Abstract
Objective
We evaluated whether ivermectin combined with doxycycline reduced the clinical recovery time in adults with COVID-19 infection.
Methods
This was a randomized, blinded, placebo-controlled trial in patients with mild-to-moderate COVID-19 symptoms randomly assigned to treatment (n = 200) and placebo (n = 200) groups. The primary outcome was duration from treatment to clinical recovery. Secondary outcomes were disease progression and persistent COVID-19 positivity by RT-PCR.
Results
Among 556 screened patients, 400 were enrolled and 363 completed follow-up. The mean patient age was 40 years, and 59% were men. The median recovery time was 7 (4–10, treatment group) and 9 (5–12, placebo group) days (hazard ratio, 0.73; 95% confidence interval, 0.60–0.90). The number of patients with a ≤7-day recovery was 61% (treatment group) and 44% (placebo groups) (hazard ratio, 0.06; 95% confidence interval, 0.04–0.09). The proportion of patients who remained RT-PCR positive on day 14 and whose disease did not progress was significantly lower in the treatment group than in the placebo group.
Conclusions
Patients with mild-to-moderate COVID-19 infection treated with ivermectin plus doxycycline recovered earlier, were less likely to progress to more serious disease, and were more likely to be COVID-19 negative by RT-PCR on day 14.
Trial Registration
ClinicalTrials.gov Identifier: NCT04523831.
Data Repository ID
Dryad. doi:10.5061/dryad.qjq2bvqf6
Keywords: Ivermectin, doxycycline, COVID-19, recovery time, infection, reverse transcription polymerase chain reaction
Introduction
On 31 December 2019, the World Health Organization announced the outbreak of an atypical pneumonia in Wuhan City, China. Within weeks, the illness became a pandemic.1,2 The presentation of the disease, named coronavirus disease 2019 (COVID-19), ranges from asymptomatic to fatal.3 Death is mainly caused by pneumonia and potentially also by hyperinflammation associated with cytokine storm syndrome.4 To date, treatment has relied mainly on supportive care.
Numerous clinical trials worldwide have explored the efficacy of existing medicines against COVID-19, including various antiviral and immunomodulatory drugs.5 Australian researchers from Monash University established the efficacy of ivermectin, a broad-spectrum antiviral drug,6,7 against severe acute respiratory syndrome-related coronavirus (SARS-CoV-2) in in vitro studies; viral replication was stopped within 24 to 48 hours.8 Tetracycline, a widely available and well-tolerated antibiotic useful against atypical infections,9 has known anti-inflammatory effects10 and, along with its synthetic derivative, doxycycline, has been shown by Mohit et al.11 to potentially be effective against COVID-19. The antiviral and anti-inflammatory properties of ivermectin combined with doxycycline may be beneficial in the treatment of COVID 19. Given that these two drugs have different modes of action, their synergistic effects may contain viral infection by targeting different sites of disease pathogenesis. In the present study, we sought to determine the efficacy of this combination in patients with mild-to-moderate COVID-19 symptoms.
Patients and methods
Patients
Patients were enrolled between 1 June 2020 and 30 August 2020. The inclusion criteria for enrollment were as follows: age >18 years, positive COVID-19 reverse transcription polymerase chain reaction (RT-PCR) test within 3 days prior to enrollment, and mild to moderately severe COVID-19 infection. Patients who were unable to receive oral medications, were pregnant or breastfeeding, had severe COVID-19 symptoms (defined as tachypnea [>30 breaths/minute] and hypoxia [oxygen saturation (SpO2) <90%] requiring supplemental oxygen), were admitted to intensive care or high-dependency units, or had known hypersensitivities to ivermectin or doxycycline were excluded from participation.
Trial design
This was an investigator-initiated, placebo-controlled trial. The independent data safety monitoring board of Dhaka Medical College monitored treatment safety and efficacy. Routine investigations were performed at the pathology laboratory of Dhaka Medical College. RT-PCR tests were performed at the virology laboratory of Dhaka Medical College and Bangabandhu Sheikh Mujib Medical University. Ivermectin, doxycycline, and placebo preparations were provided by Popular Pharmaceuticals Ltd. (Dhaka, Bangladesh).
Eligible patients were randomly assigned to the treatment group or the placebo group at a 1:1 ratio on day 1 of the trial. Group assignment was not stratified according to disease severity.
The allocation schedule was created with a list of random numbers generated using a random number generator program by the head of the Department of Medicine of Dhaka Medical College. Group assignment was concealed in sequentially numbered, opaque, sealed envelopes. The randomization code was maintained by the pharmaceutical company. Both the investigators and the patients were blinded to the treatment allocation. Decoding was performed at the end of the trial under the supervision of the principal of the institute. The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and was approved by the ethical review committee of Dhaka Medical College Hospital (ERC-DMC/ECC/2020/117). Dhaka Medical College is the largest tertiary care teaching hospital and the largest COVID-19 treatment hospital in Bangladesh. The trial was registered retrospectively at ClinicalTrials.gov (identifier: NCT04523831). Written informed consent was obtained from all patients prior to participation. The study is reported according to the Equator Network Guidelines.
Study interventions
The treatment group received a single dose of ivermectin 12 mg and doxycycline 100 mg, twice daily for 5 days, in addition to standard of care. Standard of care included administration of paracetamol, antihistamines, cough suppressants, vitamins, oxygen therapy according to indication and need, low molecular weight heparin according to indication, appropriate other broad-spectrum antibiotics, remdesivir injection, other antiviral drugs, and other drugs for associated comorbid conditions.
The placebo group received placebo in addition to standard of care.
Experimental procedures
The baseline demographic and clinical characteristics of patients were recorded. The date of random assignment was considered as day 1, and all patients received their initial study intervention on day 1. Outpatients were followed daily until at least 3 days of clinical recovery were observed. Clinical recovery was defined as a normal body temperature of 36.1°C to 37.2°C maintained for at least 3 days, significantly improved respiratory symptoms (respiratory rate <25 breaths/minute, no dyspnea), and oxygen saturation greater than 93% without assisted oxygen inhalation, as recommended by the World Health Organization and the national guidelines of Bangladesh.12,13
Hospitalized patients were followed from day 1 through day 14 or until discharge or clinical improvement, whichever occurred later. Clinical status and vital signs (including respiratory status) were recorded daily. Adverse events as defined by the Medical Dictionary for Regulatory Activities (MedDRA) were documented.
Laboratory tests were performed on day 1, and included the following: complete blood count, random blood glucose, creatinine, alanine transaminase, C-reactive protein, ferritin, and D-dimer. Chest radiography or chest CT scanning were performed as needed. For outpatients, test results were documented at the next visit. Hospitalized patients were tested on days 1 and 7 and when ordered by the treating physicians. COVID-19 RT-PCR testing was performed 14 days after the initial positive test in all patients.
Outcome measures
The primary outcome was the number of days required for clinical recovery from day 1. Clinical recovery was divided into three categories: early recovery within 7 days, intermediate recovery within 7 to 11 days, and late improvement requiring 12 or more days for recovery. Secondary outcomes were disease progression through mild, moderate, severe, or death, and the proportion of patients who continued to test positive for COVID-19 on day 14. Adverse drug reactions (adverse events assumed to be caused by the study intervention) were also recorded. Mild disease was defined as symptoms of an upper respiratory tract viral infection, including mild fever, dry cough, sore throat, nasal congestion, headache, muscle pain, anosmia, and malaise. Moderate respiratory symptoms such as cough and shortness of breath were observed without signs of severe pneumonia. Severe disease included severe dyspnea, tachypnea (>30 breaths/minute), and hypoxia (SpO2 <90% at room air). These classifications were according to the World Health Organization and national guidelines of Bangladesh.12,13 The co-investigators assessed the outcome, graded the disease, and documented adverse reactions.
Statistical methods
We sought to enroll as many patients as possible in a short time. As a result, a priori power calculations were not performed. Instead, 200 patients were enrolled in each group and it was assumed that 20% of patients in each group would be lost to follow-up. A post-hoc power calculation [2SD2 (Zα/2+ Zβ) 2/d2]14, a two-tailed test, an alpha level of 0.05, and standard deviation (SD) 6 indicated that the enrollment of 141 patients in each group (170 before 20% attrition) would result in an 80% chance of detecting a 2-day difference in median recovery time.
Intention-to-treat analysis was performed. The difference in median recovery time was determined using Kaplan–Meier analysis and a log-rank test. Details of patients who were lost to follow-up, had died, or had withdrawn from the trial owing to adverse effects were censored on the final study day. Differences in the clinical improvement in the three disease categories were analyzed using logistic regression. Hazard ratios (HRs) were calculated using Cox regression analysis. Categorical variables are presented as n (%), normally distributed continuous variables as mean (SD), and skewed continuous variables as median (interquartile range [IQR]).
Subgroup analysis was performed using the logistic regression analysis. HRs were calculated using Cox regression analysis. Age group, sex, fever, cough, respiratory distress, hypertension, diabetes, and presence of any comorbidity were adjusted. Subgroups consisted of age (less than vs. more than 60 years), sex, presence or absence of any comorbidities, mild disease, moderate disease, hospital admission, and interval between disease onset and drug application interval (less than vs. more than 4 days from the onset of the disease). A forest plot was constructed to evaluate differences in the primary outcome among the subgroups. No corrections were made for multiple comparisons. All tests were two-tailed and the alpha was set at 0.05. Data were analyzed using SPSS software, version 20 (IBM Corp., Armonk, NY, USA).
Results
Sample characteristics
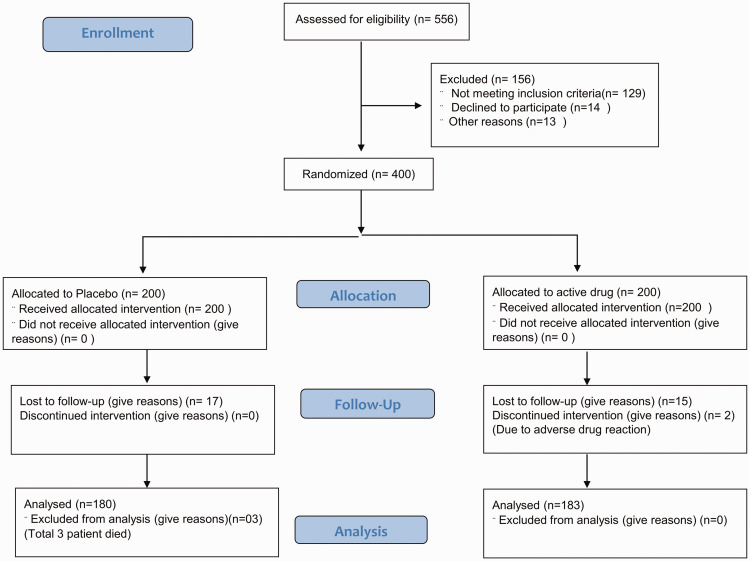
Of 556 screened patients, 400 were enrolled and randomly assigned to the treatment and placebo groups (Figure 1). Among the 200 patients in the placebo group, 17 were lost to follow-up, 3 died, and 180 completed the follow-up. Among the 200 patients in the treatment group, 15 were lost to follow-up, 2 discontinued owing to adverse effects, and 183 completed follow-up. None of the patients were receiving oxygen therapy when randomized because only patients with mild and moderate symptoms were included. A total of 132 (33%) patients required hospital admission during the study period. Among the hospitalized patients, 58% had moderate disease. The condition of 48 patients worsened during the study period: 5 patients worsened from mild disease to moderate disease but did not require oxygen and the remaining 43 patients worsened from mild/moderate to severe disease and required oxygen therapy.
Figure 1.
Enrollment, randomization, follow up, and analysis of patients according to the CONSORT 2010 flow diagram.
Patient disposition and characteristics
The two groups were balanced in terms of demographics and disease characteristics (Table 1). The mean age of all patients was 40 years, and 59% were men. Most patients presented with fever (300, 75%) or cough (247, 62%). Respiratory distress (defined as shortness of breath and respiratory rate >25 breaths/minute) was noted in 123 (31%) patients at presentation. Furthermore, 277 patients (69%) had mild disease and 41 (10%) presented with at least one comorbidity (Table 2). Patients were randomly assigned to the trial within a median (IQR) of 4 (3–5) days of symptom onset (Table 2).
Table 1.
Baseline characteristics of patients with COVID-19 treated with ivermectin and doxycycline or placebo.
| Characteristic | Total patients (n = 400) | Treatment group (n = 200) | Placebo group (n = 200) |
|---|---|---|---|
| Age, mean (SD), years | 40 (13) | 41 (14) | 38 (12) |
| Age group, n (%) | |||
| <40 years | 248 (62) | 132 (66) | 116 (58) |
| 40–60 years | 122 (31) | 56 (28) | 66 (33) |
| >60 years | 30 (8) | 12 (6) | 18 (9) |
| Men, n (%) | 235 (59) | 123 (62) | 112 (56) |
| Time between onset of symptoms and enrollment, median (IQR), days a | 4 (3–5) | 4 (3–5) | 4 (3–5) |
| Symptoms n (%) | |||
| Fever | 300 (75) | 151 (76) | 149 (75) |
| Cough | 247 (62) | 126 (53) | 121 (61) |
| Running nose | 36 (9) | 17 (9) | 19 (10) |
| Respiratory distress b | 123 (31) | 59 (30) | 64 (32) |
| Sore throat | 93 (23) | 46 (23) | 47 (24) |
| Hoarseness | 5 (1) | 2 (1) | 3 (1.5) |
| Chest pain | 25 (6) | 10 (5) | 15 (8) |
| Diarrhea | 32 (8) | 18 (11) | 14 (5) |
| Vomiting | 20 (5) | 9 (5) | 11 (6) |
| Anorexia | 119 (30) | 53 (27) | 66 (33) |
| Anosmia | 154 (39) | 73 (37) | 81 (41) |
| Headache | 80 (20) | 34 (17) | 46 (23) |
| Lethargy | 106 (27) | 55 (28) | 51 (25.5) |
| Conjunctivitis | 8 (2) | 4 (2) | 4 (2) |
| Body ache | 73 (18) | 32 (16) | 41 (20.5) |
| Co-morbidities c | 41 (10) | 19 (10) | 22 (11) |
| Hypertension | 57 (14) | 29 (15) | 28 (14) |
| Diabetes | 53 (13) | 24 (12) | 29 (15) |
| Asthma | 21 (5) | 9 (5) | 12(6) |
| Chronic kidney disease | 8 (2) | 3 (2) | 5 (3) |
| Disease severity d | |||
| Mild | 277 (69) | 141 (71) | 136 (68) |
| Moderate | 123 (31) | 59 (30) | 64 (32) |
a Time from onset of first symptoms to first dose of study intervention.
b Shortness of breath, respiratory rate >25 breaths/minute, or oxygen saturation <93%.
c Presence of any co-morbidity.
d Disease severity at presentation. Mild disease: symptoms of an upper respiratory tract viral infection including mild fever, cough (dry), sore throat, nasal congestion, malaise, headache, muscle pain, anosmia, or malaise. Moderate disease: respiratory symptoms such as cough and shortness of breath were present without signs of severe pneumonia (tachypnea >30 breaths/minute, and hypoxia: oxygen saturation <90% at room air).
SD, standard deviation; IQR, interquartile range.
Table 2.
Outcomes of patients with COVID-19 treated with ivermectin and doxycycline or placebo.
| Parameter | Total patients Number of events = 263 |
Treatment group Number of events = 183 |
Placebo group Number of events = 180 | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|---|
| Recovery, median (IQR), daysa | 7 (4–12) | 7 (4–10) | 9 (5–12) | 0.73 (0.60–0.90) | 0.003 |
| Patients responding within 7 days, n (%)b | 191 (51.9) | 111 (60.7) | 80 (44.4) | 0.06 (0.04–0.09) | <0.001 |
| Patients responding within 7–11 days, n (%)b | 68 (18.5) | 32 (47.1) | 36 (52.9) | 1.02 (0.77–1.36) | 0.90 |
| Patients remaining symptomatic after 12 days, n (%)b | 109 (29.6) | 42 (22.9) | 67 (37.2) | 0.04 (0.03–0.07) | <0.001 |
| Increase in stage of severity, n (%)c | 48 (13) | 16 (8.7) | 32 (17.8) | 0.43 (0.38–0.62) | <0.001 |
| Persistent COVID-19 RT-PCR positivity, n (%)d | 50 (13.6) | 14 (7.6) | 36 (20) | 0.61 (0.44–0.83) | 0.002 |
a Clinical recovery was defined as a normal body temperature for at least 3 days, improved respiratory symptoms defined as no shortness of breath and respiratory rate <25 breaths/minute, and oxygen saturation >93% without supplemental oxygen.
b Response criterion was the recovery of patients as defined above. The day on which clinical recovery started was considered the response day.
c Disease stages were defined as follows:
Mild: symptoms of an upper respiratory tract viral infection, including a mild fever, cough (dry), sore throat, nasal congestion, malaise, headache, muscle pain, anosmia, or malaise.
Moderate: respiratory symptoms such as cough and shortness of breath were present without signs of severe pneumonia (tachypnea >30 breaths/minute, and hypoxia: oxygen saturation <90% at room air.
Severe: tachypnea >30 breaths/minute and hypoxia: oxygen saturation <90% at room air.
d Persistent COVID-19 RT-PCR positivity: positive COVID-19 RT-PCR test at 14 days.
CI, confidence interval; COVID-19, coronavirus disease 2019; IQR, interquartile range; RT-PCR, reverse transcription polymerase chain reaction.
Primary outcome
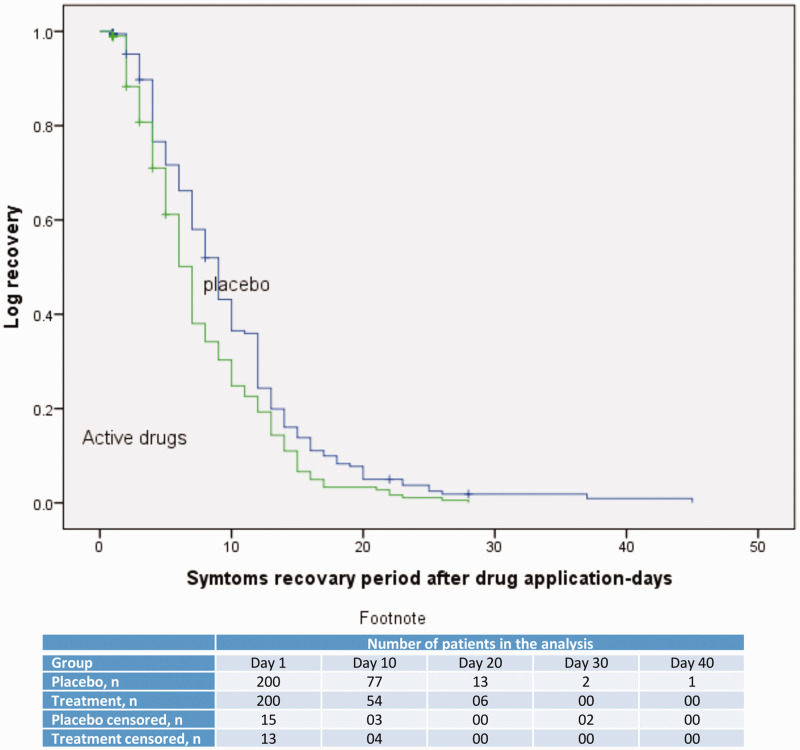
The median (IQR) recovery period was 7 (4–10) days in the treatment group and 9 (5–12) days in the placebo group (hazard ratio [HR], 0.73; 95% CI, 0.60–0.90; p = 0.003 [Figure 2; Table 2]).
Figure 2.
Time-to-recovery in the treatment and control groups, with and without censored data. Hazard ratio (95% confidence interval): 0.73 (0.60–0.90); P = 0.003.
Secondary outcomes
The proportion of patients who recovered within 7 days of treatment initiation was higher in the treatment group than in the placebo group (111 [60%] vs. 80 [44%], HR, 0.06, 95% CI, 0.04–0.09 p = <0.001). The recovery rates between 7 and 11 days were not significantly different between the groups (treatment group, 47%; placebo group, 53%). The treatment group was significantly less likely to experience symptoms that persisted for more than 12 days (42 [23%] vs. 67 [37%], HR, 0.04, 95% CI, 0.03–0.07, p = <0.001) (Table 2). Patients in the treatment group were also less likely to experience disease progression and less likely to have a positive COVID-19 RT-PCR test result after 14 days (Table 2).
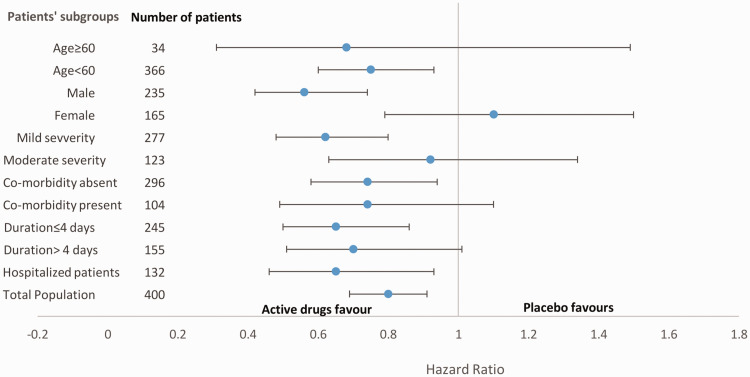
Post-hoc subgroup analysis
In the post-hoc analysis, differences in the primary outcome were determined in relation to the time interval between symptom onset and study intervention administration (<4 days vs. ≥4 days), age (<60 years vs. ≥60 years), sex, symptoms, disease severity, and presence of any comorbidities. No relationship was found between the outcome and patients who received study intervention after 4 days of symptom onset, were older than 60 years, were women, had moderately severe disease, and had comorbidities (Figure 3).
Figure 3.
Post-hoc analysis of time-to-recovery among the subgroups. Data are presented as hazard ratios and 95% confidence intervals.
Safety outcomes
Among the 400 included patients, adverse drug reactions occurred in 9 patients (2.5%); of these, 2 patients discontinued intervention owing to erosive esophagitis. Non-ulcer dyspepsia developed in seven (1.75%) patients (Appendix 1). Three patients in the placebo group died; these patients had a higher mean age than those who survived (63 years vs. 39 years) (Appendix 2) and they died 8, 22, and 28 days after randomization of respiratory failure due to COVID-19-related pneumonia.
Discussion
In the present study, patients with mild or moderate COVID-19 infection treated with ivermectin in combination with doxycycline generally recovered 2 days earlier than those treated with placebo. The proportion of patients responding within 7 days of treatment was significantly higher in the treatment group than in the placebo group. The proportions of patients who remained symptomatic after 12 days of illness and who experienced disease progression were significantly lower in the treatment group than in the placebo group. The proportion of patients who remained RT-PCR positive for COVID-19 was also lower in the treatment group than in the placebo group.
Effective vaccines and drugs for COVID-19 infection are still being researched. Potential therapies such as hydroxychloroquine15,16 and tocilizumab17 have to date been proved ineffective, while only remdesivir has shown some benefit in patients with moderate18 or severe COVID-19 infection.19 However, mortality from COVID-19 remained high, despite the use of remdesivir; therefore, treatment with an antiviral drug alone is unlikely to be sufficient. Moreover, remdesivir is expensive and can only be administered to hospitalized patients, and its effects on disease progression and viral clearance remain unclear.18 Thus, there remains an urgent need for inexpensive and effective treatment for this highly infectious and serious disease.
We were encouraged by the in vitro research findings of researchers at Monash University, Australia;6 the study revealed that ivermectin could reduce viral replication within 24 to 48 hours of treatment. There was a >5000-fold reduction in viral RNA with 5 μM ivermectin in cell culture, equating to a >99% reduction in viral RNA. Schmith et al.20 stated, “The concentration resulting in 50% inhibition (IC50; 2 µM) was >35× higher than the maximum plasma concentration (Cmax) after oral administration of the approved dose of ivermectin 200 μg/kg when given fasted,” and predicted that with the oral dose of ivermectin 200 μg/kg, lung concentrations would be approximately one-fourth of the IC50.20 The safety of higher doses has not been evaluated in humans. Therefore, we used the conventional dose of ivermectin in the present study.
Doxycycline, which may be used for the treatment of atypical bacterial pneumonia and community-acquired pneumonia,21 exerts an anti-inflammatory effect mediated by chelating zinc compounds on matrix metalloproteinases (MMPs) in mammalian cells.22 Doxycycline also has antiviral activity, especially against dengue virus23 and Chikungunya virus.24 A previous in vitro study showed that murine coronaviruses rely on MMPs for cell fusion and viral replication.25 The pathologic features of COVID-19 closely resemble those of other SARS-CoV infections, where MMPs play an important role in disease pathogenesis.26 Therefore, doxycycline may potentially be effective for the treatment of COVID-19 infection.
Ivermectin and doxycycline were co-administered in the treatment group because their synergistic action may increase the likelihood of efficacy in the treatment of COVID-19. We did not observe known drug–drug interactions between ivermectin and doxycycline;27 therefore, no drug dosage modification was required in the present study.
The effective dose of ivermectin required to reach IC50 at a pulmonary level is considerably higher than that used in this study.20 However, evaluation at higher doses requires detailed safety analysis, which was not within the scope of the present analysis. Therefore, approved dosing regimens of ivermectin and doxycycline were used in this study.
Most patients in our study received the study intervention within 3 to 5 days of symptom onset. No similar study of ivermectin for COVID-19 treatment could be identified in the literature. In the previous remdesivir trial,19 treatment benefits were prolonged in severe cases (11 vs. 15 days). In the present study, most patients recovered in both the treatment and placebo groups. However, in the treatment group, a higher proportion of patients responded within 7 days than in the placebo group. The proportions of patients whose level of disease severity worsened and who showed virus persistence beyond 14 days were also lower in the active drug group than in the placebo group. Therefore, the ivermectin and doxycycline combination might play a role in early recovery and viral clearance, thus reducing the need for hospitalization and the quarantine period of patients.
This study was performed in patients aged >18 years, and most of the included patients were relatively young (aged <40 years). Therefore, the treatment response we observed might not be generalizable to all age groups. Moreover, the subgroup analysis showed heterogeneous responses; female sex, older patients, and patients with high disease severity showed no significant responses. Early intervention also influenced the response to treatment. Early in the course of the disease, viral replication is particularly high, and in patients with severe disease, viral pathogenicity plays a less dominant role in disease pathogenesis.28 This observation explains the heterogeneity of the response in relation to the interval of drug administration and disease severity. Pharmacokinetic analysis of ivermectin29,30 showed that the drug concentration varies according to species, age, sex, and physiological conditions. The effect size of the sample might also play a role in the heterogeneous findings we observed.
Our study findings are suggestive of the efficacy of this combination treatment against COVID-19 infection. However, we speculate that earlier drug administration and a higher dose of ivermectin than that used in the present study might be more beneficial, as a higher dose will help reach the required IC5020 at the pulmonary level.
We used approved dosing regimens for both drugs and noted very few adverse reactions. Furthermore, most reactions were consistent with those associated with doxycycline,31 which supports our assumption that the combination treatment was safe to use in the study population. The death rate observed (three of 200 patients in the placebo group; 1.5%) was consistent with the COVID-19 mortality rate in Bangladesh (1.4%).32
Our study was performed at a single center over a short period. Therefore, our findings need to be carefully interpreted. Furthermore, a priori sample size calculations were not performed, limiting the strength of our findings. We could not test for viral load, and therefore could not directly assess viral clearance. It remains unclear whether the early reductions in viral load we observed should be verified by repeated RT-PCR testing during follow-up. Our testing facilities did not allow us to test patients repeatedly. Finally, we evaluated the combination of doxycycline and ivermectin but did not determine their individual effects. Therefore, further studies are needed to clarify the results observed in the present study.
Conclusions
Adult patients with mild-to-moderate COVID-19 infection treated with ivermectin combined with doxycycline recovered earlier than those receiving placebo, were less likely to progress to a serious disease, and were more likely to test negative for COVID-19 at the end of the treatment period. Although additional research on the effects of ivermectin combined with doxycycline is warranted, the safety and efficacy of this combination are favorable compared with current standard of care.
Acknowledgments
We wish to thank Popular Pharmaceuticals Bangladesh Limited for supplying the study intervention and personal protective equipment. We also thank Mr. Monir Hossain, the triage room assistant, for assistance with triage, Thomas A. Lang of Tom Lang Communication and Training International for his kind review of the manuscript, and Editage (www.editage.com) for English language editing.
Appendix
Appendix 1.
Adverse outcomes
| Attribute | Total patients (n = 400) | Treatment group (n = 200) | Placebo group (n = 200) | P-value |
|---|---|---|---|---|
| Death | 3 (0.75%) | 0 | 3 (1.5%) | 0.016 |
| Adverse drug reaction | 9 (2.25%) | 9 (2.25%) | 0 | 0.010 |
| Non-ulcer dyspepsia | 7 (1.9%) | 7 (3.8%) | 0 | |
| Erosive esophagitis | 2 (0.5%) | 2 (1.1%) | 0 |
Appendix 2.
Analysis of patients by death.
| Characteristic | Dead (n = 3) | Alive (n = 397) | P-value | Risk ratio (95% CI) |
|---|---|---|---|---|
| Treatment group, placebo (n = 200) n (%) | 3 (100) | 197 (49.6) | 0.25 | 2.0 (1.8–2.2) |
| Age, mean (SD) years | 63.7 (15) | 39.4 (13) | 0.001 | |
| Sex, male, n (%) | 03 (100) | 231 (58.4) | 0.27 | 1.7 (1.6–1.9) |
| Symptoms at presentation, patients, n (%) | ||||
| Fever | 3 (100) | 297 (74.8) | 0.58 | 1.3 (1.3–1.4) |
| Cough | 3 (100) | 244 (61.5) | 0.29 | 1.6 (1.5–1.8) |
| Running nose | 0 (0) | 36 (9.1) | >0.99 | 1.1 (1.0–1.1) |
| Respiratory distress | 2 (66.7) | 121 (30.5) | 0.22 | 2.2 (0.9–4.9) |
| Chest pain | 0 (0) | 25 (6.3) | >0.99 | 1.1 (1.0–1.1) |
| Diarrhea | 0 (0) | 32 (8.1) | >0.99 | 1.1 (1.0–1.1) |
| Vomiting | 2 (66.7) | 18 (4.5) | 0.007 | 14.7 (5.9–36.8) |
| Anorexia | 1 (33.3) | 118 (29.7) | >0.99 | 1.1 (0.22–5.6) |
| Anosmia | 1 (33.3) | 153 (38.5) | >0.99 | 0.87 (0.17–4.3) |
| Lethargy | 0 (0) | 106 (26.6) | 0.57 | 1.4 (1.3–1.4) |
| Body ache | 1 (33.3) | 72 (18.1) | 0.46 | 1.8 (0.37–9.2) |
SD, standard deviation; CI, confidence interval.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest. Popular Pharmaceuticals Limited, Bangladesh provided ivermectin, doxycycline, and placebo. The company was not involved in the planning or design of the study and had no role in the collection, analysis, or interpretation of the data.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author contributions: R. Mahmud and Md. M. Rahman had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. R. Mahmud, Md. M. Rahman, I. Alam, K. Gias Uddin Ahmed, and S.K. Jakaria Been Sayeed conceived the research and designed the study. R. Mahmud, Md. M. Rahman supervised the research. R. Mahmud, Md. M. Rahman, I. Alam, K. Gias Uddin Ahmed, A.K.M. Humayon Kabir, and Mohammad Zaid Hossain provided administrative, technical, or material support. R. Mahmud, S.K. Jakaria Been Sayeed, and M. Abdullah Yusuf performed the statistical analysis. R. Mahmud, Md. M. Rahman, S.K. Jakaria Been Sayeed, and M. Abdullah Yusuf drafted the manuscript. All authors were responsible for acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content.
ORCID iD: Reaz Mahmud https://orcid.org/0000-0002-9427-1746
References
- 1.World Health Organization. GCM teleconference – Note for the Records. 10 January 2020. Subject: Pneumonia in Wuhan, China. Accessed November 1, 2020. https://www. who. int/blueprint/10-01-2020-nfr-gcm.pdf?ua = .
- 2.WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Accessed November 1, 2020.: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19–-11-march-2020.
- 3.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020; 34: 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. International Clinical Trial Registry Platform. COVID19 trials. Accessed November 1, 2020. https://www.who.int/ictrp/en/.
- 6.Sundy NYY, Sarah CA, Chunxiao W, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport import in α/β1heterodimer. J Antiviral 2020; 177: 104760. doi: 10.1016 [DOI] [PubMed] [Google Scholar]
- 7.Canga AG, Prieto AMS, Liébana MJD, et al. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPS J 2008; 10: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caly L, Druce JD, Catton MG, et al. The FDA approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020; 178: 104787. 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin MO, Fricovsky E, Ceballos G, et al. Tetracycline: a pleiotropic family of compounds with promising therapeutic properties. Review of the literature. Am J Physiol Cell Physiol 2010; 299: C539–C548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henehan M, Montuno M, De Benedetto A. Doxycycline as an anti‐inflammatory agent: updates in dermatology. J Eur Acad Dermatol Venereol 2017; 31: 1800–1808. [DOI] [PubMed] [Google Scholar]
- 11.Sodhi M, Etminan M. Therapeutic potential for tetracycline in the treatment of COVID-19. Pharmacotherapy 2020; 40: 487–488. doi: 10.1002/phar.2395. Epub 2020 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO guidance on management of severe acute respiratory infection (SARI) when COVID19 is suspected. Accessed November 1, 2020.//www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- 13.National Guidelines on Clinical Management of Coronavirus Disease 2019 (COVID-19). 27 May, 2020. Accessed November 1, 2020. https://dghs.gov.bd/images/docs/Guideline/ COVID. Guideline. pdf.
- 14.Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med 2013; 35: 121–126. doi:10.4103/0253-7176.116232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiolet T, Guihur A, Rebeaud ME, et al. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect 2021; 27: 19–27. 10.1016/j.cmi.2020.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial. Ann Intern Med 2020; 173: 623–631. doi:10.7326/M20-4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med 2020; 383: 2333–2344. doi: 10.1056/NEJMoa2028836. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs. standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA. Published online August 21, 2020. doi:10.1001/jama.2020.16349). [DOI] [PMC free article] [PubMed]
- 19.Beigel JH, Tomashek KM, Dodd LE, et al . ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19 - Preliminary Report. N Engl J Med 2020: NEJMoa2007764. doi: 10.1056/NEJMoa2007764. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Schmith VD, Zhou JJ, Lohmer LRL. The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19. Clin Pharmacol Ther 2020; 108: 762–765. doi: 10.1002/cpt.1889. Epub 2020 Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200: e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanlaere I, Libert C. Matrix metalloproteinases as drug targets in infections caused by gram-negative bacteria and in septic shock. Clin Microbiol Rev 2009; 22: 224–39, Table of Contents. doi: 10.1128/CMR.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothan HA, Mohamed Z, Paydar M, et al . Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch Virol 2014; 159: 711–718. 10.1007/s00705-013-1880-7. [DOI] [PubMed] [Google Scholar]
- 24.Rothan HA, Bahrani H, Mohamed Z, et al. A combination of doxycycline and ribavirin alleviated chikungunya infection. PLoS One 2015; 10: e0126360. 10.1371/journal. Pone. 0126360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips JM, Gallagher T, Weiss SR. Neurovirulent Murine Coronavirus JHM.SD uses cellular zinc metalloproteases for virus entry and cell-cell fusion. J Virol 2017; 91: e01564-16. 10.1128/jvi.01564-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gralinski LE, Baric RS. Molecular pathology of emerging coronavirus infections. J Pathol 2015; 235: 185–195. 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DrugBank online. Accessed November 11, 2020. https://go.drugbank.com/drugs/DB00254.
- 28.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical therapeutic staging proposal. J Heart Lung Transplant 2020; 39: 405–407. Doi: 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.González Canga A, Sahagún Prieto AM, Diez Liébana MJ, et al. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPS J 2008; 10: 42–46. doi:10.1208/s12248-007-9000-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKellar QA, Benchaoui HA. Avermectins and milbemycins. J Vet Pharmacol Ther 1996; 19: 331–351. [DOI] [PubMed] [Google Scholar]
- 31.Holmes NE, Charles PGP. Safety and efficacy review of doxycycline. Clin Med Insights Ther 2009; 1: 471–482. doi: 10.4137/CMT.S2035. [Google Scholar]
- 32.WHO Corona virus (COVID-19) Dashboard. Accessed November 1, 2020. https://extranet. Who.int/public emergency.