Abstract
Background
Adoptive cell therapy with T cells genetically engineered to express a chimeric antigen receptor (CAR-T) or tumor-infiltrating T lymphocytes (TIL) demonstrates impressive clinical results in patients with cancer. Lymphodepleting preconditioning prior to cell infusion is an integral part of all adoptive T cell therapies. However, to date, there is no standardization and no data comparing different non-myeloablative (NMA) regimens.
Methods
In this study, we compared NMA therapies with different doses of cyclophosphamide or total body irradiation (TBI) in combination with fludarabine and evaluated bone marrow suppression and recovery, cytokine serum levels, clinical response and adverse events.
Results
We demonstrate that a cumulative dose of 120 mg/kg cyclophosphamide and 125 mg/m2 fludarabine (120Cy/125Flu) and 60Cy/125Flu preconditioning were equally efficient in achieving deep lymphopenia and neutropenia in patients with metastatic melanoma, whereas absolute lymphocyte counts (ALCs) and absolute neutrophil counts were significantly higher following 200 cGyTBI/75Flu-induced NMA. Thrombocytopenia was most profound in 120Cy/125Flu patients. 30Cy/75Flu-induced preconditioning in patients with acute lymphoblastic leukemia resulted in a minor ALC decrease, had no impact on platelet counts and did not yield deep neutropenia. Following cell infusion, 120Cy/125Flu patients with objective tumor response had significantly higher ALC and significant lower inflammatory indexes, such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR). Receiver-operating characteristics curve analysis 7 days after cell infusion was performed to determine the cut-offs, which distinguish between responding and non-responding patients in the 120Cy/125Flu cohort. NLR≤1.79 and PLR≤32.7 were associated with clinical response and overall survival. Cytokine serum levels did not associate with clinical response in patients with TIL. Patients in the 120Cy/125Flu cohort developed significantly more acute NMA-related adverse events, including thrombocytopenia, febrile neutropenia and cardiotoxicity, and stayed significantly longer in hospital compared with the 60Cy/125Flu and TBI/75Flu cohorts.
Conclusions
Bone marrow depletion and recovery were equally affected by 120Cy/125Flu and 60Cy/125Flu preconditioning; however, toxicity and consequently duration of hospitalization were significantly lower in the 60Cy/125Flu cohort. Patients in the 30Cy/75Flu and TBI/75Flu groups rarely developed NMA-induced adverse events; however, both regimens were not efficient in achieving deep bone marrow suppression. Among the regimens, 60Cy/125Flu preconditioning seems to achieve maximum effect with minimum toxicity.
Keywords: immunotherapy, adoptive, lymphocytes, tumor-infiltrating, receptors, chimeric antigen, clinical trials, phase II as topic
Introduction
Adoptive cell therapy (ACT) has become a standard treatment option for patients with cancer. T cells genetically engineered to express a chimeric antigen receptor (CAR) against CD19 obtained FDA approval for acute lymphoblastic leukemia (ALL) and non-Hodgkin’s lymphoma in 2017 and 2018.1 In addition, phase 2, phase 3 and pivotal studies of ACT with tumor-infiltrating lymphocytes (TILs) are currently conducted in patients with metastatic melanoma2–5 and other solid tumor types.6–9 A common feature of all current T cell therapies, no matter if they use gene engineered T cells or TIL, is the non-myeloablative (NMA) lymphodepleting preconditioning of patients prior to cell infusion.
It has been demonstrated that NMA preconditioning of the host with cyclophosphamide (Cy) and fludarabine (Flu) or total body irradiation (TBI)10 11 enhances the survival, persistence and antitumor activity of the infused cells12 13 by the elimination of regulatory T cells, increase of homeostatic cytokines like interleukin (IL)-7 and IL-15, and elimination of resident T cells competing for these trophic cytokines.1 13–16 Removal of cellular sinks, such as natural killer cells which compete for IL-7 and myeloid cells competing for IL-15, is required for proliferation and long-term survival of T cells.15 17 18 Anthony et al demonstrated that IL-15 secretion, induced by inflammatory signals in response to lymphodepletion, drives CD8 T cell proliferation and promotes loss of tolerance against self-antigens, thereby enhancing the antitumor activity of T cells.19 In a small series, Kochenderfer et al reported that post lymphodepletion, high serum IL-15 levels are associated with remissions of patients with lymphoma receiving CAR-T cell therapy.18
NMA-induced bone marrow suppression and recovery can be monitored by measuring absolute lymphocyte, neutrophil and platelet counts in the blood of patients. Previously, we demonstrated that absolute lymphocyte counts (ALCs) 1 and 2 weeks post-TIL infusion were significantly correlated with objective response.2 In addition, others could show that a combination of cell counts, defined as inflammatory indexes, can be used as prognostic factors for survival in different types of cancers.20–23 For example, several studies have reported that a high neutrophil-to-lymphocyte ratio (NLR) and a high platelet-to-lymphocyte ratio (PLR) predict poor survival in patients with stage I-III melanoma,24 25 in patients with diffuse large B cell lymphoma treated with the immunochemotherapy combination R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine and prednisone)26 27 and in patients with metastatic melanoma treated with checkpoint inhibitors28 or BRAF inhibitors.29
Although there is a common consensus that NMA preconditioning is required to obtain a durable objective response in patients with cancer treated with adoptive T cell therapy, there is no study comparing the different NMA lymphodepleting regimens head-to-head.
Here, we systematically compare bone marrow suppression and recovery, the impact of NMA on inflammatory biomarkers, the effect on IL-15, IL-7 and IL-21 serum levels, and related adverse events following four different NMA therapies. In addition, we evaluate the impact of NMA on clinical response.
Material and methods
Patients
Patients were enrolled to one of the three open-label, single arm, phase 2 trials. All patients were treated with T cell products manufactured in the same production facility at the Sheba Medical Center, as previously described.2 9 30 Patients signed an informed consent approved by the Israeli Ministry of Health.
Patients with stage IV melanoma were treated with TIL ACT as salvage therapy after failure of multiple treatments. The ‘120Cy/125Flu’ cohort (NCT00287131) received NMA conditioning regimen with cyclophosphamide (60 mg/kg for 2 days; days −7 and −6) and fludarabine (25 mg/m2 for 5 days; days −5 to −1) before cell infusion (day 0) and the ‘60Cy/125Flu’ cohort cyclophosphamide (30 mg/kg for 2 days; days −5 and −4) and fludarabine (25 mg/m2 for 5 days; days −5 to −1) (NCT03166397). The ‘TBI/75Flu’ cohort received TBI (200 cGy, on day −4) and fludarabine (25 mg/m2 for 3 days; days −3 to −1) (NCT03166397). TILs were administered intravenously, followed by high-dose bolus IL-2 with 720,000 IU/kg/dose, maximum of three times a day, for 5 days or up to tolerance. Patients received filgrastim for 7–10 days starting 1 day after cell infusion. Response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST V.1.1) guidelines 4 weeks following TIL administration and every 3 months thereafter or as clinically needed. Objective responders (ORs) were defined as complete and partial responders and non-responders (NRs) as patients with stable disease or progressive disease by determining the best overall response using RECIST.
Relapsed or refractory (r/r) pediatric and adult patients with ALL were treated with anti-CD19 CAR-T cells (NCT02772198). Lymphodepleting conditioning was induced by fludarabine 25 mg/m2 for 3 days (days −2 to −4) and cyclophosphamide 900 mg/m2 (which equals approximately 30 mg/kg Cy) for 1 day (day −2) (cohort ‘30Cy/75Flu’), followed by infusion of 1×106 transduced CAR-T cells per kilogram weight.9 31 Patients did not receive IL-2. For clinical evaluation of response, patients with ALL underwent a bone marrow aspiration 28 and 60 days following CAR-T cell administration, and disease assessment was performed by morphology, flow cytometry and PCR minimal residual disease based on immunoglobulin or T-cell receptor rearrangement, with a lower level of detection of 5–10 leukemia cells in 10,000–100,000 healthy cells.
Cell counts and inflammatory indexes
Absolute neutrophil, platelet and lymphocyte counts were obtained before NMA initiation and up to 3 months after cell infusion. The following inflammatory indexes were calculated: NLR, defined as neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; and systemic immune-inflammatory index (SII), defined as absolute neutrophil count×absolute platelet count/absolute lymphocyte count. All cell counts were performed in a centralized, automated laboratory at the Sheba Medical Center.
Cytokine levels
Patients’ serum was collected on day 0, before cell infusion. IL-7, IL-15 and IL-21 serum levels were determined by ELISA (IL-7, Human IL-7 Quantikine HS ELISA kit, R&D Systems, Minneapolis, Minnesota, USA; IL-15 and IL-21, ELISA MAX Deluxe Set, BioLegend, San Diego, California, USA) according to the manufacturer’s instructions. Measurements were performed in duplicates.
Statistical analysis
Significance of variation between groups was evaluated using a parametric two-tailed Student’s t-test. The differences between proportions were tested using two-sided Fisher’s exact test. Analysis of covariance was performed to examine differences in the mean values of patients’ characteristics on variable production parameters. Cell counts and inflammatory indexes were transformed to a logarithmic scale. Linear mixed models with Tukey’s adjustment were used to compare absolute cell counts and inflammatory indexes between responder groups or the different regimens. Eastern Cooperative Oncology Group (ECOG) performance status was modeled as fixed effect. Effect sizes were calculated as Cohen’s d. Linear mixed models were fit using the R statistical software (www.r-project.org). The receiver-operating characteristics (ROC) curve analysis was used to define sensitivity and specificity through the Youden’s index, and the area under the ROC curve (AUC) to find the optimal cut-off values for NLR, PLR and SII using the MedCalc V.18.11.6 (MedCalc Software). Survival curves were plotted by the Kaplan-Meier method, and differences between groups were assessed by the log-rank test using the Prism GraphPad V.8.0.1 (GraphPad Software).
Results
Cohorts
Between 2005 and 2016, 103 patients with metastatic melanoma were enrolled to a phase 2 ACT trial with TIL following preconditioning with an accumulating dose of 120 mg/kg cyclophosphamide and 125 mg/m2 fludarabine2 (120Cy/125Flu cohort; table 1). To analyze the impact of NMA on bone marrow suppression and recovery, absolute cell counts were collected, starting a few days before NMA initiation (baseline) and up to 3 months post therapy. Due to cyclophosphamide-induced toxicity (detailed later), a follow-up study was initiated. The cyclophosphamide dose was halved (60Cy/125Flu) or cyclophosphamide was replaced with 200 cGy TBI (table 1). In 2017 and 2018, nine patients with metastatic melanoma were enrolled to the TBI/75Flu group, and in 2019 and 2020, eight patients received 60Cy/125Flu-induced NMA. Patients in the 60Cy/125Flu cohort and those in the TBI/75Flu cohort did not respond to TIL therapy. The TIL manufacturing procedure was identical in all three cohorts. TILs were manufactured at the same Good Manufacturing Practice (GMP) facility and by the same staff.
Table 1.
Patient cohorts
| Cohort | ClinicalTrials identifier | N | Cyclophosphamide | Fludarabine | TBI | Disease | T cells | |||
| mg/kg | Days | mg/m2 | Days | cGy | Days | |||||
| 120Cy/125Flu | NCT00287131 | 103 | 2×60 | −7 to −6 | 5×25 | −5 to −1 | – | Melanoma | TIL | |
| 60Cy/125Flu | NCT03166397 | 8 | 2×30 | −5 to −4 | 5×25 | −5 to −1 | – | Melanoma | TIL | |
| TBI/75Flu | NCT03166397 | 9 | – | – | 3×25 | −3 to −1 | 1×200 | −4 | Melanoma | TIL |
| 30Cy/75Flu | NCT02772198 | 19 | 1×~30* | −2 | 3×25 | −4 to −2 | – | ALL | CAR-T | |
*900 mg/m2.
ALL, acute lymphocytic leukemia; CAR-T, chimeric antigen receptor T cell; Cy, cyclophosphamide; Flu, fludarabine; TBI, total body irradiation; TIL, tumor-infiltrating T lymphocyte.
The three melanoma cohorts were well matched for most baseline characteristics (eg, ECOG performance status) but were significantly different with respect to prior treatments (table 2). Most patients in the 120Cy/125Flu cohort were treated in the pre-immune checkpoint inhibitor (pre-ICI) era, whereas patients in the 60Cy/125Flu and TBI/75Flu cohorts were all treated after treatment failure with ICIs.
Table 2.
Baseline and treatment characteristics of the three metastatic melanoma cohorts
| 120Cy/125Flu patients | 60Cy/125Flu patients | TBI/75Flu patients | 120Cy/125Flu vs 60cy/125Flu | 120Cy/125Flu vs TBI/75Flu | 60Cy/125Flu vs TBI/75Flu | |
| N=103 | N=8 | N=9 | P value | P value | P value | |
| Baseline characteristics | ||||||
| Mean age, years | 51±12 | 53±9 | 51±12 | 0.646 | 1 | 1 |
| Male | 66 (64%) | 4 (50%) | 6 (67%) | 0.464 | 1 | 0.637 |
| ECOG performance status*, n (%) | ||||||
| 0 | 58 (56%) | 5 (62.5%) | 8 (89%) | 1 | 0.079 | 0.294 |
| 1 | 37 (36%) | 3 (37.5%) | 1 (11%) | 1 | 0.164 | 0.294 |
| 2 | 8 (8%) | 0 | 0 | 1 | 1 | 1 |
| M stage†, n (%) | ||||||
| IIIc | 1 (1%) | 0 | 0 | 1 | 1 | 1 |
| M1a | 7 (7%) | 0 | 0 | 1 | 1 | 1 |
| M1b | 12 (12%) | 1 (11%) | 1 (13%) | 1 | 1 | 1 |
| M1c | 83 (81%) | 8 (89%) | 7 (87%) | 1 | 1 | 1 |
| LDH level above normal, n (%) | 42 (41%) | 3 (37.5%) | 6 (67%) | 1 | 0.168 | 0.347 |
| CNS metastases at baseline, n (%) | 23 (22%) | 2 (25%) | 3 (33%) | 1 | 0.431 | 1 |
| More than 5 metastases, n (%) | 78 (76%) | 4 (50%) | 8 (89%) | 0.203 | 0.682 | 0.131 |
| HLA-A0201 positive, n (%) | 25 of 102 (25%) | 0 | 3 of 8 (38%) | 0.194 | 0.417 | 0.228 |
| Previous therapy for metastatic disease, n (%) | ||||||
| At least one prior therapy | 103 (100%) | 8 (100%) | 9 (100%) | 1 | 1 | 1 |
| Chemotherapy | 20 (19%) | 4 (50%) | 2 (22%) | 0.065 | 1 | 0.335 |
| Chemobiotherapy | 55 (53%) | 0 | 0 | 0.006 | 0.003 | 1 |
| High-dose bolus IL-2 therapy | 16 (16%) | 0 | 0 | 0.599 | 0.354 | 1 |
| Any other IL-2-based therapy | 10 (10%) | 0 | 0 | 1 | 1 | 1 |
| Anti-CTLA4 antibody single therapy | 34 (33%) | 0 | 2 (22%) | 0.104 | 0.716 | 0.471 |
| Anti-PD1 antibody single therapy | 15 (15%) | 0 | 4 (44%) | 0.595 | 0.044 | 0.082 |
| Anti-PD1+CTLA4 combo therapy | 2 (2%) | 0 | 7 (78%) | 1 | ≤0.001 | 0.002 |
| Targeted therapy (BRAFI±MEKI) | 15 (15%) | 1 (13%) | 3 (33%) | 1 | 0.157 | 0.577 |
| Other treatment | 3 (3%) | 7 (88%) | 1 (11%) | ≤0.001 | 0.288 | 0.003 |
| Infusion product characteristics | ||||||
| Total cell number (×109) | 49±24 | 40.5±19.7 | 44.9±22 | 0.332 | 0.622 | 0.672 |
| CD8 frequency (%) | 59±25 | 51.3±17.9 | 31.7±27.1 | 0.396 | 0.002 | 0.103 |
| CD4 frequency (%) | 41±25 | 48.2±17.4 | 66.3±28.3 | 0.427 | 0.005 | 0.139 |
| NMA-related adverse events, days of hospitalization and transfusion units | ||||||
| Days of hospitalization, median | 20.8±5.1 | 15.4±1.5 | 13.7±1.4 | 0.004 | ≤0.001 | 0.029 |
| Chemotherapy-related toxicity (grade 3–5) | ||||||
| Febrile neutropenia | 94 (91%) | 4 (50%) | 0 | 0.001 | ≤0.001 | 0.029 |
| Thyroid cancer | 1 (1%) | 0 | 0 | 1 | 1 | 1 |
| Fatal myelodysplastic syndrome | 1 (1%) | 0 | 0 | 1 | 1 | 1 |
| Chemotherapy-related toxicity (grade 5) | ||||||
| Cyclophosphamide-induced fatal cardiomyopathy | 3 additional, unevaluated patients | 0 | 0 | 1 | 1 | 1 |
| Transfusion data | ||||||
| No of RC units, average | 4.7±5.8 | 0.63±0.92 | 0 | 0.055 | 0.019 | 0.057 |
| No of PLT units, average | 24.8±35.4 | 0 | 0 | 0.051 | 0.039 | 1 |
*ECOG Eastern Cooperative Oncology Group (ECOG) status ranges from 0 to 5, with higher scores indicating greater impairment (5 indicates death).
†The metastasis (M) stage was classified according to the tumor-node-metastasis (TNM) categorization for melanoma of the American Joint Committee on Cancer.
CNS, central nervous system; CTLA4, cytotoxic T-lymphocyte-associated protein 4; Cy, cyclophosphamide; Flu, fludarabine; HLA, human leukocyte antigen; IL, interleukin; LDH, lactate dehydrogenase; NMA, non-myeloablative; PD1, Programmed cell death protein 1; PLT, platelets; RC, red cells; TBI, total body irradiation.
Simultaneously, CD19 CAR-T therapy was conducted at our clinical center and both TIL and CAR-T were produced at the same GMP cell production facility. Between 2016 and 2019, patients with r/r ALL were treated with CD19 CAR-T cells following NMA with fludarabine and cyclophosphamide 900 mg/m2 (which equals approximately 30 mg/kg Cy) (‘30Cy/75Flu’ cohort). Absolute cell counts were acquired from 19 patients, including 13 complete responders and 6 NRs. The patients received, on average, 4.0±1.2 prior treatments and 2 of 19 (11%) patients had brain metastasis. The 30Cy/125Flu ALL cohort included pediatric patients, thus the average age was low in this cohort. Since patients in the 30Cy/125Flu cohort had a different underlying disease and were treated with CAR-T cells instead of TIL, the results of this cohort will be demonstrated separately.
Bone marrow suppression
To evaluate the impact of the different preconditioning regimens on bone marrow suppression, absolute cell counts were monitored in the three melanoma groups (figure 1) and the ALL cohort (discussed later). Cell counts were performed in a centralized, automated laboratory. The number of patients with available cell counts per day is shown in online supplemental table 1. The day of cell infusion was defined as ‘day 0’.
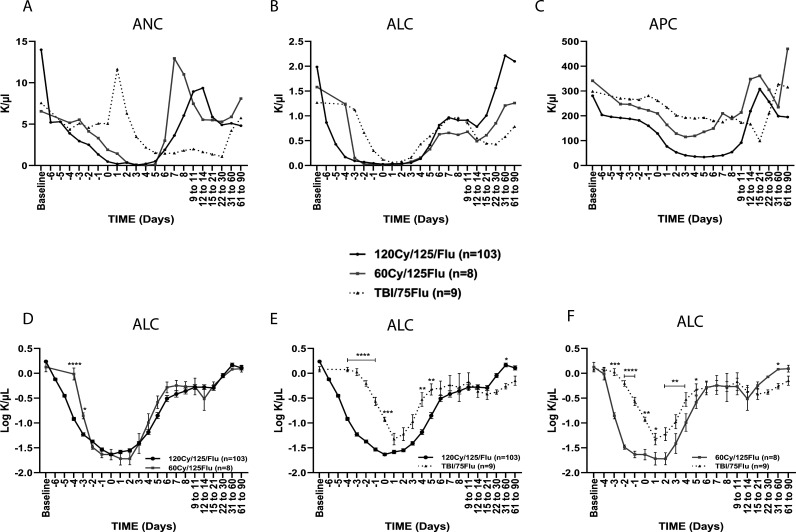
Figure 1.
Cell counts following non-myeloablative lymphodepletion. (A) Absolute neutrophil count (ANC), (B) absolute lymphocyte count (ALC) and (C) absolute platelet count (APC). (D–F) Comparison of ALC between (D) the 120Cy/175Flu and 60Cy/175Flu cohorts, (E) the 120Cy/175Flu and TBI/75Flu cohorts and (F) the 60Cy/125Flu and TBI/75Flu cohorts. Day 0, day of cell infusion. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001. Cy, cyclophosphamide; Flu, fludarabine; TBI, total body irradiation.
jitc-2020-001743supp004.pdf (14.6KB, pdf)
One hundred and one of 103 (98%) patients in the 120Cy/125Flu and 100% (8 of 8) patients in the 60Cy/125Flu cohort achieved severe neutropenia, defined as absolute neutrophil count (ANC)≤0.5 K/µL. The lowest values of ANC were found 3 days after cell infusion in the 120Cy/125Flu group (0.065±0.230 K/µL; median 0.025 K/µL) and 4 days after cell infusion in the 60Cy/125Flu cohort (0.050±0.044 K/µL; median 0.030 K/µL) (p=0.871) (figure 1A, table 3 and online supplemental figure 1). TBI/75Flu preconditioning did not result in severe neutropenia and the lowest value was 1.088±0.541 K/µL (median 1.100 K/µL) 1 month after cell infusion. In contrast, five of nine (56%) patients in the TBI/75Flu cohort demonstrated even a sharp increase of ANC, with values between 13.03 and 16.13 K/µL 1 day after cell infusion when the normal range for neutrophils is between 1.8 and 7.7 K/µL (figure 1A).
Table 3.
Lowest values of absolute cell counts
| ANC (K/µL) | ALC (K/µL) | APC (K/µL) | |||||||
| Normal level | 1.8–7.7 | 1.0–4.8 | 130–440 | ||||||
| Lowest value | Day | Lowest value | Day | Lowest value | Day | ||||
| Average±SD | Median | Average±SD | Median | Average±SD | Median | ||||
| 120Cy/125Flu | 0.065±0.230 | 0.025 | 3 | 0.029±0.036 | 0.020 | 0 | 34±26 | 26 | 5 |
| 60Cy/125Flu | 0.050±0.044 | 0.030 | 4 | 0.024±0.017 | 0.025 | 0 | 115±51 | 103 | 3 |
| TBI/75Flu | 1.088±0.541 | 1.100 | 22–30 | 0.062±0.046 | 0.050 | 1 | 99±51 | 91 | 15–21 |
ALC, absolute lymphocyte count; ANC, absolute neutrophil count; APC, absolute platelet count; Cy, cyclophosphamide; Flu, fludarabine; TBI, total body irradiation.
jitc-2020-001743supp001.pdf (162.1KB, pdf)
Similar results were observed for ALC (figure 1B and table 3). 120Cy/125Flu and 60Cy/125Flu preconditioning were equally effective in achieving deep lymphodepletion, with lowest average values of 0.029±0.036 K/µL (median 0.020 K/µL) and 0.024±0.017 K/µL (median 0.025 K/µL) on the day of cell infusion in the 120Cy/125Flu and 60Cy/125Flu cohorts, respectively (p=0.389), when normal values are between 1.0 and 4.8 K/µL. A direct comparison of the ALC in the 120Cy/125Flu and 60Cy/125Flu cohorts over time is shown in figure 1D. Administration of cyclophosphamide in the 120Cy/125Flu cohort was initiated on day −7 and in 60Cy/125Flu cohort on day −5, which explains the difference of ALC on days −4 and −3. In the TBI/Flu cohort, only a minor decrease in ALC was achieved (lowest value: 0.062±0.046 K/µL; median 0.050 K/µL; on day +1) (figure 1B). Lymphodepletion was significantly less efficient compared with the 120Cy/125Flu and 60Cy/125Flu cohorts (p values≤0.01; figure 1E and F).
Platelet counts at baseline were normal in the three melanoma cohorts (120Cy/125Flu cohort, 281±96 K/µL; 60Cy/125Flu cohort, 341±121 K/µL; TBI/75Flu cohort, 299±82 K/µL). In the 120Cy/125Flu and 60Cy/125Flu cohorts, an APC decrease was observed following NMA initiation; however, in the 120Cy/125Flu cohort, values were significantly lower (120Cy/125Flu, lowest value 34±26 K/µL, median 26 K/µL; 60Cy/125Flu cohort, lowest value 115±51 K/µL, median 103 K/µL; p≤0.001) (figure 1C and online supplemental figure 1D–F).
In conclusion, 120Cy/125Flu and 60Cy/125Flu preconditioning were equally efficient in achieving deep lymphopenia and neutropenia, which was not the case for TBI/75Flu NMA.
Bone marrow recovery
Both in the 120Cy/125Flu and 60Cy/125Flu cohorts, the patients recovered by day 6 and day 7 from their severe neutropenia, and counts within the norm (1.8–7.7 K/µL) were measured (120Cy/125Flu cohort, 3.63±5.22 K/µL, median 1.54 K/µL; 60Cy/125Flu cohort, 3.01±2.03 K/µL, median 2.73 K/µL) (figure 1A).
Interestingly, although TBI/75Flu-induced NMA caused a sharp increase of ANC 1 day after cell infusion followed by mild neutropenia, neutrophil recovery was delayed and baseline levels were only reached after 1 month.
Recovery of ALC was observed in all the cohorts 4–7 days after cell infusion and returned to the normal range of values (figure 1B). The duration of severe lymphopenia was 7 days in the 120Cy/125Flu and 60Cy/125Flu cohorts, but only 2 days in the TBI/75Flu cohort.
Platelet recovery was found 12–14 days after cell infusion in the 120Cy/125Flu cohort. In the TBI/75Flu cohort, APC steadily decreased over time and 50% (three out of six) of the patients had prolonged thrombocytopenia around 3 weeks after cell infusion (figure 1C).
Impact of NMA on clinical response
To evaluate if bone marrow suppression and recovery can serve as predictive markers of clinical response, we analyzed the cell counts in the 120Cy/125Flu melanoma patient cohort (ORs, N=29; NRs, N=74). The baseline characteristics of responders and non-responders were previously reported and multivariate logistic regression revealed that ECOG performance status was an independent factor of response in this cohort.2
Responding and non-responding patients had similar ANC, ALC and APC at baseline (p=0.953, p=0.497 and p=0.343) (figure 2A–C). Following NMA initiation, there was no significant difference between responders and non-responders regarding ANC (d=0.083, p=0.508) (figure 2A) and APC (d=0.007, p=0.972) (figure 2C). However, ALCs were significantly different (d=0.605, p=≤0.001) (figure 2B). From day 4 onwards, OR patients had significantly higher lymphocyte counts, with the highest significant difference between days 5 and 21 (p values≤0.001). Absolute cell counts were further compared between ORs (N=29) and NRs (N=29), which were matched for baseline characteristics, including ECOG and prior treatments (online supplemental figure 2A–C), as well as the entire cohort, excluding patients with ECOG 2 and prior anti-PD1 therapy (OR, N=28; NR, N=53) (online supplemental figure 2D–F) and demonstrated the same results.
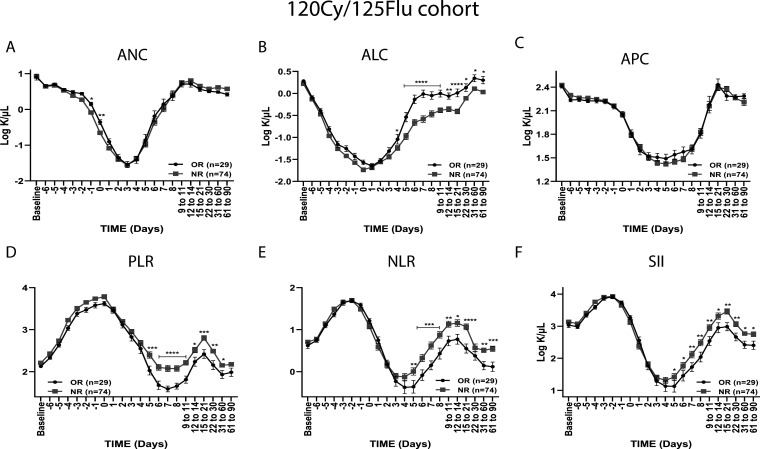
Figure 2.
Absolute cell counts and inflammatory ratios of responders and non-responders in the 120Cy/125Flu cohort. (A) Absolute neutrophil count (ANC), (B) absolute lymphocyte count (ALC) and (C) absolute platelet count (APC), (D) platelet-to-neutrophil ratio (PLR), (E) neutrophil-to-lymphocyte ratio (NLR) and (F) systemic immune-inflammatory index (SII) of objective responders (ORs) and non-responders (NRs). Data are shown as mean±SEM. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001. Cy, cyclophosphamide (Cy); Flu, fludarabine.
jitc-2020-001743supp002.pdf (100.2KB, pdf)
Inflammatory indexes as prognostic factors for response and overall survival in the 120Cy/125Flu melanoma cohort
It was shown that the combination of absolute cell counts, defined as inflammatory indexes, can be used as a prognostic factor for response and survival in different types of cancer and may be more significant than individual counts. ORs in the 120Cy/125Flu melanoma cohort had significantly lower PLR, NLR and SII from day 5 onwards (figure 2D).
ROC curves were used to obtain the optimal inflammatory indexes cut-offs on day 7, which distinguish between ORs and NRs. The optimal cut-off for NLR was 1.79, for PLR 32.7 and for SII 368 K/µL.
We found that 61% (17 of 28) of OR patients compared with 24% (16 of 66) of NR patients had an NLR ≤1.79 (p=0.002) and 61% (17 of 28) of the responders compared with 15% of the NRs had a PLR ≤32.7 (p≤0.001). SII on day 7 could not be used to distinguish between OR and NR patients.
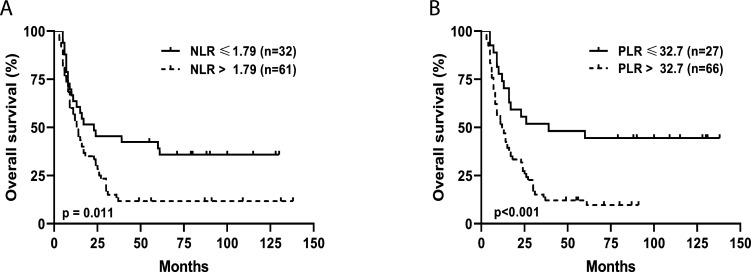
Kaplan-Meier analysis indicated that lower values of NLR and PLR were also significantly associated with prolonged overall survival (OS) in the 120Cy/125Flu cohort. As shown in figure 3, patients with NLR≤1.79 had a median OS of 23 months compared with 13.5 months for patients with NLR>1.79 months (p=0.011); patients with PLR≤32.7 had a median OS of 39 months compared with 12.5 months for patients with PLR>32.7 (p=≤0.001).
Figure 3.
Kaplan-Meier curves for assessment of median overall survival. Kaplan-Meier curves for the inflammatory indexes (A) NLR and (B) PLR according to cut-off values received by ROC curve analysis. Survival analysis was performed by the log-rank test. ROC, Receiver operating characteristics; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio;.
Impact of NMA on IL-7, IL-15 and IL-21 serum levels
To determine the impact of the different lymphodepleting regimens on cytokine levels, ELISA assays were performed with serum samples collected after completion of NMA and before cell infusion (day 0). Serum was available from patients with melanoma in the 120Cy/125Flu cohort (N=24, including 12 ORs and 12 NRs), 60Cy/125Flu cohort (N=7) and TBI/75Flu cohort (N=8).
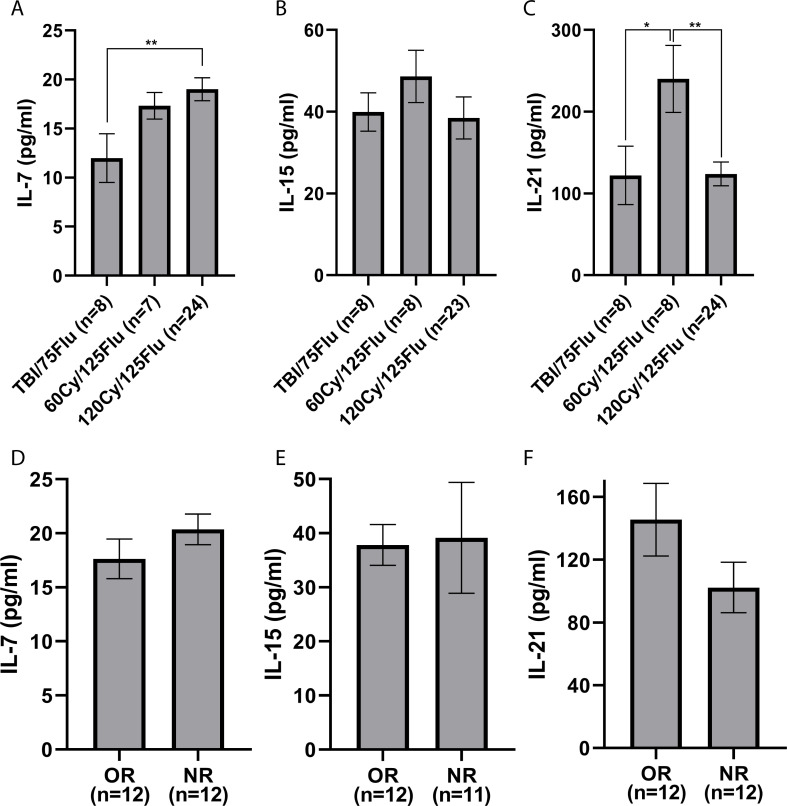
IL-7 serum levels were significantly lower following TBI/75Flu-induced lymphodepletion compared with 120Cy/125Flu NMA (TBI/75Flu cohort, 12.0±7.0 pg/mL; 120Cy/125Flu cohort, 19.0±5.7 pg/mL; p=0.008) (figure 4A). IL-15 serum levels were insignificantly different in all three cohorts (figure 4B). Patients in the 60Cy/125Flu cohort had significantly higher IL-21 serum levels (240±116 pg/mL) than patients from the TBI/75Flu cohort (122±101 pg/mL; p=0.048) and 120Cy/125Flu cohort (124±71 pg/mL; p=0.002) (figure 4C).
Figure 4.
Cytokine serum levels on the day of cell infusion. (A) IL-7, (B) IL-15 and (C) IL-21. (D–F) Comparison of cytokine serum levels between objective responders (ORs) and non-responders (NRs) in the 120Cy/125Flu cohort. Data are shown as mean±SEM analyzed using a non-parametric two-tailed Student’s t-test. *p<0.05 and **p<0.01. Cy, cyclophosphamide; Flu, fludarabine; IL, interleukin; TBI, total body irradiation.
No significant differences between IL-7 (p=0.252), IL-15 (p=0.902) and IL-21 (p=0.140) serum levels were observed between ORs (N=12) and NRs (N=12) in the 120Cy/125Flu cohort (figure 4D).
NMA-related toxicity
120Cy/125Flu cohort
The NMA, TIL and high-dose IL-2 toxicity profiles in the 120Cy/125Flu cohort were recently published.2 Table 2 shows NMA-related adverse events. In short, most 120Cy/125Flu-related toxicities were manageable, while the most common adverse effect was acute grade 3–4 neutropenic fever affecting 91% (94 of 103) of the patients. Administration of high-dose cyclophosphamide at a dose of 60 mg/kg for 2 days required antiemetic treatment adjusted for high emetogenic-risk chemotherapy, yet steroid treatment was avoided. The use of antiemetics during days −7 to −5 of hospitalization (starting prior to the cyclophosphamide infusion) increased the level of tolerability of the infusion of high-dose cyclophosphamide chemotherapy. Mild nausea, jaw discomfort or headaches during infusion were the most commonly reported immediate side effects. No hemorrhagic cystitis was noted. No acute drug-related side effects were found to be correlated with the infusion of fludarabine for 5 days. Patients reported lack of appetite, mild nausea and fatigue. Ninety-one of 103 (88%) and 79 of 102 (77%) patients required red cells (RCs) (4.7±5.8 units) and/or platelet (PLT) transfusions (24.8±35.4 units) to prevent complications from anemia or bleeding. The average duration of hospitalization from NMA admission to discharge was 20.8±5.1 days.
Of note, there were three cases of early deaths, all considered to be related to cyclophosphamide induced cardiotoxicity. All cases presented with symptoms of acute heart failure before TIL infusion. High-dose cyclophosphamide is known to cause cardiotoxicity in 7%–28% of patients and cyclophosphamide-induced mortality has been described in 11%–43% of patients at a dose of 170–180 mg/kg.32
As for late toxicity of chemotherapy, one patient developed papillary thyroid cancer 3.5 years after TIL therapy, which was successfully treated surgically, and one patient was diagnosed with myelodysplastic syndrome followed by acute leukemia 4.5 years after therapy.
60Cy/125Flu cohort
Due to the high-dose cyclophosphamide-induced toxicities, we investigated the possibility of reducing or replacing cyclophosphamide. 60Cy/125Flu-related toxicities are detailed in table 2. Decreasing the cyclophosphamide doses from 2×60 mg/kg (120Cy) to 2×30 mg/kg (60Cy) resulted in a significantly lower number of cases with grade 3–4 febrile neutropenia (60Cy/125Flu cohort, 50% vs 91%, 120Cy/125 Flu cohort; p=0.001) and shorter time of hospitalization (15.4±1.5 days vs 20.8±5.1; p=0.004). Three of eight patients developed anemia, which required RC transfusions (0.63±0.92 units vs 4.7±5.8 units; p=0.055) and no patient experienced thrombocytopenia, which required transfusion of platelets (p=0.039). There was no fatality.
TBI/75Flu cohort
Acute toxicities were in general considerably mild in this cohort, which is reflected in a significantly shorter hospital stay (TBI/75Flu cohort 13.7±1.4, 120Cy/125Flu cohort 20.8±5.1 days; p≤0.001) (table 2). No patient in the TBI/75Flu cohort experienced chemotherapy-related febrile neutropenia and no patient required RC and PLT transfusions.
30Cy/75Flu ALL cohort
The 30Cy/75Flu cohort differed in many aspects from the other three cohorts. Patients in the 30Cy/75Flu group suffered from ALL (instead of metastatic melanoma), were treated with CAR-T (instead of TIL), did not receive IL-2 administration following infusion (instead of high-dose bolus IL-2) and received prior lines of chemotherapies, which is uncommon for patients with metastatic melanoma. Therefore, this population is discussed here separately.
30Cy/75Flu preconditioning in the ALL cohort did not yield deep neutropenia (online supplemental figure 3A). The lowest ANC value was 0.835±0.966 K/µL (median 0.537 K/µL) 9–11 days after cell infusion. In addition, only a minor decrease in ALC was achieved (lowest values: 0.164±0.167 K/µL; median 0.367, on day +1) (online supplemental figure 3B). Platelet counts were already low at baseline (119±105 K/µL; median 95 K/µL) (online supplemental figure 3C). Baseline thrombocytopenia might be a result of leukemic infiltration of the bone marrow or extensive prior chemotherapy treatments. APCs were low, but unchanged from baseline and up to 3 months post therapy.
jitc-2020-001743supp003.pdf (125.9KB, pdf)
Comparing OR (N=13) and NR (N=6) patients of the 30Cy/75Flu cohort, there was no significant difference in ANC (OR vs NR; d=1.380, p=0.053) and ALC (d=−0.246, p=0.678) (online supplemental figure 2D–E). APCs were significantly lower in non-responding ALL patients 1 day after NMA initiation and throughout the entire time of monitoring (OR vs NR; d=3.910, p=0.001) (online supplemental figure 3F). In both groups, APC was not affected by 30Cy/75Flu lymphodepletion. Thus, the lower platelet counts in non-responding ALL patients seem disease dependent, rather than NMA related, which is in accordance with the existing literature.33
Patients stayed, on average, 23±8 days in the hospital and received 1.0±1.4 units of RC and 1.3±2.4 units of PLT. Febrile neutropenia was observed in 12 of 19 (63%) patients.
Discussion
Lymphodepleting preconditioning is an integral part of all adoptive T cell therapies. However, to date, there is no guideline or standardization and no data, which compare different NMA regimens.
In this study, we compared different NMA therapies in regard to bone marrow suppression and recovery, cytokine serum levels, clinical response and adverse events.
We could demonstrate that 120Cy/125Flu and 60Cy/125Flu preconditioning were equally efficient in achieving deep lymphopenia over a period of 1 week and deep neutropenia in patients with metastatic melanoma treated with TIL, whereas ALC and ANC were significantly higher following TBI/75Flu-induced NMA. In contrast, patients in the TBI/75Flu cohort had neutrophilia 1 day after cell infusion, followed by mild neutropenia. Acute thrombocytopenia was mainly observed in the 120Cy/125Flu cohort.
Despite the deep lymphopenia and neutropenia, patients in the 120Cy/125Flu and 60Cy/125Flu cohorts recovered within 6 days, whereas patients in the TBI/75Flu cohort recovered only after 1 month from their mild neutropenia.
The objective response rate in the 120Cy/125Flu cohort was 28%. Patients in the 60Cy/125Flu and TBI/Flu cohorts did not respond to TIL therapy. The lack of clinical efficacy in the 60Cy/125Flu and TBI/Flu groups may have multiple reasons. Of note, patients in the latter two cohorts were all refractory to anti-PD1 immunotherapy compared with only 17 of 103 patients (17%) in the 120Cy/125Flu group. Interestingly, among the 17 anti-PD1 refractory patients in the 120Cy/125Flu cohort, 16 patients did not respond to TIL therapy, which strengthens our hypothesis, that patients, refractory to anti-PD1 antibodies, are less likely to respond to TIL therapy compared with anti-PD1 naïve patients.
To investigate if bone marrow suppression and recovery can serve as prognostic marker of clinical response and survival, we analyzed the cell counts in the 120Cy/125Flu melanoma patient cohort. The analysis was performed on 29 responders and 74 non-responders. At baseline, there was no difference in absolute cell counts between responding and non-responding patients.
We could confirm that higher lymphocyte counts starting 4 days after cell infusion were significantly associated with clinical response. If the higher count reflects better engraftment and proliferation of infused T cells or a faster bone marrow recovery needs to be further investigated.
Previous studies have shown that the combination of cell counts, defined as inflammatory indexes, can serve as predictors of response.34–39 As anticipated, the PLR and NLR were lower in ORs from day 5 onwards.
ROC curve analysis 7 days after cell infusion was performed to determine the optimal cut-offs to distinguish between responding and non-responding patients and predict OS. Cut-offs were defined as NLR≤1.79 and PLR≤32.7. For example, 61% of patients with PLR≤32.7 responded to TIL therapy compared with 15% NRs (p≤0.001). The median OS was 39 months for patients with PLR≤32.7 and 12.5 months at PLR above 32.7.
Previous studies demonstrated that cytokine serum levels are associated with antitumor activity of the T cells and clinical response in patients undergoing adoptive T cell therapy.18 19 Therefore, we compared the cytokine serum levels following the different NMA regimens. We could show that IL-15 levels were insignificantly different, TBI/75Flu-induced lymphodepletion resulted in significantly lower IL-7 serum levels and 60Cy/125Flu-induced lymphodepletion resulted in significantly higher IL-21 serum levels compared with 120Cy/125Flu NMA. Since there were no differences between IL-7, IL-15 and IL-21 serum levels between overall responders and non-responders in the 120Cy/125Flu cohort, the clinical relevance of these findings is questionable.
We further analyzed the absolute cell counts of patients with relapsed or refractory ALL, who received a 30Cy/75Flu preparative regimen before CD19 CAR-T cell infusion. Only a minor decrease of ALC was observed. Although the contribution of leukemic blasts to ALC in patients with ALL cannot be absolutely excluded, it is very unlikely. Most patients were treated before had low or no circulating blasts at the time of lymphodepletion.31 Additionally, in the apheresis product, the starting material for CAR-T cell production, the total number of non-malignant and malignant B cells was less than 5% in most patients and 0% in the CAR-T cell product.9
Furthermore, we investigated the impact of 30Cy/75Flu-induced NMA on ANC and APC and could show that this regimen did not cause deep neutropenia and had no effect on APC.
Higher absolute platelet counts 1 day after NMA initiation (day −4) and throughout the entire time of monitoring were significantly correlated with clinical response. At baseline, there was a trend (p=0.060) of a higher platelet count in OR patients. Prolonged event-free survival following CAR-T cell therapy was previously reported in patients with ALL with higher pre-lymphodepletion platelet count (32). Thus, it should be further investigated if APC assessment could be a helpful predictive marker to make clinical decisions in this patient population.
A major aspect that must be taken into account when choosing chemotherapy for lymphodepletion is the toxicity related to it. Patients in our 120Cy/125Flu cohort developed significantly more acute adverse events, including thrombocytopenia, febrile neutropenia and cardiotoxicity. Thus, the time of hospitalization and the number of RC and PLT transfusions were significantly higher in this cohort. We reported three cases of acute cardiotoxicity following 120Cy/125Flu NMA. All three cases presented with symptoms of acute heart failure before TIL infusion and none of the patients suffered from cardiac problems before therapy. Of note, no other TIL trial reported cyclophosphamide-induced mortality, but, on the other hand, high-dose cyclophosphamide is known to cause cardiotoxicity in 7%–28% of patients.32 It was demonstrated that cyclophosphamide metabolites are responsible for cardiotoxicity due to depletion of antioxidants/ATP level, altered contractility, damaged endothelium and enhanced proinflammatory/proapoptotic activities resulting in cardiomyopathy, myocardial infarction and heart failure.32 Mortality has been reported in 11%–43% of patients at a dose of 170–180 mg/kg. Halving the doses of cyclophosphamide from 60 mg/kg for 2 days (120Cy) to 30 mg/kg for 2 days (60Cy) might prevent this rare but severe adverse effect.
In summary, bone marrow suppression and recovery were equally effected by the 120Cy/125Flu and 60Cy/125Flu preconditioning in the melanoma cohorts; however, toxicity and consequently time of hospitalization were significantly lower in the 60Cy/125Flu cohort. Patients in the 30Cy/75Flu ALL cohort and TBI/75Flu melanoma cohort rarely developed NMA-induced adverse events; however, both regimens were not efficient in achieving deep bone marrow suppression.
Acknowledgments
The authors would also like to thank the Lemelbaum family for their generous support.
Footnotes
AN, SL-A and TM contributed equally.
Contributors: AN, SL-A, and MB designed the study. EJ, NA, GB, RS-F, JS, and GM recruited and treated the patients and are investigators. AN, SL, OI, EJ, and TM collected and analyzed the data. AN and TM performed the statistical analysis. AN, SL, and MJ wrote the paper. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: No, there are no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study has been performed in accordance with the Declaration of Helsinki and was approved by the Israeli Ministry of Health. NCT numbers: NCT00287131 (TIL), NCT03166397 (TIL) and NCT02772198 (CAR-T). An informed consent to participate in the study was obtained from all participants or their legal guardian for minors.
References
- 1.Hirayama AV, Gauthier J, Hay KA, et al. The response to lymphodepletion impacts pfs in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood 2019;133:1876–87. 10.1182/blood-2018-11-887067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besser MJ, Itzhaki O, Ben-Betzalel G, et al. Comprehensive single Institute experience with melanoma TIL: long term clinical results, toxicity profile, and prognostic factors of response. Mol Carcinog 2020;59:736–44. 10.1002/mc.23193 [DOI] [PubMed] [Google Scholar]
- 3.Shapira-Frommer R, Schachter J. Adoptive immunotherapy of advanced melanoma. Curr Treat Options Oncol 2012;13:340–53. 10.1007/s11864-012-0203-7 [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 2009;21:233–40. 10.1016/j.coi.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002;298:850–4. 10.1126/science.1076514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen M, Westergaard MCW, Milne K, et al. Adoptive cell therapy with tumor-infiltrating lymphocytes in patients with metastatic ovarian cancer: a pilot study. Oncoimmunology 2018;7:e1502905. 10.1080/2162402X.2018.1502905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevanović S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol 2015;33:1543–50. 10.1200/JCO.2014.58.9093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevanović S, Helman SR, Wunderlich JR, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res 2019;25:1486–93. 10.1158/1078-0432.CCR-18-2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itzhaki O, Jacoby E, Nissani A, et al. Head-To-Head comparison of in-house produced CD19 CAR-T cell in all and NHL patients. J Immunother Cancer 2020;8:e000148. 10.1136/jitc-2019-000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008;26:5233–9. 10.1200/JCO.2008.16.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wrzesinski C, Paulos CM, Kaiser A, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother 2010;33:1–7. 10.1097/CJI.0b013e3181b88ffc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Powell DJ, Rosenberg SA, et al. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 2006;6:383–93. 10.1038/nri1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salem ML, Cole DJ. Dendritic cell recovery post-lymphodepletion: a potential mechanism for anti-cancer adoptive T cell therapy and vaccination. Cancer Immunol Immunother 2010;59:341–53. 10.1007/s00262-009-0792-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klebanoff CA, Khong HT, Antony PA, et al. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol 2005;26:111–7. 10.1016/j.it.2004.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geukes Foppen MH, Donia M, Svane IM, et al. Tumor-Infiltrating lymphocytes for the treatment of metastatic cancer. Mol Oncol 2015;9:1918–35. 10.1016/j.molonc.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antony PA, Piccirillo CA, Akpinarli A, et al. Cd8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol 2005;174:2591–601. 10.4049/jimmunol.174.5.2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005;202:907–12. 10.1084/jem.20050732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol 2017;35:1803–13. 10.1200/JCO.2016.71.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anthony SM, Rivas SC, Colpitts SL, et al. Inflammatory signals regulate IL-15 in response to Lymphodepletion. J Immunol 2016;196:4544–52. 10.4049/jimmunol.1600219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J-H, Zhai E-T, Yuan Y-J, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol 2017;23:6261–72. 10.3748/wjg.v23.i34.6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diao P, Wu Y, Li J, et al. Preoperative systemic immune-inflammation index predicts prognosis of patients with oral squamous cell carcinoma after curative resection. J Transl Med 2018;16:365. 10.1186/s12967-018-1742-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong J-H, Huang D-H, Chen Z-Y. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget 2017;8:75381–8. 10.18632/oncotarget.18856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Diao F, Ye Z, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer 2017;36:75. 10.1186/s40880-017-0243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wade RG, Robinson AV, Lo MCI, et al. Baseline neutrophil-lymphocyte and platelet-lymphocyte ratios as biomarkers of survival in cutaneous melanoma: a multicenter cohort study. Ann Surg Oncol 2018;25:3341–9. 10.1245/s10434-018-6660-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lino-Silva LS, Salcedo-Hernández RA, García-Pérez L, et al. Basal neutrophil-to-lymphocyte ratio is associated with overall survival in melanoma. Melanoma Res 2017;27:140–4. 10.1097/CMR.0000000000000333 [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Ma Y, Sun L, et al. Prognostic significance of pretreatment neutrophil/lymphocyte ratio and Platelet/Lymphocyte ratio in patients with diffuse large B-cell lymphoma. Biomed Res Int 2018;2018:1–8. 10.1155/2018/9651254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porrata LF, Ristow K, Habermann T, et al. Predicting survival for diffuse large B-cell lymphoma patients using baseline neutrophil/lymphocyte ratio. Am J Hematol 2010;85:896–9. 10.1002/ajh.21849 [DOI] [PubMed] [Google Scholar]
- 28.Khoja L, Atenafu EG, Templeton A, et al. The full blood count as a biomarker of outcome and toxicity in ipilimumab-treated cutaneous metastatic melanoma. Cancer Med 2016;5:2792–9. 10.1002/cam4.878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finon A, Zaragoza J, Maillard H, et al. A high neutrophil to lymphocyte ratio prior to BRAF inhibitor treatment is a predictor of poor progression-free survival in patients with metastatic melanoma. Eur J Dermatol 2018;28:38–43. 10.1684/ejd.2017.3167 [DOI] [PubMed] [Google Scholar]
- 30.Besser MJ, Shapira-Frommer R, Itzhaki O, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res 2013;19:4792–800. 10.1158/1078-0432.CCR-13-0380 [DOI] [PubMed] [Google Scholar]
- 31.Jacoby E, Bielorai B, Avigdor A, et al. Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. Am J Hematol 2018;93:1485–92. 10.1002/ajh.25274 [DOI] [PubMed] [Google Scholar]
- 32.Iqubal A, Iqubal MK, Sharma S, et al. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: old drug with a new vision. Life Sci 2019;218:112–31. 10.1016/j.lfs.2018.12.018 [DOI] [PubMed] [Google Scholar]
- 33.Hay KA, Gauthier J, Hirayama AV, et al. Factors associated with durable EFS in adult B-cell all patients achieving MRD-negative Cr after CD19 CAR T-cell therapy. Blood 2019;133:1652–63. 10.1182/blood-2018-11-883710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jomrich G, Gruber ES, Winkler D, et al. Systemic immune-inflammation index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg 2020;24:610–8. 10.1007/s11605-019-04187-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu B, Yang X-R, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212–22. 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 36.Cho U, Park HS, Im SY, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS One 2018;13:e0200936. 10.1371/journal.pone.0200936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Zhang H, Yang Y, et al. Prognostic value of peripheral inflammatory markers in preoperative mucosal melanoma: a multicenter retrospective study. Front Oncol 2019;9:995. 10.3389/fonc.2019.00995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson AV, Keeble C, Lo MCI, et al. The neutrophil-lymphocyte ratio and locoregional melanoma: a multicentre cohort study. Cancer Immunol Immunother 2020;69:559–68. 10.1007/s00262-019-02478-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 2018;6:74. 10.1186/s40425-018-0383-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001743supp004.pdf (14.6KB, pdf)
jitc-2020-001743supp001.pdf (162.1KB, pdf)
jitc-2020-001743supp002.pdf (100.2KB, pdf)
jitc-2020-001743supp003.pdf (125.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request.