Abstract
Sublingual immunotherapy (SLIT) has been used for more than three decades as a therapeutic strategy for the treatment of allergic diseases. Studies have demonstrated its efficacy and safety, and numerous clinical trials have evaluated these parameters. In the present study, through patient perception, we investigated the patient satisfaction with the use of house dust mite SLIT treatment. “Satisfaction Scale for Patients Receiving Allergen Immunotherapy” (ESPIA) questionnaire, a standardized and validated instrument for clinical studies evaluating allergen immunotherapy, was applied to allergic patients (N = 136). Children and adults of both sexes who received SLIT for Dermatophagoides pteronyssinus and/or Blomia tropicalis, according to the results of an immediate reading puncture test, were included. Data analysis showed that the perception of treatment effectiveness was 92%, performance improvement in the daily activities was 91%, a satisfactory cost-benefit balance was 84%, and the perception of general satisfaction was 97%. The results showed a high perception of satisfaction in allergic patients undergoing house dust mite SLIT.
Keywords: house dust mite, SLIT
Introduction
Introduced into medicine by Leonard Noon and John Freeman in 1911, allergen immunotherapy (AIT) is the only treatment strategy which is capable of altering the natural history of allergic diseases.1,2 Until 1986, AIT was administered exclusively through the subcutaneous route (SCIT). In that year, the British Committee on Safety of Medicines reported 26 deaths related to this route of administration.3 Although some fatal events related to SCIT were due to avoidable errors, such as wrong dosage, incorrect prescription, or incorrect administration have indeed been demonstrated, such reports aroused interests in trying alternative methods turning sublingual administration a promising strategy. Thus, from the late 1980s, this approach was investigated to increase the safety of AIT use.4,5
Scadding et al. in 1986, published the first randomized controlled trial with sublingual immunotherapy (SLIT) showing positive clinical results to its use through this route of administration. After this study, the number of clinical trials with SLIT has increased considerably leading the World Health Organization (WHO), in 1998, to consider SLIT as a viable therapeutic alternative to SCIT.5 Additionally, in 1998, the European Academy of Allergy and Clinical Immunology (EAACI) justified the use of SLIT due to its efficacy and safety.6
In 2001, the position paper, “Allergic rhinitis and its impact on asthma” (ARIA), approved the use of SLIT in adults and children as an alternative to SCIT. This was confirmed in its 2008 update, and then by WHO in 2009, in addition to other relevant academic institutions working in the area of Allergy and Immunology, such as the American Academy of Allergy, Asthma and Immunology (AAAAI), and the American College of Allergy, Asthma and Immunology (ACAAI).1,7–9 In the United States, the use of SLIT remained “off-label” and empirical until 2014, when the Food and Drug Administration (FDA) approved two types of SLIT in tablet form for the administration of AIT for pollens.1,5,10
Although the efficacy of SLIT has been confirmed over three decades and it has been found to provide greater safety while using AIT, data in the literature on the perception of patients about its beneficial effects and changes in their quality of life (QoL) are scarce. According to the WHO, QoL is “the individual’s perception of their position in life in the context of the culture and value system in which they live and in relation to their objectives, expectations, standards and concerns”. More broadly, the term refers to an individual’s subjective perception of his personal satisfaction, being influenced by complex interactions, intrinsic and extrinsic, such as living conditions, experiences, and personal values. Given its subjectivity, QoL is often evaluated using different questionnaires.11
In tropical countries, house dust mites correspond to the main allergens etiologically associated with allergic diseases such as rhinitis, asthma, and atopic dermatitis. The climatic characteristics with high temperatures and high humidity facilitate the proliferation of Dermatophagoides farinae (Df), Dermatophagoides pteronyssinus (Dp), and Blomia tropicalis (Bt). The latter is characteristic of Latin American countries, with few studies addressing its use in immunotherapy with this house dust mite. Vieira-Hernández et al.12 demonstrated the effectiveness of intradermal immunotherapy with low doses of Dp/Df and Bt.13
Although several questionnaires reported in the literature prioritize evaluation of symptoms and reduction in the use of medications, they do not specifically aim at the general welfare.14 The goal of the present study was to evaluate the satisfaction of the patients with SLIT treatment in a real-world setting from the patients’ own perception.
Methods
Patients
In order to evaluate the perception of treatment effectiveness and its influence on the satisfaction with SLIT treatment, a descriptive cross-sectional study was conducted using the Original Satisfaction Scale for Patients Receiving Allergen Immunotherapy (ESPIA) questionnaire among 136 allergic patients of both sexes who received SLIT in the Allergy, Asthma, and Clinical Immunology Service of the Monte Sinai Medical Center, Juiz de Fora-MG, Brazil. All allergic patients with prick test positive for house dust mites submitted to SLIT treatment during the period January to July 2018 were included in this study. The included sample has been sufficiently heterogeneous from both a sociodemographic and clinical point of view to reflect the variability of the main characteristics of the allergic patients in Brazil. The validation of ESPIA questionare.6 was performed for a cross-sectional cohort of 335 patients. ESPIA questionnaire presents high consistency and internal validity, for cross-sectional cohort studies made with patients whose clinical characteristics are heterogeneous. The size of the study sample was determined by criteria established in the literature.15 In the present study, we used a sample of 136 patients that represents 40% of the number of patients evaluated in the baseline study sample that validated ESPIA questionare allowing to observe consistent results through ESPIA questionnaire.
The inclusion criteria were a minimum age of 5 years, having a pre-established diagnosis of atopic disease by a specialist in Allergy and Immunology, having started a specific SLIT for Dermatophagoides pteronyssinus and/or Blomia tropicalis (IPI/ASAC, São Paulo, Brazil) according to the results of the immediate read puncture test (Prick Test), and being in treatment after 4 months, when the monthly maintenance dose of 5.6 micrograms of main allergens (Derp1 or Derp1+Bt) was obtained. The evaluation was performed 4 months after the start of treatment in order to achieve the maintenance dose of SLIT enabling the induction of specific allergen immunological tolerance.
According to the diagnosis, the participants in this study had persistent allergic rhinitis (79%), asthma (28%), atopic dermatitis (14%), and allergic conjunctivitis (11%). The disease overlap was 21%. All patients diagnosed with atopic dermatitis had concomitant rhinitis and/or asthma. The characteristics of this sample represent the real life of routine allergy and immunology care.
All participants or their legal guardians signed the consent form authorizing the use of SLIT. The legal guardians assisted the patients in the answers to the questionnaire, when appropriate. All participants or legal guardians provided written informed consent form and the research was approved by the Comitê de Ética em Pesquisa (Research Ethics Committee) of the Faculdade de Ciências Médicas e da Saúde—SUPREMA, Juiz de Fora, MG, Brazil (Parecer number 1481788).
Satisfaction scale for patients receiving allergen immunotherapy (ESPIA) questionnaire application
The original ESPIA is a specific validated questionnaire6 to evaluate the satisfaction of patients who receive specific immunotherapy for allergens. The ESPIA questionnaire to assess patient satisfaction with respect to AIT treatment presented satisfactory psychometric properties for its use in clinical practice.The questionnaire consists of 16 items distributed in four dimensions: Perceived effectiveness (questions 1−4), daily activities (questions 5−10), cost-benefit analysis (questions 12−14), and general satisfaction (questions 11, 15, and 16) (Table 1).
Table 1.
Specification of questions and assessment scenarios. Answers 1–5, with 1 being the worst and 5 being the best assessment.6
| Always | Many times | Half of the time | At times | Never | ||
|---|---|---|---|---|---|---|
| Q1 | Since being vaccinated for my allergy, I have fewer symptoms | 5 | 4 | 3 | 2 | 1 |
| Q2 | My vaccine works | 5 | 4 | 3 | 2 | 1 |
| Q3 | Thanks to the vaccine, I am less dependent on carrying other medication (pills, inhalers, etc) | 5 | 4 | 3 | 2 | 1 |
| Q4 | My vaccine works faster than I expected | 5 | 4 | 3 | 2 | 1 |
| Q5 | Thanks to the vaccine, I no longer avoid things or places that caused my allergy | 5 | 4 | 3 | 2 | 1 |
| Q6 | My vaccine helps me to perform my daily activities | 5 | 4 | 3 | 2 | 1 |
| Q7 | Since being vaccinated, I can go anywhere with my family and friends | 5 | 4 | 3 | 2 | 1 |
| Q8 | Thanks to the vaccine, I can work or study better | 5 | 4 | 3 | 2 | 1 |
| Q9 | Since being vaccinated, I enjoy outdoor activities more | 5 | 4 | 3 | 2 | 1 |
| Q10 | Since being vaccinated, I don’t find myself in uncomfortable or compromising situations caused by my allergy. | 5 | 4 | 3 | 2 | 1 |
| Q11 | Since being vaccinated, I have gained in quality of life | 5 | 4 | 3 | 2 | 1 |
| Q12 | The good performance of my vaccine compensates for all the things I have to do to get it (visits prescriptions, leave, etc) | 5 | 4 | 3 | 2 | 1 |
| Q13 | The good performance of my vaccine compensates for the financial burden it involves | 5 | 4 | 3 | 2 | 1 |
| Q14 | The good performance of my vaccine compensates for the discomforts it may cause me | 5 | 4 | 3 | 2 | 1 |
| Q15 | In general, I am satisfied with my allergy vaccine | 5 | 4 | 3 | 2 | 1 |
| Q16 | In general, I would recommend this vaccine treatment to other people. | 5 | 4 | 3 | 2 | 1 |
Statistical analyses
Statistical analyses were carried out with SPSS software (SPSS Institute, Cary, NC, USA). The patients answered each question in the questionnaire, and the overall score from the four dimensions was obtained by the score of each question, quantified as 1 point for “never” and 5 points for “always.” The sum of its items was transformed to a scale of 0−100 points, in which the lower the score, the lower was the degree of satisfaction. The distribution of the overall and dimension scores was analyzed by calculating mean scores
Results
The analysis of the questionnaire data showed a high percentage of positive perception on the part of patients for all the questions.
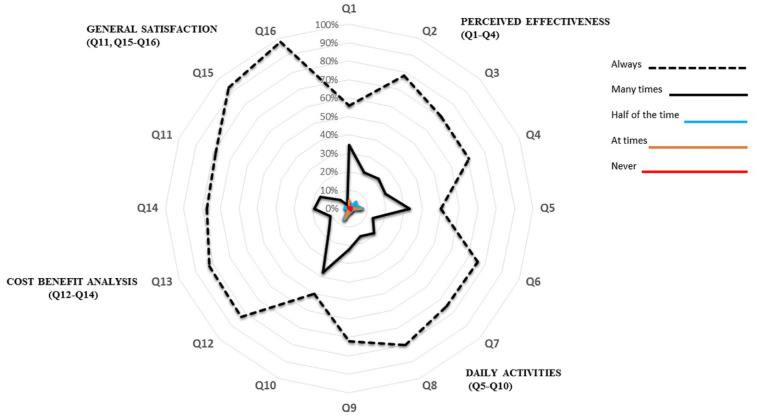
The results of the responses to each question in the questionnaire are shown in Table 2. The analysis of each dimension analyzed shows that the perception for effectiveness was 92%, for improvement of performance in daily activities was 91%, for satisfactory cost-benefit balance was 84%, and for overall satisfaction was 97%. Figure 1 shows the schematic distribution in a diagram of the values by different dimensions according to the responses obtained in this study to the questions of the ESPIA questionnaire.
Table 2.
Patient satisfaction with allergen-specific immunotherapy scale. Study patients responses (N = 136). Results expressed as punctuation and percentage (%).
| Always | Many times | Half of the time | At times | Never | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Score | % | Score | % | Score | % | Score | % | Score | % | |
| Q1 | 76 | 56 | 47 | 35 | 5 | 4 | 6 | 4 | 1 | 1 |
| Q2 | 106 | 78 | 29 | 21 | 0 | 0 | 0 | 0 | 0 | 0 |
| Q3 | 96 | 71 | 31 | 23 | 7 | 5 | 1 | 1 | 0 | 0 |
| Q4 | 96 | 71 | 29 | 21 | 6 | 4 | 1 | 1 | 2 | 1 |
| Q5 | 68 | 50 | 45 | 33 | 10 | 7 | 9 | 7 | 2 | 1 |
| Q6 | 103 | 76 | 19 | 14 | 3 | 2 | 0 | 0 | 1 | 1 |
| Q7 | 102 | 75 | 26 | 19 | 3 | 2 | 3 | 2 | 2 | 1 |
| Q8 | 109 | 80 | 22 | 16 | 3 | 2 | 0 | 0 | 0 | 0 |
| Q9 | 98 | 72 | 30 | 22 | 4 | 3 | 3 | 2 | 0 | 0 |
| Q10 | 68 | 50 | 51 | 38 | 9 | 7 | 8 | 6 | 0 | 0 |
| Q11 | 107 | 79 | 23 | 17 | 3 | 2 | 0 | 0 | 0 | 0 |
| Q12 | 113 | 83 | 21 | 15 | 2 | 1 | 0 | 0 | 0 | 0 |
| Q13 | 112 | 82 | 15 | 11 | 0 | 0 | 0 | 0 | 0 | 0 |
| Q14 | 105 | 77 | 26 | 19 | 3 | 2 | 0 | 0 | 0 | 0 |
| Q15 | 126 | 93 | 9 | 7 | 0 | 0 | 0 | 0 | 1 | 1 |
| Q16 | 133 | 98 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
Source: Authors.
Q: questions.
Figure 1.
Distribution of values by dimensions of the ESPIA questionnaire.
ESPIA: satisfaction scale for patients receiving allergen immunotherapy; Q: questions.
Discussion
The relationship between an individual’s QoL and his/her state of health is complex and can be influenced by the effects and consequences of a disease, its treatment, and the intrinsic impression of the patient on of his/her condition and prospects.11 Alvarez-Cuesta et al. have stressed that AIT is a treatment modality that can alter the natural pathological history leading to a significant reduction in the severity of allergic diseases, reducing the need of anti-allergic drugs, and permitting better a QoL for patients. Alvarez-Cuesta et al.7 have also observed the importance of starting treatment at the beginning of the disease, preventing its progression, and improving the feeling of well-being of the patient, thereby impacting the performance of their daily activities, such as work and social life.
In fact, AIT has been shown to be effective in the clinical management of allergic diseases leading to prolonged remission of symptoms, significantly reducing treatment costs, improving the prognosis, and improving the QoL of patients.8 Patel et al.8 conducted a study to evaluate the QoL and the efficacy of treatment with SCIT. Patients with rhinoconjunctivitis were divided into two groups, one received placebo and the other was treated with SCIT. The results revealed that the use of SCIT effectively reduced the symptoms and improved QoL. Schwanke et al.9 conducted a study to assess the QoL of 105 individuals who were subjected to SCIT or SLIT for respiratory allergies. Although statistically significant differences were observed in the group that received SCIT, the data revealed favorable changes in the overall scores and domains in both the groups.
A multicentric study with a prospective follow-up of 248 patients with rhinitis and asthma treated with SCIT was conducted involving the use of questionnaires that evaluated QoL, the severity of the disease, number of days with symptoms per year, and the number of days sick per year. SCIT reduces the symptoms and requirement of medications for asthma and rhinitis/rhinoconjunctivitis and improves the specific QoL. It is associated with few reactions, especially the immunotherapy regimens involving the use of one or two main allergens.10 Lemberg et al.17 observed a significant improvement in the QoL after a year of treatment, a significant improvement in the severity and control of the disease, and a reduction in the number of sick days and days with symptoms.
A recent study peformed with SLIT showed a significant improvement in the QoL of patients with seasonal allergies, improving the productivity in daily activities and their general feelings of well-being.11 In another study, 32 patients diagnosed with asthma and allergic rhinitis were treated with SLIT for 6 months. In order to evaluate the effectiveness of the treatment, symptoms, complementary medications, and QoL of the participants were analyzed. Arikan et al.14 reported significant improvement both in the symptoms and QoL. Novakova et al.16 conducted research with 191 adult patients (mean age, 27 years) diagnosed with moderate to severe allergic rhinitis. The patients were prospectively evaluated throughout the 3 years of treatment with house dust mite and grass pollen SLIT. The authors concluded that there was an improvement in the daily activities, quality of sleep, emotional state, and the nasal and ocular symptoms. In children, the use of SLIT was also able to alter the QoL. A retrospective cross-sectional study conducted with 201 pediatric patients treated with SLIT concluded that the QoL of the patients was equal to that of the general population. An interesting additional result was the observation that SLIT significantly contributed to treatment adhesion.17 The improved sense of smell associated with the control of other classical symptoms of persistent allergic rhinitis was the other reason behind the study using patient’s own self-evaluation. Katotomichelakis et al.18 used standardized questionnaires with special attention to olfactory functions in 145 patients with persistent allergic rhinitis being treated with SLIT. The data revealed a significant improvement in the sense of smell, as well as clinical improvement, that was reflected in the QoL of the patients.
Many studies have also directly addressed the QoL and clinical outcomes of allergic patients treated with SLIT.16–20 However, the assessment of patient satisfaction with SLIT treatment has been little investigated. To evaluate patient satisfaction we use the ESPIA questionnaire in a cross-sectional cohort study where a convenience sample was chosen in an Allergy and Immunology service representing a real situation of allergic patients who presented the indication for treatment with SLIT. In routine clinical applications, patient satisfaction, which indirectly represents perception in the improvement of symptoms and aspects of their quality of life, influences the success of the treatment mainly due to the commitment of patients to the proposed therapy.
The sample studied was for convenience but replicates the real life of the universe of patients with allergic diseases treated with SLIT. Additionally, the number of patients evaluated although limited is equivalent to 40% of the sample size used in the ESPIA questionnaire validation study, giving representativeness according to the characteristics of the population and objectives of the study. The ESPIA questionnaire used has high consistency and internal validity, for cross-sectional cohort studies performed with patients whose clinical characteristics of the sample are heterogeneous as occurs in this study. Therefore the characteristics of the sample although they may limit the conclusions of the study also represent a strength for representing situations observed in real life. Finally, although there are few studies with Blomia tropicalis, immunotherapy with this allergen presents similar results to those observed with other house dust mites. In the present study we did not observe any differences in the patients’ perception regarding the satisfaction with the SLIT treatment in patients only sensitized with Dermatophagoides pteronyssinus or sensitized with Blomia tropicalis and Dermatophagoides pteronyssinus concomitantly.
Conclusion
In this study, we evaluate patient satisfaction through four areas: perceived effectiveness, daily activities, general satisfaction, and cost benefit analysis.Taken together, the results showed a high perception of satisfaction in allergic patients undergoing house dust mite SLIT.
Footnotes
Author contributions: Matheus Fonseca Aarestrup participated in generating and gathering the data for the study and have approved the final version of this paper.
Paula Fonseca Aarestrup participated in generating and gathering the data for the study and have approved the final version of this paper.
Mariana Senff de Andrade participated in generating and gathering the data and have approved the final version of this paper.
Beatriz Julião V Aarestrup participated in analysis of the data, writing the paper and have approved the final version of this paper.
Akinori Cardozo Nagato participated in analysis of the data, writing the paper and have approved the final version of this paper.
Fernando Monteiro Aarestrup participated in generating and gathering the data for the study, designing the study, wrote the majority of the original draft of the paper, guarantee that all individuals who meet the Journal’s authorship criteria are included as authors of this paper, reviewed the pertinent raw data on which the results and conclusions of this study are based, and have approved the final version of this paper.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All participants or their legal guardians signed the consent form authorizing the use of SLIT. The legal guardians assisted the patients in the answers to the questionnaire, when appropriate. All participants or legal guardians provided written informed consent form and the research was approved by the Comitê de Ética em Pesquisa (Research Ethics Committee) of the Faculdade de Ciências Médicas e da Saúde – SUPREMA, Juiz de Fora, MG, Brazil (Parecer number 1481788).
Ethics approval: Not Applicable.
Informed consent: Not Applicable.
Trial registration: Not Applicable.
ORCID iD: Fernando Monteiro Aarestrup  https://orcid.org/0000-0001-9226-8271
https://orcid.org/0000-0001-9226-8271
References
- 1. Canonica GW, Bousquet J, Casale T, et al. (2009) Sublingual immunotherapy: World allergy organization position paper. World Allergy Organization Journal 2(11): 223–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Committee on the Safety of Medicines (CSM) (1986) CSM update: Desensitizing vaccines. British Medical Journal 293: 948. [PMC free article] [PubMed] [Google Scholar]
- 3. Passalacqua G, Guerra L, Pasquali M, et al. (2004) Efficacy and safety of sublingual immunotherapy. Annals of Allergy, Asthma & Immunology 93(1): 3–12. [DOI] [PubMed] [Google Scholar]
- 4. Bousquet J, Khaltaev N, Cruz AA, et al. (2008) Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA (2) LEN and AllerGen). Allergy 63(Suppl. 86): 8–160. [DOI] [PubMed] [Google Scholar]
- 5. Bousquet J, Van Cauwenberge P, Khaltaev N. (2001) Aria Workshop Group, World Health Organization. Allergic rhinitis and its impact on asthma. Journal of Allergy and Clinical Immunology 108(Suppl. 5): S147–S334. [DOI] [PubMed] [Google Scholar]
- 6. Justicia J, Cardona V, Guardia P, et al. (2013) Validation of the first treatment-specific questionnaire for the assessment of patient satisfaction with allergen-specific immunotherapy in allergic patients: The ESPIA questionnaire. Journal of Allergy and Clinical Immunology 131(6): 1539–1546. [DOI] [PubMed] [Google Scholar]
- 7. Alvarez-Cuesta, Bousquet J, Canonica GW, et al. (2006) EAACI, immunotherapy task force. Standards for practical allergen-specific immunotherapy. Allergy 61(s82): 1–20. [DOI] [PubMed] [Google Scholar]
- 8. Patel P, Holdich T, Fischer von Weikersthal-Drachenberg KJ, et al. (2014) Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. Journal of Allergy and Clinical Immunology 133(1): 121–129. [DOI] [PubMed] [Google Scholar]
- 9. Schwanke T, Carragee E, Bremberg M, et al. (2017) Quality-of-life outcomes in patients who underwent subcutaneous immunotherapy and sublingual immunotherapy in a real-world clinical setting. American Journal of Rhinology & Allergy 31(5): 310–316. [DOI] [PubMed] [Google Scholar]
- 10. Petersen KD, Kronborg C, Larsen JN, et al. (2013) Patient related outcomes in a real life prospective follow up study: Allergen immunotherapy increase quality of life and reduce sick days. World Allergy Organization Journal 6(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guilleminault L, Viala-Gastan C. (2017) Blomia tropicalis: A house dust mite in the tropics. Revue des Maladies Respiratoires 34(8): 791–801. [DOI] [PubMed] [Google Scholar]
- 12. Vieira-Hernández A, Capriles-Hulett A, Sánchez-Borges M, et al. (2018) Intradermal immunotherapy with low-dose house dust mite allergens in patients with allergic rhinitis: A proof-of-concept study. Revista Alergia México 65(1): 41–51. [DOI] [PubMed] [Google Scholar]
- 13. Rak S, Yang WH, Pedersen MR, et al. (2007) Once-daily sublingual allergen-specific immunotherapy improves quality of life in patients with grass pollen-induced allergic rhinoconjunctivitis: A double-blind, randomised study. Quality of Life Research 16: 191–201. [DOI] [PubMed] [Google Scholar]
- 14. Arikan C, Bahceciler NN, Deniz G, et al. (2004) Bacillus Calmette-Guerin-induced interleukin-12 did not additionally improve clinical and immunologic parameters in asthmatic children treated with sublingual immunotherapy. Clinical Experimental Allergy 34: 398–405. [DOI] [PubMed] [Google Scholar]
- 15. Taherdoost H. (2016) Sampling methods in research methodology; How to choose a sampling technique for research. International Journal of Academic Research in Management 5: 18–27. [Google Scholar]
- 16. Novakova SM, Staevska MT, Novakova PI, et al. (2017) Quality of life improvement after a three years course of sublingual immunotherapy in patients with house dust mite and grass pollen induced allergic rhinitis: Results from real-life. Health and Quality of Life Outcomes 15: 1892–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lemberg ML, Eberle P, Hosseini KS. (2016) Importance of quality of life for adherence to sublingual immunotherapy. BioMed Research International 2016: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katotomichelakis M, Riga M, Tripsianis G, et al. (2015) Predictors of quality of life improvement in allergic rhinitis patients after sublingual immunotherapy. Annals of Otology, Rhinology & Laryngology 124: 430–436. [DOI] [PubMed] [Google Scholar]
- 19. Soh JY, Thalayasingam M, Ong S, et al. (2016) Sublingual immunotherapy in patients with house dust mite allergic rhinitis: Prospective study of clinical outcomes over a two-year period. The Journal of Laryngology & Otology 130(3): 272–277. [DOI] [PubMed] [Google Scholar]
- 20. Rodríguez Santos O. (2008) Sublingual immunotherapy in allergic rhinitis and asthma in 2-5 year-old children sensitized to mites. Revista Alergia México 55: 71–75. [PubMed] [Google Scholar]