4) | 28.4 (17.0–66.7) |
| Passive scattering/spot scanning | 38/0 | 43/3 | 37/6 |
| Cone down +/– | 3/35 | 16/30 | 19/24 |
| Testosterone, median (range) (ng dl–1) | 403 (27–910) | 4 (3–28) | 4 (3–27) |
| Testosterone, average (range) (ng/dl) | 415 (27–910) | 7 (3–28) | 7 (3–27) |
iPSA = initial prostate-specific antigen.
Low-risk group
Figure 1 shows changes in serum testosterone levels and PSA before and after IGPT. In low-risk patients, PSA gradually decreased after IGPT, whereas serum testosterone levels gradually increased (Fig. 1A). Figure 2 shows scatter plots of testosterone versus PSA of all the data obtained during this study for each risk. No correlations were observed in the low-risk group, which may have been due to the influence of PSA from cancer cells (Fig. 2A). On the other hand, the mean EHS decreased (Fig. 3). Mean sexual domain summary scores before IGPT and 1, 6, 12, 36 and 60 months after IGPT were 46.5, 41.4, 42.1, 40.5 (P < 0.05), 36.6 (P < 0.05) and 36.1 (P < 0.05), respectively (Fig. 4A). Mean sexual function subscale scores before IGPT and 1, 6, 12, 36 and 60 months after IGPT were 28.1, 24.3, 26.4, 23.8 (P < 0.05), 16.3 (P < 0.05) and 15.0 (P < 0.05), respectively (Fig. 4B). Mean bother subscale scores before IGPT and 1, 6, 12, 36 and 60 months after IGPT were 86.9, 79.0, 76.7 (P < 0.05), 77.1 (P < 0.05), 82.1 and 83.3, respectively (Fig. 4C). Reductions to MIDs from baseline sexual domain summary scores were observed in 18, 29, 26, 47 and 47%, respectively. At 60 months, mean sexual domain summary scores almost decreased to the MID threshold.
Fig. 1.
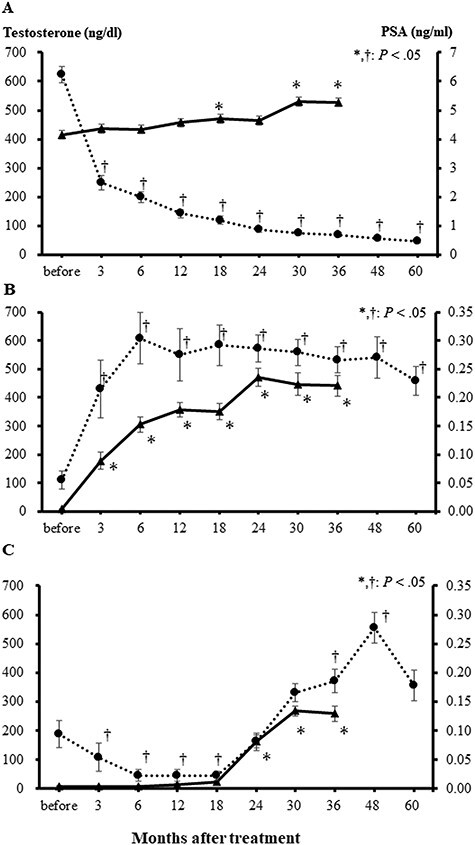
Average serum testosterone (solid line) and PSA levels (dotted line) before and after image-guided proton therapy. Serum testosterone levels gradually increased over time without CAB, and PSA values decreased in low-risk patients (A). In the intermediate- (B) and high- and very-high-risk (C) groups, serum testosterone increased after the completion of CAB therapy. PSA levels also increased as serum testosterone levels became higher (C). Bars represent standard errors.
Fig. 2.
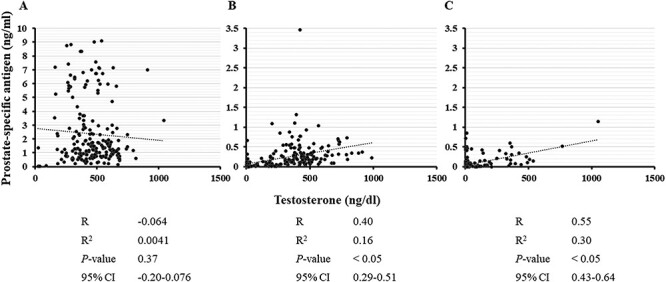
Correlation between serum testosterone and PSA levels for all data obtained during the present study (A, low risk; B, intermediate risk; C, high and very high risk). R = correlation coefficient, R2 = coefficient of determination, 95% CI = 95% confidence interval. In intermediate- and high- and very-high-risk patients, a correlation was observed between serum testosterone and PSA levels.
Fig. 3.
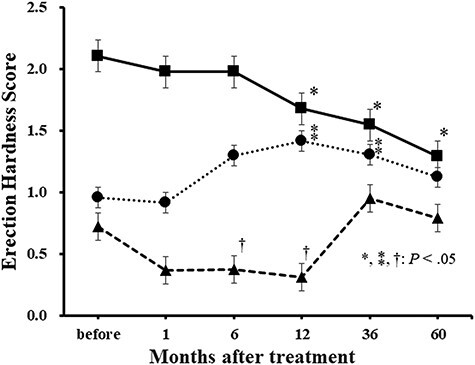
Changes in the EHS after image-guided proton therapy for each risk group (low risk, solid line; intermediate risk, dotted line; high and very high risk, dashed line). In low-risk patients, the EHS decreased over time. In intermediate-risk patients, it increased once after image-guided proton therapy. In high- and very-high-risk patients, the EHS was low while receiving combined antigen blockade (CAB). The EHS increased after the completion of CAB; however, at 60 months, it did not recover to the level in patients without CAB. Bars represent standard errors.
Fig. 4.

Changes in domains in the EPIC after image-guided proton therapy for each risk group. The averages of (A) sexual domain summary, (B) sexual function subscales and (C) sexual bother subscales of EPIC for 60 months after image-guided proton therapy are shown. Low risk, straight line; intermediate risk, dotted line; high and very high risk, dashed line. Bars represent standard errors.
Intermediate-risk group
PSA and testosterone both increased after IGPT and the termination of CAB (Fig. 1B). A correlation was observed between serum testosterone and PSA levels (Fig. 2B). The average EHS increased after IGPT (Fig. 3). The mean EHSs before IGPT and 1, 6, 12, 36 and 60 months after IGPT were 1.0, 0.9, 1.3, 1.4 (P < 0.05), 1.3 (P < 0.05) and 1.1, respectively. Mean EPIC sexual domain summary scores before IGPT and 1, 6, 12, 36 and 60 months after IGPT were 29.4, 30.4 (P < 0.05), 34.4 (P < 0.05), 35.3 (P < 0.05), 34.3 (P < 0.05) and 34.0 (P < 0.05), respectively (Fig. 4A). Mean EPIC sexual function subscale scores before PT and 1, 6, 12, 36 and 60 months after IGPT were 4.4, 6.7, 13.9 (P < 0.05), 14.8 (P < 0.05), 14.7 (P < 0.05) and 14.3 (P < 0.05), respectively (Fig. 4B). Mean bother subscale scores before IGPT and 1, 6, 12, 36 and 60 months after IGPT were 84.2, 82.8, 78.3 (P < 0.05), 80.1, 79.6 and 78.5 (P < 0.05), respectively (Fig. 4C). In intermediate-risk patients, the sexual function subscale score increased and the bother subscale decreased 6 months after the treatment. However, a slight increase was observed in the EPIC sexual domain summary score when the scores before and ≥6 months after IGPT were compared, possibly due to testosterone recovery, although the increase did not meet the MID threshold.
High- and very-high-risk groups
During adjuvant CAB after IGPT, testosterone levels were low (Fig. 1C). Following the completion of CAB 18 months after IGPT, serum testosterone levels began to increase at 24 months. Testosterone levels also reached the normal range at 30 months, but were lower than those in intermediate-risk patients. PSA increased as serum testosterone levels became higher, and this time course was similar to that in intermediate-risk patients. In the high- and very-high-risk groups, a correlation was observed between serum testosterone levels and PSA (Fig. 2C). The mean EHSs before IGPT and 1, 6, 12, 36 and 60 months after IGPT were 0.7, 0.4, 0.4 (P < 0.05), 0.3 (P < 0.05), 0.9 and 0.8, respectively (Fig. 3). Mean EPIC sexual domain summary scores before IGPT and 1, 6, 12, 36 and 60 months after IGPT were 32.9, 30.3, 31.2, 30.2, 34.5 (P < 0.05) and 35.0 (P < 0.05), respectively (Fig. 4A). Mean function subscale scores before PT and 1, 6, 12, 36 and 60 months after PT were 5.4, 2.5 (P < 0.05), 2.9, 1.8 (P < 0.05), 10.1 (P < 0.05) and 8.1 (P < 0.05), respectively (Fig. 4B). Mean bother subscale scores before IGPT and 1, 6, 12, 36 and 60 months after IGPT were 94.3, 89.5, 90.0, 89.1, 84.8 (P < 0.05) and 89.4, respectively (Fig. 4C). In high- and very-high-risk patients, sexual domain summary scores slightly increased at 60 months from 32.9 (before IGPT) to 35.1 (P <.05), possibly due to the completion of CAB. However, this did not meet the MID threshold.
DISCUSSION
Testosterone is a hormone produced in the testicles and plays a key role in reproduction and the maintenance of bone and muscle strength. Lower testosterone levels in older men may contribute to decreases in mobility, sexual function and energy [28]. A relationship has been reported between testosterone and metabolic derangement. Testosterone deficiency causes late-onset hypogonadism and promotes obesity, insulin resistance, metabolic syndrome and type 2 diabetes mellitus [29, 30]. Based on the present results and previous findings [15, 16], PT for prostate cancer is considered to be beneficial because it does not decrease serum testosterone and does not appear to have an impact on these metabolic diseases and symptoms. If the possibility of metabolic syndrome is reduced and muscle strength is maintained, the urinary and bowel QOL may also be maintained [31]. In addition, the absence of a significant decline in sexual function may reduce mental health problems and maintain a good relationship with a partner.
In the present study, testosterone levels were only obtained up to 36 months after IGPT because of changes to the national health insurance system; therefore, more long-term data are needed. In addition, a limitation associated with intermediate-, high- and very-high-risk patients was the lack of data on testosterone levels before CAB. In the low-risk patients, testosterone increased after IGPT; this might have been due to the decrease in patient number during the follow-up period and/or the differences in blood sampling times, since daily fluctuation in testosterone levels has been reported [32]. The inability to determine the reason is another limitation of this study, and it should be clarified in future studies. Based on low-risk group data, serum testosterone levels in intermediate-risk patients were considered to return to normal levels ~2 years after the end of CAB. In high-risk patients, an increase in testosterone levels was observed 18 months after the end of CAB, and its level was near the lower normal limit, suggesting the impact of the long-term administration of CAB. Further studies are needed to investigate whether testosterone recovers to normal levels with further long-term data. In patients undergoing CAB, a correlation was observed between testosterone and PSA levels, indicating that PSA from normal prostate cells is influenced by testosterone.
Although serum testosterone did not decrease after IGPT without CAB, the EHS, sexual summary scores and function subscale scores decreased over time. Sexual domain summary scores decreased to the MID ~60 months after IGPT in low-risk patients. Male sexual function is influenced not only by serum testosterone, but also by other factors, such as age, race, complications and a reduced semen volume due to atrophy of the prostate after irradiation. Sixty months after IGPT, the effects of aging may be substantial. Pre-treatment sexual domain summary scores were lower in Japanese patients than in those from other countries [33–38]. This may be in part due to the older ages of Japanese patients. In addition, Japanese individuals characteristically feel embarrassed to openly discuss sexual topics and tend to express them modestly. Sometimes they hesitate to talk about sexual function, even to medical staff. Since Japanese patients do not have the habit of using drugs for erectile dysfunction, sexual summary scores may be lower than in Western countries. In patients who did not receive androgen deprivation therapy (ADT), sexual domain summary scores decreased, which is consistent with previous findings on SBRT, IMRT, brachytherapy and PT [12, 33–38]. Although greater declines of 12–32 points were noted at 2–5 years in these studies than the decrease of 9.9 points at 36 months in the present study, it should be noted that these studies were from Western countries and the difference may be in part due to the low baseline levels of Japanese patients. As a limitation of this study, we had no QOL data after other radiotherapy modalities, so we were unable to directly compare the results of the present study with those after other treatments. However, in a Japanese study on QOL after IMRT without ADT, a decline in the EPIC sexual function subscale tended to occur earlier and the score at 24 months was lower than in our study [39]. Therefore, further comparative studies are warranted in Japanese patients to clarify the advantages of PT.
A large number of studies have reported a decline in sexual function due to surgery, and improvements are difficult even with robot-assisted radical prostatectomy [40–42]. The sexual summary domain score decreased by ~3- to 4-fold of the MID (i. e. 30–40 points) after surgery; therefore, sexual function preservation after IGPT was considered to be good. Recent PT studies also reported a similar reduction in sexual summary scores to the present study [34, 37, 38]. Few studies have investigated health-related QOL after radiation therapy for ADT-free prostate cancer in Japan and, thus, further accurate assessments are needed [39, 43]. In a Chinese study with a follow-up period of 24 months, the sexual domain scores were ~21, and no significant difference was noted before and after carbon-ion radiotherapy [44]. These results will be useful for comparisons among surgery, IMRT, carbon-ion radiotherapy and PT.
Increased sexual domain summary scores did not reach the MID threshold in intermediate- or high-risk patents. However, the EHS, sexual domain summary and function subscale scores slightly increased after the completion of CAB. This improvement in QOL occurred due to increases in testosterone, suggesting that CAB influenced QOL. The EHS, sexual domain summary and functional subscale scores at 36 months were higher in intermediate-risk than in high-risk patients. These results suggest that long-term CAB affected recovery to the normal level. Sexual bother subscale scores were higher in the intermediate- and high-risk groups than in previous American studies that reported scores of 26 (age ≥69 years) and 34 (age <69 years) with neoadjuvant ADT [45], and they did not markedly change after the completion of CAB in the present study. Sexual bother subscale scores were higher in the high-risk group than in the low- and intermediate-risk groups. In addition, a temporary decrease was observed in sexual bother subscale scores at 36 months. These results support the hypothesis that the loss of sexual function due to CAB may emancipate patients from sexual bother in contrast to the maintenance of sexual activity without CAB in Japan. In the present study, only the sexual function of the EPIC was evaluated; however, a hormonal evaluation may be necessary for intermediate- and high-risk patients. In future studies, we plan to investigate whether differences exist between conventionally fractionated and hypofractionated IGPT.
In conclusion, IGPT alone did not decrease serum testosterone levels. However, the EHS and sexual function scale scores declined. Sexual domain summary scores decreased to the MID threshold 60 months after IGPT. In patients treated with IGPT with CAB, PSA, testosterone, the EHS and sexual domain summary scores increased after the completion of CAB. Since this study did not compare testosterone levels and QOL with those after other treatment modalities, further prospective studies to directly compare IGPT with other modalities are warranted.
PRESENTATION AT A CONFERENCE
This study was partially presented at the 58th Annual Meeting of the American Society for Radiation Oncology, Boston Convention and Exhibition Center, Boston, 25–28 September 2016.
ACKNOWLEDGEMENTS
The authors thank Dr Fumiya Baba, Maho Yamada, Dr Mikiko Imai and the staff at the Nagoya Proton Therapy Center for their valuable help with this research.
Contributor Information
Yukiko Hattori, Department of Radiation Oncology, Nagoya Proton Therapy Center, Nagoya City West Medical Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya 462-8508, Japan.
Hiromitsu Iwata, Department of Radiation Oncology, Nagoya Proton Therapy Center, Nagoya City West Medical Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya 462-8508, Japan; Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
Koichiro Nakajima, Department of Radiation Oncology, Nagoya Proton Therapy Center, Nagoya City West Medical Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya 462-8508, Japan; Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
Kento Nomura, Department of Radiation Oncology, Nagoya Proton Therapy Center, Nagoya City West Medical Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya 462-8508, Japan; Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
Kensuke Hayashi, Department of Proton Therapy Technology, Nagoya Proton Therapy Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya 462-8508, Japan.
Toshiyuki Toshito, Department of Proton Therapy Physics, Nagoya Proton Therapy Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya 462-8508, Japan.
Shingo Hashimoto, Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
Yukihiro Umemoto, Department of Nephro-Urology, Nagoya City West Medical Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya 462-8508, Japan.
Jun-etsu Mizoe, Sapporo High Functioning Radiotherapy Center, Hokkaido Ohno Memorial Hospital, 2-1-16-1 Miyanosawa, Nishi-ku, Sapporo 063-0052, Japan.
Hiroyuki Ogino, Department of Radiation Oncology, Nagoya Proton Therapy Center, Nagoya City West Medical Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya 462-8508, Japan; Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
Yuta Shibamoto, Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
FUNDING
This work was supported by the Takeda Science Foundation 2018.
CONFLICT OF INTEREST
No conflicts of interest exist for any of the authors.
References
- 1. Coughlin GD, Yaxley JW, Chambers SKet al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol 2018;19:1051–60. [DOI] [PubMed] [Google Scholar]
- 2. Takemoto S, Shibamoto Y, Sugie Cet al. Long-term results of intensity-modulated radiotherapy with three dose-fractionation regimens for localized prostate cancer. J Radiat Res 2019;60:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuller DB, Falchook AD, Crabtree Tet al. Phase 2 multicenter trial of heterogeneous-dosing stereotactic body radiotherapy for low- and intermediate-risk prostate cancer: 5-year outcomes. Eur Urol Oncol 2018;1:540–7. [DOI] [PubMed] [Google Scholar]
- 4. Levin-Epstein R, Cook RR, Wong JKet al. Prostate-specific antigen kinetics and biochemical control following stereotactic body radiation therapy, high dose rate brachytherapy, and low dose rate brachytherapy: a multi-institutional analysis of 3502 patients. Radiother Oncol 2020;151:26–32. [DOI] [PubMed] [Google Scholar]
- 5. Iwata H, Ishikawa H, Takagi Met al. Long-term outcomes of proton therapy for prostate cancer in Japan: a multi-institutional survey of the Japanese Radiation Oncology Study Group. Cancer Med 2018;7:677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braide K, Kindblom J, Lindencrona Uet al. A comparison of side-effects and quality-of-life in patients operated on for prostate cancer with and without salvage radiation therapy. Scand J Urol 2020;54:393–400. [DOI] [PubMed] [Google Scholar]
- 7. Seymour ZA, Hamstra DA, Daignault-Newton Set al. Long-term follow-up after radiotherapy for prostate cancer with and without rectal hydrogel spacer: a pooled prospective evaluation of bowel-associated quality of life. BJU Int 2020;126:367–72. [DOI] [PubMed] [Google Scholar]
- 8. Giberti C, Gallo F, Schenone Met al. Robotic prostatectomy versus brachytherapy for the treatment of low risk prostate cancer. Can J Urol 2017;24:8728–33. [PubMed] [Google Scholar]
- 9. Elliott J, Kelly SE, Millar ACet al. Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open 2017;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ottarsdottir K, Nilsson AG, Hellgren Met al. The association between serum testosterone and insulin resistance: a longitudinal study. Endocr Connect 2018;7:1491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zagars GK, Pollack A. Serum testosterone levels after external beam radiation for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 1997;39:85–9. [DOI] [PubMed] [Google Scholar]
- 12. Yuan Y, Aghdam N, King CRet al. Testosterone levels and sexual quality of life after stereotactic body radiation therapy for prostate cancer: a multi-institutional analysis of prospective trials. Int J Radiat Oncol Biol Phys 2019;105:149–54. [DOI] [PubMed] [Google Scholar]
- 13. Markovina S, Weschenfelder DC, Gay Het al. Low incidence of new biochemical hypogonadism after intensity modulated radiation therapy for prostate cancer. Pract Radiat Oncol 2016;4:430–6. [DOI] [PubMed] [Google Scholar]
- 14. Farhood B, Mortezaee K, Haghi-Aminjan Het al. A systematic review of radiation-induced testicular toxicities following radiotherapy for prostate cancer. J Cell Physiol 2019;234:14828–37. [DOI] [PubMed] [Google Scholar]
- 15. Nichols RC, Morris CG, Bryant Cet al. Serum testosterone 60 months after passive-scatter proton therapy for localized prostate cancer. Cancer Invest 2019;37:85–9. [DOI] [PubMed] [Google Scholar]
- 16. Nichols RC, Hu C, Bahary JPet al. Serum testosterone changes in patients treated with radiation therapy alone for prostate cancer on NRG oncology RTOG 9408. Adv Radiat Oncol 2017;2:608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoon M, Ahn SH, Kim Jet al. Radiation-induced cancers from modern radiotherapy techniques: intensity-modulated radiotherapy versus proton therapy. Int J Radiat Oncol Biol Phys 2010;77:1477–85. [DOI] [PubMed] [Google Scholar]
- 18. Mortezaee K, Motallebzadeh E, Milajerdi Aet al. The effect of prostate cancer radiotherapy on testosterone level: a systematic review and meta-analysis. Anticancer Agents Med Chem 2020;20:636–42. [DOI] [PubMed] [Google Scholar]
- 19. Nakajima K, Iwata H, Ogino Het al. Acute toxicity of image-guided hypofractionated proton therapy for localized prostate cancer. Int J Clin Oncol 2018;23:353–60. [DOI] [PubMed] [Google Scholar]
- 20. Roach M 3rd, Hanks G, Thames H Jret al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys 2006;65:965–74. [DOI] [PubMed] [Google Scholar]
- 21. Iwata H, Ogino H, Hashimoto Set al. Spot scanning and passive scattering proton therapy: relative biological effectiveness and oxygen enhancement ratio in cultured cells. Int J Radiat Oncol Biol Phys 2016;95:95–102. [DOI] [PubMed] [Google Scholar]
- 22. Toshito T, Omachi C, Kibe Yet al. A proton therapy system in Nagoya Proton Therapy Center. Australas Phys Eng Sci Med 2016;39:645–54. [DOI] [PubMed] [Google Scholar]
- 23. Ohta K, Ogino H, Iwata Het al. Feasibility of transrectal and transperineal fiducial marker placement for prostate cancer before proton therapy. Jpn J Clin Oncol 2020, article in press. [DOI] [PubMed] [Google Scholar]
- 24. Miyake H, Miyazaki A, Yao Aet al. Significance of erection hardness score as a diagnostic tool to assess erectile function recovery in Japanese men after robot-assisted radical prostatectomy. J Robot Surg 2016;10:221–6. [DOI] [PubMed] [Google Scholar]
- 25. Skolarus TA, Dunn RL, Sanda MGet al. Minimally important difference for the expanded prostate cancer index composite short form. Urology 2015;85:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kakehi Y, Takegami M, Suzukamo Yet al. Health related quality of life in Japanese men with localized prostate cancer treated with current multiple modalities assessed by a newly developed Japanese version of the expanded prostate cancer index composite. J Urol 2007;177:1856–61. [DOI] [PubMed] [Google Scholar]
- 27. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Snyder PJ, Bhasin S, Cunningham GRet al. Effects of testosterone treatment in older men. N Engl J Med 2016;374:611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pivonello R, Menafra D, Riccio Eet al. Metabolic disorders and male hypogonadotropic hypogonadism. Front Endocrinol (Lausanne) 2019;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corona G, Bianchini S, Sforza Aet al. Hypogonadism as a possible link between metabolic diseases and erectile dysfunction in aging men. Hormones (Athens) 2015;14:569–78. [DOI] [PubMed] [Google Scholar]
- 31. Ishikawa H, Tsuji H, Murayama Set al. Particle therapy for prostate cancer: the past, present and future. Int J Urol 2019;26:971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf) 1993;39:163–71. [DOI] [PubMed] [Google Scholar]
- 33. Bhattasali O, Chen LN, Woo Jet al. Patient-reported outcomes following stereotactic body radiation therapy for clinically localized prostate cancer. Radiat Oncol 2014;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mendenhall NP, Hoppe BS, Nichols RCet al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014;88:596–602. [DOI] [PubMed] [Google Scholar]
- 35. Evans JR, Zhao S, Daignault Set al. Patient-reported quality of life after stereotactic body radiotherapy (SBRT), intensity modulated radiotherapy (IMRT), and brachytherapy. Radiother Oncol 2015;116:179–84. [DOI] [PubMed] [Google Scholar]
- 36. King CR, Collins S, Fuller Det al. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys 2013;87:939–45. [DOI] [PubMed] [Google Scholar]
- 37. Vargas CE, Hartsell WF, Dunn Met al. Image-guided hypofractionated proton beam therapy for low-risk prostate cancer: analysis of quality of life and toxicity, PCG GU 002. Rep Pract Oncol Radiother 2016;21:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bryant C, Smith TL, Henderson RHet al. Five-year biochemical results, toxicity, and patient-reported quality of life after delivery of dose-escalated image guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2016;95:422–34. [DOI] [PubMed] [Google Scholar]
- 39. Yamamoto S, Fujii Y, Masuda Het al. Longitudinal change in health-related quality of life after intensity-modulated radiation monotherapy for clinically localized prostate cancer. Qual Life Res 2014;23:1641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hashine K, Kakuda T, Iuchi Set al. Prospective longitudinal outcomes of quality of life after laparoscopic radical prostatectomy compared with retropubic radical prostatectomy. Health Qual Life Outcomes 2018;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miyake H, Miyazaki A, Furukawa Jet al. Prospective assessment of time-dependent changes in quality of life of Japanese patients with prostate cancer following robot-assisted radical prostatectomy. J Robot Surg 2016;10:201–7. [DOI] [PubMed] [Google Scholar]
- 42. Ueno S, Kitagawa Y, Onozawa Met al. Background factors and short-term health-related quality of life in patients who initially underwent radical prostatectomy or androgen deprivation therapy for localized prostate cancer in a Japanese prospective observational study (J-CaP innovative Study-1). Prostate Int 2018;6:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miyoshi Y, Morizane S, Honda Met al. Health related quality of life in Japanese patients with localized prostate cancer: comparative retrospective study of robot-assisted laparoscopic radical prostatectomy versus radiation therapy. Yonago Acta Med 2020;63:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Y, Li P, Yu Qet al. Preliminary exploration of clinical factors affecting acute toxicity and quality of life after carbon ion therapy for prostate cancer. Radiat Oncol 2019;14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hollenbeck BK, Dunn RL, Wei JTet al. Neoadjuvant hormonal therapy and older age are associated with adverse sexual health-related quality-of-life outcome after prostate brachytherapy. Urology 2002;59:480–4. [DOI] [PubMed] [Google Scholar]


