Abstract
Solid organ transplant recipients are at risk for a wide range of opportunistic infections, the most common being cytomegalovirus. These infections may occur as reactivation of latent disease, donor-derived, or de novo. In this article, we present a case of acute liver failure secondary to toxoplasmosis following orthotopic liver transplantation. Our patient presented 5 weeks after orthotopic liver transplantation with altered mental status and fatigue. She was found to have disseminated cytomegalovirus infection, which resolved with intravenous ganciclovir; however, she subsequently developed acute liver failure due to toxoplasmosis, which is hypothesized to be donor-derived. Infection with Toxoplasma may be asymptomatic in the immunocompetent host; however, in immunocompromised hosts, such as solid organ transplant recipients, this infection can be life threatening. Though prophylaxis with trimethoprim-sulfamethoxazole may prevent infections with Toxoplasma, it is often held for renal dysfunction, hyperkalemia, or other side effects, placing patients at risk. With 13 cases now reported, routine screening of donor and recipient for toxoplasma exposure may be warranted.
Keywords: acute liver failure, liver transplantation, toxoplasmosis, Toxoplasma gondii, cytomegalovirus, donor-derived infection
Introduction
Toxoplasma gondii is a parasitic infection estimated to affect 25% to 30% of the world’s population with great variability among different countries.1 Transmission is generally via ingestion or handling of undercooked or raw meat containing cysts but can also occur via fecal-oral transmission after ingestion of oocysts excreted in cat feces. Less commonly, secondary infection can occur, as is seen with congenital toxoplasmosis or in solid organ transplant (SOT). Toxoplasmosis in SOT recipients can be the result of donor-transmission, reactivation of latent infection in the host, or de novo infection.2 T gondii infection is diagnosed most often with a positive toxoplasma-specific immunoglobulin M (IgM), which appears within 1 week of infection, or serum polymerase chain reaction. Toxoplasma may also be diagnosed histologically by the identification of either tachyzoites or cysts within a pathologic tissue specimen. In a subset of patients, serology may be negative and infection is found only at autopsy.
Since T gondii encysts specially in muscle tissue, toxoplasmosis has been considered the main worry for donor-transmitted disease in the recipients who get hearts. Though seropositivity is widespread, most infections with T gondii are benign and asymptomatic in the immunocompetent host.1,3 Twelve cases of donor-derived toxoplasmosis in liver transplant patients have been reported, with 58% mortality.4 Clinical presentation is variable, and can include pneumonia, encephalitis, meningitis, ocular involvement, fulminant liver failure, and multisystem organ failure (Table 1).4-6 High mortality is likely multifactorial due to immunosuppression and delayed diagnosis. According to previous observational studies, the most important risk factor for toxoplasmosis infection in SOT recipients is a seronegative recipient receiving organs from seropositive donor [D+/R−].7 Other risk factors include cytomegalovirus (CMV) infection within 6 months preceding transplant and recent use of high-dose prednisone.7 CMV infection may indirectly affect the host by immunomodulation.8,9 Trimethoprim-sulfamethoxazole (TMP-SMX), commonly used as prophylaxis against Pneumocystis jiroveci, may prevent T gondii infection in SOT; however, a previous case has been described despite TMP-SMX prophylaxis.6 Here we present a case of acute liver failure in a liver transplant recipient due to toxoplasmosis.
Table 1.
Reported hepatic toxoplasmosis cases in the english literature.
| Case | Presenting symptoms | Donor status | Recipient status | Immunosuppression regimen | Clinical outcome |
|---|---|---|---|---|---|
| 1 | Fever, pneumonia | Azathioprine, prednisone | Died | ||
| 2 | Fever, meningitis | + | + | Cyclosporine, prednisone, and muromonab-CD3 | Survived |
| 3 | Retinitis, choroiditis | Azathioprine, cyclosporine | Survived | ||
| 4 | Fever, pneumonia | + | − | Antithymocyte globulin, azathioprine, prednisone, cyclosporine, and muromonab-CD3 | Died |
| 5 | Hypotension, pneumonia | + | Tacrolimus, mycophenolate mofetil | Died | |
| 6 | Retinitis | + | − | Survived | |
| 7 | Fever, sepsis | − | + | Mycophenolate mofetil, azathioprine, and cyclosporine | Died |
| 8 | Seizure | Cyclosporine, azathioprine, prednisone, antithymocyte globulin | Survived | ||
| 9 | Encephalitis | Cyclosporine, azathioprine, prednisone, and antithymocyte globulin | Died | ||
| 10 | Fever, pneumonia | + | Prednisone, tacrolimus, and anti-thymocyte globulin | Died | |
| 11 | Fever, pneumonia | + | Tacrolimus, prednisone, and antithymocyte globulin | Survived | |
| 12 | Fever, pneumonia | + | − | Tacrolimus, mycophenolate mofetil, and prednisone | Died |
| 13 | Fever, encephalopathy | − | − | Tacrolimus, mycophenolic acid, and prednisone | Died |
Case Report
A 61-year-old Caucasian female presented to the emergency department 5 weeks after orthotopic liver transplant with fever and altered mental status. Her posttransplant course had previously been uncomplicated. Immunosuppression included tacrolimus (trough 12.2 ng/mL), mycophenolic acid, and low-dose prednisone. In addition, she was on CMV prophylaxis with acyclovir (D−/R−). Approximately 4 weeks prior to presentation, routine laboratory results revealed worsening creatinine elevation, so prophylaxis against Pneumocystis jiroveci with TMP-SMX was held. On presentation, laboratory data revealed acute kidney injury and elevated liver tests (total bilirubin 1.2 mg/dL, alanine aminotransferase 69 U/L, aspartate aminotransferase 101 U/L, and alkaline phosphatase 286 U/L). She was found to have elevated CMV polymerase chain reaction in the blood (679 198 IU/mL) and spinal fluid (>2 000 000 IU/mL), as well as biopsy-proven CMV hepatitis (Figure 1). She was promptly treated with intravenous ganciclovir and obtained resolution of CMV infection over the following month, only to suddenly and unexpectantly develop fulminant liver failure and expire. A liver biopsy, obtained shortly prior to death, showed confluent hepatocyte necrosis (Figure 2) and intrahepatic protozoa (~1-2 µm in diameter), consistent with Toxoplasma bradyzoites (Figure 3). Given these findings, the local organ procurement organization and the Centers for Disease Control and Prevention (CDC) were contacted for confirmatory testing. Immunohistochemical stain for Toxoplasma, performed by the CDC, confirmed Toxoplasma infection (Figure 4). Review of donor data revealed Toxoplasma IgG positivity, suggesting donor-derived infection; however, lack of toxoplasma serologic testing in the recipient leaves the mode of transmission uncertain.
Figure 1.

Cytomegalovirus (CMV) viral inclusion (black arrow points to the infected cells with nuclear inclusion). CMV infected cell is enlarged (cytomegalic), containing basophilic intranuclear inclusion (Cowdry body) surrounded by a clear halo, giving the appearance of an owl’s eye (hematoxylin and eosin stain at 600× original magnification).
Figure 2.
Spotty to confluent hepatocyte necrosis (dashed line), hepatocellular swelling (A, hematoxylin and eosin stain at 400× original magnification) and cholestasis in the lobule, black arrows (B, hematoxylin and eosin stain at 200× original magnification).
Figure 3.
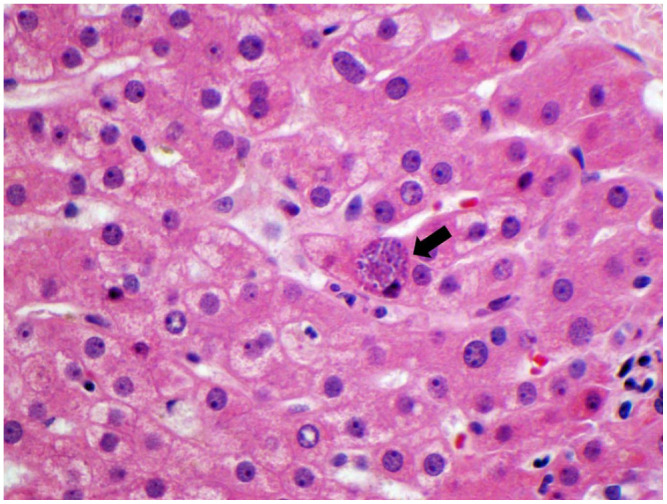
Intrahepatic protozoa (~1-2 µm in diameter) consistent with Toxoplasma cyst with bradyzoites, black arrow (hematoxylin and eosin stain at 600× original magnification).
Figure 4.

Immunohistochemical stain for Toxoplasma gondii confirms the diagnosis. It shows multiple intrahepatocytic protozoa that are arranged individually, consistent with Toxoplasma tachyzoites infection, black box/arrow (immunohistochemistry at 200×; inset box at 630× original magnification).
Discussion
While toxoplasmosis is typically asymptomatic in the immunocompetent host, infection in the immunocompromised patient can be life threatening. Given the rarity of toxoplasmosis in liver transplant patients, neither recipients nor donors routinely undergo serological testing for T gondii exposure. In this case, the donor was known to be IgG positive for T gondii, but serologies were not obtained for the recipient prior to transplant. Although our hypothesis cannot be confirmed, we suspect that our patient developed donor-derived T gondii infection after overcoming a severe case of de novo CMV infection. To our knowledge, this is the first report of allograft failure and death from toxoplasma following disseminated CMV infection.
Though prophylaxis with TMP-SMX may prevent infection with Toxoplasma, it is often held for renal dysfunction, hyperkalemia, or other side effect, placing patients at risk. With 13 cases now reported, routine screening of donor and recipient for Toxoplasma exposure may be warranted. Although disseminated toxoplasmosis is rare in the post orthotopic liver transplant patient, 61% of reported cases have resulted in death of the patient.
In summary, we present a rare case of toxoplasmosis in a patient after liver transplantation. Toxoplasmosis is a rare but serious complication that can occur after orthotropic liver transplantation. Toxoplasmosis needs to be considered in the posttransplant setting in patients with possible infectious etiology, especially in the clinical setting of renal dysfunction where TMP-SMX prophylaxis is not given.
Footnotes
Authors’ Note: The abstract of this case was presented at the American College of Gastroenterology (ACG) 2020 Virtual Annual Meeting; October 23 to 28, 2020.10
Author Contributions: Nicholas Baldwin was involved in drafting of the article, critical revision of the article for important intellectual content, and final approval of the article.
Meagan Gray was involved in critical revision of the article for important intellectual content and final approval of the article.
Chirag R. Patel was involved in the diagnosis of the case, critical revision of the article for important intellectual content, and final approval of the article.
Sameer Al Diffalha was involved in conception and design, critical revision of the article for important intellectual content, and final approval of the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Informed consent for patient information to be published in this article was not obtained because patient was expired and the autopsy was performed after obtaining appropriate consenting.
ORCID iD: Sameer Al Diffalha  https://orcid.org/0000-0002-3095-4089
https://orcid.org/0000-0002-3095-4089
References
- 1. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965-1976. [DOI] [PubMed] [Google Scholar]
- 2. Khurana S, Batra N. Toxoplasmosis in organ transplant recipients: evaluation, implication, and prevention. Trop Parasitol. 2016;6:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saadatnia G, Golkar M. A review on human toxoplasmosis. Scand J Infect Dis. 2012;44:805-814. [DOI] [PubMed] [Google Scholar]
- 4. Assi MA, Rosenblatt JE, Marshall WF. Donor-transmitted toxoplasmosis in liver transplant recipients: a case report and literature review. Transpl Infect Dis. 2007;9:132-136. [DOI] [PubMed] [Google Scholar]
- 5. Mayes JT, O’Connor BJ, Avery R, et al. Transmission of Toxoplasma gondii infection by liver transplantation. Clin Infect Dis. 1995;21:511-515. [DOI] [PubMed] [Google Scholar]
- 6. Webb GJ, Shah H, David MD, et al. Post-prophylaxis Toxoplasma chorioretinitis following donor–recipient mismatched liver transplantation. Transpl Infect Dis. 2016;18:805-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernàndez-Sabé N, Cervera C, Fariñas MC, et al. Risk factors, clinical features, and outcomes of toxoplasmosis in solid-organ transplant recipients: a matched case-control study. Clin Infect Dis. 2012;54:355-361. [DOI] [PubMed] [Google Scholar]
- 8. Herman D, Han H. Cytomegalovirus in liver transplant recipients. Curr Opin Organ Transplant. 2017;22:345-350. [DOI] [PubMed] [Google Scholar]
- 9. Jorgensen MR, Descourouez JL, Leverson GE, et al. High-dose acyclovir for cytomegalovirus prophylaxis in seropositive abdominal transplant recipients. Ann Pharmacother. 2018;52: 5-10. [DOI] [PubMed] [Google Scholar]
- 10. Baldwin N, Gray M, Al Diffalha S. S2441 acute liver failure due to toxoplasmosis after orthotopic liver transplantation. Am J Gastroenterol. 2020;115:S1293. doi: 10.14309/01.ajg.0000711812.22789.ea [DOI] [PMC free article] [PubMed] [Google Scholar]



