Abstract
Uric acid is an end product of purine metabolism in humans. An unusual and still unexplained phenomenon is that higher primates have relatively high uric acid levels in body fluids due to a combination of absence of degradation, and renal retention. The physiologic purpose of high uric acid levels is still enigmatic but the pathobiologic burden is a variety of crystallopathies due to the low aqueous solubility of uric acid such as gouty arthritis and acute uric acid nephropathy. In the urinary space, three distinct conditions result from chronic uric acid and/or urate precipitation. The first and most common variety is uric acid urolithiasis. In this condition, urate is a victim of a systemic metabolic disease where increased acid load to the kidney is coupled with diminished urinary buffer capacity due to defective ammonium excretion, resulting in titration of urate to its sparingly soluble protonated counterpart, uric acid, and the formation of stones. Uric acid is the innocent bystander of the crime. The second variety is hyperuricosuric calcium urolithiasis, where uric acid confers lithogenicity via promotion of calcium oxalate precipitation by multiple mechanisms involving soluble, colloidal, and crystalline urate salts. Uric acid is the instigator of the crime. The third and least common condition involves urate as an integral part of the urolith as an ammonium salt driven by high ammonium and high urate concentrations in urine. Here, uric acid is one of the perpetrator of the crime. Both known and postulated pathogenesis of these three types of urolithiasis are reviewed and summarized.
Keywords: kidney stones, uric acid urolithiasis, hyperuricosuric, calcium, ammonium urate
URIC ACID BIOLOGY AND PHYSICAL CHEMISTRY
Uric acid biology
Uric acid (UA) was discovered by the Swedish chemist-apothecary Carl Wilhelm Scheele from a urolith, which he aptly termed lithic acid that was subsequently renamed uric acid. The biology of UA is covered extensively in other reviews1–3 and will only be briefly highlighted here. UA is a key intermediate in purine metabolism without which there will be no deoxyribonucleic acid and no life. In humans, uric acid is a cul-de-sac metabolic end product because of the absence of uricase to further degrade it to allantoin and allantoic acid1,4. UA is one of the three nitrogen-bearing compounds to sustain excretion of nitrogenous waste; the other two being ammonia and urea. The use of uric acid to excrete nitrogen (termed uricotelism) mandates high uric acid concentrations in birds and reptiles5. Mammals who use urea to excrete nitrogen (termed ureotylism) have low UA levels. A peculiar exception are higher primates who are ureotelics but paradoxically have UA levels that are much higher than all other mammals1,2 (Figure 1A).
Figure 1: Uric acid biology.
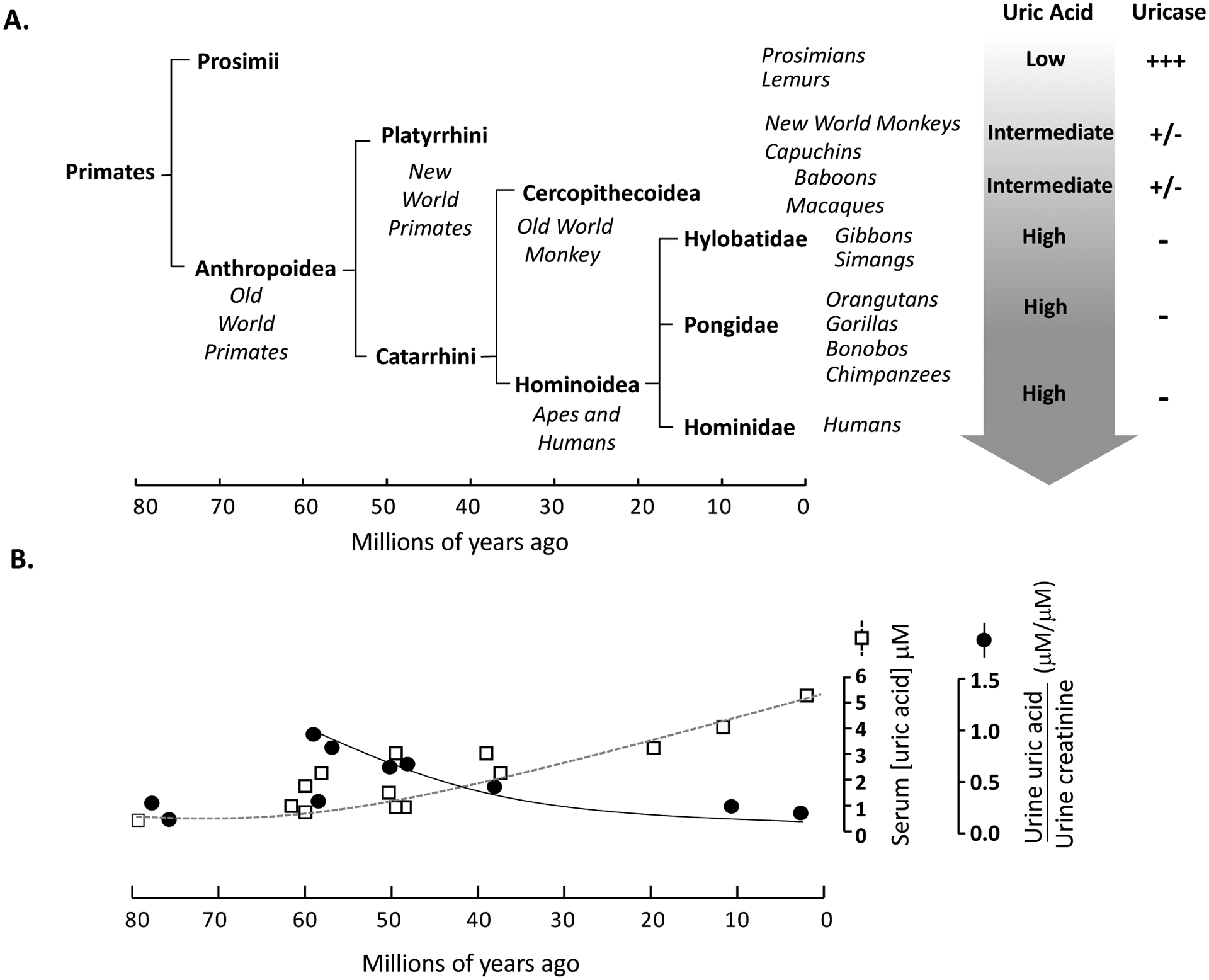
A. History of primate order evolution over 80 million years and subdivision into families. Progressive rise in uric acid levels parallels the loss of uricase activity from prosimians to humans. B. The rise in serum uric acid concentration is compared to urine uric acid-to-creatinine concentration; fractional excretion of urate was only available in very few species.
The increase in UA levels is in part due to silencing of the uricase gene in higher primates due to multiple cumulative inactivating mutations in both the coding and non-coding regions6. If these were accidental events, the organism would have evolved compensatory mechanisms to counter or at least minimize these stochastic events. The lower levels of xanthine oxidase, which catalyzes the formation of UA, may represent some form of compensation7. However, the changes in renal handling during the period of progressive silencing of uricase was exactly the opposite of what one expects for stochastic accidental inactivation of the uricase gene. Urinary excretion gradually decreased as UA degradation decreased (Figure 1B). The most plausible explanation is that the reduced metabolism and reduced excretion of UA were synergistic to achieve higher UA levels in body fluids.
While these changes are unequivocal, the purpose of having higher UA levels in higher primates remains enigmatic. Several theories abound attempting to speculate on the biologic advantage of but none has been proven or universally accepted. The low solubility of UA constitutes one distinct advantage for uricotelics as the excretion of UA in solid form negates the need for aqueous solution thus contributes to water conservation. There is clearly no need for this property in ureotelic organisms such as humans. The low solubility of uric acid ends up as the root of the crystallopathies in humans-, which are gouty arthritis, acute UA nephropathy, and several types of UA-containing urolithiasis.
Uric acid chemistry
UA is a weak acid with one dissociable H+ at physiologic pH ranges and a second one at supraphysiologic pH ranges (Figure 2A). UA and urate have vastly different solubilities. UA solubility in a urinary environment is limited to about 96 mg/L. Since excretion in humans typically exceed 600 mg/day in approximately 1–2L of urine, the risk of UA precipitation is omnipresent8. With a pKa of 5.35 at 37°C9, UA solubility is determined primarily by pH in that acidic urine (pH ≤5.5) titrates urate to UA which is sparingly soluble and hence precipitates10–13 (Figure 2B). Generally, human urine is metastably supersaturated with respect to UA. This suggests the presence of an inhibitor and the inadequacy or absence of an inhibitor may influence the propensity for UA nephrolithiasis. There is experimental evidence supporting presence of macromolecules, which enhance UA solubility and attenuate UA crystal adherence to renal tubular epithelium14.
Figure 2: Uric acid chemistry.

A. Uric acid exists in two keto-enol tautomers with the clinically relevant tautomers being the lactim (enol) form. There are two dissociable H+ giving rise to acid urate and urate (often just called urate) with pKa1 =5.4 and pKa2 = 9.8; the former being relevant in mammalian urine with the latter not present in human physiologic states. Under extreme conditions of UpH (e.g. urea-splitting organism in the urinary tract), some divalent urate may be present. Monovalent urate can pair with ambient cations (Cat+). B. Relative solubilities of uric acid and three urate salts as function of pH.
While more soluble than UA, urate (Ur) solubility is finite and is dependent on the prevailing and accompanying cation, with potassium having the highest, ammonium the lowest solubility, and sodium in between. (Figure 2B). The concentration of cations in normal human urine under most circumstances is highest for sodium, followed by potassium, and then ammonium.
URIC ACID UROLITHIASIS
In this first example, UA plays a passive role in lithogenesis even though it is the main and sometimes only component of the stone. It is the example par excellence of the “tip-of-the-iceberg” phenomenon where the UA stone is just a sentinel of much larger systemic derangements, which deserve attention. Hence, the approach to evaluation and therapy should be multi-organ in nature, and are not limited to stone removal and empirical alkalinization of urine.
Epidemiology
There is a long history of associations between insulin resistance, features of the metabolic syndrome with UA urolithiasis and low urine pH15,16; more recent findings are highlighted. An Italian study showed higher levels of serum glucose and triglycerides and an independent Chinese report found higher triglyceride levels in UA acid stone formers17,18. The inverse relationship between body mass index and 24-hour urine pH reported by multiple investigators18–24 was recently expanded to pediatric stone formers22. In adult stone formers from a single center, hyperglycemia correlated with lower pH and higher urinary UA saturation25. Greater abdominal visceral fat based in CT scan is associated with lower urine pH and greater uric acid stone risk26.
The incidence of UA nephrolithiasis is increasing globally, likely paralleling the increasing prevalence of the metabolic syndrome and diabetes. In one large center in the USA, the fraction of UA relative to total stone formers increased from 7% to 14% over the past three decades27. Another U.S. study reported that UA accounts for 12% of >4,300 urinary stones from seven states28. In a Norwegian surgical cohort, UA stones comprised of 9% of >1,200 stones from 2014–2017 compared to 2% 40 years ago29. UA, struvite, and brushite stone formers have among the highest risks of recurrence30.
Pathogenesis
Despite the often uniformity of its composition (i.e. UA), UA stones is a disease of urine pH, and not UA. The low UpH in UA stone formers was noted for decades31–33. This low pH was found to be due to increased acid load to the kidney and a concomitant inadequacy of buffering by the open buffer ammonia (NH3) to ammonium (NH4+)11,34. Unduly acidic urine is a feature in obesity, the metabolic syndrome, and diabetes15,16,19,20,35. The aciduria is constant throughout the 24-hour period leading to increase UA saturation around the clock36,37. The reduced ammonia buffering is present not only at the steady state, but also upon acute acid challenge in UA stone formers38. Part of the reasons for the impaired ammoniagenesis and excretion is steatosis and lipotoxicity of the kidney and in particular the proximal tubule39–41 (Figure 3).
Figure 3: Proposed multi-organ model of uric acid stone formation driven by aciduria.
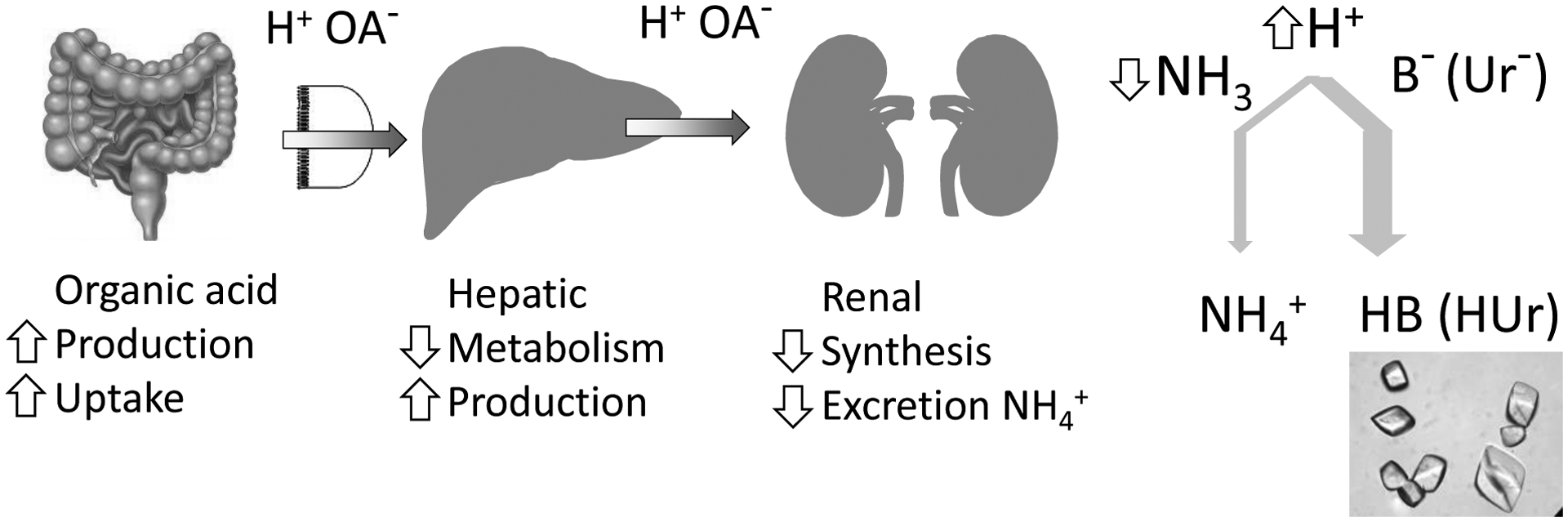
Organic (H+; OA− organic anion) acid load due to increased production and uptake by gut lumen exacerbated by decreased hepatic metabolism and increased production. This poses an increased acid load to the kidney, which is capable of excreting the acid. However, impairment of ammonia synthesis and ammonium excretion mandates carriage of H+ by alternative buffers (B); one of which is urate (Ur−) which when protonated, forms the insoluble uric acid.
The impaired buffering by the kidney is compounded by an increased acid load to the kidney, which likely originates from the gastrointestinal tract and liver (Figure 3)42. Hepatic steatosis almost invariably accompanies renal steatosis in rodent model of metabolic syndrome and aciduria40,43 and in humans, hepatic fat content on CT scan is inversely associated with 24-hour urine pH26.
Treatment
The cornerstone of treatment of UA stones have been alkali therapy since the first description by Pak and colleagues in 198631,44–46. The pathophysiologic basis of this approach is to neutralize the excess acid presented to the kidney (Figure 3), and also overcome the impaired buffering capacity by empirically raising urine pH. While this therapy does not target the fundamental systemic defects of UA urolithiasis, it is very effective; akin to treating primary hypertension with drugs that lower blood pressure without getting at the root cause of the hypertension, but nonetheless reduces cardiovascular risk. Low acid-high alkali dietary therapy has been shown to alleviate the biochemical risk but reduction in clinical events has not been shown47.
A recent study took the therapy one-step closer to the underlying etiology. Maalouf and coworkers treated UA stone formers with a thiazolidinedione, which is a peroxisome proliferator-activated receptor gamma (PPARγ) agonist that has multiple action including amelioration of steatosis in organs. After 24 weeks of therapy, UA patients exhibited higher UpH and higher fraction of net acid excretion being represented by ammonium, indicating a relief of the renal lesion48. However, an impressive and most revealing finding is the decrease in net acid excretion indicating that the high acid load presented to the kidney was reduced (Figure 3), most likely via alleviation of lipotoxicity in the liver and perhaps the intestine.
Conclusion
UA acid stone is the prime example of the “tip of the iceberg” where the stone disease provide a window to glimpse into deep-seated systemic metabolic disturbance. The utilitarian efficacy of alkali therapy is pragmatic, but it has unintentionally decreased enthusiasm and effort towards addressing and treating the underlying root of the problem. The practitioner needs to adopt the philosophy that every UA stone former deserves a multi-organ interrogation and management. Therapy of UA stones should also progress beyond empiric alkali therapy.
HYPERURICOSURIC CALCIUM UROLITHIASIS
Coe and then Pak independently described hyperuricouric calcium urolithiasis (HUCU) where the principal underlying pathogenic factor is hyperuricosuria but the stone is primarily calcium oxalate49. The pathogenesis and therapy of this condition is quite different from the other causes of calcium oxalate urolithiasis and deserves separate consideration.
Epidemiology
Mixed calcium oxalate/UA stones were noted as far back as in 189350 and many reports followed. Gutman and Yü ventured to propose pathogenicity where calcium oxalate may act as nidus for mixed CaOx/UA stones51. Coe and coworkers noted in 420 consecutive calcium stone formers that 15% have isolated hyperuricosuria and 12% have combined hyperuricosuria and hypercalciuria52 amounting to 27% of calcium stone formers having hyperuricosuria. Interestingly, the source of hyperuricouria seemed to be largely dietary-originated which is not that different from the hyperuricosuria of non-stone formers53.
Some have emphasized the inability to observe an association between urine UA excretion rate and risk of being a stone former and questioned the existence of HUCU54,55. Coe and coworkers unequivocally demonstrated that only subgroups of stone formers, those with isolated hyperuricosuria or hyperuricosuria in conjunction with hypercalciuria, respond to UA lowering drugs56. Given that hyperuricosuria is common, the strength of hyperuricosuria as a risk factor for stone formation in the general population may not be easy to demonstrate because only a small fraction of patients is actually true HUCU sufferers.
Pathogenesis
Since calcium oxalate stones are common and so is asymptomatic (clinically insignificant) hyperuricosuria, one may question whether these two conditions are in fact causally related. A very compelling evidence is the fact that treatment with xanthine oxidase inhibitor and other urate lowering agents decreased rate of stone recurrence in selected patients with calcium stones and hyperuricosuria56–59. Several physicochemical models have been described to collaboratively contribute to formation of calcium stones under hyperuricosuria (Figure 2)60–65.
“Salting out” is a process where certain solutes are less soluble at either very low or very high ambient concentrations due to the existence of an optimal window of hydration shell. This mechanism for salting out calcium oxalate was proposed and experimentally supported63,64. Patients with hypercalciuria and hyperoxaluria are more vulnerable to stone formation with increase in concentration of dissolved urate64.
Urine contains inhibitors of CaOx crystallization66. Pak et al. suggested that the colloidal form of urate could have promoted nucleation of CaOx by removing inhibitors of CaOx nucleation from urine62. The state of a solute in aqueous medium can be classified according to the size of the solute- solution <10−9 m; colloid 10−6 - 10−9 m; suspension >10−6 m (Figure 3). Sodium (Na) Ur in urine can exist in colloidal form and may remove mucopolysaccharides that inhibit crystal aggregation of CaOx65,67 but there is to date no direct experimental evidence that colloidal NaUr absorbs inhibitor has been observed.
The crystalline phase of Ur proposed that epitaxy accounts for HUCU60,61 acting via epitaxy which is the process by which one type of crystal growing upon another type of crystalline surface68 (Figure 4). Coe et al. demonstrated sodium urate can be a “seed” for nucleation of CaOx precipitation at pH 5.760 (Figure 4). Pak et al. showed NaUr can cause heterogeneous nucleation of CaOx at pH 5.7 and 6.7, and of calcium phosphate at pH 5.3, 5.7, and 6.7 from metastably supersaturated solutions in vitro61. Urine can exist with supersaturation of NaUr, and in supersaturated urine samples from patients with hyperuricosuria and calcium stones, NaUr can serve as seeds for the heterogeneous nucleation of calcium oxalate and phosphate crystals62,69. In contrast, seeds of UA had very small or no effect in both studies60,61. In vivo human studies showed that oral purine load was associated with an increased saturation of NaUr and purine deprivation and/or allopurinol therapy was found to decrease saturation with respect to NaUr70.
Figure 4: Pathophysiology of huperuricosuric calcium urolithiasis.
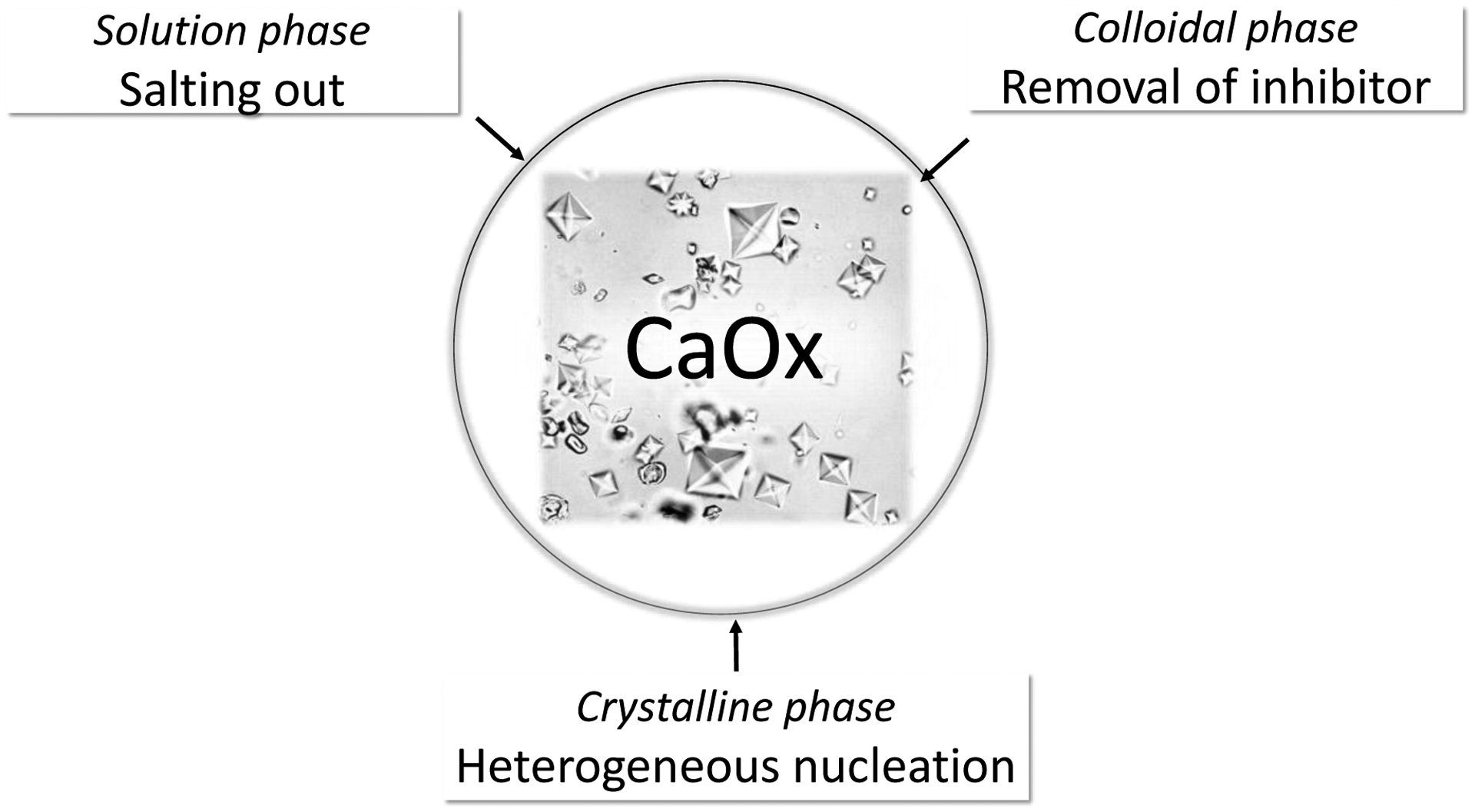
In the solution phase, sodium urate is a potent salting out agent that can increase the activity product of calcium oxalate. In the colloidal phase, sodium urate can adsorb and remove inhibitors of calcium oxalate crystallization. Finally, sodium urate crystals can initiate calcium oxalate crystallization via heterogeneous nucleation or epitaxy.
Treatment
A strong support for HUCU rests on interventional studies with UA lowering agents in calcium oxalate stone formers despite some caveats in a number of these studies including the small sample sizes, short duration, usage of historical controls, and lack of standard treatment protocols. Nonetheless, in totality, they constitute a compelling body of literature. Coe and Raisen examined calcium stone formers with no metabolic abnormalities other than hyperuricosuria, hyperuricemia, or both. Both hyperuricemia and hyperuricosuria responded well to xanthine oxidase inhibition and stone events were nearly obliterated after starting therapy57. A prospective randomized placebo-controlled did not report metabolic characterization of the patients, and the regimen was an unusual one that combined allopurinol with urinary alkalinization to pH > 6.5 with NaHCO3, but nonetheless reported reduction of stone events58. Coe did a more extensive analysis of >200 CaOx stone-formers with idiopathic hypercalciuria or hyperuricosuria, or both treated for about 2.9 years56. Hypercalciuria was treated with thiazides, hyperuricosuria was treated with allopurinol, and when neither was present, fluid was prescribed. The reduction in stone events was no less than dramatic highlighting the importance of pathophysiology-directed therapy56. In a randomized prospective randomized placebo-controlled trial in 60 calcium stone patients with hyperuricosuria but normocalciuria, there were clear benefits of xanthine oxidase inhibition59
Conclusion
In HUCU, one sees stones with mixed calcium oxalate and UA in the clear presence of hyperuricosuria and absence or very mild degrees of other risk factors for calcium oxalate stones such as hypercalciuria, hypocitraturia, hyperoxaluria, and low urine volume. If hyperuricosuria is the sole abnormality or if correction of other risk factors by conventional therapy fails, treatment of the hyperuricosuria improves clinical outcomes.
AMMONIUM ACID URATE STONES
The least known and least often encountered urate-containing stone is ammonium acid urate (AAU). While urate is a bystander in UA stones and an instigator or co-conspirator in HUCU, it is actually an integral part of the crystallization in AAU stones. Compared to the other UA/Ur-containing stones, AAU stone is unique for its epidemiology and physicochemical properties.
Epidemiology
Regarding its epidemiology, the terms “sporadic” and “endemic” are used to describe the occurrence of AAU in industrialized countries and developing countries respectively71. In industrialized nations, AAU stones are relatively uncommon72,73.
As societies become more economically advanced and industrialized, the prevalence of AAU stones actually decreases. In Norwich, England, in the eighteenth and nineteenth century, more than a third of stones contained AAU. By early twentieth century in the same region, this decreased to 2.5%73. In general, studies have not shown a consistent gender predominance.
A single-center Canadian study reported a prevalence of 0.2% with none being pure AAU stones. In most cases, AAU made up less than 10% of the total stone composition74. Soble and colleagues analyzed more than 3000 stones from the Cleveland Clinic and reported AAU in 0.3% of stones and again no pure AAU stones were found71. A more recent analysis from a Mayo Clinic cohort found a slightly higher prevalence of 0.9% with pure AUU representing 19% of the AAU-containing stones75. There are other differences between these cohorts. In the Canadian study, the predominant stones that coexisted with AAU were uric acid, struvite, calcium phosphate and calcium oxalate. In the Mayo cohort however, coexistent stones were struvite, calcium phosphate and uric acid in decreasing order of occurrence. Similar to the Canadian study, the Cleveland Clinic study also reported uric acid stone as the most predominant coexisting stone.
In Japan, uric acid, calcium phosphate, mixed uric acid-calcium phosphate, and struvite (in decreasing frequency) are the coexisting stones76. Whilst this apparent discordance may reflect different populations and periods covered in the studies, another reason might be the varied definitions used to categorize stone predominance.
The most dramatic difference is that developing countries suffer much more AAU stones which has been attributed to socioeconomic factors, like diet, hygiene and urinary tract infections72. Children in developing countries have a much higher prevalence of AAU stones. An analysis of 103 stones from Iran showed nearly a quarter of the stones from a mostly adult cohort contained AAU77. In a subgroup analysis of the stones in children, 48% of the stones were AAU. This trend of a high burden of AAU stones in children has been replicated in other Asian Countries, including India, Pakistan, Indonesia and the Uyghur region of China. However, studies from Africa, especially regions south of the Sahara have generally failed to show a high burden of AAU78–80.
It has been suggested that dietary differences between sub-Sahara Africa that tends to be maize based compared to the Asian countries where rice predominates may explain the reported low prevalence in the former. North Africa that has historic and socioeconomic differences from the Sub-Saharan region has a higher burden of AAU stones especially in children81,82. Perhaps, rather than there being a true dichotomy in the burden of AAU between northern Africa and regions south of the Sahara, under sampling of cases may explain the differences. For instance, review of stones from Ghana and Nigeria did not report AAU; Niger and Cameroon, countries in the same subregion however showed a high burden of AAU79,83–85.
Pathophysiology
Sodium and potassium urate salts are much more soluble than AAU (Figure 2). Typically, these electrolytes are present in urine in higher concentration than ammonium and thus more likely to form salt with urate. This begs the question why urate forms salt with ammonium86.
Empirical and experimental evidence provide clues on the conditions, which promote formation of AAU (Figure 5). High concentrations of both urate and ammonium are required to facilitate AAU87. A diet rich in purines satisfies the former condition. AAU is commonly found in Dalmatian dogs, a breed with congenital defect in uric acid transport and hyperuricosuria88. Bottle nose dolphins in captivity fed a diet high in purine and acidogenic protein showed post-prandial urine supersaturated with urate and ammonium respectively89.
Figure 5: Model for pathogenesis of ammonium acid urate stones.
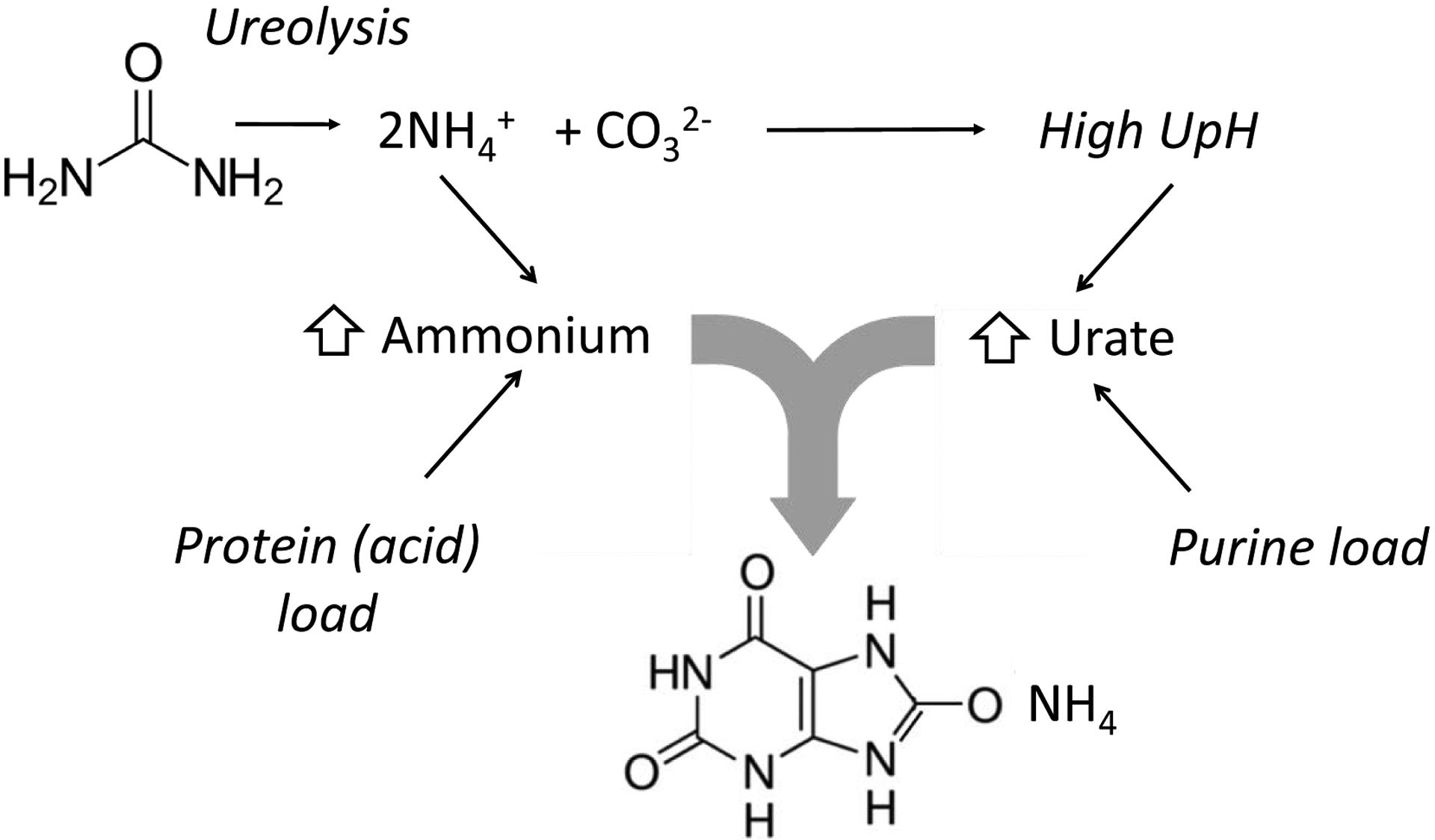
Contributors to high urine ammonium include physiologic response to acid load and intraluminal generation of ammonium from urea. One of the byproduct of ureolysis is HCO3−/CO32−, which also alkalinizes the urine. Increased endogenous or exogenous purine load and metabolism leads to increased uric acid generation, and the high urine pH (UpH) ensures that urate form is preferred.
In humans, two sources are proposed to explain the increased ammonium. UTI may lead to the elevation of ammonium through ureolysis by bacterial urease (Figure 5). Half of UAA stone formers from Taiwan had documented UTI and mostly from urease-positive organisms90. In the US, up to a third of cases AAU stones may be associated with UTI71–75. It is important to note that UTI is not a factor in some cases and in instances where UTI coexist, the organisms may not be urease-positive91.
The classic explanation for AAU in endemic countries is a cereal-based diet deficient in inorganic phosphate depletes urine of a major buffer resulting in up-regulation of ammoniagenesis and hyperammoniauria91. Compared to controls, AAU formers in endemic countries have low phosphate in their urine72. Upregulation in ammoniagenesis sets AAU stone formers apart from uric acid stone formers in whom a defect in ammoniagenesis has been implicated92.
The pH necessary to promote the formation of AAU is lower than the pH for struvite formation. In vitro experiment suggests that at pH <5.7 uric acid predominates over urate with urate occurring at higher pH93. Teotia et al. took urine of a non-stone former and found that AAU precipitated at pH between 6–7.588. The ionic strength of urine may also influence the formation of AAU with urine of low ionic strength appearing to favor the precipitation of AAU94. This may explain the association of diarrheal diseases with both sporadic and endemic stones.
In endemic countries environmental factors including a hot humid climate, diarrheal diseases in children, urinary tract infection and a diet of purine rich but poor in inorganic phosphate, may explain the occurrence of AAU. Since sporadic cases occur infrequently, studies attempting to describe them have tended to be small. Soble and colleagues studied 44 AAU stone formers and found an association with inflammatory bowel disease, laxative abuse, morbid obesity and recurrent urinary tract infection71, Kuruma et al. described a phenotype of thin young females in Japan with pure AAU distinct from overweight middle aged men who had the mixed type of AAU76. Associations were also found with lower serum potassium and protein suggesting possible gastrointestinal losses from laxative abuse and poor nutrition respectively. There have been case reports of AAU in patients with anorexia nervosa, chronic kidney disease, and prostate surgery. Similar to the situation in developing countries, gastrointestinal losses from inflammatory bowel disease and laxative abuse would result in smaller urine volumes and intracellular acidosis resulting in high urinary ammonium95. A study of 9 females with AAU stones showed low urine volumes, elevated urinary ammonium and low urine sodium, potassium and citrate96.
Treatment
Acute management of AAU is similar to the general management of stone disease. Preventing AAU stone formation requires treatment of the underlying predisposing conditions. This includes the modification of diet, fluid intake, treatment of UTI and stopping laxatives in relevant situations. Because occurrence of AAU depends on the urine pH, European guidelines recommend urine acidification with L-methionine97,98. This must be done cautiously because acidic pH may promote formation of uric acid stones. Allopurinol to decrease uric acid levels has also been proposed97,98.
Conclusion
AAU stones are a rare occurrence in economically advanced countries like the US. They may however, occur in patients with risk factors which promote a low ionic strength of urine, ammoniagenesis and supersaturation with urate. Recognition of the unique factors that promote AAU formation is important in preventing stone recurrence and guiding treatment.
EPILOGUE
While the purpose of maintaining high UA/Ur levels in human is still enigmatic, we certainly pay a price for the yet-to-be defined benefit(s). The low solubility of UA may be useful for excreting nitrogen in solid phase for uricotelics or serve as “danger sentinel signal” molecules in tissue damage to call for action, this physicochemical property is driving a variety of human crystallopathies. Using criminological analogy as in the urinary space, we visualize UA/Ur as an innocent bystander in UA acid stones where aciduria is the primary culprit, as an instigator of calcium oxalate precipitation in HUCU, and finally as part of guilty perpetrator, where Ur is actually part of the AAU stones. These are three conditions with completely different pathophysiology hat requires targeted evaluation and therapy. Therefore, simply labelling a kidney stone as a “uric acid stone” is not an adequate term in medical parlance.
Table 1:
Risk Factors for ammonium acid urate stones
Acknowledgement
The authors are supported by the National Institutes of Health (R01 DK081423, R01 DK115703, R01 DK091392, DK092461, R21HL145424), the O’Brien Kidney Research Center (P30 DK-079328), the Charles and Jane Pak Center of Mineral Metabolism and Clinical Research Endowed Professor Collaborative Research Support and Innovative Research Program. We are grateful to Ms. Yesenia Aguirre for her expertise and assistance in preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
The authors declare that they have no conflict of interest.
References
- 1.Moe OW. Uric acid nephrolithiasis: proton titration of an essential molecule? Curr Opin Nephrol Hypertens. 2006;15(4):366–373. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Lario B, Macarron-Vicente J. Uric acid and evolution. Rheumatology (Oxford). 2010;49(11):2010–2015. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RJ, Lanaspa MA, Gaucher EA. Uric acid: a danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: evolutionary considerations. Semin Nephrol. 2011;31(5):394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werner AK, Witte CP. The biochemistry of nitrogen mobilization: purine ring catabolism. Trends Plant Sci. 2011;16(7):381–387. [DOI] [PubMed] [Google Scholar]
- 5.Long S, Skadhauge E. The role of urinary precipitates in the excretion of electrolytes and urate in the domestic fowl. J Exp Biol. 1983;104:41–50. [DOI] [PubMed] [Google Scholar]
- 6.Kratzer JT, Lanaspa MA, Murphy MN, et al. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc Natl Acad Sci U S A. 2014;111(10):3763–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurosaki M, Bolis M, Fratelli M, et al. Structure and evolution of vertebrate aldehyde oxidases: from gene duplication to gene suppression. Cell Mol Life Sci. 2013;70(10):1807–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367(9507):333–344. [DOI] [PubMed] [Google Scholar]
- 9.B F, Smith A. Stability of first dissociable proton of uric acid. Journal of Chemical and Engineering Data. 1974;19(1):94–97. [Google Scholar]
- 10.Sakhaee K, Nicar M, Hill K, Pak CY. Contrasting effects of potassium citrate and sodium citrate therapies on urinary chemistries and crystallization of stone-forming salts. Kidney Int. 1983;24(3):348–352. [DOI] [PubMed] [Google Scholar]
- 11.Moe OW, Abate N, Sakhaee K. Pathophysiology of uric acid nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31(4):895–914. [DOI] [PubMed] [Google Scholar]
- 12.Sakhaee K Recent advances in the pathophysiology of nephrolithiasis. Kidney Int. 2009;75(6):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riese RJ, Sakhaee K. Uric acid nephrolithiasis: pathogenesis and treatment. J Urol. 1992;148(3):765–771. [DOI] [PubMed] [Google Scholar]
- 14.Koka RM, Huang E, Lieske JC. Adhesion of uric acid crystals to the surface of renal epithelial cells. Am J Physiol Renal Physiol. 2000;278(6):F989–998. [DOI] [PubMed] [Google Scholar]
- 15.Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K. Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17(5):1422–1428. [DOI] [PubMed] [Google Scholar]
- 16.Abate N, Chandalia M, Cabo-Chan AV Jr., Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney international. 2004;65(2):386–392. [DOI] [PubMed] [Google Scholar]
- 17.Trinchieri A, Croppi E, Simonelli G, Sciorio C, Montanari E. Anthropometric variables, physical activity and dietary intakes of patients with uric acid nephrolithiasis. Urolithiasis. 2020;48(2):123–129. [DOI] [PubMed] [Google Scholar]
- 18.Ding Q, Ouyang J, Fan B, et al. Association between Dyslipidemia and Nephrolithiasis Risk in a Chinese Population. Urol Int. 2019;103(2):156–165. [DOI] [PubMed] [Google Scholar]
- 19.Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CY. Association of urinary pH with body weight in nephrolithiasis. Kidney international. 2004;65(4):1422–1425. [DOI] [PubMed] [Google Scholar]
- 20.Pigna F, Sakhaee K, Adams-Huet B, Maalouf NM. Body fat content and distribution and urinary risk factors for nephrolithiasis. Clin J Am Soc Nephrol. 2014;9(1):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trinchieri A, Montanari E. Biochemical and dietary factors of uric acid stone formation. Urolithiasis. 2018;46(2):167–172. [DOI] [PubMed] [Google Scholar]
- 22.Murphy MO, Erpelding SG, Chishti AS, Dugan A, Ziada A, Kiessling SG. Influence of BMI in nephrolithiasis in an Appalachian pediatric population: A single-center experience. J Pediatr Urol. 2018;14(4):330.e331–330.e338. [DOI] [PubMed] [Google Scholar]
- 23.Otto BJ, Bozorgmehri S, Kuo J, Canales M, Bird VG, Canales B. Age, Body Mass Index, and Gender Predict 24-Hour Urine Parameters in Recurrent Idiopathic Calcium Oxalate Stone Formers. J Endourol. 2017;31(12):1335–1341. [DOI] [PubMed] [Google Scholar]
- 24.Wood K, Boyd C, Whitaker D, et al. Impact of Demographic Factors and Systemic Disease on Urinary Stone Risk Parameters Amongst Stone Formers. Reviews in urology. 2019;21(4):158–165. [PMC free article] [PubMed] [Google Scholar]
- 25.Maciolek KA, Penniston KL, Jhagroo RA, Best SL. Successful Diabetic Control as Measured by Hemoglobin A1c Is Associated with Lower Urine Risk Factors for Uric Acid Calculi. J Endourol. 2018;32(8):771–776. [DOI] [PubMed] [Google Scholar]
- 26.Patel ND, Ward RD, Calle J, Remer EM, Monga M. Computerized Tomography Based Diagnosis of Visceral Obesity and Hepatic Steatosis is Associated with Low Urine pH. J Urol. 2017;198(5):1085–1090. [DOI] [PubMed] [Google Scholar]
- 27.Xu LHR, Adams-Huet B, Poindexter JR, Maalouf NM, Moe OW, Sakhaee K. Temporal Changes in Kidney Stone Composition and in Risk Factors Predisposing to Stone Formation. J Urol. 2017;197(6):1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant C, Guzman G, Stainback RP, Amdur RL, Mufarrij P. Variation in Kidney Stone Composition Within the United States. J Endourol. 2018;32(10):973–977. [DOI] [PubMed] [Google Scholar]
- 29.Kravdal G, Helgo D, Moe MK. Kidney stone compositions and frequencies in a Norwegian population. Scand J Urol. 2019;53(2–3):139–144. [DOI] [PubMed] [Google Scholar]
- 30.Singh P, Enders FT, Vaughan LE, et al. Stone Composition Among First-Time Symptomatic Kidney Stone Formers in the Community. Mayo Clin Proc. 2015;90(10):1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pak CY, Sakhaee K, Fuller C. Successful management of uric acid nephrolithiasis with potassium citrate. Kidney international. 1986;30(3):422–428. [DOI] [PubMed] [Google Scholar]
- 32.Khatchadourian J, Preminger GM, Whitson PA, Adams-Huet B, Pak CY. Clinical and biochemical presentation of gouty diathesis: comparison of uric acid versus pure calcium stone formation. J Urol. 1995;154(5):1665–1669. [DOI] [PubMed] [Google Scholar]
- 33.Pak CY, Sakhaee K, Peterson RD, Poindexter JR, Frawley WH. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int. 2001;60(2):757–761. [DOI] [PubMed] [Google Scholar]
- 34.Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62(3):971–979. [DOI] [PubMed] [Google Scholar]
- 35.Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol. 2010;5(7):1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron MA, Baker LA, Maalouf NM, Moe OW, Sakhaee K. Circadian variation in urine pH and uric acid nephrolithiasis risk. Nephrol Dial Transplant. 2007;22(8):2375–2378. [DOI] [PubMed] [Google Scholar]
- 37.Cameron M, Maalouf NM, Poindexter J, Adams-Huet B, Sakhaee K, Moe OW. The diurnal variation in urine acidification differs between normal individuals and uric acid stone formers. Kidney Int. 2012;81(11):1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bobulescu IA, Maalouf NM, Capolongo G, et al. Renal ammonium excretion after an acute acid load: blunted response in uric acid stone formers but not in patients with type 2 diabetes. Am J Physiol Renal Physiol. 2013;305(10):F1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294(6):F1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW. Reduction of renal triglyceride accumulation: effects on proximal tubule Na+/H+ exchange and urinary acidification. Am J Physiol Renal Physiol. 2009;297(5):F1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bobulescu IA, Lotan Y, Zhang J, et al. Triglycerides in the human kidney cortex: relationship with body size. PLoS One. 2014;9(8):e101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bobulescu IA, Park SK, Xu LHR, et al. Net Acid Excretion and Urinary Organic Anions in Idiopathic Uric Acid Nephrolithiasis. Clin J Am Soc Nephrol. 2019;14(3):411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong F, Zhou X, Xu J, Gao L. Rodent Models of Nonalcoholic Fatty Liver Disease. Digestion. 2019:1–14. [DOI] [PubMed] [Google Scholar]
- 44.Rodman JS. Prophylaxis of uric acid stones with alternate day doses of alkaline potassium salts. J Urol. 1991;145(1):97–99. [DOI] [PubMed] [Google Scholar]
- 45.Rodman JS. Intermittent versus continuous alkaline therapy for uric acid stones and ureteral stones of uncertain composition. Urology. 2002;60(3):378–382. [DOI] [PubMed] [Google Scholar]
- 46.Moe OW, Pearle MS, Sakhaee K. Pharmacotherapy of urolithiasis: evidence from clinical trials. Kidney Int. 2011;79(4):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siener R, Hesse A. The effect of a vegetarian and different omnivorous diets on urinary risk factors for uric acid stone formation. Eur J Nutr. 2003;42(6):332–337. [DOI] [PubMed] [Google Scholar]
- 48.Maalouf NM, Poindexter JR, Adams-Huet B, Moe OW, Sakhaee K. Increased production and reduced urinary buffering of acid in uric acid stone formers is ameliorated by pioglitazone. Kidney Int. 2019;95(5):1262–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moe OW, Xu LHR. Hyperuricosuric calcium urolithiasis. J Nephrol. 2018;31(2):189–196. [DOI] [PubMed] [Google Scholar]
- 50.JC G, DE O. Sir Henry Thompson 1820–1904: Scientist, Artist, Motorist, Gourmet, Traveller, Cremationist and Subspecialist Urologist. 2004;11:91–105. [Google Scholar]
- 51.Gutman AB, Yu TF. Uric acid nephrolithiasis. Am J Med. 1968;45(5):756–779. [DOI] [PubMed] [Google Scholar]
- 52.Coe FL. Hyperuricosuric calcium oxalate nephrolithiasis. Kidney Int. 1978;13(5):418–426. [DOI] [PubMed] [Google Scholar]
- 53.Coe FL, Kavalach AG. Hypercalciuria and hyperuricosuria in patients with calcium nephrolithiasis. N Engl J Med. 1974;291(25):1344–1350. [DOI] [PubMed] [Google Scholar]
- 54.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 2008;73(4):489–496. [DOI] [PubMed] [Google Scholar]
- 55.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59(6):2290–2298. [DOI] [PubMed] [Google Scholar]
- 56.Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med. 1977;87(4):404–410. [DOI] [PubMed] [Google Scholar]
- 57.Coe FL, Raisen L. Allopurinol treatment of uric-acid disorders in calcium-stone formers. Lancet. 1973;1(7795):129–131. [DOI] [PubMed] [Google Scholar]
- 58.Smith MJ. Placebo versus allopurinol for renal calculi. J Urol. 1977;117(6):690–692. [DOI] [PubMed] [Google Scholar]
- 59.Ettinger B, Tang A, Citron JT, Livermore B, Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med. 1986;315(22):1386–1389. [DOI] [PubMed] [Google Scholar]
- 60.Coe FL, Lawton RL, Goldstein RB, Tembe V. Sodium urate accelerates precipitation of calcium oxalate in vitro. Proc Soc Exp Biol Med. 1975;149(4):926–929. [DOI] [PubMed] [Google Scholar]
- 61.Pak CY, Arnold LH. Heterogeneous nucleation of calcium oxalate by seeds of monosodium urate. Proc Soc Exp Biol Med. 1975;149(4):930–932. [DOI] [PubMed] [Google Scholar]
- 62.Pak CY, Waters O, Arnold L, Holt K, Cox C, Barilla D. Mechanism for calcium urolithiasis among patients with hyperuricosuria: supersaturation of urine with respect to monosodium urate. The Journal of clinical investigation. 1977;59(3):426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grover PK, Ryall RL. The effect of preincubation of seed crystals of uric acid and monosodium urate with undiluted human urine to induce precipitation of calcium oxalate in vitro : implications for urinary stone formation. Mol Med. 2002;8(9):525–535. [PMC free article] [PubMed] [Google Scholar]
- 64.Grover PK, Marshall VR, Ryall RL. Dissolved urate salts out calcium oxalate in undiluted human urine in vitro: implications for calcium oxalate stone genesis. Chem Biol. 2003;10(3):271–278. [DOI] [PubMed] [Google Scholar]
- 65.H F, Robertson, Smith WG, Vahlensieck LH. Physical Chemical Aspects of Calcium Stone-Formation in the Urinary Tract. Urolithiasis Research. Boston, MA; 1976. [Google Scholar]
- 66.Ryall RL. Urinary inhibitors of calcium oxalate crystallization and their potential role in stone formation. World J Urol. 1997;15(3):155–164. [DOI] [PubMed] [Google Scholar]
- 67.H F, Robertson, Smith WG, Vahlensieck LH. Urinary Acid Mucopolysaccharide Inhibitors of Calcium Oxalate Crystallisation. In: US: URS, ed.1976:331–334. [Google Scholar]
- 68.Royer L. Expermimental research on parellel growth or mutual orientation of crytals of different species. Bull Soc Fran Mineral. 1928(51):7–159. [Google Scholar]
- 69.Pak CY, Hayashi Y, Arnold LH. Heterogeneous nucleation with urate, calcium phosphate and calcium oxalate. Proc Soc Exp Biol Med. 1976;153(1):83–87. [DOI] [PubMed] [Google Scholar]
- 70.Pak CY, Barilla DE, Holt K, Brinkley L, Tolentino R, Zerwekh JE. Effect of oral purine load and allopurinol on the crystallization of calcium salts in urine of patients with hyperuricosuric calcium urolithiasis. Am J Med. 1978;65(4):593–599. [DOI] [PubMed] [Google Scholar]
- 71.Soble JJ, Hamilton BD, Streem SB. Ammonium acid urate calculi: a reevaluation of risk factors. J Urol. 1999;161(3):869–873. [DOI] [PubMed] [Google Scholar]
- 72.Klohn M, Bolle JF, Reverdin NP, Susini A, Baud CA, Graber P. Ammonium urate urinary stones. Urological research. 1986;14(6):315–318. [DOI] [PubMed] [Google Scholar]
- 73.Lonsdale K, Mason P. Uric acid, uric acid dihydrate, and urates in urinary calculi, ancient and modern. Science (New York, NY). 1966;152(3728):1511–1512. [DOI] [PubMed] [Google Scholar]
- 74.Pichette V, Bonnardeaux A, Cardinal J, et al. Ammonium acid urate crystal formation in adult North American stone-formers. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1997;30(2):237–242. [DOI] [PubMed] [Google Scholar]
- 75.Lomas DJ, Jaeger CD, Krambeck AE. Profile of the Ammonium Acid Urate Stone Former Based on a Large Contemporary Cohort. Urology. 2017;102:43–47. [DOI] [PubMed] [Google Scholar]
- 76.Kuruma H, Arakawa T, Kubo S, et al. Ammonium acid urate urolithiasis in Japan. International journal of urology : official journal of the Japanese Urological Association. 2006;13(5):498–501. [DOI] [PubMed] [Google Scholar]
- 77.Minon Cifuentes J, Pourmand G. Mineral composition of 103 stones from Iran. British journal of urology. 1983;55(5):465–468. [DOI] [PubMed] [Google Scholar]
- 78.Kambal A, Wahab EM, Khattab AH. The composition of urinary stones in the Sudan. British journal of urology. 1979;51(5):342–344. [DOI] [PubMed] [Google Scholar]
- 79.Mbonu O, Attah C, Ikeakor I. Urolithiasis in an African population. International urology and nephrology. 1984;16(4):291–296. [DOI] [PubMed] [Google Scholar]
- 80.Wathigo FK, Hayombe A, Maina D. Urolithiasis analysis in a multiethnic population at a tertiary hospital in Nairobi, Kenya. BMC research notes. 2017;10(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harrache D, Mesri A, Addou A, Semmoud A, Lacour B, Daudon M. [Urolithiasis in children in West Algeria]. Annales d’urologie. 1997;31(2):84–88. [PubMed] [Google Scholar]
- 82.Aggour A, Ziada AM, AbdelHamid AZ, AbdelRahman S, Morsi A. Metabolic stone composition in Egyptian children. J Pediatr Urol. 2009;5(2):132–135. [DOI] [PubMed] [Google Scholar]
- 83.Klufio GO, Bentsi IK, Yeboah ED, Quartey JK. Upper urinary tract stones in Accra, Ghana. West African journal of medicine. 1996;15(3):173–176. [PubMed] [Google Scholar]
- 84.Angwafo FF 3rd, Daudon M, Wonkam A, Kuwong PM, Kropp KA. Pediatric urolithiasis in sub-saharan Africa: a comparative study in two regions of Cameroon. European urology. 2000;37(1):106–111. [DOI] [PubMed] [Google Scholar]
- 85.Vanwaeyenbergh J, Vergauwe D, Verbeeck RM. Infrared spectrometric analysis of endemic bladder stones in Niger. European urology. 1995;27(2):154–159. [DOI] [PubMed] [Google Scholar]
- 86.Geng X, Meegan J, Smith C, Sakhaee K, Rimer JD. Crystallization of Hierarchical Ammonium Urate: Insight into the Formation of Cetacean Renal Stones. Crystal Growth & Design. 2019;19(11):6727–6735. [Google Scholar]
- 87.Grases F, Villacampa AI, Costa-Bauza A. Ammonium and sodium urates precipitating from synthetic urine and fine structure of urate renal calculi. Urological research. 1999;27(2):141–147. [DOI] [PubMed] [Google Scholar]
- 88.Teotia M, Sutor DJ. Crystallisation of ammonium acid urate and other uric acid derivatives from urine. British journal of urology. 1971;43(4):381–386. [DOI] [PubMed] [Google Scholar]
- 89.Smith CR, Poindexter JR, Meegan JM, et al. Pathophysiological and physicochemical basis of ammonium urate stone formation in dolphins. J Urol. 2014;192(1):260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chou YH, Huang CN, Li WM, et al. Clinical study of ammonium acid urate urolithiasis. The Kaohsiung journal of medical sciences. 2012;28(5):259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thalut K, Rizal A, Brockis JG, Bowyer RC, Taylor TA, Wisniewski ZS. The endemic bladder stones of Indonesia---epidemiology and clinical features. British journal of urology. 1976;48(7):617–621. [DOI] [PubMed] [Google Scholar]
- 92.Sakhaee K. Epidemiology and clinical pathophysiology of uric acid kidney stones. J Nephrol. 2014;27(3):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bowyer RC, McCulloch RK, Brockis JG, Ryan GD. Factors affecting the solubility of ammonium acid urate. Clinica chimica acta; international journal of clinical chemistry. 1979;95(1):17–22. [DOI] [PubMed] [Google Scholar]
- 94.Bowyer RC, Brockis JG, McCulloch RK. The role of common urinary constituents in the precipitation of ammonium acid urate. Clinica chimica acta; international journal of clinical chemistry. 1979;99(3):221–227. [DOI] [PubMed] [Google Scholar]
- 95.Wang Z, Königsberger L, Königsberger E. Solubility equilibria in the uric acid–ammonium urate–water system. Monatshefte für Chemie - Chemical Monthly. 2018;149(2):327–332. [Google Scholar]
- 96.Dick WH, Lingeman JE, Preminger GM, Smith LH, Wilson DM, Shirrell WL. Laxative abuse as a cause for ammonium urate renal calculi. J Urol. 1990;143(2):244–247. [DOI] [PubMed] [Google Scholar]
- 97.Türk C; Neisius A; Petřík A et al. EAU guidelines on urolithiasis (2017 ed). Available at: https://uroweb.org/wp-content/uploads/Guidelines_WebVersion_Complete-1.pdf Accessed (accessed on 12-15-2019).
- 98.Türk C; Neisius A; Petřík A; et al. EAU guidelines on urolithiasis (2018 ed). Available at: https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urolithiasis-2018-large-text.pdf Accessed (accessed on 12-15-2019).


