Abstract
The host immune response to infection is a well-coordinated system of innate and adaptive immune cells working in concert to prevent the colonization and dissemination of a pathogen. While this typically leads to a beneficial outcome and the suppression of disease pathogenesis, the Lyme borreliosis bacterium, Borrelia burgdorferi sensu lato, can elicit an immune profile that leads to a deleterious state. As B. burgdorferi s.l. produces no known toxins, it is suggested that the immune and inflammatory response of the host are responsible for the manifestation of symptoms, including flu-like symptoms, musculoskeletal pain, and cognitive disorders. The past several years has seen a substantial increase in the use of microarray and sequencing technologies to investigate the transcriptome response induced by B. burgdorferi s.l., thus enabling researchers to identify key factors and pathways underlying the pathophysiology of Lyme borreliosis. In this review we present the major host transcriptional outcomes induced by the bacterium across several studies and discuss the overarching theme of the host inflammatory and immune response, and how it influences the pathology of Lyme borreliosis.
Keywords: Lyme borreliosis, Borrelia burgdorferi, Host transcriptome
Introduction
In 1975 a cluster of cases originally thought to be juvenile rheumatoid arthritis were identified in the towns of Lyme and Old Lyme, Connecticut (Steere et al., 1977b). This epidemic form of arthritis was initially investigated by Steere et al. and ultimately led to the recognition of what is now known as Lyme borreliosis (Elbaum-Garfinkle, 2011). A few years later, Burgdorfer and colleagues first isolated the infectious agent now known as Borrelia burgdorferi sensu stricto and epidemiological evidence indicated transmission occurred through the bite of ticks from the genus Ixodes (Burgdorfer et al., 1982; Steere et al., 1978, 1977a). Since its identification, Lyme borreliosis has seen a consistent increase in the number of cases throughout North America, Europe, and Asia, with an estimated 300,000 cases annually in the United States, over 232,000 cases in Europe and 3,500 in Asia (Centers for Disease Control and Prevention (U.S.). Center for Surveillance, 2019; Hubálek, 2009; Rizzoli et al., 2011, Sykes and Makiello, 2016). It is now known that the disease is predominantly caused by three species of the Borrelia burgdorferi sensu lato complex: Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto (referred to as B. burgdorferi hereafter) (Wang et al., 1999). The three species vary in geographic distribution – in North America, only B. burgdorferi is found, in Europe all three species are present, though B. afzelii and B. garinii are most prevalent, and in Asia B. garinii is the most predominant species (Stanek et al., 2012). Although there are many similarities of clinical manifestations of Lyme borreliosis that is shared between species, B. burgdorferi in North America is typically associated with greater pathogenic symptoms and tends to be more arthritogenic (Cerar et al., 2016). In contrast, B. afzelii shows greater affinity to skin manifestations and B. garinii tends to be more neurotropic (Stanek et al., 2012; Strle and Stanek, 2009).
During the initial localized stages of the disease following the transmission of the bacteria from the tick, the most common and indicative symptom is the presence of the erythema migrans (EM) rash that is present in up to 70% of infected individuals and appears within 3 to 30 days, however some patients may be asymptomatic (Schwartz, 2017). Treatment during this initial phase with appropriate antibiotic therapy is effective in resolving an active infection in the vast majority of cases. However, if left untreated, B. burgdorferi s.l. will hematogenously disseminate into secondary tissue and organs within a few weeks leading to involvement of the musculoskeletal system (Biesiada et al., 2012). Common regions for the bacteria to enter are the joints, tendons, or bursae in the early disseminated stages of the disease leading to musculoskeletal pain and swelling of large joints (Bitar and Lally, 2008). During this dissemination phase, B. burgdorferi s.l. may enter cardiac tissue on rare occasions, which causes between 1-4% of patients to develop carditis (Bartůnĕk et al., 2007; Schwartz, 2017). Involvement in cardiac tissue can interfere and impair electrical signals between the atria and ventricles of the heart, termed atrioventricular block (Silver et al., 2007). Clinical manifestations of Lyme carditis present as shortness of breath, light-headedness/fainting, heart palpitations, or chest pains (Silver et al., 2007). Involvement of the central nervous system (CNS) is usually seen in the late stages of delayed or untreated Lyme borreliosis but has been observed in patients that still present with EM rash (Biesiada et al., 2012). While it is unknown how the bacteria are able to cross the blood-brain barrier and enter the CNS, B. burgdorferi can be found and has been isolated from the cerebral spinal fluid of patients diagnosed with Lyme neuroborreliosis. Neurological manifestations range from radiculoneuritis, meningitis, and facial palsy (Pachner and Steere, 1985; Schmidt et al., 2015; Thaisetthawatkul and Logigian, 2002). Additionally, during this late stage of the disease, unresolved infection and inflammatory response within joints can lead to tissue degradation and subsequently arthritis. Recent surveillance of reported cases by the CDC indicates that approximately 30% of individuals will develop arthritis (Schwartz, 2017). Although active infection may be cleared through antibiotics, some patients may experience persistent inflammation within the CNS and joints that may last for months to years (Marques, 2008; Pícha et al., 2006; Steere and Angelis, 2006). Because B. burgdorferi s.l. does not produce or secrete any known toxins that can be attributed to the manifestations of the disease, it is suggested that the host immune and inflammatory response elicited by the bacteria is the major contributing factor to the pathogenesis of the disease (Stanek et al., 2012). Figure 1 highlights the general timeline for the progression of the disease and common symptoms associated with Lyme borreliosis.
Fig. 1.
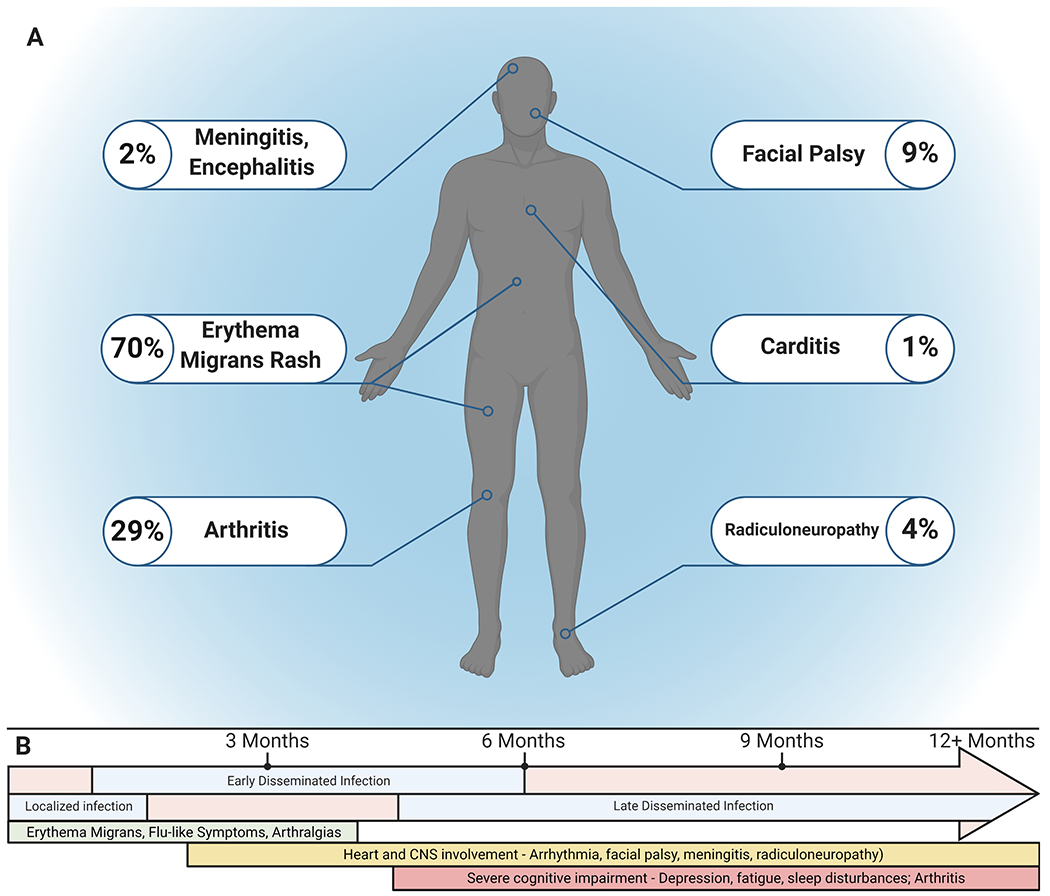
A summary of Lyme borreliosis symptoms and general timeline of the stages of the disease. A) Symptoms of Lyme borreliosis. The most common symptom is the erythema migrans (EM) rash. Arthritis is typically seen in 29 % of patients, while neurological and cardiac manifestations are less common. B) General timeline of the stages of Lyme borreliosis. The initial localized infection involves the EM rash, flu-like symptoms, and musculoskeletal pain, typically in the first month of infection. Early dissemination occurs within one to several months and can lead to heart and CNS involvement in addition to initial localized symptoms. Pathogen establishment in secondary tissue and organs during the late disseminated phase (months to years from initial infection) can lead to the addition of more severe complications including cognitive impairment and arthritis. Created with BioRender.com.
With the advent of next-generation sequencing technologies, the ability to perform transcriptome analysis has become much more accessible and affordable. The past several years has seen a dramatic increase in RNA-seq analysis that allows researchers to conduct genome-wide transcriptome profiling of both model and non-model organisms. The impact of this technological innovation is best seen in studies of host-pathogen interactions. The use of RNA-seq and microarray technologies aids in understanding the development of the host response to a disease. This can better illustrate the host immune response towards a pathogen and how the pathogen may overcome these obstacles. Particularly, regarding B. burgdorferi s.l., the host immune response and subsequent inflammation, while generally beneficial, poses a deleterious outcome for patients with Lyme borreliosis, especially if such symptoms are persistent. With such a tremendous evolution in sequencing technologies, researchers are able to better understand this dichotomy in Lyme borreliosis. However, to date, there has not been a comprehensive review that compares and summarizes the findings of studies that have utilized such technologies. The goal of this review is to summarize past and current findings that have utilized RNA-seq and microarray technologies in Lyme borreliosis models to formulate a cohesive understanding of host transcriptome alterations in response to B. burgdorferi sensu lato. A list of these studies that are highlighted in this review can be found in Table 1.
Table 1 –
List of publications reviewed
| Cell or Tissue Type | Borrelia sp. | Publication | Sequencing Platform | Dataset | Note |
|---|---|---|---|---|---|
| Dendritic cells | B. garinii | Hartiala et al., 2007 [1] | Microarray - Hum-16K cDNA | In publication | |
| Mus musculus, Macrophages | B. burgdorferi 297 | Carreras-González et al., 2018 [2] | RNA-Seq-Illumina HiScanSQ | GSE103483 | |
| Monocytes | B. burgdorferi B31-5A15 | Carreras-González et al., 2018 [2] | Microarray-Illumina Human HT12 v4 BeadChips | GSE103483 | |
| Dermal Fibroblasts | B. burgdorferi N40, Pbre, 1408 | Schramm et al., 2012 [3] | Microarray-PIQOR− Skin cDNA | GSE31740 | |
| Endothelia (HUVEC) | B. burgdorferi HBD1 | Dame et al., 2007 [4] | Microarray-Affymetrix Human U133 Expression Array Plus 2.0 | GSE6092 | |
| LD Patient PBMCs | N/A | Bouquet et al., 2016 [5] | RNA-Seq – Illumina HiSeq 2000 | GSE63085 | |
| PBMCs | B. burgdorferi B515 | Petzke et al., 2009 | Microarray – Affymetrix Human Genome U133 Plus 2.0 | GSE17103 ** | |
| Choroid Plexus Epithelium | B. burgdorferi B31 - MI-16 | Thompson et al., 2020 [6] | RNA-Seq – Illumina HiSeq 4000 | GSE153261 | |
| Mus musculus, Joint | B. burgdorferi N40 | Crandall et al., 2006 [7] | Microarray – Affymetrix Mouse Expression 430A; Affymetrix Mouse Genome 430 2.0 | GSE6055 | |
| Rhesus macaque (Macaca mulatta) – Brain parenchyma | B. burgdorferi B31 – 5A19 | Ramesh et al., 2008 [8] | Microarray – Agilent 4x44K rhesus macaque, G2519F | N/A | |
| Rhesus macaque (Macaca mulatta) – Microglia, astrocytes; human SH-SY5Y | B. burgdorferi B31 – 5A19 | Myers et al., 2009 [9] | Microarray – Agilent 4x44K rhesus macaque (G2519F); Agilent 4x44K Human (G4112F) | In publication | |
| Astrocytes | B. burgdorferi B31-MI-16 | Casselli et al., 2017 [10] | RNA-seq – Illumina HiSeq 2000 | GSE85143 |
All samples derive from human unless otherwise stated
Could not be found in GEO database
Transcriptional Response of Early Localized Infection
Lyme borreliosis begins at the site of the tick bite where B. burgdorferi s.l. and tick saliva are transmitted to the host during feeding (Spielman et al., 1987). Though many studies cited throughout this review do not utilize ticks as a route of transmission, it is important to note the role of tick saliva in Lyme borreliosis. Tick saliva contains a milieu of immunomodulatory factors that include antihistamines, antioxidants, and anti-complement proteins that promote bacterial survival (Das et al., 2001, p. 25; Hourcade et al., 2016; Kotsyfakis et al., 2006). The saliva has been shown to inhibit neutrophil functions and impedes the killing and clearing of bacteria; the proteins ISL 929 and ISL 1373 have been shown to inhibit neutrophil chemotaxis to the site of infection (Guo et al., 2009; Ribeiro et al., 1990). Furthermore, dendritic cell (DC) migration and maturation are also inhibited by tick saliva, specifically Prostaglandin E2 (Sá-Nunes et al., 2007; Skallová et al., 2008). These anti-inflammatory and immunosuppressive effects aid in the initial establishment of a localized infection at the skin of the host. Nevertheless, investigating the initial contact of B. burgdorferi s.l. with host tissue provides valuable understanding into the early pathogenesis of Lyme borreliosis. This section summarizes the transcriptional alterations within tissue and cells that first encounter B. burgdorferi s.l. during the early phase of the disease – Figure 2 highlights these interactions.
Fig. 2.
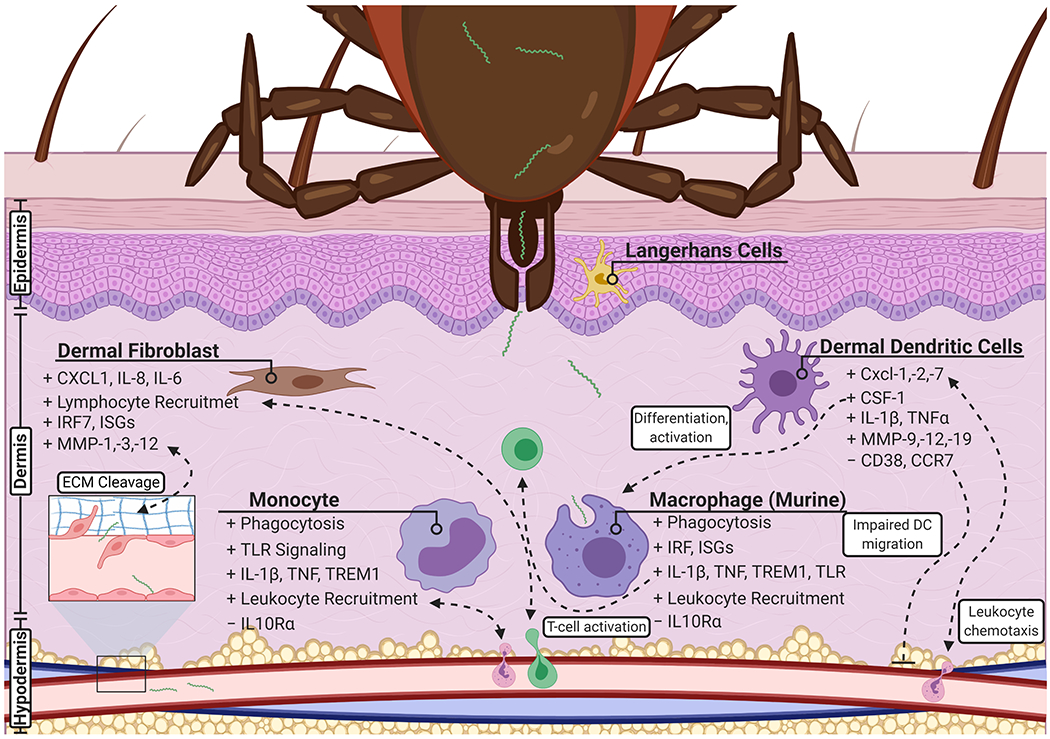
An overview of the transcriptional response of early localized infection. Infection occurs by the transmission of B. burgdorferi s.l. through the bite of a tick and is localized within the skin of the host. Following infection, several resident cells detect and initiate a transcriptional profile in response to the pathogen. Dermal fibroblasts aid in the recruitment of lymphocytes and produce matrix metalloproteinases that leads to the degradation of the extracellular matrix. Monocytes and macrophages initiate phagocytosis and play key roles in the promotion of inflammation, and activation and attraction of leukocytes. Similarly, dendritic cells aid in peripheral immune cell chemotaxis and promote their differentiation and activation. Infection may lead to an impaired migration of dendritic cells to lymph nodes. Created with BioRender.com.
Dendritic cells
Dendritic cells are phagocytic and professional antigen-presenting cells that can be found in many tissues including the skin where they monitor for and detect invading pathogens. As a part of the innate immune system, DCs act as a bridge linking the innate and adaptive immune response through their ability to activate naïve T cells following stimulation and migration to lymph nodes (Banchereau and Steinman, 1998; Patente et al., 2019). DCs, which reside in the epidermis (Langerhans cells) or dermis (dermal DCs), are one of the first types of immune cells to interact and phagocytize B. burgdorferi, allowing for the processing and presentation of Borrelia antigens for the activation of CD4+ T cells (Filgueira et al., 1996; Mason et al., 2014). There have been very few studies that have investigated the role and interactions of Langerhans cells with B. burgdorferi s.l., but one study has shown their potential role in the modulation of the T-helper cell response during tick feeding in Langerhans cell deficient mice, though this response was attenuated in the presence of B. burgdorferi (Hulínska et al., 1994; Mason et al., 2014; Vesely et al., 2009). As Langerhans cells are a major tissue-resident cell of the skin, this lack of experiment data represents a large gap in knowledge within the early stages of disease pathogenesis. Additionally, due to the limited number of DCs that can be collected for in vitro investigation, it is common to derive DCs either through bone morrow or peripheral blood monocytes (PBMCs); however, to date, only one study has investigated the host transcriptional effects between Borrelia and DCs. Hartiala et al. (2007) utilized PBMCs from healthy donors and generated DCs in vitro to determine the transcriptional response to Borrelia garinii, a member of the Borrelia burgdorferi sensu lato complex. Like B. burgdorferi, B. garinii elicits a phagocytic response from DCs, in which they are processed, induce DC maturation, and activate T cells (Suhonen et al., 2003). The microarray experiments performed by Hartiala et al. made two comparisons, B. garinii stimulated vs unstimulated DCs and B. garinii stimulated versus LPS stimulated DCs. In both comparisons, several genes that transcribe chemotactic cytokines were upregulated in response to B. garinii stimulation. The neutrophil chemoattractants CXCL1, CXCL7, and CXCL2, the latter also showing chemotactic properties for polymorphonuclear leukocytes and hematopoietic stem cells, showed increased expression compared to unstimulated and LPS-stimulated groups, and have previously been implicated in the inflammatory response in later manifestations of Lyme borreliosis (Burdon et al., 2005; Moser et al., 1990; Ritzman et al., 2010; Rouault et al., 2013). The gene encoding for CSF-1 was induced at greater levels in B. garinii stimulated DCs; CSF-1 functions as a cytokine for hematopoietic stem cell differentiation into macrophages, and activates macrophage phagocytosis as well as inflammatory and chemotactic functions (Jones and Ricardo, 2013; Nemunaitis, 1993). Transcripts for markers of DC maturation and differentiation were found to be upregulated in both the LPS and B. garinii stimulated groups – ADAM19, CD83, and SLAMF1 (Aerts-Toegaert et al., 2007; Fritsche et al., 2000; Kruse et al., 2001; Li et al., 2019). Further evidence of the DC inflammatory response to these stimuli is observed by the induction of inflammatory cytokine genes – TNF-α, IL-1α, IL-1β, and IL-6. Though it has been previously shown that B. burgdorferi s.l. can stimulate an interferon response, interestingly, such a response was not seen in DCs stimulated by B. garinii, yet interferon-inducible genes were upregulated within the LPS group – this includes IFIT1, IRF2, and IRF7. One of the principle findings by Hartiala et al. was the demonstration of a reduced response in CD38 and CCR7 expression in DCs stimulated by B. garinii when compared to LPS. During differentiation to immature DCs, CD38 is down-regulated, but expressed following DC maturation and is required for DC chemotaxis and transendothelial migration (Fedele et al., 2004; Partida-Sánchez et al., 2004). Similarly, CCR7, a chemokine receptor, is a key promoter for DC migration to the lymph nodes (MartIn-Fontecha et al., 2003). This results in an impaired and weakened humoral response and suggests a possible cause for the immune abnormalities associated with Lyme borreliosis (Hartiala et al., 2007). This is in line with similar experiments that utilize Ex Vivo skin models injected with B. burgdorferi sensu stricto, LPS, or Pam3CSK4 (TLR2 agonist) to measure dendritic cell activation and migration (Mason et al., 2016). Here, the authors observe the TLR2-mediated migration of DCs in response to B. burgdorferi, however, the expression of CCR7 and CD38 were comparable to PBS controls, while LPS and Pam3CSK4 induced significant upregulation of these markers. Infection of monocyte derived DCs with B. burgdorferi, again, did not show an upregulation of CCR7 and CD38 (Mason et al., 2016).
Macrophages (mouse)
A second prominent immune cell found in the skin are macrophages. While phenotypically and morphologically distinct, macrophages share many functional characteristics with dendritic cells. Like DCs, macrophages play a central role in the innate immune response during pathogen invasion. While both are antigen presenting, macrophages are predominantly phagocytic, clearing cellular debris and pathogens while modulating an inflammatory response through the secretion of cytokines to alert the adaptive immune system (Ferenbach and Hughes, 2008; Gottschalk and Kurts, 2015).
To study the innate immune response to B. burgdorferi, Carreras-González et al. (2018) utilized RNA-seq to investigate the transcriptional profile of primary murine bone marrow-derived macrophages (BMMs). Following infection, a large number of differentially expressed genes (DEGs) were observed, with gene expression consistent with an innate immune and inflammatory response. The pattern of gene transcription involved biological processes for the recruitment, differentiation, and activation of leukocytes. A sizable number of DEGs (317) that were induced by B. burgdorferi were directly related to the inflammatory response, and 64 DEGs were found to be involved in phagocytosis, a significant function of macrophages. The macrophage pathway profile following exposure to B. burgdorferi resembled the profile for the recognition of bacteria and viruses by pattern recognition receptors (PRRs); more specifically, pathway mediators within toll-like receptor (TLR) signaling showed a significant increase within the stimulated BMMs. TLRs are a well-recognized and studied receptor family required for the innate immune response that is activated during Borrelia infection, and TLR2 signaling has been extensively shown to be a predominant pathway in the induction of a pro-inflammatory response (Berende et al., 2010; Bernardino et al., 2008; Wooten et al., 2002). Nonetheless, studies have shown that such an immune response to B. burgdorferi is not isolated to only TLR2. TLR2-deficient mice show poor response to B. burgdorferi in which the bacteria persist at elevated levels for at least 8 weeks, and while macrophages showed no response to OspA, an outer surface protein known to signal through TLR2, sonicated spirochetes did stimulate a macrophage response (Hirschfeld et al., 1999; Yoder et al., 2003). Additionally, in TLR2 knock-out mice, macrophages were still able to activate and respond to spirochete lysates (Wooten et al., 2002). However, the role of TLRs is diminished in the current study by Carreras-González and colleagues. Though there were significant overlap in the number of genes between stimulated BMMs and TLR2 or PAM3CSK4 (a TLR2 agonist) signaling pathways, the percentage of shared genes was less than 10% - suggesting that a greater diversity in signaling receptors are responsible for the overall response, as seen with an upregulation in receptor Nod2 and overlap with triggering receptor expressed on myeloid cells-1 (TREM1) signaling. TREM-1 is a receptor that amplifies a pro-inflammatory response, and while a more robust inflammatory response could provide efficient clearing of an invading pathogen, it can lead to substantial cell death and tissue damage – a concept not unfamiliar to the Lyme borreliosis field (Colonna, 2019; Ramesh et al., 2013; Roe et al., 2014; Wang et al., 2009; Wooten and Weis, 2001). However, in a study using TREM1−/− mice infected with Leishmania major, Legionella pneumophila, or influenza virus, the mice displayed a significant attenuation of disease pathology in regards to inflammatory infiltrates and a reduction in the expression of pro-inflammatory cytokines, yet were equally capable of clearing the infection compared to controls (Weber et al., 2014). In fact, additional studies of B. burgdorferi infection implicate the TREM1 pathway as a significant contributor to the pathogen-induced inflammatory response (Bouquet et al., 2016; Myers et al., 2009). The inflammatory response by BMMs was further highlighted by the reduction in signaling intermediates induced by PPAR, an inflammatory suppressor, and the downregulation of Il10ra, the receptor for the anti-inflammatory interleukin-10. Furthermore, several pro-inflammatory cytokines were shown to be upregulated including IL-1β, IL-6, IFNβ1, and TNF (a regulator of TREM1). Importantly, protein levels were assessed using label-free mass spectrometry to determine gene expression correlation to protein translation. Although a limited number of proteins were identified to be significantly changed, several proteins/genes were differentially changed in the same direction. Moreover, some proteins were inversely correlated or showed no difference compared to gene expression, but the majority of proteins were associated with similar pathway enrichment found at the gene expression level. Overall, the study showed an inflammatory response by BMMs following B. burgdorferi infection that was characterized by an increase in pro-inflammatory cytokines, a reduction in anti-inflammatory pathways, and an increase in phagocytic related genes.
Monocytes
In addition to transcriptional profiling of murine macrophages, Carreras-González et al. (2018) further characterized the transcriptional response of the interaction between B. burgdorferi and human CD14+ peripheral blood monocytes via microarray analysis to determine their relevance in the human innate immune response. A similar number of DEGs were found in the human monocytes compared to BMMs. A significantly reduced number of DEGs were shared between the two cell types, most likely attributed to differences across species. However, similarities appeared following functional and pathway analysis in which pathways involving the recruitment of leukocytes, phagocytosis, and endocytosis were increased. Furthermore, analysis regarding upstream regulators indicated similar patterns of gene expression for receptors TLR3, TLR7, TLR9, and NOD2, and proinflammatory cytokines TNF, IL-1α, IL-1β, and IFNα2. The gene expression pattern for the inhibition of the anti-inflammatory IL10RA pathway was observed in both BMMs and human monocytes. While there were distinct differences between the two groups regarding specific gene expressions, it appears that infection with B. burgdorferi produces a pro-inflammatory response that overlaps both groups.
Many of these findings are supported by the transcriptome profiling of peripheral blood mononuclear cells (PBMCs) by Salazar et al. (2009), which contain a milieu of peripheral immune cells which include monocytes. Their study, involving human PBMCs infected with Bb 297, demonstrated that PBMCs produced a greater and more expanded inflammatory and immune response to live bacteria compared to lysates, which was induced by the phagocytosis of live Bb. Isolated human monocytes from PBMCs were then determined to be a major source of this gene expression profile through the upregulation and secretion of pro-inflammatory cytokines that include IL-1β, TNF-α, and IL-6. Furthermore, isolated monocytes were determined to be a source of a substantial type I interferon response through the upregulation of IFN-β and several type I interferon-inducible genes including IFIT1, IFIT2, IFIT3, and ISG15.
Dermal fibroblasts
The skin represents an important obstacle in the progression of Lyme borreliosis as it forms a complex physical barrier comprised of the epidermis, dermis, and hypodermis. B. burgdorferi s.l. establishes a local infection within the dermis, and aside from resident immune cells such as DCs and macrophages, dermal fibroblasts are among the first cells to come into contact with the spirochetes (Castelli et al., 2008; Schramm et al., 2012; Vasudevan and Chatterjee, 2013). Fibroblasts are the main resident cells in the dermis and play an important role in the formation and reorganization of the extracellular matrix. Moreover, fibroblasts are able to communicate and modulate local immunocompetent cells as a functional part of the innate immune response through the recruitment of leukocytes, inflammatory regulation, and the maturation of dendritic cells (Saalbach et al., 2007; Sorrell and Caplan, 2009).
To understand the role of dermal fibroblasts in skin inflammation during the early pathogenesis of Lyme borreliosis, Schramm et al. (2012) performed microarray analysis of human dermal fibroblasts in response to three different strains of Borrelia burgdorferi sensu stricto. The three strains of B. burgdorferi were isolated from different environments representative of different stages of Lyme borreliosis – a tick isolate (strain N40), an erythema migrans skin biopsy (strain Pbre), and from a skin biopsy of acrodermatitis chronica atrophicans (ACA), a late stage manifestation of Lyme borreliosis (strain 1408). Though there were distinct and common transcriptional profiles between groups, there were no discernable differences in relevant transcriptional pathways across each group. As one of the major functions of fibroblasts, ECM production and remodeling genes were found to be upregulated among the genes shared across groups. These involve the structural components that include integrin ITGA1, laminin LAMA1, microfibrils MFAP3, and collagen fibrils COL8A1. Most importantly, three matrix metalloproteinases (MMPs) were found to be upregulated – MMP-1, MMP-3, and MMP-12. The induction of MMPs by B. burgdorferi have been linked to the development of Lyme arthritis showing similar erosive and inflammatory pathologies to rheumatoid arthritis (Cawston et al., 1984; Hu et al., 2001; Steere et al., 1988). MMP-1 and MMP-3 have been shown to be elevated in the synovial fluid of Lyme arthritis patients (Hu et al., 2001; Lin et al., 2001). B. burgdorferi appears to induce the production of host MMPs to degrade and digest extracellular matrix proteins that allow for greater dissemination (Behera et al., 2005; Gebbia et al., 2001).
In addition to the upregulation of ECM remodeling pathways, the transcriptional response in fibroblasts was largely indicative of a pro-inflammatory profile. A high level of cytokines and chemokines were shown to be upregulated. Additionally, many upregulated genes were representative of signaling mediators that regulate and sustain an inflammatory response such as NF-κB transcription factors (NF-κB1, -2), interferon-related and inducible genes (IRF1, STAT1/2, OAS2, and IFIH1), and genes within the tumor necrosis factor family (TNFSF10, TNFSF13B). Through the release of secreted factors, dermal fibroblasts appear to communicate with innate immune cells that allows for their attraction, activation, and maturation to the site of infection (de Koning, 1993; Ebnet et al., 1997; Jones et al., 1994; Müllegger et al., 2007; Parsonage et al., 2005). Moreover, during the localized infection phase, dermal fibroblasts inadvertently provide a means for B. burgdorferi dissemination by the induction of ECM degradation factors like MMPs (Schramm et al., 2012).
A similar study performed by the same research group, Meddeb et al. (2016), sought to determine the transcriptional response of human dermal fibroblasts to three different Borrelia burgdorferi sensu lato strains – these species included Borrelia afzelii (IBS17), B. garinii (IBS6), and B. burgdorferi sensu stricto (IBS19). There were no significant transcriptional differences between the three species that were of relevance to the immune response, which was further characterized by similar induction and secretion of IL-8 in a dose and time dependent manner when compared to unstimulated controls. A core of 42 highly up-regulated genes were found to be common among the three strains that included genes involved in the pro-inflammatory and innate immune response. These include several chemokines and cytokines that act as inflammatory and chemotactic factors – CCL2, CXCL1, CXCL2, CXCL6, CXCL10, IL-6, IL-8. Additionally, the gene expression profile was representative of a sustained inflammatory response that involve NF-κB and Type I interferon related genes (IRF7, ISG15, IFIT1, IFIT2, IFIT3, and STAT1). Many of these genes were also shared with the B. burgdorferi s.s. strains isolated from varying clinical manifestations of Lyme borreliosis (Schramm et al., 2012). In this regard, it can be suggested that the transcriptional profile of dermal fibroblasts is not impacted by the strain or species of B. burgdorferi s.l. and that differences in species-specific clinical manifestations are indicative of a more systemic immune response during the pathogenesis of the disease (Cerar et al., 2016).
Erythema migrans
The erythema migrans rash is the manifestation of the collaborative effects of the individual cellular responses highlighted thus far. Marques et al. (2018) supports this conclusion through the transcriptome assessment of EM biopsies from early Lyme borreliosis patients. Their findings reveal upregulation of genes associated with the innate immune response, cell migration, and chemotaxis when compared to health controls. Genes associated with this response including several CXCLs (CXCL9, 10, 11, and 13) and CCLs (CCL5, 8, and 9). Furthermore, a robust interferon inducible response was the predominate profile among the immune response, as was observed in dermal fibroblasts (Meddeb et al., 2016; Schramm et al., 2012). This includes IFN-γ, which was predicted to be a top upstream regulated and previously established within EM lesions, IFIT1, IFIT3, OAS1, STAT1, and IRF7 (Salazar et al., 2003). In contrast, genes involved in tissue development and remodeling were found to be repressed. In support of the overexpression of the chemokines, CXCL9, CXCL10, and CXCL11, which are predominate T-cell chemoattractants and induced by IFN-γ, infiltrating regulatory T-cells as well as DCs were found to be present within the EM lesions (Marques et al., 2018; Metzemaekers et al., 2018).
Transcriptional Response of Early Disseminated Infection
The next phase following the initial localized infection of Lyme borreliosis is the early dissemination of B. burgdorferi s.l. to other host tissue and organs. Hematogenous dissemination is the primary mechanism for the spread of the spirochete in the host and the first barrier that the spirochetes interact with is the endothelial barrier of the blood vessels. This is also a primary barrier that must be overcome by innate and adaptive immune cells. Figure 3 illustrates the host transcriptome response during this phase of dissemination.
Fig. 3.
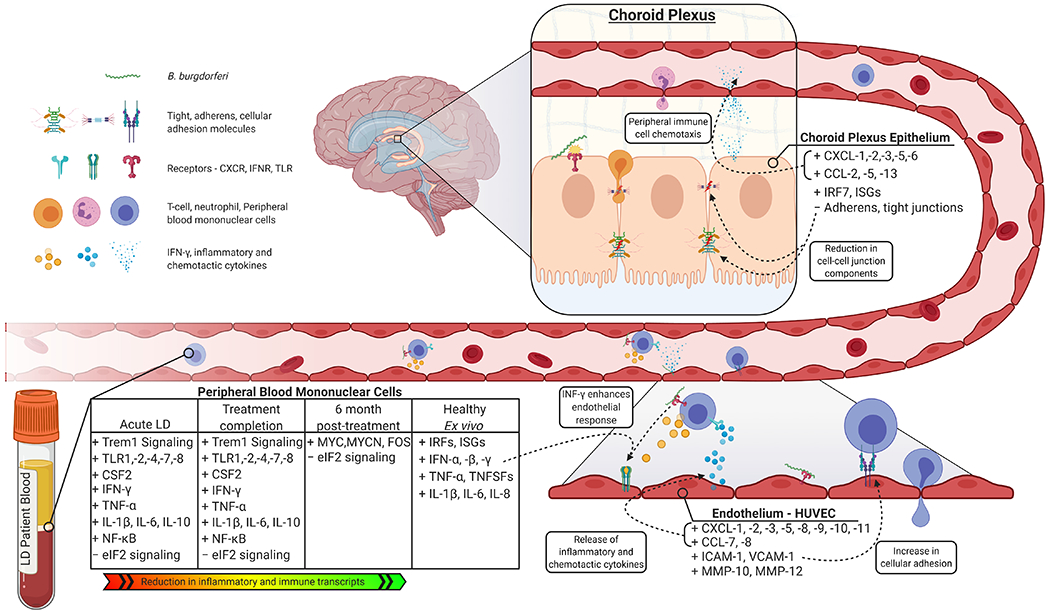
An overview of the transcriptional response of early disseminated infection. The main route of dissemination for B. burgdorferi s.l. is through the circulatory system. The endothelium of blood vessels represents a barrier that must be overcome by both B. burgdorferi and immune cells – infection induces the production of several inflammatory and chemotactic cytokines and an increase in cellular adhesion molecules that promotes that binding of leukocytes. The induction of MMPs in both endothelial cells and fibroblasts can aid in the dissemination of the bacteria. Likewise, the choroid plexus epithelium, in response to B. burgdorferi, produces a similar profile conducive to the chemotaxis of peripheral immune cell and a reduction in cell-cell junction transcripts. Peripheral blood mononuclear cells produce an inflammatory response marked by Trem1 signaling, amplifying inflammation. Key mediators of the response include TNF, NF-κB, and IFN-γ, the latter which enhances the endothelial response. Created with BioRender.com.
Endothelial cells
Dame et al. (2007a) sought to determine the role of endothelial cells in Lyme borreliosis by performing microarray analysis of a human umbilical vein endothelial cell (HUVEC) in vitro system infected with B. burgdorferi. T lymphocytes have long been implicated in the pathogenesis of Lyme borreliosis and specifically Th1 cells have been shown to be elevated in the synovial fluids of Lyme arthritis patients (Gross et al., 1998; Salazar et al., 2003). Furthermore, Th1 cells which specifically secrete IFN-γ have shown a migratory affinity towards endothelia that have been stimulated by B. burgdorferi, and a significant correlation has been shown between symptom score of LD patients and IFN-γ (Gergel and Furie, 2004; Salazar et al., 2003). To this end, Dame and colleagues further sought to identify the synergistic effects of IFN-γ and B. burgdorferi on the transcriptional profile of primary human endothelial cells.
In the presence of B. burgdorferi, HUVECs showed poor activation and minimal differential gene expression (Dame et al., 2007a). Of these genes, several have already been identified to be induced by B. burgdorferi in a number of in vitro and in vivo models – these genes include immune cell adhesion molecules (ICAM-1, VCAM-1) and leukocyte recruitment chemokines (CXCL8, CCL2), which have been specifically shown to promote the transmigration of leukocytes across endothelia during B. burgdorferi infection (Böggemeyer et al., 1994; Burns et al., 1997; Burns and Furie, 1998; Gergel and Furie, 2004; Sellati et al., 1995). Stimulation of HUVECs with IFN-γ only produced a larger transcriptional change in both magnitude and number of genes when compared to B. burgdorferi only, with a subset of genes being classified as mediators of inflammation. However, the combinatorial effects of IFN-γ and infection revealed several inflammatory and immune related genes that were significantly upregulated and showed a robust fold change compared to each individual treatment. This effect can be clearly seen in the expression of CCL8 - IFN-γ only: 17.672 FC, Bb only: 1.31 FC, and Bb+ IFN-γ: 113.58 FC. Of the genes that were markedly upregulated in response to the combinatorial effects of Bb and IFN-γ, several were involved in the recruitment of leukocytes which include specifically T lymphocyte chemoattractants CCL7, CCL8, CXCL9, CXCL10, CXCL11, CX3CL1 and also the aforementioned adhesion molecules (ICAM-1, VCAM-1), as well as the neutrophil chemoattractant CXCL2 (Bazan et al., 1997; Cole et al., 1998; Liao et al., 1995; Loetscher et al., 1994; Rouault et al., 2013; Taub et al., 1993). Furthermore, the presence of T lymphocytes has been attributed to more persistent inflammatory syndromes (Cope, 2002; Divan et al., 2015; Moro-García et al., 2018; Skapenko et al., 2005). Many of these genes are mediators in both the innate and adaptive immune systems; however, interestingly, the CIITA gene showed a downregulation in Bb only treatment, but an upregulation in IFN-γ only, and an even greater upregulation with the combination of treatments. CIITA encodes for the class II, MHC, transactivator and acts as a master regulator for MHC class II genes, involved in antigen processing and presentation. This change in gene expression brought upon by IFN-γ treatment is suggestive of a transition towards adaptive immune response. Together, the transcriptional changes observed indicate a key role of endothelial cells in conjunction with IFN-γ that leads to a greater adaptive immunological response through the selective recruitment of T lymphocytes, and as Th1 cells themselves produce and secrete IFN-γ, a positive-feedback loop would promote a more chronic inflammatory environment which is seen in Lyme borreliosis patients (Dame et al., 2007a; Duray, 1989; Duray and Steere, 1988; Forsberg et al., 1995; Gross et al., 1998, p. 1; Salazar et al., 2003).
Peripheral blood mononuclear cells
During hematogenous dissemination, B. burgdorferi s.l. encounters a wide variety of immunocompetent cells within the blood circulatory system. Due to the ease of access, availability, and as one of the predominate classes of immune cells in the blood, peripheral blood mononuclear cells (PBMCs) are commonly used in both ex vivo and in vivo analysis of Lyme borreliosis. PBMCs are defined as any peripheral blood cell that has a round nucleus, as opposed to anuclear cells such as mature erythrocytes or multi-lobed nucleated cells such as granulocytes. They are predominately comprised of lymphocytes (T cells, B cells, and NK cells) and to a lesser extent monocytes (Acosta Davila and Hernandez De Los Rios, 2019; Autissier et al., 2010).
To determine the global and temporal transcriptional pathways involved during B. burgdorferi infection in humans, Bouquet et al. (2016) performed longitudinal transcriptome analysis (RNA-seq) of PBMCs collected from patients with acute Lyme borreliosis at three time points: time of diagnosis/pre-treatment, treatment completion (3 weeks), and 6 months post-treatment to determine the transcriptional profiles of post-treatment symptoms. Twenty-nine individuals with acute Lyme borreliosis that presented with a documented EM rash and flu-like symptoms, such as fever, fatigue, and the onset of muscle or joint pains, were enrolled in addition to 13 healthy control individuals. It was observed that the longer the duration between the onset of symptoms and the time of completed treatment, the greater the correlation with PTLDS diagnosis, in which non-PTLDS patients had an average duration of 9.7 days between symptom onset and treatment completion and 19.3 days for PTLDS patients.
Transcriptional analysis showed a consistent decrease in the number of DEGs across the three time points compared to controls (Bouquet et al., 2016). Interestingly, when taking into consideration other infectious diseases such as Influenza, the gene expression of patients with Lyme borreliosis failed to return to baseline levels; however, persistent symptoms were not solely dependent on this failure to return to baseline as no DEGs were found between patients with resolved symptoms and those with persistent symptoms. Pathway analysis of the pre-treatment group indicated activation of the inflammatory response, immune cell trafficking, hematological system, as would be expected during the early dissemination phase of B. burgdorferi. However, these pathways remained activated at treatment completion and to a lesser extent at the 6-month post-treatment time point. This is reflected in the top 10 enriched pathways in that 8 pathways of the pre-treatment, 10 pathways in treatment completion group, and 4 pathways in 6-month post-treatment group were directly related to the immune response. Furthermore, TREM-1 signaling was the top activated pathway for both pre- and completed treatment groups. As mentioned previously, TREM-1 acts as an amplifier of inflammation, and also acts as a mediator for Th1 activation through factors such as DAP12, IL-6, IL-12 that were upregulated in these two groups. Inversely, the eIF2 signaling pathway was found to be downregulated in all three groups and has been found to be involved in protein synthesis during cellular stress (Park and Moon, 2013). The pathogen Legionella pneumophila was previously shown to disrupt and downregulate mediators of this pathway through the secretion of effectors, and defective signaling of eIF2 was shown to lead to greater susceptibility to intracellular invasion by Yersinia pseudotuberculosis, Listeria monocytogenes, and Chlamydia trachomatis (Fontana et al., 2011; Shrestha et al., 2012). However, the current mechanism and immunological impact of eIF2 signaling inhibition following B. burgdorferi s.l. infection is yet to be elucidated. Several TLRs were also found to be either upregulated or predicted to be activated that include TLR-1, -2, -4, -7, and -8 within the first two time points. Additionally, within these two groups, the majority of upstream regulators were proinflammatory cytokines such as CSF2, IFN-γ, TNF-α (a master regulator of eIF2, TREM1, and TLR signaling), anti-inflammatory cytokines IL-6 and IL-10, and the transcription factor NF-κB. In contrast, the top upstream regulators for 6-month group mainly involved genes associated with the regulation of gene expression such as MYCN, MYC, and FOS. Overall, Bouquet et al. found that the transcriptional profile of acute Lyme borreliosis patients prior to treatment and upon treatment completion shared many characteristics, with many overlapping DEGs involved in the inflammatory and immune process but the activation of inflammatory T-cell apoptosis and B-cell developmental pathways were inhibited when compared to other acute infection diseases. While the 6-month post-treatment group did not produce a marked inflammatory response, it failed to return to baseline levels; however, when compared to the transcriptional profiles of other immune-mediated chronic diseases such as systemic lupus erythematosus, up to 60% of the pathways overlapped. Many of the immune and inflammatory responses observed by Bouquet et al. were corroborated by a previous study by Petzke et al. utilizing ex vivo PBMC cultures, and in fact 44% of DEGs were shared between the two studies (Bouquet et al., 2016; Petzke et al., 2009). Additionally, the type I interferon response was found to be induced by TLR7 and TLR9 (Petzke et al., 2009). Although the study by Bouquet et al. lacked protein level assessment of the patients, the gene expression profiles were correlated to the demographics, clinical manifestations of the disease, and disease progression. Similarly, Petske et al. investigated minimal protein translation, but concluded an increase in IFN-α protein levels induced by TLR7 and TLR9.
Choroid plexus epithelium
In a study performed by our laboratory, Thompson et al. (2020) sought to investigate a long-standing question that occurs during the early disseminating phase of Lyme borreliosis – How and where does B. burgdorferi enter the central nervous system (CNS)? Evidence of the spirochete penetrating into the human brain parenchyma is sparse, however, it is well-known that the bacteria is capable to exist within the cerebral spinal fluid (CSF) - this is evident by the isolation of the Bb 297 strain from the CSF of a Lyme borreliosis patient as well as direct detection of Bb DNA (Luft et al., 1992; Steere et al., 1983). A common outcome of neuroborreliosis is the invasion and abundance of peripheral immune cells into the CSF, specifically lymphocytic pleocytosis (Djukic et al., 2012). With this in mind, the choroid plexus was selected as a primary candidate due to its role in the formation of the blood-CSF barrier and potential for the transmigration of both pathogens and peripheral immune cells into the CSF (Meeker et al., 2012; Schwerk et al., 2012; Steinmann et al., 2013).
As the epithelial cells of the choroid plexus form the tight blood-CSF barrier, primary human choroid plexus epithelial cells (HCPECs) were infected in vitro by B. burgdorferi B31 MI-16 for 48 hours followed by RNA-seq analysis (Thompson et al., 2020). Functional and Pathway analysis identified a strong immune and inflammatory response comprised of chemokine-mediated signaling pathway, and type I and II interferon pathways. Many of the chemokines that were upregulated, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CCL2, CCL5, and CCL13 are involved in immune cell chemotaxis specifically neutrophils, monocytes, T-helper cells, and other lymphocytes (Charo and Ransohoff, 2006; Ritzman et al., 2010). Protein analysis of CXCL1, CXCL2, CXCL5, and CXCL6 through ELISA were found to correlate to changes in gene expression. Additionally, many interferon-related and inducible genes were significantly upregulated, including OAS1, -2, -3, signaling mediators STAT1, -2, and the master regulator IRF7. Signaling pathway impact analysis provides information of the activation or inhibition of enriched pathways which indicated the activation of a number of viral pathways such as Influenza A, Measles, and Herpes simplex infection – this is indicative of an interferon response as historically, interferon-related genes were initially associated with viral infections. Of the downregulated transcripts, genes predominately clustered into regulatory, structural, and angiogenesis functions. Of interest to this study, a number of tight and adherens junction components were downregulated – CLDN14, PCDH7 and 10, and CDH2. Additionally, regulatory and scaffolding components of these junctions were downregulated.
Taken together, these data suggest that the choroid plexus functions as a key mediator in the pathogenesis of neuroborreliosis through the promotion of an inflammatory state and the upregulation of genes specific to the recruitment of peripheral immune cells. Furthermore, the choroid plexus may act as a potential entry point for B. burgdorferi and these immune cells into the CSF by the downregulation of tight and adherens junction genes.
Transcriptional Response of Late Disseminated Infection
The late disseminated phase of Lyme borreliosis is characterized by successful invasion of secondary tissue and organs, including joints, heart, and CNS, followed by subsequent clinical presentations associated with affected tissue. A common outcome of dissemination into the joints is Lyme arthritis, in which 30% of patients will develop it (Arvikar and Steere, 2015; Schwartz, 2017; Steere, 1987). Furthermore, by overcoming the tight barriers of the CNS, dissemination into this system followed by neurological manifestations of symptoms occur in up to 12% of patients with Lyme borreliosis (Koedel et al., 2015). During this late phase of the disease, symptoms become more deleterious and have a greater chance of persistence, leading to a reduction in quality of life due to musculoskeletal pain, cognitive disruption, and fatigue (Eikeland et al., 2011; Rebman et al., 2017; Wills et al., 2016). Figure 4 details the effects of B. burgdorferi s.l. on cells and tissues during this late stage of the disease.
Fig. 4.
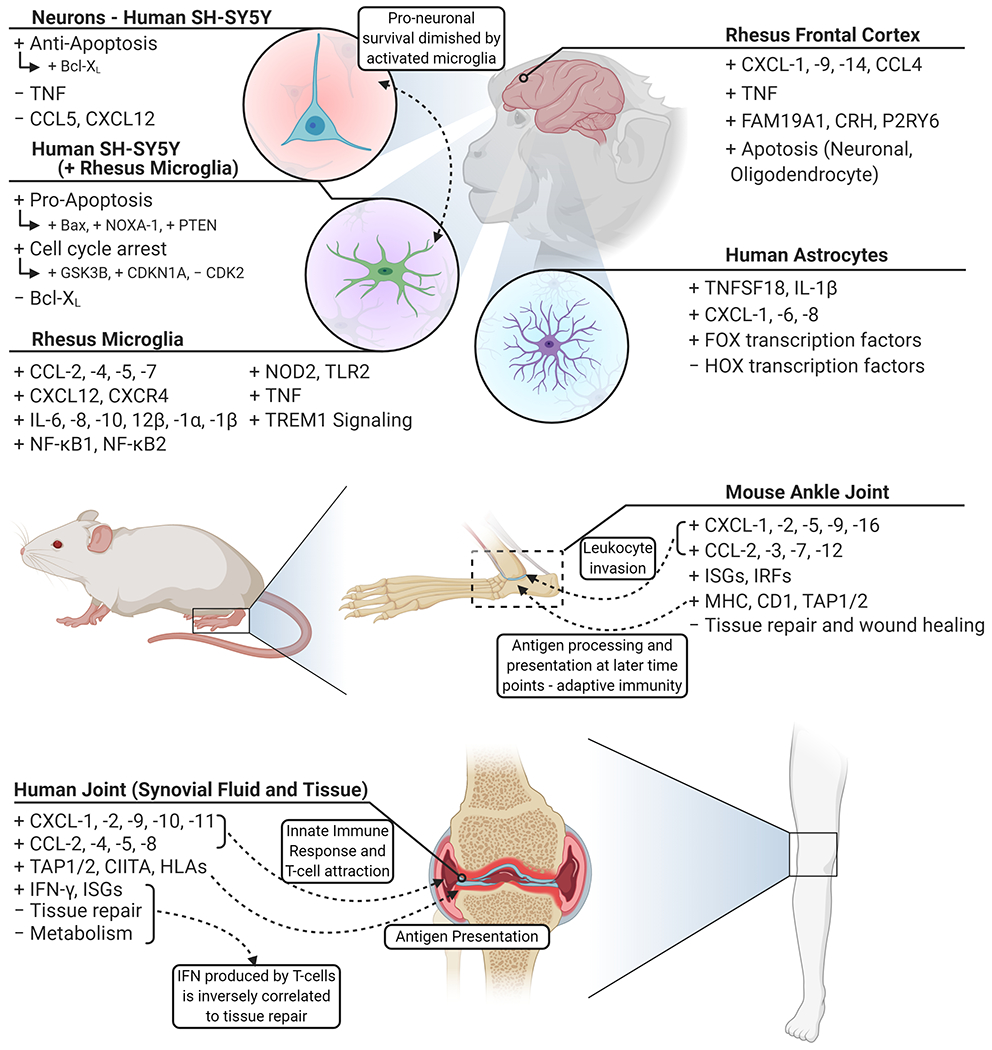
An overview of the transcriptional response of late disseminated infection. Following hematogenous dissemination, the central nervous system and joints are common regions for B. burgdorferi s.l. to colonize. Frontal cortex tissue responds in a diverse manner representative of resident cells – highlighted by inflammatory cytokines and marked by neuronal and oligodendrocyte apoptosis. SH-SY5Y neuronal cells indicate minimal response to infection and are inclined towards a pro-survival profile. Through the activation of microglia, marked by inflammatory factors, SH-SY5Y cells shift towards cell cycle arrest and pro-apoptotic profiles. Astrocytes promote a less robust immune response compared to microglia and show a shift in transcriptional regulation. B. burgdorferi invade the synovial fluid and surrounding tissue of joints, leading to the induction of chemotactic cytokines and subsequent invasion of leukocytes – an indication of Lyme arthritis. Created with BioRender.com.
Joint
Following dissemination of B. burgdorferi s.l. into the joints, a common outcome of late-disseminated infection is Lyme arthritis (LA). Like other symptoms, arthritis usually resolves following antibiotic treatment in patients; however, tissue damage and inflammation may persist within the joint and synovial tissue despite successful bacterial clearance (Arvikar and Steere, 2015; Li et al., 2011). A series of studies performed by Lochhead et al. characterized the transcriptome profile of synovial tissue and synovial fluid from patients with postinfectious LA and compared the gene expression to other forms of arthritis, including rheumatoid arthritis (RA) and osteoarthritis (OA) (Lochhead et al., 2019a). When compared, the gene expression profile of LA showed greater similarity to more chronic forms of arthritis such as RA, in contrast to OA, a more degenerative arthritis. Pathway analysis of differentially expressed genes between LA and OA further distinguish the two conditions in which pathways associated with immune disorders and immune activation were highly enriched within the LA profile. Conversely, pathway analysis of LA and other forms of chronic inflammatory arthritides show a pattern of innate and adaptive immune activation in which genes related to antigen processing, cell mediated cytotoxicity, and chemokine signaling were upregulated. Even in the absence of an active infection, the expression of pattern recognition receptors and inflammatory chemokines were consistently upregulated in LA patients. In contrast to these other forms of inflammatory conditions, the gene expression profiles of LA synovial tissue show a downregulation of genes related to tissue repair and metabolism.
A strong interferon gamma profile was observed in which 37% of all genes upregulated were interferon-regulated genes when compared to OA and 52% when compared to other chronic inflammatory arthritis conditions. Such a robust interferon related response has been observed before, however, in this study Lochhead et al. showed an inversely correlation of the interferon response to tissue repair mechanisms in LA patients. This relationship, in conjunction to a positive correlation to other inflammatory profiles, provides an explanation in disease pathology from initial infection towards tissue degradation and a more chronic inflammatory state observed in LA patients. Importantly, these findings in gene expression were confirmed at the protein level. Protein measurements from serum, synovial fluid, and synovial tissue from LA patients were found to have elevated chemokines and cytokines associated with the innate and adaptive immune response that include IFNγ, IL-6, CXCL9, and CXCL10.
IFN-γ had been previously shown to be an important factor in determining the host response to B. burgdorferi towards a more inflammatory state (Dame et al., 2007b; Gergel and Furie, 2004; Gross et al., 1998). This characteristic IFN-γ response observed in the synovial tissue of postinfectious LA patient was found to also be a predominate factor in the inflammatory determination of the disease (Lochhead et al., 2019b). Characterization of joint lesions in these patients have identified an abundance of inflammatory cell infiltrates (Gross et al., 1998; Lochhead et al., 2019a). In fact, CD4+ and CD8+ T cells are typically seen to be the most common type of infiltrating cells within joints of LA patients (Lochhead et al., 2019b). Lochhead et al. identified that subsets of these lymphocytes, including subsets of DN T cells and CD56+ NK cells, were sources of active production of IFN-γ within the synovial tissue. Synovial fluid analysis further established the presence of several chemokines and cytokines including IFN-γ, CXCL10, TNFα, and IL-6. The role of IFN-γ was previously established to be an important factor in the differentiation of fibroblast-like synoviocytes (FLS) into immune effector cells within the synovium of LA patients (Bartok and Firestein, 2010; Lochhead et al., 2012). When FLS were treated in vitro with B. burgdorferi, minimal changes in gene expression were observed (Lochhead et al., 2019b). However, when treated with IFN-γ, a robust upregulation of genes associated with chronic inflammation and autoimmune disorders were observed and, with the addition of B. burgdorferi, this was further expanded to a similar profile found within the synovial tissue of postinfectious LA patients (Lochhead et al., 2019a, 2019b).
MicroRNAs (miRNA), which are small non-coding RNAs, aid in the regulation of gene expression through the inhibition of translation. Expression of miRNAs have the ability to profoundly impact the host inflammatory and immune response in bacterial infections, as well as chronic inflammatory disorders such as RA (Kurowska-Stolarska et al., 2011; Okada et al., 2016; Zhou et al., 2018). In another study by Lochhead et al., the miRNA profile of synovial tissue from LA patients was found to be more conducive towards inflammation and cellular proliferation when compared to OA (Lochhead et al., 2017). Many of the miRNAs expressed in postinfectious LA synovial tissue was also found to be highly expressed within the synovial fluid of postantibiotic LA patients, including miR-146a and miR-155, which are anti-inflammatory and pro-inflammatory, respectively (Boldin and Baltimore, 2012; Jurkin et al., 2010; O’Connell et al., 2012, 2007; Yamasaki et al., 2009). This is supported within a murine model that found miR-146a to be upregulated specifically within the joints of B6, C3H, and B6 IL10 −/− mice, while interestingly, miR-155 was only upregulated within IL10 −/− mice (Lochhead et al., 2015, 2014). These miRNAs are associated with the TLR and NF-κB inflammatory response, while other upregulated miRNAs showed expression in association with myeloid function and acute inflammation (miR-142 and miR-223) (Boldin and Baltimore, 2012). Additionally, miR-17 and miR-20a, both oncogenic, were found to be upregulated, while the tumor suppressor miRNAs let-7a and let-7c, were downregulated (Gruszka and Zakrzewska, 2018; Lee et al., 2016).
Together, the inflammatory and immune gene expression profile, marked by a robust interferon response and cellular infiltration, as well as the dysregulation of miRNA associated with inflammatory pathways, suggests a dysregulation of the immune response and thus suggests a mechanism for the perpetuation of a chronic inflammatory state within the synovium of postinfectious LA patients. Further characterization of tissue also highlights the macroscopic outcomes of this molecular profile with the invasion of lymphocytes, specifically IFN-γ producing T-cells, and inflammation and tumor-like proliferation within the joints (Lochhead et al., 2019a, 2017; Strle et al., 2017).
The findings by Lochhead et al. share several similarities with previous experiments that utilized a murine (Mus musculus) model of Lyme arthritis to determine the gene expression within the joints of mice in response to B. burgdorferi, including an inverse correlation between IFN-inducible genes and tissue repair gene expression (Crandall et al., 2006). Crandall et al. (2006) used two strains of mice that developed either severe arthritis (C3H) or mild disease (C57BL/6), as well as C578BL/6-IL-10−/− that lacked the anti-inflammatory cytokine IL-10, to study this outcome (Brown and Reiner, 1998; Ma et al., 1998).
Across several time points, the transcriptome profile of the three mice strains showed increasing similarities determined by significant inflammatory response and immune cell infiltration that was evident through gene expression and histological analysis. A robust leukocyte chemoattractant profile was also observed: PMN-recruiting (CXCL1, CXCL2, CXCL5), mononuclear cell-recruiting (CCL2, CCL3, CCL7, CCL12), and T cell-recruiting (CXCL9, CXCL16, CCL2, CCL7). Although it has been established that B. burgdorferi activates the production of several pro-inflammatory cytokines such as TNF-α, IL-1β, or IFN-γ in humans, through the regulation of NF-κB, only IL-1β was observed in the joints of C3H and C57BL/6 mice. In contrast, many of these inflammatory cytokines were significantly induced in IL-10−/− mice; this further suggests that IL-10 plays an important role in the mediation of the immune and inflammatory response. Furthermore, several interferon-inducible genes were found to be overexpressed across all three strains, though the response was either transient (C3H) or delayed (C57BL/6) when compared to the robust response in IL-10−/− mice. Although IL-10−/− mice were able to more effectively clear the bacterial growth, the associated increase in pro-inflammatory cytokines and interferon-inducible genes correlated to more severe arthritis (Crandall et al., 2006).
Brain parenchyma (rhesus macaque)
Neuroborreliosis is defined by the invasion of the CNS by B. burgdorferi s.l., leading to an inflammatory response and subsequent neurological manifestations of the disease. Due to the disease severity and difficulty in treatment of persistent symptoms, many studies have been aimed to understand the mechanisms that lead to this pathology. The rhesus macaque has long been used as a model for neuroborreliosis due to the similarities of disease pathogenesis including CNS infection, CSF pleocytosis, and inflammation of the meninges (England et al., 1997; Roberts et al., 1998). Ramesh et al. (2008) utilized an ex vivo model of rhesus macaque brain cortex slices to determine the inflammatory profile induced by B. burgdorferi and its impact on neuronal and glial apoptosis. A large number of immune-related genes were found to be upregulated, and in contrast to the peripheral immune response, a number of these genes were found to be CNS specific: FAM19A1, a member of the C-C-chemokine family that is predominantly expressed within the brain as a regulator of immune and nervous cells; Corticotropin-releasing hormone (CRH), a hormone that is produced and secreted from the hypothalamus in response to infection and stress; and P2RY6, a receptor involved in microglial activation (Fu et al., 2014; Webster et al., 1997). Additionally, common inflammatory and immune factors including TNF-α, CXCL1, CXCL9, CXCL14, and CCL4 were upregulated. In response to B. burgdorferi, several genes related to cellular death and apoptosis were significantly upregulated within the host tissue. CHCHD6 which regulates mitochondria-mediated apoptosis, and ELAVL4, involved in inflammation and neuronal death, were found to be upregulated. Neuronal and oligodendrocyte apoptosis was further confirmed following B. burgdorferi infection through TUNEL and activated caspase-3 assays (Ramesh et al., 2008).
Microglia (rhesus macaque), and SH-SY5Y neurons
Further characterization of the apoptotic fate of neuronal cells was performed by Myers et al. (2009), in which primary rhesus macaque microglia and astrocytes, and human SH-SY5Y neuroblastoma cells were co-cultured with B. burgdorferi either alone or in combination. Astrocytes and microglia are the main resident glial cells of the brain and serve a number of supportive functions, with microglia acting as the resident macrophage. Alone, astrocytes and neuronal cells showed minimal inflammatory activation following B. burgdorferi infection. In contrast, microglia and co-culture of cells showed a robust increase in inflammatory chemokines. Microarray analysis of B. burgdorferi infected microglia indicates a similar response that is seen in peripheral immune cells with the induction of CCL and CXCL cytokines, including CCL-2, -4, -5, and -7, and CXCL12. Additionally, the inflammatory related factors IL-6, -8, -10, and -1β, and TNF were found to be significantly elevated. As many of these genes work in concert, the top enriched pathways involved TREM1 signaling, IL-6 and IL-10 signaling, and pattern recognition receptors. Co-culturing microglia and SH-SY5Y cells together following infection, led to the increase in the expression of pro-apoptotic genes (Bax, NOXA-1) and a decrease in anti-apoptotic gene expression (Bcl-XL) within the neuronal cells. These data suggest that the inflammatory state of the CNS that is generated by resident glial cells can lead to neuronal death and provides insight into the pathogenesis of neuroborreliosis.
Astrocytes
Though Myers et al. indicated that astrocytes may not exhibit a robust inflammatory response compared to microglia, astrocytes provide important trophic support to neurons, and their dysfunction could impact the homeostasis of the brain. Another study performed by our laboratory, Casselli et al. (2017), investigated the role of astrocytes in the exacerbation of neuroborreliosis through the use of RNA-seq. Astrocytes are the most abundant cell in the brain and play a role in a wide variety of functions (Pekny and Pekna, 2014). Their functions include nutrient support, neurotransmitter recycling, aiding in synaptic remodeling and plasticity, structural support which includes the formation of the blood-brain barrier, and respond to CNS injury and infection through inflammatory and immune modulation (Khakh and Sofroniew, 2015; Pekny and Pekna, 2014).
Casselli et al. used primary human astrocytes in vitro stimulated by Bb for 24 and 48 hours. A total of 275 transcripts were found to be differential expressed across both time points with significant overlap between them (221 DEGs). Tumor necrosis factor superfamily, member 18 (TNFSF18) showed the strongest induction. Additional inflammatory and immune related genes were upregulated that include chemokines CXCL1, CXCL6, and CXCL8 as well as ERAP2 which is involved in innate immunity and antigen processing and presentation (Castro and A, 2018). In line with the broad nature of astrocyte functionality, biological and functional analysis indicated genes were involved in nervous system development, angiogenesis, and cell adhesion. The role of miRNA in the regulation of gene expression was also investigated, with several miRNAs being differentially expressed that are predicted to be involved with MAPK signaling, cell adhesion, and adherens junctions. While these experiments indicated an inflammatory and immune response following B. burgdorferi infection, it seems the response was less concerted compared to more specialized immune cells. This may suggest and provide credibility to disease severity that correlates to the invasion of peripheral immune cells into the CNS, as seen in the CSF of neuroborreliosis patients. Such an outcome may lead to a dysregulation in astrocytic function and result in the exacerbation of a more persistent inflammatory environment within the CNS.
Conclusion
The use and expansion of transcriptomics approach allows researchers the ability to acquire large data sets of both cellular and systemic environments that allow for identification of novel pathways that may be associated with a specific biosignature or biomarker of a disease. This is the case with Lyme borreliosis, where transcriptome profiling is becoming more common place and provides additional insight into the mechanisms that regulate phenotypic change.
There are many different types of models utilized in the studies reviewed, including in vitro, ex vivo, and in vivo in a humans, mice, and rhesus macaque – each are unique in their benefits and limitations. Many of the in vitro studies benefit from the precision of environmental manipulation that can be performed and provide a reductionist method of research. In Thompson et al. (2020), purified cultures of choroid plexus epithelia cells can be tested to determine their specific role in the disease pathology of Lyme borreliosis and investigate its role in CNS dissemination. However, this study and others like it are severely limited due to the lack of systemic and biological context – without the positive or negative feedback provided by other cell types, it is difficult to determine the extent of alterations that may take place in vivo. This context dependent outcome is highlighted by Lochhead et al. (2019) when stimulation of FLS by B. burgdorferi only shows minimal enrichment of genes; in contrast, with the addition of IFN-γ, which would normally be produced by infiltrating T-cells, the transcriptome profile is greatly expanded and was found to be highly similar to that of postinfectious LA patients. While animal models provide this biological context, it is important to consider their limitations as well. Mice (Mus musculus) are a common model to study the pathogenesis of Lyme borreliosis but have significant differences in both innate and adaptive immunity compared to humans (Mestas and Hughes, 2004). Furthermore, several important immune mediators are absent in mice, including IL-8, CXCL11 (IFN-inducible T-cell chemoattractant), and several other chemokines. This is an important distinction when determine significance of pathways involved in disease pathology, as several processes do not occur in mice – in humans, STAT4 activation allows for the induction of Th1 development through IFN-α, however, this process does not occur in mice due to the failure of STAT4 activation (Farrar et al., 2000; Mestas and Hughes, 2004).
While this review highlighted the outcomes of transcriptome profiling in several Lyme borreliosis models, insights into the mechanisms of the immune response and disease progression can only be made when such data is correlated to protein translation within the same experimental conditions. There are many mechanisms in which gene expression and protein translation can be regulated and it is by no means a certainty that a change in gene expression would positively correlate to the increase in protein production. In fact, a recent publication utilizing integrative transcriptomic and proteomic analysis of live B. burgdorferi (Bb4680) in rhesus macaque frontal cortex implants found that the correlation between transcripts and protein levels was low (Ding et al., 2020). In other words, the abundance and presence of specific mRNAs does not indicate similar abundance of their associated proteins. Several studies mentioned in this review lacked appropriate protein level assessment – Schramm et al. (2012), Meddeb et al. (2016), Bouquet et al. (2016), Crandall et al. (2006), and as such, extrapolation to clinical relevance should be cautiously considered. It is common, however, to determine the secretion of chemokines and cytokines within the supernatant of in vitro experiments or through the utilization of immunohistochemistry as seen in Hartiala et al. (2007), Myers et al. (2009), Casselli et al. (2017), Ramesh et al. (2008) and Thompson et al. (2020); however, it is important to note that these methods are inherently limited based on the number of proteins assessed. Lochhead et al. utilized several methods to determine the validation of gene expression to protein levels through flow cytometric analysis, cytokine/chemokine quantification via multiplex assays, and immunofluorescence. Whereas Carreras-González et al. (2018) performed mass spectrometry to determine correlation between gene expression and protein levels. All methods have their limitations, but it is critical to take these methods into consideration when interpreting transcriptome data of past and future studies.
Many of these studies reviewed in this paper corroborate an overarching observation in Lyme borreliosis patients in which 1) Time between treatment and onset of symptoms predicts disease severity and 2) The host immune response, specifically a robust adaptive immunity marked by immune cell infiltration and pro-inflammatory factors, correlates to a more severe pathology. It is no surprise that these two observations can be simplified as one of the same. A prompt and effective antibiotic treatment is highly indicative of patient symptoms as it prevents the pathogen from greater dissemination and impact of secondary tissue, where many of the late stage symptoms occur i.e. arthritis, CNS inflammation and subsequent neurological pathology, and carditis. However, in many cases, the host innate immune response and delayed treatment fail to halt the dissemination of the pathogen early enough to prevent a stronger acquired immunity which may lead to these symptoms.
Omic-level studies provide significant insight into pathways affected during Lyme borreliosis that allows for theorization of targets for therapeutic research. In studies mentioned above, TNF and associated pathways such as TREM1 were abundantly observed to be enriched following infection. Previous therapeutic strategies aim for an anti-inflammatory approach, and indeed, treatments for an anti-TNF-a treatment have been proposed in the past. As these studies become even more wide-spread within the Lyme borreliosis field, as well as other infectious diseases, a greater emphasis on meta-analysis of these large data sets will become invaluable to understand the disease at a systemic level (McSweegan, 2007; Steere and Angelis, 2006). As Lyme borreliosis becomes a greater public concern, the utilization of these large datasets will become more important. Meta-analysis of all these datasets will provide a larger, systemic overview in the pathogenesis of the disease and provide information for targeted therapeutics.
References
- Acosta Davila JA, Hernandez De Los Rios A, 2019. An overview of peripheral blood mononuclear cells as a model for immunological research of toxoplasma gondii and other apicomplexan parasites. Front. Cell. Infect. Microbiol 9. 10.3389/fcimb.2019.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aerts-Toegaert C, Heirman C, Tuyaerts S, Corthals J, Aerts JL, Bonehill A, Thielemans K, Breckpot K, 2007. CD83 expression on dendritic cells and T cells: correlation with effective immune responses. Eur. J. Immunol 37, 686–695. 10.1002/eji.200636535 [DOI] [PubMed] [Google Scholar]
- Arvikar SL, Steere AC, 2015. Diagnosis and treatment of Lyme arthritis. Infect. Dis. Clin. North Am, 29, 269–280. 10.1016/j.idc.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autissier P, Soulas C, Burdo TH, Williams KC, 2010. Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans. Cytom. Part J. Int. Soc. Anal. Cytol 77, 410–419. 10.1002/cyto.a.20859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM, 1998. Dendritic cells and the control of immunity. Nature 392, 245–252. 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- Bartok B, Firestein GS, 2010. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev 233, 233–255. 10.1111/j.0105-2896.2009.00859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartůnĕk P, Gorican K, Veiser T, Táborský M, Hulínská D, 2007. Significance of Borrelia infection in development of dilated cardiomyopathy (a pilot study). Prague Med. Rep 108, 339–347. [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ, 1997. A new class of membrane-bound chemokine with a CX3C motif. Nature 385, 640–644. 10.1038/385640a0 [DOI] [PubMed] [Google Scholar]
- Behera AK, Hildebrand E, Scagliotti J, Steere AC, Hu LT, 2005. Induction of host matrix metalloproteinases by Borrelia burgdorferi differs in human and murine Lyme arthritis. Infect. Immun 73, 126–134. 10.1128/IAI.73.1.126-134.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berende A, Oosting M, Kullberg B-J, Netea MG, Joosten LA, 2010. Activation of innate host defense mechanisms by Borrelia. Eur. Cytokine Netw 21, 7–18. 10.1684/ecn.2009.0179 [DOI] [PubMed] [Google Scholar]
- Bernardino ALF, Myers TA, Alvarez X, Hasegawa A, Philipp MT, 2008. Toll-like receptors: Insights into their possible role in the pathogenesis of Lyme neuroborreliosis. Infect. Immun 76, 4385–4395. 10.1128/IAI.00394-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesiada G, Czepiel J, Leśniak MR, Garlicki A, Mach T, 2012. Lyme disease: review. Arch. Med. Sci. AMS 8, 978–982. 10.5114/aoms.2012.30948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar I, Lally EV, 2008. Musculoskeletal manifestations of Lyme disease. Med. Health R. I 91, 213–215. [PubMed] [Google Scholar]
- Böggemeyer E, Stehle T, Schaible UE, Hahne M, Vestweber D, Simon MM, 1994. Borrelia burgdorferi upregulates the adhesion molecules E-selectin, P-selectin, ICAM-1 and VCAM-1 on mouse endothelioma cells in vitro. Cell Adhes. Commun 2, 145–157. 10.3109/15419069409004433 [DOI] [PubMed] [Google Scholar]
- Boldin MP, Baltimore D, 2012. MicroRNAs, new effectors and regulators of NF-κB. Immunol. Rev 246, 205–220. 10.1111/j.1600-065X.2011.01089.x [DOI] [PubMed] [Google Scholar]
- Bouquet J, Soloski MJ, Swei A, Cheadle C, Federman S, Billaud J-N, Rebman AW, Kabre B, Halpert R, Boorgula M, Aucott JN, Chiu CY, 2016. Longitudinal transcriptome analysis reveals a sustained differential gene expression signature in patients treated for acute Lyme disease. mBio 7. 10.1128/mBio.00100-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR, Reiner SL, 1998. Clearance of Borrelia burgdorferi may not be required for resistance to experimental Lyme arthritis. Infect. Immun 66, 2065–2071. 10.1128/IAI.66.5.2065-2071.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon PCE, Martin C, Rankin SM, 2005. The CXC chemokine MIP-2 stimulates neutrophil mobilization from the rat bone marrow in a CD49d-dependent manner. Blood 105, 2543–2548. 10.1182/blood-2004-08-3193 [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP, 1982. Lyme disease-a tick-borne spirochetosis? Science 216, 1317–1319. 10.1126/science.7043737 [DOI] [PubMed] [Google Scholar]
- Burns MJ, Furie MB, 1998. Borrelia burgdorferi and interleukin-1 promote the transendothelial migration of monocytes in vitro by different mechanisms. Infect. Immun 66, 4875–4883. 10.1128/IAI.66.10.4875-4883.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns MJ, Sellati TJ, Teng EI, Furie MB, 1997. Production of interleukin-8 (IL-8) by cultured endothelial cells in response to Borrelia burgdorferi occurs independently of secreted [corrected] IL-1 and tumor necrosis factor alpha and is required for subsequent transendothelial migration of neutrophils. Infect. Immun 65, 1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras-González A, Navasa N, Martín-Ruiz I, Lavín JL, Azkargorta M, Atondo E, Barriales D, Macías-Cámara N, Pascual-Itoiz MA, Sampedro L, Tomás-Cortázar J, Peña-Cearra A, Pellón A, Prados-Rosales R, Abecia L, Elortza F, Aransay AM, Rodríguez H, Anguita J, 2018. A multi-omic analysis reveals the regulatory role of CD180 during the response of macrophages to Borrelia burgdorferi. Emerg. Microbes Infect 7. 10.1038/s41426-017-0018-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casselli T, Qureshi H, Peterson E, Perley D, Blake E, Jokinen B, Abbas A, Nechaev S, Watt JA, Dhasarathy A, Brissette CA, 2017. MicroRNA and mRNA transcriptome profiling in primary human astrocytes infected with Borrelia burgdorferi. PLoS ONE 12. 10.1371/journal.pone.0170961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli E, Caputo V, Morello V, Tomasino RM, 2008. Local reactions to tick bites. Am. J. Dermatopathol 30, 241–248. 10.1097/DAD.0b013e3181676b60 [DOI] [PubMed] [Google Scholar]
- Castro L. de, A, J., 2018. How ERAP1 and ERAP2 shape the peptidomes of disease-associated MHC-I proteins. Front. Immunol 9. 10.3389/fimmu.2018.02463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston TE, Mercer E, de Silva M, Hazleman BL, 1984. Metalloproteinases and collagenase inhibitors in rheumatoid synovial fluid. Arthritis Rheum. 27, 285–290. 10.1002/art.1780270306 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (U.S.). Center for Surveillance, E., and Laboratory Services. National Notifiable Diseases Surveillance System. 2019. Nationally Notifiable Infectious Diseases and Conditions, United States: Annual Tables.Table 2i. Listeriosis; Lyme disease; Malaria; Measles. Nationally Notifiable Infectious Diseases and Conditions, United States: Annual Tables 2018. [Google Scholar]
- Cerar T, Strle F, Stupica D, Ruzic-Sabljic E, McHugh G, Steere AC, Strle K, 2016. Differences in genotype, clinical features, and inflammatory potential of Borrelia burgdorferi sensu stricto Strains from Europe and the United States. Emerg. Infect. Dis 22, 818–827. 10.3201/eid2205.151806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM, 2006. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med 354, 610–621. 10.1056/NEJMra052723 [DOI] [PubMed] [Google Scholar]
- Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K, 1998. Interferon–inducible T cell alpha chemoattractant (I-TAC): A novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J. Exp. Med 187, 2009–2021. 10.1084/jem.187.12.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, 2019. TREM1 blockade: Killing two birds with one stone. Trends Immunol. 40, 781–783. 10.1016/j.it.2019.07.008 [DOI] [PubMed] [Google Scholar]
- Cope AP, 2002. Studies of T-cell activation in chronic inflammation. Arthritis Res. 4, S197–S211. 10.1186/ar557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall H, Dunn DM, Ma Y, Wooten RM, Zachary JF, Weis JH, Weiss RB, Weis JJ, 2006. Gene expression profiling reveals unique pathways associated with differential severity of Lyme arthritis. J. Immunol 177, 7930–7942. 10.4049/jimmunol.177.11.7930 [DOI] [PubMed] [Google Scholar]
- Dame TM, Orenzoff BL, Palmer LE, Furie MB, 2007a. IFN-γ alters the response of Borrelia burgdorferi-activated endothelium to favor chronic inflammation. J. Immunol 178, 1172–1179. 10.4049/jimmunol.178.2.1172 [DOI] [PubMed] [Google Scholar]
- Dame TM, Orenzoff BL, Palmer LE, Furie MB, 2007b. IFN-gamma alters the response of Borrelia burgdorferi-activated endothelium to favor chronic inflammation. J. Immunol. Baltim. Md 1950 178, 1172–1179. 10.4049/jimmunol.178.2.1172 [DOI] [PubMed] [Google Scholar]
- Das S, Banerjee G, DePonte K, Marcantonio N, Kantor FS, Fikrig E, 2001. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J. Infect. Dis 184, 1056–1064. 10.1086/323351 [DOI] [PubMed] [Google Scholar]
- de Koning J, 1993. Histopathologic patterns of erythema migrans and borrelial lymphocytoma. Clin. Dermatol 11, 377–383. 10.1016/0738-081x(93)90093-r [DOI] [PubMed] [Google Scholar]
- Ding Z, Sun L, Bi Y, Zhang Y, Yue P, Xu X, Cao W, Luo L, Chen T, Li L, Ji Z, Jian M, Lu L, Abi M-E, Liu A, Bao F, 2020. Integrative transcriptome and proteome analyses provide new insights into the interaction between live Borrelia burgdorferi and frontal cortex explants of the rhesus brain. J. Neuropathol. Exp. Neurol 79, 518–529. 10.1093/jnen/nlaa015 [DOI] [PubMed] [Google Scholar]
- Divan A, Budd RC, Tobin RP, Newell-Rogers MK, 2015. γδ T Cells and dendritic cells in refractory Lyme arthritis. J. Leukoc. Biol 97, 653–663. 10.1189/jlb.2RU0714-343RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic M, Schmidt-Samoa C, Lange P, Spreer A, Neubieser K, Eiffert H, Nau R, Schmidt H, 2012. Cerebrospinal fluid findings in adults with acute Lyme neuroborreliosis. J. Neurol 259, 630–636. 10.1007/s00415-011-6221-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duray PH, 1989. Histopathology of clinical phases of human Lyme disease. Rheum. Dis. Clin. North Am 15, 691–710. [PubMed] [Google Scholar]
- Duray PH, Steere AC, 1988. Clinical pathologic correlations of Lyme disease by stage. Ann. N. Y. Acad. Sci 539, 65–79. 10.1111/j.1749-6632.1988.tb31839.x [DOI] [PubMed] [Google Scholar]
- Ebnet K, Brown KD, Siebenlist UK, Simon MM, Shaw S, 1997. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J. Immunol. Baltim. Md 1950 158, 3285–3292. [PubMed] [Google Scholar]
- Eikeland R, Mygland A, Herlofson K, Ljøstad U, 2011. European neuroborreliosis: quality of life 30 months after treatment. Acta Neurol. Scand 124, 349–354. 10.1111/j.1600-0404.2010.01482.x [DOI] [PubMed] [Google Scholar]
- Elbaum-Garfinkle S, 2011. Close to home: A history of Yale and Lyme Disease. Yale J. Biol. Med 84, 103–108. [PMC free article] [PubMed] [Google Scholar]
- England JD, Bohm RP, Roberts ED, Philipp MT, 1997. Lyme neuroborreliosis in the rhesus monkey. Semin. Neurol 17, 53–56. 10.1055/s-2008-1040913 [DOI] [PubMed] [Google Scholar]
- Farrar JD, Smith JD, Murphy TL, Leung S, Stark GR, Murphy KM, 2000. Selective loss of type I interferon-induced STAT4 activation caused by a minisatellite insertion in mouse Stat2. Nat. Immunol 1, 65–69. 10.1038/76932 [DOI] [PubMed] [Google Scholar]
- Fedele G, Frasca L, Palazzo R, Ferrero E, Malavasi F, Ausiello CM, 2004. CD38 is expressed on human mature monocyte-derived dendritic cells and is functionally involved in CD83 expression and IL-12 induction. Eur. J. Immunol 34, 1342–1350. 10.1002/eji.200324728 [DOI] [PubMed] [Google Scholar]
- Ferenbach D, Hughes J, 2008. Macrophages and dendritic cells: what is the difference? Kidney Int. 74, 5–7. 10.1038/ki.2008.189 [DOI] [PubMed] [Google Scholar]
- Filgueira L, Nestlé FO, Rittig M, Joller HI, Groscurth P, 1996. Human dendritic cells phagocytose and process Borrelia burgdorferi. J. Immunol 157, 2998–3005. [PubMed] [Google Scholar]
- Fontana MF, Banga S, Barry KC, Shen X, Tan Y, Luo Z-Q, Vance RE, 2011. Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS Pathog. 7, e1001289. 10.1371/journal.ppat.1001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg P, Ernerudh J, Ekerfelt C, Roberg M, Vrethem M, Bergström S, 1995. The outer surface proteins of Lyme disease borrelia spirochetes stimulate T cells to secrete interferon-gamma (IFN-gamma): diagnostic and pathogenic implications. Clin. Exp. Immunol 101, 453–460. 10.1111/j.1365-2249.1995.tb03134.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche J, Moser M, Faust S, Peuker A, Büttner R, Andreesen R, Kreutz M, 2000. Molecular cloning and characterization of a human metalloprotease disintegrin--a novel marker for dendritic cell differentiation. Blood 96, 732–739. [PubMed] [Google Scholar]
- Fu R, Shen Q, Xu P, Luo JJ, Tang Y, 2014. Phagocytosis of Microglia in the Central Nervous System Diseases. Mol. Neurobiol 49, 1422–1434. 10.1007/s12035-013-8620-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbia JA, Coleman JL, Benach JL, 2001. Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase b (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect. Immun 69, 456–462. 10.1128/IAI.69.1.456-462.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergel EI, Furie MB, 2004. Populations of human T lymphocytes that traverse the vascular endothelium stimulated by Borrelia burgdorferi are enriched with cells that secrete gamma interferon. Infect. Immun 72, 1530–1536. 10.1128/IAI.72.3.1530-1536.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk C, Kurts C, 2015. The debate about dendritic cells and macrophages in the kidney. Front. Immunol 6. 10.3389/fimmu.2015.00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DM, Steere AC, Huber BT, 1998. T helper 1 response is dominant and localized to the synovial fluid in patients with Lyme arthritis. J. Immunol 160, 1022–1028. [PubMed] [Google Scholar]
- Gruszka R, Zakrzewska M, 2018. The oncogenic relevance of miR-17-92 cluster and its paralogous miR-106b-25 and miR-106a-363 clusters in brain tumors. Int. J. Mol. Sci 19. 10.3390/ijms19030879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Booth CJ, Paley MA, Wang X, DePonte K, Fikrig E, Narasimhan S, Montgomery RR, 2009. Inhibition of neutrophil function by two tick salivary proteins. Infect. Immun 77, 2320–2329. 10.1128/IAI.01507-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartiala P, Hytönen J, Pelkonen J, Kimppa K, West A, Penttinen MA, Suhonen J, Lahesmaa R, Viljanen MK, 2007. Transcriptional response of human dendritic cells to Borrelia garinii—defective CD38 and CCR7 expression detected. J. Leukoc. Biol 82, 33–43. 10.1189/jlb.1106709 [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Kirschning CJ, Schwandner R, Wesche H, Weis JH, Wooten RM, Weis JJ, 1999. Cutting edge: Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol 163, 2382–2386. [PubMed] [Google Scholar]
- Hourcade DE, Akk AM, Mitchell LM, Zhou H, Hauhart R, Pham CTN, 2016. Anti-complement activity of the Ixodes scapularis salivary protein Salp20. Mol. Immunol 69, 62–69. 10.1016/j.molimm.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, Eskildsen MA, Masgala C, Steere AC, Arner EC, Pratta MA, Grodzinsky AJ, Loening A, Perides G, 2001. Host metalloproteinases in Lyme arthritis. Arthritis Rheum. 44, 1401–1410. [DOI] [PubMed] [Google Scholar]
- Hubálek Z, 2009. Epidemiology of Lyme borreliosis. Lyme Borreliosis 37, 31–50. 10.1159/000213069 [DOI] [PubMed] [Google Scholar]
- Hulínska D, Barták P, Hercogová J, Hančil J, Bašta J, Schramlová J, 1994. Electron microscopy of Langerhans cells and Borrelia burgdorferi in Lyme disease patients. Zentralblatt Für Bakteriol. 280, 348–359. 10.1016/S0934-8840(11)80597-9 [DOI] [PubMed] [Google Scholar]
- Jones CV, Ricardo SD, 2013. Macrophages and CSF-1. Organogenesis 9, 249–260. 10.4161/org.25676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Germain A, Riley KE, Bautista C, Taylor W, Wells AF, 1994. Borrelia burgdorferi decreases hyaluronan synthesis but increases IL-6 production by fibroblasts. Microb. Pathog 16, 261–267. 10.1006/mpat.1994.1027 [DOI] [PubMed] [Google Scholar]
- Jurkin J, Schichl YM, Koeffel R, Bauer T, Richter S, Konradi S, Gesslbauer B, Strobl H, 2010. miR-146a is differentially expressed by myeloid dendritic cell subsets and desensitizes cells to TLR2-dependent activation. J. Immunol. Baltim. Md 1950 184, 4955–4965. 10.4049/jimmunol.0903021 [DOI] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV, 2015. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci 18, 942–952. 10.1038/nn.4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedel U, Fingerle V, Pfister H-W, 2015. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat. Rev. Neurol 11, 446–456. 10.1038/nrneurol.2015.121 [DOI] [PubMed] [Google Scholar]
- Kotsyfakis M, Sá-Nunes A, Francischetti IMB, Mather TN, Andersen JF, Ribeiro JMC, 2006. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J. Biol. Chem 281, 26298–26307. 10.1074/jbc.M513010200 [DOI] [PubMed] [Google Scholar]
- Kruse M, Meinl E, Henning G, Kuhnt C, Berchtold S, Berger T, Schuler G, Steinkasserer A, 2001. Signaling lymphocytic activation molecule is expressed on mature CD83+ dendritic cells and is up-regulated by IL-1 beta. J. Immunol. Baltim. Md 1950 167, 1989–1995. 10.4049/jimmunol.167.4.1989 [DOI] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Alivernini S, Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna M, Fraser AR, Stolarski B, McSharry C, Hueber AJ, Baxter D, Hunter J, Gay S, Liew FY, McInnes IB, 2011. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc. Natl. Acad. Sci. U. S. A 108, 11193–11198. 10.1073/pnas.1019536108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Han S, Kwon CS, Lee D, 2016. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell 7, 100–113. 10.1007/s13238-015-0212-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, Steere AC, 2011. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum. 63, 2238–2247. 10.1002/art.30384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ju X, Silveira PA, Abadir E, Hsu W-H, Hart DNJ, Clark GJ, 2019. CD83: activation marker for antigen presenting cells and its therapeutic potential. Front. Immunol 10. 10.3389/fimmu.2019.01312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM, 1995. Human Mig chemokine: biochemical and functional characterization. J. Exp. Med 182, 1301–1314. 10.1084/jem.182.5.1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Kidder JM, Noring R, Steere AC, Klempner MS, Hu LT, 2001. Differences in synovial fluid levels of matrix metalloproteinases suggest separate mechanisms of pathogenesis in Lyme arthritis before and after antibiotic treatment. J. Infect. Dis 184, 174–180. 10.1086/322000 [DOI] [PubMed] [Google Scholar]
- Lochhead RB, Arvikar SL, Aversa JM, Sadreyev RI, Strle K, Steere AC, 2019a. Robust interferon signature and suppressed tissue repair gene expression in synovial tissue from patients with postinfectious, Borrelia burgdorferi-induced Lyme arthritis. Cell. Microbiol 21, e12954. 10.1111/cmi.12954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead RB, Ma Y, Zachary JF, Baltimore D, Zhao JL, Weis JH, O’Connell RM, Weis JJ, 2014. MicroRNA-146a provides feedback regulation of Lyme arthritis but not carditis during infection with Borrelia burgdorferi. PLoS Pathog. 10. 10.1371/journal.ppat.1004212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead RB, Ordoñez D, Arvikar SL, Aversa JM, Oh LS, Heyworth B, Sadreyev R, Steere AC, Strle K, 2019b. Interferon-gamma production in Lyme arthritis synovial tissue promotes differentiation of fibroblast-like synoviocytes into immune effector cells. Cell. Microbiol 21, e12992. 10.1111/cmi.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead RB, Sonderegger FL, Ma Y, Brewster JE, Cornwall D, Maylor-Hagen H, Miller JC, Zachary JF, Weis JH, Weis JJ, 2012. Endothelial cells and fibroblasts amplify the arthritogenic type I IFN response in murine Lyme disease and are major sources of chemokines in Borrelia burgdorferi-infected joint tissue. J. Immunol 189, 2488–2501. 10.4049/jimmunol.1201095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead RB, Strle K, Kim ND, Kohler MJ, Arvikar SL, Aversa JM, Steere AC, 2017. MicroRNA expression shows inflammatory dysregulation and tumor-like proliferative responses in joints of patients with post-infectious Lyme arthritis. Arthritis Rheumatol. n/a-n/a. 10.1002/art.40039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead RB, Zachary JF, Dalla Rosa L, Ma Y, Weis JH, O’Connell RM, Weis JJ, 2015. antagonistic interplay between MicroRNA-155 and IL-10 during Lyme carditis and arthritis. PLoS ONE 10. 10.1371/journal.pone.0135142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B, 1994. Monocyte chemotactic proteins MCP-1, MCP-2, and MCP-3 are major attractants for human CD4+ and CD8+ T lymphocytes. FASEB J. 8, 1055–1060. 10.1096/fasebj.8.13.7926371 [DOI] [PubMed] [Google Scholar]
- Luft BJ, Steinman CR, Neimark HC, Muralidhar B, Rush T, Finkel MF, Kunkel M, Dattwyler RJ, 1992. Invasion of the central nervous system by Borrelia burgdorferi in acute disseminated infection. JAMA 267, 1364–1367. [PubMed] [Google Scholar]
- Ma Y, Seiler KP, Eichwald EJ, Weis JH, Teuscher C, Weis JJ, 1998. Distinct characteristics of resistance to Borrelia burgdorferi-Induced arthritis in C57BL/6N mice. Infect. Immun 66, 161–168. 10.1128/IAI.66.1.161-168.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques A, 2008. Chronic Lyme disease: An appraisal. Infect. Dis. Clin. North Am 22, 341–360. 10.1016/j.idc.2007.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques A, Schwartz I, Wormser GP, Wang Y, Hornung RL, Demirkale CY, Munson PJ, Turk S-P, Williams C, Lee C-CR, Yang J, Petzke MM, 2018. Transcriptome assessment of erythema migrans skin lesions in patients with early Lyme disease reveals predominant interferon signaling. J. Infect. Dis 217, 158–167. 10.1093/infdis/jix563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MartIn-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F, 2003. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med 198, 615–621. 10.1084/jem.20030448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason LMK, Veerman CC, Geijtenbeek TBH, Hovius JWR, 2014. Ménage à trois: Borrelia, dendritic cells, and tick saliva interactions. Trends Parasitol. 30, 95–103. 10.1016/j.pt.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Mason LMK, Wagemakers A, Veer C. van ‘t, Oei A, Pot W.J. van der, Ahmed K, Poll T. van der, Geijtenbeek TBH, Hovius JWR, 2016. Borrelia burgdorferi induces TLR2-mediated migration of activated dendritic cells in an ex vivo human skin model. PLOS ONE 11, e0164040. 10.1371/journal.pone.0164040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweegan E, 2007. Anti-tumor necrosis factor-alpha treatment for Lyme borreliosis. J. Infect. Dis 196, 1866; author reply 1866-1867. 10.1086/523828 [DOI] [PubMed] [Google Scholar]
- Meddeb M, Carpentier W, Cagnard N, Nadaud S, Grillon A, Barthel C, De Martino SJ, Jaulhac B, Boulanger N, Schramm F, 2016. Homogeneous inflammatory gene profiles induced in human dermal fibroblasts in response to the three main species of Borrelia burgdorferi sensu lato. PLoS ONE 11. 10.1371/journal.pone.0164117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker RB, Bragg DC, Poulton W, Hudson L, 2012. Transmigration of macrophages across the choroid plexus epithelium in response to the feline immunodeficiency virus. Cell Tissue Res. 347, 443–455. 10.1007/s00441-011-1301-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CCW, 2004. Of Mice and Not Men: Differences between mouse and human immunology. J. Immunol 172, 2731–2738. 10.4049/jimmunol.172.5.2731 [DOI] [PubMed] [Google Scholar]
- Metzemaekers M, Vanheule V, Janssens R, Struyf S, Proost P, 2018. Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Front. Immunol 8. 10.3389/fimmu.2017.01970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro-García MA, Mayo JC, Sainz RM, Alonso-Arias R, 2018. Influence of inflammation in the process of t lymphocyte differentiation: proliferative, metabolic, and oxidative changes. Front. Immunol 9. 10.3389/fimmu.2018.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B, Ckark-Lewis I, Zwahlen R, Baggiolini M, 1990. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J. Exp. Med 171, 1797–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllegger RR, Means TK, Shin JJ, Lee M, Jones KL, Glickstein LJ, Luster AD, Steere AC, 2007. Chemokine signatures in the skin disorders of Lyme borreliosis in Europe: predominance of CXCL9 and CXCL10 in erythema migrans and acrodermatitis and CXCL13 in lymphocytoma. Infect. Immun 75, 4621–4628. 10.1128/IAI.00263-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers TA, Kaushal D, Philipp MT, 2009. Microglia Are Mediators of Borrelia burgdorferi–Induced Apoptosis in SH-SY5Y Neuronal Cells. PLoS Pathog. 5. 10.1371/journal.ppat.1000659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemunaitis J, 1993. Macrophage function activating cytokines: potential clinical application. Crit. Rev. Oncol. Hematol 14, 153–171. 10.1016/1040-8428(93)90022-V [DOI] [PubMed] [Google Scholar]
- O’Connell RM, Rao DS, Baltimore D, 2012. microRNA regulation of inflammatory responses. Annu. Rev. Immunol 30, 295–312. 10.1146/annurev-immunol-020711-075013 [DOI] [PubMed] [Google Scholar]
- O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D, 2007. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. U. S. A 104, 1604–1609. 10.1073/pnas.0610731104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Muramatsu T, Suita N, Kanai M, Kawakami E, Iotchkova V, Soranzo N, Inazawa J, Tanaka T, 2016. Significant impact of miRNA–target gene networks on genetics of human complex traits. Sci. Rep 6, 22223. 10.1038/srep22223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachner AR, Steere AC, 1985. The triad of neurologic manifestations of Lyme disease. Neurology 35, 47. 10.1212/WNL.35.1.47 [DOI] [PubMed] [Google Scholar]
- Park S-H, Moon Y, 2013. Integrated stress response-altered pro-inflammatory signals in mucosal immune-related cells. Immunopharmacol. Immunotoxicol 35, 205–214. 10.3109/08923973.2012.742535 [DOI] [PubMed] [Google Scholar]
- Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, Salmon M, Buckley CD, 2005. A stromal address code defined by fibroblasts. Trends Immunol. 26, 150–156. 10.1016/j.it.2004.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida-Sánchez S, Goodrich S, Kusser K, Oppenheimer N, Randall TD, Lund FE, 2004. Regulation of dendritic cell trafficking by the ADP-ribosyl cyclase CD38: impact on the development of humoral immunity. Immunity 20, 279–291. 10.1016/s1074-7613(04)00048-2 [DOI] [PubMed] [Google Scholar]
- Patente TA, Pinho MP, Oliveira AA, Evangelista GCM, Bergami-Santos PC, Barbuto JAM, 2019. Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy. Front. Immunol 9. 10.3389/fimmu.2018.03176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M, 2014. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol. Rev 94, 1077–1098. 10.1152/physrev.00041.2013 [DOI] [PubMed] [Google Scholar]
- Petzke MM, Brooks A, Krupna MA, Mordue D, Schwartz I, 2009. Recognition of Borrelia burgdorferi, the Lyme disease spirochete, by TLR7 and TLR9 induces a type I IFN response by human immune cells. J. Immunol 183, 5279–5292. 10.4049/jimmunol.0901390 [DOI] [PubMed] [Google Scholar]
- Pícha D, Moravcova L, Lasikova S, Holeckova D, Maresova V, 2006. Symptoms of post-Lyme syndrome in long-term outcome of patients with neuroborreliosis. Scand. J. Infect. Dis 38, 747–748. 10.1080/00365540600810000 [DOI] [PubMed] [Google Scholar]
- Ramesh G, Borda JT, Dufour J, Kaushal D, Ramamoorthy R, Lackner AA, Philipp MT, 2008. Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am. J. Pathol 173, 1415–1427. 10.2353/ajpath.2008.080483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, Santana-Gould L, Inglis FM, England JD, Philipp MT, 2013. The Lyme disease spirochete Borrelia burgdorferi induces inflammation and apoptosis in cells from dorsal root ganglia. J. Neuroinflammation 10, 88. 10.1186/1742-2094-10-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebman AW, Bechtold KT, Yang T, Mihm EA, Soloski MJ, Novak CB, Aucott JN, 2017. The clinical, symptom, and quality-of-life characterization of a well-defined group of patients with posttreatment Lyme disease syndrome. Front. Med 4. 10.3389/fmed.2017.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM, Weis JJ, Telford SR, 1990. Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp. Parasitol 70, 382–388. 10.1016/0014-4894(90)90121-r [DOI] [PubMed] [Google Scholar]
- Ritzman AM, Hughes-Hanks JM, Blaho VA, Wax LE, Mitchell WJ, Brown CR, 2010. The chemokine receptor CXCR2 Ligand KC (CXCL1) mediates neutrophil recruitment and is critical for development of experimental Lyme arthritis and carditis. Infect. Immun 78, 4593–4600. 10.1128/IAI.00798-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli A, Hauffe HC, Carpi G, Vourc’h GI, Neteler M, Rosà R, 2011. Lyme borreliosis in Europe. Eurosurveillance 16, 19906. 10.2807/ese.16.27.19906-en [DOI] [PubMed] [Google Scholar]
- Roberts ED, Bohm RP, Lowrie RC, Habicht G, Katona L, Piesman J, Philipp MT, 1998. Pathogenesis of Lyme neuroborreliosis in the rhesus monkey: the early disseminated and chronic phases of disease in the peripheral nervous system. J. Infect. Dis 178, 722–732. 10.1086/515357 [DOI] [PubMed] [Google Scholar]
- Roe K, Gibot S, Verma S, 2014. Triggering receptor expressed on myeloid cells-1 (TREM-1): a new player in antiviral immunity? Front. Microbiol 5. 10.3389/fmicb.2014.00627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault C, Pellegrinelli V, Schilch R, Cotillard A, Poitou C, Tordjman J, Sell H, Clément K, Lacasa D, 2013. Roles of Chemokine Ligand-2 (CXCL2) and neutrophils in influencing endothelial cell function and inflammation of human adipose tissue. Endocrinology 154, 1069–1079. 10.1210/en.2012-1415 [DOI] [PubMed] [Google Scholar]
- Saalbach A, Klein C, Sleeman J, Sack U, Kauer F, Gebhardt C, Averbeck M, Anderegg U, Simon JC, 2007. Dermal fibroblasts induce maturation of dendritic cells. J. Immunol. Baltim. Md 1950 178, 4966–4974. 10.4049/jimmunol.178.8.4966 [DOI] [PubMed] [Google Scholar]
- Salazar JC, Duhnam-Ems S, Vake CL, Cruz AR, Moore MW, Caimano MJ, Velez-Climent L, Shupe J, Krueger W, Radolf JD, 2009. Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-β. PLOS Pathog. 5, e1000444. 10.1371/journal.ppat.1000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar JC, Pope CD, Sellati TJ, Feder HM, Kiely TG, Dardick KR, Buckman RL, Moore MW, Caimano MJ, Pope JG, Krause PJ, Radolf JD, 2003. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J. Immunol 171, 2660–2670. 10.4049/jimmunol.171.5.2660 [DOI] [PubMed] [Google Scholar]
- Sá-Nunes A, Bafica A, Lucas DA, Conrads TP, Veenstra TD, Andersen JF, Mather TN, Ribeiro JMC, Francischetti IMB, 2007. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J. Immunol. Baltim. Md 1950 179, 1497–1505. 10.4049/jimmunol.179.3.1497 [DOI] [PubMed] [Google Scholar]
- Schmidt H, Djukic M, Jung K, Holzgraefe M, Dechent P, Steinbüchel N. von, Blocher J, Eiffert H, Schmidt-Samoa C, 2015. Neurocognitive functions and brain atrophy after proven neuroborreliosis: a case-control study. BMC Neurol. 15. 10.1186/s12883-015-0386-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm F, Kern A, Barthel C, Nadaud S, Meyer N, Jaulhac B, Boulanger N, 2012. Microarray analyses of inflammation response of human dermal fibroblasts to different strains of Borrelia burgdorferi sensu stricto. PLoS ONE 7. 10.1371/journal.pone.0040046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AM, 2017. Surveillance for Lyme Disease — United States, 2008–2015. MMWR Surveill. Summ 66. 10.15585/mmwr.ss6622a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerk C, Papandreou T, Schuhmann D, Nickol L, Borkowski J, Steinmann U, Quednau N, Stump C, Weiss C, Berger J, Wolburg H, Claus H, Vogel U, Ishikawa H, Tenenbaum T, Schroten H, 2012. Polar invasion and translocation of Neisseria meningitidis and Streptococcus suis in a novel human model of the blood-cerebrospinal fluid barrier. PLOS ONE 7, e30069. 10.1371/journal.pone.0030069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellati TJ, Burns MJ, Ficazzola MA, Furie MB, 1995. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect. Immun 63, 4439–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha N, Bahnan W, Wiley DJ, Barber G, Fields KA, Schesser K, 2012. Eukaryotic Initiation Factor 2 (eIF2) signaling regulates proinflammatory cytokine expression and bacterial invasion. J. Biol. Chem 287, 28738–28744. 10.1074/jbc.M112.375915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver E, Pass RH, Kaufman S, Hordof AJ, Liberman L, 2007. Complete heart block due to Lyme carditis in two pediatric patients and a review of the literature. Congenit. Heart Dis 2, 338–341. 10.1111/j.1747-0803.2007.00122.x [DOI] [PubMed] [Google Scholar]
- Skallová A, Iezzi G, Ampenberger F, Kopf M, Kopecky J, 2008. Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J. Immunol. Baltim. Md 1950 180, 6186–6192. 10.4049/jimmunol.180.9.6186 [DOI] [PubMed] [Google Scholar]
- Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H, 2005. The role of the T cell in autoimmune inflammation. Arthritis Res. Ther 7, S4–S14. 10.1186/ar1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell JM, Caplan AI, 2009. Fibroblasts-a diverse population at the center of it all. Int. Rev. Cell Mol. Biol 276, 161–214. 10.1016/S1937-6448(09)76004-6 [DOI] [PubMed] [Google Scholar]
- Spielman A, Ribeiro JMC, Mather TN, Piesman J, 1987. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae). J. Med. Entomol 24, 201–205. 10.1093/jmedent/24.2.201 [DOI] [PubMed] [Google Scholar]
- Stanek G, Wormser GP, Gray J, Strle F, 2012. Lyme borreliosis. The Lancet 379, 461–473. 10.1016/S0140-6736(11)60103-7 [DOI] [PubMed] [Google Scholar]
- Steere AC, 1987. The clinical evolution of Lyme arthritis. Ann. Intern. Med 107, 725. 10.7326/0003-4819-107-5-725 [DOI] [PubMed] [Google Scholar]
- Steere AC, Angelis SM, 2006. Therapy for Lyme arthritis: Strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 54, 3079–3086. 10.1002/art.22131 [DOI] [PubMed] [Google Scholar]
- Steere AC, Broderick TF, Malawista SE, 1978. Erythema chronicum migrans and Lyme arthritis: epidemiologic evidence for a tick vector. Am. J. Epidemiol 108, 312–321. 10.1093/oxfordjournals.aje.a112625 [DOI] [PubMed] [Google Scholar]
- Steere AC, Duray PH, Butcher EC, 1988. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis. Comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 31, 487–495. 10.1002/art.1780310405 [DOI] [PubMed] [Google Scholar]
- Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE, 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med 308, 733–740. 10.1056/NEJM198303313081301 [DOI] [PubMed] [Google Scholar]
- Steere AC, Malawista SE, Hardin JA, Ruddy S, Askenase W, Andiman WA, 1977a. Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann. Intern. Med 86, 685–698. 10.7326/0003-4819-86-6-685 [DOI] [PubMed] [Google Scholar]
- Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, Steele FM, 1977b. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 20, 7–17. 10.1002/art.1780200102 [DOI] [PubMed] [Google Scholar]
- Steinmann U, Borkowski J, Wolburg H, Schröppel B, Findeisen P, Weiss C, Ishikawa H, Schwerk C, Schroten H, Tenenbaum T, 2013. Transmigration of polymorphonuclear neutrophils and monocytes through the human blood-cerebrospinal fluid barrier after bacterial infection in vitro. J. Neuroinflammation 10, 832. 10.1186/1742-2094-10-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle F, Stanek G, 2009. Clinical manifestations and diagnosis of Lyme borreliosis. Lyme Borreliosis 37, 51–110. 10.1159/000213070 [DOI] [PubMed] [Google Scholar]
- Strle K, Sulka KB, Pianta A, Crowley JT, Arvikar SL, Anselmo A, Sadreyev R, Steere AC, 2017. T-Helper 17 cell cytokine responses in Lyme disease correlate with Borrelia burgdorferi antibodies during early infection and with autoantibodies late in the illness in patients with antibiotic-refractory Lyme arthritis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 64, 930–938. 10.1093/cid/cix002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhonen J, Komi J, Soukka J, Lassila O, Viljanen MK, 2003. Interaction between Borrelia burgdorferi and immature human dendritic cells. Scand. J. Immunol 58, 67–75. 10.1046/j.1365-3083.2003.01284.x [DOI] [PubMed] [Google Scholar]
- Sykes RA, Makiello P, 2016. An estimate of Lyme borreliosis incidence in Western Europe. J. Pub. Health 39, 74–81. Doi: 10.1093/pubmed/fdw017. [DOI] [PubMed] [Google Scholar]
- Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ, 1993. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J. Exp. Med 177, 1809–1814. 10.1084/jem.177.6.1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaisetthawatkul P, Logigian E, 2002. Peripheral nervous system manifestations of Lyme borreliosis. J. Clin. Neuromuscul. Dis 3, 165–171. [DOI] [PubMed] [Google Scholar]
- Thompson D, Sorenson J, Greenmyer J, Brissette CA, Watt JA, 2020. The Lyme disease bacterium, Borrelia burgdorferi, stimulates an inflammatory response in human choroid plexus epithelial cells. PLOS ONE 15, e0234993. 10.1371/journal.pone.0234993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan B, Chatterjee M, 2013. Lyme borreliosis and skin. Indian J. Dermatol 58, 167–174. 10.4103/0019-5154.110822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely DL, Fish D, Shlomchik MJ, Kaplan DH, Bockenstedt LK, 2009. Langerhans cell deficiency impairs Ixodes scapularis suppression of Th1 responses in mice. Infect. Immun 77, 1881–1887. 10.1128/IAI.00030-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, van Dam AP, Schwartz I, Dankert J, 1999. Molecular Typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev 12, 633–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-M, Hamza M, Wu T-X, Dionne RA, 2009. Up-Regulation of IL-6, IL-8 and CCL2 gene expression after acute inflammation: correlation to clinical pain. Pain 142, 275–283. 10.1016/j.pain.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Schuster S, Zysset D, Rihs S, Dickgreber N, Schürch C, Riether C, Siegrist M, Schneider C, Pawelski H, Gurzeler U, Ziltener P, Genitsch V, Tacchini-Cottier F, Ochsenbein A, Hofstetter W, Kopf M, Kaufmann T, Oxenius A, Reith W, Saurer L, Mueller C, 2014. TREM-1 deficiency can attenuate disease severity without affecting pathogen clearance. PLOS Pathog. 10, e1003900. 10.1371/journal.ppat.1003900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster EL, Elenkov IJ, Chrousos GP, 1997. The role of corticotropin-releasing hormone in neuroendocrine-immune interactions. Mol. Psychiatry 2, 368–372. 10.1038/sj.mp.4000305 [DOI] [PubMed] [Google Scholar]
- Wills AB, Spaulding AB, Adjemian J, Prevots DR, Turk S-P, Williams C, Marques A, 2016. Long-term follow-up of patients with Lyme Disease: Longitudinal analysis of clinical and quality-of-life measures. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 62, 1546–1551. 10.1093/cid/ciw189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, Kirschning CJ, Weis JJ, 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. Baltim. Md 1950 168, 348–355. 10.4049/jimmunol.168.1.348 [DOI] [PubMed] [Google Scholar]
- Wooten RM, Weis JJ, 2001. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr. Opin. Microbiol 4, 274–279. 10.1016/s1369-5274(00)00202-2 [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Nakasa T, Miyaki S, Ishikawa M, Deie M, Adachi N, Yasunaga Y, Asahara H, Ochi M, 2009. Expression of microRNA-146 in osteoarthritis cartilage. Arthritis Rheum. 60, 1035–1041. 10.1002/art.24404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder A, Wang X, Ma Y, Philipp MT, Heilbrun M, Weis JH, Kirschning CJ, Wooten RM, Weis JJ, 2003. Tripalmitoyl-S-Glyceryl-Cysteine-dependent OspA vaccination of toll-like receptor 2-deficient mice results in effective protection from Borrelia burgdorferi challenge. Infect. Immun 71, 3894–3900. 10.1128/IAI.71.7.3894-3900.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Li X, Wu M, 2018. miRNAs reshape immunity and inflammatory responses in bacterial infection. Signal Transduct. Target. Ther 3, 1–13. 10.1038/s41392-018-0006-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


