Abstract
Background:
The purpose of this meta-analysis was to analyze the influence of sarcopenia, defined as low skeletal muscle mass, on clinical outcomes in patients with head and neck squamous cell carcinoma (HNSCC) based on a large sample.
Methods:
The MEDLINE, EMBASE, and SCOPUS databases were screened for associations between sarcopenia and clinical outcomes in HNSCC up to December 2020. Overall, 27 studies met the inclusion criteria. The methodological quality of the studies involved was checked according to the QUADAS instrument. The meta-analysis was undertaken using RevMan 5.3 software. DerSimonian and Laird random-effects models with inverse-variance weights were used to account for heterogeneity between the studies.
Results:
The 27 included studies comprised 7704 patients with different HNSCCs. The cumulative calculated frequency among the studies was 42.0% [95% confidence interval (CI) 35.34–48.65]. Sarcopenia was associated with occurrence of severe postoperative complications, odds ratio (OR) 4.79, 95% CI (2.52–9.11), p < 0.00001. Sarcopenia predicted disease-free survival (DFS), simple regression: hazard ratio (HR) 2.00, 95% CI (1.63–2.45), p < 0.00001, multiple regression: HR 1.64, 95% CI (1.33–2.03), p < 0.00001. Also, sarcopenia was associated with lower overall survival (OS), simple regression: HR 1.96, 95% CI (1.71–2.24), p < 0.00001, multiple regression: HR = 1.87, 95% CI (1.53–2.29), p < 0.00001. In patients who underwent definitive chemotherapy and/or radiation, sarcopenia predicted lower OS (simple regression), HR 1.95, 95% CI (1.61–2.36), p < 0.00001, multiple regression: HR = 1.51, 95% CI (1.17–1.94), p < 0.002). In patients with primary surgical strategy with or without adjuvant radio-chemotherapy, sarcopenia was associated with lower OS (simple regression), HR 2.21, 95% CI (1.72–2.84), p < 0.00001, multiple regression: HR = 2.05, 95% CI (1.55–2.72), p < 0.00001).
Conclusion:
The cumulative prevalence of sarcopenia in HNSCC is 42.0%. Sarcopenia is an independent risk factor for OS and DFS in patients with HNSCC who undergo curative therapy. Sarcopenia is associated with the occurrence of severe postoperative complications.
Keywords: head and neck cancer, overall survival, sarcopenia
Introduction
Sarcopenia is a condition defined as a syndrome associated with loss of muscle mass and strength as well as decreased physical performance.1 In clinical practice, low skeletal muscle mass (LSMM) on computed tomography (CT) is used as a surrogate marker of sarcopenia.2–4 LSMM is a prognostic biomarker predicting disease outcome in different malignancies.2–7 So far, it has been shown that sarcopenic patients have higher rates of postoperative major cardiac and/or pulmonary complications in gastric cancer.2 In breast cancer, patients with sarcopenia had more grade 3–5 toxicity under chemotherapy compared with non-sarcopenic patients.3 In surgically treated non-small cell lung cancer, patients with sarcopenia had a lower 5-year overall survival (OS) rate [risk ratio (RR) = 1.63, 95% confidence interval (CI) = (1.13, 2.33); p = 0.008].4 In addition, sarcopenia was associated with a lower 5-year disease-free survival (DFS) rate [RR = 1.59, 95% CI = (1.01, 2.52); p = 0.046].4 Similar results were also reported for pancreatic cancer,5 hepatocellular carcinoma,6 urothelial carcinoma,7 hematological malignancies,8 and ovarian cancer.9 Loss of skeletal muscle mass during neoadjuvant radiochemotherapy in rectal cancer patients is an independent prognostic factor for DFS and distant metastasis-free survival following curative intent resection.10 Some authors indicated that sarcopenia defined as LSMM can also play an essential role also in HNSCC.11,12
The purpose of this meta-analysis was to analyze the influence of LSMM on OS in patients with HNSCC based on a large sample.
Materials and methods
Data acquisition
The MEDLINE library, and Cochrane, EMBASE, and SCOPUS databases were screened for the presence of sarcopenia in HNSCC and associations between LSMM and clinically relevant outcomes like survival, occurrence of complications, and therapy toxicity up to December 2020 (Figure 1).
Figure 1.
PRISMA flow chart of the data acquisition.
HNSCC, head and neck squamous cell carcinoma; PRISMA, preferred reporting items for systematic reviews and meta-analyses.
For data acquisition, the following search criteria were used: “sarcopenia OR low skeletal muscle mass OR body composition AND head neck cancer OR head and neck squamous cell carcinoma OR neck cancer”
The primary search identified 1366 items. Inclusion criteria for the meta analysis were:
- human studies including patients with HNSCC of different origins;
- investigation of pretreatment status of the skeletal musculature by staging computed tomography (CT);
- English language.
Exclusion criteria were:
- Duplicate articles;
- review articles;
- experimental studies used animal models;
- case reports;
- non-English language.
Overall, 1339 articles were excluded and 27 items were included in the analysis. The included 27 articles provided information regarding prevalence of sarcopenia and/or the influence of sarcopenia on complications and survival in patients with HNSCC.13–39
The following data were extracted from the included studies: authors, year of publication, diagnosis, number of patients, prevalence of sarcopenia, and statistical data about influence of sarcopenia on clinical outcomes [hazard ratio (HR) and 95% CI]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used for this research.40
Meta-analysis
The methodological quality of the 27 included studies was checked by one observer (AS) using the Quality Assessment of Diagnostic Studies (QUADAS) instrument.41 Figure 2 shows the QUADAS results.
Figure 2.
QUADAS-2 quality assessment of the included studies.
QUADAS, quality assessment of diagnostic studies.
The meta-analysis was undertaken using RevMan 5.3 (Computer program, version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014).42,43 Heterogeneity was calculated by means of the inconsistency index I2. Furthermore, DerSimonian and Laird random-effects models with inverse-variance weights were performed without corrections, as reported previously.44
Results
Included studies and patients
The 27 studies collected were published predominantly in the years 2019–2020 (n = 20, 74%). Most were retrospective (n = 24, 89%), with only three studies (11%) of prospective design. The included studies comprised 7704 patients (Table 1). There were 1666 women (21.6%) and 5847 men (75.9%) with a mean age of 62.4 ± 24.8 years. In 191 (2.5%) patients, gender was not reported. The patients had different HNSCC (Table 2). Most frequently, HNSCC of the nasopharynx occurred (n = 3633, 47.1%).
Table 1.
Details of included studies.
| Authors | Design | Patients (n) | Analyzed clinical values |
|---|---|---|---|
| Achim et al.13 | Retrospective | 70 | Prevalence |
| Alwani et al.14 | Retrospective | 168 | Prevalence, postoperative complications |
| Ansari et al.15 | Retrospective | 78 | Prevalence, DFS, OS, postoperative complications |
| Bril et al.16 | Retrospective | 235 | Prevalence, postoperative complications, OS |
| Caburet et al.17 | Retrospective | 68 | Prevalence |
| Chargi et al.18 | Retrospective | 85 | Prevalence, OS |
| Cho et al.19 | Retrospective | 221 | Prevalence, OS |
| Choi et al.20 | Retrospective | 79 | Prevalence, OS |
| Fattouh et al.21 | Retrospective | 114 | OS |
| Findlay et al.22 | Retrospective | 79 | Prevalence, OS |
| Ganju et al.23 | Retrospective | 246 | Prevalence, OS |
| Grossberg et al.24 | Retrospective | 190 | Prevalence, OS |
| He et al.25 | Prospective | 1767 | Prevalence, OS |
| Hua et al.26 | Retrospective | 862 | Prevalence, OS |
| Huang et al.27 | Prospective | 394 | Prevalence |
| Huiskamp et al.28 | Retrospective | 91 | Prevalence, DFS, OS |
| Jung et al.29 | Retrospective | 258 | Prevalence, DFS, OS |
| Nakamura et al.30 | Retrospective | 106 | Prevalence, OS |
| Nishikawa et al.31 | Retrospective | 85 | Prevalence |
| Olson et al.32 | Retrospective | 245 | Prevalence |
| Pai et al.33 | Retrospective | 881 | Prevalence, OS |
| Schodo et al.34 | Retrospective | 41 | Prevalence |
| Stone et al.35 | Retrospective | 260 | Prevalence, OS |
| Tamaki et al.36 | Retrospective | 113 | Prevalence, DFS, OS |
| van Rijn-Dekker et al.37 | Prospective | 744 | Prevalence, DFS, OS |
| Wendrich et al.38 | Retrospective | 112 | Prevalence |
| Zwart et al.39 | Retrospective | 112 | Prevalence |
DFS, disease-free survival; OS, overall survival.
Table 2.
Data regarding patients and tumors.
| Patients | n (%) |
|---|---|
| Total | 7704 |
| Female | 1666 (21.6) |
| Male | 5847 (75.9) |
| nr | 191 (2.5) |
| Tumor localization | n (%) |
| Oral cavity | 463 (6.0) |
| Nasopharynx | 3633 (47.1) |
| Oropharynx | 1555 (20.2) |
| Hypopharynx | 490 (6.4) |
| Larynx | 813 (10.6) |
| Salivary glands | 21 (0.3) |
| Paranasal sinuses | 19 (0.2) |
| Other (non specified) | 710 (9.2) |
| Tumor stage | n (%) |
| 1 | 302 (3.9) |
| 2 | 693 (9.0) |
| 3 | 2092 (27.1) |
| 4 | 2655 (34.5) |
| nr | 1962 (25.5) |
nr, not reported.
In all cases, pretreatment CT images were analyzed for estimation of muscle mass. In most cases (27 studies, 93%), pretreatment skeletal muscle index (SMI) was calculated as a relation: skeletal muscle area divided by the square of the height (cm2/m2). In detail, in 18 studies (62%), skeletal muscle area was estimated at the third lumbar vertebra. In nine cases (31%), skeletal muscle area was estimated at the third cervical vertebra, and, thereafter, it was converted via a special equation to the skeletal muscle area at L3. Different threshold values of SMI were used for the definition of sarcopenia (Table 3). In the remaining two studies (7%), only skeletal muscle areas were estimated.
Table 3.
Thresholds of LSMM and treatment strategies performed in the included studies.
| Authors | Performed treatment | Threshold values for LSMM |
|
|---|---|---|---|
| Men | Women | ||
| Achim et al.13 | Surgery alone (total laryngectomy) | 52.4 cm2/m2 | 38.5 cm2/m2 |
| Alwani et al.14 | Surgery alone | 41.6 cm2/m2 | 32.0 cm2/m2 |
| Ansari et al.15 | Surgery alone | 43.2 cm2/m2 | 43.2 cm2/m2 |
| Bril et al.16 | Surgery alone (total laryngectomy) | 43.2 cm2/m2 | 43.2 cm2/m2 |
| Caburet et al.17 | Surgery alone | 52.4 cm2/m2 | 38.5 cm2/m2 |
| Chargi et al.18 | Curative treatments, non specified | 43.2 cm2/m2 | 43.2 cm2/m2 |
| Cho et al.19 | Concurrent CRT or definitive radiotherapy alone | 55 cm2/m2 | 39 cm2/m2 |
| Choi et al.20 | Definitive RT | 605.77 cm3 | 445.42 cm3 |
| Fattouh et al.21 | Surgery and CRT | 52.4 cm2/m2 | 38.5 cm2/m2 |
| Findlay et al.22 | Curative treatments: definitive RT, surgery and adjuvant CRT or RT; definitive CRT | 43 cm2/m2 | 41 cm2/m2 |
| Ganju et al.23 | Curative treatment: surgery and adjuvant CRT or RT | 43 cm2/m2 | 41 cm2/m2 |
| Grossberg et al.24 | Curative treatment: definitive RT, surgery and adjuvant CRT or RT; definitive CRT | 52.4 cm2/m2 | 38.5 cm2/m2 |
| He et al.25 | Definitive RT, surgery and adjuvant CRT or RT; definitive CRT | BMI adjusteda | BMI adjusteda |
| Hua et al.26 | Concurrent CRT | 18.82 cm2/m2 | 18.82 cm2/m2 |
| Huang et al.27 | Concurrent CRT | 42.4 cm2/m2 | 42.4 cm2/m2 |
| Huiskamp et al.28 | Concomitant cetuximab and RT | 45.2 cm2/m2 | 45.2 cm2/m2 |
| Jung et al.29 | Definitive treatments: surgery alone; surgery and RT/CRT; RT alone/CRT | 52.4 cm2/m2 | 38.5 cm2/m2 |
| Nakamura et al.30 | Surgery alone | 36.16 cm2/m2 | 31.02 cm2/m2 |
| Nishikawa et al.31 | Definitive treatments: surgery alone; RT alone; CRT | 46.7 cm2/m2 | 30.3 cm2/m2 |
| Olson et al.32 | Definitive treatments: surgery alone; RT alone | 52.4 cm2/m2 | 38.5 cm2/m2 |
| Pai et al.33 | Definitive treatments: RT alone; CRT | 51.74 cm2/m2 | 34.3 cm2/m2 |
| Schodo et al.34 | Concurrent CRT | 39.7 cm2/m2 | 39.7 cm2/m2 |
| Stone et al.35 | Surgery alone | 52.4 cm2/m2 | 38.5 cm2/m2 |
| Tamaki et al.36 | Curative treatment: definitive RT, surgery and adjuvant CRT or RT; definitive CRT | BMI adjustedb | 41 cm2/m2 |
| van Rijn-Dekker et al.37 | Concurrent CRT or definitive RT alone | 42.4 cm2/m2 | 30.6 cm2/m2 |
| Wendrich et al.38 | CRT | 43.2 cm2/m2 | 43.2 cm2/m2 |
| Zwart et al.39 | not reported | 43.2 cm2/m2 | 43.2 cm2/m2 |
For patients with BMI < 30 kg/m2, sarcopenia was defined as an SMI of <52 cm2/m2. For men and <38 cm2/m2 for women. For patients with BMI ⩾ 30 kg/m2, sarcopenia was defined as an SMI of <54 cm2/m2 for men and <47 cm2/m2 for women.
For males, SMI < 43 cm2/m2 is defined as sarcopenic if the patient is in the BMI category of underweight (<20.0 kg/m2) or normal weight (20.0–24.9 kg/m2). Overweight (25.0–29.9 kg/m2) and obese (>30.0 kg/m2) men are considered sarcopenic with an SMI < 41 cm2/m2. For females, all BMI categories are defined as sarcopenic if SMI is <41 cm2/m2.
BMI, body mass index; CRT, chemo-radiotherapy; LSMM, low skeletal muscle mass; RT, radiotherapy; SMI, skeletal muscle index.
In most cases (26 studies, 7619 patients) different curative treatments were performed (Table 3). In one study (85 patients), a heterogeneous cohort with both curative and palliative treatment strategies was analyzed.
Prevalence of sarcopenia
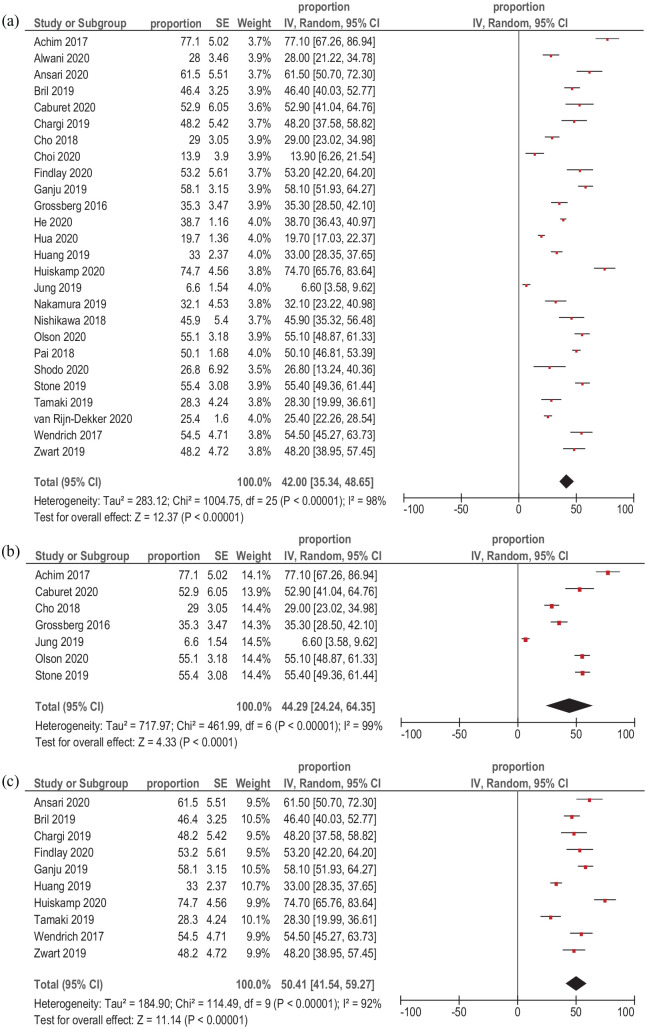
The prevalence of sarcopenia was reported in 26 studies (7590 patients). It ranged from 6.6% to 77%. The cumulative calculated prevalence among all included studies was 42.0% CI95% (35.34–48.65) (Figure 3a).
Figure 3.
Forest plots of reported prevalences of sarcopenia in patients with HNSCC. (a) Cumulative calculated prevalence among all studies. (b) Cumulative calculated prevalence among studies that used thresholds of 52.4 cm2/m2 for male patients and 38.5 cm2/m2 for female patients. (c) Cumulative calculated prevalence among studies that used thresholds of 41.0–45.2 cm2/m2.
CI, confidence interval; HNSCC, head and neck squamous cell carcinoma; SE, standard error.
At the next step, the prevalence of sarcopenia in dependency on the reported SMI thresholds was calculated. In the subgroups that used thresholds of 52.4 cm2/m2 for male patients and 38.5 cm2/m2 for female patients (seven studies, 1312 patients), the cumulative calculated prevalence among the studies was 44.29% I95%C (24.24–64.35) (Figure 3b). In the subgroups that used thresholds of 41.0–45.2 cm2/m2 for all patients (10 studies, 1545 patients), the cumulative calculated prevalence among the studies was 50.41% 95% CI (41.54–59.27) (Figure 3c).
The remaining studies used different threshold values and, therefore, no other subgroups could be composed.
Postoperative complications
For this subanalysis, only reported data on the occurrence of severe complications according to the Clavien–Dindo classification of surgical complications were collected. Associations between the presence of preoperative sarcopenia and occurrence of postoperative complications were analyzed in three studies (481 patients with HNSCC). Simple regression of the collected data showed that sarcopenia was associated with occurrence of severe (three or more points according to the Clavien-Dindo classification) postoperative complications, OR 4.79, 95% CI (2.52–9.11), p < 0.00001 (Figure 4). Heterogeneity between the studies was low (I2 = 19%).
Figure 4.
Forest plots of reported HRs of sarcopenia regarding to occurrence of severe postoperative complications (three or more points according to the Clavien–Dindo classification) in patients with HNSCC.
CI, confidence interval; HNSCC, head and neck squamous cell carcinoma; HR, hazard ratio; SE, standard error.
Disease-free survival
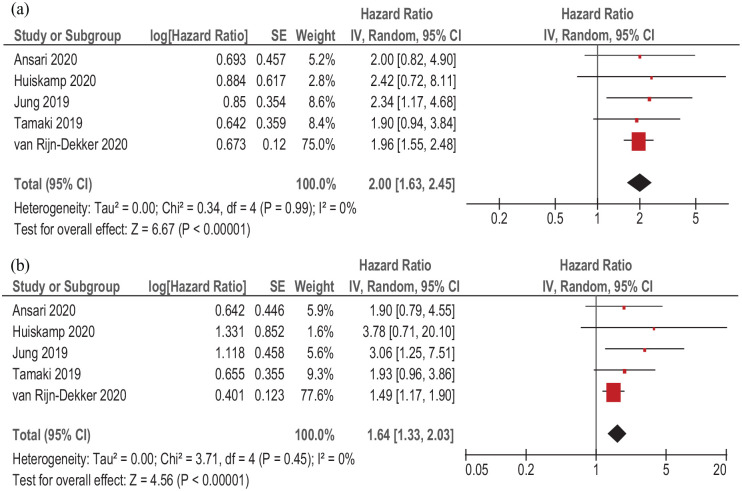
Associations between sarcopenia and DFS were investigated in five studies (1284 patients). Different curative treatment strategies were performed in the acquired studies. Simple regression of the acquired data showed that sarcopenia predicted DFS in patients with HNSCC, HR 2.00, 95% CI (1.63–2.45), p < 0.00001 (Figure 5a). There was no heterogeneity between the included studies (I2 = 0%).
Figure 5.
Forest plots of reported HRs of sarcopenia relating to DFS in patients with HNSCC. (a) Unadjusted HRs. (b) Adjusted HRs.
CI, confidence interval; DFS, disease-free survival; HNSCC, head and neck squamous cell carcinoma; HR, hazard ratio; SE, standard error.
Also, multiple regression identified that sarcopenia predicted DFS, HR 1.64, 95% CI (1.33–2.03), p < 0.00001 (Figure 5b). There was no heterogeneity between the acquired studies (I2 = 0%).
Overall survival
In 18 studies (6388 patients), relationships between sarcopenia and OS in HNSCC were analyzed. Sarcopenia was associated with lower OS (simple regression), HR 1.96, 95% CI (1.71–2.24), p < 0.00001 (Figure 6a). Heterogeneity between the studies was low (I2 = 24%).
Figure 6.
Forest plots of reported HRs of sarcopenia with regard to OS in patients with HNSCC. (a) Unadjusted HRs. (b) Adjusted HRs.
CI, confidence interval; HNSCC, head and neck squamous cell carcinoma; HR, hazard ratio; OS, overall survival; SE, standard error.
Furthermore, adjusted HRs of sarcopenia were studied. Meta-analysis (multiple regression) identified that adjusted sarcopenia was also associated with lower OS, HR = 1.87, 95% CI (1.53–2.29), p < 0.008 (Figure 6b). Heterogeneity among the studies was 52%.
On the next step, associations between pretreatment sarcopenia and OS in dependency on treatment strategy were analyzed. In six studies (2878 patients), definitive chemotherapy and/or radiation was performed. In this subgroup, sarcopenia was associated with lower OS (simple regression), HR 1.95, 95% CI (1.61–2.36), p < 0.00001 (Figure 7a). Heterogeneity between the studies was 31%.
Figure 7.
Forest plots of reported HRs of sarcopenia with regard to OS in patients with HNSCC treated by curative radio-chemotherapy. (a) Unadjusted HRs. (b) Adjusted HRs.
CI, confidence interval; HNSCC, head and neck squamous cell carcinoma; HR, hazard ratio; OS, overall survival; SE, standard error.
Adjusted sarcopenia (multiple regression) was also associated with lower OS, HR = 1.51, 95% CI (1.17–1.94), p < 0.002) (Figure 7b). Heterogeneity among the studies was 48%.
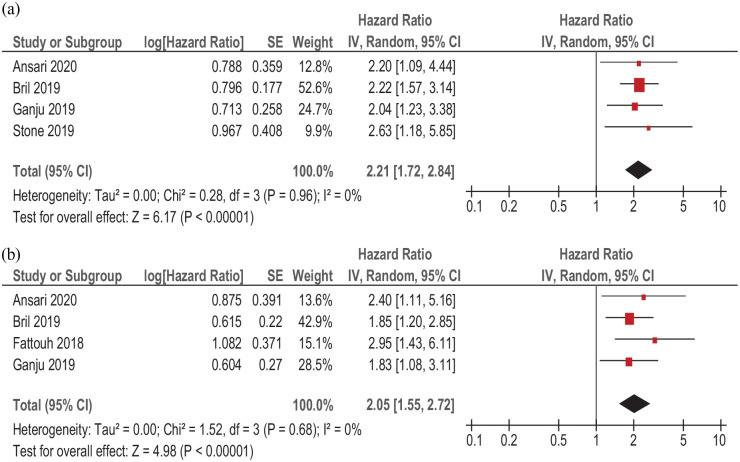
In five studies (933 patients), primary surgical strategy with/or without adjuvant radiochemotherapy was performed. Sarcopenia was associated with lower OS (simple regression), HR 2.21, 95% CI (1.72–2.84), p < 0.00001 (Figure 8a). There was no heterogeneity between the studies (I2 = 0%). Adjusted sarcopenia (multiple regression) was also associated with lower OS, HR = 2.05, CI95% (1.55–2.72), p < 0.00001), without heterogeneity (I2 = 0%) among the studies (Figure 8b).
Figure 8.
Forest plots of reported HRs of sarcopenia with regard to OS in patients with HNSCC treated by surgery with or without adjuvant radio-chemotherapy. (a) Unadjusted HRs. (b) Adjusted HRs.
CI, confidence interval; HNSCC, head and neck squamous cell carcinoma; HR, hazard ratio; OS, overall survival; SE, standard error.
In the other studies, different treatment strategies were performed. Therefore, no further subgroups in regard to treatment could be composed.
Discussion
Our data suggest that LSMM plays an important role in patients with HNSCC. Although numerous previous studies have investigated the role of sarcopenia in HNSCC, the data reported are inconsistent. In fact, the true prevalence of sarcopenia in HNSCC is unknown. As shown, prevalence ranges significantly among the reported studies. The present meta-analysis shows that it occurs in 42.0% of patients with HNSCC. This frequency is high and is caused by several factors. Firstly, HNSCC can mechanically impede the intake of nourishment. Secondly, HNSCC can also cause odynophagia and/or dysphagia. Thirdly, frequent alcohol and tobacco abuse in patients with HNSCC provokes malnutrition.
We hypothesize that sarcopenia can also influence short-term postoperative complications in HNSCC. Our results confirm this assumption. As shown, sarcopenia is associated with occurrence of severe (three or more points according to the Clavien–Dindo classification) postoperative complications in patients with HNSCC. Previously, similar results were published for patients with other malignant tumor. So far, in gastric cancer, sarcopenia also predicts postoperative complications.45 Also in colorectal cancer, sarcopenia is associated with high risk of postoperative complications.46 As mentioned by Xue, sarcopenia might be a marker of a clinically distinct “frailty syndrome” characterized by declines in physiological reserves, which result in an inability to manage acute stressors.47
Importantly, our results show that sarcopenia can predict DFS in HNSCC. Interestingly, no heterogeneity among the studies involved was observed. These stable findings covering simple and multiple regression analyses suggest that sarcopenia really predicts DFS in HNSCC and that the calculated HRs are not influenced by study heterogeneity or other factors.
The principle question is, however, whether sarcopenia can predict OS in HNSCC. If so, it can be used as a biomarker in this tumor entity. Some studies have indicated that low skeletal muscle mass also predicted OS in HNSCC.11,12 In agreement with these reports, the present meta-analysis based on a large cohort shows that sarcopenia was associated with lower OS. Remarkably, the calculated HRs among the studies do not differ largely. Moreover, also adjusted HRs of sarcopenia are well comparable with those of simple regression. Our data are in agreement with recently published smaller series.11,12 Importantly, sarcopenia can be used as a predictor for OS independent of treatment strategy. As shown, LSMM is associated with OS both in the subgroup treated with curative radio-chemotherapy and in the subgroup treated by surgery.
Overall, the present results have high clinical relevance because the fact that sarcopenia is potentially a modifiable factor, and because identification of sarcopenic patients may allow for early interventions to minimize treatment delays and improve outcomes. In fact, it has been shown that a preoperative exercise and nutritional support program can reduce sarcopenia and improve postoperative outcomes in elderly sarcopenic patients with gastric cancer.48 Also, in patients with HNSCC, additive nutrition programs can improve clinical outcomes.49
Our analysis has some limitations. Firstly, it is based only on results in the English language. Secondly, there are some methodological problems in the included studies; most were retrospective. Some included studies also had high patient selection bias. Thirdly, different approaches were used among the studies to estimate sarcopenia. Most frequently, a measure at the level of L3 from CT images was performed. However, some authors performed a measure at the level of C3 from CT images. Furthermore, we included in the analysis only studies that estimated LSMM on CT. Recently, some reports indicated that ultrasound can also be used successfully to estimate skeletal muscle mass.50,51
Fourth, the adjustment variables in the multiple Cox regression models differed in the considered studies. Unfortunately, in all studies, tumors of different origins were pooled and, therefore, no sub-analyses in regard to tumor site and/or stage could be performed. Similarly, no analysis could be performed in regard to tumor grade. Clearly, further studies are needed to overcome the limitations mentioned.
In conclusion, in HNSCC, the cumulative prevalence of sarcopenia defined as LSMM is 42.0%. Sarcopenia is an independent risk factor of OS and DFS in patients with HNSCC who underwent curative therapy. Furthermore, sarcopenia is also associated with occurrence of postoperative complications in HNSCC.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alexey Surov  https://orcid.org/0000-0002-9273-3943
https://orcid.org/0000-0002-9273-3943
Andreas Wienke  https://orcid.org/0000-0001-5871-2586
https://orcid.org/0000-0001-5871-2586
Contributor Information
Alexey Surov, Department of Radiology and Nuclear Medicine, Otto-von-Guericke-University of Magdeburg, Leipziger Str. 44, Magdeburg, 39112, Germany.
Andreas Wienke, Institute of Medical Epidemiology, Biostatistics, and Informatics, Martin-Luther-University Halle-Wittenberg, Halle, Sachsen-Anhalt, Germany.
References
- 1. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kamarajah SK, Bundred J, Tan BHL. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2019; 22: 10–22. [DOI] [PubMed] [Google Scholar]
- 3. Aleixo GFP, Williams GR, Nyrop KA, et al. Muscle composition and outcomes in patients with breast cancer: meta-analysis and systematic review. Breast Cancer Res Treat 2019; 177: 569–579. [DOI] [PubMed] [Google Scholar]
- 4. Deng HY, Hou L, Zha P, et al. Sarcopenia is an independent unfavorable prognostic factor of non-small cell lung cancer after surgical resection: a comprehensive systematic review and meta-analysis. Eur J Surg Oncol 2019; 45: 728–735. [DOI] [PubMed] [Google Scholar]
- 5. Mintziras I, Miligkos M, Wächter S, et al. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: systematic review and meta-analysis. Int J Surg 2018; 59: 19–26. [DOI] [PubMed] [Google Scholar]
- 6. Chang KV, Chen JD, Wu WT, et al. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer 2018; 7: 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu X, Dou WC, Shao YX, et al. The prognostic value of sarcopenia in patients with surgically treated urothelial carcinoma: a systematic review and meta-analysis. Eur J Surg Oncol 2019; 45: 747–754. [DOI] [PubMed] [Google Scholar]
- 8. Surov A, Wienke A. Sarcopenia predicts overall survival in patients with malignant hematological diseases: a meta-analysis. Clin Nutr 2021; 40: 1155–1160. [DOI] [PubMed] [Google Scholar]
- 9. Ubachs J, Ziemons J, Minis-Rutten IJG, et al. Sarcopenia and ovarian cancer survival: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2019; 10: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levolger S, van Vledder MG, Alberda WJ, et al. Muscle wasting and survival following pre-operative chemoradiotherapy for locally advanced rectal carcinoma. Clin Nutr 2018; 37: 1728–1735. [DOI] [PubMed] [Google Scholar]
- 11. Wong A, Zhu D, Kraus D, et al. Radiologically defined sarcopenia affects survival in head and neck cancer: a meta-analysis. Laryngoscope 2021; 131: 333–341. [DOI] [PubMed] [Google Scholar]
- 12. Hua X, Liu S, Liao JF, et al. When the loss costs too much: a systematic review and meta-analysis of sarcopenia in head and neck cancer. Front Oncol 2020; 9: 1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Achim V, Bash J, Mowery A, et al. Prognostic indication of sarcopenia for wound complication after total laryngectomy. JAMA Otolaryngol Head Neck Surg 2017; 143: 1159–1165. [DOI] [PubMed] [Google Scholar]
- 14. Alwani MM, Jones AJ, Novinger LJ, et al. Impact of sarcopenia on outcomes of autologous head and neck free tissue reconstruction. J Reconstr Microsurg 2020; 36: 369–378. [DOI] [PubMed] [Google Scholar]
- 15. Ansari E, Chargi N, van Gemert JTM, et al. Low skeletal muscle mass is a strong predictive factor for surgical complications and a prognostic factor in oral cancer patients undergoing mandibular reconstruction with a free fibula flap. Oral Oncol 2020; 101: 104530. [DOI] [PubMed] [Google Scholar]
- 16. Bril SI, Pezier TF, Tijink BM, et al. Preoperative low skeletal muscle mass as a risk factor for pharyngocutaneous fistula and decreased overall survival in patients undergoing total laryngectomy. Head Neck 2019; 41: 1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caburet C, Farigon N, Mulliez A, et al. Impact of nutritional status at the outset of assessment on postoperative complications in head and neck cancer. Eur Ann Otorhinolaryngol Head Neck Dis. Epub ahead of print 20 December 2019. DOI: 10.1016/j.anorl.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 18. Chargi N, Bril SI, Emmelot-Vonk MH, et al. Sarcopenia is a prognostic factor for overall survival in elderly patients with head-and-neck cancer. Eur Arch Otorhinolaryngol 2019; 276: 1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho Y, Kim JW, Keum KC, et al. Prognostic significance of sarcopenia with inflammation in patients with head and neck cancer who underwent definitive chemoradiotherapy. Front Oncol 2018; 8: 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi Y, Ahn KJ, Jang J, et al. Prognostic value of computed tomography-based volumetric body composition analysis in patients with head and neck cancer: feasibility study. Head Neck 2020; 42: 2614–2625. [DOI] [PubMed] [Google Scholar]
- 21. Fattouh M, Chang GY, Ow TJ, et al. Association between pretreatment obesity, sarcopenia, and survival in patients with head and neck cancer. Head Neck 2019; 41: 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Findlay M, Brown C, De Abreu Lourenço R, et al. Sarcopenia and myosteatosis in patients undergoing curative radiotherapy for head and neck cancer: impact on survival, treatment completion, hospital admission and cost. J Hum Nutr Diet 2020; 33: 811–821. [DOI] [PubMed] [Google Scholar]
- 23. Ganju RG, Morse R, Hoover A, et al. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol 2019; 137: 117–124. [DOI] [PubMed] [Google Scholar]
- 24. Grossberg AJ, Chamchod S, Fuller CD, et al. Association of body composition with survival and locoregional control of radiotherapy-treated head and neck squamous cell carcinoma. JAMA Oncol 2016; 2: 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He WZ, Jiang C, Liu LL, et al. Association of body composition with survival and inflammatory responses in patients with non-metastatic nasopharyngeal cancer. Oral Oncol 2020; 108: 104771. [DOI] [PubMed] [Google Scholar]
- 26. Hua X, Liao JF, Huang X, et al. Sarcopenia is associated with higher toxicity and poor prognosis of nasopharyngeal carcinoma. Ther Adv Med Oncol 2020; 12: 1758835920947612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang X, Ma J, Li L, et al. Severe muscle loss during radical chemoradiotherapy for non-metastatic nasopharyngeal carcinoma predicts poor survival. Cancer Med 2019; 8: 6604–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huiskamp LFJ, Chargi N, Devriese LA, et al. The predictive and prognostic value of low skeletal muscle mass for dose-limiting toxicity and survival in head and neck cancer patients receiving concomitant cetuximab and radiotherapy. Eur Arch Otorhinolaryngol 2020; 277: 2847–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jung AR, Roh JL, Kim JS, et al. Prognostic value of body composition on recurrence and survival of advanced-stage head and neck cancer. Eur J Cancer 2019; 116: 98–106. [DOI] [PubMed] [Google Scholar]
- 30. Nakamura H, Makiguchi T, Yamaguchi T, et al. Impact of sarcopenia on postoperative surgical site infections in patients undergoing flap reconstruction for oral cancer. Int J Oral Maxillofac Surg 2020; 49: 576–581. [DOI] [PubMed] [Google Scholar]
- 31. Nishikawa D, Hanai N, Suzuki H, et al. The impact of skeletal muscle depletion on head and neck squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec 2018; 80: 1–9. [DOI] [PubMed] [Google Scholar]
- 32. Olson B, Edwards J, Stone L, et al. Association of sarcopenia with oncologic outcomes of primary surgery or definitive radiotherapy among patients with localized oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg 2020; 146: e201154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pai PC, Chuang CC, Chuang WC, et al. Pretreatment subcutaneous adipose tissue predicts the outcomes of patients with head and neck cancer receiving definitive radiation and chemoradiation in Taiwan. Cancer Med 2018; 7: 1630–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shodo R, Yamazaki K, Ueki Y, et al. Sarcopenia predicts a poor treatment outcome in patients with head and neck squamous cell carcinoma receiving concurrent chemoradiotherapy. Eur Arch Otorhinolaryngol. Epub ahead of print 8 August 2020. DOI: 10.1007/s00405-020-06273-4. [DOI] [PubMed] [Google Scholar]
- 35. Stone L, Olson B, Mowery A, et al. Association between sarcopenia and mortality in patients undergoing surgical excision of head and neck cancer. JAMA Otolaryngol Head Neck Surg 2019; 145: 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tamaki A, Manzoor NF, Babajanian E, et al. Clinical significance of sarcopenia among patients with advanced oropharyngeal cancer. Otolaryngol Head Neck Surg 2019; 160: 480–487. [DOI] [PubMed] [Google Scholar]
- 37. van Rijn-Dekker MI, van den Bosch L, van den Hoek JGM, et al. Impact of sarcopenia on survival and late toxicity in head and neck cancer patients treated with radiotherapy. Radiother Oncol 2020; 147: 103–110. [DOI] [PubMed] [Google Scholar]
- 38. Wendrich AW, Swartz JE, Bril SI, et al. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol 2017; 71: 26–33. [DOI] [PubMed] [Google Scholar]
- 39. Zwart AT, van der Hoorn A, van Ooijen PMA, et al. CT-measured skeletal muscle mass used to assess frailty in patients with head and neck cancer. J Cachexia Sarcopenia Muscle 2019; 10: 1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 42. Leeflang MM, Deeks JJ, Gatsonis C, et al. Systematic reviews of diagnostic test accuracy. Ann Intern Med 2008; 149: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006; 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 45. Rinninella E, Cintoni M, Raoul P, et al. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical outcomes in patients with gastric cancer: a systematic review and meta-analysis. Clin Nutr 2020; 39: 2045–2054. [DOI] [PubMed] [Google Scholar]
- 46. van der Kroft G, Bours DMJL, Janssen-Heijnen DM, et al. Value of sarcopenia assessed by computed tomography for the prediction of postoperative morbidity following oncological colorectal resection: a comparison with the malnutrition screening tool. Clin Nutr ESPEN 2018; 24: 114–119. [DOI] [PubMed] [Google Scholar]
- 47. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011; 27: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamamoto K, Nagatsuma Y, Fukuda Y, et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer 2017; 20: 913–918. [DOI] [PubMed] [Google Scholar]
- 49. Kabarriti R, Bontempo A, Romano M, et al. The impact of dietary regimen compliance on outcomes for HNSCC patients treated with radiation therapy. Support Care Cancer 2018; 26: 3307–3313. [DOI] [PubMed] [Google Scholar]
- 50. Mueller N, Murthy S, Tainter CR, et al. Can sarcopenia quantified by ultrasound of the rectus femoris muscle predict adverse outcome of surgical intensive care unit patients as well as frailty? A prospective, observational cohort study. Ann Surg 2016; 264: 1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Galli A, Colombo M, Carrara G, et al. Low skeletal muscle mass as predictor of postoperative complications and decreased overall survival in locally advanced head and neck squamous cell carcinoma: the role of ultrasound of rectus femoris muscle. Eur Arch Otorhinolaryngol 2020; 277: 3489–3502. [DOI] [PubMed] [Google Scholar]