Abstract
Tenacissoside H (TEH), which has anti-inflammatory and anti-tumor effects, is a major active ingredient extracted from the stem of Marsdenia tenacissima. However, the effect of TEH on hepatocellular carcinoma (HCC) as well as the underlying mechanisms are still indistinct. Presently, HCC cells (including Huh-7 and HepG2) were dealt with different concentrations of TEH. The proliferation and apoptosis of HCC cells were determined via Cell Counting Kit-8 (CCK8) assay and flow cytometry. In addition, Western blot was conducted to evaluate the expressions of autophagy—and apoptosis-related proteins. Tissue immunofluorescence was carried out to evaluate LC3B expression in the tumor tissues. The data showed that TEH suppressed the growth of HCC cells in a concentration-dependent manner. Besides, TEH enhanced radiosensitivity and promoted the apoptosis of HCC cells. Moreover, the mRNA and protein levels of autophagy-related genes (LC3-II/LC2-I, ATG5, Beclin-1) were significantly promoted by TEH. Mechanistically, TEH attenuated the activation of PI3K/Akt/mTOR signaling pathway. However, inhibition of PI3 K pathway abolished the anti-tumor effects of TEH in HCC cells. Collectively, this study suggested that TEH increases the radiosensitivity of HCC cells via inducing autophagy and apoptosis through downregulating PI3K/Akt/mTOR signaling pathway.
Keywords: tenacissoside H, hepatocellular carcinoma, radiosensitivity, autophagy, signaling pathway
Introduction
Hepatocellular carcinoma (HCC) ranks the sixth most common malignant tumors, and has a mortality rate ranked third worldwide.1 Moreover, HCC often proceedes fast, and the 5 year survival rate of HCC patients is less than 30%.2 Surgical treatment is the preferred method for early HCC. In addition, chemotherapy and radiotherapy are also vital therapeutic methods for patients with advanced HCC, but the patients’ life quality after traditional treatments remains unsatisfactory.3 Even worse, during radiotherapy, certain tumor cells gradually adapt to the radiation and develop radiation resistance, leading to treatment failure (recurrence or metastasis).4 -5 Therefore, it is necessary to study the mechanism of radiotherapy resistance and enhance the radiosensitivity of HCC.
Autophagy, a highly conserved process of material turnover in cells, exists in eukaryotes. Autophagy can fulfill the needs of cell metabolism, renew organelles and maintain cell homeostasis, but aberrant autophagy may lead to the emergence of cancer cells, such as enhanced autophagy in breast cancer6 and esophageal cancer.7 Under normal conditions, autophagy is maintained at a low level, but in case of stress, such as ionizing radiation, autophagy is activated to protect cells from environmental damage, thus generating radiation resistance.8 Moreover, the phosphoinositol 3-kinase (PI3 K)/protein kinase B (Akt)/rapamycin (mTOR) signaling pathway acts as a crucial regulator of autophagy and takes part in the occurrence development of many tumors. For example, resveratrol, a natural polyphenol plant antitoxin, promotes autophagy by restraining the PI3K/Akt/mTOR signaling pathway, thereby suppressing the activity and migration of melanoma cells.9 Besides, curcumin can induce the apoptosis and autophagy of human lung cancer cell A549 by inhibiting the PI3K/Akt/mTOR pathway, thus effectively suppressing tumor cells’ growth.10 Those researches all prove that targeting PI3K/Akt/mTOR pathway restrains the progression of malignant tumors by modulating autophagy.
Tenacissoside H (molecular formula: C42H66O14, TEH) is a Chinese medicine monomer extracted from the dried stem of Marsdenia tenacissima. Emerging evidence has proved that TEH has anti-inflammatory11 and anti-tumor12 effects. For instance, TEH suppresses the proliferation, and arrests the cell cycle in S phase of esophageal cancer by inhibiting the PI3K/Akt pathway.13 However, the mechanism of TEH in HCC needs further exploration.
Presently, it was discovered that TEH could not only inhibit HCC cells’ proliferation and promote cell apoptosis, but also improve tumor cells’ sensitivity to radiotherapy. Moreover, TEH markedly repressed the activation of PI3K/Akt/mTOR pathway. Therefore, we hypothesized that TEH could affect the development of HCC through the PI3K/Akt/mTOR pathway via inducing autophagy, which is possible to be a potential therapeutic drug for HCC.
Materials and Methods
Cell Culture and Treatment
HCC cell line Huh-7 and HepG2 were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were cultured in Dulbeccos modified Eagle’s medium/Ham’s Nutrient mixture F12 (DMEM/F12, Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, HyClone, Logan, UT, USA) and 100 U/mL penicillin (Sigma, St. Louis, MO, USA). They were then incubated in an incubator at 37°C, with 5% CO2 and saturated humidity. When the cell fusion rate reached 70%∼80%, the cells were treated with 0.25% trypsin and went through cell passage. Tenacissoside H (CAS No.: 191729-45-0) and PI3 K pathway inhibitor PI-103 (CAS No.: 371935-74-9) were both obtained from MedChemExpress (NJ, USA). For cell treatment, both Huh-7 and HepG2 were dealt with 0-20 μM Tenacissoside H and 1 μM PI-103 for 24 hours.
CCK8 Assay
Huh-7 and HepG2 cells (2 × 103) were inoculated in 96-well plates with 100 µL cell suspension per well and cultured in an incubator at 37 °C with 5% CO2 saturated humidity. After the cells were adherent to the wall, TEH at different concentrations (0, 2.5, 5, 10 and 20 μM) was added. After continuous culturing for 0, 24, 48, and 72 hours, we added 10 µL CCK8 solution (Beyotime Biotechnology, Shanghai, China) to each well, gently shook well and incubated the culture plate in the incubator for 2 hours. Microplate Reader determined the absorbance value (A) of each well at 450 nm. Cell survival rate (%) = (experimental group A/ control group A) ×100%.
Radiosensitivity Test
The radiosensitivity experiment consists of the blank control group (DMSO) and the TEH group with different concentrations (0, 5, 10, 20 μM). Huh-7 and HepG2 cells were cultured for 48 h. The cells were digested into a single-cell suspension, which was divided into 2-ml culture bottles and exposed to different doses. Irradiation conditions: 8 MV linear accelerator (varianclinicl8, USA), with the addition of 2 cm equivalent tissue filling material, irradiated 0, 2, 4, 6 and 8 Gy/min, respectively. To analyze the radiosensitivity of the cells, the cell proliferation was detected via CCK8 assay after irradiation. Huh-7 and HepG2 cells were inoculated in 96-well plates (2 × 103 cells, 100 µL cell suspension per well). After 24 hours culture, the cells were treated in accordance with the previous grouping for 48 h. With the addition of 10 µL CCK8 reagent (Beyotime Biotechnology, Shanghai, China) in each cell well, the cells underwent further incubation (37 °C, 4 h). Finally, a Microplate Reader was used to determine the absorbance value (A value) of each group at 570 nm.
Flow Cytometry
According to the manufacturer’s instructions, AnnexinV/7-AADA apoptosis Detection Kit (Southern Biotechnology, Birmingham, Al, USA) was applied to examine the degree of cell apoptosis. The treated cells were trypsinized, centrifuged, washed twice with cold PBS and resuspended using binding buffer till a final concentration of 1.5 ×104 cell/mL. Then, they went through double-staining with AnnexinV and 7-AAD, and incubated 15 minutes in darkness (15-25 °C, room temperature). Finally, we performed the flow cytometry to analyze the stained cells with a FACS Calibur Flow cytometry (BD Biosciences, SanJose, CA, USA). We duplicated the experiment 3 times. In the subsequent experiments, after adherent culture, the cells were treated with TEH group (5 μM), gamma-ray (4 Gy) and PI3 K inhibitor (1 μM PI-103), and the rest steps were the same as the above.
Western Blot
After cell treatment, the culture medium was discarded, the protein lysate (Roche) was added (Beyotime Biotechnology, Shanghai, China) and the total protein was isolated. 50 µg total protein was added to 12% polyacrylamide gel for 2-hour electrophoresis at 100 V. Then, it was electrotransferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After being sealed with 5% skim milk powder at room temperature for 1 h, the membranes went through TBST wash 3 times (10 min each) and incubated overnight with primary Anti-Bax antibody (ab32503, 1: 1000, abcam, USA), Anti-Bcl-2 antibody (ab59348, 1: 1000, abcam, USA), Anti-LC3I/II antibody (ab128025, 1: 1000, abcam, USA)), Anti-LC3 antibody (ab48394, 1: 1000, abcam, USA), Anti-APG5 L / ATG5 antibody (ab108327, 1:1000, abcam, USA), Anti-Beclin 1 antibody (ab62557, 1:1000, abcam, USA), Anti-PI3 Kinase catalytic subunit gamma / PI3K-gamma antibody (ab32089, 1: 1000, abcam, USA), Anti-pan-AKT antibody (ab8805, 1: 1000, abcam, USA), Anti-mTOR antibody (ab109268, 1:1000, abcam, USA) at 4 °C. Next, after TBST wash, the membranes were incubated for 1 h at room temperature with horseradish peroxidase (HRP) labeled anti-goat anti-rabbit (ab150077, 1:300, abcam, USA). Afterward, they were rinsed with TBST 3 times with 10 minutes each time. At last, Western blot reagent (Invitrogen) was performed for color imaging, and Image J was employed to analyze the gray values of each protein.
Xenograft Tumor Experiment
Forty nude female BALB/c mice (6 8 weeks old, 20 ± 2 g) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China). Human HepG2 cells were used to test the effect of TEH on HCC cell growth in vivo. The xenograft model was established by subcutaneous injection of 1 × 107 cells/100 µL cell suspension. When the tumors were formed with a volume of 75 100 mm3, the nude mice were randomly divided into 4 groups, including control group, TEH group (5, 10, 20 μmol/kg body weight). PBS (100 µl) were used as negative control in the veh group. TEH was administered via intraperitoneal injection 3 times a week for 4 weeks. During the treatment, the weights of the mice and the volume of the tumors were measured every 4 days. The volume of the tumors was calculated according to the following formula: V = 0.5×length ×width2. Finally, the mice were sacrificed at the fourth week of xenograft tumor experiment. Then the tumors were carefully resected and the weight of the tumors were counted. All the animal procedures were approved by the Animal Care and Use Committee of Peking University Shenzhen Hospital.
Immunofluorescence Staining
The paraffin-embedded sections (4 µm in thickness) were dewaxed, and got antigen repaired, then blocked with normal goat serum, incubated with anti-LC3B antibody (ab192890, 1:200, Abcam, USA) for 12 hours at 4 °C and Alexa Fluor® 488 Anti-LC3B antibody (ab225382, 1:200, Abcam, USA) at 37 °C for 60 minutes, respectively. After the nucleus was stained by DAPI (Beyotime, Shanghai, China), the fluorescence signals were observed under fluorescence microscope (Olympus, Japan).
Statistical Analysis
We took SPSS17.0 statistical software (SPSS Inc, Chicago, IL, USA) for data analysis, t-test for comparison of the mean between 2 sample groups and one-way analysis of variance (ANOVA) for the difference between multiple groups. The measurement data were expressed as mean ± standard deviation (x ± s). We considered P < 0.05 as statistically valuable.
Results
TEH Inhibited the Proliferation of HCC Cells
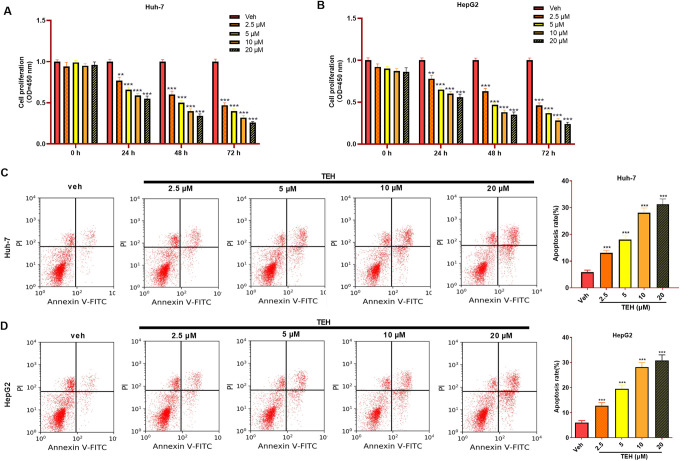
For the purpose of detecting the function of TEH in the growth of HCC cells, different concentrations of TEH (0, 2.5, 5, 10, 20 μM) were used to treat Huh-7 and HepG2 cells for 0, 24, 48 and 72 hours, and cell proliferation and apoptosis were detected via CCK8 assay and flow cytometry. The data illustrated that TEH markedly repressed the proliferation of HCC cells with a dose-dependent manner (Figure 1A and B). In addition, the apoptosis of HCC cells was significantly accelerated with the increase of TEH’s doses (Figure 1C and D). Therefore, the results implied that TEH limits the growth of HCC cells in a dose-dependent manner and promotes cell apoptosis.
Figure 1.
TEH inhibited the proliferation and aggravated apoptosis of HCC cells. Huh-7 and HepG2 cells were treated with different doses of TEH. (A-B) Cell proliferation of Huh-7 and HepG2 cells that were treated with different concentrations of TEH were measured via CCK8 assay. (C-D) Flow cytometry was used to detect apoptosis of Huh-7 and HepG2 cells. **P < 0.01, ***P < 0.001 (vs. veh group).
TEH Repressed the Growth of HCC Cells In Vivo and Induced Autophagy
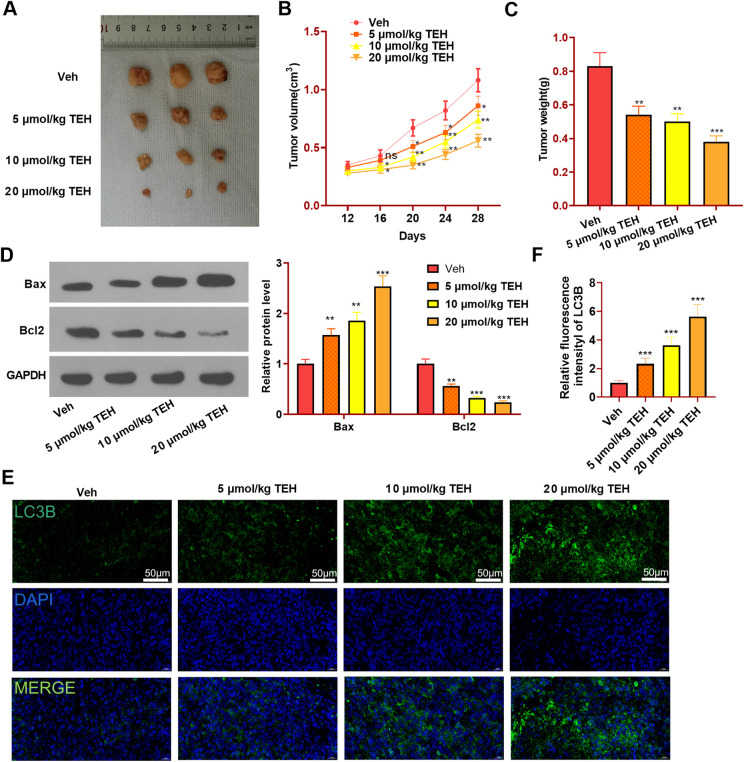
To further confirm the effects of TEH on HCC cells, we performed in vivo experiment. Our data showed that compared with veh group the volume and weight of the xenograft tumors in the TEH group were significantly reduced (Figure 2A-C). Moreover, with the increase of TEH’s concentration, the inhibitive effect on tumor growth became more significant (Figure 2A-C). By detecting the apoptosis-realted markers (including Bax and Bcl2), we found that TEH promoted Bax and inhibited Bcl2 expressions in a dose-dependent manner (in comparison to veh group, Figure 2D). Further, tissue immunofluorescence was conducted to evaluate the autophagy marker LC3B in the tumor tissues. It was found that TEH markedly promoted LC3B expression in the tumor tissues (Figure 2E-F). Therefore, the in-vivo experiment further confirmed that TEH has an anti-tumor role in HCC cells via enhancing apoptosis and autophagy.
Figure 2.
TEH reduced HCC cells growth in vivo and induces apoptosis and autophagy. Human HepG2 cells were used to test the effect of TEH on HCC cell growth in vivo. HepG2 cells were subcutaneously injected in to the nude mice, which were then dealt with different doses of TEH (5-20 μmol/kg body weight). The tumor volume was measured every 4 days since the twelfth day of modeling. After a total of 28-day incubation, the mice were sacrificed and the tumors were isolated and weighted. (A) Tumor images. (B) Tumor volume. (C) Tumor weight. (D) Westrn blot was conducted to evaluate the apoptosis markers (including Bax and Bcl2) in the tumor tissues. (E-F) Tissue immunofluorescence was performed to detect LC3B (green marker) expression in the tumor tissues, and the fluorescence intensity of LC3B was analyzed. N = 5. **P < 0.01, ***P < 0.001 (vs. veh group).
TEH Enhanced the Radiosensitivity of HCC Cells
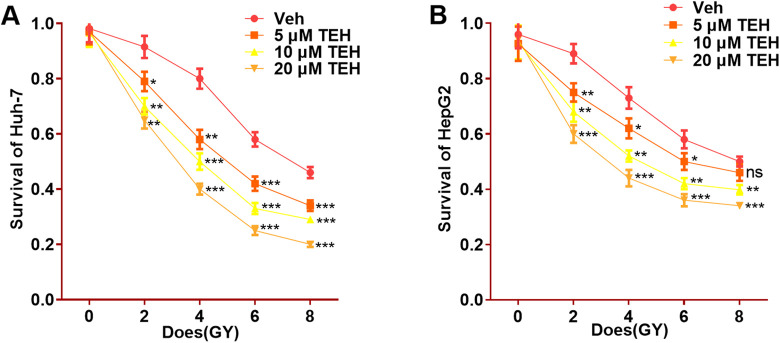
To verify TEH’s effect on the radiosensitivity of HCC cells, different concentrations (0, 5, 10, 20 μM) of TEH were added to Huh-7 and HepG2 cells and exposed to different doses (0, 2, 4, 6, 8 Gy) of ionizing radiation. The results of radiosensitivity experiment revealed that, the cell survival rate significantly decreased with the increase of TEH concentration and radiation dose, and the higher the concentration of TEH, the lower the cell survival rate, compared with the veh group (0 μM TEH) (P < 0.05, Figure 3A-B). The statistics suggest that TEH enhances the sensitivity of HCC cells to ionizing radiation.
Figure 3.
TEH enhanced the radiosensitivity of HCC cells. Huh-7 and HepG2 cells were treated with different doses of TEH and ionizing radiation. (A-B) The proliferation of the 2 cells were detected by CCK8 assay. ns P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001 (vs. veh group).
TEH Promoted the Apoptosis of HCC Cells Induced by Ionizing Radiation
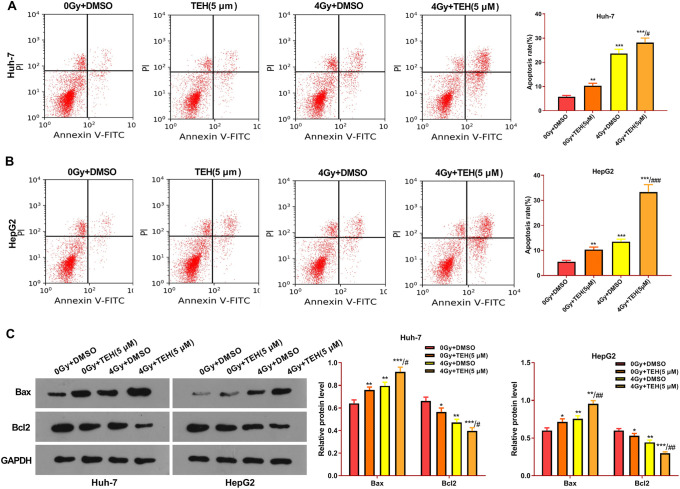
To explore TEH’s effect on the apoptosis of irradiated HCC cells, Huh-7 and HepG2 cells were dealt with TEH (5 μM) and gamma-ray (4 Gy). Flow cytometry results represented that, compared with the control group (0Gy+DMSO), the cell apoptosis rates after TEH intervention alone and after radiation intervention alone were increased. Interestingly, the combination of TEH with ionizing radiation prominently enhanced the apoptotic level of HCC cells (compared with 0Gy+ TEH group or 4 Gy+DMSO group) (Figure 4A and B). Furthermore, the Western bolt was applied to detect apoptosis-related proteins. The results illustrated that, compared with the control group, the pro-apoptotic protein Bax was upregualted and the anti-apoptotic protein Bcl2 was downregulated in 0Gy+ TEH group or 4 Gy+DMSO group. Interestingly, the combination of TEH and radiation led to higher level of Bax and lower level of Bcl2 (compared with 0Gy+ TEH group or 4 Gy+DMSO group) (P < 0.05, Figure 4C). These findings indicated that combining radiation and TEH aggravated the apoptosis HCC cells.
Figure 4.
TEH promoted the apoptosis of HCC cells. Huh-7 and HepG2 were treated with TEH (5 μM) and then subjected to irradiation (4 Gy) for 24 hours. (A-B) The apoptosis rate of Huh-7 and HepG2 cells was examined by flow cytometry. (C) The expression of apoptosis-related proteins (including Bax and Bcl2) were evaluated via Western blot. *P < 0.05, **P < 0.01, ***P < 0.001 (vs. 0 Gy + DMSO group); # P < 0.001, ## P < 0.001, ### P < 0.001 (vs. 4 Gy + DMSO group).
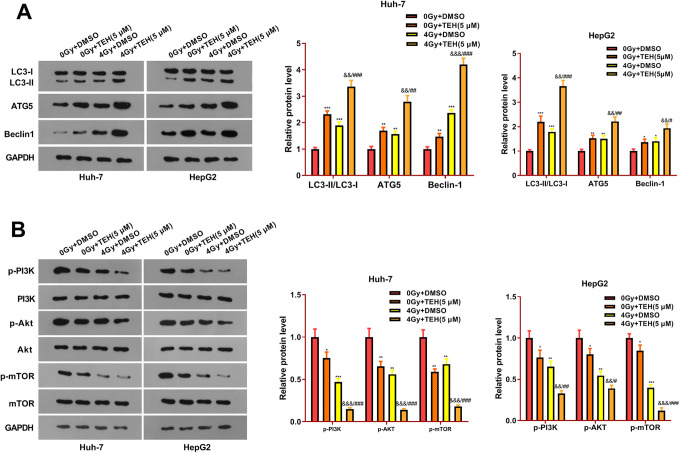
TEH Promoted Autophagy and Inhibited the PI3K/Akt/mTOR Signaling Pathway in HCC Cells
To approach the mechanism of TEH on HCC cells, Western bolt was performed to evaluate the expression of autophagy-related proteins LC3-I/II, AGT5 and Beclin1 in HCC cells. The results displayed that, compared with the control group (0Gy+DMSO), autophagy-related proteins LC3-II/I, AGT5 and Beclin1 were up-regulated after TEH intervention alone. At the same time, LC3-II/I, ATG5 and Beclin1 expressions were up-regulated after radiation treatment (Figure 5A). Interestingly, the combination of TEH and radiation treatment promoted autophagy (compared with 0Gy+ TEH group or 4 Gy+DMSO group) (Figure 5A). Further, Western bolt was employed to measure the effect of TEH on the PI3K/Akt/mTOR pathway. The result showed that compared with the control group, the expressions of p-PI3 K, p-Akt and p-mTOR were both down-regulated in the 0Gy+ TEH group and 4 Gy+DMSO group. However, the addition of TEH significantly inhibited the activation of PI3K/Akt/mTOR pathway (P < 0.05, Figure 5B). These data suggested that TEH suppresses the PI3K/Akt/mTOR signaling pathway by activating autophagy of HCC cells.
Figure 5.
TEH inhibited autophagy of HCC cells and PI3K/Akt/mTOR signaling pathway. Huh-7 and HepG2 cells were treated with TEH (5 μM) and then subjected to irradiation (4 Gy). (A) Western bolt was used for the detection of autophagy-related protein expression (LC3-I/II, ATG5 and Beclin1). (B) Western bolt experiment was carried out to analyze the expression of PI3 K, Akt, and mTOR. *P < 0.05, **P < 0.01, ***P < 0.001 (vs. 0 Gy + DMSO group); ### P < 0.001 (vs. 4 Gy + DMSO group).
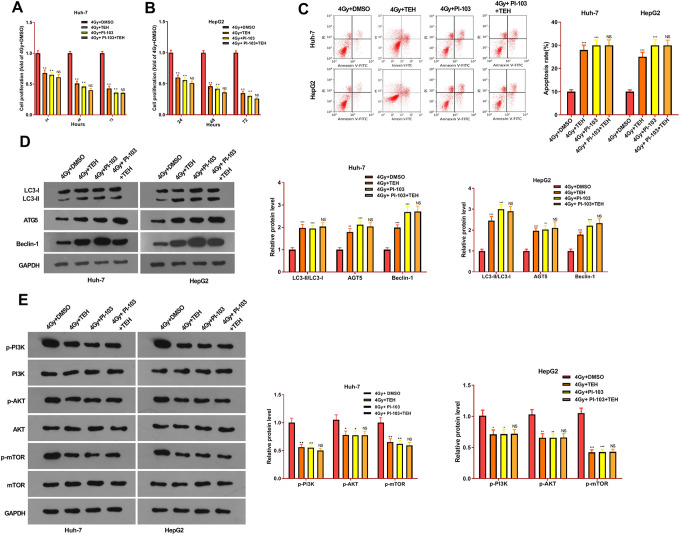
Inhibiting PI3K/Akt/mTOR Signaling Pathway Dampened TEH-Induced Effects in HCC Cells
To further study whether TEH could affect the progression of HCC via PI3 K pathway, we gave PI-103 (1 μM) to intervene the PI3 K pathway activation in Huh-7 and HepG2 cells. CCK8 assay was taken to detect cell proliferation, and flow cytometry was performed to examine cell apoptosis. The results demonstrated that, compared with the control group (4Gy+DMSO), the cell proliferation in the 4Gy+TEH group and the 4Gy+ PI-103 group were remarkably decreased, and the cell apoptosis considerably increased (Figure 6A-C). However, the proliferative and apoptotic levels in the 4Gy+PI-103+TEH group were not significantly changed in contrast to those in the 4Gy+PI-103 group (Figure 6A-C). Moreover, Western blot was used to detect the expressions of autophagy-related proteins and PI3K/Akt/mTOR pathway. The results indicated that compared with the control group, autophagy-related proteins LC3-II/I, AGT5 and Beclin1 were notably up-regulated with the addition of PI-103, while the expressions of p-PI3 K, p-Akt and p-mTOR were notably suppressed (Figure 6D and E). However, the combination of TEH and PI-103 had no significant effects on the autophagy and PI3K/Akt/mTOR pathway expression (compared with 4Gy+PI-103 group). Taken together, the above studies indicated that TEH reduced the proliferation of HCC cells, promoted cell apoptosis, promoted cell autophagy and radiosensitivity dependently via inhibiting the PI3K/AKt/mTOR pathway.
Figure 6.
TEH inhibited the progression of HCC through PI3K/Akt/mTOR signaling pathway. Huh-7 and HepG2 cells were treated with TEH (5 μM) and/or PI-103 (1 μM) and then subjected to irradiation (4 Gy). (A-B) CCK8 experiment was carried out to examine the proliferation of Huh-7 and HepG2 cells. (C) The apoptosis rate of Huh-7 and HepG2 cells was evaluated via flow cytometry. (D-E) Western bolt was applied to measure autophagy-related proteins and PI3 K / Akt / mTOR pathway expression. *P < 0.05, **P < 0.01, ***P < 0.001 (vs.4 Gy + DMSO group); ns P > 0.05 (vs.4 Gy + PI-103 group).
Discussion
In this study, we investigated the biofunctions of TEH in HCC development. The results revealed that TEH inhibits HCC cell growth by aggravating apoptosis and autophagy dependently through repressing the PI3K/AKt/mTOR pathway.
The development of HCC is rooted in uncontrolled cancer cell growth and metastasis. Inhibiting the proliferation of HCC cells and inducing cell apoptosis are the main strategies for its treatment.14-15 Radiotherapy causes radioactive damage when rays directly act on biomolecules, indirectly damages DNA by producing free radicals to interact with biological macromolecules, blocks cell division and proliferation, and causes irreversible damage in cells, which is the first choice for treatment of locally advanced HCC.16 However, radiation resistance is the major challenge during radiotherapy. After long-term radiation irradiation, tumors show radiation resistance similar to chemotherapy drug tolerance, which reduces the sensitivity of the tumor to radiation and causes tumor metastasis or recurrence.17 Overcoming or eliminating radiation resistance during radiotherapy is crucial to improving the survival rate of HCC patients.
In recent years, abundant literatures have manifested that some plant extracts make great contribution to the inhibition of HCC development. For instance, the licorice roots extract can induce apoptosis and cell cycle arrest of HCC cells via modulating tumor suppressor miRNAs, inhibiting HIF1α, PI3 K, C-Myc and activating AMPK and p53 pathway.18 As another example, Oleanolic acid attenuates the development of HCC by inducing autophagy and promoting apoptosis.19 TEH, along with the other 2 ingredients (tenacissoside I and G), is extracted form Marsdenia tenacissima and has been found with anti-inflammatory and anti-tumor effects.20 Interestingly, a recent study proved that the Marsdenia tenacissima extract (MTE) promotes gefitinib accumulation in lung cancer tissues via fasciliating the activities of ABCG2, which is the primary drug transporter for gefitinib.21 Our study found that with the increase of TEH concentration, the proliferative ability of HCC cells decreased and cell apoptosis increased. Additionally, TEH reduced the growth of HCC cells under irradiation treatment, indicating that TEH is a promising anti-tumor drug in HCC.
Autophagy, a self-digestion phenomenon in eukaryotes, is a double-edged sword that can promote or inhibit cell apoptosis. In the general state, autophagy is maintained at a low level. When stimulated by radiation, the autophagy level is sharply up-regulated, and even type II programed cell death occurs, resulting in radiotherapy resistance.22 However, several studies have proven that autophagy may reduce chem—or radio-resistance.23-24 For example, inhibition of autophagy in esophageal carcinoma can improve the radiosensitivity of cancer cells.25 Similarly, 3-methyladenine (3-MA), an autophagy inhibitor, sensitizes radio-resistant HepG2 cells to radiation-induced cytotoxicity,26 proving that modulating autophagy is a powerful method in treating HCC. Here, our data showed that both TEH and irradiation enhanced the autophagy of HCC cells. More interestingly, the combination of the 2 treatments significantly enhances the autophagic level, suggesting that TEH facilitating irradiation induced cell growth inhibition by modulating autophagic cell death.
The PI3K/Akt/mTOR signaling pathway takes part in the development of various tumors, which not only regulates autophagy, but also is closely related to radiation resistance. Studies have found that the use of PI3K/Akt/mTOR pathway inhibitor in prostate cancer can induce apoptosis of cancer cells, inhibit cell proliferation, and improve the radiosensitivity of prostate cancer.27 Other studies have shown that rapamycin combined with cisplatin inhibits the expressions of p-PI3 K, p-Akt and p-mTOR in pancreatic cancer cells, which leads to a great increase in cell apoptosis rate, indicating that it can regulate the PI3K/ Akt /mTOR pathway to mediate the sensitivity of pancreatic cancer cells to cisplatin.28 TEH exerts a prominent effect on tumor growth by regulating the signal transduction pathway. For example, TEH suppresses the development of Esophageal Cancer by regulating the PI3K/Akt/NF-κB pathway.13 Moreover, TEH also induced apoptosis and reduced the invasion of colon cancer cells through downregulating GOLPH3 expression and activity of PI3K/AKT/mTOR and Wnt/β-catenin signaling pathways.29 Our results suggest that TEH suppresses the expression of PI3K/Akt/mTOR signaling pathway and promoted autophagy-related proteins and cell apoptosis. And inhibiting PI3K/Akt/mTOR signaling pathway obviously dampened the anti-tumor role of TEH in HCC cells.
Collectively, TEH limits the proliferation of HCC cells and enhances radiosensitivity by suppressing the PI3K/Akt/mTOR signaling pathway and ultimately activating death-related autophagy and apoptosis. All over, this study confirms that TEH is an anti-tumor agent in HCC and provides a new reference for radiotherapy of HCC.
Supplemental Material
Supplemental Material, sj-pdf-1-dos-10.1177_15593258211011023 for Tenacissoside H Induces Autophagy and Radiosensitivity of Hepatocellular Carcinoma Cells by PI3K/Akt/mTOR Signaling Pathway by Jiatian Lin, Jiyin Ruan, Hao Zhu, Zaizhong Chen, Junhui Chen and Hongjian Yu in Dose-Response
Footnotes
Authors’ Note: Conceived and designed the experiments: Hongjian Yu, Jiatian Lin. Performed the experiments: Jiatian Lin, Jiyin Ruan. Statistical analysis: Hao Zhu, Zaizhong Chen, Junhui Chen. Wrote the paper: Jiatian Lin. All authors read and approved the final manuscript.
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request. Our study was approved by the Ethics Review Board of Peking University Shenzhen Hospital.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Sanming Project of Medicine in Shenzhen (SZSM201612071).
ORCID iD: Jiatian Lin  https://orcid.org/0000-0001-5662-7644
https://orcid.org/0000-0001-5662-7644
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2. Li D, Sedano S, Allen R, Gong J, Cho M, Sharma S. Current treatment landscape for advanced hepatocellular carcinoma: patient outcomes and the impact on quality of life. Cancers (Basel). 2019;11(6):841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schlachterman A, Craft WW, Jr, Hilgenfeldt E, Mitra A, Cabrera R. Current and future treatments for hepatocellular carcinoma. World J Gastroenterol. 2015;21(28):8478–8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen ZT, Chen Y, Huang GC, Zhu XX, Wang R, Chen LB. Aurora-a confers radioresistance in human hepatocellular carcinoma by activating NF-κB signaling pathway. BMC Cancer. 2019;19(1):1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin LC, Chen CF, Ho CT, Liu JJ, Liu TZ, Chern CL. γ-Glutamylcysteine synthetase (γ-GCS) as a target for overcoming chemo—and radio-resistance of human hepatocellular carcinoma cells. Life Sci. 2018;198:25–31. [DOI] [PubMed] [Google Scholar]
- 6. Espina V, Mariani BD, Gallagher RI, et al. Malignant precursor cells pre-exist in human breast DCIS and require autophagy for survival. PLoS One. 2010,5(4):e10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whelan KA, Chandramouleeswaran PM, Tanaka K, et al. Autophagy supports generation of cells with high CD44 expression via modulation of oxidative stress and Parkin-mediated mitochondrial clearance. Oncogene. 2017;36(34):4843–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tam SY, Wu VW, Law HK. Influence of autophagy on the efficacy of radiotherapy. Radiat Oncol. 2017;12(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gong C, Xia H. Resveratrol suppresses melanoma growth by promoting autophagy through inhibiting the PI3K/AKT/mTOR signaling pathway. Exp Ther Med. 2020;19(3):1878–1886. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Liu F, Gao S, Yang Y, et al. Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol Rep. 2018,39(3):1523–1531. [DOI] [PubMed] [Google Scholar]
- 11. Li JJ, Zhang Y, Han LW, et al. Tenacissoside H exerts an anti-inflammatory effect by regulating the NF-κb and p38 pathways in zebrafish. Fish Shellfish Immunol. 2018;83:205–212. [DOI] [PubMed] [Google Scholar]
- 12. Ye B, Yang J, Li J, Niu T, Wang S. In vitro and in vivo antitumor activities of tenacissoside C from Marsdenia tenacissima. Planta Med. 2014;80(1):29–38. [DOI] [PubMed] [Google Scholar]
- 13. Jia YS, Hu XQ, Gabriella H, Qin LJ, Meggyeshazi N. Antitumor activity of Tenacissoside H on esophageal cancer through arresting cell cycle and regulating PI3K/Akt-NF-κB Transduction Cascade. Evid Based Complement Alternat Med. 2015;2015:464937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang HF, Wang YC, Han YD. MicroRNA 34a inhibits liver cancer cell growth by reprogramming glucose metabolism. Mol Med Rep. 2018;17(3):4483–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shan QL, Chen NN, Meng GZ, Qu F. Overexpression of lncRNA MT1JP mediates apoptosis and migration of hepatocellular carcinoma cells by regulating miR-24-3p. Cancer Manag Res. 2020;12:4715–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Isaksson LJ, Pepa M, Zaffaroni M, et al. Machine learning-based models for prediction of toxicity outcomes in radiotherapy. Front Oncol. 2020;10:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim JY, Lee HY, Park KK, Choi YK, Nam JS, Hong IS. CWP232228 targets liver cancer stem cells through Wnt/β-catenin signaling: a novel therapeutic approach for liver cancer treatment. Oncotarget. 2016;7(15):20395–20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdel-Wahab AA, Effat H, Mahrous EA, Ali MA, Al-Shafie TA. A licorice roots extract induces apoptosis and cell cycle arrest and improves metabolism via regulating MiRNAs in liver cancer cells. Nutr Cancer. 2020;1–12. [DOI] [PubMed] [Google Scholar]
- 19. Zhou W, Zeng X, Wu X. Effect of oleanolic acid on apoptosis and autophagy of SMMC-7721 hepatoma cells. Med Sci Monit. 2020;26:e921606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Long G, Zhao C, Zhao P, Zhou C, Ntirenganya E, Zhou Y. Transcriptomic response to cold of thermophilous medicinal plant Marsdenia tenacissima. Gene. 2020;742:144602. [DOI] [PubMed] [Google Scholar]
- 21. Zhao C, Hao H, Zhao H, et al. Marsdenia tenacissima extract promotes gefitinib accumulation in tumor tissues of lung cancer xenograft mice via inhibiting ABCG2 activity. J Ethnopharmacol. 2020;255:112770. [DOI] [PubMed] [Google Scholar]
- 22. Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16(8):461–472. [DOI] [PubMed] [Google Scholar]
- 23. Sheng J, Qin H, Zhang K, Li B, Zhang X. Targeting autophagy in chemotherapy-resistant of hepatocellular carcinoma. Am J Cancer Res. 2018;8(3):354–365. [PMC free article] [PubMed] [Google Scholar]
- 24. Ke Y, Wu C, Zeng Y, et al. Radiosensitization of clioquinol combined with zinc in the nasopharyngeal cancer stem-like cells by inhibiting autophagy in vitro and in vivo . Int J Biol Sci. 2020;16(5):777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen Y, Li X, Guo L, et al. Combining radiation with autophagy inhibition enhances suppression of tumor growth and angiogenesis in esophageal cancer. Mol Med Rep. 2015;12(2):1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tseng HC, Liu WS, Tyan YS, Chiang HC, Kuo WH, Chou FP. Sensitizing effect of 3-methyladenine on radiation-induced cytotoxicity in radio-resistant HepG2 cells in vitro and in tumor xenografts. Chem Biol Interact. 2011;192(3):201–208. [DOI] [PubMed] [Google Scholar]
- 27. Chang L, Graham PH, Hao J, et al. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014;5(10):e1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li B, Yang J, Lu Z, Liu B, Liu F. A study on the mechanism of rapamycin mediating the sensitivity of pancreatic cancer cells to cisplatin through PI3K/AKT/mTOR signaling pathway. J BUON. 2018;24(2):739–745. [PubMed] [Google Scholar]
- 29. Hong ZS, Zhuang HB, Qiu CZ, et al. Tenacissoside H induces apoptosis and inhibits migration of colon cancer cells by downregulating expression of GOLPH3 Gene. Evid Based Complement Alternat Med. 2020;2020:2824984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-dos-10.1177_15593258211011023 for Tenacissoside H Induces Autophagy and Radiosensitivity of Hepatocellular Carcinoma Cells by PI3K/Akt/mTOR Signaling Pathway by Jiatian Lin, Jiyin Ruan, Hao Zhu, Zaizhong Chen, Junhui Chen and Hongjian Yu in Dose-Response