ABSTRACT
The aim of this study was to assess the feasibility of planning dose–volume histogram (DVH) parameters in computed tomography-based 3D image-guided brachytherapy for locally advanced cervical cancer. In a prospective multi-institutional study, 60 patients with stage IIA2–IVA cervical cancer from eight institutions were treated with external beam radiotherapy using central shielding and intracavitary or hybrid (combined intracavitary/interstitial) brachytherapy (HBT). The dose constraints were set as a cumulative linear quadratic equivalent dose (EQD2) of at least 60 Gy for high-risk clinical target volume (HR-CTV) D90, D2cc ≤ 75 Gy for rectum, D2cc ≤ 90 Gy for bladder and D2cc ≤ 75 Gy for sigmoid. The median HR-CTV D90 was 70.0 Gy (range, 62.8–83.7 Gy) in EQD2. The median D2cc of rectum, bladder and sigmoid was 57.1 Gy (range, 39.8–72.1 Gy), 68.9 Gy (range, 46.5–84.9 Gy) and 57.2 Gy (range, 39.2–71.2 Gy) in EQD2, respectively. In 76 of 233 sessions (33%), 23 patients underwent HBT, and the median number of interstitial needles was 2 (range, 1–5). HBT for a bulky HR-CTV (≥40 cm3) significantly improved the HR-CTV D90 compared with intracavitary brachytherapy alone (P = 0.010). All patients fulfilled the dose constrains for target and at risk organs by undergoing HBT in one-third of sessions. We conclude that the planning DVH parameters used in our protocol are clinically feasible.
Keywords: cervical cancer, image-guided brachytherapy, hybrid brachytherapy, dose constraints, dose–volume histogram parameters
INTRODUCTION
3D image-guided brachytherapy (3D-IGBT) for locally advanced cervical cancer is gradually becoming the standard of care. This approach is replacing 2D brachytherapy and spreading throughout the world [1]. The recommendation of dose–volume histogram (DVH) parameters for 3D-IGBT planning was published by the Groupe Européen de Curiethérapie and the European Society for Radiotherapy & Oncology (GEC-ESTRO) working group [2] and the American Brachytherapy Society (ABS) [3]. The new brachytherapy technique demonstrated an improvement in local control without increasing the risk of severe complications in a multi-international prospective study [4]. Despite this, the acceptance of 3D-IGBT for cervical cancer in Japan is slower compared with Western countries [5]. One possible reason for this is that consensus-based dose constraints for high-risk clinical target volume (HR-CTV) and organs at risk (OARs) have not been well established. Since several treatment conditions in Japan are different, including the use of a central shield (CS), dose and fractionation schedule, and type of applicator [6–8], the treatment planning parameters recommended by the GEC-ESTRO working group and ABS cannot be directly applied to Japanese clinical practice. Further, GEC-ESTRO guidelines require magnetic resonance imaging (MRI) for HR-CTV and OAR delineation with applicators in situ at every treatment fraction [9]. Although MRI is the gold standard for 3D-IGBT planning for cervical cancer, frequent use of MRI is difficult at most institutions because of absence of MRI equipment in radiation oncology departments and because of time-consuming operational issues. Several surveys, including Japan, on the use of 3D-IGBT for cervical cancer demonstrated that computed tomography (CT) is the most commonly used imaging modality for dose specification in clinical practice [10–12].
Recently, data on the relationship between clinical outcomes and DVH parameters for 3D-IGBT based on retrospective studies on cervical cancer have been accumulated in Japan [13, 14]. In 2014, we conducted a multi-institutional prospective study (Japanese study on CT-based brachytherapy in locally advanced cervical cancer; JCBRACE) to assess the effectiveness and safety of CT-based 3D-IGBT for cervical cancer. The present study also aims to establish a consensus on the dose constraints for HR-CTV and OARs using the Japanese protocol for 3D-IGBT. Herein, we evaluated the feasibility of treatment planning DVH parameters, which were obtained based on our retrospective analyses of clinical outcomes in patients with locally advanced cervical cancer undergoing CT-based 3D-IGBT. Specifically, we attempted to assess the usefulness of newly proposed DVH parameters for brachytherapy planning.
MATERIALS AND METHODS
Patients
A total of 60 patients were prospectively recruited between November 2014 and November 2017 from eight institutions in Japan. Patients with histologically proven locally advanced cervical cancer, Federation of Gynecology and Obstetrics (FIGO 2008) stage IIA2–IVA, were considered suitable for curative treatment. Eligibility criteria also included patients with a tumor diameter ≥ 4 cm at initial diagnosis and no para-aortic lymph nodes ≥1 cm in minimum diameter on CT. The institutional ethical review board of each institution approved the study. Written informed consent for data acquisition was obtained from each patient. This trial is registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR; number 000016140) [15].
Radiotherapy
The radiotherapy protocol consisted of a combination of whole-pelvis external beam radiotherapy (WP-EBRT) and high-dose-rate brachytherapy (HDR-BT). After 30 or 40 Gy of WP-EBRT, a 3–4-cm width CS was inserted [16]. For WP-EBRT, the four-field technique was utilized, and intensity-modulated radiotherapy was not allowed. Three radiotherapy schedules were provided for the protocol, which is standard in Japan [17, 18], and schedule selection was left to the attending radiation oncologist (Table 1). Boost EBRT of 6–10 Gy/3–5 fractions was performed for patients with nodal metastasis. Weekly cisplatin (40 mg/m2, five courses) was concurrently given with radiotherapy.
Table 1.
Radiotherapy schedulesa
| Treatment schedule | External beam radiotherapy | HDR-BTb | Total EQD2 10 Gy | |
|---|---|---|---|---|
| WP | CS | WP + HDR-BT | ||
| 1 | 30 Gy/15 fr | 20 Gy/10 fr | 24 Gy/4 fr | 62 Gy |
| 2 | 40 Gy/20 fr | 10 Gy/5 fr | 18 Gy/3 fr | 64 Gy |
| 3 | 40 Gy/20 fr | 10 Gy/5 fr | 24 Gy/4 fr | 72 Gy |
aWP = whole-pelvis radiotherapy; CS = pelvis irradiation with central shielding; fr = fraction; EQD2, equivalent dose in 2 Gy per fraction (the EQD2 is calculated using α/β = 10). bPlanning aim doses of HR-CTV D90.
HDR-BT was performed once a week using the 192Ir afterloading system (microSelectron-HDR; Nucletron, Elekta AB, Stockholm, Sweden). After applicator implantation, CT data were acquired with the patient in a lithotomy position or a supine position. CT slice thickness was < 3.0 mm, and CT-based treatment planning was performed. T2-weighted MR images acquired at diagnosis and just before the first brachytherapy session without an applicator were routinely used as reference images for tumor extension. There was no restriction on the type or size of applicator. Hybrid brachytherapy (HBT) combining a uterine applicator and free interstitial needles was allowed [19, 20], but interstitial brachytherapy alone was not. Ultrasound-guided insertion of interstitial needles was recommended. The contouring for HR-CTV was performed with the same definitions as the Japanese Radiation Oncology Study Group recommendations [21]. For the rectum, bladder and sigmoid, the outer organ contours were delineated. Delineation of the rectum included all regions from the anorectal junction to the rectosigmoid flexure. The dose prescription was performed according to our schedule (Table 1), whereas the source dwell patterns (i.e. times and positions) were determined based on institutional practice. In principle, plan optimization was performed according to the planning aims for HR-CTV and OARs during each brachytherapy session (Table 2).
Table 2.
Planning aims for HR-CTV and OARs in each brachytherapy session
| Treatment schedule | Dose constraint | HR-CTV D90 | Rectum D2cc | Bladder D2cc | Sigmoid D2cc |
|---|---|---|---|---|---|
| 1, 2 | Preferable | ≥7.0 Gy | <5.5 Gy | <6.5 Gy | <5.5 Gy |
| limit | ≥6.0 Gy | 5.5–6.0 Gy | 6.5–7.6 Gy | 5.5–6.0 Gy | |
| 3 | Preferable | ≥7.0 Gy | <5.0 Gy | <6.0 Gy | <5.0 Gy |
| limit | ≥6.0 Gy | 5.0–5.2 Gy | 6.0–6.5 Gy | 5.0–5.2 Gy |
Total treatment doses were calculated as a cumulative linear quadratic equivalent dose (EQD2) using α/β = 10 Gy for HR-CTV and α/β = 3 Gy for OARs. The cumulative EQD2 dose was the summation of the EBRT doses (without the CS) and HDR-BT doses according to Japan Society of Gynecologic Oncology (JSGO) guidelines [18]. The final objective was a total dose in EQD2 of at least 60 Gy corresponding to HR-CTV D90, D2cc ≤ 75 Gy for rectum, D2cc ≤ 90 Gy for bladder and D2cc ≤ 75 Gy for sigmoid. Table 2 shows the DVH parameters as planning aims for HR-CTV and OARs in each brachytherapy session. Dose optimization was performed manually and targeted to fulfil the ‘preferable’ or ‘limit’ values. The first priority for dose optimization was to fulfill a total dose in EQD2 of D2cc ≤ 75 Gy for rectum, D2cc ≤ 90 Gy for bladder and D2cc ≤ 75 Gy for sigmoid based on retrospective studies using the Japanese standard schedule and the EMBRACE study [4–5, 22–26]. The threshold value of each group was determined by an expert discussion based on the guidelines and the results of the retrospective study in Japan.
Quality assurance
To improve the quality of this study, workshops and annual monitoring were established. The workshop was conducted to homogenize treatments between institutions. A radiation oncologist, a medical physicist and a radiation technologist from each institution participated in the workshop. A dummy run was performed to ensure that the selected medical professionals were suitable for 3D-IGBT treatment planning. All case report forms were checked at the data center. If there was any doubt, the data center queried the relevant institution and consulted with the study office. Moreover, to ensure treatment plan integrity, the data center checked the relationship between the total reference air kerma and isodose surface volume.
Analysis
DVH parameters for target and OARs were analyzed as a total treatment dose and each 3D-IGBT dose. The treatment plan quality of each brachytherapy session was evaluated and classified according to the criteria shown in Table 2. When a dose for OARs did not meet the ‘limit’ level, it was classified as ‘unsatisfied’. All treatment plans were divided into two volume groups: the non-bulky group (HR-CTV < 40 cm3) and the bulky group (HR-CTV ≥ 40 cm3). Moreover, the relationship between the HR-CTV volume groups and DVH parameters (HR-CTV D90, bladder D2cc, rectum D2cc and sigmoid D2cc) was evaluated in intracavitary brachytherapy (ICBT) and HBT. Statistical analyses were performed using JMP® 14 software (SAS Institute Inc., Cary, NC, USA) and P values were calculated using the Wilcoxon rank-sum test. The level of statistical significance was defined as P < 0.05. For HBT, the relationship between the number of needles and the HR-CTV and the percentage of needle dwell time to total dwell time were evaluated.
RESULTS
Patient demographics
Patient demographics and tumor characteristics are shown in Table 3. The HR-CTV width as defined on MRI had a median size at initial presentation of 5.2 cm (range, 3.1–7.3 cm) and a median size just before the first brachytherapy session of 3.6 cm (range, 0–6.3 cm). Two patients had a complete response on MRI just before the first brachytherapy session.
Table 3.
Patient demographicsa
| Variable | Median (range) | n (%) |
|---|---|---|
| Age (years) | 53 (26–73) | |
| BMI (kg/m2) | 21.0 (16.3–35.1) | |
| Performance score (ECOG) | ||
| 0 | 43 (72) | |
| 1 | 16 (27) | |
| 2 | 1 (2) | |
| Histology | ||
| Squamous cell carcinoma | 56 (93) | |
| Adenocarcinoma | 3 (5) | |
| Adenosquamous carcinoma | 1 (2) | |
| FIGO Stage (2008) | ||
| IIA2 | 4 (7) | |
| IIB > 4 cm | 39 (65) | |
| IIIA | 1 (2) | |
| IIIB | 16 (27) | |
| Pelvic lymph node | ||
| Negative | 31 (52) | |
| Positive | 29 (48) | |
| Tumor size at initial presentation (cm) | 5.2 (3.1–7.3) | |
| Width < 5 cm | 24 (40) | |
| Width ≥ 5 cm | 36 (60) | |
| Tumor size just before first brachytherapy (cm) | 3.6 (0–6.3) | |
| Width < 5 cm | 50 (83) | |
| Width ≥ 5 cm | 10 (17) | |
| Tumor volume at first brachytherapy (cm3) | 32.4 (12.3–117.4) | |
| <40 cm3 | 36 (60) | |
| ≥40 cm3 | 24 (40) |
aBMI = body mass index; ECOG = Eastern Cooperative Oncology Group.
Treatment characteristics
Table 4 shows the treatment characteristics. A total of 50 patients (83%) were treated with schedule 1 (WP 30 Gy + CS 20 Gy + HDR-BT 4 fractions). All patients received at least one cycle of cisplatin with EBRT, and 27 patients had a radiation boost to involved lymph nodes. A total of 233 brachytherapy sessions were performed, in which 76 treatment sessions (33%) were HBT. One or two needles were used for 53 treatment sessions (70%).
Table 4.
Treatment characteristics
| Variable | n (%) |
|---|---|
| Radiotherapy schedulea | |
| Schedule 1 (WP 30 Gy + CS 20 Gy + HDR-BT 4 fr) | 50 (83) |
| Schedule 2 (WP 40 Gy + CS 10 Gy + HDR-BT 3 fr) | 7 (12) |
| Schedule 3 (WP 40 Gy + CS 10 Gy + HDR-BT 4 fr) | 3 (5) |
| Central shield width | |
| 3 cm | 57 (95) |
| 4 cm | 3 (5) |
| EBRT nodal boost | |
| Yes | 27 (45) |
| No | 33 (55) |
| Brachytherapy applicators | |
| Tandem + ovoid | 214 (92) |
| Tandem | 11 (5) |
| Ovoid | 2 (1) |
| Cylinder | 6 (3) |
| Brachytherapy technique | |
| Intracavitary (ICBT) | 157 (67) |
| Intracavitary/interstitial (HBT) | 76 (33) |
| Number of needles used in HBT | |
| 1 | 26 (34) |
| 2 | 27 (36) |
| 3 | 14 (18) |
| 4 | 7 (9) |
| 5 | 2 (3) |
| Number of HBT for each session | |
| First | 19 (32) |
| Second | 22 (37) |
| Third | 20 (33) |
| Fourth | 15 (28) |
aWP = whole-pelvis external beam radiotherapy; fr =fraction.
DVH parameters of the treatment plan
Table 5 shows the total treatment doses for each DVH parameter. The median HR-CTV D90 was 70.0 Gy (range, 62.8–83.7 Gy) in EQD2 (α/β = 10). The median D2cc of rectum, bladder and sigmoid was 57.1 Gy (range, 39.8–72.1 Gy), 68.9 Gy (range, 46.5–84.9 Gy) and 57.2 Gy (range, 39.2–71.2 Gy) in EQD2 (α/β = 3), respectively. All patients fulfilled the final objective of a total dose in EQD2 of at least 60 Gy for HR-CTV D90, D2cc ≤ 75 Gy for rectum, D2cc ≤ 90 Gy for bladder and D2cc ≤ 75 Gy for sigmoid.
Table 5.
DVH parameters for HR-CTV and OARs. Doses are shown as WP + HDR-BT in EQD2 dose. The EQD2 is calculated using α/β = 10 for HR-CTV and α/β = 3 for OAR
| Variablea | Median (range) |
|---|---|
| HR-CTV (EQD2 10 Gy) | |
| D98 | 62.4 Gy (51.6–76.2) |
| D90 | 70.0 Gy (62.8–83.7) |
| D50 | 102.6 Gy (89.4–119.3) |
| Rectum (EQD2 3 Gy) | |
| D0.1cc | 74.7 Gy (42.9–105.7) |
| D2cc | 57.1 Gy (39.8–72.1) |
| Bladder (EQD2 3 Gy) | |
| D0.1cc | 89.9 Gy (57.4–119.3) |
| D2cc | 68.9 Gy (46.5–84.9) |
| Sigmoid (EQD2 3 Gy) | |
| D0.1cc | 71.9 Gy (45.2–89.7) |
| D2cc | 57.2 Gy (39.2–71.2) |
| Point A (EQD2 10 Gy) | 67.2 Gy (60.9–95.0) |
| Rectum DICRU (EQD2 3 Gy) | 61.3 Gy (40.2–121.1) |
| Bladder DICRU (EQD2 3 Gy) | 50.7 Gy (35.5–89.4) |
aWP = whole-pelvis external beam radiotherapy; EQD2 = equivalent dose in 2 Gy per fraction; ICRU = International Commission on Radiation Units and Measurements.
The treatment plan quality in each brachytherapy session is shown in Figure 1. The median HR-CTV in each brachytherapy session was 32.4, 30.2, 28.3 and 28.8 cm3 for the first, second, third and fourth session, respectively. As the brachytherapy session progressed, the proportion of HR-CTV D90 ≥ 7.0 Gy increased. The proportion of ‘preferable’ and ‘limit’ levels accounted for 219 of 233 sessions (94%). The proportion of ‘unsatisfied’ in each brachytherapy session was 2.6% (6 of 233) for rectum, 0.4% (1 of 233) for bladder and 3.0% (7 of 233) for sigmoid. Two or more ‘unsatisfied’ did not occur in the same session. In sigmoid, the proportion of ‘unsatisfied’ of 6% [4 of 67] in the HR-CTV ≥ 40 cm3 group was higher than that in the HR-CTV < 40 cm3 group 1.8% [3 of 166] and higher than that in the other OARs (rectum 3.0% [2 of 67] and bladder 0% [0 of 67]).
Fig. 1.

Evaluation of treatment plan quality among each brachytherapy session, which was classified according to the criteria shown in Table 2. When the ‘limit’ dose level was not met in organs at risk, it was classified as ‘unsatisfied’.
Figure 2 shows the relationships between HR-CTV and DVH parameters for ICBT and HBT. For ICBT, the HR-CTV D90 in the HR-CTV ≥ 40 cm3 group was significantly lower compared with the HR-CTV D90 in the HR-CTV < 40 cm3 group (P = 0.005; Fig. 2a). In addition, the dose ratios of rectum D2cc/HR-CTV D90 and bladder D2cc/HR-CTV D90 were significantly higher in the HR-CTV ≥ 40 cm3 group compared with the HR-CTV < 40 cm3 group (P < 0.001; Fig 2b and c). For HBT, the dose ratio of sigmoid D2cc/HR-CTV D90 was significantly higher in the HR-CTV ≥ 40 cm3 group compared with the HR-CTV < 40 cm3 group (P = 0.008; Fig. 2d). There was no significant difference in the HR-CTV D90 between the HR-CTV ≥ 40 cm3 group and the HR-CTV < 40 cm3 group (P = 0.625). The dose ratios of rectum D2cc/HR-CTV D90 and bladder D2cc/HR-CTV D90 were not significantly different between the groups. The HR-CTV D90 of HBT in the HR-CTV ≥ 40 cm3 group was significantly higher compared with that of ICBT (P = 0.010).
Fig. 2.
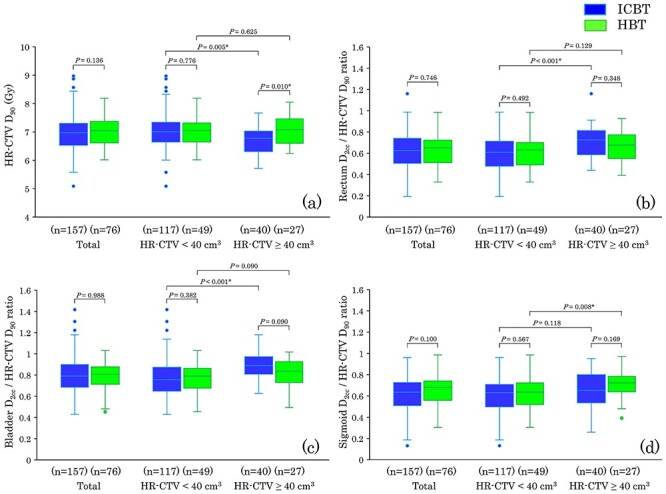
A box plot shows the DVH parameter relationships among ICBT, HBT and HR-CTV. The 50% interquartile range, median and range of data are displayed. All treatment plans were divided into two volume groups: the non-bulky group (HR-CTV < 40 cm3) and the bulky group (HR-CTV ≥ 40 cm3). (a) HR-CTV D90 and HR-CTV volume. (b) The dose ratio of rectum D2cc/HR-CTV D90 and HR-CTV volume. (c) The dose ratio of bladder D2cc/HR-CTV D90 and HR-CTV volume. (d) The dose ratio of sigmoid D2cc/HR-CTV D90 and HR-CTV volume.
Contribution of interstitial needles
In 76 HBT sessions undergone by 23 patients, the median number of inserted needles was 2 (range, 1–5). The relationships among HR-CTV, number of needles, and percentage of needle dwell time to total dwell time are shown in Fig. 3. The median percentage dose delivered from the needles was 12.7% (range, 0.8–71.5%). The contribution of the percentage of needle dwell time to total dwell time tended to increase with the number of needles. By contrast, a clear relationship was not confirmed between the number of needles and HR-CTV.
Fig. 3.
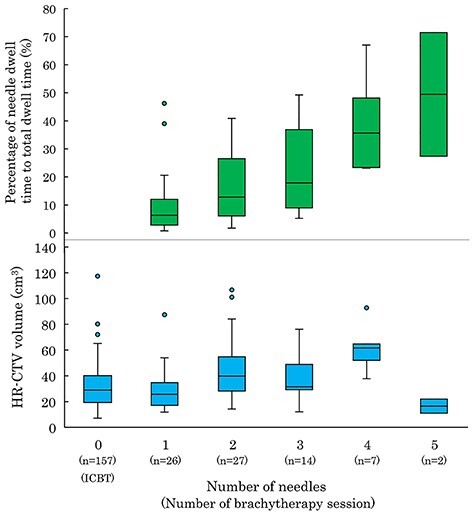
A box plot showing the relationship between HR-CTV, needle contribution and number of needles. The 50% interquartile range, median and range of data are displayed.
DISCUSSION
The present study was designed to assess the feasibility of treatment planning DVH parameters and to establish a consensus on the dose constraints for target and OARs in CT-based 3D-IGBT for cervical cancer among Japanese institutions. All patients achieved the final objective total dose in EQD2 of at least 60 Gy for HR-CTV D90, D2cc ≤ 75 Gy for rectum, D2cc ≤ 90 Gy for bladder and D2cc ≤ 75 Gy for sigmoid by undergoing HBT for 32.6% of 3D-IGBT sessions. ‘Preferable’ and ‘limit’ dose levels accounted for 219 of all 233 sessions (94%). Therefore, our results confirm that DVH parameters based on the retrospective analyses for target and OARs in CT-based 3D-IGBT for locally advanced cervical cancer are feasible in a prospective setting and that the proposed planning aims in each 3D-IGBT session appear to be useful in clinical practice. Importantly, clinical effectiveness and safety will be confirmed by the emerging clinical results of this study in the near future.
In the dose prescription of 3D-IGBT, the physician must decide on a specific balance between HR-CTV and OARs doses. Although dose constraints of total dose in EQD2 for the rectum, bladder and sigmoid are important to avoid severe late complications, the significance of planning aims for HR-CTV and OARs in each 3D-IGBT session has not previously been sufficiently clarified. Okazaki et al. suggested that 90 or 98% of the HR-CTV should be covered with at least 6.5 Gy (~9 Gy in EQD2) or 5.5 Gy (~7 Gy in EQD2) per session to obtain favorable local control using a Japanese treatment regimen [14]. The dose–response relationship between dosimetric parameters and local control should be analyzed in our study. The planning aims used in the current study (Table 2) were flexible because physicians had three to four opportunities for dose optimization over the course of 3D-IGBT. For example, even when the rectal dose resulted in an overdose beyond the ‘limit’ dose level in the first session, the physician could reduce the rectal dose below the ‘preferable’ dose level from the second session onward with tumor shrinkage. With this approach, total dose constraints were kept within D2cc ≤ 75 Gy for the rectum. The clinical significance and flexibility in our planning aims should be further evaluated.
The threshold dose of HR-CTV D90 in our study was determined based on the clinical outcome of Japanese studies. Previous retrospective studies from Japanese institutions have shown that the median HR-CTV D90 ranged from 65 to 74 Gy in EQD2 [14, 23]. Murakami et al. reported that both local control and progression-free survival were significantly favorable in patients receiving ≥60 Gy for HR-CTV D90 in EQD2 [23]. In our study, the median HR-CTV D90 was 70.0 Gy (range, 62.8–83.7 Gy) in EQD2, which is comparable to previous Japanese studies, but lower than other studies [27–30]. One of the major reasons for this was that the dose contribution from the CS was completely ignored according to JSGO guidelines [18]. Tamaki et al. demonstrated that 24–56% of CS doses contributed to the HR-CTV D90 when the CS had a width of 3 cm, and 13–35% of CS doses contributed to HR-CTV D90 when the CS had a width of 4 cm in a phantom study [31]. Also, Tamaki et al. suggested that the addition of 5–7 Gy to the D90 value may account for the dose contribution from the CS. Since the actual tumor volume, shape and location may vary during treatment, precise estimation of the dose contribution of the CS is difficult. Another possible reason for the lower HR-CTV D90 is the difference in the imaging modality (MRI vs CT) used for treatment planning. Viswanathan et al. reported that CT-based tumor contours can overestimate the tumor width significantly compared with MRI, resulting in significant differences in the HR-CTV D90 as well as volume treated with respect to the prescription dose or greater for the HR-CTV [32]. In our study, however, T2-weighted MR images acquired at diagnosis and just before the first brachytherapy session without an applicator were routinely used as reference images for tumor extension. Comprehensive discussions of the reason for the relatively lower total dose of HR-CTV D90 in the Japanese treatment regimen will be required to accompany the emerging clinical outcomes.
Compared with ICBT, HBT is advantageous in that it can optimize the anatomy-oriented dose, which results in an improvement in DVH parameters. As shown in Fig. 2, HBT maintained target coverage for bulky tumors (HR-CTV ≥ 40 cm3) without increasing the relative dose of the bladder D2cc and rectum D2cc to HR-CTV D90. Anderson et al. reported that DVH parameter improvements in the HR-CTV volume ≥ 40 cm3 group were diminished even with 3D-IGBT when a conventional intracavitary applicator is used without supplementary needles [33]. Chargari et al. also reported that a lower ability to reach target HR-CTV D90 planning and an HR-CTV volume ≥ 40 cm3 correlated with a high propensity of relapse, with these factors being interrelated [34]. Therefore, HBT has an essential role in achieving the final objective total dose, especially in the HR-CTV volume ≥ 40 cm3 group.
One of the limitations of our study is that the decision criteria for HBT were not provided in the protocol and the selection of ICBT or HBT was left to the attending radiation oncologist. Several authors have summarized the needle position and number of needles for applicators with needle holes, such as Utrecht applicators [35, 36]. Due to the limited availability of applicators dedicated to HBT, free needles are commonly used in Asian countries [19, 37–39]. Liu et al. claimed that HBT using free needles is clinically feasible for large-volume tumors (>5 cm) [38]. Yoshida et al. performed a simulation analysis and proposed that HBT should be considered for a HR-CTV > 4 × 3 × 3 cm (36 cm3) [39]. In our study, HBT was performed even in the group with a small HR-CTV, and there was no clear regularity in the number of needles (Fig. 3). It is assumed that there was inter-physician variability. Further study of HBT standardization is needed in terms of the volume and shape of HR-CTV and the anatomical relationship between HR-CTV and OARs.
In conclusion, in this multi-institutional prospective study on CT-based 3D-IGBT for locally advanced cervical cancer, all 60 patients fulfilled the dose constrains for HR-CTV D90 and D2cc for the rectum, bladder and sigmoid, indicating that the treatment planning DVH parameters were feasible. Additionally, our planning aims for HR-CTV and OARs, which were achievable in > 90% of 3D-IGBT sessions, would be useful in brachytherapy planning. Indications and the dosimetric impact of HBT should be further evaluated to effectively balance HR-CTV and OAR doses.
ACKNOWLEDGMENTS
The authors would like to express our deep gratitude to Takuya Kaminuma, MD, Yuya Yoshimoto, MD, Masahiro Onishi, MD, Mototaro Iwanaga, MD, Daijiro Kobayashi, MD, Tomoaki Tamaki, MD, Shohei Okazaki, MD, Takanori Abe, MD, Haruko Numajiri, MD, Reiko Kanuma, MD, Tomoko Kazumoto, MD, Toru Kojima, MP, Yu Ohkubo, MD, for their assistance with the collection of clinical data. This work was presented at the 31st Annual Meeting of the Japanese Society for Radiation Oncology, Kyoto, 11–13 October 2018.
Contributor Information
Yuki Otani, Department of Radiation Oncology, Osaka University Graduate School of Medicine, 2-2 (D10) Yamada-oka, Suita, Osaka 565-0871, Japan; Department of Radiology, Kaizuka city hospital, 3-10-20 Hori, Kaizuka, Osaka, 597-0015, Japan.
Tatsuya Ohno, Department of Radiation Oncology, Gunma University Graduate School of Medicine, 3-39-22 Showa-machi, Maebashi, Gunma 371-8511, Japan.
Ken Ando, Department of Radiation Oncology, Gunma University Graduate School of Medicine, 3-39-22 Showa-machi, Maebashi, Gunma 371-8511, Japan; Department of Radiation Oncology, Gunma Prefectural Cancer Center, 617-1 Takabayashinishi-machi, Ota, Gunma 373-8550, Japan.
Kazutoshi Murata, Department of Radiation Oncology, Gunma University Graduate School of Medicine, 3-39-22 Showa-machi, Maebashi, Gunma 371-8511, Japan.
Shingo Kato, Department of Radiation Oncology, Saitama Medical Univercity International Medical Center, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Shin-ei Noda, Department of Radiation Oncology, Saitama Medical Univercity International Medical Center, 1397-1 Yamane, Hidaka, Saitama 350-1298, Japan.
Keiko Murofushi, Department of Radiation Oncology, University of Tsukuba Hospital, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576, Japan; Department of Radiation Oncology, Tokyo Metropolitan Cancer and Infectious diseases Center Komagome Hospital, Honkomagome 3-18-22 Bunkyo, Tokyo 113-8677, Japan.
Hiroki Ushijima, Department of Radiation Oncology, Saitama Cancer Center, 780 Komuro, Ina, Kita Adachi-gun, Saitama 362-0806, Japan.
Daisaku Yoshida, Department of Radiation Oncology, Saku Central Hospital Advanced Care Center, 3400-28 Nakagomi, Saku, Nagano 385-0051, Japan; Department of Radiation Oncology, Kanagawa Cancer Center, 2-3-2 Nakao, Asahi-ku, Yokohama 241-8515, Japan.
Noriyuki Okonogi, QST Hospital, National Institutes for Quantum and Radiological Science and Technology, 4-9-1 Anagawa, Inage-ku, Chiba 263-8555, Japan.
Fumiaki Isohashi, Department of Radiation Oncology, Osaka University Graduate School of Medicine, 2-2 (D10) Yamada-oka, Suita, Osaka 565-0871, Japan.
Masaru Wakatsuki, QST Hospital, National Institutes for Quantum and Radiological Science and Technology, 4-9-1 Anagawa, Inage-ku, Chiba 263-8555, Japan.
Takashi Nakano, Department of Molecular Imaging and Theranostics, National Institutes for Quantum and Radiological Science and Technology, 4-9-1 Anagawa, Inage-ku, Chiba 263-8555, Japan.
CONFLICT OF INTEREST
None declared.
FUNDING
This work was supported by JSPS KAKENHI (grant number 19 K08103 to M.W.) and Japan mHDR Research Fund.
REFERENCES
- 1. Harkenrider MM, Alite F, Silva SRet al. Image-based brachytherapy for the treatment of cervical cancer. Int J Radiat Oncol Biol Phys 2015;92:921–34. [DOI] [PubMed] [Google Scholar]
- 2. Pötter R, Haie-Meder C, Van Limbergen Eet al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77. [DOI] [PubMed] [Google Scholar]
- 3. Viswanathan AN, Beriwal S, De Los Santos JFet al. American brachytherapy society consensus guidelines for locally advanced carcinoma of the cervix. Part II: High-dose-rate brachytherapy. Brachytherapy 2012;11:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. EMBRACE : An international study on MRI-guided BRachytherapy in locally advanced cervical cancer. Available at: https://www.embracestudy.dk(4 November 2020, date last accessed).
- 5. Toita T, Ohno T, Ikushima Het al. National survey of intracavitary brachytherapy for intact uterine cervical cancer in Japan. J Radiat Res 2018;59:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toita T, Kitagawa R, Hamano Tet al. Phase II study of concurrent chemoradiotherapy with high-dose-rate intracavitary brachytherapy in patients with locally advanced uterine cervical cancer: Efficacy and toxicity of a low cumulative radiation dose schedule. Gynecol Oncol 2012;126:211–6. [DOI] [PubMed] [Google Scholar]
- 7. Kawashima A, Isohashi F, Mabuchi Set al. A 3-year follow-up study of radiotherapy using computed tomography-based image-guided brachytherapy for cervical cancer. J Radiat Res 2019;60:264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohno T, Noda SE, Okonogi Net al. In-room computed tomography-based brachytherapy for uterine cervical cancer: Results of a 5-year retrospective study. J Radiat Res 2017;58:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haie-Meder C, Pötter R, Van Limbergen Eet al. Recommendations from gynaecological (GYN) GEC-ESTRO working group (I): Concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005;74:235–45. [DOI] [PubMed] [Google Scholar]
- 10. Grover S, Harkenrider MM, Cho LPet al. Image guided cervical brachytherapy: 2014 survey of the American brachytherapy society. Int J Radiat Oncol Biol Phys 2016;94:598–604. [DOI] [PubMed] [Google Scholar]
- 11. Phan T, Mula-Hussain L, Pavamani Set al. The changing landscape of brachytherapy for cervical cancer: A Canadian practice survey. Curr Oncol 2015;22:356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan LT. Implementation of image-guided brachytherapy for cervix cancer in the UK: Progress update. Clin Oncol 2011;23:681–4. [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi D, Okonogi N, Wakatsuki Met al. Impact of CT-based brachytherapy in elderly patients with cervical cancer. Brachytherapy 2019;18:771–9. [DOI] [PubMed] [Google Scholar]
- 14. Okazaki S, Murata K, Noda SEet al. Dose-volume parameters and local tumor control in cervical cancer treated with central-shielding external-beam radiotherapy and CT-based image-guided brachytherapy. J Radiat Res 2019;60:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. A prospective multicenter study on 3D image-guided brachytherapy for locally advanced cervical cancer. Available at: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_his_list.cgi?recptno=R000018670. (in Japanese) (4 November 2020, date last accessed).
- 16. Japan Society of Obstetrics and Gynaecology, the Japanese Society of Pathology, the Japan Radiological Society . General Rules for Clinical and Pathological Study of Uterine Cervical Cancer in Japan. Tokyo: Kanehara and Co. Ltd, 1999. [Google Scholar]
- 17. Toita T. Current status and perspectives of brachytherapy for cervical cancer. Int J Clin Oncol 2009;14:25–30. [DOI] [PubMed] [Google Scholar]
- 18. Ebina Y, Yaegashi N, Katabuchi Het al. Japan Society of Gynecologic Oncology guidelines 2011 for the treatment of uterine cervical cancer. Int J Clin Oncol 2015;20:240–8. [DOI] [PubMed] [Google Scholar]
- 19. Wakatsuki M, Ohno T, Yoshida Det al. Intracavitary combined with CT-guided interstitial brachytherapy for locally advanced uterine cervical cancer: Introduction of the technique and a case presentation. J Radiat Res 2011;52:54–8. [DOI] [PubMed] [Google Scholar]
- 20. Kirisits C, Lang S, Dimopoulos Jet al. The Vienna applicator for combined Intracavitary and interstitial brachytherapy of cervical cancer: Design, application, treatment planning, and Dosimetric results. Int J Radiat OncolBiol Phys 2006;65:624–30. [DOI] [PubMed] [Google Scholar]
- 21. Ohno T, Wakatsuki M, Toita Tet al. Recommendations for high-risk clinical target volume definition with computed tomography for three-dimensional image-guided brachytherapy in cervical cancer patients. J Radiat Res 2017;58:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terahara A, Nakano T, Ishikawa Aet al. Dose-volume histogram analysis of high dose rate intracavitary brachytherapy for uterine cervix cancer. Int J Radiat Oncol Biol Phys 1996;35:549–54. [DOI] [PubMed] [Google Scholar]
- 23. Murakami N, Kasamatsu T, Wakita Aet al. CT based three dimensional dose-volume evaluations for high-dose rate intracavitary brachytherapy for cervical cancer. BMC Cancer 2014;14:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kato S, Tran DN, Ohno Tet al. CT-based 3D dose-volume parameter of the rectum and late rectal complication in patients with cervical cancer treated with high-dose-rate intracavitary brachytherapy. J Radiat Res 2010;51:215–21. [DOI] [PubMed] [Google Scholar]
- 25. Isohashi F, Yoshioka Y, Koizumi Met al. Rectal dose and source strength of the high-dose-rate iridium-192 both affect late rectal bleeding after intracavitary radiation therapy for uterine cervical carcinoma. Int J Radiat Oncol Biol Phys 2010;77:758–64. [DOI] [PubMed] [Google Scholar]
- 26. Ohno T, Noda S, Tamaki Tet al. In room CT-guided adaptive brachytherapy for cervical cancer at Gunma University. Proceeding of the 3rd biennial meeting of Asian Society of Gynecologic Oncology. Kyoto 2013;P226. [Google Scholar]
- 27. Pötter R, Tanderup K, Kirisits Cet al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol 2018;9:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamran SC, Manuel MM, Cho LPet al. Comparison of outcomes for MR-guided versus CT-guided high-dose-rate interstitial brachytherapy in women with locally advanced carcinoma of the cervix. Gynecol Oncol 2017;145:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koh V, Choo BA, Lee KMet al. Feasibility study of toxicity outcomes using GEC-ESTRO contouring guidelines on CT based instead of MRI-based planning in locally advanced cervical cancer patients. Brachytherapy 2017;16:126–32. [DOI] [PubMed] [Google Scholar]
- 30. Choong ES, Bownes P, Musunuru HBet al. Hybrid (CT/MRI based) vs. MRI only based image-guided brachytherapy in cervical cancer: Dosimetry comparisons and clinical outcome. Brachytherapy 2016;15:40–8. [DOI] [PubMed] [Google Scholar]
- 31. Tamaki T, Noda SE, Ohno Tet al. Dose-volume histogram analysis of composite EQD2 dose distributions using the central shielding technique in cervical cancer radiotherapy. Brachytherapy 2016;15:598–606. [DOI] [PubMed] [Google Scholar]
- 32. Viswanathan AN, Dimopoulos J, Kirisits Cet al. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: Results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys 2007;68:491–8. [DOI] [PubMed] [Google Scholar]
- 33. Anderson JW, Xia J, Flynn RTet al. High resolution (3 tesla) MRI-guided conformal brachytherapy for cervical cancer: Consequences of different high-risk CTV sizes. J Contemp Brachytherapy 2013;5:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chargari C, Mazeron R, Escande Aet al. Image-guided adaptive brachytherapy in cervical cancer: Patterns of relapse by brachytherapy planning parameters. Brachytherapy 2016;15:456–62. [DOI] [PubMed] [Google Scholar]
- 35. Smolic M, Sombroek C, Bloemers MCWMet al. Needle use and dosimetric evaluation in cervical cancer brachytherapy using the Utrecht applicator. Radiother Oncol 2018;126:411–6. [DOI] [PubMed] [Google Scholar]
- 36. Fokdal L, Tanderup K, Hokland SBet al. Clinical feasibility of combined intracavitary/interstitial brachytherapy in locally advanced cervical cancer employing MRI with a tandem/ring applicator in situ and virtual preplanning of the interstitial component. Radiother Oncol 2013;107:63–8. [DOI] [PubMed] [Google Scholar]
- 37. Murakami N, Kato S, Nakano Tet al. A phase I/II clinical trial for the hybrid of intracavitary and interstitial brachytherapy for locally advanced cervical cancer. BMC Cancer 2016;16:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu ZS, Guo J, Lin Xet al. Clinical feasibility of interstitial brachytherapy using a "hybrid" applicator combining uterine tandem and interstitial metal needles based on CT for locally advanced cervical cancer. Brachytherapy 2016;15:562–9. [DOI] [PubMed] [Google Scholar]
- 39. Yoshida K, Yamazaki H, Kotsuma Tet al. Simulation analysis of optimized brachytherapy for uterine cervical cancer: Can we select the best brachytherapy modality depending on tumor size? Brachytherapy 2016;15:57–64. [DOI] [PubMed] [Google Scholar]


