Abstract
We performed a systematic literature review to summarize the underlying pathogenic mechanisms by which adipokines influence rheumatological diseases and the resulting clinical manifestations. Increasing evidence display that numerous adipokines may significantly influence the development or clinical course of various rheumatological diseases. Despite the normal anti- or pro-inflammatory role of the cytokines, the serum level varies enormously in various rheumatological diseases. The expression of high levels of pro-inflammatory cytokines such as leptin or visfatin, respectively in systemic lupus erythematosus and in rheumatoid arthritis, represents a negative prognostic factor; other adipokines such as adiponectin, broadly known for their anti-inflammatory effects, showed a correlation with disease activity in rheumatoid arthritis. In the near future pro-inflammatory cytokines may represent a potential therapeutic target to restrain the severity of rheumatological diseases. Further studies on adipokines may provide important information on the pathogenesis of these diseases, which are not yet fully understood. The mechanisms by which adipokines induce, worsen, or suppress inflammatory and degenerative musculoskeletal pathologies and their clinical significance will be discussed in this review.
Keywords: adipokines, adiponectin, autoimmune, rheumatology, leptin
Introduction
Nowadays adipocytes are not anymore considered a passive storage of fat tissue and their metabolic functions are not limited to energetic homeostasis and thermogenesis alone. Indeed, it has been widely demonstrated that adipocytes produce a complex cohort of cytokines, called adipokines, which perform endocrine, paracrine, and autocrine effects. Adipokines are neuromodulators, growth factors, proteins of the complement system, acute phase proteins, stress hormones, and proteins involved in glucose homeostasis.1 Circulating levels of specific adipokines correlate with the amount of adipose tissue in the human body. Changes in adipokine titers define an impairment in the balance between proinflammatory and antiinflammatory cytokines. This is in keeping with the hypothesis of a complex homeostatic function performed by adipocytes, which have also been defined as an endocrine organ.2
It is widely known that obesity impairs the physiological balance of cytokines. Hypertrophy of adipocytes in obese individuals leads to a state of local hypoxia which subsequently causes necrosis associated changes in local immune response.3,4 Obesity defines a shift in the anti-inflammatory/pro-inflammatory balance due to migration of monocytes to necrotic clusters in the center of adipose tissue.5 The resulting low grade inflammation, whether due to obesity or to autoimmune diseases, leads to an increase in cardiovascular risk.6,7 High-fat diet and obesity lead to CD4+ Foxp3+ regulatory T cells depletion in men, which defines reduced insulin sensitivity.8,9 iNK (invariant natural killer) is a subset of NK lymphocytes that is widely expressed in white adipose tissue; these lymphocytes are thought to promote an anti-inflammatory effect by producing cytokines belonging to the Th2 group, such as IL-4, IL-13,10 and IL-10, which can avoid the insulin-desensitizing effects of TNF-a on adipocytes.11 Lynch et al.12 observed that hypertrophic adipose tissue expressed a decreased concentration of iNK, while the number of proinflammatory macrophages increased.
A specific subset of lymphocytes, called B regulatory lymphocytes, are constitutively expressed in white adipose tissue. Their cytokine pattern includes IL-10 and TGF-beta which exert an antiinflammatory effect and in the obese population this subset is less expressed leading to a progressive proinflammatory condition.13 It is important to emphasize the action performed by macrophages as scavenger cells of necrotic adipocytes, as it leads to a local increase of IL-17 and TNF-alfa.14 Patients with autoimmune rheumatic diseases such as rheumatoid arthritis (RA), osteoarthritis (OA), systemic lupus erythematosus (SLE), systemic sclerosis (Ssc), and vasculitis among others, display a high prevalence of obesity and consequently an altered balance of adipokines toward a pro-inflammatory condition,15 compared to general population.16 For example, obesity has been shown to be associated with an increased incidence of RA.17
Adipokines may play a central role in controlling the immune response and have been shown to play a crucial role importance in the pathogenesis of arthritic diseases.18 The divergence between the adipokine concentration in serum and synovial fluid depends on many factors: the difference of molecular weight may play a role in this process, although adipokines with a similar molecular weight such as leptin and resistin showed different concentrations in the synovium and serum, which is in keeping with a local production of adipokines, as has been shown for leptin in cortical bone.19 The distribution undergoes many adjustments influenced by multiple factors such as the presence of inflamed synovium or serum binding proteins.20
This review aims to summarize the evidences on the importance of adipokines in inflammatory and degenerative musculoskeletal diseases.
Adiponectin
Adiponectin is a 30 kDa protein characterized by a structure similar to that of the complement protein C1q secreted by adipocytes. Various forms of adiponectin have been described: the globular form, the trimeric form (low molecular weight, LMW), the hexameric form (middle molecular weight, MMW) and the multimeric (high molecular weight, HMW) adiponectin, and each of these form exerts a specific biological effect.21 Adiponectin has shown anti-diabetic, anti-inflammatory and anti-atherogenic effects,22 indeed it is a TNF-alfa inhibitor and vice versa.23,24 But the actual role of adiponectin with respect to diseases typically featured by a high level of pro-inflammatory cytokines such as autoimmune diseases is still unclear. In addition, cardiovascular diseases, such as hypertension, have been shown to express an altered balance of adipokines including adiponectin.25,26 In vitro studies showed anti-inflammatory effects of adiponectin, as a decreased expression of IL-8 in aortic endothelial cells stimulated by TNF-alfa was shown,27 conversely a ceased expression of the anti-inflammatory cytokine IL-10 in human macrophage, has been observed.28 In a case series of patients with sarcoidosis, high levels of serum adiponectin were observed and it was associated with clinical features such as arthritis or arthralgia, suggesting an anti-inflammatory role in attenuating the inflammatory cascade of the disease.29 In addition, exposure to adiponectin has been shown to reduce the adhesion of monocytes to endothelial cells and the conversion of macrophages to foam cells,30 thus hampering the genesis of atherosclerosis. Despite the positive in vitro effects demonstrated by adiponectin, several studies have shown that low doses of the cytokine did not show any future cardiovascular event.31 Low serum levels of adiponectin have also been found in a series of cases with chronic low back pain and could take on clinical significance as a marker between the acute and the chronic phase of the disease, although more studies are needed to clarify this finding.32 Serum adiponectin levels were found to be lower in obese subjects, conversely weight loss and reduction in the average size of adipocytes is associated with an increase in serum adiponectin levels.33,34 The salivary glands in patients with Sjogren’s disease showed stronger expression of adiponectin and adiponectin receptor. The significance for this altered expression is still unknown, but it may be linked to the aberrant production of cytokines by the glandular cells.35
Adiponectin and rheumatoid arthritis
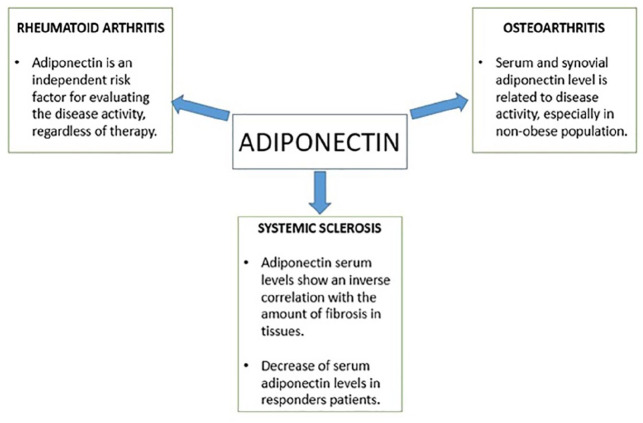
Rheumatoid arthritis (RA) is a chronic, ankylosing, and progressive inflammatory polyarthritis characterized by an autoimmune pathogenesis, mainly affecting the synovial joints. In addition to the joints, RA can become a systemic disease that defines lung fibrosis, uveitis, pleuropericarditis, or a small-medium size vessel vasculitis. The effect of biological immunosuppressive drugs used in RA lacks mutual agreement regarding the influence exerted on serum adipokine levels as many studies show conflicting data. A recent work has shown that serum adiponectin level is an independent risk factor for assessing disease activity in R since it is directly associated with the DAS28-ESR score and is not affected by biological disease-modifying anti-rheumatic drugs, jak-inhibitors, and the patient’s BMI.36 Other papers showed lower serum adiponectin level after 4 months treatment with tocilizumab,37 while some authors did not observe any difference after treatment with anti IL-6.38 In recent years, many authors have focused attention on possible connections between adiponectin gene polymorphisms and susceptibility to autoimmune diseases. A Chinese study showed a significantly higher prevalence of a specific adiponectin gene polymorphism (rs1063539) in RA patients positive to anticyclic citrullinated peptide (anti-CCP), than in anti-CCP negative patients.39 In patients with severe rheumatoid arthritis, refractory to conventional disease modifying anti-rheumatic drugs, who were undergoing anti-TNF therapy, adiponectin concentrations inversely correlated with triglycerides/HDL cholesterol ratios, total cholesterol/HDL cholesterol ratios, and high fasting plasma glucose levels. Therefore, in this inflammatory arthritis low adiponectin levels clustered with metabolic syndrome features that are implicated in the development of accelerated atherosclerosis.40 Furthermore, in non-diabetic patients with ankylosing spondylitis, another chronic inflammatory arthritis that, unlike RA, has a predominant spinal involvement, a significant correlation between adiponectin concentrations and insulin sensitivity was revealed. In these patients, a marginally significant negative correlation between adiponectin serum levels and the body mass index was also observed.41
Adiponectin and ankylosing spondylitis
Ankylosing spondylitis (AS) is a chronic systemic inflammatory disease that primarily affects the axial skeleton (cervical, dorsal, lumbar, and sacroiliac joints), but also peripheral joints (e.g. hip and shoulder), leading to progressive fibrosis and ossification (ankylosis) of the involved structures. Adiponectin serum levels have been found higher in AS patients with hip involvement and synovitis and/or enthesitis of peripheral joints than patients who did not have these features.41 Some authors found significantly increased serum adiponectin levels in AS patients receiving infliximab than controls, but others did not found differences in serum adiponectin levels between controls and AS patients.42 Miranda-Filloy et al.41 disclosed a positive correlation between insulin sensitivity and adiponectin serum levels in AS patients. This effect is due to the action of adiponectin on glucose uptake by skeletal and cardiac muscle, and inhibition of glucose production by the liver43
Adiponectin and osteoarthritis
Osteoarthritis is a chronic disease typical of elder age, which causes damage to the cartilage and surrounding tissues; it is characterized by pain, stiffness, and loss of function. The correlation between serum and synovial levels of adiponectin in osteoarthritis patients has been investigated in several studies. Adiponectin titer in both serum and in synovial fluid was shown to be related to disease activity. Orellana et al.44 observed a positive correlation between synovial adiponectin levels, local inflammation markers, and the WOMAC (Western Ontario and McMaster Universities Osteoarthritis) disease activity index in elderly women with knee osteoarthritis. Surprisingly, the serum adiponectin level was lower in the obese population: in this group it was a weaker indicator of disease activity than in the non-obese population. But the role of this cytokine in osteoarthritis is still unclear as different works have shown contrasting data. In fact, while some studies account adiponectin as one of the main local anti-inflammatory factors,67 other authors have observed opposite results, supporting a potential protective role exerted by adiponectin.45
Adiponectin and systemic sclerosis
Adiponectin plays an important role in preventing the fibrotic proliferation occurring during systemic sclerosis, and this effect appears to be linked to several molecular mechanisms. As a matter of fact this cytokine suppresses Th2 CD4+ lymphocytes polarization and their pro-fibrotic effects; furthermore a low serum level of adiponectin has been observed in many patients with systemic sclerosis during the early stages of the disease.46,47 Marangoni et al.48 observed that skin biopsies from patients with systemic sclerosis express a lower cellular phosphorylated AMPK level, which in turn reflects lower serum adiponectin levels. The expression of adiponectin in lung and gastric tissue subjected to biopsy in patients with end-stage systemic sclerosis, showed an inverse correlation with the amount of fibrosis of these tissues; while healthy controls showed strong cytokine expression, especially in the larger and thinner vessels of the lungs.49 This finding may be partially explained by the anti-fibrotic effect of adiponectin, as it has been shown to attenuate liver fibrosis and prevent myocardial fibrosis.50,51 In cutaneous systemic sclerosis, a negative correlation between clinical activity and disease duration has been observed.52,53 Evidence that IL-17 serum titer is linked to the extent of skin fibrosis54 implies a connection between adipokines and systemic sclerosis. Zhang et al.55 demonstrated that adiponectin suppresses the differentiation of Th17 and Th1 cells, while in murine models it has been observed that a deficiency of the resistin-Like Molecule (RELM) causes a decrease in the population of Th17 and Th1 cells (Figure 1).
Figure 1.
Simplified graphic showing adiponectin implications in autoimmune diseases.
Leptin
In 1994, following the discovery of a new protein, the first of the adipokine family called leptin,56 many hopes raised for the obese population as this new protein seemed to be the missing link for a comprehensive understanding of the pathophysiological mechanisms and energy balance process in this population.57 This discovery drew the attention of the scientific community and in the following years many other adipokines were described.58 Leptin is a 16 kDa glycosylated protein that exerts its functions by binding to the leptin receptor, a class I cytokine receptor. This protein has two isoforms: the first is soluble and characterized by a short cytoplasmatic domain, while the second is represented in almost every organ and is involved in the JAK/STAT pathway.59 This cytokine plays a role in the hypothalamic–pituitary–adrenal (HPA) axis by inducing hypothalamic cells to increase anorexigenic factors and lower orexigenic factors,60 in order to regulate appetite and food intake. The HPA axis is responsible for the production of glucocorticoids and responds to the secretion of pro-inflammatory factors such as IL-6 or TNF-alfa. It was observed that mice with a leptin receptor deficiency showed a dramatic lowering of CD4+ T lymphocytes.61 The expression of this receptor by almost all immune cells, as well as its influence on HPA axis, suggests an important role played by leptin in regulating immune system.62,63 Recently Caso et al.64 showed that the serum level of leptin in patients with psoriatic arthritis (PsA) sine psoriasis correlates positively with C reactive protein and BMI, while in PsA with psoriasis the serum level of leptin correlates with IL-6 serum level but not with CRP (C reactive protein).
Leptin and rheumatoid arthritis
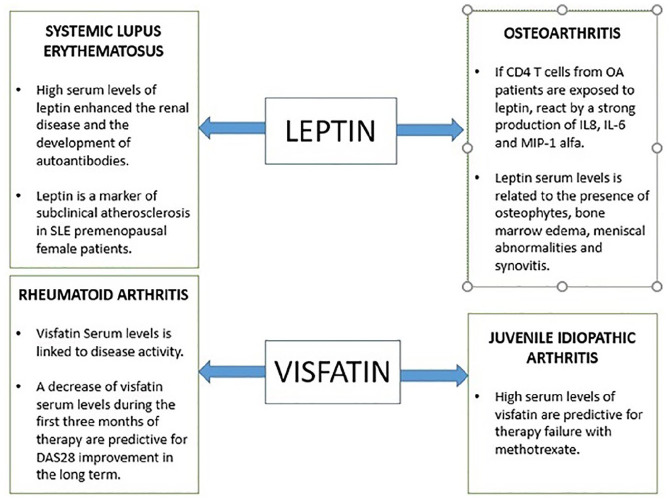
The direct association of serum leptin level and clinical activity index in RA is unclear as several studies have shown contrasting data, for example Taghadosi et al. compared the serum leptin levels of a group of healthy people to that of a group of RA patients. They observed a positive association between BMI and serum leptin levels in RA patients, but this was not true between leptin levels and DAS 28, while a direct proportionality between the disease activity score-28 (DAS-28) and the BMI in patients with rheumatoid arthritis. Remarkably, leptin levels were higher in obese and overweight population compared to RA patients.65 On the other hand, some studies support the reliability of serum leptin levels in order to describe the extent of disease activity. Several studies have shown that serum leptin concentration was independently associated with the DAS-28 score.66 This evidence is also valid for the synovial leptin concentration as well, in fact the synovial/serum leptin ratio was significantly higher in erosive RA than in non-erosive RA, which in turn was higher than the synovial leptin concentration in healthy controls.67 A study in patients treated with anti-TNF-alfa also showed a positive correlation between the serum level of leptin and DAS 28 and, in conjunction with a good clinical response, both leptin and DAS 28 lower their score after treatment with anti-TNF-alfa.68 As noted above, there is no general agreement on the clinical significance of leptin, in fact Hoffman et al.37 did not observe any change in leptin levels after treatment with anti-IL-6 drugs. Other studies have hypothesized the possible increase of IL 6 and IL 8 production by leptin via the JAK2/STAT3 pathway in patients with rheumatoid arthritis.69 Leptin has been shown to have a certain influence on the immune system through the effect exerted on lymphocytes, In fact a leptin deficiency causes a lower CD4+ T lymphocytes count with a consequent reduced proliferation.70 Adipokines may acquire significance as regards the response to treatment. In fact Xibillé-Friedmann et al.71 have shown that leptin serum level at the baseline may predict the clinical response in RA patients that initiate DMARD treatment. In RA patients undergoing anti-TNF therapy due to disease severity, there was found a strong positive correlation between body mass index of the patients and serum levels of leptin.72 It was also the case for patients with RA undergoing intravenous therapy with the anti-IL-6 receptor-tocilizumab. Moreover, a significant reduction of leptin levels was observed in these RA patients following one single intravenous infusion of the anti-IL-6 receptor tocilizumab.73 However, no statistically significant differences in the genotype or allele frequencies of the LEP rs2167270 gene polymorphism between RA patients and controls were seen.74 Also, in patients with moderate-to-severe cutaneous psoriasis undergoing anti-TNF therapy, leptin correlated with metabolic syndrome features and inflammation. In this regard, in these patients with moderate-to-severe psoriasis, leptin concentration correlated with correlated with C-reactive protein and with systolic and diastolic blood pressure before the onset of the anti-TNF-adalimumab therapy. A negative correlation with insulin sensitivity was also found.75
Leptin and ankylosing spondylitis
Some authors described higher leptin serum levels in AS patients with active disease than in healthy controls,76 while others found lower circulating levels of the adipokine than in controls.42 Similarly, some studies did not find any correlation between Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and leptin serum levels in a group of AS patients41 while in another study, a positive correlation between BASDAI and serum levels of leptin has been observed in AS patients.76 Treatment with anti-TNF a did not lead to significant variations in the circulating levels of leptin41 even after a 6 months of infliximab treatment.77 Interestingly, when AS patients were stratified according to sex, women showed higher serum leptin levels than men.41
Leptin and osteoarthritis
Recent works show that adipokines may play an important role in linking obesity, adipose tissue, and inflammation in diseases such as osteoarthritis.78 More and more evidence support an important role played by the presence of a low-grade inflammation in the pathogenesis of osteoarthritis,79 whose pathogenesis is no longer attributable to mechanical damage alone. Initially, OA was thought to be due solely to mechanical wear damage, but has subsequently also been linked to an immune pathogenesis, which is in keeping with the chronic and relapsing behavior of the disease. This behavior would be sustained by the altered Th1 lymphocytes activity80 which in turn is influenced by the circulating levels of leptin. Leptin has shown to enhance the Th1 phenotype by stimulating the production of TNF-alfa and IL-2.81 There is still conflicting evidence regarding the pathogenic mechanism by which leptin causes cartilage damage. Leptin itself does not seem to damage joint tissue, but a double face role was evidenced. It has been shown that Leptin synovial titer enhances the local expression of inflammatory cytokines such as IL-6, which potentiates proteoglycan catabolism82 and IL-1, which in turn stimulates cartilage degradation by increasing the expression of metalloproteases.83 Interestingly, CD4 T cells from OA patients, when exposed to leptin react with a strong production of pro-inflammatory cytokines such as IL8, IL-6, and MIP-1 alfa, while this reaction was absent in CD4 T cells from heathy subjects.84 On the other hand low concentrations of leptin have shown to improve the production of proteoglycan and type II collagen while higher concentrations stimulate articular cartilage cells.85 Although some correlation was observed between the level of synovial leptin and the disease activity during osteoarthritis,86 a study that included obese women pointed out that the level of synovial leptin was related to the level of IL-6 and serum leptin but not with the disease activity.44 Several studies found higher levels of leptin in the serum of patients with OA than in healthy controls87 and, furthermore, it was related to the presence of osteophytes, bone marrow edema, meniscal abnormalities, and synovitis,88 but this result was absent in similar studies.89
Leptin and systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disease occurring predominantly in younger women. Lourenço et al.90 observed an interesting correlation between leptin and SLE. The serum leptin level was assessed in two groups of mices with pristine induced-SLE; the first group was deficient in leptin while the second was wild-type. The second group developed lupus like disease followed by an increase in IgG, ANA, and ENA; while the first group did not encounter the development of such autoantibodies. This evidence showed that that leptin may play a role in the pathogenesis and progression of the disease; furthermore, high levels of leptin worsen kidney disease and the development of autoantibodies. Demir et al.91 pointed out that leptin serum level may play a potential role in premenopausal female patients with SLE as a marker of subclinical atherosclerosis, since a direct correlation with the carotid intima media thickness was demonstrated. However, other studies showed that leptin titer was correlated with BMI and CRP in SLE patients, but not with coronary atherosclerosis.92
Visfatin
Visfatin is a novel adipokine broadly known as nicotinamide phosphoribosyltransferase (NAMPT), studied for its implications in oncology and neurology.93,94 Visfatin is mainly expressed in visceral fat versus subcutaneous fat, but it is not limited to fat tissue as it is expressed in the lungs, kidney, bone marrow, and heart.95,96 The homology of the highly conserved protein sequence in multiple species such as the human, mouse, dog, and invertebrate sponge suggests an important role of this adipokine,97–99 whose expression in the cartilage and synovium100 plays a potential role in the physiopathology of musculoskeletal diseases.101 Visfatin exerts a pro-inflammatory role by inducing the production of TNF-alfa, IL-6, IL1-beta in CD14+ monocytes and modulating the MAPK pathway and the activation of NF-kB signaling.102 Recently the expression of visfatin following local hypoxia has been mainly linked to the activation of the Janus kinase pathway.103
Visfatin serum levels in RA patients has been linked to disease activity, while a decrease in visfatin levels during the first 3 months of therapy has been shown to be predictive for a long-term DAS28 improvement.104 Conversely, the serum level of visfatin has not shown any correlation with disease activity in juvenile idiopathic arthritis (JIA) despite effective treatment, but a higher visfatin level may have a potential role as a negative predictor of treatment, identifying patients more likely to cease methotrexate, which is a first line treatment in JIA; this evidence could thus lead to earlier use of second-line treatments.105 Hulejová et al.106 did not found any correlation between visfatin levels and disease activity and acute phase reactants in AS patients. Similarly, AS patients on long term treatment with infliximab did not show any significant correlation between visfatin levels and disease activity.41 Behcet’s syndrome patients had lower serum levels of visfatin in both the active and quiescent phases of the disease compared to healthy controls, likely due to genetic suppression by pro-inflammatory cytokines such as TNF-alfa and IL-6107 (Figure 2).
Figure 2.
Simplified graphic showing leptin and visfatin implications in autoimmune diseases.
MIP: Macrophage inflammatory protein; SLE: systemic lupus erythematosus.
Chemerin-1
Chemerin, known as retinoic acid receptor responder protein 2 (RARRES2), has been shown to be expressed in various tissues. Initially it was found in stimulated cultures of psoriatic skin,108 in exudate from synovial fluids of RA patients with,109 but then it was also found in white adipose tissue. Chemerin recruits dendritic cells to migrate to visceral adipose tissue, where they produce interferon-1 which triggers the pro-inflammatory response of macrophages.110 This is in keeping with the high levels of chemerin observed in autoimmune inflammatory diseases such as psoriasis, SLE, or Crohn’s disease.111 The chemerin receptor (ChemR23) is expressed in endothelial cells and is significantly up-regulated by pro-inflammatory cytokines112 and chemerin serum titers was associated with I-cam and E-selectin levels,113 which in turn are expressed on endothelial cells after activation by pro-inflammatory cytokines, such as IL-1 or TNF-alfa.
Chemerin and kawasaki disease
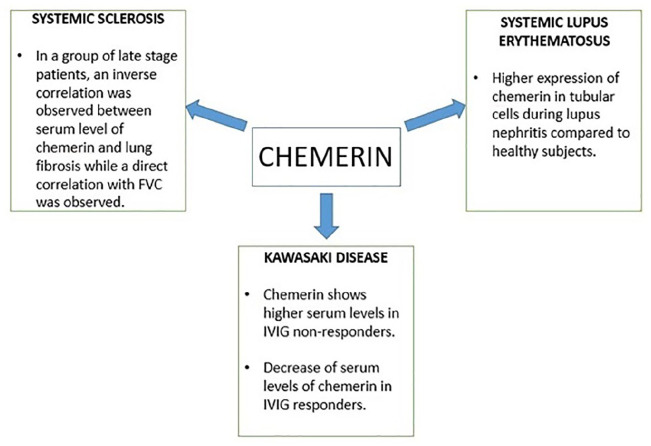
Zhang et al.114 showed that chemerin serum levels are higher during the acute stage of Kawasaki disease compared to febrile and healthy controls of the same age; in a recent work, chemerin serum levels were observed to be higher in Kawasaki disease patients who do not respond to intravenous immunoglobulins (IVIG) treatment than in responders. After 48 h of IVIG treatment, the responder group showed a significant reduction in serum chemerin levels, unlike the non-responder group. This evidence could support the therapeutic choice in Kawasaki disease.115
Chemerin and SLE
It has been shown that skin lesions observed in SLE selectively express chemerin, unlike the skin of healthy individuals.116 ChemR23 is expressed on plasmacytoid dendritic cells (P-DC), which are claimed to play an important role in the pathogenesis of SLE.117 Tubular epithelial cells from SLE patients have been shown to express chemerin, deviant from healthy subjects, which may play a role and recruit immune cells during lupus nephritis.118
Chemerin and systemic sclerosis
Recently Sawicka et al.119 studied the serum levels of visfatin and chemerin in patients with systemic sclerosis in terms of clinical manifestations and duration of the disease and compared them with the levels in the healthy population. Serum levels of chemerin were significantly elevated in patients with systemic sclerosis, especially in the diffuse cutaneous subgroup, compared with the heathy population. No changes in serum levels of the cytokine were found in relation to the duration of the disease. The same was observed in patients with decreased forced vital capacity (FVC) measured by spirometry and the presence of interstitial lung disease (ILD), but interestingly, the chemerin level patients in the late stage group correlated positively with FVC when patients were divided into two groups, one early- and middle-stage group and one late-stage group; similarly, the late-stage group showed an inverse correlation between serum chemerin levels and fibrosis observed in high-resolution computed tomography (HRCT) of the lung (Figure 3).
Figure 3.
Simplified graphic showing chemerin implications in autoimmune diseases.
FVC: forced vital capacity; IVIG: intravenous immunoglobulin.
Omentin
Omentin/intelectin-1 is an adipokine that was first described in Paneth cells, but subsequently was also expressed in lung, placenta, heart, and ovary.120,121 In vitro studies demonstrated an inhibitory effect that omentin exerts through modulation of TNF-alfa phosphorylation, Janus kinase pathway activation122,123 and NF-kB pathway through suppression of nuclear accumulation of p65, NF-kB promoter activity, and attenuation of LPS-induced inflammation in macrophages.124 This anti-inflammatory effect is consistent with the inverse correlation found between synovial omentin-1 and radiographic severity of osteoarthritis.125 On the other hand, omentin levels in the synovial fluid of patients with osteoarthritis were higher than in RA patients,126 suggesting a possible role of omentin in OA development. Serum levels of omentin are inversely correlated with inflammatory cytokines such as IL-6 and TNF-alfa.127 The determined serum levels of omentin were elevated in patients with juvenile idiopathic arhtrtis.128 The same evidence was found in patients with PsA129 compared to healthy and psoriatic controls. A recent study has shown that low omentin serum levels are associated with cardiovascular (CV) risk factors in axial spondyloarthritis (axSpA). Also, the omentin rs12409609 minor allele seems to downregulate the expression of omentin in these patients with this inflammatory arthritis. These data support a potential role of omentin as a cardiovascular risk biomarker in axSpA.130
Omentin and SLE
Zhang et al.131 investigated the possible association between specific adipokine polymorphisms and susceptibility to SLE, but no significant result was found, except for the omentin-1 rs13376023 A allele, which showed that it defined susceptibility to oral ulcers; for resistin rs3745367 AA genotype and A allele frequencies, which were more frequent in renal involvement.
Meteorin-Like
IL-41 or meteorin-like is a cytokine discovered in 2004 that was initially studied mainly for its neurotrophic role,132 but it was later found that this cytokine is expressed by activated macrophages in skin and mucous membranes, while IL-17 and TNF-alfa increase its production.133 Recently Bridgewood et al.134 demonstrated the presence of high levels of IL-41 in the synovial fluid of patients with psoriatic arthritis and in rheumatoid arthritis, while normal levels were assessed in patients affected by osteoarthritis. They also showed that the main source of local IL-41 is the stromal cells of enthesis, although other cells may be involved in this process in inflammatory conditions.
Vaspin
Vaspin (visceral adipose tissue-derived serine protease inhibitor) is a novel cytokine, that belongs to the serine-protease inhibitor group. Initially, it was shown that the intake of vaspin defined a reduction in insulin resistance and improved glucose tolerance in obese mice.135 Recently, it was shown that vaspin is adipocyte-specific and that its expression in subcutaneous adipose tissue decreases with increasing fat mass136 and it may define a protective effect exerted on white adipose tissue, by influencing gene expression toward improved insulin resistance.135 Zieger et al.137 compared the expression of the NfKb inflammatory pathway of a native adipocyte cell line with another line overexpressing visfatin, and it was found both in vitro and in vivo that when stimulated with IL-1 beta almost no increases in IL-6 and TNF-alfa were observed in the cell line overexpressing visfatin, whereas the native cell line responded with an increase in pro-inflammatory cytokines.
Vaspin and Psoriatic Arthritis
Although vaspin serum levels have been shown to be higher in PsA patients than in the healthy population, clinical activity in these patients is not significantly correlated with serum vaspin levels.138 On the other hand, patients affected by psoriasis showed a positive correlation between serum levels of vaspin and PASI, while during clinical remission, serum vaspin levels were lower than in healthy controls.139
Retinol Binding Protein 4
Retinol Binding protein 4 was initially identified as an adipocyte factor involved in the pathogenesis of type 2 diabetes.140 The exact role of this protein is not yet known as conflicting data on its purported are available. While Young et al.141 observed a direct link between insulin resistance, obesity, and serum levels of this cytokine, other studies have not obtained the same result.142 The serum and synovial level of RBP-4 was recently assessed for the first time in a series of patients with osteoarthritis, resulting in a high level of the cytokine either in synovial fluid or in serum, and a correlation with the level of MMP-1 was pointed out.143 However, in a study that included 101 patients with rheumatoid arthritis, retinol-binding protein 4 serum levels did not correlate with the presence of insulin resistance and β-cell function. Therefore, the mechanisms leading to insulin resistance in patients with this chronic inflammatory disease may be different from those occurring in obesity or diabetes.144 Interestingly, in non-diabetic patients with ankylosing spondylitis, a single infusion of the anti-TNF monoclonal antibody infliximab led to significant reduction of retinol-binding protein 4 serum levels (Figure 4).145
Figure 4.
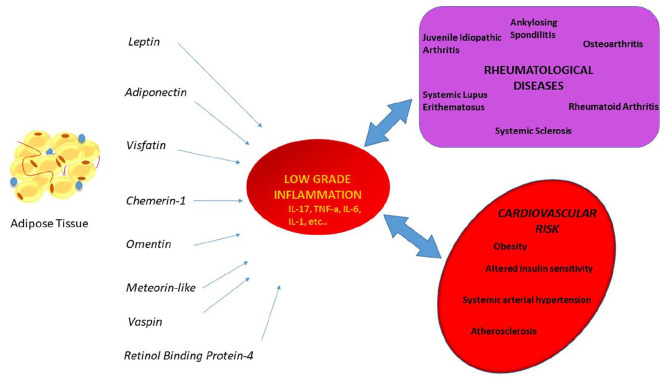
Schematic representation of the role of adipokines in rheumatological diseases and cardiovascular risk. Depending on the rheumatological disease, adipokines may increase or restrain the low grade inflammation.
Irisin
A recently discovered myokine/adipokine called irisin is drawing more and more attention because of multiple effects exerted on several tissues such as fat, brain, muscle, and bone. The expression of PGC1α (PPARγ coactivator-1 α) by muscle cells increases as a response to physical activity, which in turn enhances the expression of Fndc5, a membrane protein cleaved, and secreted as a hormone called irisin. This hormone influences the phenotype of adipose cells toward a “browning differentiation,” but it is believed that the beneficial effects of this adipokine go well beyond the only fat metabolism. In vivo work assessed the effects of a direct injection of the hormone in mice, resulting in reduced diet-induced obesity and improved glucose homeostasis,146,147 although such effects could also result from browning differentiation of fat cells. Lavrova et al.148 assessed the serum level of irisin in a case series of patients affected by RA and they observed that a lower serum level is associated to a higher DAS28 score, extra-articular manifestations, and lower 25-OH vitamin D serum level. Another study disclosed significant lower levels of serum irisin in RA patients with sleep disturbances, while an inverse correlation with the duration of morning stiffness duration and the DAS28 score.149
Conclusions
Adipokines exert their biological effects by several mechanisms which are still not fully understood. The complex network and the different concentration of pro and anti-inflammatory cytokines in the serum depends on many factors such as underlying disease, ethnic group, treatment, etc. While some adipokines have shown an anti-inflammatory effect, others have been shown to increase the expression of pro-inflammatory cytokines such as IL-17 or TNF-alfa. Population heterogeneity and different methods used in the determination of cytokines could lead to conflicting data. The serum level of various adipokines has been shown to predict disease severity index, as observed for leptin in SLE or chemerin in KD. In several papers leptin has been shown to be independently correlated with the RA disease activity index, also reflecting the improvement in clinical condition after anti-TNF treatment; nevertheless, it was not true for anti-IL-6. Leptin has been shown to exert a pro-inflammatory role which in turn defines clinical worsening in SLE and in OA. This group of adipokines could become a therapeutic target in the near future, although a comprehensive understanding of the underlying pathogenic mechanisms is required. Other adipokines, such as visfatin, have been shown to predict long-term response to therapy in RA patients, when reduced serum levels were observed during the first 3 months of therapy. Adipokines may represent a great opportunity in the near future for understanding autoimmune and rheumatological diseases, for choosing more individualized and effective treatments, and as a possible follow-up tool. Further research is needed to elucidate the influence of cytokines in degenerative musculoskeletal diseases. The lack of previous papers and the limited case series are important limitations of current knowledge, especially with regard to recently discovered adipokines.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This paper/manuscript/study has been published with the financial support of the Dept. of Medical and Surgical Sciences of the University of Foggia.
ORCID iD: Liberato Giardullo  https://orcid.org/0000-0003-1655-0608
https://orcid.org/0000-0003-1655-0608
References
- 1. Cooper DA, Merigan TC. (1996) Clinical treatment. AIDS 10: S133–S134. [DOI] [PubMed] [Google Scholar]
- 2. Kershaw EE, Flier JS. (2004) Adipose tissue as an endocrine organ. The Journal of Clinical Endocrinology and Metabolism 89(6): 2548–2556. [DOI] [PubMed] [Google Scholar]
- 3. Westendorf A, Skibbe K, Adamczyk A, et al. (2017) Hypoxia enhances immunosuppression by inhibiting CD4+ Effector T cell function and promoting treg activity. Cellular Physiology and Biochemistry 41(4): 1271–1284. [DOI] [PubMed] [Google Scholar]
- 4. Rausch ME, Weisberg S, Vardhana P, et al. (2008) Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. International Journal of Obesity 32(3): 451–463. [DOI] [PubMed] [Google Scholar]
- 5. Lumeng CN, DelProposto JB, Westcott DJ, et al. (2008) Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57(12): 3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castañeda S, Martín-Martínez MA, González-Juanatey C, et al. (2015) Erratum to “Cardiovascular morbidity and associated risk factors in Spanish patients with chronic inflammatory rheumatic diseases attending rheumatology clinics: Baseline data of the CARMA Project” [Semin Arthritis Rheum 44 (2015) 618–626]. Seminars in Arthritis and Rheumatism 45(2): e7–e8. [DOI] [PubMed] [Google Scholar]
- 7. Izaola O, Luis D, Sajoux I, Domingo JC, Vidal M. (2015) Inflammation and obesity (Lipoinflammation). Nutrición Hospitalaria 31(6): 2352–2358. [DOI] [PubMed] [Google Scholar]
- 8. Nishimura S, Manabe I, Nagasaki M, et al. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Natural Medicines 15(8): 914–920. [DOI] [PubMed] [Google Scholar]
- 9. Winer S, Chan Y, Paltser G, et al. (2009) Normalization of obesity-associated insulin resistance through immunotherapy. Natural Medicines 15(8): 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schipper HS, Rakhshandehroo M, van de Graaf SFJ, et al. (2012) Natural killer T cells in adipose tissue prevent insulin resistance. Journal of Clinical Investigation 122(9): 3343–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hong EG, Ko HJ, Cho YR, et al. (2009) Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes 58(11): 2525–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lynch L, Nowak M, Varghese B, et al. (2012) Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37(3): 574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishimura S, Manabe I, Takaki S, et al. (2013) Adipose natural regulatory B cells negatively control adipose tissue inflammation. Cell Metabolism 18(5): 759–766. [DOI] [PubMed] [Google Scholar]
- 14. O’Rourke RW, White AE, Metcalf MD, et al. (2011) Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia 54(6): 1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Unamuno X, Gómez-Ambrosi J, Rodríguez A, et al. (2018) Adipokine dysregulation and adipose tissue inflammation in human obesity. European Journal of Clinical Investigation 48(9): e12997. [DOI] [PubMed] [Google Scholar]
- 16. Gremese E, Tolusso B, Gigante MR, et al. (2014) Obesity as a risk and severity factor in rheumatic diseases (autoimmune chronic inflammatory diseases). Frontiers in Immunology 5. DOI: 10.3389/fimmu.2014.00576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Symmons DPM, Bankhead CR, Harrison BJ, et al. (1997) Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis. Results from a primary care-based incident case-control study in Norfolk, England. Arthritis & Rheumatism 40(11): 1955–1961. [DOI] [PubMed] [Google Scholar]
- 18. Francisco V, Pino J, Gonzalez-Gay MA, et al. (2018) Adipokines and inflammation: Is it a question of weight? British Journal of Pharmacology 175(no. 10): 1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dumond H, Presle N, Terlain B, et al. (2003) Evidence for a key role of leptin in osteoarthritis. Arthritis & Rheumatism 48(11): 3118–3129. [DOI] [PubMed] [Google Scholar]
- 20. Presle N, Pottie P, Dumond H, et al. (2006) Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis and Cartilage 14(7): 690–695. [DOI] [PubMed] [Google Scholar]
- 21. Sargolzaei J, Chamani E, Kazemi T, et al. (2018) The role of adiponectin and adipolin as anti-inflammatory adipokines in the formation of macrophage foam cells and their association with cardiovascular diseases. Clinical Biochemistry 54: 1–10. [DOI] [PubMed] [Google Scholar]
- 22. Achari AE, Jain SK. (2017) Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. International Journal of Molecular Sciences 18(6): 1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bruun JM, Lihn AS, Verdich C, et al. (2003) Regulation of adiponectin by adipose tissue-derived cytokines: In vivo and in vitro investigations in humans. American Journal of Physiology: Endocrinology and Metabolism 285(3): E527–E533. [DOI] [PubMed] [Google Scholar]
- 24. Lihn AS, Richelsen B, Pedersen SB, et al. (2003) Increased expression of TNF-α, IL-6, and IL-8 in HALS: Implications for reduced adiponectin expression and plasma levels. American Journal of Physiology: Endocrinology and Metabolism 285(5): E1072–E1080. [DOI] [PubMed] [Google Scholar]
- 25. Chukaeva II, Gankovskaya LV, Orlova NV, et al. (2018) [Study of cytokine profile in men with hypertension] Klinicheskaia Laboratornaia Diagnostika 63(7): 439–444. [DOI] [PubMed] [Google Scholar]
- 26. Tian N, Moore RS, Braddy S, et al. (2007) Interactions between oxidative stress and inflammation in salt-sensitive hypertension. American Journal of Physiology: Heart and Circulatory Physiology 293(6): H3388–H3395. [DOI] [PubMed] [Google Scholar]
- 27. Kobashi C, Urakaze M, Kishida M, et al. (2005) Adiponectin inhibits endothelial synthesis of interleukin-8. Circulation Research 97(12): 1245–1252. [DOI] [PubMed] [Google Scholar]
- 28. Kumada M, Kihara S, Ouchi N, et al. (2004) Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 109(17): 2046–2049. [DOI] [PubMed] [Google Scholar]
- 29. Kobak S, Semiz H, Akyildiz M, et al. (2020) Serum adipokine levels in patients with sarcoidosis. Clinical Rheumatology 39(7): 2121–2125. [DOI] [PubMed] [Google Scholar]
- 30. Ouchi N, Kihara S, Arita Y, et al. (2001) Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 103(8): 1057–1063. [DOI] [PubMed] [Google Scholar]
- 31. Wu Z, Cheng Y, Aung LHH, et al. (2013) Association between adiponectin concentrations and cardiovascular disease in diabetic patients: A systematic review and meta-analysis. PLoS One 8(11): e78485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klyne DM, Hodges PW. (2020) Circulating adipokines in predicting the transition from acute to persistent low back pain. Pain Medicine 21(11): 2975–2985. [DOI] [PubMed] [Google Scholar]
- 33. De Rosa A, Monaco ML, Capasso M, et al. (2013) Adiponectin oligomers as potential indicators of adipose tissue improvement in obese subjects. European Journal of Endocrinology 169(1): 37–43. [DOI] [PubMed] [Google Scholar]
- 34. Varady KA, Tussing L, Bhutani S, et al. (2009) Degree of weight loss required to improve adipokine concentrations and decrease fat cell size in severely obese women. Metabolism 58(8): 1096–1101. [DOI] [PubMed] [Google Scholar]
- 35. Katsiougiannis S, Kapsogeorgou EK, Manoussakis MN, et al. (2006) Salivary gland epithelial cells: A new source of the immunoregulatory hormone adiponectin. Arthritis & Rheumatism 54(7): 2295–2299. [DOI] [PubMed] [Google Scholar]
- 36. Minamino H, Katsushima M, Yoshida T, et al. (2020) Increased circulating adiponectin is an independent disease activity marker in patients with rheumatoid arthritis: A cross-sectional study using the KURAMA database. PLoS One 15(3): e0229998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoffman E, Rahat MA, Feld J, et al. (2019) Effects of tocilizumab, an anti-interleukin-6 receptor antibody, on serum lipid and adipokine levels in patients with rheumatoid arthritis. International Journal of Molecular Sciences 20(18): 4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tournadre A, Pereira B, Dutheil F, et al. (2017) Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. Journal of Cachexia Sarcopenia and Muscle 8(4): 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao Y-L, Zhang TP, Wu J, et al. (2020) Association of adiponectin and adiponectin receptor gene polymorphisms with rheumatoid arthritis in a Chinese population. Postgraduate Medical Journal 96(1133): 149–155. [DOI] [PubMed] [Google Scholar]
- 40. Gonzalez-Gay MA, Llorca J, Garcia-Unzueta MT, et al. (2008) High-grade inflammation, circulating adiponectin concentrations and cardiovascular risk factors in severe rheumatoid arthritis. Clinical and Experimental Rheumatology 26: 596–603. [PubMed] [Google Scholar]
- 41. Miranda-Filloy JA, López-Mejias R, Genre F, et al. (2013) Leptin and visfatin serum levels in non-diabetic ankylosing spondylitis patients undergoing TNF-α antagonist therapy. Clinical and Experimental Rheumatology 31(4): 538–545. [PubMed] [Google Scholar]
- 42. Toussirot É, Streit G, Nguyen NU, et al. (2007) Adipose tissue, serum adipokines, and ghrelin in patients with ankylosing spondylitis. Metabolism 56(10): 1383–1389. [DOI] [PubMed] [Google Scholar]
- 43. Yamauchi T, Kamon J, Minokoshi Y, et al. (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Natural Medicines 8(11): 1288–1295. [DOI] [PubMed] [Google Scholar]
- 44. Orellana C, Calvet J, Berenguer-Llergo A, et al. (2020) Synovial adiponectin was more associated with clinical severity than synovial leptin in women with knee osteoarthritis. Cartilage. Epub ahead of print 20 February 2020. DOI: 10.1177/1947603520904776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ibrahim SM, Hamdy MS, Amer N. (2008) Plasma and synovial fluid adipocytokines in patients with rheumatoid arthritis and osteoarthritis. The Egyptian Journal of Immunology/Egyptian Association of Immunologists 15(1):159–170. [PubMed] [Google Scholar]
- 46. Żółkiewicz J, Stochmal A, Rudnicka L. (2019) The role of adipokines in systemic sclerosis: A missing link? Archives for Dermatological Research Archiv fur Dermatologische Forschung 311(4): 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stochmal A, Czuwara J, Zaremba M, et al. (2020) Altered serum level of metabolic and endothelial factors in patients with systemic sclerosis. Archives for Dermatological Research Archiv fur Dermatologische Forschung 312(6): 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marangoni RG, Masui Y, Fang F, et al. (2017) Adiponectin is an endogenous anti-fibrotic mediator and therapeutic target. Scientific Reports 7(1): 4397. DOI: 10.1038/s41598-017-04162-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Neumann E, Lepper N, Vasile M, et al. (2019) Adipokine expression in systemic sclerosis lung and gastrointestinal organ involvement. Cytokine 117: 41–49. [DOI] [PubMed] [Google Scholar]
- 50. Sam F, Walsh K. (2010) What can adiponectin say about left ventricular function? Heart 96(5): 331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang W, Zhao C, Wang Y-D, et al. (2011) Adiponectin inhibits the activation of hepatic stellate cells induced by TGFb1 via up-regulating the expression of eNOS]. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese Journal of Hepatology 19: 917–922. [DOI] [PubMed] [Google Scholar]
- 52. Masui Y, Asano Y, Shibata S, et al. (2012) Serum adiponectin levels inversely correlate with the activity of progressive skin sclerosis in patients with diffuse cutaneous systemic sclerosis. Journal of the European Academy of Dermatology and Venereology 26(3): 354–360. [DOI] [PubMed] [Google Scholar]
- 53. Arakawa H, Jinnin M, Muchemwa FC, et al. (2011) Adiponectin expression is decreased in the involved skin and sera of diffuse cutaneous scleroderma patients. Experimental Dermatology 20(9): 764–766. [DOI] [PubMed] [Google Scholar]
- 54. Truchetet ME, Brembilla NC, Montanari E, et al. (2013) Interleukin-17A+ cell counts are increased in systemic sclerosis skin and their number is inversely correlated with the extent of skin involvement. Arthritis & Rheumatism 65(5): 1347–1356. [DOI] [PubMed] [Google Scholar]
- 55. Zhang K, Guo Y, Ge Z, et al. (2017) Adiponectin suppresses T helper 17 cell differentiation and limits autoimmune CNS inflammation via the SIRT1/PPARγ/RORγt pathway. Molecular Neurobiology 54(7): 4908–4920. [DOI] [PubMed] [Google Scholar]
- 56. Zhang Y, Proenca R, Maffei M, et al. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372(6505): 425–432. [DOI] [PubMed] [Google Scholar]
- 57. Jeet Singh H. (2001) The unfolding tale of leptin. Malaysian Journal of Medical Sciences 8(2): 1–6. [PMC free article] [PubMed] [Google Scholar]
- 58. Scherer PE, Williams S, Fogliano M, et al. (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. Journal of Biological Chemistry 270(45): 26746–26749. [DOI] [PubMed] [Google Scholar]
- 59. Frühbeck G. (2006) Intracellular signalling pathways activated by leptin. Biochemical Journal 393(1): 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Swieten MM, Pandit R, Adan RA, et al. (2014) The neuroanatomical function of leptin in the hypothalamus. Journal of Chemical Neuroanatomy 61-62: 207–220. [DOI] [PubMed] [Google Scholar]
- 61. Bennett BD, Solar GP, Yuan JQ, et al. (1996) A role for leptin and its cognate receptor in hematopoiesis. Current Biology 6(9): 1170–1180. [DOI] [PubMed] [Google Scholar]
- 62. Carlton ED, Demas GE, French SS. (2012) Leptin, a neuroendocrine mediator of immune responses, inflammation, and sickness behaviors. Hormones and Behavior 62(3): 272–279. [DOI] [PubMed] [Google Scholar]
- 63. Procaccini C, De Rosa V, Galgani M, et al. (2012) Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. Journal of Immunology 189(6): 2941–2953. [DOI] [PubMed] [Google Scholar]
- 64. Caso F, Postiglione L, Covelli B, et al. (2019) Pro-inflammatory adipokine profile in psoriatic arthritis: Results from a cross-sectional study comparing PsA subset with evident cutaneous involvement and subset “sine psoriasis”. Clinical Rheumatology 38(9): 2547–2552. [DOI] [PubMed] [Google Scholar]
- 65. Taghadosi M, Samimi Z, Assar S, et al. (2020) Plasma leptin does not reflect the effect of high body mass index on disease activity in rheumatoid arthritis. Immunological Investigations 49(1–2): 32–45. [DOI] [PubMed] [Google Scholar]
- 66. Batún-Garrido JADJ, Salas-Magaña M, Juárez-Rojop IE, et al. (2018) Relationship between leptin concentrations and disease activity in patients with rheumatoid arthritis. Medicina Clinica 150(9): 341–344. [DOI] [PubMed] [Google Scholar]
- 67. Olama SM, Senna MK, Elarman M. (2012) Synovial/serum leptin ratio in rheumatoid arthritis: The association with activity and erosion. Rheumatology International 32(3): 683–690. [DOI] [PubMed] [Google Scholar]
- 68. Corrado A, Colia R, Rotondo C, et al. (2019) Changes in serum adipokines profile and insulin resistance in patients with rheumatoid arthritis treated with anti-TNF-α. Current Medical Research and Opinion 35(12): 2197–2205. [DOI] [PubMed] [Google Scholar]
- 69. Wang M, Wei J, Li H, et al. (2018) Leptin Upregulates peripheral CD4+CXCR5+ICOS+ T cells via increased IL-6 in rheumatoid arthritis patients. Journal of Interferon & Cytokine Research 38(2): 86–92. [DOI] [PubMed] [Google Scholar]
- 70. Farooqi IS, Matarese G, Lord GM, et al. (2002) Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. Journal of Clinical Investigation 110(8): 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xibillé-Friedmann DX, Ortiz-Panozo E, Bustos Rivera-Bahena C, et al. (2015) Leptin and adiponectin as predictors of disease activity in rheumatoid arthritis. Clinical and Experimental Rheumatology 33(4): 471–477. [PubMed] [Google Scholar]
- 72. Gonzalez-Gay MA, Garcia-Unzueta MT, Berja A, et al. (2009) Anti-TNF-α therapy does not modulate leptin in patients with severe rheumatoid arthritis. Clinical and Experimental Rheumatology 27(2): 222–228. [PubMed] [Google Scholar]
- 73. Pulito-Cueto V, Remuzgo-Martínez S, Genre F, et al. (2020) Anti-IL-6 therapy reduces leptin serum levels in patients with rheumatoid arthritis. Clinical and Experimental Rheumatology 38: 1201–1205. [PubMed] [Google Scholar]
- 74. García-Bermúdez M, González-Juanatey C, Rodríguez-Rodríguez L, et al. (2011) Lack of association between LEP rs2167270 (19 G>A) polymorphism and disease susceptibility and cardiovascular disease in patients with rheumatoid arthritis. Clinical and Experimental Rheumatology 29: 293–298. [PubMed] [Google Scholar]
- 75. Pina T, Genre F, Lopez-Mejias R, et al. (2015) Relationship of Leptin with adiposity and inflammation and Resistin with disease severity in Psoriatic patients undergoing anti-TNF-alpha therapy. Journal of the European Academy of Dermatology and Venereology 29(10): 1995–2001. [DOI] [PubMed] [Google Scholar]
- 76. Park M-C, Chung S-J, Park Y-B, et al. (2009) Pro-inflammatory effect of leptin on peripheral blood mononuclear cells of patients with ankylosing spondylitis. Joint Bone Spine 76(4): 447–175. [DOI] [PubMed] [Google Scholar]
- 77. Derdemezis CS, Filippatos TD, Voulgari PV, et al. (2010) Leptin and adiponectin levels in patients with ankylosing spondylitis. The effect of infliximab treatment. Clinical and Experimental Rheumatology 28(6): 880–883. [PubMed] [Google Scholar]
- 78. Azamar-Llamas D, Hernández-Molina G, Ramos-Ávalos B, et al. (2017) Adipokine contribution to the pathogenesis of osteoarthritis. Mediators of Inflammation 2017: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Robinson WH, Lepus CM, Wang Q, et al. (2016) Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nature Reviews Rheumatology 12(10): 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Harrell CR, Markovic BS, Fellabaum C, et al. (2019) Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomedicine & Pharmacotherapy 109: 2318–2326. [DOI] [PubMed] [Google Scholar]
- 81. Martín-Romero C, Santos-Alvarez J, Goberna R, et al. (2000) Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cellular Immunology 199(1): 15–24. [DOI] [PubMed] [Google Scholar]
- 82. Sui Y, Lee JH, DiMicco MA, et al. (2009) Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor α in immature bovine and adult human articular cartilage. Arthritis & Rheumatism 60(10): 2985–2996. [DOI] [PubMed] [Google Scholar]
- 83. Troeberg L, Nagase H. (2012) Proteases involved in cartilage matrix degradation in osteoarthritis. Biochimica et Biophysica Acta (BBA): Proteins and Proteomics 1824(1): 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Scotece M, Pérez T, Conde J, et al. (2017) Adipokines induce pro-inflammatory factors in activated Cd4+ T cells from osteoarthritis patient. Journal of Orthopaedic Research 35(6): 1299–1303. [DOI] [PubMed] [Google Scholar]
- 85. Loeser RF. (2003) Systemic and local regulation of articular cartilage metabolism: Where does leptin fit in the puzzle? Arthritis & Rheumatism 48(11): 3009–3012. [DOI] [PubMed] [Google Scholar]
- 86. Richter M, Trzeciak T, Rybka JD, et al. (2017) Correlations between serum adipocytokine concentrations, disease stage, radiological status and total body fat content in the patients with primary knee osteoarthritis. International Orthopaedics 41(5): 983–989. [DOI] [PubMed] [Google Scholar]
- 87. Corrado A, Sanpaolo ER, Rotondo C, et al. (2019) Pattern of adipokine expression in osteoblasts from osteoporotic and osteoarthritic bone. Journal of Gerontology and Geriatrics 67(4). [Google Scholar]
- 88. Karvonen-Gutierrez CA, Harlow SD, Jacobson J, et al. (2014) The relationship between longitudinal serum leptin measures and measures of magnetic resonance imaging-assessed knee joint damage in a population of mid-life women. Annals of the Rheumatic Diseases 73(5): 883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Berry PA, Jones SW, Cicuttini FM, et al. (2011) Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis. Arthritis & Rheumatism 63(3): 700–707. [DOI] [PubMed] [Google Scholar]
- 90. Lourenço EV, Liu A, Matarese G, et al. (2016) Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proceedings of the National Academy of Sciences of the United States of America 113(38): 10637–10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Demir S, Erten G, Artım-Esen B, et al. (2018) Increased serum leptin levels are associated with metabolic syndrome and carotid intima media thickness in premenopausal systemic lupus erythematosus patients without clinical atherosclerotic vascular events. Lupus 27(9): 1509–1516. [DOI] [PubMed] [Google Scholar]
- 92. Dahl TB, Yndestad A, Skjelland M, et al. (2007) Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: Possible role in inflammation and plaque destabilization. Circulation 115(8): 972–980. [DOI] [PubMed] [Google Scholar]
- 93. Beauparlant P, Bédard D, Bernier C, et al. (2009) Preclinical development of the nicotinamide phosphoribosyl transferase inhibitor prodrug GMX1777. Anti-Cancer Drugs 20(5): 346–354. [DOI] [PubMed] [Google Scholar]
- 94. Wang G, Han T, Nijhawan D, et al. (2014) P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 158(6): 1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Samal B, Sun Y, Stearns G, et al. (1994) Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Molecular and Cellular Biology 14(2): 1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Revollo JR, Grimm AA, Imai SI. (2007) The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Current Opinion in Gastroenterology 23(2): 164–170. [DOI] [PubMed] [Google Scholar]
- 97. Kitani T, Okuno S, Fujisawa H. (2003) Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor 1. FEBS Letters 544(1-3): 74–78. [DOI] [PubMed] [Google Scholar]
- 98. McGlothlin JR, Gao L, Lavoie T, et al. (2005) Molecular cloning and characterization of canine Pre-B-Cell colony-enhancing factor. Biochemical Genetics 43(3-4): 127–141. [DOI] [PubMed] [Google Scholar]
- 99. Muller WEG, Perovic S, Wilkesman J, et al. (1999) Increased gene expression of a cytokine-related molecule and profilin after activation of suberites domuncula cells with xenogeneic sponge molecule(s). DNA and Cell Biology 18(12): 885–893. [DOI] [PubMed] [Google Scholar]
- 100. Laiguillon M-C, Houard X, Bougault C, et al. (2014) Expression and function of visfatin (Nampt), an adipokine-enzyme involved in inflammatory pathways of osteoarthritis. Arthritis Research & Therapy 16(1): R38–R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gosset M, Berenbaum F, Salvat C, et al. (2008) Crucial role of visfatin/pre–B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: Possible influence on osteoarthritis. Arthritis & Rheumatism 58(5): 1399–1409. [DOI] [PubMed] [Google Scholar]
- 102. Moschen AR, Kaser A, Enrich B, et al. (2007) Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. Journal of Immunology 178(3): 1748–1758. [DOI] [PubMed] [Google Scholar]
- 103. Chiu C, Wang B, Yu Y, et al. (2020) Hyperbaric oxygen activates visfatin expression and angiogenesis via angiotensin II and JNK pathway in hypoxic human coronary artery endothelial cells. Journal of Cellular and Molecular Medicine 24(4): 2434–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sglunda O, Mann H, Hulejová H, et al. (2014) Decreased circulating visfatin is associated with improved disease activity in early rheumatoid arthritis: Data from the PERAC cohort. PLoS One 9(7): e103495–e103495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Funk R, Singh R, Pramann L, et al. (2016) Nicotinamide phosphoribosyltransferase attenuates methotrexate response in juvenile idiopathic arthritis and in vitro. Clinical and Translational Science 9(3): 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hulejová H, Levitová A, Kuklová M, et al. (2012) No effect of physiotherapy on the serum levels of adipocytokines in patients with ankylosing spondylitis. Clinical Rheumatology 31(1): 67–71. [DOI] [PubMed] [Google Scholar]
- 107. Sezen H, Okumus S, Pehlivan Y, et al. (2012) Visfatin levels in Behcet’s disease. Inflammation 35(2): 405–408. [DOI] [PubMed] [Google Scholar]
- 108. Nagpal S, Patel S, Jacobe H, et al. (1997) Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. Journal of Investigative Dermatology 109(1): 91–95. [DOI] [PubMed] [Google Scholar]
- 109. Wittamer V, Franssen JD, Vulcano M, et al. (2003) Specific Recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. Journal of Experimental Medicine 198(7): 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Helfer G, Wu QF. (2018) Chemerin: A multifaceted adipokine involved in metabolic disorders. Journal of Endocrinology 238(2): R79–R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Weigert J, Obermeier F, Neumeier M, et al. (2010) Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn’s disease. Inflammatory Bowel Diseases 16(4): 630–637. [DOI] [PubMed] [Google Scholar]
- 112. Kaur J, Adya R, Tan BK, et al. (2010) Identification of chemerin receptor (ChemR23) in human endothelial cells: Chemerin-induced endothelial angiogenesis. Biochemical and Biophysical Research Communications 391(4): 1762–1768. [DOI] [PubMed] [Google Scholar]
- 113. Glowinska B, Urban M, Peczynska J, et al. (2005) Soluble adhesion molecules (sICAM-1, sVCAM-1) and selectins (sE selectin, sP selectin, sL selectin) levels in children and adolescents with obesity, hypertension, and diabetes. Metabolism 54(8): 1020–1026. [DOI] [PubMed] [Google Scholar]
- 114. Zhang XY, Yang TT, Hu XF, et al. (2018) Circulating adipokines are associated with Kawasaki disease. Pediatric Rheumatology 16(1): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Xiang H, Chang M, Wang QX, et al. (2020) Changes in serum levels of adipokine after treatment in children with Kawasaki disease. Chinese Journal of Contemporary Pediatrics 22(1): 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vermi W, Riboldi E, Wittamer V, et al. (2005) Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. Journal of Experimental Medicine 201(4): 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Banchereau J, Pascual V, Palucka AK. (2004) Autoimmunity through cytokine-induced dendritic cell activation. Immunity 20(5): 539–550. [DOI] [PubMed] [Google Scholar]
- 118. De Palma G, Castellano G, Del Prete A, et al. (2011) The possible role of ChemR23/Chemerin axis in the recruitment of dendritic cells in lupus nephritis. Kidney International 79(11): 1228–1235. [DOI] [PubMed] [Google Scholar]
- 119. Sawicka K, Michalska-Jakubus M, Potembska E, et al. (2019) Visfatin and chemerin levels correspond with inflammation and might reflect the bridge between metabolism, inflammation and fibrosis in patients with systemic sclerosis. Postepy Dermatologii i Alergologii 36(5): 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Schäffler A, Neumeier M, Herfarth H, et al. (2005) Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochimica et Biophysica Acta: Gene Structure and Expression 1732(1-3): 96–102. [DOI] [PubMed] [Google Scholar]
- 121. Yang RZ, Lee MJ, Hu H, et al. (2006) Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. American Journal of Physiology: Endocrinology and Metabolism 290(6): E1253–E1261. [DOI] [PubMed] [Google Scholar]
- 122. Kazama K, Usui T, Okada M, et al. (2012) Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. European Journal of Pharmacology 686(1–3): 116–123. [DOI] [PubMed] [Google Scholar]
- 123. de Souza Batista CM, Yang RZ, Lee MJ, et al. (2007) Omentin plasma levels and gene expression are decreased in obesity. Diabetes 56(6): 1655–1661. [DOI] [PubMed] [Google Scholar]
- 124. Wang J, Gao Y, Lin F, et al. (2020) Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Archives of Biochemistry and Biophysics 679: 108187. [DOI] [PubMed] [Google Scholar]
- 125. Xu L, Zhu GB, Wang L, et al. (2012) Synovial fluid omentin-1 levels are inversely correlated with radiographic severity of knee osteoarthritis. Journal of Investigative Medicine 60(3): 583–586. [DOI] [PubMed] [Google Scholar]
- 126. Senolt L, Polanska M, Filkova M, et al. (2010) Vaspin and omentin: New adipokines differentially regulated at the site of inflammation in rheumatoid arthritis. Annals of the Rheumatic Diseases 69(7): 1410–1411. [DOI] [PubMed] [Google Scholar]
- 127. Tan YL, Zheng XL, Tang CK. (2015) The protective functions of omentin in cardiovascular diseases. Clinica Chimica Acta 448: 98–106. [DOI] [PubMed] [Google Scholar]
- 128. Cantarini L, Simonini G, Fioravanti A, et al. (2011) Paediatric rheumatology Circulating levels of the adipokines vaspin and omentin in patients with juvenile idiopathic arthritis, and relation to disease activity. Clinical and Experimental Rheumatology 29(6): 1044–1048. [PubMed] [Google Scholar]
- 129. Xue Y, Jiang L, Cheng Q, et al. (2012) Adipokines in psoriatic arthritis patients: The correlations with osteoclast precursors and bone erosions. PLoS One 7(10): e46740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Genre F, Rueda-Gotor J, Remuzgo-Martínez S, et al. (2020) Omentin: A biomarker of cardiovascular risk in individuals with axial spondyloarthritis. Scientific Reports 10(1): 9636. DOI: 10.1038/s41598-020-66816-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang TP, Li HM, Li R, et al. (2020) Association of omentin-1, adiponectin, and resistin genetic polymorphisms with systemic lupus erythematosus in a Chinese population. International Immunopharmacology 83: 106343. [DOI] [PubMed] [Google Scholar]
- 132. Jørgensen JR, Thompson L, Fjord-Larsen L, et al. (2009) Characterization of meteorin: An evolutionary conserved neurotrophic factor. Journal of Molecular Neuroscience 39(1-2): 104–116. [DOI] [PubMed] [Google Scholar]
- 133. Ushach I, Arrevillaga-Boni G, Heller GN, et al. (2018) Meteorin-like/Meteorin-β is a novel immunoregulatory cytokine associated with inflammation. Journal of Immunology 201(12): 3669–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bridgewood C, Russell T, Weedon H, et al. (2019) The novel cytokine Metrnl/IL-41 is elevated in psoriatic arthritis synovium and inducible from both entheseal and synovial fibroblasts. Clinical Immunology 208: 108253. [DOI] [PubMed] [Google Scholar]
- 135. Hida K, Wada J, Eguchi J, et al. (2005) Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proceedings of the National Academy of Sciences of the United States of America 102(30): 10610–10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lee JA, Park HS, Song YS, et al. (2011) Relationship between vaspin gene expression and abdominal fat distribution of Korean women. Endocrine Journal 58(8): 639–646. [DOI] [PubMed] [Google Scholar]
- 137. Zieger K, Weiner J, Krause K, et al. (2018) Vaspin suppresses cytokine-induced inflammation in 3T3-L1 adipocytes via inhibition of NFκB pathway. Molecular and Cellular Endocrinology 460: 181–188. [DOI] [PubMed] [Google Scholar]
- 138. Maijer KI, Neumann E, Müller-Ladner U, et al. (2015) Serum vaspin levels are associated with the development of clinically manifest arthritis in autoantibody-positive individuals. PLoS One 10(12): e0144932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ataseven A, Kesli R. (2016) Novel inflammatory markers in psoriasis vulgaris: Vaspin, vascular adhesion protein-1 (VAP-1), and YKL-40. Giornale Italiano di Dermatologia e Venereologia. [PubMed] [Google Scholar]
- 140. Yang Q, Graham TE, Mody N, et al. (2005) Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436(7049): 356–362. [DOI] [PubMed] [Google Scholar]
- 141. Cho YM, Youn BS, Lee H, et al. (2006) Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 29(11): 2457–2461. DOI: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 142. Gmez-Ambrosi J, Rodrguez A, Cataln V, et al. (2008) Serum retinol-binding protein 4 is not increased in obesity or obesity-associated type 2 diabetes mellitus, but is reduced after relevant reductions in body fat following gastric bypass. Clinical Endocrinology 69(2): 208–215. [DOI] [PubMed] [Google Scholar]
- 143. Scotece M, Koskinen-Kolasa A, Pemmari A, et al. (2020) Novel adipokine associated with OA: Retinol binding protein 4 (RBP4) is produced by cartilage and is correlated with MMPs in osteoarthritis patients. Inflammation Research 69(4): 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ferraz-Amaro I, González-Gay MA, Diaz-González F. (2014) Retinol-binding protein 4 in rheumatoid arthritis-related insulin resistance and β-cell function. The Journal of Rheumatology 41(4): 658–665. [DOI] [PubMed] [Google Scholar]
- 145. Genre F, López-Mejías R, Miranda-Filloy JA, et al. (2014) Antitumour necrosis factor α treatment reduces retinol-binding protein 4 serum levels in non-diabetic ankylosing spondylitis patients. Annals of the Rheumatic Diseases 73(5): 941–943. [DOI] [PubMed] [Google Scholar]
- 146. Ruth M. (2012) A PGC1–α–dependent myokine that drives brown–fat–like development of white fat and thermogenesis. Nature 2012: 114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cederberg A, Grønning LM, Ahrén B, et al. (2001) FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106(5): 563–573. [DOI] [PubMed] [Google Scholar]
- 148. Lavrova DP, Zavodovsky BV, Akhverdyan YR, et al. (2018) Irjsin as a new marker of early diagnostics of low-traumatic fractures in rheumatoid arthritis. Klinicheskaia Laboratornaia Diagnostika 63(11): 702–706. [DOI] [PubMed] [Google Scholar]
- 149. Gamal RM, Mohamed ME, Hammam N, et al. (2020) Preliminary study of the association of serum irisin levels with poor sleep quality in rheumatoid arthritis patients. Sleep Medicine 67: 71–76. [DOI] [PubMed] [Google Scholar]