Abstract
Background:
Autologous matrix-induced chondrogenesis (AMIC) has been shown to result in favorable clinical outcomes in patients with osteochondral lesions of the talus (OLTs). Though, the influence of ankle instability on cartilage repair of the ankle has yet to be determined.
Purpose/Hypothesis:
To compare the clinical and radiographic outcomes in patients with and without concomitant lateral ligament stabilization (LLS) undergoing AMIC for the treatment of OLT. It was hypothesized that the outcomes would be comparable between these patient groups.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
Twenty-six patients (13 with and 13 without concomitant ankle instability) who underwent AMIC with a mean follow-up of 4.2 ± 1.5 years were enrolled in this study. Patients were matched 1:1 according to age, body mass index (BMI), lesion size, and follow-up. Postoperative magnetic resonance imaging and Tegner, American Orthopaedic Foot & Ankle Society (AOFAS), and Cumberland Ankle Instability Tool (CAIT) scores were obtained at a minimum follow-up of 2 years. A musculoskeletal radiologist scored all grafts according to the MOCART (magnetic resonance observation of cartilage repair tissue) 1 and MOCART 2.0 scores.
Results:
The patients’ mean age was 33.4 ± 12.7 years, with a mean BMI of 26.2 ± 3.7. Patients with concomitant LLS showed worse clinical outcome measured by the AOFAS (85.1 ± 14.4 vs 96.3 ± 5.8; P = .034) and Tegner (3.8 ± 1.1 vs 4.4 ± 2.3; P = .012) scores. Postoperative CAIT and AOFAS scores were significantly correlated in patients with concomitant LLS (r = 0.766; P = .002). A CAIT score >24 (no functional ankle instability) resulted in AOFAS scores comparable with scores in patients with isolated AMIC (90.1 ± 11.6 vs 95.3 ± 6.6; P = .442). No difference was seen between groups regarding MOCART 1 and 2.0 scores (P = .714 and P = .371, respectively).
Conclusion:
Concurrently performed AMIC and LLS in patients with OLT and ankle instability resulted in clinical outcomes comparable with isolated AMIC if postoperative ankle stability was achieved. However, residual ankle instability was associated with worse postoperative outcomes, highlighting the need for adequate stabilization of ankle instability in patients with OLT.
Keywords: articular cartilage, AMIC, ankle, ankle instability, lateral ligament stabilization, osteochondral lesion, talus
Osteochondral lesions of the talus (OLTs) commonly present after ankle sprains in young, active patients and may lead to early osteoarthritis.9,17 Aiming to prevent adverse long-term outcomes, numerous surgical techniques have been proposed, including simple debridement of the joint surface with or without microfracturing,11 osteochondral autograft transfer (OAT; mosaicplasty),15,18 osteochondral allograft transplantation,28 and matrix-induced autologous chondrocyte transplantation (MACI).2
Whereas results after microfracturing seem to deteriorate in the long term,11 good mid- to long-term outcomes were reported after the other cartilage-restoring procedures.13,18 However, these techniques are associated with some potential risks and disadvantages, such as donor-site morbidity, graft mismatch, or the need for a 2-stage procedure.
Therefore, the autologous matrix-induced chondrogenesis (AMIC) procedure was introduced in 2005,3 which provides a 1-step cartilage repair in which microfracturing of the subchondral bone leads to the release of mesenchymal stem cells that are stabilized with a collagen matrix (ChondroGide; Geistlich Surgery). Depending on the depth of the bony defect, the lesion may first be filled with autologous spongiosa graft. Recently, excellent mid- to long-term results were published, demonstrating a significant reduction in pain and successful return to sport.34 As OLTs are often associated with ankle instability, concurrent surgical treatment, such as lateral ligament stabilization (LLS), is warranted to regain ankle stability.7,23 Interestingly, Choi et al5 reported that the presence of an OLT in patients who underwent LLS for chronic ankle instability is an independent predictor of unfavorable postoperative outcomes.
Thus far, studies investigating clinical outcome after AMIC either excluded patients with instability entirely34 or did not distinguish between patients with and without concomitant procedures or patients with and those without ankle instability, respectively.32,35 Nevertheless, a study showed that LLS with concomitant treatment of OLT utilizing retrograde drilling and microfracture is a safe procedure that leads to favorable clinical outcomes at a mean of 7.3 years postoperatively.14 Moreover, a recently published article21 highlighted the fact that the presence of concomitant ankle instability worsens the quality of life of patients with OLT. Thus, patients with OLT should be evaluated for ankle instability to integrate appropriate treatment for ankle instability if required. Yet, there is paucity of existing literature reporting clinical and radiographic outcomes after cartilage repair in the setting of ankle instability. Consequently, clinical evidence is warranted to elucidate outcomes after cartilage repair for the treatment of OLTs, namely AMIC, with concomitant LLS in patients with ankle instability.
This study sought to compare the clinical and radiographic outcomes in patients with and without concomitant LLS undergoing AMIC for the treatment of OLT. We hypothesized that patients with ankle instability and consequently concomitant LLS would show comparable results with patients who underwent isolated AMIC for the treatment of OLT.
Methods
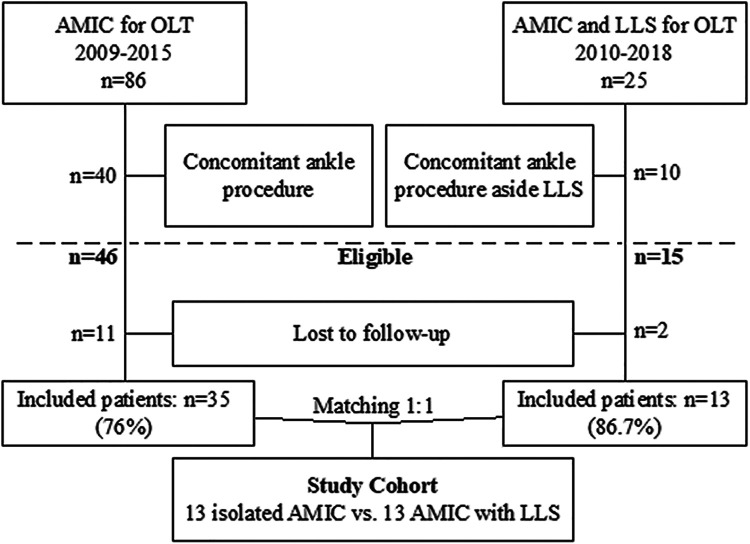
The study protocol was approved by the regional ethics committee. A total of 25 patients who underwent AMIC for OLT with concomitant LLS for the treatment of coexistent symptomatic ankle instability in our institution between April 2010 and April 2018 were identified and reviewed. Exclusion criteria for study participation comprised patients younger than 18 years of age, any concomitant ankle procedure aside from AMIC and LLS, and a follow-up less than 2 years. Of the 25 patients who underwent AMIC with concomitant LLS for OLT in the setting of ankle instability, 10 patients also underwent concomitant, ipsilateral procedures, including corrective osteotomies and tendon repairs. Thus, 15 patients were eligible for the current study. Of these, 2 patients (13.3%) declined to participate in the study, and thus, 13 patients (86.7%) were finally enrolled. A patient cohort that was studied previously to evaluate clinical and magnetic resonance imaging (MRI) outcomes after isolated AMIC for the treatment of OLT at our institution served as a control group. This study34 reviewed 86 consecutive patients who underwent isolated AMIC for the treatment of OLT between 2009 and 2015. Patients were excluded if they had a follow-up <2 years or concomitant surgery to the affected ankle.34 Patients with and without concomitant LLS were matched 1:1 for body mass index (BMI), osteochondral lesion size, follow-up, and age (Figure 1).
Figure 1.
Flowchart showing patient enrollment and recruitment. AMIC, autologous matrix-induced chondrogenesis; LLS, lateral ligament stabilization; OLT, osteochondral lesion of the talus.
Surgical treatment with AMIC was indicated in patients with symptomatic focal OLT after failed nonoperative therapy. Contraindications for AMIC comprised inflammatory arthritis and/or advanced osteoarthritis. Concomitant LLS was performed in patients with concurrent symptomatic ankle instability evaluated during preoperative clinical assessment.
Patients were invited for a clinical examination at our institution, including the assessment of standardized patient-reported outcome measures and postoperative MRI. The clinical outcome was documented with the American Orthopaedic Foot & Ankle Society (AOFAS; 0-100 points) score,24 the Cumberland Ankle Instability Tool (CAIT) score,16 and the Tegner activity scale.31 Additionally, Tegner scores were retrospectively obtained for time points before the start of initial symptoms (preinjury) and before the surgery (presurgery).
Surgical Technique
All OLTs in patients who underwent isolated AMIC were accessed with a medial (n = 11) or lateral (n = 2) malleolar osteotomy, while OLTs in patients with concomitant ankle instability were addressed through either a medial (n = 4) or lateral (n = 4) osteotomy or a direct approach without the need for osteotomy (n = 5).
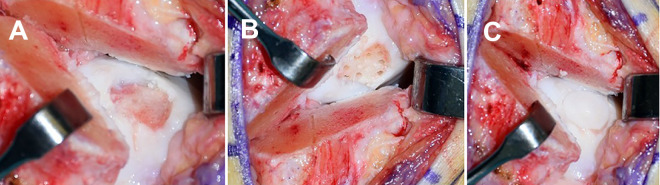
The AMIC procedure to address the OLT was performed as previously reported.4,34 Briefly, the damaged cartilage was identified and resected with a scalpel or curettage. Then, the subchondral bone was debrided until vital bleeding bone tissue was visualized. If the defect exceeded a depth of 5 mm, it was filled with cancellous bone autograft from the osteotomy site. Before implanting the cut-to-size AMIC bilayer type 1/3 collagen matrix (Chondro-Gide; Geistlich Pharma) into the defect and securing it with fibrin glue, we microfractured the bone surface to promote healing by discharging stem cell–rich bone marrow (Figure 2, A-C).
Figure 2.
(A) Visualization and debridement of the osteochondral defect of the medial talus. (B) Microfracturing of the subchondral bone to create channels through which stem cell–rich bone marrow can be discharged. (C) Implantation of the autologous matrix-induced chondrogenesis bilayer type 1/3 collagen matrix into the defect.
Patients who experienced concomitant symptomatic ankle instability underwent additional LLS with either lateral ligament repair or anatomic lateral ligament reconstruction. The decision for lateral ligament reconstruction was made when the anterior talofibular ligament (AFTL) and calcaneofibular ligament (CFL) were deemed to be markedly deficient with inferior tissue quality at the time of surgery. In the case of sufficient tissue quality, the ATFL was repaired with a modified Broström technique by reinserting its fibular attachment using 1.4-mm Juggerknots (Zimmer Biomet). The anatomic lateral ligament reconstruction was performed using a free tendon allograft (gracilis), thus reconstructing the ATFL and CFL.27
If the OLT was accessed through a medial approach, an additional incision from the anterior border of the fibula curving obliquely toward the sinus tarsi was established. Blunt dissection was performed to the anatomic talar attachment of the ATFL. After drilling of the bone tunnel, one end of the graft was fixed with an interference screw (diameter, 6 mm; length, 19 mm; Mega Fix; Karl Storz). Next, two fibular tunnels were drilled from anterior to posterior, starting from the anatomic fibular footprint of the ATFL and the CFL. The free end of the graft was then passed through both fibular bone tunnels, thus re-creating the ATFL. Finally, the free end of the graft was shuttled underneath the peroneal tendons and secured to the calcaneus with another interference screw, thus re-creating the CFL. During the final fixation, the ankle was brought into neutral position and maximal graft tension was applied to optimize lateral ankle stability. The malleolar osteotomy was fixed with either two 3.5-cm malleolar screws (medial) or compression plating (lateral). In the case of lateral osteotomy, the fibula was fixed before performing LLS.
Postoperative Rehabilitation
Regardless of concomitant ankle instability, all patients were immobilized in a lower-leg cast for 6 weeks with partial weightbearing of 15 kg. Protected weightbearing was then gradually increased in a lower-leg cast to full weightbearing within weeks 10 to 12. Ankle joint mobilization was started after wound healing in patients with isolated AMIC. In contrast, patients with concomitant LLS were not allowed to mobilize their ankle joint until 6 weeks postoperatively.
MRI Evaluation
All patients underwent a dedicated postoperative MRI (MAGNETOM Avanto Fit system [isolated AMIC] or MAGNETOM Prisma [AMIC with LLS]; Siemens Healthcare) protocol including 3-mm slice thickness with an 8-channel (for MAGNETOM Avanto Fit) or 16-channel (for MAGNETOM Prisma) foot and ankle coil with coronal, sagittal, and axial turbo spin-echo intermediate-weighted sequences with fat visualization using the Dixon technique.
A musculoskeletal fellowship-trained radiologist (C.G.) assessed the cartilage repair tissue utilizing the previously published and validated comprehensive MOCART (magnetic resonance observation of cartilage repair tissue)25 and MOCART 2.029 scores, which have been frequently used to evaluate cartilage repair in OLT.1,22,32,34
Statistical Analysis
Sociodemographic and clinical characteristics of patients were determined using descriptive statistics. All data were assessed for normality utilizing the Shapiro-Wilk test. Subsequently, continuous variables were analyzed with the independent t test or Mann-Whitney U test. Categorical variables were assessed with the chi-square or Fisher exact test. Pearson correlation was used to assess the relationship of CAIT and AOFAS scores. Lesion size was calculated utilizing the ellipse formula used for OLT (sagittal length × coronal length × 0.79).6 All statistical analyses were performed in IBM SPSS for Mac (IBM). Significance was set at P < .05. A post hoc power analysis showed that with a sample size of 13 per group, the study had a power of more than 0.70 to detect a difference of 11 points in postoperative AOFAS scores at a level of significance of .05. In order to reach a power of 0.80 for the MRI analysis, a minimum sample size of 750 and 125 patients would have been required to detect a statistical significance in the differences seen in MOCART and MOCART 2.0 scores between both groups (2.4 and 5.0 points), respectively. Thus, the power values for the MOCART analysis in the current study were 0.06 and 0.15. Power calculation was performed with G*Power Version 3.1 (Heinrich Heine University Düsseldorf, Germany).
Results
Of the 13 patients with LLS, 9 patients (69.2%) received lateral ligament repair, while 4 patients (30.8%) underwent anatomic lateral ligament reconstruction with gracilis allograft. MRI assessment at the final follow-up confirmed the intact integrity of each lateral ligament repair and reconstruction. Mean postoperative and clinical follow-up MRI scans were obtained at 4.2 ± 1.5 years. The patients’ mean age was 33.4 ± 12.7 years, with a mean BMI of 26.2 ± 3.7. Patient and lesion characteristics are presented in Table 1.
Table 1.
Patient Characteristics Stratified by Whether Patients Received LLSa
| AMIC + LLS (n = 13) | AMIC (n = 13) | P Value | |
|---|---|---|---|
| Age, y | 33.3 ± 13.5 | 33.4 ± 12.5 | .994 |
| BMI | 26.2 ± 3.9 | 26.1 ± 3.7 | .724 |
| Lesion size, cm2 | 0.9 ± 0.6 | 0.8 ± 0.4 | .699 |
| Follow-up, y | 4.2 ± 1.4 | 4.2 ± 1.6 | .801 |
| Smoker, n | 7 | 7 | >.999 |
| Female sex, n | 6 | 3 | .411 |
| Lesion location, medial/lateral | 8/5 | 11/2 | .378 |
| Bone grafting, n | 6 | 4 | .688 |
aAMIC, autologous matrix-induced chondrogenesis; BMI, body mass index; LLS, lateral ligament stabilization.
At the final follow-up, patients who underwent AMIC with concomitant LLS showed significantly worse clinical outcomes compared with patients after isolated AMIC, as measured by the AOFAS score and Tegner activity scale (Table 2). Patients who underwent AMIC with concomitant LLS were evaluated with a mean CAIT score of 23.2 ± 7.3. When correlating postoperative CAIT and AOFAS scores in patients with ankle instability, both scores were significantly associated with each other (r = 0.766; P = .002). In fact, patients who underwent concomitant LLS and were evaluated with a postoperative CAIT score >24 points (n = 8) had AFOAS scores comparable with those of patients with isolated AMIC (90.1 ± 11.6 vs 95.3 ± 6.6; P = .442).
Table 2.
Clinical Outcomes After AMIC With and Without Concomitant LLSa
| AMIC + LLS (n = 13) | AMIC (n = 13) | P Value | |
|---|---|---|---|
| Tegner score | |||
| Preinjury | 6.2 ± 1.8 | 5.8 ± 1.6 | .653 |
| Presurgery | 2.5 ± 2.5 | 4 ± 2.3 | .081 |
| Final follow-up | 3.8 ± 1.1 | 4.4 ± 2.3 | .012 |
| AOFAS score, final follow-up | 85.1 ± 14.4 | 96.3 ± 5.8 | .034 |
aBoldface type indicates statistical significance (P < .05). AMIC, autologous matrix-induced chondrogenesis; AOFAS, American Orthopaedic Foot & Ankle Society; LLS, lateral ligament stabilization.
Analyzing postoperative MOCART scores, no statistically significant difference was observed between groups with AMIC and LLS evaluated with a MOCART 1 score of 60.8 ± 15.4 and a MOCART 2.0 score of 64.2 ± 15.1 compared with 63.1 ± 16.3 and 69.2 ± 12.7 in patients with isolated AMIC (P = .714 and P = .371, respectively).
Discussion
The key finding of the present study is that patients who underwent AMIC with concomitant LLS were evaluated as having a worse clinical outcome than patients with isolated AMIC for the treatment of OLT at the midterm follow-up. However, if LLS restored ankle stability in those patients, similar results were seen when compared with isolated AMIC. Interestingly, ankle instability had no influence on postoperative imaging morphology as scored by MOCART 1 and 2.0.
Patients with ankle instability often present with concurrent OLT, with a reported incidence of up to 60% to 95%.30,33 In a recently published study, Kim et al20 showed that patients with chronic ankle instability and medial ankle osteoarthritis significantly benefit from LLS, with AOFAS scores increasing from 61.9 ± 14.2 to 89.7 ± 6.2 (P < .001) postoperatively. Conversely, Jiang et al19 investigated whether concurrent arthroscopic OLT treatment with abrasion, curettage, drilling, or microfracture has an adverse effect on the clinical outcome of LLS in patients with chronic ankle instability, as it might compromise the rehabilitation protocol. Yet, no substantial effect was seen in the studied cohort, which highlights the possibility of performing the procedures concomitantly. Further, prior studies showed favorable results in patients who underwent microfracturing or retrograde drilling for the treatment of OLT with concomitant LLS.14,36 Yet, Gregush and Ferkel14 reported worse overall outcome in patients with a concurrently treated OLT when compared with patients who underwent LLS as an isolated procedure. Aside from these case series focusing on bone marrow stimulation, there is paucity of current literature reporting the clinical outcomes of patients who underwent LLS and more sophisticated cartilage repair procedures such as MACI, OAT, or AMIC.
Recently, data from the German Cartilage Registry were published emphasizing the need for studies evaluating clinical outcomes of patients with ankle instability and concurrent cartilage repair for OLT.21 The authors assessed and compared preoperative demographics and lesion characteristics of patients who underwent AMIC and concurrent LLS (group A) with those of patients who received isolated AMIC (group B). They reported that patients in group A tended to be older (median, 34 years [range, 20-65 years] vs 28.5 years [range, 18-72 years]) with smaller OLT lesion size (median, 100 mm2 [range, 15-600 mm2] vs 150 mm2 [range, 25-448 mm2]) than group B, yet neither reached statistical significance. Additionally, patients in group A showed worse preoperative quality-of-life measures than patients in group B. This is in accordance with the results of the current study. While it did not reach statistical significance, patients with OLT in the setting of ankle instability showed worse preoperative Tegner scores than patients with isolated OLT, suggesting that ankle instability has a substantial effect on quality of life, regardless of demographics and lesion size, as patients were matched to their controls.
To date, only one study has reported clinical and radiographic outcomes after isolated AMIC for the treatment of OLT with encouraging results.34 A total of 33 patients were assessed with AOFAS and Tegner scores and MRI at a follow-up between 2 and 8 years postoperatively. Patients were evaluated as having excellent AOFAS scores, with a mean of 93.0 ± 7.5 points, and a mean Tegner score of 5.2 ± 1.7, regardless of follow-up, as patients with longer than a 5-year follow-up showed similar results to patients with a follow-up between 2 and 5 years. In the current study, patients with isolated AMIC had a mean postoperative AOFAS score of 96.3 ± 5.8 with a mean Tegner score of 4.4 ± 2.3. Conversely, patients with concurrent ankle instability were evaluated with significantly worse postoperative outcomes, resulting in mean postoperative AOFAS and Tegner scores of 85.1 ± 14.4 (P = .0.34) and 3.8 ± 1.1 (P = .012), respectively. Furthermore, patients who underwent AMIC and LLS also completed postoperative CAIT, which is frequently used to quantify the perceived ankle instability during both sports and activities of daily life,8,12 because of its valid and reliable assessment of functional ankle instability.16 Interestingly, patients with clinically stable postoperative ankle joints, as indicated by a CAIT score >24,10 showed similar clinical outcome when compared with their controls (90.1 ± 11.6 vs 95.3 ± 6.6; P = .442), thus highlighting the fact that achieving postoperative ankle stability is essential to attain favorable results in patients with OLT and concurrent ankle instability.
The current study also assessed postoperative MRI outcome as measured by both MOCART 1 and 2.0 scores. Remarkably, ankle instability seems to not influence cartilage repair tissue morphology when assessed with MRI, as both groups showed similar MOCART scores at final follow-up. In a recently published study, Casari et al4 investigated the role of postoperative MRI in patients who underwent AMIC for the treatment of OLT. While osteochondral lesion size was the only factor influencing postoperative overall MOCART scores, no significant association between MOCART scores or its subscales and clinical outcome (AOFAS and Tegner scores) was detected. Accordingly, the authors concluded that MOCART scores should not routinely be implemented in the postoperative care of asymptomatic patients who underwent AMIC for OLT, as they hold little clinical relevance. This seems applicable to the current study since no difference in MOCART score was observed, yet clinical outcome was worse in patients with AMIC and LLS.
The following limitations have to be acknowledged. First, preoperative clinical scores were unavailable, as the study was retrospective in its design. Consequently, the clinical improvement from pre- to postoperatively in patients with and without concomitant ankle instability can only be assumed. Second, patients who received isolated AMIC did not complete postoperative CAIT scores; hence, postoperative ankle stability was not objectivized in these patients. However, neither pre-/postoperative clinical and imaging evaluation nor subjective perception of these patients indicated ankle instability at any time point. Third, two different surgical techniques were used to address ankle instability, depending on the quality of the remaining lateral ligaments. While it would have been desirable to investigate homogeneous operative techniques, stratifying groups by LLS technique did not seem appropriate, as both techniques eventually intend to restore ankle instability. In fact, lateral ligament repair and reconstruction have been shown to result in equally favorable clinical outcomes in patients with ankle instability.26 However, we tried to increase homogeneity by excluding concomitantly performed interventions such as calcaneal osteotomy, peroneus tendon repair, flexor digitorum longus transfer, or medial ligament repair.
A fourth limitation was that this study did not compare 2 concurrent groups but rather compared patients who were treated between 2009 and 2015 (isolated AMIC) with patients who underwent surgery between 2014 and 2018 (AMIC with LLS). Thus, it cannot be excluded that this introduced some form of bias regarding the surgical learning curve of the AMIC procedure, imaging, and rehabilitation, since AMIC with LLS was first performed years after isolated AMIC at our institution. Yet, AMIC is a relatively simple surgery based on the principles of microfracturing, which was implemented in our treatment algorithm before AMIC, thus lowering the learning curve. Further, while MR systems were different, both groups underwent the identical imaging protocol, and all images were assessed (and reassessed for isolated AMIC) by the same musculoskeletal radiologist to ensure comparability. Regarding the rehabilitation process, both protocols are inherently different because of the additional LLS in patients with OLT, yet both aim to fully restore ankle stability and functionality. Last, the insufficient power for the MRI analysis did not allow for a conclusive statement regarding the influence of ankle instability on postoperative MOCART scores. Yet, the observed effect size suggests a rather small influence of ankle instability on postoperative radiographic outcomes.
Conclusion
Concurrently performed AMIC and LLS in patients with OLT and ankle instability results in a clinical outcome that is comparable with isolated AMIC if postoperative ankle stability is achieved. Residual ankle instability, however, was associated with worse postoperative outcome, highlighting the need for adequate stabilization of ankle instability in patients with OLT.
Footnotes
Final revision submitted December 14, 2020; accepted January 26, 2021.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the regional ethics committee of Zürich Canton.
References
- 1. Apprich S, Trattnig S, Welsch GH, et al. Assessment of articular cartilage repair tissue after matrix-associated autologous chondrocyte transplantation or the microfracture technique in the ankle joint using diffusion-weighted imaging at 3 Tesla. Osteoarthritis Cartilage. 2012;20(7):703–711. [DOI] [PubMed] [Google Scholar]
- 2. Aurich M, Bedi HS, Smith PJ, et al. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39(2):311–319. [DOI] [PubMed] [Google Scholar]
- 3. Behrens P. Matrixgekoppelte Mikrofrakturierung. Ein neues Konzept zur Knorpeldefektbehandlung. Arthroskopie. 2005;18(3):193–197. [Google Scholar]
- 4. Casari FA, Germann C, Weigelt L, Wirth S, Viehöfer A, Ackermann J. The role of magnetic resonance imaging in autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: analyzing MOCART 1 and 2.0. Cartilage. Published online August 1, 2020. doi:10.1177/1947603520946382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi WJ, Lee JW, Han SH, Kim BS, Lee SK. Chronic lateral ankle instability: the effect of intra-articular lesions on clinical outcome. Am J Sports Med. 2008;36(11):2167–2172. [DOI] [PubMed] [Google Scholar]
- 6. Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974–1980. [DOI] [PubMed] [Google Scholar]
- 7. Coughlin MJ, Schenck RC, Jr, Grebing BR, Treme G. Comprehensive reconstruction of the lateral ankle for chronic instability using a free gracilis graft. Foot Ankle Int. 2004;25(4):231–241. [DOI] [PubMed] [Google Scholar]
- 8. Delahunt E, Coughlan GF, Caulfield B, Nightingale EJ, Lin CW, Hiller CE. Inclusion criteria when investigating insufficiencies in chronic ankle instability. Med Sci Sports Exerc. 2010;42(11):2106–2121. [DOI] [PubMed] [Google Scholar]
- 9. DiGiovanni BF, Fraga CJ, Cohen BE, Shereff MJ. Associated injuries found in chronic lateral ankle instability. Foot Ankle Int. 2000;21(10):809–815. [DOI] [PubMed] [Google Scholar]
- 10. Donahue M, Simon J, Docherty CL. Critical review of self-reported functional ankle instability measures. Foot Ankle Int. 2011;32(12):1140–1146. [DOI] [PubMed] [Google Scholar]
- 11. Ferkel RD, Zanotti RM, Komenda GA, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750–1762. [DOI] [PubMed] [Google Scholar]
- 12. Gehring D, Faschian K, Lauber B, Lohrer H, Nauck T, Gollhofer A. Mechanical instability destabilises the ankle joint directly in the ankle-sprain mechanism. Br J Sports Med. 2014;48(5):377–382. [DOI] [PubMed] [Google Scholar]
- 13. Giannini S, Battaglia M, Buda R, Cavallo M, Ruffilli A, Vannini F. Surgical treatment of osteochondral lesions of the talus by open-field autologous chondrocyte implantation: a 10-year follow-up clinical and magnetic resonance imaging T2-mapping evaluation. Am J Sports Med. 2009;37(suppl 1):112S–118S. [DOI] [PubMed] [Google Scholar]
- 14. Gregush RV, Ferkel RD. Treatment of the unstable ankle with an osteochondral lesion: results and long-term follow-up. Am J Sports Med. 2010;38(4):782–790. [DOI] [PubMed] [Google Scholar]
- 15. Hangody L, Kish G, Modis L, et al. Mosaicplasty for the treatment of osteochondritis dissecans of the talus: two to seven year results in 36 patients. Foot Ankle Int. 2001;22(7):552–558. [DOI] [PubMed] [Google Scholar]
- 16. Hiller CE, Refshauge KM, Bundy AC, Herbert RD, Kilbreath SL. The Cumberland Ankle Instability Tool: a report of validity and reliability testing. Arch Phys Med Rehabil. 2006;87(9):1235–1241. [DOI] [PubMed] [Google Scholar]
- 17. Holmer P, Sondergaard L, Konradsen L, Nielsen PT, Jorgensen LN. Epidemiology of sprains in the lateral ankle and foot. Foot Ankle Int. 1994;15(2):72–74. [DOI] [PubMed] [Google Scholar]
- 18. Imhoff AB, Paul J, Ottinger B, et al. Osteochondral transplantation of the talus: long-term clinical and magnetic resonance imaging evaluation. Am J Sports Med. 2011;39(7):1487–1493. [DOI] [PubMed] [Google Scholar]
- 19. Jiang D, Ao YF, Jiao C, et al. Concurrent arthroscopic osteochondral lesion treatment and lateral ankle ligament repair has no substantial effect on the outcome of chronic lateral ankle instability. Knee Surg Sports Traumatol Arthrosc. 2018;26(10):3129–3134. [DOI] [PubMed] [Google Scholar]
- 20. Kim SW, Jung HG, Lee JS. Ligament stabilization improved clinical and radiographic outcomes for individuals with chronic ankle instability and medial ankle osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2020;28(10):3294–3300. [DOI] [PubMed] [Google Scholar]
- 21. Korner D, Ateschrang A, Schroter S, et al. Concomitant ankle instability has a negative impact on the quality of life in patients with osteochondral lesions of the talus: data from the German Cartilage Registry (KnorpelRegister DGOU). Knee Surg Sports Traumatol Arthrosc. 2020;28(10):3339–3346. [DOI] [PubMed] [Google Scholar]
- 22. Kubosch EJ, Erdle B, Izadpanah K, et al. Clinical outcome and T2 assessment following autologous matrix-induced chondrogenesis in osteochondral lesions of the talus. Int Orthop. 2016;40(1):65–71. [DOI] [PubMed] [Google Scholar]
- 23. Li X, Killie H, Guerrero P, Busconi BD. Anatomical reconstruction for chronic lateral ankle instability in the high-demand athlete: functional outcomes after the modified Broström repair using suture anchors. Am J Sports Med. 2009;37(3):488–494. [DOI] [PubMed] [Google Scholar]
- 24. Lieshout E, Boer S, Meuffels D, et al. American Orthopaedic Foot and Ankle Society (AOFAS) Ankle-Hindfoot Score: a study protocol for the translation and validation of the Dutch language version. BMJ Open. 2017;7:e012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marlovits S, Striessnig G, Resinger CT, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310–319. [DOI] [PubMed] [Google Scholar]
- 26. Matheny LM, Johnson NS, Liechti DJ, Clanton TO. Activity level and function after lateral ankle ligament repair versus reconstruction. Am J Sports Med. 2016;44(5):1301–1308. [DOI] [PubMed] [Google Scholar]
- 27. McGarvey WC, Clanton TO. Lateral ankle ligament reconstruction using allograft and interference screw fixation. In: Easley ME, Weisel SW, eds. Operative Techniques in Foot and Ankle Surgery. Wolters Kluwer, Lippincott Williams & Wilkins; 2010:851–859. [Google Scholar]
- 28. Raikin SM. Fresh osteochondral allografts for large-volume cystic osteochondral defects of the talus. J Bone Joint Surg Am. 2009;91(12):2818–2826. [DOI] [PubMed] [Google Scholar]
- 29. Schreiner MM, Raudner M, Marlovits S, et al. The MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) 2.0 knee score and atlas. Cartilage. Published online August 17, 2019. doi:10.1177/1947603519865308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taga I, Shino K, Inoue M, Nakata K, Maeda A. Articular cartilage lesions in ankles with lateral ligament injury. An arthroscopic study. Am J Sports Med. 1993;21(1):120–127. [DOI] [PubMed] [Google Scholar]
- 31. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 32. Valderrabano V, Miska M, Leumann A, Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2013;41(3):519–527. [DOI] [PubMed] [Google Scholar]
- 33. van Dijk CN, Bossuyt PM, Marti RK. Medial ankle pain after lateral ligament rupture. J Bone Joint Surg Br. 1996;78(4):562–567. [PubMed] [Google Scholar]
- 34. Weigelt L, Hartmann R, Pfirrmann C, Espinosa N, Wirth SH. Autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a clinical and radiological 2- to 8-year follow-up study. Am J Sports Med. 2019;47(7):1679–1686. [DOI] [PubMed] [Google Scholar]
- 35. Wiewiorski M, Werner L, Paul J, Anderson AE, Barg A, Valderrabano V. Sports activity after reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2016;44(10):2651–2658. [DOI] [PubMed] [Google Scholar]
- 36. Yasui Y, Takao M, Miyamoto W, Matsushita T. Simultaneous surgery for chronic lateral ankle instability accompanied by only subchondral bone lesion of talus. Arch Orthop Trauma Surg. 2014;134(6):821–827. [DOI] [PubMed] [Google Scholar]