Major advancements in antiretroviral therapy (ART) have been accomplished in the last two decades for the treatment of persons with HIV (PWH). This is largely the result of newer and more potent antiretroviral drugs that effectively suppress viral replication and are associated with fewer side effects.
Despite this, the success of modern ART still relies on overall patient adherence. Therefore, accurately quantifying ART adherence remains of critical importance in clinical practice and research settings.
Currently, a wide range of methods are used to assess adherence, each with specific advantages and limitations.1 Subjective measures of adherence (e.g., self-report) are easy to implement, but are subject to both reporting and recall biases.2 Pharmacy refills and pill counts are usually considered more objective, but are difficult to implement in clinical practice and do not confirm drug ingestion.1 Since high adherence (i.e., >95%) was initially required to achieve and sustain viral suppression due to less potent drugs,3 HIV viral load (VL) has been used as a surrogate for high ART adherence for many years. However, with the increased potency of new regimens, lower adherence may be sufficient to achieve viral suppression.4 While this “forgiveness” to more potent drugs is advantageous, it results in less-than-optimal adherence that cannot be identified by relying on HIV VL alone, and has been found to have deleterious consequences, as it has been associated with heightened inflammation, immune activation, coagulopathy, and mortality.5–7
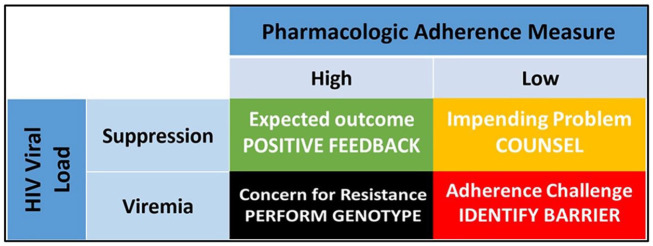
Pharmacologic measures of adherence are objective and can avoid subjective biases.1 However, they are dependent on the pharmacokinetics of the parent drug or metabolite that is quantified in the body fluid or matrix of interest. Short-term measures of recent dosing (i.e., drug concentration in plasma or urine and emtricitabine triphosphate in dried blood spots), are limited by the short half-lives of antiretrovirals in those matrices, thus translating into qualitative (i.e., yes/no for a recent dose) adherence information that is subject to white coat bias.1 In comparison, pharmacologic measures of cumulative (i.e., long-term) adherence, such as drug (or metabolite) concentrations in dried blood spots and hair samples, can provide a more informative picture of cumulative drug intake over the preceding 4–12 weeks,1 similar to the information provided by hemoglobin A1c (HbA1c) in persons with diabetes mellitus. In particular, tenofovir diphosphate (TFV-DP) in dried blood spots has been associated with viral suppression,8,9 and is predictive of future viremia in PWH who are virologically suppressed.10–12 Thus, this – and other – pharmacologic adherence biomarkers could be used to inform about ART adherence beyond HIV VL, allowing for a more comprehensive understanding of the level of adherence that resulted in viremia or suppression. In this context, TFV-DP in DBS as a marker of cumulative adherence, measured in conjunction with HIV VL, would result in four unique clinical interpretations as described below (Figure 1):
Figure 1.
Interpreting concomitant HIV viral suppression and drug concentrations in PWH. The green and red squares represent expected clinical scenarios in patients on ART in whom the pharmacologic adherence measures (i.e., drug concentrations) match the clinical outcome (i.e., viral suppression or viremia) and in whom positive feedback (green) or the identification of an adherence barrier (red) are indicated. The black square represents a mismatch scenario where HIV resistance testing is indicated in the setting of high viremia despite high drug concentrations that are expected to be suppressive. The yellow square represents a mismatch scenario in a patient who is virologically suppressed despite having low drug concentrations and in whom an adverse outcome (i.e., viremia, HIV transmission, heightened inflammation) might be imminent, requiring immediate counseling intervention. Adapted with permission from Spinelli et al.1
ART, antiretroviral therapy; PWH, persons with HIV.
Viral suppression with high drug concentrations
This is an expected – and very likely – scenario where cumulative adherence (i.e., high drug concentrations) match the clinical outcome (i.e., undetectable HIV VL), informing PWH and medical providers that the patient is adhering to ART and that treatment is effective (Figure 1, green square). As such, this could serve as positive reinforcement to encourage continued adherence and to reassure the patient of the treatment’s efficacy, as is commonly done in other chronic medical conditions, such as diabetes mellitus.13 This feedback could also reinforce the view that, by remaining highly adherent and virally suppressed, PWH greatly reduce their risk of progression to AIDS and of HIV transmission.
Viremia with low drug concentrations
For PWH in this scenario, the quantification of low cumulative adherence would match an unsuppressed HIV VL, informing patients and providers that treatment failure is most likely due to low adherence (Figure 1, red square). In this case, the clinician could use this information to initiate an open and honest discussion with the patient and identify challenges or barriers to adherence (side effects, cost of medication, competing events, etc.) and seek out an appropriate solution.14 In addition, this could be an opportunity to remind PWH that low adherence and viremia are associated with the development of AIDS, non-AIDS comorbidities, and transmission.
Viremia with high drug concentrations
This is a mismatch scenario where HIV VL is elevated despite high cumulative adherence as measured by high drug concentrations (Figure 1, black square). Low-level or residual viremia have been associated with lower cumulative adherence,15 but can also occur in patients who are highly adherent.16 However, residual viremia has been found mostly to predict development of drug resistance and virologic failure only in patients with lower adherence.15,17 More informative, however, would be high viremia in patients with high cumulative adherence, which is most likely due to antiretroviral resistance.18–20 Thus, when facing this clinical presentation (i.e., high adherence and high viremia), providers could be more inclined to order HIV drug resistance testing rather than have repeated discussions about adherence (with the potential of further perpetuating the accumulation of drug resistance).
Viral suppression with low drug concentrations
The last scenario, another mismatch, is also highly informative – and perhaps the most impactful – as it encompasses PWH who are virally suppressed despite having low ART adherence (Figure 1, yellow square). Currently, interventions aimed at improving ART adherence are implemented only in PWH who are already viremic and might have already transmitted HIV, overlooking PWH in this fourth category. Since low cumulative adherence is predictive of future viremia,10–12 even in PWH who are virally suppressed at the time of testing,10 identifying PWH at high risk of viremia before it occurs could trigger an early intervention to prevent adverse health outcomes. In addition, it could help identify PWH who are undergoing frequent ART interruptions, which have also been associated with heightened residual inflammation, immune activation, and coagulopathy,7 and identify early adherence barriers.
In conclusion, pharmacologic measures of ART adherence can provide important clinical information beyond HIV VL, with the potential of better predicting outcomes in HIV treatment. The above-mentioned case-scenarios assume a rapid turnaround time for drug level assessment, which is currently approximately 1–2 weeks for laboratory-based assays. However, point-of-care assays of cumulative adherence are in development.21 As data on the clinical utility of these measures continues to accumulate, further research on their implementation and their utility to provide constructive feedback will be required.
Footnotes
Author contributions: MK, PLA, JCM: idea; MK and JCM: first draft; PLA: edits and corrections.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health [R01 AI145453 to J.C.M.; R01 AI122298 to P.L.A.]. The other authors reported no conflicts of interest.
Conflict of interest statement: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. PLA received personal fees and received research support from Gilead Sciences paid to his institution. Other authors reported no conflict of interest.
ORCID iD: Jose R. Castillo-Mancilla  https://orcid.org/0000-0003-1242-1745
https://orcid.org/0000-0003-1242-1745
Contributor Information
Michael Kristofich, Colorado Antiviral Pharmacology Laboratory and Department of Pharmaceutical Sciences, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of Colorado-AMC, Aurora, CO, USA.
Peter L. Anderson, Colorado Antiviral Pharmacology Laboratory and Department of Pharmaceutical Sciences, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of Colorado-AMC, Aurora, CO, USA
Jose R. Castillo-Mancilla, Jose R. Castillo-Mancilla Division of Infectious Diseases, Department of Medicine, School of Medicine, University of Colorado, Anschutz Medical Campus, 12700 E 19th Ave., B168, Aurora, CO 80045, USA.
References
- 1. Spinelli MA, Haberer JE, Chai PR, et al. Approaches to objectively measure antiretroviral medication adherence and drive adherence interventions. Curr HIV/AIDS Rep 2020; 17: 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simoni JM, Kurth AE, Pearson CR, et al. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav 2006; 10: 227–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133: 21–30. [DOI] [PubMed] [Google Scholar]
- 4. Byrd KK, Hou JG, Hazen R, et al. Antiretroviral adherence level necessary for HIV viral suppression using real-world data. J Acquir Immune Defic Syndr 2019; 82: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castillo-Mancilla JR, Cavassini M, Schneider MP, et al. Association of incomplete adherence to antiretroviral therapy with cardiovascular events and mortality in virologically suppressed persons with HIV: the Swiss HIV Cohort Study. Open Forum Infect Dis 2021; 8: ofab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castillo-Mancilla JR, Brown TT, Erlandson KM, et al. Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis 2016; 63: 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Musinguzi N, Castillo-Mancilla JR, Morrow M, et al. Antiretroviral therapy adherence interruptions are associated with systemic inflammation among Ugandans who achieved viral suppression. J Acquir Immune Defic Syndr 2019; 82: 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castillo-Mancilla JR, Morrow M, Coyle RP, et al. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with human immunodeficiency virus infections. Clin Infect Dis 2018; 68: 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Phillips TK, Sinxadi P, Abrams EJ, et al. A comparison of plasma efavirenz and tenofovir, dried blood spot tenofovir-diphosphate, and self-reported adherence to predict virologic suppression among South African women. J Acquir Immune Defic Syndr 2019; 81: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morrow M, MaWhinney S, Coyle RP, et al. Predictive value of tenofovir diphosphate in dried blood spots for future viremia in persons living with HIV. J Infect Dis 2019; 220: 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Odayar J, Phillips TK, Hu N-C, et al. Tenofovir diphosphate to predict future viraemia in postpartum women living with HIV. In: CROI virtual conference, 6–10 March 2021. Abstract 93. [Google Scholar]
- 12. Robbins R, Jennings L, Nguyen N, et al. Tenofovir diphosphate in dried blood spots predicts future viremia in South Africa. In: CROI virtual conference, 6–10 March 2021. Abstract 398. [Google Scholar]
- 13. Gopalan A, Tahirovic E, Moss H, et al. Translating the hemoglobin A1C with more easily understood feedback: a randomized controlled trial. J Gen Intern Med 2014; 29: 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med 2016; 13: e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li JZ, Gallien S, Ribaudo H, et al. Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS 2014; 28: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palich R, Wirden M, Peytavin G, et al. Persistent low-level viraemia in antiretroviral treatment-experienced patients is not linked to viral resistance or inadequate drug concentrations. J Antimicrob Chemother 2020; 75: 2981–2985. [DOI] [PubMed] [Google Scholar]
- 17. Pasternak AO, de Bruin M, Jurriaans S, et al. Modest nonadherence to antiretroviral therapy promotes residual HIV-1 replication in the absence of virological rebound in plasma. J Infect Dis 2012; 206: 1443–1452. [DOI] [PubMed] [Google Scholar]
- 18. Yager JL, Coyle RP, Coleman SS, et al. Moderately high tenofovir diphosphate in dried blood spots indicates drug resistance in viremic persons living with HIV. J Int Assoc Provid AIDS Care 2019; 18: 2325958219888457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gandhi M, Ameli N, Bacchetti P, et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS 2009; 23: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castillo-Mancilla J, Zhao Y, Brijkumar J, et al. Tenofovir diphosphate in dried blood spots predicts virologic filuare and resistance. In: Virtual conference on retroviruses and opportunistic infections, 8–11 March 2020. Poster 520. [Google Scholar]
- 21. Pu F, Pandey S, Bushman LR, et al. Direct quantitation of tenofovir diphosphate in human blood with mass spectrometry for adherence monitoring. Anal Bioanal Chem 2020; 412: 1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]