Abstract
Hypoxic zones in solid tumors contribute to radioresistance, and pharmacological agents that increase tumor oxygenation prior to radiation, including anti-angiogenic drugs, can enhance treatment response to radiotherapy. Although such strategies have been applied, imaging assessments of tumor oxygenation to identify an optimum time window for radiotherapy have not been fully explored. In this study, we investigated the effects of alpha-sulfoquinovosylacyl-1,3-propanediol (SQAP; a synthetic derivative of an anti-angiogenic agent) on the tumor microenvironment in terms of oxygen partial pressure (pO2), oxyhemoglobin saturation (sO2), blood perfusion, and microvessel density using electron paramagnetic resonance imaging, photoacoustic imaging, dynamic contrast-enhanced MRI with Gd-DTPA injection, and T2*-weighted imaging with ultrasmall superparamagnetic iron oxide (USPIO) contrast. SCCVII and A549 tumors were grown by injecting tumor cells into the hind legs of mice. Five days of daily radiation (2 Gy) combined with intravenous injection of SQAP (2 mg/kg) 30 min prior to irradiation significantly delayed growth of tumor xenografts. Three days of daily treatment improved tumor oxygenation and decreased tumor microvascular density on T2*-weighted images with USPIO, suggesting vascular normalization. Acute effects of SQAP on tumor oxygenation were examined by pO2, sO2, and Gd-DTPA contrast-enhanced imaging. SQAP treatment improved perfusion and tumor pO2 (ΔpO2: 3.1±1.0 mmHg) and was accompanied by decreased sO2 (20–30% decrease) in SCCVII implants 20–30 min after SQAP administration. These results provide evidence that SQAP transiently enhances tumor oxygenation by facilitating oxygen dissociation from oxyhemoglobin and improving tumor perfusion. Therefore, SQAP-mediated sensitization to radiation in vivo can be attributed to increased tumor oxygenation.
Keywords: Sulfoglycolipid, SQAP, CG-0321, radiosensitizer, oxygen
Introduction
Solid tumor growth is accompanied by increased vasculature through a process known as angiogenesis (1). Extensive research has led to the identification and characterization of several pro- and anti-angiogenic regulators, which represent plausible therapeutic targets. In tumors, an imbalance between pro- and anti-angiogenic factors exists because of the hypoxic tumor microenvironment (2). This imbalance triggers the growth of an abnormally structured and leaky tumor vasculature (3). Consequently, tissue oxygenation remains inadequate, which not only causes continuous stimulation of angiogenesis, but also interferes with response to chemotherapy and radiotherapy. In the last two decades, various combinations of ionizing radiation (XRT) with different anti-angiogenic drugs, nitroimidazoles, and hypoxia-activated prodrugs (HAPs) have been evaluated for their abilities to enhance therapeutic efficacy. Our group has developed quantitative noninvasive electron paramagnetic resonance (EPR) imaging (EPRI) capabilities to serially map tumor oxygen in vivo and determine changes in tumor partial oxygen (pO2) distribution in response to treatment (4,5). Such imaging techniques have found that tumors display both spatial and temporal heterogeneities in pO2 status (6–9). The clinical importance of hypoxia and its potential modification has been one of the most investigated issues in radiotherapy, resulting in the identification of novel tumor hypoxia-modifying agents.
Sulfoquinovosylacylglycerols (SQAGs) are sulfoglycolipids originally isolated from natural sources such as higher plants (10,11), sea urchins (12), and marine algae (13). They fall into two groups, monoacyl forms (SQMG) and diacyl forms (SQDG), depending on the number of fatty acids. Several structural isomers of SQAG exist, although naturally they are only known as alpha-isoforms. SQAGs exhibit various biological effects, including inhibition of HIV reverse transcriptase and DNA polymerase activity (10,13–15). Synthetic -SQMG has also demonstrated anti-angiogenic and tumor-radiosensitizing properties (16–18). However, apart from the upregulation of thrombospondin 1 (19), the downregulation of Tie-2 (20), and its direct interaction with angiogenic growth factors (21) and mitotic centromere-associated kinesin (MCAK) (22), the molecular mechanisms of its anti-angiogenic and radiosensitizing effects remain elusive. A synthetic analog of SQAG, alpha-sulfoquinovosylacyl-1,3-propanediol (SQAP or CG-0321, Toyo Suisan Kaisha Ltd.) was synthesized in preparation for a Phase 1 study (23) (Fig. 1A). Sawada et al. (24) demonstrated that the tumor volume in xenograft models of the human prostate cell lines DU145 and PC3 was reduced after a combination of SQAP administration and radiotherapy. PC3 tumors were fully eradicated by radiation alone, and therefore did not benefit from the addition of SQAP, while DU145 tumors showed little if any response to XRT, but substantial response to SQAP. The vascular normalization index calculated according to positive CD34 and alpha-smooth muscle actin (α-SMA) cells in DU145-derived tumors was significantly increased by the combination therapy, whereas such effects were absent in the PC3-derived tumors (24). However, the radiobiological mechanisms of SQAP radiosensitizing actions have not been completely elucidated. Currently, the only study investigating the mechanisms of the radiosensitizing action was by Izaguirre-Carbonell et al. (25), which utilized a T7 phage display technique to identify proteins that bind to SQAP in cell-free extracts to elucidate its mechanism of action. This approach identified five SQAP-binding proteins: sterol carrier protein 2, multifunctional enzyme type 2, proteasomal ubiquitin receptor, UV excision repair protein, and focal adhesion kinase (FAK). SQAP decreased FAK phosphorylation and cell migration in human umbilical vein endothelial cells (HUVECs) and A549 lung cancer cells. In summary, the physiological basis of the radiosensitizing actions of SQAP in vivo requires further investigation.
Figure 1.

Effect of SQAP on tumor cell and tissue growth inhibition by XRT. A, Structure of SQAP or CG-0321 (C27H51O10S1/2Ca, Mw 587.79). B, C, In vitro cell survival after various doses of XRT in the presence or absence of SQAP (10 μM). Effects on murine SCCVII (B) and human A549 (C). D–G, Growth kinetics of mice tumor xenografts and mice body weight (BW) changes after each treatment. D, C3H mice bearing murine SCCVII tumors. E, Athymic nude mice bearing human A549 tumors. F, G, BW changes in C3H mice (F) or athymic nude mice (G) in each treatment group. + signifies treatment. Data are means ± SE (SCCVII: n = 5, A549: n = 7–8). **, P < 0.01, ***, P < 0.001.
To evaluate the role of SQAP as a potential novel in vivo radiosensitizer, the current study utilized a multimodal molecular imaging approach to characterize tumor physiological changes after treatment with SQAP and XRT. Mice bearing murine squamous cell carcinoma (SCCVII) tumors or human lung adenocarcinoma (A549) tumors were used. The oxygen status, perfusion, and hemoglobin saturation on SQAP treatment were monitored in each tumor. Based on the results from EPR oxygen imaging, additional studies were conducted, including photoacoustic (PA)-micro-ultrasound (US) imaging and dynamic contrast-enhanced MRI (DCE-MRI), to evaluate the effect of SQAP on hemoglobin-oxygen saturation (sO2), tumor perfusion, and vascular permeability. PA imaging was previously used to examine the effect of vascular disrupting agents (26–28); however, in this study it was used to monitor drug-induced increases in sO2 and total hemoglobin concentration (HbT). Findings from our imaging studies identified a temporal window of increased oxygenation after SQAP administration, which results in in vivo radiosensitization of tumors and is optimal for a synergistic interaction with XRT.
Materials and Methods
Cell survival studies
Murine squamous cell carcinoma (SCCVII) cells were derived from established SCCVII tumors (obtained from Dr. T. Phillips, UCSF, San Francisco, CA), while human non-small-cell lung carcinoma (NSCLC) A549 and colon cancer HT29 cell lines were purchased from the American Type Culture Collection (ATCC, Manassas,VA). Both cell lines were grown in RPMI 1640 supplemented with 10% fetal calf serum and antibiotics. Human umbilical vein endothelial cells (HUVECs) were obtained from Lonza (Lonza.com, Allendale, NJ, C2517A, Cryopreserved cells) and cultured using EGM™−2 BulletKit™ medium (CC-3162, Lonza). All cells were tested for any cross-species contamination in 2017 and authenticated by IDEXX RADIL (Columbia, MO) using a panel of microsatellite markers (Supplementary data Figure S1). All cell lines used in this study were maintained in culture for 4 to 5 weeks, regularly monitored for mycoplasma contamination by MycoAlert™ detection kit (Lonza) and discarded in case of positive results. The survival of cells exposed to SQAP and/or XRT was assessed by clonogenic assays. Cells (2.5 × 105) were plated into 6 cm culture dishes, and incubated at 37°C overnight. Medium containing varying concentrations of SQAP was added, and the cells were further incubated for 1 h at 37°C. After the treatment, cells were exposed to varying doses of XRT using an XRAD 320 (Precision X-ray Inc. North Branford, CT). Cells were then rinsed, trypsinized, counted, plated, and incubated for 10 to 14 days for macroscopic colony formation. Colonies were fixed with methanol/acetic acid (3:1) and stained with crystal violet. Colonies with > 50 cells were scored, and cell survival was determined. Experiments were repeated two to three times, and the error bars shown in the figures represent the standard error of the mean (SEM).
Animal studies
Animal experiments were carried out in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996), and were approved by the National Cancer Institute (NCI) Animal Care and Use Committee (29). Female 5–8-week-old C3H/Hen mice (15–23 g) and athymic nude mice (19–27 g) were supplied by the Animal Production Department, Frederick Cancer Research Center. Mouse SCCVII and human A549 solid tumor formation, and management of the mice during imaging, were carried out as previously described (7). SQAP (Toyo Suisan Kaisha Ltd. Tokyo, Japan) was dissolved in DPBS (Dulbecco’s Phosphate-Buffered Saline) and intravenously injected to provide a dose of 2 mg/kg body weight. The tumor-bearing mice were then exposed to X-rays (2 Gy) 30 min after SQAP administration. Treatment was performed five times, on days 6 to 10 after tumor implantation for SCCVII lines, and days 19 to 23 for A549 lines, when the tumor size had reached 300 mm3.
EPR oxygen imaging
Details of our homebuilt EPR scanner operating at 300 MHz, the data acquisition, the image reconstruction, and the oxygen mapping procedure are described in an earlier report (30). A parallel coil resonator (17 mm i.d. and 25 mm long) was constructed for sequential EPRI and MRI of the tumor-bearing leg. For the EPRI and MRI measurements, mice were anesthetized by isoflurane (4% for induction and 1.5% for maintenance of anesthesia) in medical air (750 ml/min), and positioned prone with their tumor-bearing legs placed inside the resonator. The breathing rate of the mice was monitored with a pressure transducer (SA Instruments Inc. Stony Brook, NY) and maintained at 80 ± 20 breaths per minute. Core body temperature was monitored and maintained at 36 ± 1°C with a flow of warm air. After the resonator was placed in the cradle and inserted into the scanner, the paramagnetic tracer Oxo63 (GE Healthcare, Chicago, IL) was injected intravenously through a cannula (30-gauge needle with extended polyethylene tube [PE-10]) placed into the tail vein. Oxo63 was given as a 1.125 mmol/kg bolus followed by 0.04 mmol/kg/min continuous injection. After acquiring the first EPR dataset, a 2 mg/kg bolus (0.4 mg/ml SQAP in DPBS, 5 μl × mouse body weight in g) of SQAP solution was injected intravenously through the cannula. EPRI scans were then acquired after SQAP administration (15, 30, 45, and 60 min after SQAP administration for imaging, 10, 20, 30, 45, 60, and 75 min for pO2 quantification). The spatial resolution of the pO2 images measured using EPRI was 1.8 mm2. The pixel resolution was digitally enhanced by cubic interpolation during co-registration to the MR images.
MRI for anatomy and blood volume (BV)
MRI scanning was conducted on a 7-T scanner controlled with ParaVision 5.1 (Bruker BioSpin MRI, Billerica MA). T2*-weighted anatomical images were obtained as described previously (5). For blood volume (BV) calculation, spoiled gradient echo sequence images were collected before and 5 min after injection of ultrasmall superparamagnetic iron oxide (USPIO) contrast (1.2 μl/g of body weight). The imaging parameters included the following: matrix = 256 × 256; echo time (TE) = 5.4 ms; and TR = 250 ms. The percentage tumor BV was estimated as previously described (30). The co-registration of EPR and MR images was accomplished using code written in MATLAB (MathWorks, Natick, MA), as previously described (6).
DCE-MRI of Gd-DTPA uptake
DCE-MRI studies were performed on a 7-T scanner (Bruker BioSpin MRI GmbH). T1-weighted fast low-angle shot (FLASH) images were obtained with TR = 156 ms; TE = 4 ms; flip angle = 45°; four slices; 0.44 × 0.44 mm resolution; 20 s acquisition time per image; and 98 repetitions. Gd-DTPA solution (4 μl/g of body weight of 50 mM Gd-DTPA) was injected through a tail vein cannula 2 min after the start of the dynamic FLASH sequence. To determine the local concentrations of Gd-DTPA, T1 maps were calculated from three sets of RARE images obtained with TR = 300, 2000, and 6000 ms, with the acquisitions being made before running the FLASH sequence.
PA imaging
The effects of SQAP on sO2 and total BV were visualized using a VisualSonics Vevo2100 LAZR PA-micro-US imaging system (FUJIFILM VisualSonics, Inc., Toronto, Canada) with a 40 MHz center frequency probe (28,31). The SCCVII tumor-bearing mice were anesthetized using 1.5% isoflurane with medical air. A depilatory cream was used (for a short duration to avoid chemical burns) to remove hair over the area of the right hind leg bearing the SCCVII tumor. US gel was then applied over the region of interest (ROI). To collect anatomical information at high resolution, B-mode imaging was acquired at 40 MHz.
During the PA imaging, a tunable laser (680–970 nm) was used as described previously (31). The pulse-to-pulse energy fluctuation was continuously monitored, with fluctuations above 25% requiring recalibration of the system before further imaging. Oxygen saturation and hemoglobin concentration were measured at 21 MHz (LZ250, VisualSonics). In Oxy-Hemo mode, PA dual-wavelength imaging at 750 and 850 nm creates images of oxygenated hemoglobin and deoxygenated hemoglobin, which are then co-registered with gray scale B-mode imaging (a detailed description of the algorithms can be found in (31)). The sO2 within a ROI was calculated as the percentage of oxygenated hemoglobin against total hemoglobin. Total hemoglobin and oxygen saturation were quantified using the HemoMeaZure™ (VisualSonics) and OxyZated™ tools (VisualSonics), respectively.
Histochemical analysis
Tumor tissues were excised 1 h after intravenous injection of pimonidazole, as per manufacturer’s instructions. Tumors were frozen by ultra-cold ethanol and sectioned to 10 μm using a cryostat, with the sections being thaw-mounted on glass slides. After fixing with 4% paraformaldehyde, sections were treated with cold acetone for 15 min. After blocking nonspecific binding sites on sections with Protein Block Serum-Free reagent (Dako North America Inc., Carpinteria, CA) for 30 min, the slides were covered by CD31 antibody (BD Biosciences, Billerica, MA; 1:250) combined with α-SMA antibody (Abcam, Inc. Cambridge, MA; 1:250) or Hypoxyprobe 4.3.11.3 mouse MAb (Hypoxyprobe.inc. Burlington MA; 1:100) for pimonidazole staining overnight at 4°C. The sections were then incubated with Alexa Fluor 488 anti-mouse and the Alexa Fluor 546 F(ab’)2 fragment of goat anti-rabbit IgG (H+L) (Invitrogen Carlsbad, CA; 1:2000) for 1 h at room temperature, before being mounted with Prolong Gold antifade reagent with DAPI (Invitrogen). Fluorescence microscopy was performed using a BZ-9000 BIOREVO (Keyence, Itasca, IL), and images were captured using a BZ-9000E viewer. To quantify the pimonidazole-positive area, digital images of stained sections at 10× magnification were assembled using a BZ-II Analyzer (Keyence) to create an image of the whole tumor, and the pixels of the positive area were counted and shown as a percentage of the whole tumor area. To evaluate the therapeutic response, the area of the CD31-positive pathological microvessels (green) or α-SMA pericyte (red) in the optical field was measured and shown as percent (n = 3).
Statistical analysis
All results are expressed as the mean ± SE. The statistical significance of differences in the means of groups was determined using two-tailed Student’s t-tests.
Results
Effect of SQAP on tumor radiotherapy
The radiosensitizing effect of SQAP (Fig. 1A) was evaluated using an in vitro clonogenic cell survival assay and the in vivo growth kinetics of the tumor xenografts in mice. Figure 1B and C show cell survival according to colony formation efficiency 10–14 days after treatment with various doses of XRT, and in the presence or absence of 10 μM SQAP. Compared with the XRT alone, the SQAP combination showed no radiosensitizing effect in SCCVII (Fig. 1B) and A549 (Fig. 1C) tumor cells in vitro. The growth of HUVECs was minimally affected by XRT in the presence of 10 μM SQAP (Supplementary data Figure S2). These results suggest that SQAP at a concentration of up to 10 μM shows no radiosensitizing effect on murine SCCVII or human A549 tumor cells in vitro.
The murine SCCVII implants or human A549 tumor xenografts that were subcutaneously grown in the hind legs of mice were tested with radiation alone or in combination with SQAP. In these experiments, a SQAP dose of 2 mg/kg/day was used for a better comparison with a previous study showing the radiosensitizing effect of SQAP (24). Compared with the non-treated control, SQAP alone (open triangles), or radiation alone (open squares), the growth of the C3H mice bearing SCCVII tumors was significantly delayed by 5 days by daily fractionated XRT (2 Gy) in combination with a 30 min prior treatment with SQAP (filled circles Fig. 1D; n = 5, P < 0.001 vs control, P < 0.01 vs daily fractionated XRT alone, P < 0.01 vs SQAP alone on day 14). This is in contrast to the observation that no radiosensitizing effect on SCCVII tumors was observed in vitro (Fig. 1B). In particular, this combination therapy, which was effective only with a 2 Gy radiation dose and 2 mg/kg intravenous administration of SQAP per treatment, showed no observable side effects such as a decrease in the body weight of the mice. Furthermore, SQAP administration 30 min before the XRT significantly delayed tumor growth (filled circles), although it was not effective 60 (filled triangles) or 120 min before (filled squares) XRT (Fig. 1D). Following the results obtained with SCCVII tumors, the effect of XRT on A549 xenografts 30 min after SQAP treatment was examined. A similar growth inhibitory effect was also observed in the athymic nude mice bearing A549 tumors: although SQAP monotherapy or XRT alone did not suppress the growth of A549 tumors, 5 days of daily fractionated XRT (2 Gy) in combination with pretreatment with SQAP 30 min before XRT significantly delayed the growth of A549 tumors (n = 7, P < 0.01 vs control on day 45; Fig. 1E). As with the SCCVII-bearing C3H mice (Fig. 1F), no observable body weight changes appeared in A549-bearing nude mice (Fig. 1G). These results demonstrate that SQAP has no effect on tumor cell survival in vitro, but it modulates extracellular events or the physiological status of in vivo tumor implants, influencing the tumor microenvironment within a specific time window.
Transient increase in tumor oxygenation after intravenous administration of SQAP
Tumor oxygenation is an important physiological factor influencing the tumor microenvironment and governing radiosensitization (32). To investigate the influence of intravenous SQAP administration on tumor oxygenation (pO2), EPR oxygen imaging was performed in tumor-bearing mice using OXO63, and pO2 maps were serially obtained every 15 or 20 min after SQAP administration. Figure 2A and B shows the anatomical images and corresponding time-dependent changes in pO2 (upper panels) and levels of OXO63 tracer (lower panel) measured by EPRI in squamous cell carcinoma (SCCVII) tumors at the indicated times (min) after intravenous administration of SQAP (2 mg/kg) or saline. The median pO2 of the SCCVII tumors (10.0 ± 3.5 mmHg, n = 3) transiently increased 20–30 min after the SQAP administration (Fig. 2A), while no time-dependent changes in pO2 (upper panels) or OXO63 tracer (lower panels) were observed after intravenous administration of saline only (Fig. 2B). Similar results were obtained when SQAP was tested in A549 xenografts (16.1 ± 2.6 mmHg, n = 3) (Supplementary data Figure S3A–D). The region of higher oxygen distribution significantly increased for 30 min, then returned to the preinjection level 1 h after injection, while the tracer level during this time window remained relatively stable. As shown in Fig. 2C, median pO2, which was calculated as an average of three slices from the center of the SCCVII tumor, transiently increased for 20–30 min (~Δ4 mmHg), and then recovered 1 h after SQAP treatment, whereas no significant changes in tracer level were observed across the time course. Significant changes in the pO2 level were not observed in the control experiment with saline injection (Fig. 2D). These results indicate that intravenously administered SQAP transiently increased SCCVII tumor pO2 20–30 min after injection.
Figure 2.

Monitoring of the tumor oxygen status by EPR oxygen imaging. A, B, T2-weighted anatomy of C3H mice bearing SCCVII tumors, and tumor pO2 and EPR tracer level maps in the tumor scanned every 15 min after intravenous administration of SQAP (A) or saline (B). C, D, Plots of median pO2 value (mmHg) and tracer level as image intensity (arbitrary units; a.u.) after the SQAP (C) or saline (D) injection. E–H, T2-weighted anatomy and EPRI pO2 maps of SCCVII/C3H and A549/nude mice before (0 min) and 30 min after SQAP injection. I, Changes in median pO2 value (ΔpO2 mmHg) 30 min after injection.
Figure 2E and F show MR anatomical images and EPR oxygen images of C3H mice bearing SCCVII (Fig. 2E) tumors, and athymic nude mice bearing A549 (Fig. 2F) xenografts, taken at 0 and 30 min post-SQAP administration. Compared with the preinjection pO2 map (0 min), the local oxygen distribution in SCCVII tumors (Fig. 2G) significantly increased 30 min after intravenous administration of SQAP. Similar results were obtained for the A549 xenografts implanted in nude mice (Fig. 2H). The increases in median pO2 value in SCCVII and A549 tumors were 3.1 ± 1.0 mmHg (n = 3) and 4.9 ± 2.9 mmHg (n = 3), respectively (Fig. 2I). These results suggest that intravenously administered SQAP transiently increases tumor pO2 in two different tumor models grown in different strains of mice.
Effect of SQAP on sO2 and erythrocyte flux in SCCVII tumors
To evaluate the mechanisms underlying the transient increase in tumor oxygenation caused by SQAP administration, sO2 measurements were conducted using PA imaging (31). The SCCVII tumor-bearing C3H mice underwent an intra-tumoral US scan with co-registered PA imaging using a dual-wavelength scan. The sO2 intensity (%) in the SCCVII tumors started to decrease immediately after the intravenous SQAP administration (Supplementary data Movie S1). Figure 3A shows a representative anatomical US image co-registered to a PA functional image, with the 2D ROIs 1 and 2 indicating SCCVII tumor (500 mm3), where a high sO2 intensity was observed, potentially due to the location of tumor vasculature. Compared with the preinjection levels, ROIs 1 and 2 displayed a marked decrease in sO2 (%) 30 or 60 min after intravenous administration of 10 mg/kg SQAP, suggesting that SQAP lowers sO2 in SCCVII tumors. As shown in Fig. 3B, the sO2 in ROIs 1 (■) and 2 (▲) showed a rapid and significant decrease immediately after the SQAP treatment, and then only gradually dropped further over the 60 min period. The sO2 average in the whole SCCVII tumor, calculated from the 3D PA image, decreased from 42.9% to 30.6% 60 min after the treatment (●). Furthermore, after 25 min post-injection, the average sO2 (%) decreased in a dose-dependent manner (Fig. 3C), suggesting that the decrease in tumor sO2 could be attributed to the SQAP injection.
Figure 3.
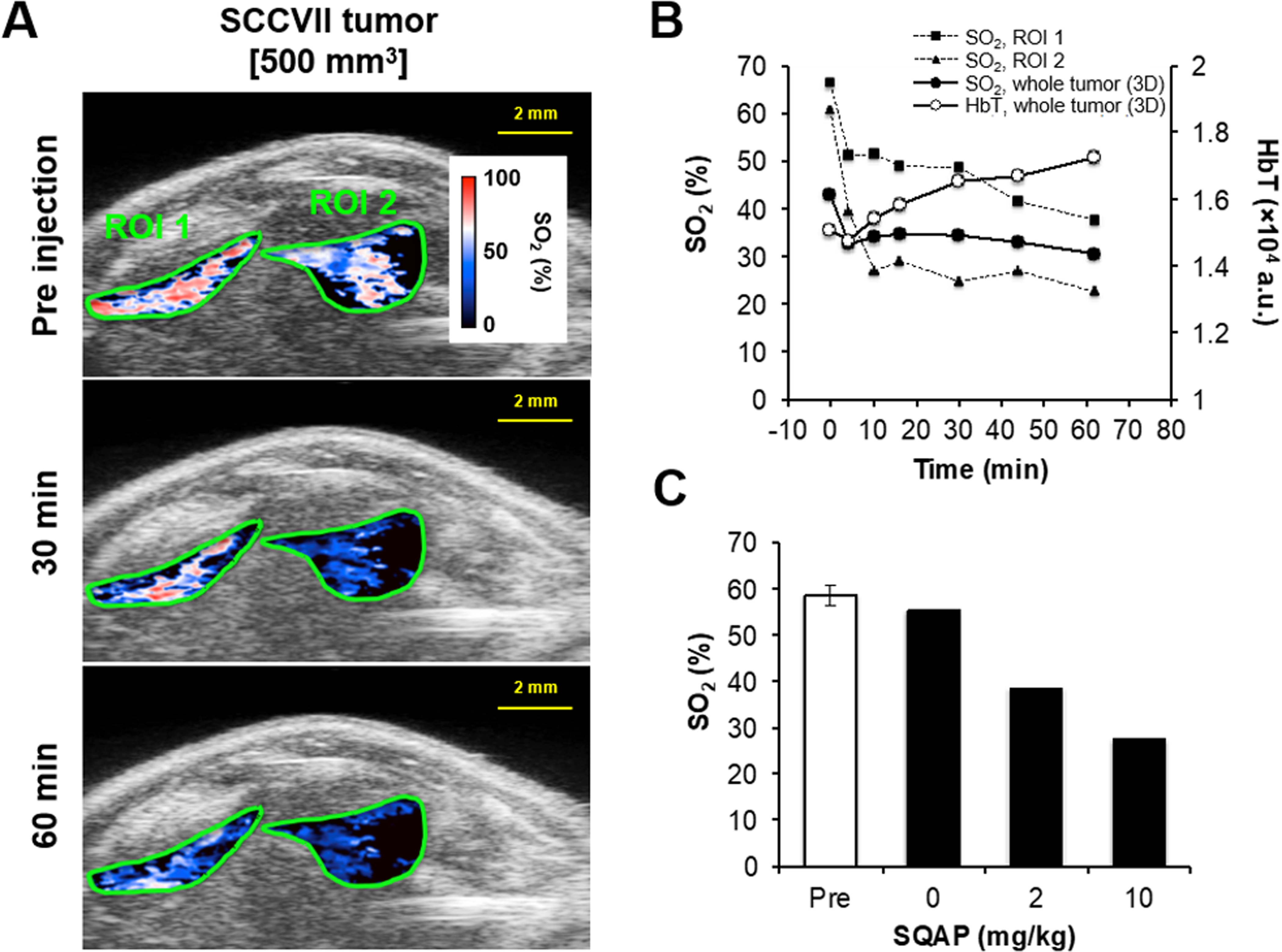
Noninvasive monitoring and quantification of tumor vascular oxygen saturation by photoacoustic-micro-ultrasound imaging before and after treatment with SQAP in SCCVII-bearing C3H mice. A, SCCVII anatomy on a photoacoustic image with sO2 levels in the ROIs collected by photoacoustic dual-wavelength imaging at 750 and 850 nm before (0 min) and 30 or 60 min after intravenous administration of SQAP (10 mg/kg). B, Changes in sO2 and HbT in ROIs 1 and 2 of Fig. 3A or 3D whole tumors. C, Decrease in tumor sO2 (%) before (Pre) and after (average of 25–60 min) the SQAP administration. sO2 (%) = oxyhemoglobin / total hemoglobin × 100.
In addition, the HbT in the tumor was also calculated from these PA images. The HbT gradually increased after SQAP treatment, and reached 110% by 60 min (Fig. 3B, ○), suggesting an increase in BV following SQAP administration. Taken together, these results suggest that intravenously administered SQAP transiently decreased tumor sO2 by facilitating oxygen dissociation from oxyhemoglobin in the tumor, as well as increasing the tumor BV, thereby increasing the tumor pO2.
SQAP preinjection increases tumor Gd-chelate uptake
Tumor perfusion changes in response to SQAP treatments were investigated by DCE-MRI. Figure 4A shows T2-weighted anatomical images and dynamic T1-weighted MR images of SCCVII tumors (within ROIs) collected at 20 s intervals for the first minute and then at 300 s after Gd-DTPA injection. The 30 min prior treatment with SQAP (upper panel) significantly enhanced Gd-DTPA uptake in comparison with untreated controls (lower panel). The time-intensity kinetic curve of intravenously injected Gd-DTPA in the tumor region is shown in Fig. 4B. Compared with the controls (○, n = 3), Gd-DTPA uptake was significantly increased by pre-treatment with SQAP (●, n = 4). The increase in Gd-DTPA uptake at early time points (0–60 s) in test mice, as calculated using the area under the curve (AUC) of the Gd-DTPA concentration, was significantly higher than in the control mice (2.4-fold increase, Fig. 4C), while the AUC total (0–30 min) was not (Fig. 4D). These results suggest that SQAP administration has minimal effect on vascular permeability, but that it improves tumor perfusion, in agreement with the PA data from the US imaging experiment (Fig. 3B).
Figure 4.

Effect of SQAP on Gd-DTPA uptake in tumors (DCE-MRI). A, T2-weighted anatomical images of SCCVII tumors and monitoring of the Gd-DTPA intensity with or without SQAP treatment 30 min prior to Gd-DTPA injection. Images were acquired every 20 s for the first 60 s and then at 300 s after Gd-DTPA injection. B, Kinetics of the Gd-DTPA incorporation into tumors. The concentration of Gd was obtained from the maximal slice of whole tumors scanned every 20 s and plotted for 30 min. Data are means ± SE (SQAP: n = 4, Cont: n = 3). C, D, Area under the curve (AUC) with or without SQAP pretreatment 30 min prior to the Gd-DTPA injection. C, AUC for the first 60 s after Gd-DTPA injection, which mainly reflects tumor perfusion. D, AUC for the whole 30 min of scanning (every 20 s), which reflects Gd-DTPA uptake into tumor tissues through vascular permeability. Data are means ± SE (SQAP: n = 4, Cont: n = 3). *, P < 0.05, N.S., not significant.
Physiological properties of tumors after the SQAP/XRT combination therapy
The imaging experiments described above support the notion that the SQAP-induced increase in tumor perfusion and decrease in hemoglobin-oxygen affinity cause the transient increase in tumor tissue pO2. To evaluate the tumor oxygen status and vascular density changes after the combination therapy with SQAP + XRT, EPR oxygen imaging and BV imaging were conducted with MRI using USPIO. The local pO2 (mmHg) and BV (%) in tumors were quantitatively measured from each image of the SCCVII tumors. The experimental design is schematically shown in Fig. 5A. Treatments were started on day 6 with a SCCVII tumor size of ~300 mm3. During the 3 days of daily treatment, images were obtained from SCCVII tumors at 0 (before), 24, and 72 h. As shown in Fig. 5B, the median pO2 in the ~300 mm3 SCCVII tumors did not change 24 h after the treatment with SQAP, XRT, or SQAP/XRT combination therapy. Even after 3 continuous days of SQAP or XRT monotherapy, the SCCVII tumors (72 h) showed a similar decrease in tumor pO2 to the non-treated controls, indicating the emergence of tumor hypoxia, while the median pO2 levels in SCCVII tumors remained at the pretreatment level 3 days after the daily treatment with SQAP/XRT (n = 4, P < 0.01 vs day 9 control). This observation suggests that the combination therapy of SQAP with XRT delays hypoxia onset. Likewise, tumor BV did not change at 24 or 72 h in the daily SQAP or XRT monotherapy groups, while it significantly dropped after 3 days of daily treatment with SQAP/XRT (n = 4, P < 0.05 vs day 9 control; Fig. 5C, Supplementary data Figure S4). These results indicate that anti-angiogenic effects were observed only with the combination therapy, as was the case with the natural product α-SQMG (17,18).
Figure 5.
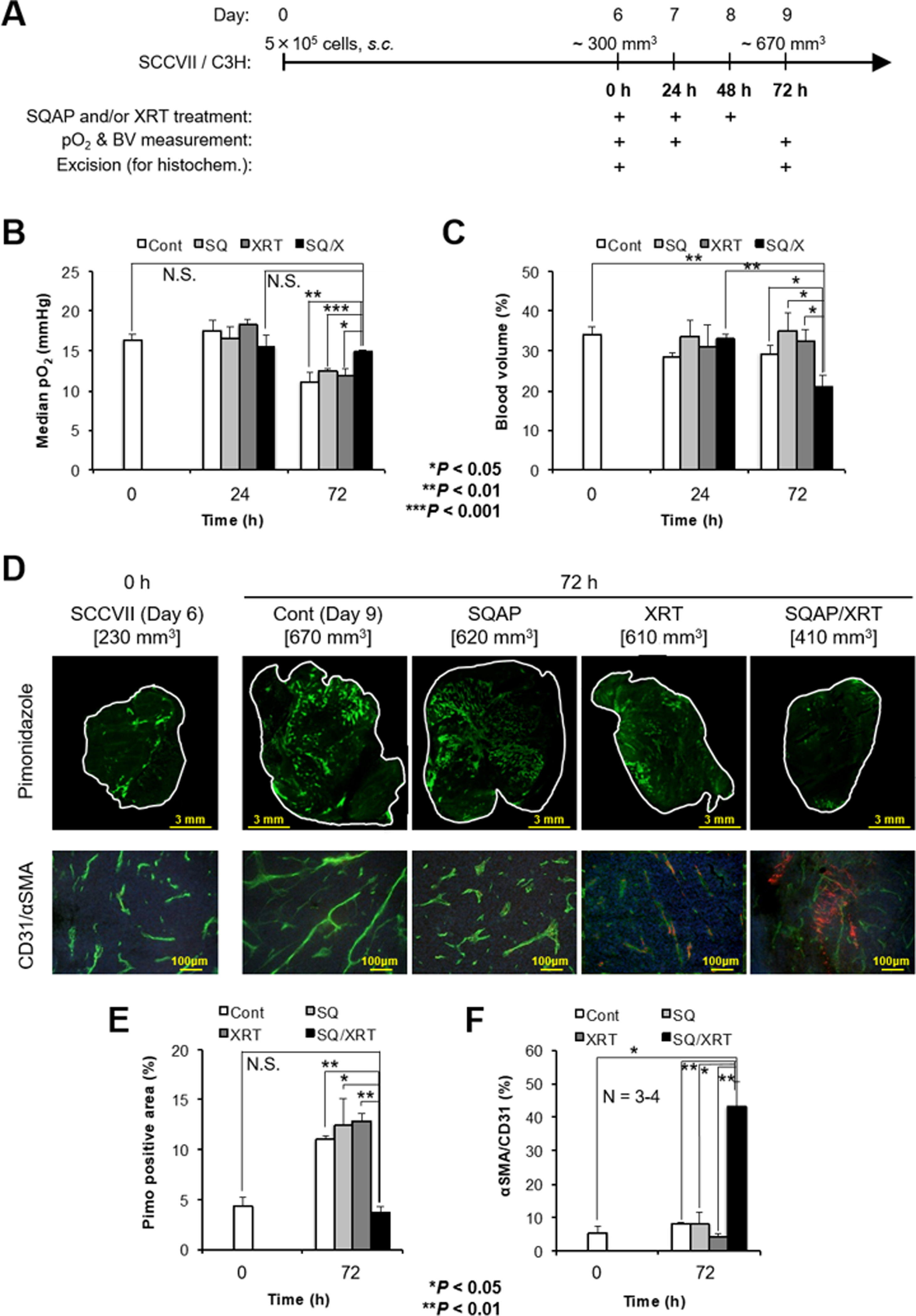
Monitoring of physiological properties in the tumors before and after 3 days of daily SQAP combination therapy with XRT. A, Schematic representation of the experiments. The 3 days (0, 24, and 48 h) of daily treatment with SQAP and/or XRT started at day 6, with a SCCVII tumor size of ~300 mm3. B, C, Noninvasive monitoring of tumor median pO2 (B) and blood volume (BV) changes (C) in SCCVII tumors before (0 h), and 24 or 72 h during or after the three treatments. B, The tumor median pO2 (mmHg) obtained from the EPRI pO2 map. C, BV (%) changes calculated using MRI with the blood pooling T2 contrast agent USPIO. Each pO2 and BV value was calculated from three 2 mm thick center images from each tumor (Supplementary data Figure S4). D–F, Immunohistochemical analysis of tumor slices before (0 h) or after (72 h) the 3 days of daily treatment. A dose of 60 mg/kg pimonidazole was intravenously injected as a hypoxic marker, and mice were excised 45–60 min after injection. Anti-CD31 antibody was used as a marker of vascular endothelial cells in the pathological tumor vasculature. Anti-α-SMA antibody was used as a marker for pericytes. E, Quantitative measurement of the pimonidazole-positive area (%) in whole center slices before (0 h) or after (72 h) three treatments. F, The ratio of α-SMA/CD31 expression (%) as a normalization index. Data are means ± SE (n = 3). *, P < 0.05, **, P < 0.01.
Histochemical analysis of SCCVII slices (days 6 and 9) further supported the in vivo observation. As shown in Fig. 5D, the pimonidazole-positive area (green in the upper panels), which indicates the hypoxic region, was 4.5% of the SCCVII tumor slice size (230 mm3) on day 6, with this increasing to 10% by day 9 (~670 mm3) in the non-treated SCCVII tumors (Fig. 5E). Although neither SQAP nor XRT monotherapy inhibited the formation of a hypoxic region, the combination therapy of XRT with SQAP significantly suppressed hypoxia onset, with the 4.5% pimonidazole-positive area at day 9 (410 mm3) covering the same tumor proportion as on day 6 (Fig. 5E). The therapeutic benefit of the combination therapy was also observed in the tumor blood vessel formation. Figure 5D shows the CD31-positive area in SCCVII slices (green in lower panels, CD31 is a vascular endothelial cell marker) observed at day 6 or 9. Although the density of CD31 did not change with 3 days of daily SQAP or XRT monotherapy, the CD31-positive area was significantly reduced by the SQAP + XRT combination therapy, similar to the effect observed using sunitinib (6). This result suggests that the combination therapy of SQAP + XRT was effective in vascular re-normalization (2,6). Further studies with α-SMA staining to quantify pericyte coverage support the conclusion of a vascular re-normalization window after 3 days of daily SQAP + XRT therapy. The CD31/α-SMA ratio, considered to be a normalization index for tumor vasculature, significantly increased 72 h after the 3 days of daily treatment (Fig. 5F). Thus, these results suggest that the combination of SQAP with radiotherapy significantly suppressed tumor hypoxia formation, thereby improving normalization of the tumor vasculature. These observations are in agreement with those on the combination therapy of α-SQMG with XRT (16).
Discussion
The radiation response of a tumor is governed by multiple factors. As novel radiosensitizing agents are evaluated in pre-clinical models, factors that influence the tumor microenvironment such as the physiologic and metabolic profile are examined. Several strategies were evaluated to overcome the radiation resistance imposed by tumor hypoxia, including oxygen treatment to increase tumor oxygenation and oxygen mimetics such as the electron affinic hypoxic radiosensitizers (9,33–37). In addition to these strategies, HAPs have been developed to target hypoxic regions in tumors (38). The mechanisms of radiosensitization with these agents have been attributed to vascular re-normalization, which is a transient phenomenon where increased tumor pO2 may be detected, with this being caused by pruning of immature neovasculature during a post-treatment time window, resulting in improved tumor vessel function and delivery of oxygen and nutrients (2,6,39). Radiotherapy during this temporal window resulted in enhanced tumor control (8).
Compounds from the SQAG class were found to have anti-angiogenic effects when tested using in vivo models, although they had no cytotoxic effects in vitro. They also displayed in vivo radiosensitizing effects, but no such sensitizing effects in the same cell lines in vitro. In this study, the tumor-radiosensitizing effect induced by α-SQAP was shown to be specific to in vivo tumors, but not in tumor cells in vitro (Fig. 1B–E). The mechanism underlying the in vivo-specific tumor radiosensitization by SQAP was explored using several molecular imaging techniques.
Multimodal imaging studies revealed that SQAP can transiently increase tumor oxygenation in murine SCCVII/C3H and human A549/nude mice xenografts immediately after intravenous administration. As this phenomenon took place in a short period of time, it may be minimally related to vascular normalization. This assumption was supported by PA imaging experiments, where decreased sO2 accompanied by increased HbT was observed in SQAP-treated tumors (Fig. 3B). This result suggests that the therapeutic effect is not just a hemodynamic change caused by vascular disrupting agents, as such agents are reported to induce decreased HbT (32). Figure 5D and Supplementary data Figure S4 also show that emergence of the hypoxic tumor fraction detected by the pimonidazole-positive area (Fig. 5D, upper) and visible on the EPR pO2 image (Supplementary data Figure S4, middle) was significantly delayed by the 3 days of daily SQAP/XRT treatments. Vascular normalization was not observed for at least 3 days after treatment when SQAP was used as a monotherapy (Fig. 5D, lower). The opposite effects on local sO2 and pO2 imply an enhancement of oxygen dissociation from hemoglobin in regions where an increase in oxygen release from hemoglobin (sO2 decrease) and an increase in BV are involved in a rapid increase in tumor pO2 immediately after injection of SQAP. The dose-dependent decrease in sO2 observed in Fig. 3C implies that a higher dose of SQAP may be more beneficial when combined with radiotherapy, because of enhanced oxygen release. The median pO2 reached a maximum 20–30 min after the administration (Fig. 2C, D), which explains why the combined use of SQAP and XRT is effective for suppression of SCCVII tumor growth when the XRT is applied 30 min after administration of SQAP, but not after 60 or 120 min (Fig. 1D).
Importantly, tumor pO2 elevation by SQAP did not depend on tumor cell type or the host mouse strains. The biological effect directly resulted in tumor radiosensitization in vivo, and was attributed to hemoglobin allosteric ligand binding causing a release of O2 from oxyhemoglobin and increasing tumor pO2 (40–43). Such biological effects have not been reported for sulfoglycolipids. Further experiments will be required to clarify the molecular mechanism of this compound’s action.
Previously, Ohta et al. reported that α-SQMG showed remodeling of tumor tissues when used in combination therapy with radiation (16). Sakimoto et al. demonstrated that the combination of α-SQMG and radiation suppressed tumor angiogenesis, promoting vascular endothelial cells to adopt a senescence-like phenotype (18). In this study, an increase in tumor oxygen level and suppression of pathological microvascularization was observed after 3 days of daily treatment with SQAP + radiation. This was accompanied by an increase in α-SMA, a marker for vascular normalization by improved pericyte coverage (2,16,24). Taking these reports into consideration, the in vivo-specific radiosensitizing effect of sulfoglycolipid can be attributed to improved tumor oxygenation through the release of oxygen from hemoglobin, with the resultant damage in vascular endothelial cells also leading to a less hypoxic tumor microenvironment.
Given the significant benefit of the SQAP/XRT combination therapy without any observable side effects, the transient increase in tumor oxygenation caused by the low dose of sulfoglycolipid could become an important part of the mechanism for creating an in vivo-specific radiosensitizing effect. While EPRI and PA imaging will be useful in pre-clinical drug discovery research for quantitatively evaluating agents that may alter tumor physiology, DCE-MRI is the only technique that can be used clinically to monitor perfusion changes.
Supplementary Material
Acknowledgments
We thank Mr. Andrew Heinmiller (Fuji VisualSonics) and Daryl Despres (Mouse Imaging Facility, NMR Research Center, NIH) for technical assistance with the PA system.
Financial support: This research was supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, NIH. (Grant number 1ZIABC010476-15).
Disclosure of potential conflicts of interest
Masahiro Ishima, Hiroshi Murata, and Keisuke Ohta are employed by Toyo Suisan Kaisha Ltd. CG-0321 was received from Toyo Suisan Kaisha Ltd., through a materials transfer agreement with NIH.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182–6 [DOI] [PubMed] [Google Scholar]
- 2.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005;307:58–62 [DOI] [PubMed] [Google Scholar]
- 3.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res 1998;58:1408–16 [PubMed] [Google Scholar]
- 4.Takakusagi Y, Kishimoto S, Naz S, Matsumoto S, Saito K, Hart CP, et al. Radiotherapy Synergizes with the Hypoxia-Activated Prodrug Evofosfamide: In Vitro and In Vivo Studies. Antioxid Redox Signal 2018;28:131–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takakusagi Y, Matsumoto S, Saito K, Matsuo M, Kishimoto S, Wojtkowiak JW, et al. Pyruvate induces transient tumor hypoxia by enhancing mitochondrial oxygen consumption and potentiates the anti-tumor effect of a hypoxia-activated prodrug TH-302. PLoS One 2014;9:e107995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto S, Saito K, Takakusagi Y, Matsuo M, Munasinghe JP, Morris HD, et al. In vivo imaging of tumor physiological, metabolic, and redox changes in response to the anti-angiogenic agent sunitinib: longitudinal assessment to identify transient vascular renormalization. Antioxid Redox Signal 2014;21:1145–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasui H, Matsumoto S, Devasahayam N, Munasinghe JP, Choudhuri R, Saito K, et al. Low-field magnetic resonance imaging to visualize chronic and cycling hypoxia in tumor-bearing mice. Cancer Res 2010;70:6427–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diepart C, Karroum O, Magat J, Feron O, Verrax J, Calderon PB, et al. Arsenic trioxide treatment decreases the oxygen consumption rate of tumor cells and radiosensitizes solid tumors. Cancer Res 2012;72:482–90 [DOI] [PubMed] [Google Scholar]
- 9.Hallac RR, Zhou H, Pidikiti R, Song K, Stojadinovic S, Zhao D, et al. Correlations of noninvasive BOLD and TOLD MRI with pO2 and relevance to tumor radiation response. Magn Reson Med 2014;71:1863–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizushina Y, Watanabe I, Ohta K, Takemura M, Sahara H, Takahashi N, et al. Studies on inhibitors of mammalian DNA polymerase alpha and beta: sulfolipids from a pteridophyte, Athyrium niponicum. Biochem Pharmacol 1998;55:537–41 [DOI] [PubMed] [Google Scholar]
- 11.Benson AA, Daniel H, Wiser R. A Sulfolipid in Plants. Proc Natl Acad Sci U S A 1959;45:1582–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahara H, Ishikawa M, Takahashi N, Ohtani S, Sato N, Gasa S, et al. In vivo anti-tumour effect of 3’-sulphonoquinovosyl 1’-monoacylglyceride isolated from sea urchin (Strongylocentrotus intermedius) intestine. Br J Cancer 1997;75:324–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohta K, Mizushina Y, Hirata N, Takemura M, Sugawara F, Matsukage A, et al. Sulfoquinovosyldiacylglycerol, KM043, a new potent inhibitor of eukaryotic DNA polymerases and HIV-reverse transcriptase type 1 from a marine red alga, Gigartina tenella. Chem Pharm Bull (Tokyo) 1998;46:684–6 [DOI] [PubMed] [Google Scholar]
- 14.Ohta K, Mizushina Y, Hirata N, Takemura M, Sugawara F, Matsukage A, et al. Action of a new mammalian DNA polymerase inhibitor, sulfoquinovosyldiacylglycerol. Biol Pharm Bull 1999;22:111–6 [DOI] [PubMed] [Google Scholar]
- 15.Takakusagi K, Takakusagi Y, Ohta K, Aoki S, Sugawara F, Sakaguchi K. A sulfoglycolipid beta-sulfoquinovosyldiacylglycerol (betaSQDG) binds to Met1-Arg95 region of murine DNA polymerase lambda (Mmpol lambda) and inhibits its nuclear transit. Protein Eng Des Sel 2010;23:51–60 [DOI] [PubMed] [Google Scholar]
- 16.Ohta K, Murata H, Mori Y, Ishima M, Sugawara F, Sakaguchi K, et al. Remodeling of the tumor microenvironment by combined treatment with a novel radiosensitizer, {alpha}-sulfoquinovosylmonoacylglycerol ({alpha}-SQMG) and X-irradiation. Anticancer Res 2010;30:4397–404 [PubMed] [Google Scholar]
- 17.Miura M, Sakimoto I, Ohta K, Sugawara F, Sakaguchi K. Sulfoglycolipids as candidate antiangiogenic radiosensitizers. Anti-cancer drugs 2007;18:1–5 [DOI] [PubMed] [Google Scholar]
- 18.Sakimoto I, Ohta K, Yamazaki T, Ohtani S, Sahara H, Sugawara F, et al. Alpha-sulfoquinovosylmonoacylglycerol is a novel potent radiosensitizer targeting tumor angiogenesis. Cancer Res 2006;66:2287–95 [DOI] [PubMed] [Google Scholar]
- 19.Matsuki K, Tanabe A, Hongo A, Sugawara F, Sakaguchi K, Takahashi N, et al. Anti-angiogenesis effect of 3’-sulfoquinovosyl-1’-monoacylglycerol via upregulation of thrombospondin 1. Cancer Sci 2012;103:1546–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori Y, Sahara H, Matsumoto K, Takahashi N, Yamazaki T, Ohta K, et al. Downregulation of Tie2 gene by a novel antitumor sulfolipid, 3’-sulfoquinovosyl-1’-monoacylglycerol, targeting angiogenesis. Cancer Sci 2008;99:1063–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takakusagi Y, Takakusagi K, Ida N, Takami M, Matsumoto Y, Kusayanagi T, et al. Binding region and interaction properties of sulfoquinovosylacylglycerol (SQAG) with human vascular endothelial growth factor 165 revealed by biosensor-based assays. Medchemcomm 2011;2:1188–93 [Google Scholar]
- 22.Aoki S, Ohta K, Yamazaki T, Sugawara F, Sakaguchi K. Mammalian mitotic centromere-associated kinesin (MCAK): a new molecular target of sulfoquinovosylacylglycerols novel antitumor and immunosuppressive agents. FEBS J 2005;272:2132–40 [DOI] [PubMed] [Google Scholar]
- 23.Hanashima S, Mizushina Y, Yamazaki T, Ohta K, Takahashi S, Sahara H, et al. Synthesis of sulfoquinovosylacylglycerols, inhibitors of eukaryotic DNA polymerase alpha and beta. Bioorg Med Chem 2001;9:367–76 [DOI] [PubMed] [Google Scholar]
- 24.Sawada Y, Omoto K, Kohei N, Sakaguchi K, Miura M, Tanabe K. Sulfoquinovosylacylpropanediol is a novel potent radiosensitizer in prostate cancer. Int J Urol 2015;22:590–5 [DOI] [PubMed] [Google Scholar]
- 25.Izaguirre-Carbonell J, Kawakubo H, Murata H, Tanabe A, Takeuchi T, Kusayanagi T, et al. Novel anticancer agent, SQAP, binds to focal adhesion kinase and modulates its activity. Sci Rep 2015;5:15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dey S, Kumari S, Kalainayakan SP, Campbell J 3rd, Ghosh, Zhou H, et al. The vascular disrupting agent combretastatin A-4 phosphate causes prolonged elevation of proteins involved in heme flux and function in resistant tumor cells. Oncotarget 2018;9:4090–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNally LR, Mezera M, Morgan DE, Frederick PJ, Yang ES, Eltoum IE, et al. Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology. Clin Cancer Res 2016;22:3432–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rich LJ, Seshadri M. Photoacoustic imaging of vascular hemodynamics: validation with blood oxygenation level-dependent MR imaging. Radiology 2015;275:110–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Research council. Guide for the care and use of laboratory animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- 30.Matsumoto S, Hyodo F, Subramanian S, Devasahayam N, Munasinghe J, Hyodo E, et al. Low-field paramagnetic resonance imaging of tumor oxygenation and glycolytic activity in mice. J Clin Invest 2008;118:1965–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Needles A, Heinmiller A, Sun J, Theodoropoulos C, Bates D, Hirson D, et al. Development and initial application of a fully integrated photoacoustic micro-ultrasound system. IEEE Trans Ultrason Ferroelectr Freq Control 2013;60:888–97 [DOI] [PubMed] [Google Scholar]
- 32.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953;26:638–48 [DOI] [PubMed] [Google Scholar]
- 33.Saunders M, Dische S. Clinical results of hypoxic cell radiosensitisation from hyperbaric oxygen to accelerated radiotherapy, carbogen and nicotinamide. Br J Cancer Suppl 1996;27:S271–8 [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell JB, Wink DA, Degraff W, Gamson J, Keefer LK, Krishna MC. Hypoxic Mammalian-Cell Radiosensitization by Nitric-Oxide. Cancer Res 1993;53:5845–8 [PubMed] [Google Scholar]
- 35.Hall EJ, Miller R, Astro M, Rini F. The nitroimidazoles as radiosensitizers and cytotoxic agents. Br J Cancer Suppl 1978;3:120–3 [PMC free article] [PubMed] [Google Scholar]
- 36.Adams GE. Chemical radiosensitization of hypoxic cells. Br Med Bull 1973;29:48–53 [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues LM, Howe FA, Griffiths JR, Robinson SP. Tumor R2* is a prognostic indicator of acute radiotherapeutic response in rodent tumors. J Magn Reson Imaging 2004;19:482–8 [DOI] [PubMed] [Google Scholar]
- 38.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11:393–410 [DOI] [PubMed] [Google Scholar]
- 39.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 2014;26:605–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol (R Coll Radiol) 2007;19:397–417 [DOI] [PubMed] [Google Scholar]
- 41.Song CW, Hasegawa T, Kwon HC, Lyons JC, Levitt SH. Increase in tumor oxygenation and radiosensitivity caused by pentoxifylline. Radiat Res 1992;130:205–10 [PubMed] [Google Scholar]
- 42.Hirst DG, Wood PJ. The influence of haemoglobin affinity for oxygen on tumour radiosensitivity. Br J Cancer 1987;55:487–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siemann DW, Macler LM. Tumor radiosensitization through reductions in hemoglobin affinity. Int J Radiat Oncol Biol Phys 1986;12:1295–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


