Abstract
The electromechanical function of the heart involves complex, coordinated activity over time and space. Life-threatening cardiac arrhythmias arise from asynchrony in these space–time events; therefore, therapies for prevention and treatment require fundamental understanding and the ability to visualize, perturb and control cardiac activity. Optogenetics combines optical and molecular biology (genetic) approaches for light-enabled sensing and actuation of electrical activity with unprecedented spatiotemporal resolution and parallelism. 2020 marks a decade of developments in cardiac optogenetics since this technology was adopted from neuroscience and applied to the heart. In this Review, we appraise a decade of advancements that define near-term (immediate) translation based on all-optical electrophysiology, including high-throughput screening, cardiotoxicity testing and personalized medicine assays, and long-term (aspirational) prospects for clinical translation of cardiac optogenetics, including new optical therapies for rhythm control. The main translational opportunities and challenges for optogenetics to be fully embraced in cardiology are also discussed.
ToC blurb
In this Review, Entcheva and Kay discuss a decade of important developments and applications of optogenetics to the heart, focusing on near-term and longer-term clinical translation of this technology in cardiology.
Introduction
The year 2020 marks a decade of developments in cardiac optogenetics since the publication of the first studies that adopted this technology from neuroscience and applied it to the heart to optically control its function1-4 (Fig. 1). Optogenetics uses light-sensitive proteins, which are genetically expressed in the cells or tissues of interest, and optical components for contactless control and monitoring of biological function in a highly precise, spatiotemporal manner. To date, no other approach (electrical, mechanical, chemical or non-optogenetic optical approaches) has matched the combination of space–time resolution, specificity and richness of control afforded by optogenetic tools (Fig. 2a).
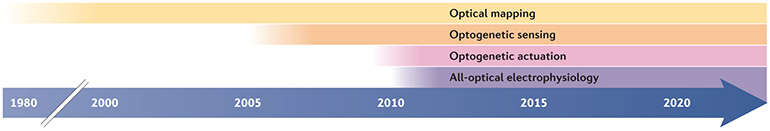
Fig. 1 ∣. Timeline of optical tools applied to the heart.
Beginning with optical mapping over 40 years ago, optical tools have been used extensively for cardiac research. In the past decade, optogenetics enabled the combination of optical sensing and optical actuation for the development of all-optical cardiac electrophysiology systems. See Table 1 for specific applications of cardiac optogenetics, including translational aspects, with the relevant publications since 2010, including Review articles.
Fig. 2 ∣. Advantages of all-optical electrophysiology and the optogenetic toolkit.
a ∣ Advantages of optogenetics and all-optical electrophysiology in enabling cardiac applications through spectral compatibility of optogenetic actuators and optogenetic sensors. Unique features include inherent parallelism, scalability, capacity for long-term monitoring of function, bidirectional multimodal imaging and perturbation of cardiac function, and closed-loop feedback control of electrical events and wave parameters, as well as cell-specific and organelle-specific targeting with high spatiotemporal resolution. Taken together, optogenetic technology is superior to electrical and chemical methods for actuation and sensing. b ∣ All-optical electrophysiology draws upon an extensive toolkit of optogenetic actuators of voltage, including depolarizing (excitatory) opsins (such as channelrhodopsin 2 (ChR2), CheRiff, Crimson and ReaChR) and hyperpolarizing (inhibitory) opsins (such as BLINK-1, PAC-K, GtACR1, archaerhodopsin T (ArchT), halorhodopsin (Halo) and Jaws) that are activatable across a wide band of wavelengths. Relevant optogenetic sensors include genetically encoded voltage indicators (GEVIs), such as VSFP2.3, ArcLight, ASAP, Voltron525, FlicR1, Quasars, Archon1 and near-infrared (NIR)-Butterfly, and genetically encoded calcium indicators (GECIs), such as GCaMPs, R-CaMPs, R-GECOs and NIR-GECO, with peak excitation wavelengths ranging from 450 nm to 660 nm. These spectral properties and biophysical performance have enabled various combinations of actuators and sensors to be deployed in cardiac research.
Cardiac optogenetics emerged after 30 years of research using optical tools to track cardiac arrhythmias, starting with early optical mapping in the 1970s5,6, and following in the footsteps of neuroscientists who first utilized this technique to control neuronal activity. Cardiologists have embraced the power of optical mapping, which enables improved mechanistic understanding of cardiac electrical disturbances with unprecedented space–time resolution, to test hypotheses about arrhythmia development and termination as well as to inform the development of new device technologies and treatments. Optogenetic sensing with genetically encoded calcium indicators (GECIs) was a natural progression for cardiac optical mapping, with early adoption (before 2010) for long-term monitoring of conduction abnormalities in transgenic mice and for tracking the integration of transplanted cells for cardiac repair in animal models7-9. The genetic aspect of these optical measurements empowered cell-specific probing in the heart and overcame the typically acute nature of traditional optical-mapping experiments with small-molecule dyes. Around the time of publication of the studies using GECIs (2006), the term optogenetics was coined10, after the first demonstrated utility of fast-kinetics optogenetic actuators to perturb neural activity and brain function11-13 on the basis of the newly discovered, light-sensitive ion channels from green algae14,15.
The broadest utility of using light to perturb and to monitor biological processes is captured in a brief review16 and early work by Miesenbock and colleagues17,18. These ideas (the combination of optogenetic actuators and sensors for control of function) are also pertinent to cardiac research, particularly for developing new strategies to control cardiac arrhythmias, which are complex and dynamic life-threatening, space–time events. Fig. 1 and Table 1 summarize the timeline of experimental and computational studies that have contributed to various aspects of cardiac optogenetics, including reviews19-51. This Review reflects on a decade of important developments and applications of optogenetics to the heart, focusing on both near-term (immediate) translation and longer-term (aspirational) clinical translation of this technology in cardiology.
Table 1 ∣.
References for key aspects of cardiac optogenetics
| Aspect of cardiac optogenetics |
2010– 2011 |
2012– 2013 |
2014–2015 | 2016–2017 | 2018–2019 | 2020 |
|---|---|---|---|---|---|---|
| All-optical cardiac electrophysiology | 1,4 | 120 | 106,111,121-128 | 60,129-135 | 136-142 | 48,62,63,110,143,144 |
| All-optical electrophysiology: high-throughput drug screening | – | – | – | 60,61,129,133 | 65,70,140 | 62,63,164 |
| Applications to advancement of induced pluripotent stem cell-derived cardiomyocytes | – | 153 | 64,72,73,154,155 | 60,61,129,132,133, 156-159 | 70,71,113,138,140, 160-163 | 62,63,144,164 |
| Cell-specific control: neuro-cardiology | – | 212 | 127 | 210,211,213,215 | 142,203,204 | 51,110 |
| Cell-specific control: other | 1,4 | 146 | 124,214 | 39,74,132,180,182, 216 | 191,217,218 | 143,181 |
| Computational cardiac optogenetics | 150 | 102,145,146 | 103,107,108 | 147,148 | 149 | 143 |
| Optogenetic sensing of cardiac activitya | – | – | 59,64,72,73,75,83,154,197,220 | 60,61,74,133,157 | 70,71,86,138 | 62,63 |
| Optical cardiac pacing | 1-4 | 102,153 | 91,103,106,124-126,128,170-173 | 60,61,129,132,133,135,156-159,174,175 | 141,161-163,176 | 62,63,110,144,178 |
| Optical cardioversion–defibrillation, ablation | – | – | 111,121,122,128,197,198 | 96,130,131,147,174,190,199 | 95,112,136,137,149,179,191,194-196 | 48,114,189 |
| Optical control of cardiac ion channels, G-protein-coupled receptor signalling, energetics | – | 116 | 117 | 115,119 | 65,84,118 | – |
| Optical control of excitation waves | – | – | 111 | 131,190,199,200 | 112,137,138 | 48,110,143,181 |
| Optical control of action potential shape | – | – | 103,106,108 | – | 113 | 143 |
| Optical closed-loop feedback control | – | – | 111,126 | 29,190,199 | 112,113,137,139,191 | 48,114 |
| Towards an optical voltage clamp | – | – | 107 | – | 33,65,113 | 114 |
| Towards low-energy optical pacing and defibrillation | 4 | 102,146 | 103,124,173 | 131 | 112,136,149 | 48,114 |
| Towards in vivo deployment: considerations | – | 146 | 85,128,171 | 130,147 | 136,176,179,191 | 178,192,193 |
| Review articles | – | 19,20 | 21-25,27 | 28-39 | 40-47,50 | 49-51 |
Papers from before 2010 used genetically-encoded calcium probes.
Optogenetic tools
Optogenetic sensors: GECI and GEVI
Optical tracking of changes in membrane voltage and intracellular calcium allows a comprehensive assessment of cardiac activity with very high spatiotemporal resolution. Optogenetic sensors add both cell and organelle specificity as well as the ability to perform in vitro and in vivo longitudinal studies. The cameleon proteins were the first GECIs to be developed and used calmodulin as a sensing element fused to fluorescent proteins excitable with blue–green light52. Various kinetic properties were optimized in the GCaMP family of GECIs (consisting of green fluorescent protein, calmodulin and Ml 3 protein), with GCaMP6 variants53 being the latest member. Following the first cardiac applications of GCaMPs around 20067-9, these GECIs remain by far the most widely used optogenetic sensors for in vivo experiments, especially for cardiac applications. The red-shifted variants, including the R-CaMP54,55 and the R-GECO56 family of GECIs, were developed to be used in combination with blue-excitable optogenetic actuators. One study developed improved-sensitivity versions (JR-CaMP and jR-GECO) that were better suited for use with channelrhodopsin 2 (ChR2) actuation57. Finally, a near-infrared (NIR) fluorescent GECI, NIR-GECO58, was developed in 2019, with improved spectral separation from optogenetic actuators and potential voltage sensors, while also providing deeper probing owing to NIR light penetration. Some of these GECIs, such as jR-GECO, come close in performance (in terms of kinetics and quantum efficiency) to the best synthetic calcium indicators when used in vitro. For long-term, repeated probing in vitro, as well as for in vivo measurements, GECIs are extremely valuable and reasonably well tolerated. Additional advantages of GECIs include organelle-specific targeting, such as nuclear, mitochondrial and sarcoplasmic reticulum targeting, to study compartment-specific changes in calcium levels37,59. In cardiac applications, red-shifted GECIs have been used in combination with optogenetic actuators60-63, optogenetic voltage sensors that are blue-shifted or NIR60,64,65 or with excellent-performance NIR synthetic dyes, such as BeRST1 or di-4ANBDQBS62.
Genetically encoded voltage indicators (GEVIs) have lagged behind the GECIs in development and deployment in mammalian cells, including cardiomyocytes, owing to difficulties in achieving fast kinetics of sensing as well as their potential to interfere with native electrophysiology (as many large membrane proteins would). However, great progress towards this goal has been achieved in the past decade, and some of these developments have been used in cardiac research. The first, more-widely used GEVIs were VSFP2.366,67 and ArcLight68. VSFP2.3, ArcLight and the red-shifted variant FlicR169 (excitable at 570 nm) are all based on a phosphatase voltage-sensing domain (Ci-VSD). These GEVIs have been validated for use in cardiomyocytes in vitro64,70-73, as well as to monitor cardiac electrical activity in transgenic mice in a cell-specific manner74,75. VSFP2.3 and ArcLight have relatively slow kinetics compared with synthetic voltage-sensitive dyes and cannot accurately capture the upstroke and high-frequency components of the action potential; however, these indicators are useful in reporting rate responses and relative changes in action potential duration. The faster ASAP family of GEVIs76-78 now includes the latest variant, ASAP3, with high response to two-photon excitation in vivo (in the brains of mice)76. The GEVI Voltron, developed in 2019, uses a Halo-tag and a synthetic dye as a fluorophore instead of a fluorescent protein, thereby representing an optogenetic–chemogenetic hybrid GEVI for in vivo use79. Voltron525 showed the highest sensitivity to voltage, although a wide spectrum of other sensors, excitable between 525 nm and 635 nm, was generated with the Voltron modular design, leveraging the spectral properties of fluorophore partners79. ASAP and Voltron have not yet been used to record cardiac action potentials. The motivation to obtain optical sensors designed for all-optical electrophysiology (considering the shorter-wavelength excitatory and silencing opsins) led to the development of a range of NIR-absorbing GEVIs, starting with the Quasars80 and, more recently, Archon181, NIR-Butterfly82 and Voltron63579 (Fig. 2b). The drive to generate these NIR sensors is also motivated by their expected superior performance in vivo to shorter-wavelength indicators for probing deep within the tissue by avoiding haemoglobin absorption.
CaViar80, a combined GEVI–GECI construct, was designed and applied to study the zebrafish heart in vivo83. In later studies, CaViar was used in combination with the actuator CheRiff for all-optical electrophysiology studies in human induced pluripotent stem cell (iPSC)-derived cardiomyocytes (iPSC-CMs)60. In addition to voltage and calcium, cytosol and mitochondria redox state have been recorded using an optogenetic sensor (the glutaredoxin 1 (Grx1)–roGFP2 fusion protein) during human cardiomyocyte differentiation84.
Unresolved challenges exist for the routine deployment of GECIs and GEVIs and other optogenetic sensors in whole hearts, especially in vivo. Robust expression of the sensors without interference with native function is a desirable target; to date, the work has been primarily done in transgenic mice. Optical measurements are confounded by the mechanical motion of the heart, the dense muscle tissue and the high absorbance of haemoglobin21,85. For in vivo measurements, ratiometric sensors or other methods for motion correction are necessary. Continuous monitoring of in vitro cardiac function is more straightforward. The optogenetic toolbox (actuators and sensors) is ever-expanding and efforts are focused towards the development of high-throughput discovery systems with rational design of features, as exemplified by some new GEVIs76,81.
Comprehensive reviews on optogenetic sensors (GECIs and GEVIs) offer details on the spectral and biophysical properties of these proteins and their use in generating transgenic animals for research24,27,34,37,38,86-91; those with specific relevance to the heart are shown in Table. 1.
Excitatory and inhibitory optogenetic actuators
Optogenetic actuators of voltage are light-sensitive proteins that can be expressed in mammalian cells and can transform photon flux into transmembrane ion flux, thereby manipulating transmembrane potential on a millisecond scale. The discovery and cloning of ChR2, a cation-selective, light-sensitive ion channel, in 200314 led to the development of an extensive toolkit of optogenetic actuators (opsins). This toolkit includes depolarizing (excitatory) opsins (such as ChR214, CheRiff80, Crimson92 and ReaChR93) and hyperpolarizing (inhibitory) opsins (such as BLINK-194, PAC-K95, GtACR196, archaerhodopsin T (ArchT)97, halorhodopsin (Halo)98 and Jaws99) that are activatable across a wide spectrum of wavelengths (Fig. 2b). All these optical actuators are microbial opsins that use all-trans retinal as a chromophore, with which the opsins form covalent bonds.
The most widely used optogenetic actuator is a larger-photocurrent H134R mutant of ChR213. A vast number of other ChR2-based, depolarizing opsins have been developed to change their spectrum, kinetics or dynamic range14,100,101. Fig. 2b shows the blue-shifted CheRiff80, red-shifted Crimson92 and red-shifted ReaChR93. These optogenetic actuators are both voltage-dependent and light-dependent, which means that they are non-linear transducers of light to voltage. These actuators have voltage rectification, that is, the ChR2 current is able to limit itself after the membrane potential becomes depolarized, even if the light remains on during longer pulses. Understanding the non-linearity of the function of ChR2-based opsins (through computer modelling102) is essential. These properties (the self-closing through voltage rectification) can be beneficial for optimizing the energy for stimulation103 and can be leveraged for more intelligent control of actuator function. Opsins can also be modified through rational design104 or metagenomic discovery105 to tailor them to cardiac research.
Of note, depending on irradiance and pulse duration, excitatory opsins can act as complex modulators of cardiac function, beyond simple excitation and pacing. As discussed later, excitatory opsins can modulate the shape of the action potential106-108, as well as cardiac rhythm even when constant light is applied48,103,109, and the conduction properties and wave dynamics48,110-112 of cardiac tissue. Excitatory opsins can also provide inhibition of activity during strong illumination and long-duration pulses109. Unlike electrical current input, optogenetic actuation can be long-lasting and can provide feedback-controlled, complex modulation over time113,114 and over time and space111,112.
Fast, bidirectional, optical modulation of voltage is particularly attractive, but the hyperpolarizing (inhibitory) opsins have been less robust than ChR2 and derivatives. Cardiac research has adopted the early tools ArchT97 (a proton pump) and Halo98 (a chloride pump). Fig. 2b shows Jaws99 (a red-shifted version of a chloride pump), GtARC196 (an anion-selective ion channel), BLINK-194 (a potassium channel-based inhibitory opsin) and PAC-K95 (a newly developed, two-component potassium channel system). The search for improved hyperpolarizing opsins continues because the currently available pump-based tools lack sufficient photocurrent; in particular, the GtARC1-based tools are highly dependent on the cellular chloride reversal potential and the potassium channel opsin systems are difficult to express in cells or cannot be deployed on the same millisecond time scale as their depolarizing counterparts.
Unique applications of voltage optogenetic actuators or other optogenetic actuators (that modulate signalling pathways) include mitochondria-specific targeting of ChR2, which led to the development of optometabolic tools for cardiac research115. Early deployment of optogenetic tools provided insights into L-type calcium channel clustering and its effects on channel gating116. Optogenetic actuators for G-protein-coupled receptor (GPCR) signalling (known as opto-XRs or opto-GPCRs) have been used for precise space–time manipulation of rhythm by very low light intensities via the Gq signalling pathway with the use of genetically expressed melanopsin117 or via Gs signalling with the use of JellyOp (an opsin photopigment) expression118. Further discussion of the variety and properties of the opto-XR or opto-GPCRs classes of optogenetic actuators can be found in previous reviews38,119. Comprehensive reviews on optogenetic actuators and their biophysical properties are listed in Table 120,38,41,100,101
A large number of cardiac studies have used all-optical electrophysiology1,4,60,62,63,106,110,111,120-144 by combining optogenetic actuators with optogenetic sensors, synthetic voltage-sensitive and calcium-sensitive dyes, or dye-free optical methods to image cardiac activity. Computational modelling of cardiac optogenetics has complemented experiments by providing insights into optimal design and interpretation of findings in cardiac cells and tissues102,103,107,108,143,145-150 (summarized in Table 1).
Near-term translation of optogenetics
Clinical trials using optogenetics for correction of retinal disorders are underway and, so far, the approach has been safe. However, gene and light delivery to the human heart is much more challenging than delivery to the localized, immune-privileged setting of the eye. Therefore, the immediate translation of cardiac optogenetics is for outside-the-body deployment and is likely to draw upon some of the advantages of optogenetics (Fig. 2a), such as scalability, parallelism (the ability to observe and control a large number of locations simultaneously in an easily reconfigurable manner) and the ability to perform repeated measurements in long-term or longitudinal studies. At the time that optogenetics was first used to control neurons, another scalable technology emerged, that of human iPSCs. Human iPSC-CMs have been used in cardiology for disease modelling and cardiotoxicity testing151,152. Since 2013, the combination of optogenetics (optogenetic sensing, actuation or all-optical electrophysiology) and human iPSC-CMs has proved to be a fruitful union60-64,70-73,113,129,132,133,138,140,144,153-164 (Table 1). Previous publications, illustrated in Figs 3 and 4, present four areas of application that we consider an immediate and important translation of optogenetics for the cardiovascular field.
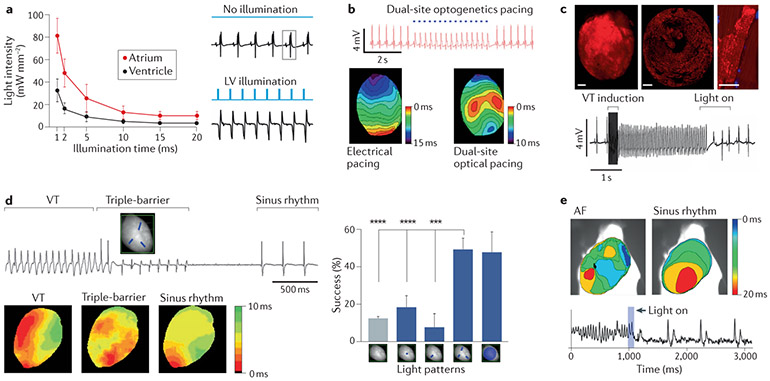
Fig. 3 ∣. Near-term translation for high-throughput drug screening and cardiotoxicity testing.
a ∣ High-throughput drug screening. Optogenetic voltage sensors (QuasAr1) and bidirectional optogenetic actuation (depolarization via channelrhodopsin (ChR2) and hyperpolarization via archaerhodopsin T (ArchT)) can be used in an all-optical electrophysiology setup to reveal drug effects on voltage-gated ion channels in heterologous systems. Sodium channel hNav1.5 (hNav1.5) and responses to lidocaine are shown. Light-induced electrophysiology (LiEp) and classic voltage clamp (VC) both capture functional cellular responses to lidocaine for light-induced voltages within the range of operation (before rectification) of the optogenetic actuators, b ∣ High-throughput cardiotoxicity testing. CheRiff (an optogenetic actuator) and CaViar (an optogenetic construct for dual-voltage and calcium imaging) were expressed in human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs), and cell populations (expressing the actuator or expressing the sensor) were intermixed in a connected network (top-left panels). This approach was applied to perform dose–response testing with several cardiotoxic drugs. The top-right panel shows the voltage responses to cisapride for spontaneous activity and in response to 1 Hz optical pacing; early afterdepolarizations are induced at 1 μmol/l. Cisapride is a gastrokinetic drug that was removed from the US market in 2000 because of cardiotoxicity. The bottom-left panel shows OptoDyCE62,129, an automated, high-throughput, all-optical electrophysiology setup for optogenetic actuation and optical–optogenetic sensing of voltage and calcium with the use of spectrally compatible proteins and/or small-molecule probes. The bottom-right panel shows the responses of human iPSC-CMs in 384-format plates to 0.1% dimethyl sulfoxide (DMSO) versus 10 μmol/l azimilide; the spontaneous and optically-paced activity is shown (the blue dots are the optical pulses). Azimilide prolongs the action potential duration and can induce small, localized, spontaneous calcium-release events, seen in the calcium records (marked by *). Although azimilide is a class III antiarrhythmic drug, azimilide can be cardiotoxic and cause torsade de pointes. aLED, light-emitting diode used for actuation; sLED, light-emitting diode used for excitation in sensing; DM, dichroic mirror; F, optical filter; L, lens, eGFP, enhanced green fluorescent protein. Part a modified with permission from REF.65. Part b top panels modified with permission from REF.60. Part b bottom panels modified with permission from REF.62.
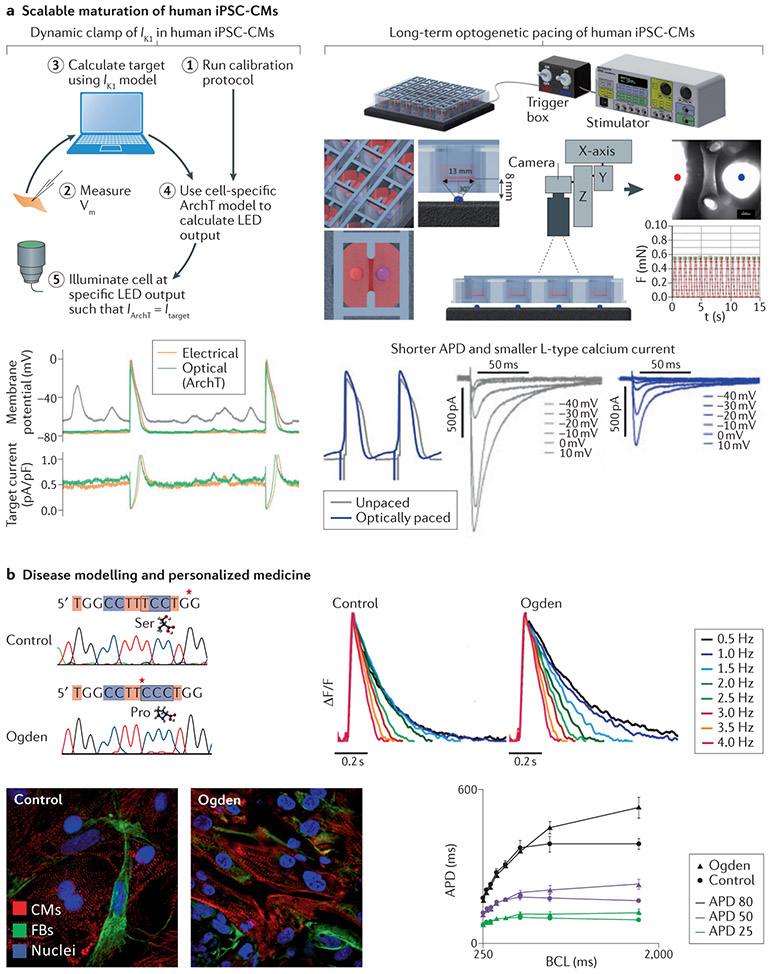
Fig. 4 ∣. Near-term translation to enhance stem-cell technology and personalized medicine.
a ∣ Scalable maturation of human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). Left: an ‘optical dynamic clamp’ for computer-controlled, real-time manipulation of live cardiomyocytes (CMs) was used to ‘inject’ the desired ion current by light-emitting diode (LED) light when an optogenetic inhibitor ArchT was expressed in human iPSC-CMs. The dynamically clamped current was the inward-rectifier potassium current (Ik1) and its ‘optical injection’ yielded more hyperpolarized resting potential, consistent with mature CMs. The bottom-left panels show records without the dynamic clamp (depolarized resting membrane potential (grey) and wandering baseline), electrical dynamic clamp (orange) and optical dynamic clamp (green). This more mature CM ‘phenotype’ was used for studying drug responses. Right: the figure shows a system for long-term optogenetic stimulation of engineered human tissue constructs and tracking the induced force responses. The system was applied over 3 weeks and the optically paced constructs (3-Hz, 30-ms pulses at 0.3 mW/mm2, 15 s on and 15 s off) showed electromechanical remodelling with faster contractions, shorter action potential durations (APDs) and lower L-type calcium current. In this study, the optically tachypaced engineered human tissues were more susceptible to arrhythmias than unpaced engineered human tissues, but the arrhythmias could be prevented by pharmacological means. b ∣ Disease modelling and personalized medicine. The example of disease modelling shown is of a rare X-linked genetic disorder, Ogden syndrome165, with a point S37P mutation in NAA10 (left panel), which encodes the catalytic subunit of N-alpha-acetyltransferase 10 (involved in N-terminal acetylation of proteins). Cells from patients with Odgen syndrome and cells from age-matched and sex-matched healthy individuals (control) were transformed into human iPSCs and then differentiated into CMs (the bottom-left panels show immunofluorescence images with labelling for CMs, fibroblast (FBs) and nuclei). After 3 months, the iPSC-CMs were transduced with ChR2 adenovirus and their function was characterized with all-optical electrophysiology. The patient-derived cells showed APD prolongation selectively at lower pacing rates or longer basic cycle lengths (BCLs) compared with control cells, consistent with clinical findings that patients with Ogden syndrome have cardiac problems and arrhythmias, including bradycardia and torsades de pointes, and eventually die young. APD25, APD50 and APD80 are APDs at 25%, 50% and 80% repolarization. Part a left panels modified with permission from REF.113. Part a right panels modified with permission from REF.163. Panel b modified with permission from REF.156.
High-throughput drug discovery
The drug-development process is lengthy and costly and relies on high-throughput screening for target identification, target validation, compound discovery and hit validation. All-optical electrophysiology20,80,129 could augment each of these steps in the drug-development pipeline, as reviewed in detail previously33. Fig. 3a illustrates how bidirectional, optogenetic voltage control (with the use of depolarizing ChR2 and hyperpolarizing ArchT opsins), combined with an optogenetic voltage readout (Quasar 1) can produce similar results to the classic (electrical) voltage clamp when studying ion channel responses to drugs65. Considering the non-linear light–opsin photocurrent relationships, this voltage control applies for certain dynamic range, depending on the opsins. The main benefit of the optogenetic approach compared with the automated planar-patch systems, which underwent rapid development in the past decade, is the non-contact nature of actuation and sensing by light, lending this technique to scalability and enabling work with a variety of cell types. Ultimately this motivates rigorous experimental design with a greater number of testing conditions.
High-throughput cardiotoxicity testing
Preclinical testing for all drugs involves a cardiotoxicity assay, which includes the detection of electrophysiological abnormalities. This assessment is currently based exclusively on compound testing in heterologous systems for potassium voltage-gated channel subfamily H member 2 (also known as hERG) channel blocking, a prime target for drugs. However, ongoing efforts are underway to consider more comprehensive cardiotoxicity assays with the use of human iPSC-CMs152. Given that drug actions are rate-dependent, it is important to capture cellular electromechanical responses under controlled, paced conditions. Electrical pacing is not easily scalable, therefore contact-free optical methods offer an attractive alternative. Similarly, optical sensing has proved to be valuable in increasing throughput as well as enabling long-term observations (Fig. 3b). The utility and limitations of all-optical electrophysiology (optogenetic actuators and optogenetic sensors) to address the needs of drug testing was investigated computationally and experimentally by Klimas and colleagues129. An alternative implementation of optogenetic pacing without genetically modifying the cardiomyocytes is to use dedicated, light-sensitive, non-excitable cell constructs that can couple to cardiomyocytes, known as the tandem cell unit [G] approach4. To date, experimental studies have not reported interference with native electrophysiology or notable limitations of optogenetic tools in drug testing.
After early reports on the use of optogenetic pacing of stem-cell-derived cardiomyocytes153,155, several groups focused on developing automated high-throughput all-optical platforms for cardiotoxicity testing. Optopatch, a fully optogenetic construct (GEVI–GECI CaViar sensor in tandem with an optogenetic actuator CheRiff) was developed by Cohen and colleagues80 and combined with high-end macroscopic systems for cardiotoxicity testing with the use of human iPSC-CMs60,133 (Fig. 3b). Variants of the system have been translated to an industrial setting for drug discovery140. Our group developed a simpler approach for automated, all-optical, dynamic cardiac electrophysiology (OptoDyCE)62,129, which integrates an optogenetic actuator (ChR2) with spectrally compatible, high-performance, synthetic dyes for calcium and voltage or red-shifted, optogenetic calcium sensors (Fig. 3b). The system is low-cost, using LEDs and temporal multiplexing to simultaneously record voltage and calcium responses or cell contraction with a single camera. The high-content, drug-response measurements in human iPSC-CMs have been used to construct populations of human iPSC-CM computer models for personalized medicine164. Such work has been extended to include long-term, high-throughput microfluidic perfusion63 and to allow for long-term pacing and recording to enable chronic drug testing, while other research groups used some of these all-optical electrophysiology tools for iPSC-CM phenotyping and drug screening61,157. Some studies have specifically leveraged optogenetic pacing, pairing it with high-throughput microelectrode recordings to capture drug responses158,159,161. These approaches have sparked interest from industry and the FDA and are likely to influence drug development in the near-term.
Short-term and long-term actuation and sensing in human iPSC-CMs
Human iPSC-CMs have transformed cardiac research, but concerns about the immature state of the iPSC-CMs have prevented their rapid translation into the clinic151. Optogenetic tools can have a crucial role in phenotyping, long-term monitoring and maturation of these cells. The main advantage of optogenetic sensors compared with synthetic voltage or calcium dyes is the opportunity to acquire repeated measurements over long periods of time. Since 2014, multiple studies have used iPSC lines with constitutively expressed GEVI ArcLight to monitor cell changes during the differentiation and maturation into cardiomyocytes and cellular responses to pharmacological agents64,70-73,154. Both control cells and cells from patients with inherited arrhythmogenic disorders have been characterized in detail with the use of ArcLight imaging71,72, GCaMP calcium monitoring72 and with a combination of ArcLight and a GECI (R-GECO)64. Over the past 5 years, efforts involve linking phenotypes to single-cell transcriptomic signatures70 and leveraging optogenetics to monitor redox states in engineered human tissues84.
Optogenetic actuation has been applied in creative ways to address iPSC-CM immaturity. Fig. 4a illustrates work by Quach and colleagues to transform a classic (electrical) dynamic clamp assay into an optical dynamic clamp [G] to yield a more hyperpolarized and mature resting membrane potential in human iPSC-CMs113, to be closer to that of an adult cardiomyocyte. Viral expression of ArchT in human iPSC-CMs and computer-controlled, real-time manipulation by light (LED) was used to perform ‘optical injection’ of the inward-rectifier potassium current (Ik1) to make the resting membrane potential more negative, thereby shifting the electrophysiological phenotype of human iPSC-CMs to be more adult-like. Furthermore, a study from 2020 applied the same approach (optical dynamic clamp) to inject a synthetic, antiarrhythmic, frequency-dependent current in atrial cardiomyocytes114 Extending this idea to an all-optical clamp would enable multicellular and tissue-level applications. However, more work is needed to calibrate voltage responses and to implement real-time processing for multiple cells.
Dynamic stimulation (electrical or mechanical) of human iPSC-CMs has been identified as a potential approach to induce a more mature cellular phenotype. Several studies in 2019 leveraged optogenetic actuators to explore chronic optical stimulation162,163 (Fig. 4a). One study used closed-loop control for overdrive-pacing of human iPSC-CMs during weeks in culture and quantified cell remodelling162. Another study used optogenetic pacing of engineered human tissues over 3 weeks and monitored their contractile performance163. The stimulated tissue constructs showed electromechanical remodelling, with faster contractions, shorter action potentials and lower L-type calcium current than unstimulated samples. The optically tachypaced engineered human tissues were more susceptible to arrhythmias163. Overall, optogenetics enables scale up and accelerates the development of more mature and physiologically-relevant human iPSC-CMs for drug screening and for applications in regenerative medicine.
Applications for disease modelling and personalized medicine
Optogenetic tools have been used in several studies on patient-specific disease modelling with human iPSC-CMs. For example, optogenetic sensors (GEVI and GECI) were used to monitor responses in Timothy syndrome, a calcium-channel genetic disorder64. Another study used control iPSC-CMs and iPSC-CMs derived from patients with catecholaminergic polymorphic ventricular tachycardia or long QT syndrome type 2 to phenotype and test the iPSC-CMs for pharmacological interventions72. A 2019 study leveraged optogenetic pacing to phenotype catecholaminergic polymorphic ventricular tachycardia subtypes with the use of engineered human tissues160. Fig. 4b shows an example of an all-optical electrophysiology platform used to phenotype a rare genetic disorder with cardiac abnormalities156. Ogden syndrome165 involves a point S37P mutation in NAA10 (encoding the catalytic subunit of N-α-acetyltransferase 10, responsible for the N-terminal acetylation of certain proteins). Patients with Ogden syndrome die young and some develop torsade de pointes, which is an extremely rare presentation of abnormal heart rhythm in children. Overall, the ability to phenotype patient-specific iPSC-CMs quickly and to quantify cellular responses to therapeutic interventions is at the core of personalized medicine. Optogenetic tools offer an enabling technology for such interventions and might help to provide better stratification during preclinical testing of drugs, taking into account patient sex, race, age and other aspects through increased throughput and improved experimental design.
Long-term translational prospects
Arrhythmia management
Implantable electronic pacemakers and implantable cardioverter–defibrillators are the gold-standard for arrhythmia management166. Despite their established value as safe devices, they still have substantial deficiencies. In particular, high-voltage shocks damage myocardial tissue, and device battery life is limited. Batteries are drained when shocks are frequent, such as in patients with ventricular electrical storm167. Shocks are also painful, causing patient anxiety and depression168. Optogenetics has the potential to address these deficiencies by offering routes for non-electrical, low-energy pacing and cardioversion that can be cell-specific and painless.
Optogenetic pacing for rhythm control
Optical pacing would be an attractive alternative to electronic pacemakers if, by leveraging the advantages of optogenetics (Fig. 2a), this approach could be achieved at lower energy, thereby extending battery life20,22, while providing the additional advantages of cell specificity and distributed actuation. In 2010–2011, the first reports demonstrated the utility of optogenetic pacing of cardiomyocytes with the use of blue-light pulses1-4. Over the past decade, multiple other studies have used optogenetic pacing in cultured cell systems in vitro, in perfused hearts ex vivo and in anaesthetized animals60-63,91,102,106,124-126,128,129,132,133,135,141,143,153,156-159,161-163,169-178 (Table 1). The hearts of transgenic species have been optically paced, including zebrafish, Drosophila and mice, as well as mouse and rat hearts virally transduced to express optogenetic actuators. Although long-term optogenetic pacing with an implantable device has not yet been demonstrated in an awake animal, in this section, we summarize crucial advances towards this goal.
A study in 2010 was the first to use optogenetics to pace the mammalian heart with the use of light2 (Fig. 5a). Transgenic mice expressing ChR2 within the cell membrane of cardiomyocytes enabled optical pacing of the ventricles and atria at physiologically relevant rates with the use of short (<10 ms) pulses and well-tolerated light levels. Prolonged depolarization was observed during longer pulses, both in vitro and in mouse hearts in vivo, suggesting that optical termination of arrhythmias was possible, as demonstrated later by this group and others130,131,179. Among the pioneering studies of optogenetic pacing, Arrenberg and colleagues demonstrated cell-specific expression of opsins within the specialized conduction system of zebrafish hearts1. They used transgenically expressed halorhodopsin and ChR2 either to optically block intrinsic activation or to optically pace zebrafish hearts at specific rates. Using patterned illumination to switch rapidly between a normal activation sequence and an arrhythmia, they demonstrated for the first time the utility of optogenetic tools for spatiotemporal control of cardiac activity. Their optogenetic interrogation of zebrafish hearts provided new insights into endogenous sinus node electrophysiology.
Fig. 5 ∣. Long-term translation of cardiac optogenetics for rhythm control.
The figure shows proof-of-concept results from multiple studies. a ∣ Photostimulation intensity and duration required for in vivo pacing of transgenic channelrhodopsin 2 (ChR2) mice hearts2. Electrocardiogram (ECG) signal before and after pulsed photostimulation indicating 1:1 capture during optical pacing of the left ventricle (LV). b ∣ ECG indicating dual-site, optical pacing of the ventricles in a perfused rat heart at locations of myocardial injections of adeno-associated virus (AAV)-CAG-ChR2-green fluorescence protein (GFP), and activation sequences generated by electrical pacing at the apex (left panel) and by dual-site optical pacing of the ventricles (right panel)128. c ∣ Optogenetic pacing and defibrillation in hearts after in vivo gene transfer147. Stable expression of ChR2 within a mouse heart 16 months after systemic injection of AAV9-ChR2-mCherry; epicardial surface (top-left panel), left ventricular cross-section (top-middle panel) and ventricular cardiomyocyte (top-right panel). The bottom panel shows an ECG with termination of ventricular tachycardia (VT) by epicardial illumination of the ventricles. d ∣ ECG showing cardioversion after VT onset using a triple-barrier pattern (top-left panel)131. Activation sequences relative to the ECG; VT activation (bottom-left panel), photostimulation (bottom-middle panel) and restored sinus rhythm (bottom-right panel). The right panel shows the percentage of spontaneous cardioversion of VTs (grey bar) and VTs interrupted with each of the four light patterns shown on the x-axis (blue bars); P values (***P < 0.001, ****P < 0.0001) determined by one-way ANOVA. e ∣ The top panels show activation maps with re-entrant conduction during atrial fibrillation (AF) (left panel) and subsequent restoration of sinus rhythm after optical cardioversion (right panel)191. The bottom panel is the optical voltage signal showing chaotic AF that converts to sinus rhythm after exposing the right atrium to a 100-ms light pulse. Panel a modified with permission from REF.2. Panel b modified with permission from REF.128. Panel c modified with permission from REF.147. Panel d modified with permission from REF.131. Panel e modified with permission from REF.191.
Clinical deployment of optogenetic pacing will involve methods beyond transgenic approaches, such as gene therapy, because viral vectors (including lentiviruses, adenoviruses and the safer adeno-associated viruses (AAVs)) provide the most efficient way to inscribe light sensitivity to the heart and are suitable for clinical use102,106,155,170,176. Experiments in 2015 demonstrated how discrete regions of the adult rat heart could be enabled for optical pacing by local ChR2–AAV9 viral injections128 (Fig. 5b). Several weeks after the injections, hearts were responsive to optical pacing in both open-chest and ex vivo studies. Multisite optical pacing further demonstrated the application of optogenetics for ventricular resynchronization therapy128. Another study from 2015 demonstrated optogenetic transduction of the heart that did not require open-chest surgery and myocardial injections. Instead, ChR2–AAV9 was systemically delivered to mice171, capitalizing on the cardiac tropism of this AAV serotype and achieving robust myocardial responsiveness to optical pacing a year after transduction.
Delivery of non-excitable, light-sensitized cells could be an alternative to viral transduction for optical cardiac pacing. In 2011, Jia and colleagues termed this approach the tandem cell unit strategy4,124 and validated it in vitro using ChR2–HEK cells coupled to either adult dog cardiomyocytes or monolayers of neonatal rat cardiomyocytes. In a hypothetical tandem cell unit strategy, patient-derived cells would be induced to express an opsin to provide robust depolarizing current upon photoactivation. After delivery to a patient’s heart, those cells would couple to cardiomyocytes to enable optical control of heart rhythm. Although exogenous delivery of light-sensitized cells has not yet been implemented, in vitro and in vivo proof-of-concept results of the tandem cell unit concept have been reported for non-excitable cells of the heart, including cardiac fibroblasts, myofibroblasts132,143,169,180,181, cardiac progenitor cells132 and macrophages182, all of which can electrically couple to cardiomyocytes to entrain the myocardium after optical stimulation.
Low-energy optogenetic pacing could emerge as a disruptive technology if optogenetic transduction and light delivery were safe and minimally invasive20,22,23. Strategies for translating optogenetic pacing to the clinic include obtaining a better understanding of opsin operation and function in cardiomyocytes (atrial, ventricular and Purkinje cells)102,103,146,175, optimizing optogenetic actuators for cardiac cells, obtaining a deeper understanding of cell-resident cofactors (for example, all-trans retinal at the right doses considerably lowers the threshold for stimulation)173 and selectively engaging the specialized conduction system of the heart1,126,146. Computational modelling is an effective platform for testing these strategies and has provided new insights into the energetic benefits of longer stimulation pulses102,103 and constant ultra-low illumination103. Such long-duration pulses are not feasible using electrical stimulation owing to the generation of cytotoxic electrochemical by-products103,183. Results from human heart computational models have revealed that the energy required to pace the heart is substantially reduced when ChR2 is exclusively expressed within the Purkinje system and light is delivered to the bundle of His146. Cell delivery of designated, non-excitable ‘spark’ cells, as in the tandem cell unit approach, could also lower the energy required to pace the heart if the cells are clustered124. Finally, low-energy solutions for rhythm control might also arise from optogenetic modulation of intracellular GPCR signalling, such as the Gq117 and Gs118 pathways.
Optogenetic termination of cardiac arrhythmias
The critical dependence on coordinated space–time actuation is a major challenge in arrhythmia termination. Focused actuation in either space or time alone is often inadequate for termination of complex arrhythmias. Conventional defibrillation is a high-energy termination strategy that depolarizes a large myocardial mass (>95%)184 to block fibrillatory wavefronts, thereby preventing the formation of new re-entrant waves185,186. Low-energy, electrical strategies rely on the proper timing of low-amplitude depolarizing stimuli to extinguish arrhythmic wavefronts and include anti-tachycardia pacing187 and low-energy anti-fibrillation pacing188. Anti-tachycardia pacing is a feature in current implantable cardioverter–defibrillators but is limited to actuation of specific locations and, therefore, is effective in terminating only non-complex, re-entrant arrhythmias. By contrast, optogenetics enables low-energy strategies that can be distributed over large areas or deliver long pulses, capturing multiple phases of the arrhythmic activity or having light that is patterned, possibly in real-time, for mechanistic termination of fibrillatory wavefronts. The light intensity could also be titrated to terminate rotors with the use of subthreshold illumination48. By avoiding engagement of the cardiac nociceptors and preventing skeletal muscle hypercontractures, the cell-specificity of optogenetics ensures painless termination of an arrhythmia.
In an early study of optical cardioversion to terminate re-entrant arrhythmias in healthy mouse hearts, illumination of the anteroseptal epicardium with one light pulse had an 85% success rate147 (Fig. 5c), and the success during acute infarction was 88%. In computer simulations of an infarcted human heart, epicardial illumination successfully terminated infarct-related ventricular tachycardia by initiating transmural depolarization to block the re-entrant wavefront147. In a later study, ChR2–AAV9 constructs were used in combination with rapamycin (to suppress immune response) in rats to demonstrate ventricular cardioversion130. Application of a single epicardial light pulse achieved 97% cardioversion for monomorphic ventricular tachycardia and 57% for polymorphic ventricular tachycardia. Another study also successfully terminated ventricular tachycardia by open-chest application of successive light pulses in rats after myocardial infarction189.
An ex vivo study from 2018 found that ventricular tachycardia was terminated in transgenic ChR2-expressing mice after global illumination of the epicardial surface with a single light pulse136. Optical mapping revealed two primary cardioversion mechanisms that were motivated by optical depolarization of the entire epicardium; the termination of a re-entrant core and subsequent refractoriness, and an unpinning and drift of the re-entrant core until collision with refractory tissue136. Although global epicardial depolarization by light excels as a low-energy solution to cardioversion, it is not easy to implement in vivo where it is necessary to perform cardioversion with minimal area of illumination. A study in ChR2-transgenic mouse hearts addressed this issue using patterned light to annihilate arrhythmic wavefronts using mechanistic, multi-barrier illumination patterns131 (Fig. 5d). Cardioversion [G] of ventricular tachycardia required triple-barrier optical depolarization, where light was restricted to three lines oriented approximately 120° apart. This illumination pattern yielded an optical cardioversion success rate similar to that of illuminating the entire left ventricle, but used only 25% of the light energy.
Atrial cardioversion is a mid-future opportunity for translating cardiac optogenetics to the clinic. After early proof-of-principle in vitro work in monolayers and slices of rat atrial cardiomyocytes122,175,190, followed by computational studies149, two rodent studies demonstrated optogenetic termination of atrial fibrillation 179,191. One study used mice with cardiac expression of ChR2 as well as a loss-of-function mutation in Cx40 that promoted atrial fibrillation179. In open-chest experiments, the success rate of terminating atrial fibrillation was >90% using focused, low-light illumination (<0.5 mW/mm2). Optical atrial cardioversion was still possible 8 months after systemic viral delivery of ChR2, showing promise for the clinical translation of this approach. In other studies in rats, ReaChR–AAV9 with the atria-specific promoter from human NPPA was topically applied to the right atrium using the gene painting [G] technique191. After 4 weeks, cholinergic-mediated atrial fibrillation was induced in the anaesthetized animals and a computer-controlled, implanted LED delivered optical pulses to the right atrium when PR intervals consistent with atrial fibrillation were detected, providing a cardioversion success rate of 96%191 (Fig. 5e).
Light penetration into the myocardium is an important consideration for the clinical translation of optogenetic cardioversion or defibrillation, especially for the ventricles85. Illumination with longer-wavelength NIR light, in combination with red-shifted opsins, would provide deeper myocardial penetration of optical depolarization, thereby increasing the probability of capturing a critical mass of tissue. Upconversion nanoparticles [G] administered deep within the myocardium could also be engaged by NIR light, enabling the activation of optogenetic actuators via nanoparticle-assisted light conversion192. These innovations could be optimized for the clinic using computational models28,147-149. Interestingly, results from a recent study suggest that simple photon transport models could underestimate the depth of light penetration into the myocardium and its effect on opsin-expressing cells193. Additional work is certainly needed to better understand light–matter interactions within the context of using distributed light for optogenetic cardioversion.
Cardioversion using well-controlled, regional excitation or inhibition is difficult to achieve using electric fields but is a unique feature of optogenetics. Indeed, several studies have used hyperpolarizing opsins to modulate cardiac activity95,96,169,194-196. However, some hyperpolarizing opsins, especially anion-conducting opsins, such as ACR, have had controversial results in cardiac muscle194. Furthermore, termination of complex arrhythmias using optical hyperpolarization would require global actuation (expression and illumination) to prevent enduring wavefronts from re-activating hyperpolarized regions. One study used transgenic mice expressing the hyperpolarizing opsin ArchT to optically terminate ventricular arrhythmias195. Although cardioversion success was higher than in control hearts, actuation of the inhibitory opsin was inferior to actuation of an excitatory opsin, such as ChR2. A comprehensive list of studies using optogenetic tools for cardioversion or defibrillation48,95,96,111,112,114,121,122,128,130,131,136,137,147,149,174,179,189-191,194-199 is shown in Table 1.
Excitation wave control and feedback for rhythm management
The optogenetic approaches described above for pacing and cardioversion or defibrillation are guided by concepts used in present-day cardiac devices. Optogenetics also enables new innovations for rhythm management, including the control of action potential morphology and control of the speed and direction of targeted wavefronts, a concept known as optical wave steering [G]29. These rhythm-management innovations could neither be realized using present-day devices nor be rigorously tested in living tissue before the advent of cardiac optogenetics. Indeed, multiple experiments have demonstrated that wavefronts can be modulated by light48,110-112,131,137-139,143,181,190,199,200 (Fig. 6a,b), among which some have implemented quasi-real-time, closed-loop control whereby the trajectory of a wavefront is sensed and then altered using patterned illumination (Fig. 6c). This approach is particularly promising for ultra-low-energy cardioversion. However, the implementation could be challenging due to the substantial spatiotemporal data that would need to be processed in quasi-real-time by an implanted device. Even so, closed-loop sensing and actuation of cellular function is a powerful feature of optogenetics that is useful not only in cardiology but also neuroscience201.
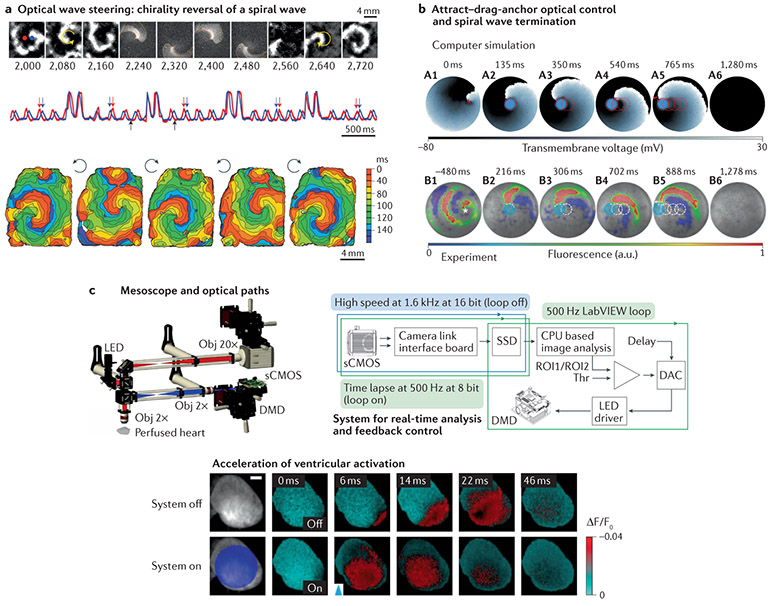
Fig. 6 ∣. Long-term translation of cardiac optogenetics for wave control and feedback control.
The figure shows proof-of-concept results from multiple studies. a ∣ The top images show an anticlockwise spiral wave, an optically applied, computer-generated clockwise spiral wave and the persisting spiral wave after chirality reversal111. The activity signals from the red and blue pixels indicated in the top panel show four light-controlled chirality reversals. Computer-generated, blue-light spirals were imposed at random phases for just over a cycle, as seen in the four higher-intensity transients (middle panel). Black arrows indicate the time period shown in the top panel. Red and blue arrows indicate the switch in order of excitation at the chosen locations due to chirality reversal. The bottom panel shows activation maps for the initial spiral wave and the four resultant spirals after each of the chirality reversals. b ∣ Attract–drag–anchor control of a spiral wave core towards termination112. The top panel shows the successful termination of a spiral wave in silico by capturing the core by using circular depolarizing light pulses and ‘dragging’ the core to the left boundary in a stepwise fashion. The bottom panel shows the successful termination of a spiral wave in vitro in a manner similar to that shown above. For each light spot, the current location of the applied light is indicated with a filled blue circle. The movement of the tip of the spiral wave, as it is anchored to the location of the light spot at previous time points, is indicated in each frame as a dashed red (in silico) or white (in vitro) line, c ∣ The top-left panel shows a fluorescence mesoscope used for optical mapping and photostimulation. Voltage-sensitive dye fluorescence was imaged using a scientific complementary metal oxide semiconductor (sCMOS) camera, and a digital micromirror device provided patterned light for photostimulation. The top-right panel shows the hardware used for real-time analysis of fluorescence and feedback control. The bottom panel shows optical membrane potential images acquired before and after photostimulation (blue arrowhead), revealing acceleration of ventricular activation after large-field illumination of the epicardium of a perfused mouse heart137. CPU, central processing unit; DAC, digital-to-analogue conversion; DMD, digital micromirror device; LED, light-emitting diode; Obj, objective; ROI, region of interest; SSD, solid state drive; Thr, threshold. Panel a modified with permission from REF.111. Panel b modified with permission from REF.112. Panel c modified with permission from REF.137.
An early experiment demonstrated optical wave steering in cardiomyocyte monolayers by combining dye-free optical imaging for wave sensing with patterned light for localized optogenetic actuation111 (Fig. 6a). A digital micromirror device was driven by sequences of programmed images to project light for the modulation of conduction velocity, to induce unidirectional block, to initiate rotors and to instantaneously reverse the chirality of rotors. This study demonstrated how optical wave steering could provide insights into a range of excitable tissue disorders, including functional and anatomical re-entry29,111. In other experiments, opsins were expressed in non-cardiomyocytes, including 3T3 fibroblasts181, cardiac myofibroblasts143 and stellate ganglion neurons110, to modulate conduction speed. Excitation wave dynamics have also been studied and manipulated within geometrically patterned cultures of human iPSC-CMs and engineered excitable cells using all-optical sensing and actuation138.
Optical wave steering using patterned light is particularly useful for investigating and implementing mechanism-based termination of re-entry. For example, mechanisms of atrial rotor termination have been revealed by illuminating ChR2-expressing rat atrial myocyte layers or slices with specific patterns of light190. Stable rotors were extinguished when an optically positioned, temporary conduction block intersected both the rotor core and an unexcitable boundary. A 2018 study used optogenetics to guide spiral waves towards termination along well-defined trajectories by optically positioning functional conduction blocks with high spatiotemporal precision112 (Fig. 6b). Results from these mechanistic studies and other studies using optogenetics111,136,190 have provided new insights into guided rotor termination. Extending this approach to perfused, ChR2-expressing mouse hearts, Scardigli and colleagues designed and tested a novel system engineered for real-time, optical sensing and stimulation to control excitation waves137 (Fig. 6c). A region-averaged fluorescence signal of electrical activity was monitored, and patterned light pulses were applied to the epicardium when a threshold was exceeded. The system reacted to non-stationary wave dynamics within 2 ms. This system could accelerate bundle-branch-mediated ventricular depolarization, restore atrioventricular conduction after atrioventricular node inhibition, and generate stable, apex-to-base re-entrant circuits, whereby sensing at the base and stimulation at the apex provided a virtual excitation pathway137,139.
Although these innovations for rhythm control have been informed by cardiac optogenetics, their clinical implementation does not necessarily require optogenetics. Some control strategies could be deployed by distributed arrays of electrodes, but optical methods remain superior in their spatiotemporal capacity for precise wave control. Furthermore, optogenetic tools developed for research, such as the optical dynamic clamp113, have enabled experimental testing of novel in silico strategies to design synthetic antiarrhythmic ion channels that have gating properties that are modulated by the rate of electrical excitation, thereby enabling the potential for fully autonomous in-situ termination of arrhythmias114.
Leveraging cell-specific control of cardiac function
Cardiac function cannot be controlled in a cell-specific manner using electrical current. Pharmacological agents provide cellular specificity but have inferior resolution of spatiotemporal actuation. Optogenetics uniquely provides both the spatiotemporal resolution of cellular activation, or inhibition, as well as the cell and organelle specificity necessary to bring about new cardiac therapy paradigms (Fig. 7).
Fig. 7 ∣. Long-term translation of cardiac optogenetics for cell-specific control.
The figure shows proof-of-concept results from multiple studies, a ∣ The top-left panel shows an atrioventricular node section from a CX3CR1+ channelrhodopsin 2(ChR2) mouse, showing endogenous ChR2–yellow fluorescent protein (YFP) signal (green) expressed in macrophages and additional staining for HCN4+ cardiomyocytes (red) and nuclei (blue)182. Bar graphs of control and CX3CR1+ChR2 hearts showing the number of conducted atrial stimuli between two non-conducted impulses of a Wenckebach period during light-off and light-on cycles (top-right panel). The bottom panel is an electrocardiogram (ECG) from a CX3CR1+ChR2 mouse heart, illustrating an increased number of conducted atrial stimuli during a light-on cycle. Arrows indicate failure of conduction leading to a missing QRS complex. Numbers indicate AV delay [ms]. b ∣ Evidence of selective expression of ChR2 in the conduction system of a mouse heart by the presence of ChR2-expressing bundles in the interventricular septum (IVS), as well as in the right ventricle (RV) subendocardium126 (top panel). ECG signal showing ectopic beats originated by epicardial photostimulation of the IVS, where light penetration through the myocardium activated Purkinje fibres (bottom panel). c ∣ Energy requirements of optogenetic pacing measured using a computer model of human ventricles146. The left panel shows the response to illumination of ChR2 gene delivery sites (blue circles) in regions of dense Purkinje system arborization. The middle panel shows the response to the same illumination pattern as in the left panel, but with gene delivery specific to the Purkinje system only. The right panel shows the response to His bundle illumination for the Purkinje system-specific gene delivery with light delivered at a single strategic site, requiring approximately four times lower energy (threshold for excitation, Ee,thr) for ventricular pacing than the approach shown in the left panel. d ∣ Cell-specific expression of ChR2 within the cardiac autonomic nervous system. Choline acetyltransferase (ChAT) is an opsin expression promoter for parasympathetic neurons whereas tyrosine hydroxylase (TH) is a promoter for sympathetic neurons (top-left panel). The top-middle panel shows expression of enhanced yellow fluorescent protein (EYFP)–ChR2 (blue) in TH-expressing sympathetic neurons (red) of the left ventricle (LV) of a mouse heart127. The middle-bottom panel shows the heart rate response during photostimulation of intrinsic cardiac TH-expressing neurons51. The top-right panel shows the co-localization of ChAT (green) with EYFP–ChR2 (red) within the nerve bundles of the right atrium142. The bottom-right panel shows the heart rate response during photostimulation of intrinsic cardiac ChAT-expressing neurons142. e ∣ The top panel shows an image of the right paravertebral chain of a mouse stained with TH (red) and green fluorescent protein (GFP; green), with expression of ChR2 in the sympathetic neurons of the stellate ganglion (SG)204. The insets show single-plane images of the SG. Blue dashed boxes indicate the location of higher magnification images shown in the blue boxes. The bottom panel is a representative heart rate response during photostimulation of the craniomedial right SG. T2G, second thoracic ganglion. Panel a modified with permission from REF.182. Panel b modified with permission from REF.126. Panel c modified with permission from REF.146. Part d top-middle panel modified with permission from REF.127. Part d bottom panels modified with permission from REF.51. Part d top-right panel modified with permission from REF.142. Part e top panel modified with permission from REF.204.
Cre–lox recombination and cell-specific promoters are used to target opsin expression within specific cell types202, for example, macrophages (Fig. 7a). In a Cre–loxP transgenic approach, a Cre-responder animal would have a loxP-flanked STOP cassette upstream of the opsin gene, preventing its expression. This animal would be crossed with a Cre driver animal expressing Cre recombinase under the control of a cell-specific promoter, such as the Myh6 promoter for cardiomyocytes126,131, the Th promoter for sympathetic neurons127,203 or the Chat promoter for parasympathetic neurons142,204. In the mice progeny, the opsin would then be expressed only within cells in which the promoter is active. Alternatively, with viral transduction, cell specificity is determined by the site of delivery (if localized), by tissue tropism of the viral vector and by the promoter used to drive expression. For example, for both localized and systemic vector delivery, the use of ubiquitous promoters, such as the cytomegalovirus or the CAG promoters, in combination with the cardiotropic AAV9 vector result in preferential ventricular myocyte transduction, with much lower expression in atrial myocytes and nonmyocytes. To drive more robust atrial myocyte expression, one study used local gene painting and the NPPA promoter191 (Fig. 5e).
A 2015 study used transgenic mice and Cre–lox recombination to express ChR2 in either cardiomyocytes (Myh6 promoter) or cells of the specialized conduction system (Cx40 promoter)126 (Fig. 7b). This cell-specific expression provided insight into the arrhythmogenic role of the myocardium and the specialized conduction system during local ischaemia. In open-chest experiments, optical stimulation (photostimulation) of the cardiomyocytes triggered sustained arrhythmias after coronary artery ligation, but photostimulation of the specialized conduction system did not. Similar viral vector-based transduction of the Purkinje network could yield low-energy optogenetic pacing146 (Fig. 7c), as discussed above. In addition, specific cell populations or myocardial regions prone to arrhythmogenic activity could be optically targeted to suppress arrhythmias.
Neurocardiology is a classic area that has benefited from the cell-specificity of optogenetics. The complexity of the intrinsic cardiac autonomic network has been revealed using Cre–lox recombination in transgenic mice or viral delivery of fluorescent protein vectors, with impressive resolution142, particularly after tissue clearing204. Neuron-specific expression of opsins enables precise functional mapping of cardioneural circuits, similar to what has been done for the brain205. Original studies in this area used transgenic mice with ChR2 expression targeted to either catecholaminergic neurons (using a Th promoter)127 or cholinergic neurons (using a Chat promoter)142. Selective photostimulation of intrinsic catecholaminergic neurons in perfused mouse hearts initiated sudden and dramatic increases in heart rate and contractility127 (Fig. 7d), consistent with downstream activation of β-adrenergic pathways and increases in cytosolic cAMP levels203.
Another study used a silicon-encapsulated micro LED placed on the right atrium of perfused mouse hearts to photostimulate cholinergic neurons142 (Fig. 7d). The RR interval quickly increased after illuminating the right atrium, consistent with activation of the Gαi/o-coupled cholinergic M2 muscarinic receptors of sinus node cells206. LED positioning altered the responses between heart rate slowing without atrioventricular delay and atrioventricular delay without heart rate slowing, confirming a cholinergic neural network whereby the sinoatrial node and the atrioventricular node are innervated by distinct axons207,208. Photostimulation also maintained substantially low heart rates for up to 30 min of continuously pulsed illumination142, indicating that continuous photostimulation of the cholinergic network could be an approach for in vivo heart rate control. In other studies, photostimulation of autonomic neurons in the stellate ganglion and the vagus nerve increased and decreased heart rate, respectively204 (Fig. 7e), supporting efficacious optical neuromodulation for the treatment of rhythm disorders209. Beyond rhythm control, in vitro studies with co-cultured cardiomyocytes and optogenetically enabled sympathetic neurons have revealed the effects of neuron–cardiomyocyte interactions on electrical wave dynamics110 as well as the effect of cardiomyocytes on neuronal maturation210.
Optogenetic suppression of sympatho-mediated ventricular arrhythmias has been reported in canine studies of acute ischaemia211. AAV9–ArchT was injected locally in the left stellate ganglion neurons, which were subsequently illuminated by an LED for an hour after coronary artery ligation. The resulting neuronal hyperpolarization greatly reduced the incidence of ventricular tachycardia and fibrillation211, providing proof-of-concept results of optogenetic arrhythmia therapy in a large-animal model. Earlier studies expressed excitatory opsins within specific neural populations of the cardiac centres of the brain stem. Those neural populations were then photostimulated to provide myocardial protection during ischaemia–reperfusion injury in rats212 and to increase exercise capacity in healthy rats213. Optogenetic neuromodulation to improve cardiac function and the associated devices that would deliver light to the cardiac ganglia and peripheral nerves, including the vagus nerve, are important mid-term future translational opportunities. Cell-specific control provided by optogenetics currently does not exist for electrical neuromodulation therapies, such as electrical stimulation of the vagus nerve.
Any cardiovascular cell type could likely be inscribed with light-sensitivity, especially as new Cre-expressing animal models become available and viral vectors are optimized. For example, one study used Cre–lox recombination in mice to target the expression of ChR2 to resident cardiac macrophages with the use of the Cx3cr1 promoter182 (Fig. 7a). Atrioventricular conduction was improved after macrophage photostimulation, underscoring the importance of connexin 43 coupling between macrophages and cardiomyocytes for normal atrioventricular node conduction. Other studies used targeted optogenetic sensor expression to assess the electrotonic coupling of excitable and non-excitable cells74 and to modulate coronary artery vasomotor tone214. A comprehensive list of studies that have applied optogenetic tools for cell-specific control of neurocardiac function110,127,142,203,204,210-213,215 and control of myocardial function via non-myocyte cells1,4,39,74,124,126,132,143,146,169,180-182,191,214,216-218 is provided in Table 1.
Conclusions
Over the past 10 years, the potential of cardiac optogenetics to empower basic and translational research has been demonstrated by an impressive growth of published reports (Table 1). These include fundamental developments in translating optogenetics from the brain to the heart, the development of optogenetically-enabled, high-throughput screening technologies for cardiotoxicity and successful proof-of-concept applications of optogenetics for cardiac rhythm control and defibrillation. Immediate translational opportunities reside in all-optical platforms that use iPSC-CMs for pharmaceutical discovery, development and toxicity screening because this strategy does not require incorporation of optogenetic tools in patients (Box 1). All-optical platforms are inherently high-throughput, offering rapid testing, revision and re-testing of new drugs and therapies, particularly those involving patient-derived cells and tissues for personalized medicine and myocardial regeneration.
Box 1 ∣. Translational opportunities and challenges for cardiac optogenetics.
Translational opportunities
Optical platforms are inherently high-throughput, offering immediate near-term translation in cardiotoxicity testing, drug discovery and optimization of patient-derived cells and tissues for personalized medicine and cardiac regeneration.
Long-term clinical translation can include fundamentally new methods for painless and ultra-low-energy (ventricular) cardioversion or defibrillation and rhythm control, enabled by the cell-specificity of optogenetics and its exquisite level of spatiotemporal feedback control, such as ‘wave steering’ rather than a brute-force shock.
Targeted optogenetic disruption of re-entrant circuits can offer non-destructive, tissue-sparing methods for diagnosis and control of high-prevalence atrial arrhythmias compared with current ablation methods.
Optogenetic methods can yield a range of ‘optoceuticals’ for cardiac-specific neuromodulation, control of inflammation, signalling and gene expression to improve heart function.
Cardiac applications can be informed and empowered by fundamental discoveries enabled by optogenetics, including a version of an ‘optical clamp’.
Translational challenges
Gene therapy is part of optogenetic approaches, and all the technical and regulatory challenges that come with gene therapy affect the clinical translation of optogenetics. Cardiac optogenetics-inspired disruptive technology solutions have to out-compete the well-established and fairly safe electrical therapy devices for the heart.
Adeno-associated virus-based viral vectors, which are the most common gene-therapy tools, face challenges in cardiac use, including specificity and physical accessibility to the heart to get site-specific expression of the target gene, obtaining good efficiency when patients have neutralizing antibodies against the viral vectors, and potential cardiotoxicity of the viral capsid or the opsins.
Major challenges for implantable light-delivering and/or light-sensing devices exist due to lack of easy access and/or stabilizing surface, limited light penetration in the thick, dense cardiac muscle, and the mechanical motion of the heart.
The limited palette of well-characterized promoters does not allow as extensive cell selectivity of optogenetic targeting in the cardiovascular system as in the brain.
Challenges in translating cardiac optogenetics to the clinic are substantial and confounded by the obvious need for new therapies to be better than the current standard of care (Box 1). Present-day cardiac devices have reliable efficacy and are fairly safe, setting a high barrier for optogenetics-inspired therapies. Nevertheless, the desire to improve patient quality of life is a strong motivation for further device innovations. Technical challenges for translation reside in the methods used to deliver opsins and light to the heart. To achieve opsin expression in patients would require gene therapy via AAV-based vectors or similar, and a minimally-invasive procedure would be required for cardiac site-specific transduction of these vectors. Efficient expression of opsins in the presence of neutralizing antibodies in the host against the viral vectors is a challenge as well as the risk of cardiotoxicity associated with viral capsids and opsins, as covered in a previous review on the current state of human gene therapy219. The possibility for the algae-derived opsins to trigger an immune response when expressed in the human heart deserves serious investigation before clinical deployment. Engaging resident opsins with light is further confounded by the vigorous contraction of the heart and the lack of a stabilizing surface to anchor an optical device. Visible light absorption by the blood remains a challenge that has motivated recent work to develop long-wavelength opsins, upconversion nanoparticle mediators for blue-light opsins, and other performance-boosting strategies. Finally, to leverage fully the cell-specific optogenetic control of cardiac function, the palette of well-characterized, cell-specific promoters would need to be expanded, at least to the level of what is currently available for neuroscience.
Even with these challenges, the envisioned clinical opportunities for cardiac optogenetics are plentiful (Box 1). Exquisite spatiotemporal control provided by light and the cell-specific expression provides new fundamental methods for painless rhythm management. These methods include ‘wave steering’, ultra-low-energy cardioversion or defibrillation, the shaping of action potentials with the use of both depolarizing and hyperpolarizing opsins, and long-term hyperpolarization to inhibit cell excitability. Optical therapy for atrial arrhythmias is likely to be on the horizon in the mid-future. As the general population ages, the market potential for optogenetic treatment of atrial arrhythmias grows. Patients with arrhythmias could receive cardioversion repeatedly with the use of light without the pain and tissue damage that occurs with current ablation therapy. Upon eventual translation into humans, optogenetics could be the specific therapy, whereby ‘optoceuticals’, through cell-specific expression, would modulate the activity of cardiac neurons, control inflammation and activate or suppress cell signalling and gene expression. Continued research and development in cardiac optogenetic technologies will undoubtedly inform and guide the development of a new generation of cardiac therapies.
Key points.
Cardiac optogenetics leverages genetically encoded optical sensors and actuators to empower basic and translational research, as evidenced by an impressive growth of published reports over the past decade.
Immediate translational opportunities reside in all-optical platforms that are inherently high-throughput, offering rapid testing and discovery of new drugs and therapies with the use of patient-derived cells for personalized medicine and myocardial regeneration.
Optogenetics offers technical innovations for cardiac rhythm control in patients and long-term opportunities for clinical translation for ultra-low-energy rhythm control, precise autonomic neuromodulation and painless defibrillation, which are based on the cell-specificity and space-time resolution of optogenetics.
Challenges in translating cardiac optogenetics to the clinic are mainly linked to safe opsin delivery to the heart, cell-specific expression and engaging the opsins with light deep within the tissue.
Acknowledgements
In this Review, we have attempted a comprehensive coverage of all work in the area over the past 10 years; we apologize for any potentially missing publications. We greatly appreciate the critical contributions of scientists and trainees in the Entcheva lab and of the Kay lab at The George Washington University, USA, for their many questions and discussions regarding numerous scientific aspects of cardiac optogenetics. For the figures in the initial submission of the manuscript, former trainees Aleks Klimas prepared a draft of parts of Fig. 2 and Yiyang Wu prepared parts of Fig. 4 (disease modelling), and current trainee Bridget Alber prepared parts of Fig. 7. The authors are supported in part by grants from the NIH (R01-HL144157, R01-HL133862, R01-HL146169, R01-HL147279 and R21-EB026152) and the National Science Foundation (EFMA 1830941, CBET1705645 and PFI 1827535).
Glossary terms
- Tandem cell unit
A multicellular unit comprised of light-sensitive, non-excitable cells that are electrically coupled to non-transduced excitable cells.
- Optical dynamic clamp
A real-time feedback system to ‘inject’ a desired current into light-sensitized cells or tissue according to a computer model and voltage measurements.
- Cardioversion
A procedure that restores normal sinus rhythm to a heart that has an arrhythmia.
- Gene painting
Transduction of tissue with a gene by ‘painting’ it with adenovirus, using a solution containing the vector, an adhering polymer and a weak protease to promote vector penetration.
- Upconversion nanoparticles
Optical nanomaterials, typically doped with lanthanide ions, that up-convert two or more lower-energy photons (longer wavelengths) into one high-energy photon (shorter wavelength).
- Optical wave steering
Specific space–time dynamic light patterns are used to control the speed and direction of an electrical wavefront within excitable tissue that expresses an opsin.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Cardiology thanks L. Sacconi and D. Pijnappels and the other, anonymous, reviewer for their contribution to the peer review of this work.
References
- 1.Arrenberg AB, Stainier DY, Baier H & Huisken J Optogenetic control of cardiac function. Science 330, 971–974 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Bruegmann T et al. Optogenetic control of heart muscle in vitro and in vivo. Nat. Methods 7, 897–900 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Hofmann B et al. Light induced stimulation and delay of cardiac activity. Lab Chip 10, 2588–2596 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Jia Z et al. Stimulating cardiac muscle by light: Cardiac optogenetics by cell delivery. Circ. Arrhythm. Electrophysiol 4, 753–760 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salama G & Morad M Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science 48, 485–487 (1976). [DOI] [PubMed] [Google Scholar]
- 6.Morad M & Salama G Optical probes of membrane potential in heart muscle. J. Physiol 292, 267–295 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tallini YN et al. Imaging cellular signals in the heart in vivo. Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc. Natl Acad. Sci. USA 103, 4753–4758 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roell W et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature 450, 819–824 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Chi NC et al. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoSBiol. 6, e109 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deisseroth K et al. Next-generation optical technologies for illuminating genetically targeted brain circuits. J. Neurosci 26, 10380–10386 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyden ES, Zhang F, Bamberg E, Nagel G & Deisseroth K Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci 8, 1263–1268 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Li X et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl Acad. Sci. USA 102, 17816–17821 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagel G et al. Light activation of channelrhodopsin-2 in excitable cells of caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol 15, 2279–2284 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Nagel G et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl Acad. Sci. USA 100, 13940–13945 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagel G et al. Channelrhodopsin-1: A light-gated proton channel in green algae. Science 296, 2395–2398 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Miesenbock G The optogenetic catechism. Science 326, 395–399 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Zemelman BV, Lee GA, Ng M & Miesenbock G Selective photostimulation of genetically charged neurons. Neuron 33, 15–22 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Zemelman BV, Nesnas N, Lee GA & Miesenbock G Photochemical gating of heterologous ion channels: Remote control over genetically designated populations of neurons. Proc. Natl Acad. Sci. USA 100, 1352–1357 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abilez OJ Cardiac optogenetics. Conf. Proc. IEEE Eng. Med. Biol. Soc 2012, 1386–1389 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Entcheva E Cardiac optogenetics. Am. J. Physiol. Heart Circ. Physiol 304, H1179–H1191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrosi CM, Klimas A, Yu J & Entcheva E Cardiac applications of optogenetics. Prog. Biophys. Mol. Biol 115, 294–304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambrosi CM & Entcheva E Optogenetics' promise: Pacing and cardioversion by light? Future Cardiol. 10, 1–4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle PM, Entcheva E & Trayanova NA See the light: Can optogenetics restore healthy heartbeats? And, if it can, is it really worth the effort? Expert Rev. Cardiovasc. Ther 12, 17–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaestner L et al. Genetically encoded Ca2+ indicators in cardiac myocytes. Circ. Res 114, 1623–1639 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Bruegmann T & Sasse P Optogenetic cardiac pacemakers: Science or fiction? Trends Cardiovasc. Med 25, 82–83 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Boyle PM, Karathanos TV & Trayanova NA "Beauty is a light in the heart": The transformative potential of optogenetics for clinical applications in cardiovascular medicine. Trends Cardiovasc. Med 25, 73–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyle PM, Karathanos TV, Entcheva E & Trayanova NA Computational modeling of cardiac optogenetics: Methodology overview & review of findings from simulations. Comput. Biol. Med 65, 200–208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karathanos TV, Boyle PM & Trayanova NA Light-based approaches to cardiac arrhythmia research: From basic science to translational applications. Clin. Med. Insights Cardiol 10, 47–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Entcheva E & Bub G All-optical control of cardiac excitation: Combined high-resolution optogenetic actuation and optical mapping. J. Physiol 594, 2503–2510 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richter C, Christoph J, Lehnart SE & Luther S Optogenetic light crafting tools for the control of cardiac arrhythmias. Methods Mol. Biol 1408, 293–302 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Montgomery KL, Iyer SM, Christensen AJ, Deisseroth K & Delp SL Beyond the brain: Optogenetic control in the spinal cord and peripheral nervous system. Sci. Transl Med 8, 337rv5 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Pianca N, Zaglia T & Mongillo M Will cardiac optogenetics find the way through the obscure angles of heart physiology? Biochem. Biophys. Res. Commun 482, 515–523 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Zhang H & Cohen AE Optogenetic approaches to drug discovery in neuroscience and beyond. Trends Biotechnol. 35, 625–639 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Zou P & Cohen AE Voltage imaging with genetically encoded indicators. Curr. Opin. Chem. Biol 39, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gepstein L & Gruber A Optogenetic neuromodulation of the heart. J. Am. Coll. Cardiol 70, 2791–2794 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Crocini C, Ferrantini C, Pavone FS & Sacconi L Optogenetics gets to the heart: A guiding light beyond defibrillation. Prog. Biophys. Mol. Biol 130, 132–139 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Koopman CD, Zimmermann WH, Knopfel T & de Boer TP Cardiac optogenetics: Using light to monitor cardiac physiology. Basic Res. Cardiol 112, 56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pomeroy JE, Nguyen HX, Hoffman BD & Bursac N Genetically encoded photoactuators and photosensors for characterization and manipulation of pluripotent stem cells. Theranostics 7, 3539–3558 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston CM et al. Optogenetic targeting of cardiac myocytes and non-myocytes: Tools, challenges and utility. Prog. Biophys. Mol. Biol 130, 140–149 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Boyle PM, Karathanos TV & Trayanova NA Cardiac optogenetics: 2018. JACC Clin. Electrophysiol 4, 155–167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider-Warme F The power of optogenetics: Potential in cardiac experimental and clinical electrophysiology. Herzschrittmacherther. Elektrophysiol 29, 24–29 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Gruber A, Edri O & Gepstein L Cardiac optogenetics: The next frontier. Europace 20, 1910–1918 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Sasse P, Funken M, Beiert T & Bruegmann T Optogenetic termination of cardiac arrhythmia: Mechanistic enlightenment and therapeutic application? Front. Physiol 10, 675 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Shea C et al. Cardiac optogenetics and optical mapping – overcoming spectral congestion in all-optical cardiac electrophysiology. Front. Physiol 10, 182 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferenczi EA, Tan X & Huang CL-H Principles of optogenetic methods and their application to cardiac experimental systems. Front. Physiol 10, 1096 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joshi J, Rubart M & Zhu W Optogenetics: Background, methodological advances and potential applications for cardiovascular research and medicine. Front. Bioeng. Biotechnol 7, 466 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaglia T, Di Bona A & Mongillo M A light wand to untangle the myocardial cell network. Methods Protoc. 2, 34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hussaini S et al. Drift and termination of spiral waves in optogenetically modified cardiac tissue at sub-threshold illumination. Preprint at https://www.biorxiv.org/content/10.1101/2020.06.12.148734v1 (2020). [DOI] [PMC free article] [PubMed]
- 49.Richter C & Bruegmann T No light without the dark: Perspectives and hindrances for translation of cardiac optogenetics. Prog. Biophys. Mol. Biol 154, 39–50 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Bub G & Daniels MJ Feasibility of using adjunctive optogenetic technologies in cardiomyocyte phenotyping - from the single cell to the whole heart. Curr. Pharm. Biotechnol 21, 752–764 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreno A, Kowalik G & Kay MW Optogenetic control of cardiac autonomic neurons in transgenic mice. Methods Mol. Biol 2191, 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyawaki A et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388, 882–887 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Chen TW et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inoue M et al. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nat. Methods 12, 64–70 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Akerboom J et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci 6, 2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y et al. An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dana H et al. Sensitive red protein calcium indicators for imaging neural activity. eLife 5, e12727 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qian Y et al. A genetically encoded near-infrared fluorescent calcium ion indicator. Nat. Methods 16, 171–174 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakao S, Wakabayashi S & Nakamura TY Stimulus-dependent regulation of nuclear Ca2+ signaling in cardiomyocytes: A role of neuronal calcium sensor-1. PLoS One 10, 1–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dempsey GT et al. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J. Pharmacol. Toxicol. Methods 81, 240–250 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Björk S et al. Evaluation of optogenetic electrophysiology tools in human stem cell-derived cardiomyocytes. Front. Physiol 8, 884 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klimas A, Ortiz G, Boggess SC, Miller EW & Entcheva E Multimodal on-axis platform for all-optical electrophysiology with near-infrared probes in human stem-cell-derived cardiomyocytes. Prog. Biophys. Mol. Biol 154, 62–70 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei L, Li W, Entcheva E & Li Z Microfluidics-enabled 96-well perfusion system for high-throughput tissue engineering and long-term all-optical electrophysiology. Lab Chip 20, 4031–4042 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song L et al. Dual optical recordings for action potentials and calcium handling in induced pluripotent stem cell models of cardiac arrhythmias using genetically encoded fluorescent indicators. Stem Cells TranslMed. 4, 468–475 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Streit J & Kleinlogel S Dynamic all-optical drug screening on cardiac voltage-gated ion channels. Sci. Rep 8, 1153–1153 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lundby A, Akemann W & Knopfel T Biophysical characterization of the fluorescent protein voltage probe vsfp2.3 based on the voltage-sensing domain of ci-vsp. Eur. Biophys. J 39, 1625–1635 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Lundby A, Mutoh H, Dimitrov D, Akemann W & Knopfel T Engineering of a genetically encodable fluorescent voltage sensor exploiting fast Ci-VSP voltage-sensing movements. PLoS One 3, e2514 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin L et al. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron 75, 779–785 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdelfattah AS et al. A bright and fast red fluorescent protein voltage indicator that reports neuronal activity in organotypic brain slices. J. Neurosci 36, 2458–2472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biendarra-Tiegs SM et al. Single-cell RNA-sequencing and optical electrophysiology of human induced pluripotent stem cell-derived cardiomyocytes reveal discordance between cardiac subtype-associated gene expression patterns and electrophysiological phenotypes. Stem Cells Dev. 28, 659–673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shaheen N et al. Human induced pluripotent stem cell-derived cardiac cell sheets expressing genetically encoded voltage indicator for pharmacological and arrhythmia studies. Stem Cell Reports 10, 1879–1894 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shinnawi R et al. Monitoring human-induced pluripotent stem cell-derived cardiomyocytes with genetically encoded calcium and voltage fluorescent reporters. Stem Cell Reports 5, 582–596 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leyton-Mange JS et al. Rapid cellular phenotyping of human pluripotent stem cell-derived cardiomyocytes using a genetically encoded fluorescent voltage sensor. Stem Cell Reports 2, 163–170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quinn TA et al. Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proc. Natl Acad. Sci. USA 113, 14852–14857 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liao MLC et al. Sensing cardiac electrical activity with a cardiac myocyte-targeted optogenetic voltage indicator. Circ. Res 117, 401–412 (2015). [DOI] [PubMed] [Google Scholar]
- 76.Villette V et al. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice. Cell 179, 1590–1608.e23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chamberland S et al. Fast two-photon imaging of subcellular voltage dynamics in neuronal tissue with genetically encoded indicators. eLife 6, e25690 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.St-Pierre F et al. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat. Neurosci 17, 884–889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdelfattah AS et al. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 365, 699–704 (2019). [DOI] [PubMed] [Google Scholar]
- 80.Hochbaum DR et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 11, 825–833 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piatkevich KD et al. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat. Chem. Biol 14, 352–360 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Monakhov MV et al. Bright near-infrared genetically encoded voltage indicator for all-optical electrophysiology. Preprint at https://www.biorxiv.org/content/10.1101/536359v1 (2019).
- 83.Hou JH, Kralj JM, Douglass AD, Engert F & Cohen AE Simultaneous mapping of membrane voltage and calcium in zebrafish heart in vivo reveals chamber-specific developmental transitions in ionic currents. Front. Physiol 5, 1–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trautsch I et al. Optogenetic monitoring of the glutathione redox state in engineered human myocardium. Front. Physiol 10, 272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klimas A & Entcheva E Toward microendoscopy-inspired cardiac optogenetics in vivo: Technical overview and perspective. J. Biomed. Opt 19, 080701 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Opbergen CJM et al. Optogenetic sensors in the zebrafish heart: A novel in vivo electrophysiological tool to study cardiac arrhythmogenesis. Theranostics 8, 4750–4764 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mutoh H & Knopfel T Probing neuronal activities with genetically encoded optical indicators: From a historical to a forward-looking perspective. Pflugers Arch. 465, 361–371 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Alford SC, Wu J, Zhao Y, Campbell RE & Knopfel T Optogenetic reporters. Biol. Cell 105, 14–29 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Knopfel T & Boyden ES Tools for observing and controlling specific molecular or physiological pathways in intact cells and tissues. Preface. Prog. Brain Res 196, vii–viii (2012). [DOI] [PubMed] [Google Scholar]
- 90.Dugue GP, Akemann W & Knöpfel T A comprehensive concept of optogenetics. Prog. Brain Res 196, 1–28 (2012). [DOI] [PubMed] [Google Scholar]
- 91.Shui B, Lee JC, Reining S, Lee FK & Kotlikoff MI Optogenetic sensors and effectors: CHROMus—the Cornell Heart Lung Blood Institute resource for optogenetic mouse signaling. Front. Physiol 5, 428 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klapoetke NC et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin JY, Knutsen PM, Muller A, Kleinfeld D & Tsien RY Reachr: A red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci 16, 1499 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cosentino C et al. Optogenetics. Engineering of a light-gated potassium channel. Science 348, 707–710 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Bernal Sierra YA et al. Potassium channel-based optogenetic silencing. Nat. Commun 9, 4611 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Govorunova EG, Cunha SR, Sineshchekov OA & Spudich JL Anion channelrhodopsins for inhibitory cardiac optogenetics. Sci. Rep 6, 33530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han X et al. A high-light sensitivity optical neural silencer: Development, and application to optogenetic control of nonhuman primate cortex. Front. Syst. Neurosci 5, 18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gradinaru V, Thompson KR & Deisseroth K Enphr: A natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 36, 129–139 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chuong AS et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci 17, 1123–1129 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yizhar O, Fenno L, Zhang F, Hegemann P & Diesseroth K Microbial opsins: A family of single-component tools for optical control of neural activity. Cold Spring Harb. Protoc 2011, top102 (2011). [DOI] [PubMed] [Google Scholar]
- 101.Schneider F, Grimm C & Hegemann P Biophysics of channelrhodopsin. Annu. Rev. Biophys 44, 167–186 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Williams JC et al. Computational optogenetics: Empirically-derived voltage- and light-sensitive channelrhodopsin-2 model. PLoS Comput. Biol 9, e1003220 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williams JC & Entcheva E Optogenetic versus electrical stimulation of human cardiomyocytes: Modeling insights. Biophys. J 108, 1934–1945 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kato HE et al. Atomistic design of microbial opsin-based blue-shifted optogenetics tools. Nat. Commun 6, 7177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oppermann J et al. Mermaids: A family of metagenomically discovered marine anion-conducting and intensely desensitizing channelrhodopsins. Nat. Commun 10, 3315 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park SA, Lee SR, Tung L & Yue DT Optical mapping of optogenetically shaped cardiac action potentials. Sci. Rep 4, 6125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Entcheva E & Williams JC Channelrhodopsin2 current during the action potential: "Optical AP clamp" and approximation. Sci. Rep 4, 5838 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karathanos TV, Boyle PM & Trayanova NA Optogenetics-enabled dynamic modulation of action potential duration in atrial tissue: Feasibility of a novel therapeutic approach. Europace 16 (Suppl. 4), iv69–iv76 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ambrosi CM, Williams JC & Entcheva E Optogenetic modulation of pacemaking, arrhythmia generation and inhibition with sustained (non-pulsed) light [abstract]. Circulation 130, A19856 (2014). [Google Scholar]
- 110.Burton RB et al. Optical interrogation of sympathetic neuronal effects on macroscopic cardiomyocyte network dynamics. iScience 23, 101334 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burton RAB et al. Optical control of excitation waves in cardiac tissue. Nat. Photonics 9, 813–816 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Majumder R et al. Optogenetics enables real-time spatiotemporal control over spiral wave dynamics in an excitable cardiac system. eLife 7, e41076 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Quach B, Krogh-Madsen T, Entcheva E & Christini DJ Light-activated dynamic clamp using ipsc-derived cardiomyocytes. Biophys. J 115, 2206–2217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Majumder R et al. Self-restoration of cardiac excitation rhythm by anti-arrhythmic ion channel gating. eLife 9, e55921 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tkatch T et al. Optogenetic control of mitochondrial metabolism and Ca2+ signaling by mitochondria-targeted opsins. Proc. Natl Acad. Sci. USA 114, E5167–E5176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dixon RE, Yuan C, Cheng EP, Navedo MF & Santana LF Ca2+ signaling amplification by oligomerization of L-type Cav1.2 channels. Proc. Natl Acad. Sci. USA 109, 1749–1754 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beiert T, Bruegmann T & Sasse P Optogenetic activation of Gq signalling modulates pacemaker activity of cardiomyocytes. Cardiovasc. Res 102, 507–516 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Makowka P et al. Optogenetic stimulation of Gs-signaling in the heart with high spatio-temporal precision. Nat. Commun 10, 1281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kleinlogel S Optogenetic user's guide to opto-gpcrs. Front. Biosci 21, 794–805 (2016). [DOI] [PubMed] [Google Scholar]
- 120.Yakushenko A et al. On-chip optical stimulation and electrical recording from cells. J. Biomed. Opt 18, 111402 (2013). [DOI] [PubMed] [Google Scholar]
- 121.Mickoleit M et al. High-resolution reconstruction of the beating zebrafish heart. Nat. Methods 11, 919–922 (2014). [DOI] [PubMed] [Google Scholar]
- 122.Bingen BO et al. Light-induced termination of spiral wave arrhythmias by optogenetic engineering of atrial cardiomyocytes. Cardiovasc. Res 104, 194–205 (2014). [DOI] [PubMed] [Google Scholar]
- 123.Weber M & Huisken J In vivo imaging of cardiac development and function in zebrafish using light sheet microscopy. Swiss Med. Wkly 145, w14227 (2015). [DOI] [PubMed] [Google Scholar]
- 124.Ambrosi CM, Boyle PM, Chen K, Trayanova NA & Entcheva E Optogenetics-enabled assessment of viral gene and cell therapy for restoration of cardiac excitability. Sci. Rep 5, 17350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alex A, Li A, Tanzi RE & Zhou C Optogenetic pacing in drosophila melanogaster. Sci. Adv 1, e1500639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zaglia T et al. Optogenetic determination of the myocardial requirements for extrasystoles by cell type-specific targeting of channelrhodopsin-2. Proc. Natl Acad. Sci. USA 112, E4495–E4504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wengrowski AM et al. Optogenetic release of norepinephrine from cardiac sympathetic neurons alters mechanical and electrical function. Cardiovasc. Res 105, 143–150 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nussinovitch U & Gepstein L Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat. Biotechnol 33, 750–754 (2015). [DOI] [PubMed] [Google Scholar]
- 129.Klimas A et al. Optodyce as an automated system for high-throughput all-optical dynamic cardiac electrophysiology. Nat. Commun 7, 11542 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nyns ECA et al. Optogenetic termination of ventricular arrhythmias in the whole heart: Towards biological cardiac rhythm management. Eur. Heart J 38, 2132–2136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Crocini C et al. Optogenetics design of mechanistically-based stimulation patterns for cardiac defibrillation. Sci. Rep 6, 35628 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu J et al. Optogap: An optogenetics-enabled assay for quantification of cell-cell coupling in multicellular cardiac tissue. Preprint at https://www.biorxiv.org/content/10.1101/171397v1 (2017). [DOI] [PMC free article] [PubMed]
- 133.Werley CA, Chien MP & Cohen AE Ultrawidefield microscope for high-speed fluorescence imaging and targeted optogenetic stimulation. Biomed. Opt. Express 8, 5794–5813 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weber M et al. Cell-accurate optical mapping across the entire developing heart. eLife 6, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Q et al. Electrophysiological properties and viability of neonatal rat ventricular myocyte cultures with inducible ChR2 expression. Sci. Rep 7, 1531 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Quinoñez Uribe RA, Luther S, Diaz-Maue L & Richter C Energy-reduced arrhythmia termination using global photostimulation in optogenetic murine hearts. Front. Physiol 9, 1651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scardigli M et al. Real-time optical manipulation of cardiac conduction in intact hearts. J. Physiol 596, 3841–3858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McNamara HM et al. Geometry-dependent arrhythmias in electrically excitable tissues. Cell Syst. 7, 359–370.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Giardini F et al. A software architecture to mimic a ventricular tachycardia in intact murine hearts by means of an all-optical platform. Methods Protoc. 2, 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nguyen C et al. Simultaneous voltage and calcium imaging and optogenetic stimulation with high sensitivity and a wide field of view. Biomed. Opt. Express 10, 789–806 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dong R et al. A protocol for dual calcium-voltage optical mapping in murine sinoatrial preparation with optogenetic pacing. Front. Physiol 10, 954 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moreno A et al. Sudden heart rate reduction upon optogenetic release of acetylcholine from cardiac parasympathetic neurons in perfused hearts. Front. Physiol 10, 16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kostecki G et al. Optogenetic currents in myofibroblasts acutely alter electrophysiology and conduction of co-cultured cardiomyocytes. Preprint at https://www.biorxiv.org/content/10.1101/2020.06.02.124529v1 (2020). [DOI] [PMC free article] [PubMed]
- 144.Li W, Han JL & Entcheva E Syncytium cell growth increases Kir2.1 contribution in human iPSC-cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol 10.1152/ajpheart.00148.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wong J, Abilez OJ & Kuhl E Computational optogenetics: A novel continuum framework for the photoelectrochemistry of living systems. J. Mech. Phys. Solids 60, 1158–1178 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boyle PM, Williams JC, Ambrosi CM, Entcheva E & Trayanova NA A comprehensive multiscale framework for simulating optogenetics in the heart. Nat. Commun 4, 2370 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bruegmann T et al. Optogenetic defibrillation terminates ventricular arrhythmia in mouse hearts and human simulations. J. Clin. Invest 126, 3894–3904 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Karathanos TV, Bayer JD, Wang D, Boyle PM & Trayanova NA Opsin spectral sensitivity determines the effectiveness of optogenetic termination of ventricular fibrillation in the human heart: A simulation study. J. Physiol 594, 6879–6891 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boyle PM et al. Termination of re-entrant atrial tachycardia via optogenetic stimulation with optimized spatial targeting: Insights from computational models. J. Physiol 596, 181–196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abilez OJ et al. Multiscale computational models for optogenetic control of cardiac function. Biophys. J 101, 1326–1334 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shi Y, Inoue H, Wu JC & Yamanaka S Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov 16, 115–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gintant G et al. Use of human induced pluripotent stem cell-derived cardiomyocytes in preclinical cancer drug cardiotoxicity testing: A scientific statement from the American Heart Association. Circ. Res 125, e75–e92 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Abilez OJ Optogenetic led array for perturbing cardiac electrophysiology. Conf. Proc. IEEE Eng. Med. Biol. Soc 2013, 1619–1622 (2013). [DOI] [PubMed] [Google Scholar]
- 154.Leyton-Mange JS & Milan DJ Pluripotent stem cells as a platform for cardiac arrhythmia drug screening. Curr. Treat. Options Cardiovasc. Med 16, 334 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhuge Y et al. Human pluripotent stem cell tools for cardiac optogenetics. Conf. Proc. IEEE Eng. Med. Biol. Soc 2014, 6171–6174 (2014). [DOI] [PubMed] [Google Scholar]
- 156.Klimas A et al. Disease modeling in human induced pluripotent stem cell derived cardiomyocytes using high-throughput all-optical dynamic cardiac electrophysiology [abstract FF3A.3]. Front. Optics 10.1364/FIO.2016.FF3A.3 (2017). [DOI] [Google Scholar]
- 157.Chang YF et al. Non-invasive phenotyping and drug testing in single cardiomyocytes or beta-cells by calcium imaging and optogenetics. PLoS One 12, e0174181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lapp H et al. Frequency-dependent drug screening using optogenetic stimulation of human ipsc-derived cardiomyocytes. Sci. Rep 7, 9629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rehnelt S et al. Frequency-dependent multi-well cardiotoxicity screening enabled by optogenetic stimulation. Int. J. Mol. Sci 18, 2634 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Park SJ et al. Insights into the pathogenesis of catecholaminergic polymorphic ventricular tachycardia from engineered human heart tissue. Circulation 140, 390–404 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Patel D, Stohlman J, Dang Q, Strauss DG & Blinova K Assessment of proarrhythmic potential of drugs in optogenetically paced induced pluripotent stem cell-derived cardiomyocytes. Toxicol. Sci 170, 167–179 (2019). [DOI] [PubMed] [Google Scholar]
- 162.Dwenger M et al. Chronic optical pacing conditioning of h-ipsc engineered cardiac tissues. J. Tissue Eng 10, 2041731419841748 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lemme M et al. Chronic intermittent tachypacing by an optogenetic approach induces arrhythmia vulnerability in human engineered heart tissue. Cardiovasc. Res 116, 1487–1499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paci M et al. All-optical electrophysiology refines populations of in silico human iPSC-CMs for drug evaluation. Biophys. J 118, 2596–2611 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rope AF et al. Using vaast to identify an X-linked disorder resulting in lethality in male infants due to N-terminal acetyltransferase deficiency. Am. J. Hum. Genet 89, 28–43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mond HG & Proclemer A The 11th world survey of cardiac pacing and implantable cardioverter–defibrillators: calendar year 2009 — a World Society of Arrhythmias project. Pacing Clin. Electrophysiol 34, 1013–1027 (2011). [DOI] [PubMed] [Google Scholar]
- 167.Huang DT & Traub D Recurrent ventricular arrhythmia storms in the age of implantable cardioverter defibrillator therapy: A comprehensive review. Prog. Cardiovasc. Dis 51, 229–236 (2008). [DOI] [PubMed] [Google Scholar]
- 168.Baumert J, Schmitt C & Ladwig KH Psychophysiologic and affective parameters associated with pain intensity of cardiac cardioverter defibrillator shock discharges. Psychosom. Med 68, 591–597 (2006). [DOI] [PubMed] [Google Scholar]
- 169.Nussinovitch U, Shinnawi R & Gepstein L Modulation of cardiac tissue electrophysiological properties with light-sensitive proteins. Cardiovasc. Res 102, 176–187 (2014). [DOI] [PubMed] [Google Scholar]
- 170.Ambrosi CM & Entcheva E Optogenetic control of cardiomyocytes via viral delivery. Methods Mol. Biol 1181, 215–228 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vogt CC et al. Systemic gene transfer enables optogenetic pacing of mouse hearts. Cardiovasc. Res 106, 338–343 (2015). [DOI] [PubMed] [Google Scholar]
- 172.Chan V et al. Fabrication and characterization of optogenetic, multi-strip cardiac muscles. Lab Chip 15, 2258–2268 (2015). [DOI] [PubMed] [Google Scholar]
- 173.Yu J, Chen K, Lucero RV, Ambrosi CM & Entcheva E Cardiac optogenetics: Enhancement by all-trans-retinal. Sci. Rep 5, 16542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhu YC, Uradu H, Majeed ZR & Cooper RL Optogenetic stimulation of Drosophila heart rate at different temperatures and Ca2+ concentrations. Physiol. Rep 4, e12695 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Feola I, Teplenin A, de Vries AA & Pijnappels DA Optogenetic engineering of atrial cardiomyocytes. Methods Mol. Biol 1408, 319–331 (2016). [DOI] [PubMed] [Google Scholar]
- 176.Ambrosi CM, Sadananda G, Han JL & Entcheva E Adeno-associated virus mediated gene delivery: Implications for scalable in vitro and in vivo cardiac optogenetic models. Front. Physiol 10, 168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kopton RA et al. Electromechanical assessment of optogenetically modulated cardiomyocyte activity. J. Vis. Exp 157, e60490 (2020). [DOI] [PubMed] [Google Scholar]
- 178.Zgierski-Johnston CM et al. Cardiac pacing using transmural multi-LED probes in channelrhodopsin-expressing mouse hearts. Prog. Biophys. Mol. Biol 154, 51–61 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bruegmann T, Beiert T, Vogt CC, Schrickel JW & Sasse P Optogenetic termination of atrial fibrillation in mice. Cardiovasc. Res 114, 713–723 (2018). [DOI] [PubMed] [Google Scholar]
- 180.Yu J & Entcheva E Inscribing optical excitability to non-excitable cardiac cells: Viral delivery of optogenetic tools in primary cardiac fibroblasts. Methods Mol. Biol 1408, 303–317 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Simone SA et al. The role of membrane capacitance in cardiac impulse conduction: An optogenetic study with non-excitable cells coupled to cardiomyocytes. Front. Physiol 11, 194 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hulsmans M et al. Macrophages facilitate electrical conduction in the heart. Cell 169, 510–522.e20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Merrill DR, Bikson M & Jefferys JG Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 141, 171–198 (2005). [DOI] [PubMed] [Google Scholar]
- 184.Zipes D, Fischer J, King R, Nicoll A & Jolly W Termination of ventricular fibrillation in dogs by depolarizing a critical amount of myocardium. Am. J. Cardiol 36, 37–44 (1975). [DOI] [PubMed] [Google Scholar]
- 185.Chattipakorn N, Banville I, Gray RA & Ideker RE Effects of shock strengths on ventricular defibrillation failure. Cardiovasc. Res 61, 39–44 (2004). [DOI] [PubMed] [Google Scholar]
- 186.Kay MW, Walcott GP, Gladden JD, Melnick SB & Rogers JM Lifetimes of epicardial rotors in panoramic optical maps of fibrillating swine ventricles. Am. J. Physiol. Heart Circ. Physiol 291, H1935–H1941 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rickard J & Wilkoff BL Advances in implantable cardioverter defibrillator therapy. Expert Rev. Cardiovasc. Ther 14, 291–299 (2016). [DOI] [PubMed] [Google Scholar]
- 188.Luther S et al. Low-energy control of electrical turbulence in the heart. Nature 475, 235–239 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cheng Y et al. Optogenetic approaches for termination of ventricular tachyarrhythmias after myocardial infarction in rats in vivo. J. Biophotonics 13, e202000003 (2020). [DOI] [PubMed] [Google Scholar]
- 190.Feola I et al. Localized optogenetic targeting of rotors in atrial cardiomyocyte monolayers. Circ. Arrhythm. Electrophysiol 10, e005591 (2017). [DOI] [PubMed] [Google Scholar]
- 191.Nyns ECA et al. An automated hybrid bioelectronic system for autogenous restoration of sinus rhythm in atrial fibrillation. Sci. Transl Med 11, 481 (2019). [DOI] [PubMed] [Google Scholar]
- 192.Rao P et al. Near-infrared light driven tissue-penetrating cardiac optogenetics via upconversion nanoparticles in vivo. Biomed. Opt. Express 11, 1401–1416 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Johannsmeier S et al. Evaluation of a model for deep tissue optogenetic stimulation. SPIE BiOS 10.1117/12.2544837 (2020). [DOI] [Google Scholar]
- 194.Kopton RA et al. Cardiac electrophysiological effects of light-activated chloride channels. Front. Physiol 9, 1806 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Funken M, Malan D, Sasse P & Bruegmann T Optogenetic hyperpolarization of cardiomyocytes terminates ventricular arrhythmia. Front. Physiol 10, 498 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stanley CE, Mauss AS, Borst A & Cooper RL The effects of chloride flux on drosophila heart rate. Methods Protoc. 2, 73 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tallini YN et al. Genetically encoded probes provide a window on embryonic arrhythmia. Methods Mol. Biol 1092, 195–219 (2014). [DOI] [PubMed] [Google Scholar]
- 198.Nussinovitch U & Gepstein L Optogenetics for suppression of cardiac electrical activity in human and rat cardiomyocyte cultures. Neurophotonics 2, 031204 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Watanabe M et al. Optogenetic manipulation of anatomical re-entry by light-guided generation of a reversible local conduction block. Cardiovasc. Res 113, 354–366 (2017). [DOI] [PubMed] [Google Scholar]
- 200.McNamara HM, Zhang H, Werley CA & Cohen AE Optically controlled oscillators in an engineered bioelectric tissue. Phys. Rev. X 6, 031001 (2016). [Google Scholar]
- 201.Grosenick L, Marshel JH & Deisseroth K Closed-loop and activity-guided optogenetic control. Neuron 86, 106–139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sauer B Inducible gene targeting in mice using the cre/loxsystem. Methods 14, 381–392 (1998). [DOI] [PubMed] [Google Scholar]
- 203.Prando V et al. Dynamics of neuroeffector coupling at cardiac sympathetic synapses. J. Physiol 596, 2055–2075 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rajendran PS et al. Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nat. Commun 10, 1944 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Packer AM, Russell LE, Dalgleish HW & Hausser M Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat. Methods 12, 140–146 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mighiu AS & Heximer SP Controlling parasympathetic regulation of heart rate: A gatekeeper role for RGS proteins in the sinoatrial node. Front. Physiol 3, 204–204 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Randall WC, Milosavljevic M, Wurster RD, Geis GS & Ardell JL Selective vagal innervation of the heart. Ann. Clin. Lab. Sci 16, 198–208 (1986). [PubMed] [Google Scholar]
- 208.Sampaio KN, Mauad H, Spyer KM & Ford TW Differential chronotropic and dromotropic responses to focal stimulation of cardiac vagal ganglia in the rat. Exp. Physiol 88, 315–327 (2003). [DOI] [PubMed] [Google Scholar]
- 209.Waldron NH, Fudim M, Mathew JP & Piccini JP Neuromodulation for the treatment of heart rhythm disorders. JACC Basic Transl Sci. 4, 546–562 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Oh Y et al. Functional coupling with cardiac muscle promotes maturation of hPSC-derived sympathetic neurons. Cell Stem Cell 19, 95–106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yu L et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J. Am. Coll. Cardiol 70, 2778–2790 (2017). [DOI] [PubMed] [Google Scholar]
- 212.Mastitskaya S et al. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc. Res 95, 487–494 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Machhada A et al. Vagal determinants of exercise capacity. Nat. Commun 8, 15097 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wu Y et al. Optogenetic approach for functional assays of the cardiovascular system by light activation of the vascular smooth muscle. Vascul. Pharmacol 71, 192–200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Malloy C et al. Using optogenetics to assess neuroendocrine modulation of heart rate in Drosophila melanogaster larvae. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol 203, 791–806 (2017). [DOI] [PubMed] [Google Scholar]
- 216.Wang Y et al. Optogenetic control of heart rhythm by selective stimulation of cardiomyocytes derived from PNMT+ cells in murine heart. Sci. Rep 7, 40687 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Felker A et al. Continuous addition of progenitors forms the cardiac ventricle in zebrafish. Nat. Commun 9, 2001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rorsman NJG, Ta CM, Garnett H, Swietach P & Tammaro P Defining the ionic mechanisms of optogenetic control of vascular tone by channelrhodopsin-2. Br. J. Pharmacol 175, 2028–2045 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ishikawa K, Weber T & Hajjar RJ Human cardiac gene therapy. Circ. Res 123, 601–613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shang W et al. Imaging Ca2+ nanosparks in heart with a new targeted biosensor. Circ. Res 114, 412–420 (2014). [DOI] [PubMed] [Google Scholar]