Abstract
Background:
Metabolic factors have been linked to tendinopathies, yet few studies have investigated the association between metabolic factors and lateral epicondylitis.
Purpose:
To evaluate risk factors for lateral epicondylitis, including several metabolic factors.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
We evaluated 1 elbow in each of 937 volunteers from a rural region that employs many agricultural laborers. Each participant received a questionnaire, physical examinations, blood tests, simple radiographic evaluations of both elbows, magnetic resonance imaging of bilateral shoulders, and an electrophysiological study of bilateral upper extremities. Lateral epicondylitis was diagnosed using 3 criteria: (1) pain at the lateral aspect of the elbow, (2) point tenderness over the lateral epicondyle, and (3) pain during resistive wrist dorsiflexion with the elbow in full extension. Multivariable logistic regression analysis was used to calculate the odds ratios (ORs) and 95% CIs for various demographic, physical, and social factors, including age, sex, waist circumference, dominant-side involvement, smoking habit, alcohol intake, and participation in manual labor; the comorbidities of diabetes, hypertension, thyroid dysfunction, metabolic syndrome, ipsilateral biceps tendon injury, ipsilateral rotator cuff tear, and ipsilateral carpal tunnel syndrome; and the serologic parameters of serum lipid profile, glycosylated hemoglobin A1c, level of thyroid hormone, and high-sensitivity C-reactive protein.
Results:
The prevalence of lateral epicondylitis was 26.1% (245/937 participants). According to the multivariable logistic regression analysis, female sex (OR, 2.47; 95% CI, 1.78-3.43), dominant-side involvement (OR, 3.21; 95% CI, 2.24-4.60), manual labor (OR, 2.25; 95% CI, 1.48-3.43), and ipsilateral rotator cuff tear (OR, 2.77; 95% CI, 1.96-3.91) were significantly associated with lateral epicondylitis (P < .001 for all). No metabolic factors were significantly associated with lateral epicondylitis.
Conclusion:
Female sex, dominant-side involvement, manual labor, and ipsilateral rotator cuff tear were found to be risk factors for lateral epicondylitis. The study results suggest that overuse activity is more strongly associated with lateral epicondylitis than are metabolic factors.
Keywords: epicondylitis, risk factors, sex, dominant elbow, dyslipidemia
Lateral epicondylitis is a common tendinopathy, with a 0.3% to 12.2% prevalence in the adult population,11,14,38,44 yet little is known regarding its causes and risk factors. Several factors, such as overuse, repetitive activity, and age, have been proposed as causes of lateral epicondylitis, but no factor has been clearly established. Several risk factors have been described as associated with lateral epicondylitis: rotator cuff pathology, De Quervain disease, carpal tunnel syndrome (CTS), oral corticosteroid therapy, smoking, obesity, rheumatoid arthritis,27,36,38,41 intensive manual occupations, and occupations involving vibratory machines.37,38 A particularly strong association of rotator cuff pathology with lateral epicondylitis has been described.12,41,45 Recently, studies have linked various tendinopathies to metabolic factors such as hyperglycemia, obesity, hypertension, dyslipidemia, and metabolic syndrome (MetS).¶ Hyperglycemia (diabetes) has been reported to be associated with rotator cuff tendon tear,1,25 obesity with rotator cuff tear47 and Achilles tendinopathy,20 and dyslipidemia with several tendinopathies, including those of the Achilles tendon,19,28,33 rotator cuff tendon,2,26 patellar tendon,6,7 and biceps tendon.8 Dyslipidemia has also been evaluated as a possible risk factor for lateral epicondylitis, but that relationship remains unproved.38 Despite increased interest in the relationships of metabolic factors to tendinopathies, few reported studies have investigated the association of metabolic factors with lateral epicondylitis. Therefore, the purpose of this study was to evaluate risk factors for lateral epicondylitis, including several metabolic factors.
Methods
This study included a cohort of 1149 volunteers from the same rural region who participated between June 2013 and December 2015. The volunteers were recruited for a survey of upper extremity morbidity in a rural area; they did not receive compensation. The volunteer participants completed a questionnaire and underwent physical examinations of both elbows by an orthopaedic surgeon (J.-Y.G.), fasting blood testing, simple radiographic evaluations of bilateral elbows (anteroposterior and lateral views), bilateral shoulder magnetic resonance imaging (MRI), and bilateral upper extremity electrophysiological assessment. For each volunteer, all physical examinations, as well as blood, imaging, and electrophysiological tests, were performed on the same day. This study received institutional review board approval.
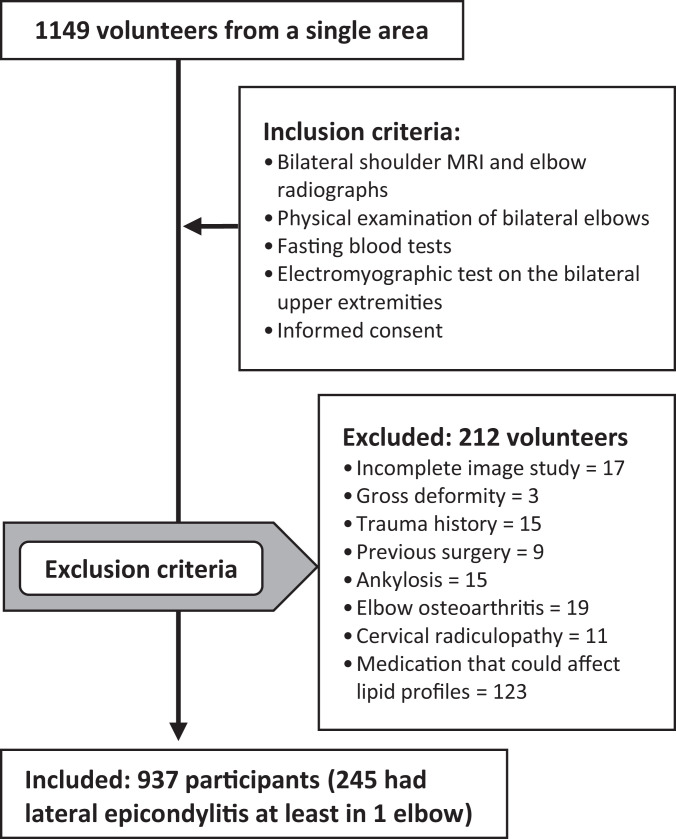
A total of 212 volunteers were excluded from the study for the following reasons: (1) 17 volunteers (32 elbows) did not have a complete imaging study; (2) 72 volunteers (144 elbows) had at least 1 elbow affected by gross deformity, a history of trauma or surgery around the elbow, ankylosis, elbow arthritis, or a cervical radiculopathy confirmed by an electromyography study; and (3) 123 volunteers (246 elbows) were taking medication that might have affected their serum lipid profiles. The total number of participants included in our final analysis was 937 (937 elbows, as only 1 elbow per participant was included in our analysis) (Figure 1). In participants with either bilateral lateral epicondylitis or no lateral epicondylitis, 1 elbow was randomly excluded (using random-number generation). For participants with unilateral lateral epicondylitis, only the involved elbow was included.
Figure 1.
Flowchart showing exclusion and inclusion criteria for this study. MRI, magnetic resonance imaging.
The 937 study participants had a mean ± SD age of 59.7 ± 8.6 years. Among the 449 male participants (47.9%), the mean age was 60.8 ± 8.7 years; among the 488 female participants (52.1%), the mean age was 58.6 ± 8.4 years. Age- and sex-related data are summarized in Table 1.
Table 1.
Data on Age and Sex According to Occupation of Enrolled Participants
| No. (% Within Group) | Age, y, Mean ± SD | |
|---|---|---|
| Total enrolled | 937 (100) | 59.7 ± 8.6 |
| Male | 449 (47.9) | 60.8 ± 8.7 |
| Female | 488 (52.1) | 58.6 ± 8.4 |
| Agricultural workers | 689 (73.5) | 59.8 ± 8.8 |
| Male | 327 (47.5) | 60.9 ± 8.7 |
| Female | 362 (52.5) | 58.9 ± 8.7 |
| Office workers | 248 (26.5) | 59.2 ± 8.2 |
| Male | 122 (49.2) | 60.6 ± 8.6 |
| Female | 126 (50.8) | 57.7 ± 7.5 |
Lateral epicondylitis was diagnosed based on the following symptoms and signs observed during physical examination: (1) pain at the lateral aspect of the elbow, (2) point tenderness over the lateral epicondyle, and (3) pain during resistive wrist dorsiflexion with the elbow in full extension.39 No further diagnostic imaging (ultrasonography or MRI) was performed at the elbow.
The evaluated physical factors were age, sex, waist circumference, and dominant-side involvement. The evaluated social factors were smoking habit, alcohol intake, and participation in manual labor, based on occupation. The investigated comorbidities were diabetes, hypertension, hyper- and hypothyroidism, MetS, ipsilateral biceps tendon injury, ipsilateral rotator cuff tear, and ipsilateral CTS. The evaluated serologic factors were serum lipid profile, glycosylated hemoglobin A1c (HbA1c), and levels of thyroid hormones and high-sensitivity C-reactive protein (hs-CRP).
Patients with prior diagnoses of diabetes were accepted; new diagnoses were made upon finding serum HbA1c levels of ≥6.5%.5 Diagnoses of hypertension were based on medical history and the detection of at least 140 mm Hg systolic blood pressure or at least 90 mm Hg diastolic pressure.13 Prior diagnoses of hyper- and hypothyroidism were accepted. New diagnoses were based on the results of thyroid function tests, in which serum-free T4 levels >1.70 ng/dL indicated hyperthyroidism and levels <0.93 ng/dL indicated hypothyroidism.17 Clinical identification of MetS was based on meeting at least 3 of the following criteria: (1) fasting plasma glucose ≥100 mg/dL or patient taking antidiabetes medication; (2) systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg or patient taking antihypertensive medication; (3) a serum triglyceride (TG) level of ≥150 mg/dL; (4) high-density lipoprotein (HDL) <40 mg/dL in men or <50 mg/dL in women; and (5) a waist circumference of ≥90 cm for men or ≥85 cm for women.3 The waist circumference criteria for obesity were adjusted according to Korean norms.24
Biceps tendon injuries and rotator cuff tendon tears were diagnosed primarily on the basis of individual MRI findings. Biceps tendon injuries were classified according to MRI findings: partial tear was determined based on attenuation of the tendon and increased T2-weighted intratendinous signal intensity; full-thickness biceps tear was identified by the absence of the long head of the biceps tendon intra-articularly or within the groove.35 Rotator cuff tears were diagnosed as either partial-thickness tears or full-thickness tears. High signal intensity within the cuff tendon that extended to its bursal or articular surface indicated a partial-thickness tendon tear. High signal intensity that passed through the entire thickness of the tendon indicated a full-thickness tear.43 CTS was diagnosed based on electrophysiological findings, after consideration of symptoms and findings on physical examination. Electrophysiological diagnoses were based on established criteria: median nerve distal motor latency of >4.0 milliseconds, distal sensory latency of >3.6 milliseconds, or distal latency delay of >0.5 milliseconds, as compared with conduction measurements of the median nerve and the ulnar nerve in the fourth digit.18
Serum lipid profiles were evaluated as scale and categorical variables. The scale variables were total cholesterol, TG, low-density lipoprotein (LDL), HDL, and non-HDL levels. The categorical variables included the following dyslipidemias: hypercholesterolemia (total cholesterol ≥200 mg/dL), hypertriglyceridemia (TG ≥150 mg/dL), hyper-LDLemia (LDL ≥100 mg/dL), hypo-HDLemia (HDL <40 in men and <50 in women), and hyper non–HDLemia (non-HDL of ≥130 mg/dL).16 Serum hs-CRP was also evaluated.
Statistical Analysis
The strength of associations between lateral epicondylitis and the evaluated factors was determined by calculating the odds ratios (ORs) with 95% CIs using logistic regression analyses. Univariate logistic regression analyses were performed for all variables; multiple forward stepwise logistic regression analysis was then performed on variables demonstrating significant associations with clinically significant effect size. A multivariable logistic regression analysis was performed after assessment of multicollinearity, using factors with a variance inflation factor (VIF) and a condition index. Multicollinearity was considered absent when both VIF and condition index were <10.9 Goodness of fit for the multivariable logistic regression model was determined by the Hosmer-Lemeshow test. The model with the lowest Akaike information criterion was selected from among the combinations of associated factors included in the multivariable analysis. In this study, the population of the dependent variable to be included in the final model was at least 15 per independent variable in order to avoid the problem of overfitting.34 Finally, the C-statistic was used in the final multivariable logistic regression model to determine the model’s accuracy.10
All statistical analyses were performed using the SPSS software program (Version 24.0; IBM SPSS Statistics for Windows). Significance of the logistic analyses was set at P < .05. Significance of the Hosmer-Lemeshow test was set at P > .05.
Results
In the 937 participants, the prevalence of participants with lateral epicondylitis in at least 1 elbow was 26.1% (95% CI, 23.3%-29.1%). These 245 participants had a mean age of 58.8 ± 8.9 years. The 165 female participants (67.3%) had a mean age of 58.4 ± 8.9 years; the 80 male participants (32.7%) had a mean age of 59.7 ± 8.9 years. Dominant-side involvement was 79.6% (195/245).
The prevalence of comorbidities in the participants who had lateral epicondylitis was as follows: diabetes, 8.2% (20/245); hypertension, 53.5% (131/245); hyperthyroidism, 1.2% (5/245); hypothyroidism, 1.6% (6/245); metabolic syndrome, 42.0% (103/245); ipsilateral biceps tendon injury, 15.5% (38/245); ipsilateral full-thickness rotator cuff tear, 19.6% (48/245); ipsilateral partial-thickness rotator cuff tear, 28.6% (70/245); and ipsilateral CTS, 34.7% (86/245). Information regarding the serum lipid levels, comorbidities, and other epidemiological factors is summarized in Table 2.
Table 2.
Prevalence of Studied Variables According to Presence or Absence of Lateral Epicondylitisa
| Characteristic | Presence of LE (n = 245)b | Absence of LE (n = 692)b | P | OR (95% CI) |
|---|---|---|---|---|
| Age, y | 58.8 ± 8.9 | 60.0 ± 8.5 | .151 | 0.99 (0.97-1.01) |
| Female sex | 165 (67.3) | 323 (46.7) | <.001 | 2.36 (1.74-3.20) |
| Waist circumference, cmc | 83.4 ± 8.3 | 82.9 ± 8.0 | .221 | 1.02 (0.98-1.04) |
| Dominant-side involvement | 195 (79.6) | 349 (50.4) | <.001 | 3.83 (2.72-5.41) |
| Smoking habit | 61 (24.9) | 195 (28.2) | .322 | 0.85 (0.61-1.18) |
| Alcohol intake | 160 (65.3) | 461 (66.6) | .588 | 0.71 (0.69-1.28) |
| Manual labor | 211 (86.1) | 478 (69.1) | <.001 | 2.78 (1.87-4.13) |
| Diabetes | 20 (8.2) | 79 (11.4) | .157 | 0.69 (0.41-1.15) |
| Hypertension | 131 (53.5) | 362 (52.3) | .755 | 1.05 (0.78-1.40) |
| Hyperthyroidism | 3 (1.2) | 6 (0.9) | .624 | 1.42 (0.35-5.71) |
| Hypothyroidism | 4 (1.6) | 12 (1.7) | .916 | 0.94 (0.30-2.94) |
| Metabolic syndrome | 103 (42.0) | 268 (38.7) | .362 | 1.15 (0.85-1.54) |
| Ipsilateral biceps tendon injury | 38 (15.5) | 103 (14.9) | .814 | 1.05 (0.70-1.57) |
| Ipsilateral rotator cuff tear | 118 (48.2) | 165 (23.8) | <.001 | 2.97 (2.19-4.03) |
| Ipsilateral CTS | 85 (34.7) | 184 (26.6) | .016 | 1.47 (1.07-2.01) |
| Cholesterol, mg/dLc | 198.7 ± 37.3 | 193.7 ± 36.8 | .084 | 1.00 (1.00-1.01) |
| Triglyceride, mg/dLc | 110.5 [80.0-163.3] | 107.0 [77.5-148.0] | .273 | 1.00 (1.00-1.00) |
| LDL, mg/dLc | 130.5 ± 34.2 | 128.8 ± 35.3 | .511 | 1.00 (1.00-1.01) |
| HDL, mg/dLc | 55.0 [43.0-66.0] | 54.0 [46.0-65.0] | .689 | 1.00 (0.99-1.01) |
| Non-HDL, mg/dLc | 142.4 ± 37.5 | 137.5 ± 36.8 | .119 | 1.00 (1.00-1.01) |
| Prevalence of dyslipidemia | 215 (87.8) | 597 (86.3) | .558 | 1.14 (0.74-1.77) |
| Hypercholesterolemia | 105 (42.9) | 290 (41.9) | .796 | 1.04 (0.77-1.40) |
| Hypertriglyceridemia | 76 (31.0) | 167 (24.1) | .035 | 1.41 (1.03-1.95) |
| Hyper-LDLemia | 199 (81.2) | 552 (79.8) | .624 | 1.10 (0.76-1.59) |
| Hypo-HDLemia | 65 (26.5) | 157 (22.7) | .225 | 1.23 (0.88-1.72) |
| Hyper–non HDLemia | 156 (63.7) | 400 (57.8) | .108 | 1.28 (0.95-1.73) |
| Hemoglobin A1cc | 5.8 ± 0.3 | 5.7 ± 0.3 | .624 | 1.39 (0.35-5.71) |
| Free T4c | 1.3 ± 0.3 | 1.2 ± 0.2 | .588 | 1.28 (0.81-1.70) |
| hs-CRP, mg/L | 0.6 [0.3-1.1] | 0.6 [0.3-1.2] | .220 | 1.02 (0.99-1.06) |
aBolded P values indicate statistically significant difference between groups (P < .05). CTS, carpal tunnel syndrome; HDL, high-density lipoprotein; hs-CRP, high sensitivity C-reactive protein; LDL, low-density lipoprotein; LE, lateral epicondylitis; OR, odds ratio.
bData are reported as mean ± SD, median [interquartile range], or n (%).
cCategorical values are presented in Appendix Table A1.
In the univariate analyses, female sex, dominant-side involvement, manual labor, ipsilateral rotator cuff tear, ipsilateral CTS, and hypertriglyceridemia were significantly associated with lateral epicondylitis. The ORs with 95% CIs and the P values for all of the variables are summarized in Table 3.
Table 3.
Strength of Associations Between Lateral Epicondylitis and Various Factorsa
| Studied Variable | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | |
| Female sex | <.001 | 2.36 (1.74-3.20) | <.001 | 2.47 (1.78-3.43) |
| Dominant-side involvement | <.001 | 3.83 (2.72-5.41) | <.001 | 3.21 (2.24-4.60) |
| Manual labor | <.001 | 2.78 (1.87-4.13) | <.001 | 2.25 (1.48-3.43) |
| Ipsilateral rotator cuff tear | <.001 | 2.97 (2.19-4.03) | <.001 | 2.77 (1.96-3.91) |
| Ipsilateral carpal tunnel syndrome | .016 | 1.47 (1.07-2.01) | — | — |
| Hypertriglyceridemia | .035 | 1.41 (1.03-1.95) | — | — |
aVariance inflation factor = 2.012; condition index = 2.581; P value (Hosmer-Lemeshow test) = .512. Dashes indicate no statistical significance. OR, odds ratio.
According to the multivariable analysis, female sex (OR, 2.47; 95% CI, 1.78-3.43), dominant-side involvement (OR, 3.21; 95% CI, 2.24-4.60), manual labor (OR, 2.25; 95% CI, 1.48-3.43), and ipsilateral rotator cuff tear (OR, 2.77; 95% CI, 1.96-3.91) were significantly associated with lateral epicondylitis. The result of C-statistic analysis was 0.71 (95% CI, 0.67-0.75), which indicates a good model. The VIF, condition index, P value of Hosmer-Lemeshow test, and ORs with 95% CIs and the P values for all the variables are summarized in Table 3.
Discussion
The results of the current study indicated that lateral epicondylitis is significantly associated with female sex, dominant-side involvement, manual labor, and ipsilateral rotator cuff tear.
The prevalence of lateral epicondylitis in this study was relatively high at 26.1%. Previous studies reported prevalence of lateral epicondylitis that ranged from 0.3% to 12.2%, depending on their populations’ characteristics and their definitions of lateral epicondylitis.11,14,38,44 Because this study was performed in a rural area, many participants were agricultural workers whose jobs involved high-intensity labor and variable operating postures. Several previous studies have reported a relationship of lateral epicondylitis to the degree of heaviness of labor, which would explain the relatively high prevalence found in our study.23,36,38,46
Female sex was highly associated with lateral epicondylitis in this study. Several previous studies have reported a higher prevalence of lateral epicondylitis among female participants.21,29,38,45 However, other studies have reported that patient sex is not significantly associated with lateral epicondylitis, thereby leaving the question open to debate.23,39 Although some research has been conducted on gene-based risk factors for lateral epicondylitis, the possibility that sex is associated with those investigated genes has not been completely evaluated.4 Therefore, the possibility remains that sex is a cofactor related to genes associated with lateral epicondylitis. Further study is also necessary to determine whether any sex-specific activities are associated with lateral epicondylitis.
Dominant-side involvement has been reported as a risk factor for lateral epicondylitis in previous studies, lending support to the results of the current study.15,23 However, 1 study reported that hand dominance was not a risk factor for lateral epicondylitis.39 The differences in these studies’ findings come, at least in part, from their different populations and from their participants’ different occupations and patterns of manual labor exposure and activity. Therefore, further study must be done to evaluate the association of lateral epicondylitis with activity patterns and activity exposure according to occupation.
Lateral epicondylitis has been reported as a factor significantly associated with rotator cuff tear,41 which supports our study results. Laban et al22 reported that patients with lateral epicondylitis had previously unrecognized internal rotation deficit in their shoulders, a common finding in chronic rotator cuff tear.40 Based on their finding, those authors postulated the biomechanical explanation that an internal rotation deficit could encourage eccentric movements of the hand and forearm, resulting in lateral epicondylitis. Chard et al12 reported that histologic features of biopsy samples of common extensor tendons were the same as those seen in aged supraspinatus tendons. Nirschl31 suggested that a “mesenchymal syndrome” was involved in the origin of lateral epicondylitis via a predisposition to tendinosis at multiple sites. These conditions included medial epicondylitis, CTS, De Quervain disease, trigger finger, and rotator cuff tendinosis. Many of these conditions, including rotator cuff pathology, have since been associated with lateral epicondylitis in other studies.41,42
Previous studies have reported that CTS is associated with lateral epicondylitis,30,41 although those studies did not mention specifically whether the affected side was an influential variable. In the current study, CTS was significantly associated with lateral epicondylitis regardless of the affected side, according to the univariate analyses. However, CTS was not a significant independent variable predictive of lateral epicondylitis, according to the multivariable analysis.
Several previous studies have reported an association between tendinopathies and serum lipid abnormalities.2,6–8,19,26,28,33 Using commonly indexed serum lipid profiles, we evaluated the possibility of significant associations between serum lipid abnormalities and lateral epicondylitis but were unable to find any significant association. One earlier study searched for associations of lateral epicondylitis with both HDL and LDL without finding any significant association between lateral epicondylitis and those lipoproteins.38 Another study investigating any association between hyperlipidemia and lateral epicondylitis determined that hyperlipidemia is not a risk factor for lateral epicondylitis.32 The current study supports the results of previous studies that dyslipidemia is not associated with lateral epicondylitis. However, because the current study and those previous studies were cross-sectional, the long-term or cumulative effects of serum lipid abnormalities associated with lateral epicondylitis could not be evaluated. Therefore, further research involving long-term follow-up is needed.
In the current study, we evaluated several metabolic factors, including hyperglycemia, obesity, thyroid abnormalities, and MetS, which have been reported as possible risk factors for tendinopathy, usually in studies of the rotator cuff tendon. However, we could not find any association between these metabolic variables and lateral epicondylitis. We suggest that lateral epicondylitis has a greater association with overuse activity than with metabolic factors, while also noting that the high prevalence of lateral epicondylitis is limited to a specific age range and does not increase with age.38,39,41 This finding suggests that lateral epicondylitis is not an age-related degenerative process, inclusive of metabolic disorders, but rather a process associated with overuse.
This study has several limitations. Although this was a cohort study, we could not include all residents in the studied region, so we relied on volunteers. There may have been a bias toward symptomatic patients being willing to volunteer. Because this was a cross-sectional study, we could not evaluate the cumulative effects of serum lipid abnormalities on lateral epicondylitis. In addition, because we did not evaluate the tendon status of lateral epicondylitis using MRI, we could not evaluate any association between dyslipidemia and lateral epicondylitis according to the grade or severity of the tendinopathy. However, because lateral epicondylitis is a clinical disease entity whose diagnosis does not require MRI scans, the results of this study have practical application in clinical situations. Finally, we did not evaluate the type of occupation or sports activity or the intensity of labor of the participants.
This study also has several strengths. Lateral epicondylitis was diagnosed using specific diagnostic criteria depending on physical examination, not on patient recall or on data based on a patient coding system. Rotator cuff tear was diagnosed using MRI findings. CTS was diagnosed using not only patient symptoms and signs but also objective examination using electrophysiological studies.
Conclusion
Female sex, dominant-side involvement, manual labor, and ipsilateral rotator cuff tear were found to be risk factors for lateral epicondylitis. The results of this study suggest that overuse activity is more strongly associated with lateral epicondylitis than are metabolic factors.
Acknowledgment
The authors express their gratitude to Nok-Bum Kim, MD, PhD, who supported the statistical aspects of this project throughout the entire process.
Appendix
Table A1.
Prevalence of Stratified Continuous Variables According to Presence or Absence of Lateral Epicondylitisa
| Characteristic | Presence of LE (n = 245)b | Absence of LE (n = 692)b | P | OR (95% CI) |
|---|---|---|---|---|
| Waist circumference, cm | 83.4 ± 8.3 | 82.9 ± 8.0 | .221 | 1.02 (0.98-1.04) |
| <80 (men) or <75 (women) | 61 (24.9) | 173 (25.0) | .597 | 1.05 (0.88-1.26) |
| 80-89 (men) or 75-84 (women) | 106 (43.3) | 314 (45.5) | ||
| 90-99 (men) or 85-94 (women) | 67 (27.3) | 180 (26.0) | ||
| ≥100 (men) or ≥95 (women) | 11 (4.5) | 25 (3.6) | ||
| Serum lipid levels | ||||
| Cholesterol, mg/dL | 198.7 ± 37.3 | 193.7 ± 36.8 | .084 | 1.00 (1.00-1.01) |
| <200 | 140 (57.1) | 402 (58.1) | .227 | 1.15 (0.92-1.45) |
| 200-249 | 79 (32.2) | 249 (36.0) | ||
| ≥250 | 26 (10.6) | 41 (5.9) | ||
| Triglycerides, mg/dL | 110.5 [80.0-163.3] | 107.0 [77.5-148.0] | .273 | 1.00 (1.00-1.00) |
| <150 | 169 (69.0) | 525 (75.9) | .033 | 1.25 (1.02-1.52) |
| 150-199 | 39 (15.9) | 91 (13.2) | ||
| ≥200 | 37 (15.1) | 76 (11.0) | ||
| LDL, mg/dL | 130.5 ± 34.2 | 128.8 ± 35.3 | .511 | 1.00 (1.00-1.01) |
| <100 | 46 (18.8) | 140 (20.2) | .696 | 1.05 (0.82-1.35) |
| 100-149 | 132 (53.9) | 377 (54.5) | ||
| ≥150 | 67 (27.3) | 175 (25.3) | ||
| HDL, mg/dL | 55.0 [43.0-66.0] | 54.0 [46.0-65.0] | .689 | 1.00 (0.99-1.01) |
| ≥40 (men) or ≥50 (women) | 183 (74.7) | 525 (75.9) | .696 | 1.05 (0.82-1.35) |
| 30-39 (men) or 40-49 (women) | 47 (19.2) | 128 (15.8) | ||
| <30 (men) or <40 (women) | 15 (6.1) | 39 (5.6) | ||
| Non-HDL, mg/dL | 142.4 ± 37.5 | 137.5 ± 36.8 | .119 | 1.00 (1.00-1.01) |
| <130 | 89 (36.3) | 292 (42.2) | .085 | 1.25 (0.97-1.61) |
| 130-199 | 141 (57.6) | 368 (53.2) | ||
| ≥200 | 15 (6.1) | 32 (4.6) | ||
| Hemoglobin A1c, % | 5.8 ± 0.3 | 5.7 ± 0.3 | .624 | 1.39 (0.35-5.71) |
| <5.70 | 112 (45.7) | 344 (49.7) | .145 | 1.23 (0.93-1.64) |
| 5.70-6.49 | 128 (52.2) | 345 (49.9) | ||
| ≥6.50 | 5 (2.0) | 3 (0.4) | ||
| Free T4, ng/dL | 1.3 ± 0.3 | 1.2 ± 0.2 | .588 | 1.28 (0.81-1.70) |
| <0.93 | 5 (2.0) | 26 (3.8) | .734 | 0.88 (0.43-1.82) |
| 0.93-1.70 | 222 (90.6) | 656 (94.8) | ||
| >1.70 | 18 (7.3) | 10 (1.4) |
aBolded P value indicates statistically significant difference between groups (P < .05). HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; LE, lateral epicondylitis; OR, odds ratio.
bData are reported as mean ± SD, median [interquartile range], or n (%).
Footnotes
Final revision submitted August 5, 2020; accepted August 25, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by a grant from the Farmers’ Musculoskeletal Disease Investigation of the Korean Rural Development Administration. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Gyeongsang National University Hospital (study No. GNUH 2015-02-001).
References
- 1. Abate M, Schiavone C, Salini V. Sonographic evaluation of the shoulder in asymptomatic elderly subjects with diabetes. BMC Musculoskelet Disord. 2010;11:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res. 2010;468(6):1493–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alberti KG, Zimmet P, Shaw J; Group IDFETFC. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–1062. [DOI] [PubMed] [Google Scholar]
- 4. Altinisik J, Meric G, Erduran M, Ates O, Ulusal AE, Akseki D. The BstUI and DpnII variants of the COL5A1 gene are associated with tennis elbow. Am J Sports Med. 2015;43(7):1784–1789. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl_1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beason D, Abboud J, Bassora A, Kuntz A, Soslowsky L. Hypercholesterolemia is detrimental to tendon properties and healing in a mouse injury model. Paper presented at: 55th Annual Meeting of the Orthopaedic Research Society; February 22-25, 2009; Las Vegas, NV. [Google Scholar]
- 7. Beason DP, Abboud JA, Kuntz AF, Bassora R, Soslowsky LJ. Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J Orthop Res. 2011;29(3):380–383. [DOI] [PubMed] [Google Scholar]
- 8. Beason DP, Kuntz AF, Hamamdzic D, et al. High cholesterol adversely affects biceps tendon mechanical properties in a porcine model. Paper presented at: 55th Annual Meeting of the Orthopaedic Research Society; February 22-25, 2009; Las Vegas, NV. [Google Scholar]
- 9. Belsley DA. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. Wiley-Interscience; 2004. [Google Scholar]
- 10. Caetano SJ, Sonpavde G, Pond GR. C-statistic: a brief explanation of its construction, interpretation and limitations. Eur J Cancer. 2018;90:130–132. [DOI] [PubMed] [Google Scholar]
- 11. Calfee RP, Patel A, DaSilva MF, Akelman E. Management of lateral epicondylitis: current concepts. J Am Acad Orthop Surg. 2008;16(1):19–29. [DOI] [PubMed] [Google Scholar]
- 12. Chard MD, Cawston TE, Riley GP, Gresham GA, Hazleman BL. Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis. 1994;53(1):30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. [DOI] [PubMed] [Google Scholar]
- 14. De Smedt T, de Jong A, Van Leemput W, Lieven D, Van Glabbeek F. Lateral epicondylitis in tennis: update on aetiology, biomechanics and treatment. Br J Sports Med. 2007;41(11):816–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dimberg L. The prevalence and causation of tennis elbow (lateral humeral epicondylitis) in a population of workers in an engineering industry. Ergonomics. 1987;30(3):573–579. [DOI] [PubMed] [Google Scholar]
- 16. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. [DOI] [PubMed] [Google Scholar]
- 17. Jameson JL, Mandel SJ, Weetman AP. Disorders of the thyroid gland. In: Kasper DL, Fauci AS, Longo DL, et al. , eds. Harrison’s Principles of Internal Medicine. McGraw-Hill; 2015:2283–2308. [Google Scholar]
- 18. Johnson EW, Kukla RD, Wongsam PE, Piedmont A. Sensory latencies to the ring finger: normal values and relation to carpal tunnel syndrome. Arch Phys Med Rehabil. 1981;62(5):206–208. [PubMed] [Google Scholar]
- 19. Kiortsis DN, Argyropoulou MI, Xydis V, Tsouli SG, Elisaf MS. Correlation of Achilles tendon thickness evaluated by ultrasonography with carotid intima-media thickness in patients with familial hypercholesterolemia. Atherosclerosis. 2006;186(1):228–229. [DOI] [PubMed] [Google Scholar]
- 20. Klein EE, Weil L, Jr, Weil LS, Sr, Fleischer AE. Body mass index and Achilles tendonitis: a 10-year retrospective analysis. Foot Ankle Spec. 2013;6(4):276–282. [DOI] [PubMed] [Google Scholar]
- 21. Kurppa K, Viikari-Juntura E, Kuosma E, Huuskonen M, Kivi P. Incidence of tenosynovitis or peritendinitis and epicondylitis in a meat-processing factory. Scand J Work Environ Health. 1991;17(1):32–37. [DOI] [PubMed] [Google Scholar]
- 22. Laban MM, Iyer R, Tamler MS. Occult periarthrosis of the shoulder: a possible progenitor of tennis elbow. Am J Phys Med Rehabil. 2005;84(11):895–898. [DOI] [PubMed] [Google Scholar]
- 23. Leclerc A, Landre MF, Chastang JF, Niedhammer I, Roquelaure Y; Study Group on Repetitive Work. Upper-limb disorders in repetitive work. Scand J Work Environ Health. 2001;27(4):268–278. [DOI] [PubMed] [Google Scholar]
- 24. Lee SY, Park HS, Kim DJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75(1):72–80. [DOI] [PubMed] [Google Scholar]
- 25. Longo UG, Franceschi F, Ruzzini L, Spiezia F, Maffulli N, Denaro V. Higher fasting plasma glucose levels within the normoglycaemic range and rotator cuff tears. Br J Sports Med. 2009;43(4):284–287. [DOI] [PubMed] [Google Scholar]
- 26. Longo UG, Franceschi F, Spiezia F, Forriol F, Maffulli N, Denaro V. Triglycerides and total serum cholesterol in rotator cuff tears: do they matter? Br J Sports Med. 2010;44(13):948–951. [DOI] [PubMed] [Google Scholar]
- 27. Malmivaara A, Viikari-Juntura E, Huuskonen M, et al. Rheumatoid factor and HLA antigens in wrist tenosynovitis and humeral epicondylitis. Scand J Rheumatol. 1995;24(3):154–156. [DOI] [PubMed] [Google Scholar]
- 28. Mathiak G, Wening JV, Mathiak M, Neville LF, Jungbluth K. Serum cholesterol is elevated in patients with Achilles tendon ruptures. Arch Orthop Trauma Surg. 1999;119(5-6):280–284. [DOI] [PubMed] [Google Scholar]
- 29. McCormack RR, Jr, Inman RD, Wells A, Berntsen C, Imbus HR. Prevalence of tendinitis and related disorders of the upper extremity in a manufacturing workforce. J Rheumatol. 1990;17(7):958–964. [PubMed] [Google Scholar]
- 30. Murray-Leslie CF, Wright V. Carpal tunnel syndrome, humeral epicondylitis, and the cervical spine: a study of clinical and dimensional relations. Br Med J. 1976;1(6023):1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nirschl RP. Mesenchymal syndrome. Va Med Mon (1918). 1969;96(11):659–662. [PubMed] [Google Scholar]
- 32. Otoshi K, Takegami M, Sekiguchi M, et al. Chronic hyperglycemia increases the risk of lateral epicondylitis: the Locomotive Syndrome and Health Outcome in Aizu Cohort Study (LOHAS). Springerplus. 2015;4:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ozgurtas T, Yildiz C, Serdar M, Atesalp S, Kutluay T. Is high concentration of serum lipids a risk factor for Achilles tendon rupture? Clin Chim Acta. 2003;331(1-2):25–28. [DOI] [PubMed] [Google Scholar]
- 34. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. [DOI] [PubMed] [Google Scholar]
- 35. Razmjou H, Fournier-Gosselin S, Christakis M, Pennings A, ElMaraghy A, Holtby R. Accuracy of magnetic resonance imaging in detecting biceps pathology in patients with rotator cuff disorders: comparison with arthroscopy. J Shoulder Elbow Surg. 2016;25(1):38–44. [DOI] [PubMed] [Google Scholar]
- 36. Roquelaure Y, Ha C, Rouillon C, et al. Risk factors for upper-extremity musculoskeletal disorders in the working population. Arthritis Rheum. 2009;61(10):1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shiri R, Viikari-Juntura E. Lateral and medial epicondylitis: role of occupational factors. Best Pract Res Clin Rheumatol. 2011;25(1):43–57. [DOI] [PubMed] [Google Scholar]
- 38. Shiri R, Viikari-Juntura E, Varonen H, Heliovaara M. Prevalence and determinants of lateral and medial epicondylitis: a population study. Am J Epidemiol. 2006;164(11):1065–1074. [DOI] [PubMed] [Google Scholar]
- 39. Tajika T, Kobayashi T, Yamamoto A, Kaneko T, Takagishi K. Prevalence and risk factors of lateral epicondylitis in a mountain village in Japan. J Orthop Surg (Hong Kong). 2014;22(2):240–243. [DOI] [PubMed] [Google Scholar]
- 40. Tauro JC. Stiffness and rotator cuff tears: incidence, arthroscopic findings, and treatment results. Arthroscopy. 2006;22(6):581–586. [DOI] [PubMed] [Google Scholar]
- 41. Titchener AG, Fakis A, Tambe AA, Smith C, Hubbard RB, Clark DI. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2013;38(2):159–164. [DOI] [PubMed] [Google Scholar]
- 42. Titchener AG, White JJ, Hinchliffe SR, Tambe AA, Hubbard RB, Clark DI. Comorbidities in rotator cuff disease: a case-control study. J Shoulder Elbow Surg. 2014;23(9):1282–1288. [DOI] [PubMed] [Google Scholar]
- 43. Tuite MJ, Yandow DR, DeSmet AA, Orwin JF, Quintana FA. Diagnosis of partial and complete rotator cuff tears using combined gradient echo and spin echo imaging. Skeletal Radiol. 1994;23(7):541–545. [DOI] [PubMed] [Google Scholar]
- 44. Viikari-Juntura E, Kurppa K, Kuosma E, et al. Prevalence of epicondylitis and elbow pain in the meat-processing industry. Scand J Work Environ Health. 1991;17(1):38–45. [DOI] [PubMed] [Google Scholar]
- 45. Walker-Bone K, Palmer KT, Reading I, Coggon D, Cooper C. Prevalence and impact of musculoskeletal disorders of the upper limb in the general population. Arthritis Rheum. 2004;51(4):642–651. [DOI] [PubMed] [Google Scholar]
- 46. Walker-Bone K, Palmer KT, Reading I, Coggon D, Cooper C. Occupation and epicondylitis: a population-based study. Rheumatology (Oxford). 2012;51(2):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wendelboe AM, Hegmann KT, Gren LH, Alder SC, White GL, Jr, Lyon JL. Associations between body-mass index and surgery for rotator cuff tendinitis. J Bone Joint Surg Am. 2004;86(4):743–747. [DOI] [PubMed] [Google Scholar]