Abstract
Maximal voluntary inspiratory breath-holding time (MVIBHT) has proved to be of clinical utility in some obstructive ventilatory defects. This study aims to correlate the breath-holding time with pulmonary function tests in patients with chronic obstructive pulmonary disease (COPD) and to determine the feasibility of using a breath-holding test in assessing the severity of COPD.
A cross-sectional study including male patients with stable COPD were conducted. Patients with respiratory comorbidities and severe or unstable cardiac diseases were excluded. Patients were interviewed and examined. Six-minute walk test (6MWT) and plethysmography were performed.For MVIBHT collection, the subject was asked to inspire deeply and to hold the breath as long as possible at the maximum inspiratory level. This maneuver was repeated three times. The best value was used for further analysis.
A total of 79 patients (mean age: 64.2 ± 8) were included in this study. The mean value of MVIBHT was 24.2 ± 8.5 s. We identified a positive and significant correlations between MVIBHT and forced vital capacity (r = .630; p < .001) as well as MVIBHT and forced expiratory volume in 1 s (FEV1%) (r = .671; p < .001). A significant inverse correlation with total lung capacity (r = −.328; p = .019) and residual volume to total lung capacity ratio (r = −.607; p < .001) was noted. MVIBHT was significantly correlated to the distance in the 6MWT (r = .494; p < .001). The mean MVIBHT was significantly different within spirometric grades (p < .001) and GOLD groups (p = .002). At 20.5 s, MVIBHT had a sensitivity of 72% and specificity of 96% in determining COPD patients with FEV1 <50%.
Our results provide additional evidence of the usefulness of MVIBHT in COPD patients as a pulmonary function parameter.
Keywords: breathing test, pulmonary function, chronic obstructive pulmonary disease, apnea, spirometry, six minute walk test
Chronic obstructive pulmonary disease (COPD) is a persistent, usually progressive lung disease due to airway or alveolar abnormalities caused by exposure to noxious particles or gases (Vogelmeier et al., 2017). COPD is a common disease, with an estimated prevalence of 9% in East Asia and 15.2% in America in 2015 (Adeloye et al., 2015). In Tunisia, its prevalence is estimated at 3.7% in the population aged over 40 years in 2011 (Tageldin et al., 2012).
Apnea is defined as a temporary cessation of gas exchange between the lungs and the atmosphere. The example of voluntary apnea is unique in physiology because of the human ability to consciously interrupt a vegetative process that is submitted to the regulation of a neurological vital system. The maximal duration of voluntary apnea varies from subject to subject. It depends on chemical and non-chemical stimuli and is reduced by increased feedback from diaphragm afferents or an increase in central stimuli.
The maximal voluntary breath-holding tele-inspiratory test (MVIBHT) is a quick and simple test that consists of measuring the apnea time after a deep inspiration. The clinical utility of this test has been proved in some respiratory pathologies. It has been reported that this test is an interesting tool to detect asthmatic subjects with a poor perception of dyspnea, which is a risk factor for fatal asthma (Nannini et al., 2007). In adult subjects with cystic fibrosis, MVIBHT is correlated with oxygen consumption at the anaerobic threshold, providing information about the exercise capacity of these subjects (Barnai et al., 2005).
However, there are little data on the utility of MVIBHT in COPD. Vieicili and al. investigated the relationship between MVIBHT and spirometric parameters in patients with obstructive respiratory disease. A significant correlation was identified between MVIBHT and the degree of bronchial obstruction during COPD (Viecili et al., 2012).
The main objective of this study is to assess the relationship between and COPD severity. The secondary objectives are to determine its correlation with six minute walking test (6MWT) and to study its utility in the detection of severe bronchial obstruction in COPD.
Methods
A cross-sectional study was carried in the department of pneumology and allergology of LaRabta University Hospital in Tunis, during the period from September 2018 to December 2019.
Patients with stable COPD at different stages of severity were recruited. The diagnosis of COPD was made according to the GOLD 2017 classification (Vogelmeier et al., 2017).
Inclusion criteria were male gender, age between 45 and 85 years, smoking more than 10 packs per year, and stable COPD for at least 3 months. We excluded patients with associated respiratory diseases (bronchiectasis, asthma, sleep apnea syndrome, interstitial lung disease, pulmonary tuberculosis), severe coronary heart disease, cardiac arrhythmias, glaucoma, unstable angina, retinal detachment, uncontrolled hypertension, and patients on treatments that might interfere with the respiratory system such as Benzodiazepine.
The design and conduct of the study were in accordance with the general principles outlined in the Declaration of Helsinki (World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects, 2001). The ethics committee of La Rabta Hospital approved the study and all subjects have given signed and informed consent in our study.
Study Conduct
All patients were interviewed and the following data were collected in a standardized questionnaire: sociodemographic data, smoking habits, symptoms (Medical Research Council (mMRC)), dyspnea, number of exacerbations in the previous year, COPD treatment, and quality of life assessment using the validated Arabic version of the COPD Assessment Test (CAT) (Nannini et al., 2007). The following anthropometric parameters were recorded: Height in meters (m), weight in kg, body mass index (BMI) weight/height² (Kg/m²). Normal BMI values were between 20 and 24.9 Kg/m². Obesity was defined by a BMI ≥ 30 Kg/m² (Barnai et al., 2005).
Patients had respiratory function tests including:
-Body plethysmography using a plethysmograph (Medisoft Bodybox 5500). The following parameters were noted: tidal volume, inspiratory capacity, expiratory capacity, Forced vital capacity (FVC), Expiratory reserve volume (ERV), Inspiratory reserve volume (IRV), Peak expiratory flow, Forced Expiratory Volume in the First minute (FEV1), Total Lung Capacity(TLC), vital capacity and Residual Volume(RV).
- Six-minute walk test (6MWT) was conducted according to the American thoracic society recommendations (ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories, 2002).
- Blood gases were performed using an automated system (Nova Biomedical Stat Profile Prime plus). The parameters studied were: blood pH, oxygen partial pressure (PaO2), carbon dioxide partial pressure (PaCO2), oxygen saturation (SaO2), bicarbonate level in the blood (HCO3-). Hypoxemia is defined by a PaO2 lower than 100-0.03 × age.
For MVIBHT collection, patients were asked to not perform a vigorous exercise in the two hours before the test. They were seated comfortably in a chair with the nose pinched by a nose clip. After 1 min of normal breathing, they were asked to do a deep exhalation followed by deep inhalation and then to remain in apnea as long as possible. No encouragement or indication of elapsed time were given to the subjects. This procedure was repeated three times with 5 min intervals between tests. The best test was taken for further analysis.
The 6MWT and the MVIBHT were performed by the same operator for all patients. The order of performance was drawn at random. There was a 2-hr interval between the two tests.
Statistical Analysis
The statistical study was conducted using IBM® SPSS Statistics® version 22.0 software. The normality of all continuous variables was tested using a Shapiro-Wilk test. These were expressed as mean and standard deviation if their distribution was normal or as median and interquartile range if not. Quantitative variables were expressed as frequencies.
For analysis of qualitative variables, we used the Fisher’s test when applicable, otherwise, we used the χ2 test.
Pearson’s correlation test was used to find a correlation between apnea time and the different data from the functional explorations.
We used the Student t test to compare two means and the Wilcoxon-Mann-Whitney test to compare two medians. We applied the ANOVA test for the comparison of several means.To compare the correlation coefficients, we used the Fisher transformation.
The analysis of the second objective consisted of performing the unilateral test with 5% risk: The 95% confidence interval (95% CI) of the area under the ROC curve was calculated.
An optimal threshold was determined by maximizing the Yuden index, which is equal to sensitivity+specificity-1. We then calculated the following parameters for the optimal threshold: sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios from the contingency table.
A two-sided p-value < .05 was considered significant for all analyses.
The calculation of the number of subjects required to estimate the AUC of the ROC curve was performed using the method defined by Rick Zepp. The area under the assumed curve was estimated to be 0.8. Considering a first species risk of .05, a one-sided test, and a ratio of 1 between the positive and negative groups, 26 subjects had to be recruited. However, 47 subjects were required to obtain a Pearson “r” correlation of .4 with a first-species risk of .05.
Results
During the study period, a total of 254 patients with COPD were initially recruited. Seventy-nine subjects met the inclusion criteria and were included in the analysis after having given signed, informed consent in our study.
The mean age was 64.1 ± 8 years with extremes ranging from 49 to 77 years. All patients were smokers, with a mean cumulative dose of 56.77 ± 28 pack/year and a maximum dose of 150 pack/year. Among the cases, four patients (7.7%) had comorbidities. Three patients had stable hypertension under treatment and one patient had cardiac rhythm disorder (Atrial Fibrillation). The median BMI was 21.34 kg/m2 with an interquartile range of 4.95 and values ranging from 14.4 kg/m to 33.2 kg/m. Five patients (5.7%) were obese (table 1). Twenty-one (26.9%) patients were classified as GOLD A, 17 patients (21.1%) as GOLD B, nine patients (11.5%) as GOLD C, and 32 patients (40.4%) as GOLD D. Dyspnea was reported in 67 patients (84.6%), with an mMRC score ≥2 in 44 patients (55.8%). The CAT score was ≥10 in 40 patients (50%). The mean FEV1/FVC ratio was 51.18 ± 9.36% with a mean FEV1 of 1.51 ± 0.72 L (52.15 ± 21.41%). GOLD stage 1 was noted in 12 patients (15.4%), GOLD 2 in 27 patients (34.6%), GOLD C in 32 patients (40.4%); and GOLD 4 in 8 patients (9.6%).
Table 1.
Anthropometric Parameters of the Study Population.
| BMI | N | % |
|---|---|---|
| <20 Kg/m² | 14 | 17.3 |
| 20–25 Kg/m² | 50 | 63.4 |
| 25–30 Kg/m² | 10 | 13.6 |
| >30 Kg/m² | 5 | 5.7 |
Note. BMI = body mass index.
Blood gases revealed hypoxemia in 23 patients (44.23%) with a mean PaO2 of 79.8 ± 7.5 mmHg. The median oxygen saturation taken at the end of the test was 94%. During the 6MWT, the mean distance was 483.95 ± 106.86 meters with extremes ranging from 220 to 660 m. The 6-min walking distance was decreased in 12 patients (15.38%) and 8 patients (9.8%) walked a distance less than 350 m.
The mean recorded apnea time was 25 ± 8.7 s with values ranging from 10 s to 50 s. The mean heart rate after the test was 89.56 ± 15.19 bpm. The median oxygen saturation was 95%.
Classification of patients according to BODE score was: BODE 0–2 in 33 patients (42.31%), BODE 3–4 in 26 patients (32.67%), BODE 5–6 in 15 patients (19.23%), and BODE 7–10 in 5 patients (5.77%).
The respiratory parameters of the study population are presented in Table 2.
Table 2.
Pulmonary Function Parameters of the Study Population.
| Average | Standard deviation | Extreme values | |
|---|---|---|---|
| FEV1 (L) | 1.51 | 0.72 | 0.56–4.33 |
| FEV1 (%) | 52.15 | 21.41 | 21–98 |
| FVC (L) | 2.87 | 0.91 | 1.37–6.25 |
| FVC(%) | 77.60 | 20.19 | 42–132 |
| FEV1/FVC(%) | 51.18 | 9.36 | 32–67 |
| RV (L) | 4.66 | 1.37 | 1–8 |
| RV (%) | 194.37 | 61.86 | 7–304 |
| TLC (L) | 7.55 | 1.13 | 5–10 |
| TLC (%) | 118 | 16.81 | 85–150 |
| RV/TLC (%) | 62.46 | 11.46 | 28.49–83.01 |
| 6MWT distance(m) | 483.95 | 106.86 | 220–260 |
Note. FEV1 = forced expiratory volume in the first minute; FVC = forced expiratory volume in the first minute; RV = residual volume; TLC = total lung capacity; 6MWT = six-minute walk test.
The MVIBHT was not correlated with age (r = −0.69; p = .626), tobacco consumption (r = −0.81; p = .566), BMI (r = −.006; p = .964), dyspnea stage (p = 0.305) or CAT score (r = −0.219; p = .119). However, the mean apnea time in patients with a CAT ≥10 (26.58 s) was significantly higher than in patients with a CAT <10 (21.77 s); (p = .041).
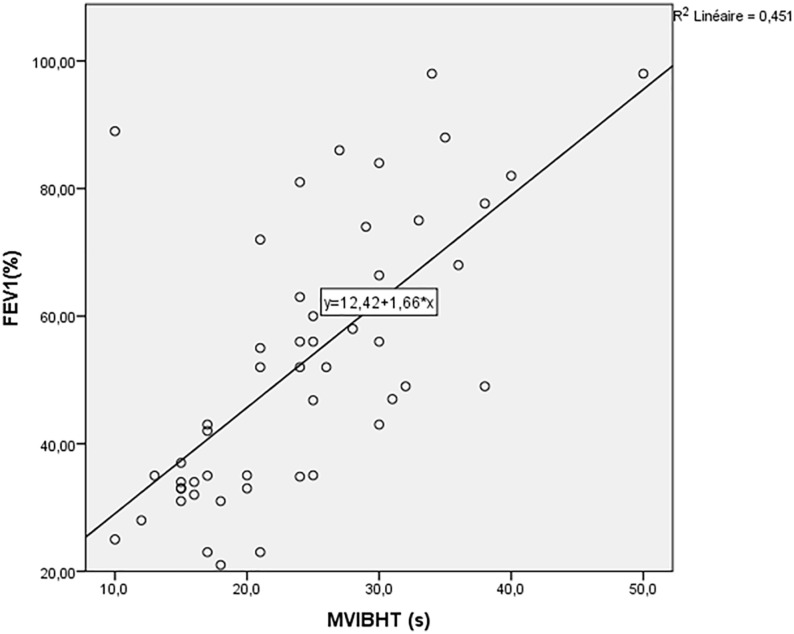
A significant correlation was identified between MVIBHT and FEV1 (r = .686; p < .0001) (Figure 1), FVC (r = .632) and FEV1/FVC ratio (r = .645). Besides, MVIBHT was significantly and inversely correlated with TLC (r = −0.328) and RV/TLC ratio (r = −.607). The correlations between the spirometry parameters and the MVIBHT is shown in Table 3.
Figure 1.
Variation of maximal voluntary inspiratory breath-holding time according to the FEV1 (%).
MVIBHT, maximal voluntary inspiratory breath-holding time.
FEV1, forced expiratory volume in the first second.
Description: MVIBHT was significantly correlated with FEV1 (r = .686; p < .001).
Table 3.
Correlations of Maximal Apnea Time with Spirometric Parameters.
| Pearson correlation (r) | Significance tests (p) | Confidence interval 95% | |
|---|---|---|---|
| FEV1 (L) | .686 | <.001 | [0.508; 0.808] |
| FEV1 (%) | .671 | <.001 | [0.487; 0.798] |
| FVC (L) | .632 | <.001 | [0.433; 0.772] |
| FVC(%) | .630 | <.001 | [0.431; 0.770] |
| FEV1/FVC | .645 | <.001 | [0.451; 0.780] |
| RV (L) | −.406 | .003 | [−0.612; −0.147] |
| RV (%) | −.311 | .026 | [−0.540; −0.039] |
| TLC (L) | −.226 | .111 | |
| TLC (%) | −.328 | .190 | |
| RV/TLC | −.607 | <.001 | [−0.757; −0.395] |
| IRV (L) | .404 | .004 | [0.141; 0.613] |
| ERV (L) | .451 | .001 | [0.197; 0.648] |
| IC (L) | .510 | <.001 | [0.272; 0.688] |
| EC(L) | .451 | .001 | [0.200; 0.646] |
| IC/TLC | .514 | <.001 | [0.277; 0.691] |
FEV1 = forced expiratory volume in the first minute; FVC = forced expiratory volume in the first minute; RV = residual volume; TLC = total lung capacity; IRV = inspiratory reserve volume; ERV = expiratory reserve volume; IC = inspiratory capacity; EC = expiratory capacity.
The MVIBHT was positively correlated with PaO2 (r = .415; p = .003) and SpO2 (r = .320; p = .023) and inversely correlated with alkaline reserve (r = −0.287; p = .043). There was a significant positive correlation between MVIBHT and 6MWT distance (r = .494; p < .0001).
The MVIBHT was statistically different among the COPD severity groups according to the degree of bronchial obstruction (p <.001), GOLD groups (p = .002) and BODE index groups (p = .002) (Figure 2). Indeed MVIBHT was lower in patients with severe COPD.
Figure 2.
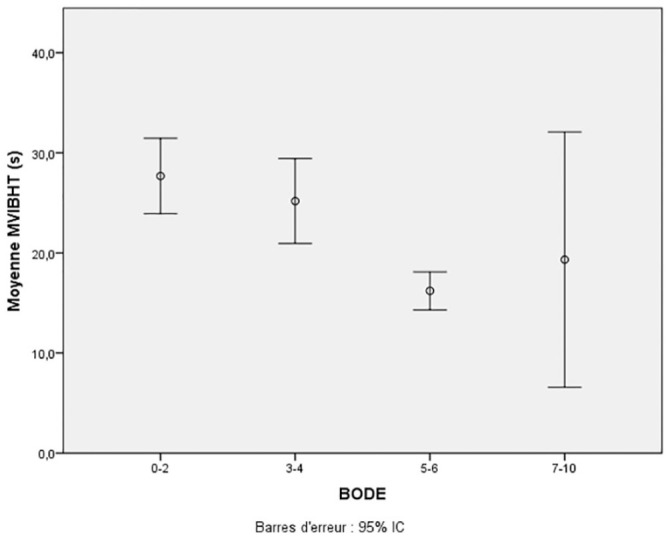
Variation of maximal voluntary inspiratory breath-holding time according to BODE score.
MVIBHT = maximal voluntary inspiratory breath-holding time.
Description: The MVIBHT was statistically different among the mean of MVIBHT for BODE index groups (p = .002).
In multivariate analysis, several models were used. The parameters that were significantly correlated with MVIBHT were the RV/TLC ratio (p = .006) and FEV1.
Assessment of the Discriminating Power of MVIBHT in the Detection of Severe Bronchial Obstruction
The area under the curve was 0.822 with a 95% confidence interval [0.7; 0.945].
The cut-off value chosen for an optimal Youden’s index of 0.65 is 20.5 s. Thus, a MVIBHT fewer than 20.5 s was considered to be the threshold for severe obstruction of COPD and therefore a “positive test.”
The contingency table, which counts the number of patients having the two characters “MVIBHT >20.5” and “FEV1 <50%” is presented in Table 4.
Table 4.
Contingency Table.
| FEV1<50% | FEV1>50% | Total | |
|---|---|---|---|
| MVIBHT <20.5 s | 18 | 1 | 19 |
| MVIBHT >20. s | 8 | 25 | 33 |
| Total | 26 | 26 | 52 |
Note. FEV1 = forced expiratory volume in the first minute; MVIBHT = maximal voluntary inspiratory breath-holding time.
The calculated χ2 was 23.97. If we set a risk of error alpha = 5%, the theoretical χ2 value is 3.84 (χ2 × (1.0.05)). The relationship between the two characters is then statistically significant. The different performance indicators have been deduced in Table 5.
Table 5.
Maximal Voluntary Inspiratory Breath-Holding Time Performance Indicator.
| Value | Confidence interval 95% | |
|---|---|---|
| Sensibility | 0.69 | [0.5; 0.835] |
| Spécificity | 0.96 | [0.811; 0.993] |
| Positive likelihood ratio | 18 | [2.589; 125.121] |
| Negative likelihood ratio | 0.32 | [0.179; 0.572] |
Discussion
We conducted a cross-sectional study including patients with confirmed and stable COPD. Our results showed that MVIBHT was significantly correlated with FEV1 (r = .686; p < .0001), FVC (r = .632), FEV1/FVC ratio (r = .645), COPD GOLD stage, and 6MWT distance.
Besides, MVIBHT was shown to have good discriminating power for severe forms of COPD with an AUC of 0.822 (95% CI 0.7–0.945). Thus, an MVIBHT fewer than 20.5 s allows the detection of FEV1 <50% with a specificity of 96% and a sensitivity of 72%.
In our study, the mean age was 64.1 ± 8 years with extremes ranging from 49 to 77 years. In most studies, the mean age was higher and ranged from 68.8 ± 9 years to 74.2 ± 9.4 years (Charles et al., 2017; Shingai & Kanezaki, 2014). Patients with chronic conditions associated with COPD that may interfere with respiratory functional abilities were not included and this would explain the young age of our population.
No significant correlation was reported between apnea time and age. These results were also observed in healthy subjects (Trembach & Zabolotskikh, 2017). Moreover, no significant correlation was identified between apnea maximal time and in the study population. Hurewitz and Sampson reported that PaO2 decreased to 70 mmHg in obese subjects after 15 s of apnea while this level of hypoxia was only observed in non-obese subjects after 30 s of apnea (Hurewitz & Sampson, 1987).
In our study, COPD patients were classified as GOLD C and D in 51.9% of cases. Severe grades of COPD were associated with poorer perceived quality of life. We reported a significant difference in MVIBHT between groups of subjects classified by the CAT score. The MVIBHT was significantly correlated with FEV1(%), FVC(%), and FEV1/FVC ratio. In a comparative study aimed to evaluate the relationship of tele-inspiratory and tele-expiratory apnea times with spirometric parameters (Viecili et al., 2012), a positive correlation was identified between tele-inspiratory apnea time and FEV1 (L) and FVC (L). These results are not statistically different from those deduced in the present study. Furthermore, the apnea time was correlated with the RV and the RV/TLC ratio. These findings highlight the role of thoracic distension in the genesis of dyspnea in COPD patients.
Mechanical disadvantages include a reduction in the appositional component of diaphragmatic action and a reduction in the insertional component. Chronic pulmonary hyperinflation is associated with the foreshortening of the diaphragm. Distension also changes its spatial conformation as it leads to the flattening of the muscle, thus reducing the capacity of the muscle to undergo length changes (Cassart et al., 1997; Decramer, 1989; De Troyer, 1997).
In our study, resting blood gases revealed hypoxemia in 44.23% of subjects with a mean PaO2 of 79.8 ± 7.5 mmHg. The means of MVIBHT for subjects subdivided by resting blood gases were not significantly different. Besides, we reported a positive correlation between resting PaO2 and MVIBHT. No relationship was identified with resting capnia.
The ventilatory response to hypoxemic and hypercapnic stimuli in COPD patients is the subject of many studies. It was conventionally claimed that in these patients, chronic hypercapnia desensitizes the chemoreceptors to PaCO2 changes and therefore the hypoxic stimulus would be preponderant. However, some studies have shown that hypercapnic patients have a ventilatory control similar to normocapnic subjects (Scano et al., 1995).
In our study, the mean distance covered during a 6MWT was 483.95 ± 106.86 m. We reported a positive correlation between apnea time and the walking distance. The study carried out by (Charles et al., 2017) also showed a positive correlation between the two parameters.
In case of expiratory flow limitation during exercise in COPD patients, a resetting of the respiratory system’s volume to a higher level is necessary. During exercise, due to the increased respiratory rate and tidal volume, there is insufficient expiratory time for complete lung emptying. This leads to a gradual increase in tele-expiratory volume with each respiratory cycle. This phenomenon is called dynamic hyperinflation. Thus, with exercise, the rise in tidal volume occurs in the COPD patient at the cost of a decrease in inspiratory capacity (O’Donnell, 2008). Some studies have demonstrated statistical relationships between dyspnea intensity, walking capacity, and dynamic hyperinflation measured with exercise (Laveneziana et al., 2011; O’Donnell et al., 2012; Puente-Maestu et al., 2005). The sensation of dyspnea could result from the mismatch between increased ventilatory control and decreased afferent return due to the fixed tidal volume that is a direct consequence of dynamic lung hyperinflation (O’Donnell et al., 2006, 2007). In COPD, the loss of elasticity is the main cause of dynamic and static distension. This may explain the relationship reported between MVIBHT and 6MWT during our study.
Indeed, voluntary control of breathing is similar to other voluntary movements and requires the activation of an integrated network of cortical and subcortical areas. Numerous brain imaging studies of dyspnea have recorded predominant neuronal activity in the regions of the insula, frontal cortex, anterior and posterior cingulate cortex, cerebellum, thalamus, and tonsil (von Leupoldt et al., 2008; von Leupoldt & Dahme, 2005).
The common predominant activity during static exercise was identified in the primary sensorimotor cortex and the insular cortex (Sander et al., 2010). Shingai et al. also showed that the perception of dyspnea induced by breath-holding impairs maximal muscular strength in stable patients with COPD (Shingai & Kanezaki, 2014).
Indeed, dyspnea specific receptors remain unclear. However, one theory suggests that dyspnea results from an imbalance between the central motor respiratory control and the peripheral response of the respiratory system. A mismatch between discharge and respiratory afferents is the subject of negative cognitive-affective treatment and leads to dyspneic sensation (O’Donnell et al., 2006; Stock et al., 2013).
During the apnea test, the relationship between the dyspneic sensation and the stimulus, which in our case is voluntary apnea, is linear. and is modeled by a straight line whose slope reflects the sensitivity of the process (Siomopoulos, 1975) If a normal subject holds his or her breath from total lung capacity, he or she experiences no respiratory discomfort until the onset of the dyspneic sensation. The period between the onset of apnea and the onset of dyspneic sensation can be considered a “comfort phase.” At the end of this phase, the first electromyographic pulses of the diaphragm are recorded (Bain et al., 2018). This phase is followed by a “struggle phase” which ends with the rupture of the apnea. In COPD subjects, even a slight increase in respiratory stimulus may cause a shortening of both the duration of the comfort phase and the duration of total apnea (Nishino, 2009).
The mean apnea time reported during our study was 25 ± 8.7 s with values ranging from 10 s to 50 s. This value was not statistically different from that reported by Viecili et al. which was 21.6 ± 12.6 s (t = 1.245; p = .214) (Viecili et al., 2012). Maximum apnea time in COPD subjects has been shown to be significantly shorter than in normal subjects (Trembach & Zabolotskikh, 2017). Indeed, the maximum apnea time is shortened by any factors increasing the tonic activity of the diaphragm or repressing the activity of the respiratory centers such as hypoxemia, hypercapnia, and reduced lung volume (Parkes, 2006). In fact, during COPD, static distension is the result of the loss of elastic recoil pressure from the pulmonary parenchyma. This induces an increase in compliance leading to an increase in total lung capacity and the establishment of a new state of equilibrium between chest expansion pressure and parenchymal retraction pressure associated with an increased functional residual capacity (Palecek, 2001).Other experiments have shown the implication of non-mechanical factors, as the duration of voluntary apnea doubled after inhaling a hyperoxic mixture or after pre-hyperventilation (Klocke & Rahn, 1959). Besides, the duration of apnea has been reduced under conditions of hypoxemia and hypercapnia (Godfrey & Campbell, 1969).
Our study had some limitations related to the external validity of the results obtained due to:
Small size of the study sample.
Exclusion of female patients: in our contry, women are less affected by COPD. Besides the number of women treated for COPD is minor (we only included two female patients) compared with men in our department. Hence, their inclusion can be a source of bias.
Low prevalence of co-morbidities associated with COPD among recruited subjects.
Anxiety depression questionnaires could disclose whether there exists a relationship between dyspnea perception and emotional factors that were not contemplated in this trial.
Breath-holding duration is heavily influenced by starting lung volume and arterial PCO2 at the onset of apnea. These values were not measured, and neither was PetCO2.
Therefore, the results cannot be generalized or transferred without adaptability conditions.
The lack of recommendations and the lack of literature on MVBIHT did not allow us to have a pre-established protocol.
Investigator intervention was minimized by prohibiting any encouragement or timing information during the performance of the test.
When carrying out our study, we explained to the participants that they will successively perform two tests, one of which, implicitly, requires relatively physical effort. This may then influence the results as the patient will tend to save himself for the “most painful” ordeal. It is for this reason that the measurements were taken in random order and a rest period was observed between tests regardless of the order in which they were performed, which decreases the risk of obtaining biased results.
Conclusion
Our study suggests that the voluntary MVIBHT serves as a useful index to the severity of COPD and its impact. Few studies have evaluated the apnea test during COPD. It is a simple method which allowed to have an idea on the severity of the COPD with a good sensitivity and specificity. However, it would be interesting to correct the biases and weaknesses of the study, especially the sample size, to show if the correlation relationships could be even stronger, and would allow the hypotheses to be asserted with more certainty. Including healthy control subjects and female patients in future studies will provide more information on the diagnostic performance of the tele-inspiratory apnea test.
The evaluation of the reproducibility of this test is important to establish recommendations or a protocol for its implementation, which will facilitate the comparison of results obtained in different studies. Nevertheless, new studies are necessary to prove the usefulness of the breath-holding test especially as a tool forscreening in the evaluation of dyspnea, perceiving patient improvement, and to determine its minimal clinically significant variation to quantify the effectiveness of a therapeutic intervention.
Footnotes
Abbreviations: BMI : body mass index
CAT: COPD assessment test
COPD: chronic obstructive pulmonary disease
EC: expiratory capacity
ERV: expiratory reserve volume
FEV1: forced expiratory volume in the first minute
FVC: forced vital capacity
GOLD: global initiative for chronic obstructive lung disease
IC: inspiratory capacity
IRV: inspiratory reserve volume
MVIBHT: maximal voluntary inspiratory breath-holding time
PaCO2: carbon dioxide partial pressure
PaO2: oxygen partial pressure
HCO3-: bicarbonate level in the blood
RV: residual volume
SaO2: oxygen saturation
TLC: total lung capacity
Author Contributions: Abir Hedhli
Study design and development
Data analysis and interpretation
Writing the article
Drafting the work or revising it critically for important intellectual content
Final approval of the submitted version
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Yassine Ouahchi
Study design and development
Data analysis and interpretation
Writing the article
Drafting the work or revising it critically for important intellectual content
Final approval of the submitted version
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Meriem Mjid
Study design and development
Data analysis and interpretation
Writing the article
Drafting the work or revising it critically for important intellectual content
Final approval of the submitted version
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Jamel Koumenji
Study design and development
Data analysis and interpretation
Writing the article
Drafting the work or revising it critically for important intellectual content
Final approval of the submitted version
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Sana Cheikh Rouhou
Study design and development
Data analysis and interpretation
Writing the article
Drafting the work or revising it critically for important intellectual content
Final approval of the submitted version
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Sonia Toujani
Study design and development
Data analysis and interpretation
Writing the article
Drafting the work or revising it critically for important intellectual content
Final approval of the submitted version
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Besma Dhahri
Study design and development
Data analysis and interpretation
Writing the article
Drafting the work or revising it critically for important intellectual content
Final approval of the submitted version
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Azza Slim
Study design and development
Data analysis and interpretation
Writing the article
Drafting the work or revising it critically for important intellectual content
Final approval of the submitted version
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Azza Slim  https://orcid.org/0000-0002-7664-7391
https://orcid.org/0000-0002-7664-7391
References
- Adeloye D., Chua S., Lee C., Basquill C., Papana A., Theodoratou E., Nair H., Gasevic D., Sridhar D., Campbell H., Chan K. Y., Sheikh A., Rudan I., & Global Health Epidemiology Reference Group (GHERG). (2015). Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. Journal of Global Health, 5(2), 020415. 10.7189/jogh.05-020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. (2002). ATS statement: Guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine, 166(1), 111–117. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- Bain A. R., Drvis I., Dujic Z., MacLeod D. B., Ainslie P. N. (2018). Physiology of static breath holding in elite apneists. Experimental Physiology, 103(5), 635–651. 10.1113/EP086269 [DOI] [PubMed] [Google Scholar]
- Barnai M., Laki I., Gyurkovits K., Angyan L., Horvath G. (2005). Relationship between breath-hold time and physical performance in patients with cystic fibrosis. European Journal of Applied Physiology, 95(2), 172–178. 10.1007/s00421-005-1350-3 [DOI] [PubMed] [Google Scholar]
- Cassart M., Pettiaux N., Gevenois P. A., Paiva M., Estenne M. (1997). Effect of chronic hyperinflation on diaphragm length and surface area. American Journal of Respiratory and Critical Care Medicine, 156, 504–508. 10.1164/ajrccm.156.2.9612089 [DOI] [PubMed] [Google Scholar]
- Charles M., Colbrant C., Renaud J., Caty G., Pieters T., Liistro G. (2017). L’apnée volontaire, alternative au test de marche de six minutes pour l’évaluation de la capacité fonctionnelle de nos patients BPCO ? Revue des Maladies Respiratoires, 34, A59. 10.1016/j.rmr.2016.10.124 [DOI] [Google Scholar]
- Decramer M. (1989). Effects of hyperinflation on the respiratory muscles. European Respiratory Journal, 2(4), 299–302. [PubMed] [Google Scholar]
- De Troyer A. (1997). Effect of hyperinflation on the diaphragm. The European Respiratory Journal, 10(3), 708–713. [PubMed] [Google Scholar]
- Godfrey S., Campbell E. J. M. (1969). Mechanical and chemical control of breath holding. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences, 54(2), 117–128. 10.1113/expphysiol.1969.sp002011 [DOI] [PubMed] [Google Scholar]
- Hurewitz A. N., Sampson M. G. (1987). Voluntary breath holding in the obese. Journal of Applied Physiology, 62(6), 2371–2376. 10.1152/jappl.1987.62.6.2371 [DOI] [PubMed] [Google Scholar]
- Klocke F. J., Rahn H. (1959). Breath holding after breathing of oxygen. Journal of Applied Physiology. 10.1152/jappl.1959.14.5.689 [DOI] [PubMed]
- Laveneziana P., Webb K., Ora J., Wadell K., O’Donnell D. (2011). Evolution of dyspnea during exercise in chronic obstructive pulmonary disease impact of critical volume constraints. American Journal of Respiratory and Critical Care Medicine, 184, 1367–1373. 10.1164/rccm.201106-1128OC [DOI] [PubMed] [Google Scholar]
- Nannini L. J., Zaietta G. A., Guerrera A. J., Varela J. A., Fernández O. M., Flores D. M. (2007). Breath-holding test in subjects with near-fatal asthma. A new index for dyspnea perception. Respiratory Medicine, 101(2), 246–253. 10.1016/j.rmed.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Nishino T. (2009). Pathophysiology of dyspnea evaluated by breath-holding test: Studies of furosemide treatment. Respiratory Physiology & Neurobiology, 167(1), 20–25. 10.1016/j.resp.2008.11.007 [DOI] [PubMed] [Google Scholar]
- O’Donnell D. E. (2008). Implications cliniques de la distension thoracique, ou quand laphysiopathologie change la prise en charge thérapeutique. Revue des Maladies Respiratoires, 25(10), 1305–1318. 10.1016/S0761-8425(08)75094-0 [DOI] [PubMed] [Google Scholar]
- O’Donnell D. E., Banzett R. B., Carrieri-Kohlman V., Casaburi R., Davenport P. W., Gandevia S. C., Gelb A. F., Mahler D. A., Webb K. A. (2007). Pathophysiology of dyspnea in chronic obstructive pulmonary disease: A roundtable. Proceedings of the American Thoracic Society, 4(2), 145–168. 10.1513/pats.200611-159CC [DOI] [PubMed] [Google Scholar]
- O’Donnell D. E., Guenette J. A., Maltais F., Webb K. A. (2012). Decline of resting inspiratory capacity in COPD: The impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. CHEST, 141(3), 753–762. 10.1378/chest.11-0787 [DOI] [PubMed] [Google Scholar]
- O’Donnell D. E., Hamilton A. L., Webb K. A. (2006). Sensory-mechanical relationships during high-intensity, constant-work-rate exercise in COPD. Journal of Applied Physiology, 101(4), 1025–1035. [DOI] [PubMed] [Google Scholar]
- Palecek F. (2001). Hyperinflation: Control of functional residual lung capacity. Physiological Research, 50(3), 221–230. [PubMed] [Google Scholar]
- Parkes M. (2006). Breath-holding and its breakpoint. Experimental Physiology, 91, 1–15. 10.1113/expphysiol.2005.031625 [DOI] [PubMed] [Google Scholar]
- Puente-Maestu L., Garcia de Pedro J., Martínez-Abad Y., Oña J., Llorente D., Cubillo J. (2005). Dyspnea, ventilatory pattern, and changes in dynamic hyperinflation related to the intensity of constant work rate exercise in COPD. Chest, 128, 651–656. 10.1378/chest.128.2.651 [DOI] [PubMed] [Google Scholar]
- Sander M., Macefield V. G., Henderson L. A. (2010). Cortical and brain stem changes in neural activity during static handgrip and postexercise ischemia in humans. Journal of Applied Physiology, 108(6), 1691–1700. 10.1152/japplphysiol.91539.2008 [DOI] [PubMed] [Google Scholar]
- Scano G., Spinelli A., Duranti R., Gorini M., Gigliotti F., Goti P., Milic-Emili J. (1995). Carbon dioxide responsiveness in COPD patients with and without chronic hypercapnia. The European Respiratory Journal : Official Journal of the European Society for Clinical Respiratory Physiology, 8, 78–85. 10.1183/09031936.95.08010078 [DOI] [PubMed] [Google Scholar]
- Shingai K., Kanezaki M. (2014). Effect of dyspnea induced by breath-holding on maximal muscular strength of patients with COPD. Journal of Physical Therapy Science, 26(2), 255–258. 10.1589/jpts.26.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomopoulos V. (1975). On the psychophysical law: An information theory interpretation. Perceptual and Motor Skills, 40(1), 8–10. 10.2466/pms.1975.40.1.8 [DOI] [PubMed] [Google Scholar]
- Stock A.-K., Wascher E., Beste C. (2013). Differential effects of motor efference copies and proprioceptive information on response evaluation processes. PLoS ONE, 8(4). 10.1371/journal.pone.0062335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tageldin M. A., Nafti S., Khan J. A., Nejjari C., Beji M., Mahboub B., Obeidat N. M., Uzaslan E., Sayiner A., Wali S., Rashid N., El Hasnaoui A., & BREATHE Study Group. (2012). Distribution of COPD-related symptoms in the Middle East and North Africa: Results of the BREATHE study. Respiratory Medicine, 106 Suppl 2, S25–S32. 10.1016/S0954-6111(12)70012-4 [DOI] [PubMed] [Google Scholar]
- Trembach N., Zabolotskikh I. (2017). The influence of age on interaction between breath-holding test and single-breath carbon dioxide test. BioMed Research International, 2017, 1010289. 10.1155/2017/1010289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viecili R. B., Silva D. R., Sanches P. R. S., Müller A. F., Silva D. P., da Barreto S. S. M., Barreto S. M. (2012). Real-time measurement of maximal voluntary breath-holding time in patients with obstructive ventilatory defects and normal controls. Pulmonary & Respiratory Medicine, 2(5), 1–3. 10.4172/2161-105X.1000127 [DOI] [Google Scholar]
- Vogelmeier C. F., Criner G. J., Martinez F. J., Anzueto A., Barnes P. J., Bourbeau J., Celli B. R., Chen R., Decramer M., Fabbri L. M., Frith P., Halpin D. M. G., López Varela M. V., Nishimura M., Roche N., Rodriguez-Roisin R., Sin D. D., Singh D., Stockley R., Agustí A. (2017). Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. American Journal of Respiratory and Critical Care Medicine, 195(5), 557–582. 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- Von Leupoldt A., Dahme B. (2005). Cortical substrates for the perception of dyspnea. Chest, 128(1), 345–354. 10.1378/chest.128.1.345 [DOI] [PubMed] [Google Scholar]
- Von Leupoldt A., Sommer T., Kegat S., Baumann H. J., Klose H., Dahme B., Büchel C. (2008). The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. American Journal of Respiratory and Critical Care Medicine, 177(9), 1026–1032. 10.1164/rccm.200712-1821OC [DOI] [PubMed] [Google Scholar]
- World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. (2001). Bulletin of the World Health Organization, 79(4), 373–374. [PMC free article] [PubMed] [Google Scholar]