Abstract
Cyclic nucleotide phosphodiesterase type 4 (PDE4), which controls the intracellular level of cyclic adenosine monophosphate (cAMP), has aroused scientific attention as a suitable target for anti-inflammatory therapy of respiratory diseases. This work describes the development and characterization of pyridazinone derivatives bearing an indole moiety as potential PDE4 inhibitors and their evaluation as anti-inflammatory agents. Among these derivatives, 4-(5-methoxy-1H-indol-3-yl)-6-methylpyridazin-3(2H)-one possesses promising activity, and selectivity towards PDE4B isoenzymes and is able to regulate potent pro-inflammatory cytokine and chemokine production by human primary macrophages.
This work describes the development of pyridazinone derivatives bearing an indole moiety as PDE4B inhibitors and their evaluation as anti-inflammatory agents.
Introduction
Chronic obstructive pulmonary disease (COPD) and asthma are among the most common chronic diseases of the twenty-first century. They are becoming a major global health problem with high morbidity and mortality.1 Asthma is one of the most common chronic conditions in children, while more than 3 million people died from COPD worldwide in 2015.2 These lung pathologies are characterized by a progressive airflow diminution accompanied by inflammatory response. Even if combined inhaled corticosteroid and long-acting beta agonist treatments play a central role in the management of asthma and COPD, poor treatment adherence and/or inhaler techniques have been reported.3
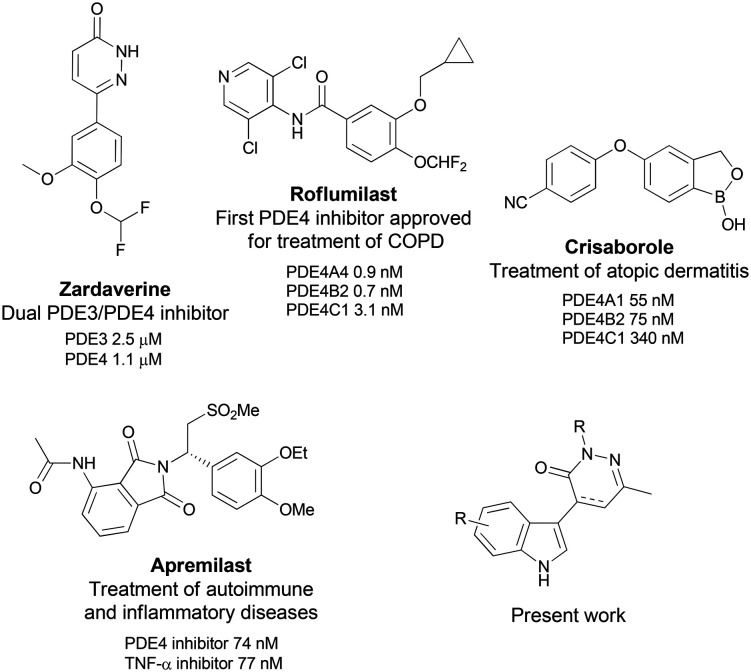
In the recent years, phosphodiesterases (PDEs), a superfamily of eleven intracellular enzymes, responsible for the hydrolysis of cyclic adenosine and/or guanosine monophosphate (ubiquitous intracellular second messengers), have emerged as new therapeutic targets with special attention given to phosphodiesterase type-4 (PDE4) for the treatment of respiratory diseases. From a biological point of view, PDE4 is the predominant isoenzyme found to be overexpressed in airway cells such as epithelial and immune cells including neutrophils, T-cells, and macrophages.4 By its inhibition, both airway smooth muscle relaxation and inflammatory modulator release can be managed; therefore, PDE4 may be considered as a valuable biological target for the treatment of inflammatory or pulmonary diseases.5 More recently, PDE4 inhibition also demonstrated positive effects against aberrant immune response diseases such as atopic dermatitis, rheumatoid arthritis or psoriasis or in the modulation of metabolic disorders such as obesity or type 2 diabetes.6 Although a large number of PDE4 inhibitors have been clinically evaluated, the use of first-generation compounds has been hampered by cardiac and emetic mechanism-associated side effects. Second-generation selective inhibitors like roflumilast and apremilast were generally better-tolerated and are currently approved for the treatment of COPD and plaque psoriasis, respectively (Scheme 1).7,8
Scheme 1. Structures and affinities of PDE4 inhibitors zardaverine and approved drugs and the targeted family.
Macrophages are cells belonging to the innate immune system. In several inflammatory diseases, macrophages orchestrate the inflammatory response as in COPD.9 Actually, a series of studies have evidenced an increase in macrophage number in COPD patients.10–12 These cells are recruited from blood flow in response to chemokine (CCL2, CXCL1) gradients.13 Their activation leads to pro-inflammatory cytokine release, among them TNF-α, IL-1β, IL-8 and IL-6, which are pivotal to ensure the recruitment and activation of more inflammatory cells like polynuclear neutrophils that become a major cell population in COPD patient lungs.14 To avoid an exacerbated inflammatory process, some therapeutics have been tested to modulate macrophage activation: macrolides, exhibiting both anti-microbial and anti-inflammatory properties,15 and PDE4 inhibitors.16,17 For example, roflumilast induces (at a dose of 5 mg kg−1 per day) reduction of macrophages in the lung of mice exposed to tobacco smoke and have demonstrated clinical benefits in COPD patients.18,19
In continuation of our efforts to develop selective inhibitors of PDE4 for the treatment of respiratory diseases possessing fewer side effects than marketed compounds,20 we have previously identified a hit compound possessing the dihydropyridazinone scaffold and bearing a heterocyclic moiety.21 We have designed this hit by introducing a degree of conformational freedom between the pyridazinone scaffold and heterocyclic moiety in order to study if these conformations can influence potency and selectivity over PDE4. This hit possessed neither cytotoxic effects nor abnormal pro-inflammatory roles in polymorphonuclear leukocyte cells and we highlighted that this PDE4 inhibitor was able to reduce IL-8 production. Based on this preliminary data, we describe herein the synthesis of new pyridazinone derivatives bearing an indole moiety in position 4 (structure in Scheme 1), their evaluation as potential PDE4B-inhibitors and their anti-inflammatory potential by regulating cytokine and chemokine production by macrophages.
Results and discussion
1. Synthesis
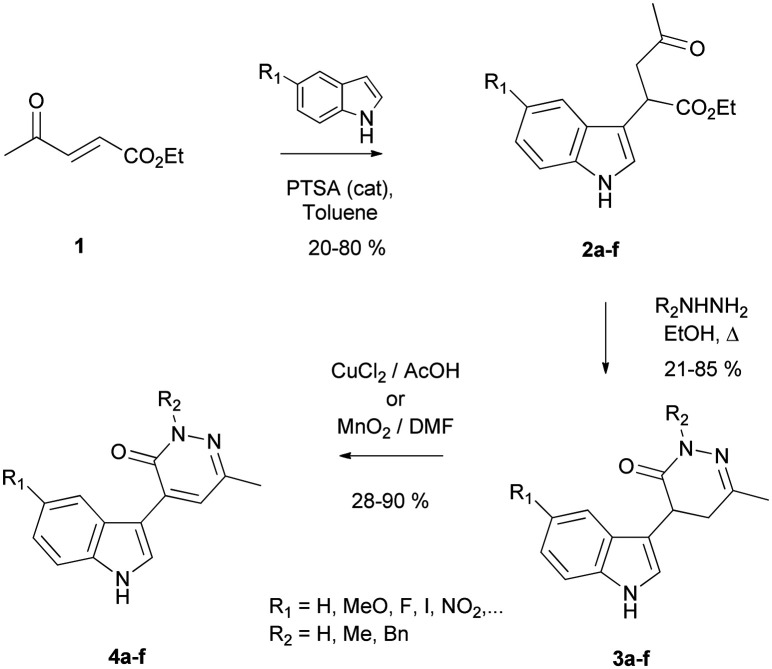
Preparation of the targeted pyridazinone derivatives was carried out through a flexible three-step sequence starting from the α,β-unsaturated levulinate 1 (Scheme 2).22 Regioselective introduction of the indole moiety was carried out by a Michael type reaction leading to intermediate compounds 2a–f.23 Different indole derivatives (5-halogeno, 5-nitro and 5-methoxyindole) were used in this reaction in order to initiate a preliminary structure–activity relationship (SAR) study by comparison with our preliminary hit. As shown in Table 1, formation of the corresponding compounds proceeded in moderate to good yields in about 36 h. Subsequent condensation of the intermediates 2a–f with hydrazine derivatives led to the formation of the corresponding 4,5-dihydropyridazinones 3a–f with good yields. The desired pyridazinones 4a–f functionalized with an indole moiety in position 4 were then obtained using oxidative conditions (copper(ii) chloride (method A) or manganese dioxide (method B)) depending on the solubility of the dihydropyridazinone derivatives.24
Scheme 2. Synthesis of (dihydro)pyridazinones bearing an indole moiety in position 4.
Synthesis of pyridazinone derivatives bearing an indole moiety in position 4.
| Entry | Heterocycle | Compound (yield)a | R2 | Compound (yield)a | Oxidative methodb | Compound (yield)a |
|---|---|---|---|---|---|---|
| 1 |

|
2a (61%) | H | 3aa (61%) | B | 4aa (50%) |
| 2 | Me | 3ab (81%) | A | 4ab (36%) | ||
| 3 | Bn | 3ac (63%) | A | 4ac (34%) | ||
| 4 |
|
H | 3ba (76%) | B | 4ba (60%) | |
| 5 | 2b (55%) | Me | 3bb (25%) | B | 4bb (35%) | |
| 6 | Bn | 3bc (41%) | B | 4bc (46%) | ||
| 7 |

|
2c (39%) | H | 3ca (77%) | B | 4ca (90%) |
| 8 | Bn | 3cc (44%) | B | 4cc (50%) | ||
| 9 |

|
2d (80%) | H | 3da (81%) | B | 4da (60%) |
| 10 | Bn | 3dc (43%) | B | 4dc (40%) | ||
| 11 |

|
2e (33%) | H | 3ea (68%) | B | 4ea (28%) |
| 12 | Bn | 3ec (40%) | B | 4ec (45%) | ||
| 13 |

|
2f (20%) | H | 3fa (73%) | B | 4fa (80%) |
Yield of isolated product.
Method A: CuCl2/AcOH; method B: MnO2/THF.
2. PDE4 inhibition
The effects of (dihydro)pyridazinones 3 and 4 on modulation of the intracellular level of cyclic AMP were then examined and compared to those of a non-specific PDE inhibitor 3-isobutyl-1-methylxanthine (IBMX), zardaverine (pyridazinone scaffold comparison) and a therapeutic reference, roflumilast. Taking into account the fact that drugs that preferentially inhibit the PDE4B isoform over PDE4D should retain many of their anti-inflammatory activity benefits with fewer unwanted central nervous system side-effects,25 all synthesized compounds were first screened for their potential inhibitory activity on this subtype. The evaluation approach, based on a “nonradioactive” assay developed in our laboratory, could be directly applied for the selection of privileged pyridazinone derivatives.21 Results were expressed as inhibition percentage of PDE4B at an initial screening concentration of 20 μM. First, in vitro results (collected in Table 2) showed that all the pyridazinones, bearing an indole moiety on the 4-position of the heterocyclic scaffold, systematically exhibited greater percentages of PDE4B inhibition (1.5–3 fold) compared to the corresponding 4,5-dihydropyridazinones (compounds 4vs.3). This observation is probably due to the more planar character of the pyridazinone scaffold (by comparison to a dihydro structure) allowing better interactions with the relatively closed and hydrophobic oblong pocket of the active site. Our attention turned to the N-substituent on the pyridazinone ring (R2). It was confirmed that the hydrogen bond donor function (R2 = H) was optimal for the PDE4B affinity in so far as N-methyl derivatives 3ab, 4ab, 3bb and 4bb appeared up to 2.5-fold less potent than their corresponding NH analogue. Interestingly, contrary to our initial expectations, increasing the hydrophobic character of the pyridazinone derivatives (R2 = Bn) slightly decreased the inhibitory effect but might have an impact on selectivity. Concerning the substitution on position 5′ of the indole moiety, modification of the nature of the halogen could drastically reduce the affinity for PDE4 receptors (F > Br ∼ I); thus, iodo- and bromo-derivatives were found to be practically inactive at screening concentration. By comparison with 5′-fluoro derivative 4ea, compound 4fa possessing a nitro function seems to interact more favorably with PDE4 but remains about three times less active than pyridazinone 4ba. Additional hydrogen-bond interaction with key glutamine (Gln) could explain the observed increase in inhibitory activity of the methoxy-functionalized compounds (see the Molecular modeling section).21
Biochemical evaluation of pyridazinone derivatives as PDE4B inhibitors.

|
Inhibition at an initial concentration of 20 μM.
Mean of two or three experiments, each performed in triplicate; protocols are given in the ESI† experimental section.
Percentage of inhibition range:  /
/ /
/ />
/> .
.
In order to explore the potentiality of compound 4ba and to reinforce the preliminary SAR results (vs. compounds 4aa and 4bc), we submitted these pyridazinones to a selectivity study versus PDE subtypes by comparison with references. We selected PDEs which demonstrate different capabilities for degrading cAMP/cGMP and regulating cell functions: PDE1 (a Ca2+ and calmodulin dependent PDE), PDE10, an isoform mainly expressed in the brain capable of hydrolyzing both cAMP and cGMP (but with a higher affinity for cAMP than cGMP), and PDE7, highly selective for cAMP isoenzymes.26 Based on previous studies suggesting that the emesis side-effects result from the inhibition of the PDE4D isoform and that knocking out PDE4B but not PDE4A/PDE4D significantly inhibited the synthesis of TNF-α in LPS-mediated monocyte and macrophages, we also evaluated the capability of our pyridazinones to modulate the three distinct PDE4 subtypes (Fig. 1).27 The results of this study confirms the potency of our scaffold enhanced by the selectivity over the PDE4 isoenzyme compared to PDE1, PDE10 and mostly PDE7. If, as previously expected, the introduction of an N-benzyl substient in our pyridazinone scaffold (compound 4bc) affects the profile, clearly, the PDE4B selectivity index value is much better for our pyridazinone 4ba than those of references.
Fig. 1. PDE inhibition (% of inhibition) and PDE4B (vs. 4D)-selectivity index values. [Inhibition at a concentration of 20 μM; mean of two or three experiments, each performed in triplicate; protocols are given in the ESI† experimental section].
Comparison of the different pharmacological data showed that the more active pyridazinone is the compound 4ba, functionalized with the 5-methoxyindole moiety, exhibiting 64% inhibition of PDE4B at 20 μM and IC50 = 251 ± 18 nM (log P 0.43). In our study, this compound is slightly less active in vitro than therapeutic reference compound roflumilast (75% of inhibition at 20 μM) but displays a better selectivity profile towards the PDE4B isoform. This pyridazinone 4ba was selected for further investigations.
3. Molecular modeling
In a complementary way to the experimental investigations and hit-detection, a challenging task to explain the preliminary SAR was to address the binding mode of our ligands by means of theoretical tools. Here, molecular docking simulations together with IGM analysis were carried out to identify, characterize and quantify ligand–protein interactions as well as atomic contributions to these interactions (see computational details).28 The PDE4B and PDE4D isoforms show a high structural similarity (98%) making it extremely difficult to develop a selective inhibitor. But, previous authors have shown that the C-terminal control region (CR3) present in the PDE4 isoforms can be exploited to generate PDE4B-selective inhibitors.29,30 Actually, some ligands cause the CR3 helix to close over the PDE4 active site, thereby, locking the enzyme in an inactive conformation. In that case, the PDE4B selectivity is due to a single amino acid of the CR3 helix (Leu in PDE4B vs. Gln in PDE4D). This so-called CR3 gating helix (GH) has been introduced in our models during the docking simulations and its associated residues will be referred hereafter with the subscript GH.
From the literature and from our previous work,21,31 although interactions vary from one inhibitor to another, the two key features of PDE4–ligand binding are: i) hydrogen bonding with Gln and ii) van der Waals interactions on both sides of a ligand possessing flat structure and fitting an oblong cavity, as illustrated in Fig. 2. Also, a shallow groove can be taken advantage of, present at the foot of the active site, to design new ligands. Upon binding the PDE4B active site, the ligand 4ba fulfills the above-mentioned key criteria, and a good complementarity shape is obtained (see Fig. 2 and 3). Its interaction with the Gln615 residue through hydrogen-bonding is limited in the reported pose. It should however be noticed that we found many poses displaying this hydrogen-bond between the methoxy group of 4ba and this Gln residue, showing that the methoxy pattern plays a key role in the ligand biorecognition by the PDE4B protein. The interactions between 4ba and the glutamine (Gln615) and phenylalanine (Phe586 and Phe618) residues of the protein are well recovered by the IGM analysis as evidenced by the large associated isosurface (Fig. 3 [upper line, central panel]). It should be noted that the methyl group on the pyridazinone moiety exploits a small hydrophobic cavity at the back of the active site. The interactions between the methoxy group of 4ba and the leucine (Leu674_GH) residue of the gating helix CR3, as well as those involving the indole moiety of 4ba and the lysine (Lys677_GH) and phenylalanine (Phe678_GH) groups, highlight the relevance of the CR3 gating helix segment in the PDE4B–ligand recognition. This is confirmed by a significant Δginter score of 1.44 a.u. (see Table S1 in the ESI†) for the CR3–4ba interaction. According to the IGM atomic decomposition, the indole pattern (42%) and pyridazinone moiety (42%) equally contribute to the total interaction with the protein PDE4B, whilst the methoxy group contribution amounts to 16% of the ligand–protein PDE4B interaction.
Fig. 2. Presentation of the PDE4B active site complexed with ligand 4ba. The glutamine residue (Gln) plays a key role in the biomolecular recognition, as well as hydrophobic residues on both sides of the ligand; a hydrophobic groove exists at the foot of the active site.
Fig. 3. Molecular docking pose and IGM analysis of 4ba [upper line] and 4ea [lower line] inside the PDE4B-CR3 cavity (left panel). IGM 0.007 a.u. δginter isosurface and a BGR color code in the range −0.05 < ρ sign (λ2) < 0.05 a.u. (center panel). IGM atomic decomposition with the ligand colored according to the Δginter/At score using a BGryR color scale (right panel). Non-polar hydrogen atoms of the protein are not displayed for the sake of clarity.
In PDE4D–4ba, the key interactions are still found with Gln (Gln535 in this isoform) and hydrophobic residues. However, it is worth noting that the pyridazinone moiety can no longer exploit the hydrophobic pocket in the back of the active site. In this pose, the pyridazinone is twisted relative to the indole heterocycle and is rather directed towards the shallow groove and partially towards the CR3 helix. A Δginter score of 1.03 a.u. (Table S1†), reflecting the interaction between ligand 4ba and the PDE4D CR3 helix, is lower in comparison with that of PDE4B–4ba (1.44 a.u.), suggesting a reduced ability of the ligand to hold the active site closed in this case. Removal of the methoxy group from 4ba (PDE4B–4aa, see Fig. S1 in the ESI†) leads to a loss of the PDE4B recognition through hydrogen-bonding to the Gln615 residue. In other respects, the pose of 4aa–PDE4B looks very similar to the pose of 4ba–PDE4B. The impact of the methoxy group on the CR3–ligand interaction is demonstrated when the 4ba–CR3(PDE4B) and 4aa–CR3(PDE4D) Δginter scores are compared (Table S1 in the ESI†). In the latter, a lower Δginter (1.08 a.u.) relative to the former (1.44 a.u.) is found, revealing the positive effect of the methoxy group incorporation in terms of CR3 gating helix recognition. In PDE4B–4bc where a benzyl substituent is present on the pyridazinone moiety, we still observe the well-known interaction between the methoxy group of 4bc and the glutamine (Gln615) as well as interactions with hydrophobic residues (see Fig. S2 in the ESI†). However, the methylpyridazinone moiety is no longer directed towards the hydrophobic pocket in the back of the active site but, once more, it is slightly twisted relative to the indole heterocycle and oriented so that the benzyl group points towards the shallow groove. Then, an additional attractive noncovalent interaction between the methylpyridazinone group and a methionine (Met519) belonging the protein cavity is observed.
It is noteworthy that some unexpected results were obtained in PDE4B–4ea with the 5′-fluorinated compound. As in PDE4B–4aa (without the methoxy group on the indole), the hydrogen-bonding recognition by Gln615 is lost (see Fig. 3 [lower line, left panel]). Also, the pyridazinone moiety is slightly twisted and pointing towards the shallow groove. But, in return, the fluorine atom is directed towards the gating helix CR3, favoring new interactions closing the active site. After inspecting very carefully the pose of 4ea in the PDE4B model (Fig. 3 [lower line, left panel]), there is no indication of halogen bond formation between the fluorine atom of 4ea and the gating helix residues. However, the IGM analysis reveals attractive vdW contacts between this fluorine atom and the lysine (Lys677_GH)/phenylalanine (Phe678_GH) residues of the CR3 helix, as illustrated in Fig. 3 [central panel]. Although the overall CR3–4ea interaction score is small (0.76 a.u.) compared to that of the other ligands, it is worth noting that the single fluorine atom interaction with the CR3 gating helix amounts to 4.6%.
4. Biology
The selected compound 4ba was then evaluated for in vitro anti-inflammatory activity. We first evaluated the morphology of the cells we used (Fig. 4). PBMCs cultured in the presence of M-CSF are intended to become macrophages.32 SEM analyses demonstrated that under basal conditions (DMSO, Fig. 4), we obtained individual cells which are spherical, exhibiting limited adhesion-related structures and few vesicles at the cell membrane. After LPS stimulation, cells tended to cluster together, increasing in size and exhibiting a more ameboid shape. They also exhibited numerous cytoplasmic extensions towards other cells, filopodia and developed lamellipodia. A high number of vesicles have also been observed and all these criteria are classical hallmarks of activated macrophage morphology.33 When treated with zardaverine, cells exhibited a morphology close to that observed under control conditions whereas in the presence of roflumilast, they were much more flattened, showing a reduced vesicle number and highly intricate cytoplasms, but were bigger in size. When treated with compound 4ba, cells display an alternative phenotype with common features from both zardaverine and roflumilast treatments.
Fig. 4. Representative scanning electron microscopy photographs showing human primary macrophages after 24 h of stimulation with DMSO alone (A) or with added LPS 10 ng mL−1 (B) and treated with zardaverine 10 μM (C), roflumilast 10 μM (D) or compound 4ba 50 μM (E). Magnification ×5000; scale bar = 1 μm. Inserts show representative micrographs under the same conditions at magnification ×2000; scale bar = 10 μm.
In order to assess that morphological changes are not attributable to cell suffering and death, we measured lactate dehydrogenase (LDH) release in cell-stimulated supernatants. As evidenced in Fig. 5, no statistical accumulation of LDH was observed in the presence of any of the tested compounds. We then measured the inhibition of pro-inflammatory cytokine secretion in the macrophage culture supernatants (Fig. 6). Here, we confirmed that in our hands LPS was able to induce an increased secretion of IL-1β (30 fold increase of average value p = 0.008), TNF-α (6.25 fold increase of average value p = 0.008), IL-6 (26.5 fold increase of average value p = 0.008), and IL-8 (18.9 fold increase of average value p = 0.008) by human primary macrophages (Fig. 6A) as has been also evidenced and reviewed elsewhere.32 We highlighted that zardaverine was unable to limit such an increase for all the 4 cytokines studied (Fig. 6B–E), while roflumilast only induced an average 10% decrease in IL-8 measurement (p = 0.047, Fig. 6E) in LPS-stimulated macrophage supernatants. Compound 4ba demonstrated a clear dose effect on IL-1β concentration decrease that reached the statistical significance threshold for concentrations of 20 μM and 50 μM (58% and 54% reduction of average values, p = 0.023 and p = 0.039, respectively, Fig. 6B). TNF-α concentration regulation by compound 4ba followed a similar pattern but only the highest concentration (50 μM) demonstrated a significant 47% decrease (p = 0.008). Interestingly, the 5 μM concentration failed to significantly reduce the LPS-induced TNF-α production despite a 43% average decrease (p = 0.2422), but it was the only condition in which LPS stimulation did not lead to a significant increase as compared to the control conditions (p = 0.219, Fig. 6C). The IL-6 concentration was reduced by all the tested concentrations of compound 4ba (average reduction of 21%, 28%, 23%, and 16% for 50 μM, 20 μM, 10 μM and 5 μM respectively; p = 0.039, p = 0.008, p = 0.023 and p = 0.008, Fig. 6D). IL-8 production by macrophages has been reduced only when cells were treated by 50 μM and 20 μM (16% and 25% average reduction respectively, p = 0.039 and p = 0.008, Fig. 6E). These results highlight the ability of our compound 4ba to regulate potent pro-inflammatory cytokine and chemokine production by macrophages at a starting concentration of 20 μM. In addition, the anti-inflammatory effects evidenced in this work are better than those demonstrated by zardaverine or roflumilast, two reference molecules for anti-PDE4 activity in inflammatory disease models.34–36 On the one hand, as compared to the literature, major discrepancies in the zardaverine and roflumilast effects may come from the incubation time (4 h most of the time) and cell source, which is very often lung-related and from rodents in the literature, as compared to human whole blood in our work.37 On the other hand, such an observation is in line with recent data from our group highlighting in human primary polymorphonuclear cells that zardaverine exerted modest regulation of chemokine production following LPS stimulation exhibiting thus a small impact on cell recruitment in vitro.21
Fig. 5. Cell death evaluation by measurement of LDH activity in human primary macrophage supernatants after 24 h treatment with zardaverine (10 μM), roflumilast (10 μM) and compound 4ba (n = 8). The red bar represents the median value. Black bars represent the 1st and 9th deciles and the limits of rectangles represent the 1st and 3rd quartiles.
Fig. 6. ELISA measurements of IL-1β, TNF-α, IL-6 and IL-8 concentrations in unexposed or LPS-exposed cell supernatants after 24 h (A). IL-1β (B), TNF-α (C), IL-6 (D) and IL-8 (E) concentrations in LPS-exposed cell supernatants after 24 h of treatment with zardaverine (10 μM), roflumilast (10 μM) or compound 4ba. The red bar represents the median value. Black bars represent the 1st and 9th deciles and the limits of rectangles represent the 1st and 3rd quartiles. n = 8 independent experiments. *p < 0.05 when compared with non-stimulated (DMSO) cells. #p < 0.05 when compared with LPS-stimulated cells.
To assess the 4ba effect on monocyte/macrophage recruitment in vivo, we used the murine air pouch model that creates a delineated cavity inside which the 4ba effect was tested. Initially dedicated to acute inflammation studies,38–39 this model has also been evidenced to allow the study of monocyte/macrophage populations.40 In response to LPS stimulation, we demonstrated an increase in monocyte and macrophage recruitment in the air pouch exudate (1.9 and 1.7 fold increase of average values, p = 0.044 and p = 0.019 respectively, Fig. 7A and B). As compared to LPS or control condition, Zardaverine failed to induce any modulation of monocyte recruitment (Fig. 7A). Interestingly, zardaverine exhibited a clear dose effect on macrophage recruitment with a significant increase as compared to the control conditions (average increase of 2 fold, p = 0.020) at a concentration of 2 μM, the absence of any variation at 20 μM, and a significant reduction of macrophage recruitment as compared to LPS at 200 μM (26% average reduction, p = 0.006, Fig. 7B). Roflumilast had no effect at 2 μM but increased monocyte recruitment at 20 μM and 200 μM as compared to the control conditions (3.5 and 2 fold increase of average values, p = 0.001 and p = 0.042 respectively), and also as compared to LPS stimulation alone at 20 μM (average increase of 1.8 fold, p = 0.020). In contrast, roflumilast exhibited no effect on macrophage recruitment (Fig. 7B). In this in vivo model, our hit compound 4ba statistically impacted monocyte recruitment at a concentration of 200 μM for which it was the only compound able to reduce the cell infiltrate in response to LPS (average reduction of 69%, p = 0.002) and tended to decrease, at the same concentration, macrophage recruitment after stimulation. Importantly, at a concentration of 2 or 20 μM, compound 4ba also blocks both the monocyte and macrophage LPS-recruitment (p > 0.05 vs. control). These results highlighted a few significant effects of anti-PDE4 molecules possessing a pyridazinone scaffold (vs. roflumilast) on monocyte/macrophage recruitment, but some interesting trends in dose effect may be observed, especially for monocytes.
Fig. 7. Quantification of monocytes (A) and macrophages (B) in murine air-pouch exudate after 6 h of inflammation induction and treatment with DMSO (control conditions), zardaverine at 2 μM, 20 μM, and 200 μM, roflumilast at 2 μM, 20 μM, and 200 μM or compound 4ba at 2 μM, 20 μM and 200 μM. n = 8–15, *p < 0.05 vs. control, #p < 0.05 vs. LPS.
Taking together our results, we can speculate that pyridazinone scaffold-based derivatives and more specifically our derivative 4ba are interesting molecules to limit inflammatory processes, fighting against both immune cell activation (via IL-1, TNF or IL-6) and also inflammatory cell recruitment (via IL8) thanks to inflammatory mediator regulated production.
Conclusions
In conclusion, a new family of molecules possessing a pyridazinone scaffold, bearing an indole moiety in position 4, were obtained in a few steps from a levulinic acid derivative and were evaluated for their potential anti-inflammatory activity. After screening, hit-detection and docking/IGM analysis to rationalize the binding mode of our ligands, we have identified 4-(5-methoxy-1H-indol-3-yl)-6-methylpyridazin-3(2H)-one 4ba to possess promising activity and selectivity towards the PDE4B isoenzyme and be able to regulate potent pro-inflammatory cytokine and chemokine production by macrophages. Of physiological relevance is that our hit compound possesses neither a cytotoxic effect nor an abnormal pro-inflammatory role and we highlighted that this PDE4 inhibitor 4ba was able to impact monocyte recruitment in vivo. While other structural modifications are currently under investigation to modulate the physico-chemical properties of this class of compounds, our hit should be useful to investigate the role of PDE4 in various pulmonary disease models.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
Financial support from the French National Centre for Scientific Research (CNRS), the Regional Council of Champagne-Ardenne (France), the French Ministry of Higher Education and Research (MESR) and the European Regional Development Fund (FEDER) to the PlAneT and URCACyt CPER projects is acknowledged. A. M.'s Ph.D. fellowship was co-funded by Reims-Metropole and the European Union (Europe invests in Champagne-Ardenne with the European Regional Development Fund). Thanks are given to the Maison de la simulation de Champagne-Ardenne (Reims, France) and the Centre Régional Informatique et d'Applications Numériques de Normandie (CRIANN, Rouen, France) for the provision of computational facilities. The authors also thank the PICT-URCA platform (University of Reims Champagne-Ardenne) for imaging core facilities.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0md00423e
Notes and references
- Centers for disease Control and Prevention: Atlanta 2018; https//www.cdc.gov/copd/ (accessed November 6, 2020) and https//www.cdc.gov/asthma/ (accessed November 6, 2020)
- Burney P. G. Patel J. Newson R. Minelli C. Naghavi M. Eur. Respir. J. 2015;45:1239–1247. doi: 10.1183/09031936.00142414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche N. Plaza V. Backer V. van der Palen J. Cerveri I. Gonzalez C. Safioti G. Scheepstra I. Patino O. Singh D. NPJ Prim. Care Respir. Med. 2020;30:1–7. doi: 10.1038/s41533-019-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews on PDE4 inhibitors in airways disease, see: ; (a) Houslay M. Schafer P. Zhang K. Drug Discovery Today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]; (b) Kodimuthali A. Jabaris S. S. L. Pal M. J. Med. Chem. 2008;71:5471–5489. doi: 10.1021/jm800582j. [DOI] [PubMed] [Google Scholar]; (c) Calverley P. M. A. Lancet. 2009;374:685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]; (d) De Ninno M. Bioorg. Med. Chem. Lett. 2012;22:6794–6800. doi: 10.1016/j.bmcl.2012.09.028. [DOI] [PubMed] [Google Scholar]; (e) Gavaldà A. Roberts R. S. Expert Opin. Ther. Pat. 2013;23:997–1016. doi: 10.1517/13543776.2013.794789. [DOI] [PubMed] [Google Scholar]; (f) Martinez A. Gil C. Expert Opin. Ther. Pat. 2014;24:1311–1321. doi: 10.1517/13543776.2014.968127. [DOI] [PubMed] [Google Scholar]; (g) Mulhall A. M. Droege C. A. Ernst N. E. Panos R. J. Zafar M. A. Expert Opin. Invest. Drugs. 2015;24:1597–1611. doi: 10.1517/13543784.2015.1094054. [DOI] [PubMed] [Google Scholar]
- Michalski J. M. Golden G. Ikari J. Rennard S. I. Clin. Pharmacol. Ther. 2012;91:134–142. doi: 10.1038/clpt.2011.266. [DOI] [PubMed] [Google Scholar]
- Press N. J. Banner K. H. Prog. Med. Chem. 2009;47:37–74. doi: 10.1016/S0079-6468(08)00202-6. [DOI] [PubMed] [Google Scholar]
- (a) Cazzola M. Calzetta L. Rogliani P. Matera M. G. Expert Opin. Drug Discovery. 2016;11:733–744. doi: 10.1080/17460441.2016.1184642. [DOI] [PubMed] [Google Scholar]; (b) Tashkin D. P. Expert Opin. Pharmacother. 2014;15:85–96. doi: 10.1517/14656566.2013.837159. [DOI] [PubMed] [Google Scholar]
- Abdulrahim H. Thistleton S. Adebajo A. O. Shaw T. Edwards C. Wells A. Expert Opin. Pharmacother. 2015;16:1099–1108. doi: 10.1517/14656566.2015.1034107. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. Chronic Obstr. Pulm. Dis. 2004;1:59–70. doi: 10.1081/COPD-120028701. [DOI] [Google Scholar]
- Di Stefano A. Capelli A. Lusuardi M. Balbo P. Vecchio C. Maestrelli P. Mapp C. E. Fabbri L. M. Donner C. Saetta M. Am. J. Respir. Crit. Care Med. 1998;158:1277–1285. doi: 10.1164/ajrccm.158.4.9802078. [DOI] [PubMed] [Google Scholar]
- Akata K. van Eeden S. F. Int. J. Mol. Sci. 2020;21:pii: E853. doi: 10.3390/ijms21030853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. J. J. Allergy Clin. Immunol. 2016;138:16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Traves S. L. Culpitt S. V. Russell R. E. Barnes P. J. Donnelly L. E. Thorax. 2002;57:590–595. doi: 10.1136/thorax.57.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W. Wells J. M. Blalock J. E. Curr. Opin. Pulm. Med. 2016;22:91–99. doi: 10.1097/MCP.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y. Luo L. Li C. Chen P. Chen Y. Int. J. Chronic Obstruct. Pulm. Dis. 2018;13:3813–3829. doi: 10.2147/COPD.S181246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D. Adams D. R. Biochem. J. 2003;370:1–18. doi: 10.1042/bj20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz M. J. Cortijo J. Morcillo E. J. Pharmacol. Ther. 2005;106:269–297. doi: 10.1016/j.pharmthera.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Matera M. G. Page C. P. Cazzola M. Trends Pharmacol. Sci. 2011;32:495–506. doi: 10.1016/j.tips.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Chung K. F. Eur. J. Pharmacol. 2006;533:110–117. doi: 10.1016/j.ejphar.2005.12.059. [DOI] [PubMed] [Google Scholar]
- (a) Sukhorukov A. Boyko Y. Nelyubina Y. Gerard S. Ioffe S. Tartakovsky V. Malleret L. Belaaouaj A. J. Org. Chem. 2012;77:5465–5469. doi: 10.1021/jo300955n. [DOI] [PubMed] [Google Scholar]; Gérard S., Bouillon J.-P., Hénon E., Belaaouaj A., WO Pat., 2016066973A1, 2016
- Barberot C. Moniot A. Allart-Simon I. Malleret L. Yegorova T. Laronze-Cochard M. Bentaher A. Médebielle M. Bouillon J.-P. Hénon E. Sapi J. Velard F. Gérard S. Eur. J. Med. Chem. 2018;146:139–146. doi: 10.1016/j.ejmech.2018.01.035. [DOI] [PubMed] [Google Scholar]
- Raoul M. Gérard S. Sapi J. Eur. J. Org. Chem. 2006;10:2440–2445. [Google Scholar]
- Zubkov I. Romanov A. Ushakov I. Rulev A. Tetrahedron. 2020;76:130884–130888. doi: 10.1016/j.tet.2019.130884. [DOI] [Google Scholar]
- Sircar I. Weishaar R. E. Kobylarz D. Moos W. H. Bristol J. A. J. Med. Chem. 1987;30:1955–1962. doi: 10.1021/jm00394a005. [DOI] [PubMed] [Google Scholar]
- Jin S.-L. C. Lan L. Zoudilova M. Conti M. J. Immunol. 2005;175:1523–1531. doi: 10.4049/jimmunol.175.3.1523. [DOI] [PubMed] [Google Scholar]
- Peng T. Gong J. Jin Y. Zhou Y. Tong R. Wei X. Bai L. Shi J. Eur. J. Med. Chem. 2018;150:742–756. doi: 10.1016/j.ejmech.2018.03.046. [DOI] [PubMed] [Google Scholar]
- Peng T. Qi B. He J. Ke H. Shi J. J. Med. Chem. 2020;63:10594–10617. doi: 10.1021/acs.jmedchem.9b02170. [DOI] [PubMed] [Google Scholar]
- (a) Lefebvre C. Rubez G. Khartabil H. Boisson J.-C. Contreras-García J. Hénon E. Phys. Chem. Chem. Phys. 2017;19:17928–17936. doi: 10.1039/C7CP02110K. [DOI] [PubMed] [Google Scholar]; (b) Ponce-Vargas M. Lefebvre C. Boisson J.-C. Hénon E. J. Chem. Inf. Model. 2020;60:268–278. doi: 10.1021/acs.jcim.9b01016. [DOI] [PubMed] [Google Scholar]
- Hagen T. J. Mo X. Burgin A. B. Fox D. Zhang Z. Gurney M. E. Bioorg. Med. Chem. Lett. 2014;24:4031–4034. doi: 10.1016/j.bmcl.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma K. Omura A. Maekawara N. Saitoh M. Ohkawa N. Kubota T. Nagumo H. Kodama T. Takemura M. Ohtsuka Y. Nakamura J. Tsujita R. Kawasaki K. Yokoi H. Kawanishi M. Bioorg. Med. Chem. Lett. 2009;19:3174–3176. doi: 10.1016/j.bmcl.2009.04.121. [DOI] [PubMed] [Google Scholar]
- Barberot C. Boisson J.-C. Gérard S. Khartabil H. Thiriot E. Monard G. Hénon E. Comput. Theor. Chem. 2014;1028:7–18. doi: 10.1016/j.comptc.2013.11.020. [DOI] [Google Scholar]
- Murray P. J. Allen J. E. Biswas S. Fisher E. A. Gilroy D. W. Goerdt S. Gordon S. Hamilton J. A. Ivashkiv L. B. Lawrence T. Locati M. Mantovani A. Martinez F. O. Mege J. L. Mosser D. M. Natoli G. Saeij J. P. Schultze J. L. Shirey K. A. Sica A. Suttles J. Udalova I. van Ginderachter J. A. Vogel S. Wynn T. A. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. S. Stachelek S. J. Tomczyk N. Finley M. J. Composto R. Eckmann D. M. J. Biomed. Mater. Res., Part A. 2013;101:203–212. doi: 10.1002/jbm.a.34309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade F. U. Schudt C. Eur. J. Pharmacol. 1993;230:9–14. doi: 10.1016/0014-2999(93)90403-5. [DOI] [PubMed] [Google Scholar]
- Crilly A. Robertson S. E. Reilly J. H. Gracie J. A. Lai W. Q. Leung B. Life P. F. McInnes I. B. Ann. Rheum. Dis. 2011;70:1130–1137. doi: 10.1136/ard.2010.134825. [DOI] [PubMed] [Google Scholar]
- Buenestado A. Grassin-Delyle S. Guitard F. Naline E. Faisy C. Israël-Biet D. Sage E. Bellamy J. F. Tenor H. Devillier P. Br. J. Pharmacol. 2012;165:1877–1890. doi: 10.1111/j.1476-5381.2011.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoni T. Salvator H. Abrial C. Brollo M. Porto L. C. S. Lagente V. Naline E. Grassin-Delyle S. Devillier P. Respir. Res. 2017;18:126–127. doi: 10.1186/s12931-017-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. W. Stevens A. J. Brennan B. Davies D. Rowland M. Houston J. B. J. Pharmacol. Toxicol. Methods. 1994;32:139–147. doi: 10.1016/1056-8719(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Duarte D. B. Vasko M. R. Fehrenbacher J. C. Curr. Protoc. Pharmacol. 2016;72:5.6.1–5.6.9. doi: 10.1002/0471141755.ph0506s72. [DOI] [PubMed] [Google Scholar]
- Stubelius A. Andersson A. Islander U. Carlsten H. Immunobiology. 2017;222:878–883. doi: 10.1016/j.imbio.2017.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.