Abstract
The mammalian cell nucleus contains different types of membrane-less nuclear bodies (NBs) consisting of proteins and RNAs. Microscopic imaging has been widely applied to study the organization and structure of NBs. However, current fixation methods are not optimized for such imaging: When a fixation method is chosen to maximize the quality of the RNA fluorescence in situ hybridization (FISH), it often limits the labeling efficiency of proteins or affects the ultrastructure of NBs. Here, we report that addition of glyoxal (GO) into the classical paraformaldehyde (PFA) fixation step not only improves FISH signals for RNAs in NBs via augmented permeability of the fixed nucleus and enhanced accessibility of probes, but also largely preserves protein fluorescent signals during fixation and immunostaining. We also show that GO/PFA fixation enables the covisualization of different types of nuclear bodies with minimal impact on their ultrastructures under super-resolution microscopy.
Keywords: glyoxal, paraformaldehyde, nuclear body, RNA fluorescence in situ hybridization, immunofluorescence
INTRODUCTION
The mammalian cell nucleus is highly organized into distinct membrane-less nuclear bodies (NBs) such as nucleoli, nuclear speckles, and paraspeckles (Stanek and Fox 2017). These microscopically visible compartments concentrate a variety of RNAs and proteins, providing a microenvironment for different biological processes including gene regulation, RNP biogenesis and stress responses (Sawyer et al. 2019). In the past decades, an increasing number of RNAs have been found to regulate the integrity and function of NBs (Stanek and Fox 2017). While some RNAs are defined as markers of NBs or required for their assembly, others accumulate in NBs to regulate their functions (Stanek and Fox 2017; Yao et al. 2019a). Deciphering the RNA composition and localization in NBs is key to understand regulatory roles of NBs.
Microscopy-related techniques including RNA fluorescence in situ hybridization (FISH) and protein immunofluorescence have been widely used to study the structure and function of NBs (West et al. 2016; Wu et al. 2016; Xing et al. 2017; Wang et al. 2018; Yao et al. 2019b). Conventional RNA FISH methods use nonionic detergents to improve the signal. Due to the compacted nature of NBs, sample preparations to detect RNA in NBs have been modified to enhance RNA FISH signals. These methods include digestion with proteinase K and treatment with acid or alcohol (Yin et al. 2012; Dunagin et al. 2015; Xing et al. 2017; Shah et al. 2018). Although these treatments could enhance RNA signals to some extent, such additional treatments also potentially disrupt ultrastructures of NBs, damage epitopes of proteins, and bleach endogenously knocked-in fluorescent proteins, making it difficult to combine multiple imaging modalities in the same sample. A method that can enhance RNA FISH signals without affecting signals from different means of protein staining or ultrastructures of NBs is needed.
Glyoxal (GO) is a low-toxicity, commercially available aldehyde that contains only two carbon atoms. The GO-mediated RNA denaturation is via introducing an additional ring onto guanosine residues, thus sterically hindering the formation of G–C base pairs (Nakaya et al. 1968; Broude and Budowsky 1971; McMaster and Carmichael 1977). It has been used in sample preparation for electron microscopy (Sabatini et al. 1963) and for tissue fixation during histological imaging (Umlas and Tulecke 2004; Paavilainen et al. 2010). A recent study showed that the combination of 3% GO and 20% ethanol serves as an alternative fixative to the most commonly used paraformaldehyde (PFA) in detecting both protein and RNA targets (Richter et al. 2018).
Here, we evaluated whether GO could benefit the imaging of NB structures and their RNA and protein components. We found that mixing 0.4% GO, 4% PFA and 0.1% methanol during fixation yielded much improved RNA FISH signals in NBs, possibly due to the increased permeability of the fixed nucleus and enhanced accessibility of probes to target RNAs, without affecting the signal acquisition of protein immunostainings. We also showed that GO could be combined with different staining methods to covisualize different types of NBs with minimal impact on their ultrastructures.
RESULTS
Addition of GO to PFA fixation provides a brighter smFISH signal than PFA alone without introducing autofluorescence
Addition of 3% GO to 20% ethanol could enhance immunostainings (Richter et al. 2018). Since PFA is the most widely used fixative, we asked whether addition of GO to the commonly used 4% PFA could increase RNA FISH signals. Given that the amount of RNAs in nuclear bodies is uncertain and cannot be accurately quantified, we chose NORAD, a lncRNA localized both in the cytoplasm and the nucleus, to optimize the GO concentration in RNA single-molecule FISH (RNA smFISH). We found that introducing low-concentration GO into 4% PFA enhanced RNA smFISH signals with 0.4% as the optimal concentration (Fig. 1A). Due to the ability of alcohol to act as an accelerator in GO-based fixation (Richter et al. 2018), we included 0.1% methanol into the 0.4% GO + 4% PFA formula and found it further improved NEAT1 signals, while addition of 0.1% methanol to 4% PFA alone had no detectable effect (Supplemental Fig. S1A), suggesting that methanol facilitates GO-based fixation rather than acting alone. Next, we evaluated the performance of the optimized GO/PFA formula (0.4% GO + 0.1% methanol + 4% PFA) in quantitative smFISH to measure different RNA species, including polyadenylated RNAs (localized in both nuclear speckles and the cytoplasm), MALAT1 (mainly localized in nuclear speckles), NEAT1 (localized in paraspeckles), and the pre-rRNA internal transcribed spacer 2, ITS2 (localized in nucleoli). Quantitative imaging showed that GO/PFA significantly raised the intensity of FISH signals of all the RNAs examined (Fig. 1B). For example, both cytoplasmic and nuclear polyadenylated RNAs could be readily detected by GO/PFA fixation, whereas cytoplasmic polyadenylated RNAs could be barely detected by PFA alone (Fig. 1B). Further comparison to other commonly used fixation methods, including 0.1% glutaraldehyde + 4% PFA (GA/PFA), 3% glyoxal + 20% ethanol (GO/EtOH), and 10% acetic acid + 3.6% PFA (AA/PFA), for the detection of PNCTR lncRNA localized in the perinucleolar compartment (PNC) showed that GO/PFA yielded signals as strong as AA/PFA, but outperformed GA/PFA and GO/EtOH (Supplemental Fig. S1B).
FIGURE 1.
Addition of glyoxal improves RNA FISH signals in nuclear bodies. (A) Introducing 0.4% glyoxal into 4% PFA improves the RNA FISH signals. Statistics were derived from over 4000 NORAD molecules counted across three independent experiments under each condition. Mean ± SEM and Mann–Whitney test are shown. Scale bar: 10 µm. (B) GO/PFA fixation dramatically enhances the FISH signals for different kinds of RNAs (cyan) in nuclei (magenta). Note that cytoplasmic polyadenylated RNAs could be readily detected by GO/PFA fixation; but these RNAs were barely detected by the PFA alone fixation (first panel, enlarged view). Statistics were counted from more than 100 cells under each condition. Mean ± SD and Mann–Whitney test are shown across three independent experiments for each RNA. Scale bar: 5 µm. (C) Glyoxal does not cause additional autofluorescence in all commonly used channels. The heat map was generated from 30 images for each channel under each fix condition. (D) Glyoxal provides a uniform background in all commonly used channels. The heat map was generated from 30 images for each channel in each fix condition. (E) GO/PFA fixation provides high-quality FISH images for different types of RNAs. Nuclei were labeled by DAPI and shown in magenta. Scale bar: 5 µm.
A successful RNA FISH requires not only strong signals but also low and uniform backgrounds. Since aldehydes often introduce autofluorescence, the addition of GO may also increase background fluorescence. To exclude this concern, we fixed HeLa cells with PFA or GO/PFA and then imaged these cells directly without staining. We found that no additional autofluorescence was introduced by GO in all commonly used channels and GO/PFA yielded even lower autofluorescence compared with PFA alone or other fixatives (Fig. 1C; Supplemental Fig. S1C,D). Meanwhile, GO/PFA fixation resulted in a more uniform background, shown by the lowest standard deviation (SD) values of the fluorescence intensity in all examined channels (Fig. 1D; Supplemental Fig. S1C,E). Furthermore, we used GO/PFA fixation to the smFISH detection of additional types of RNAs localized in different cellular compartments and obtained high-quality images in all cases (Fig. 1E).
GO improves cell permeability and probe accessibility for RNA FISH
A possible reason for the improved RNA imaging in the presence of GO for smFISH probes (Fig. 1; Supplemental Fig. S1) is that GO can increase cell permeability. To test this idea, we examined the penetration of DAPI with different fixation methods (Fig. 2A). Time-lapse imaging showed that GO/PFA fixation increased the penetration rate of DAPI as well as its intensity at the plateau stage (Fig. 2B,C).
FIGURE 2.
Glyoxal/PFA fixation improves RNA FISH efficiency by improving nuclear permeability and probe accessibility. (A) Schematic of the process to detect nuclear permeability. (B) GO/PFA fixation augments the nuclear permeability shown as a faster DAPI penetration under GO/PFA than PFA alone. Scale bar: 5 µm. (C) Statistics of B. Statistics were counted from more than 50 cells across three independent experiments under each condition. Mean ± SD and extra sum-of-squares F-test are shown. (D) An illustration of the effect of glyoxalation on RNA denaturation and the probe hybridization. Glyoxal reacts with nucleic acids and could introduce an additional ring onto guanosine residues to block the formation of G–C pairing (Nakaya et al. 1968; Broude and Budowsky 1971; McMaster and Carmichael 1977) while the exogenously introduced probes may still possibly bind to the glyoxalated-RNA. (E) Dot blots show that glyoxalation of RNA has minimal effect on probes hybridization. (F) Schematic of the process for detection of probe accessibility. (G) GO/PFA fixation augments the probe accessibility. Note that pRNA is a type of RNA polymerase I transcribed ncRNA that is tightly associated with rDNA promoter and related RBPs (Strohner et al. 2001; Zhou and Grummt 2005; Mayer et al. 2006). Statistics were quantified from more than 20 cells across three independent experiments under each condition. Mean ± SD and paired t-test are shown. Scale bar: 10 µm.
GO reacts with nucleic acids by introducing an additional ring onto guanosine residues, thus hindering the formation of G–C pairing and blocking the renaturation of native structure (Fig. 2D; Nakaya et al. 1968; Broude and Budowsky 1971; McMaster and Carmichael 1977). Next, we analyzed the effect of GO on the RNA-probe hybridization in vitro. We found that GO pretreatment of the target RNA did not alter the hybridization efficiency (Fig. 2E, lanes 1,2), while GO pretreatment of both the probe and the target RNA resulted in decreased association (Fig. 2E, lane 3). This observation prompted us to monitor the entire hybridization process during RNA smFISH for both highly abundant 18S rRNAs (Supplemental Fig. S2) and lowly abundant pRNAs (Fig. 2F). Of note, pRNAs are tightly associated with rDNA promoters and RNA binding proteins at individual FC/DFC regions within the dense nucleoli (Strohner et al. 2001; Zhou and Grummt 2005; Mayer et al. 2006). In both cases, a faster appearance of the RNA signal with stronger intensity was observed under the GO/PFA fixation compared to the PFA alone (Fig. 2G; Supplemental Fig. S2). Considering the ability of GO to enhance cell permeability (Fig. 2A–C), but not to promote the association of target RNA with the probe (Fig. 2E), we conclude that the improved RNA smFISH quality conferred by GO/PFA fixation is due to enhanced probe accessibility. It is worthwhile noting that GO/PFA fixation is unlikely to improve RNA FISH signals with long probes produced from nick translation (Supplemental Fig. S3). In this setting, two long probes, ∼3 kb for NEAT1 (Supplemental Fig. S3A; Wang et al. 2018) and ∼8 kb for SPA1 (Supplemental Fig. S3B; Wu et al. 2016) were examined and the length of these probes significantly exceeds that of smFISH probes (∼20–22 nt in length). We suspect that such long DNA probes are insensitive to cell permeability or probe accessibility.
GO/PFA fixation produces strong and accurate protein immunostaining signals
Having verified that GO is a promising additive to improve FISH of RNAs including NB-associated RNAs, we proceeded to investigate its efficiency in immunostaining with antibodies. We first tested SC35, the marker protein of nuclear speckles (Spector and Lamond 2011) and obtained the highest immunostaining intensity when using GO/PFA fixation among all examined methods (Fig. 3A). However, the brightest signal is not indicative of the highest accuracy of the immunofluorescence. To test whether GO/PFA yields accurate immunostaining signals, we stained the RPA194-EGFP knocked-in HeLa cell lines (Yao et al. 2019b), where the nucleolar FC regions were labeled by EGFP, with anti-RPA194 antibodies to visualize the colocalization of endogenous RPA194-EGFP and antibody-labeled RPA194. The intensity of anti-RPA194 signal was noticeably higher in cells with GO/PFA fixation than those with PFA alone (Fig. 3B). Importantly, the Pearson's colocalization value between the endogenous RPA194 and antibody-labeled RPA194 in the GO/PFA fixation group was much higher than that in the PFA fixation group (Fig. 3B), showing that GO/PFA fixation provides a higher accuracy in immunostainings.
FIGURE 3.
Introducing glyoxal largely preserves protein epitopes and fluorescent protein signals. (A) GO/PFA fixation preserves the protein epitopes during immunostaining. Statistics were counted from more than 30 cells across three independent experiments under each condition. Mean ± SD and paired t-test are shown. Scale bar: 5 µm. (B) GO/PFA fixation provides a better colocalization accuracy in immunostaining shown by higher P's colocalization value between the CRISPR/Cas9-mediated EGFP KI at the endogenous RPA194 locus and the antibody labeled RPA194 proteins. Statistics were counted from more than 1500 RPA194 condensates (the FC regions in nucleoli) across three independent experiments under each condition. Mann–Whitney test is shown. Scale bar: 1 µm and 400 nm for zoomed regions. (C) GO/PFA fixation largely preserves the fluorescent protein signals during RNA FISH, represented by the slower decreasing rate of fluorescent protein signals during FISH process under GO/PFA fixation than PFA alone. Statistics were counted from more than 20 cells across three independent experiments under each condition. Mean ± SD and paired t-test are shown. Scale bar: 10 µm.
GO preserves fluorescent protein signals during RNA FISH and immunostaining
Harsh RNA FISH conditions often reduce signals of fluorescent proteins. We found that GO/PFA better preserved the fluorescent protein signals than PFA alone, shown by visualizing the change of EGFP in RPA194-EGFP knocked-in HeLa cell lines after RNA FISH procedures carried out with different hybridization times (Fig. 3C). Although the 6-h hybridization drastically reduced EGFP signals in both GO/PFA and PFA groups, the EGFP signal in the GO/PFA group after 16-h hybridization were twice as bright as that in the PFA group (Fig. 3C). Meanwhile, we measured the preservation of fluorescent protein (FP) signals after immunostaining under different fixation methods (Supplemental Fig. S4A). The FP intensity after GO/PFA fixation indeed was better preserved compared with PFA, AA/PFA or GO/EtOH fixation (Supplemental Fig. S4A). It should be also noted, although GA/PFA fixation also preserves the FP fluorescence signal well, this formula introduced strong autofluorescence (Supplemental Fig. S1C,D), which is not suitable for RNA FISH.
Further comparing the effects of pH conditions (Supplemental Fig. S5A) and fixation durations (Supplemental Fig. S5B) on RNA and protein in NBs showed that an acidic or a neutral pH condition displayed the similar level of NEAT1 signals for FISH; however, an acidic fixation led to reduced signals of the examined NB marker protein, SC35 (Supplemental Fig. S5A). Prolonged GO/PFA fixation duration from 10 to 60 min did not further improve the signals of examined IF or smFISH signals (Supplemental Fig. S5B).
GO improves multiple staining methods with little effect on NB ultrastructure
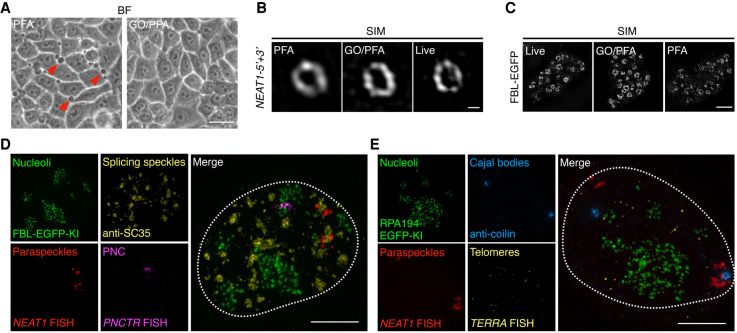
Remarkably, GO/PFA fixation led to fewer membrane blebbing areas than PFA alone, suggesting its better performance in preserving cell morphology (Fig. 4A). It should be noted that membrane blebbing is a common phenomenon when cells are insufficiently fixed (Huebinger et al. 2018). The observation of fewer membrane blebbing (Fig. 4A) indicated that GO/PFA had a stronger fixation ability than PFA alone. Consistently, structured illumination microscopy (SIM) showed that GO/PFA well-preserved the structures of chromatin domains and interchromatin compartments (Supplemental Fig. S6A; Miron et al. 2020). Furthermore, using the live-cell images as the positive control, we found that GO/PFA fixation led to a more intact and detailed morphology of the shell structure in paraspeckles labeled by the 5′ and 3′ ends of NEAT1 (Fig. 4B) and the DFC regions in nucleoli labeled by FBL (Fig. 4C) than PFA fixation alone. In addition, consistent with our previous finding (Yao et al. 2019b), RPA194 and FBL present a core-shell-like structure with 5′ ETS-1 in DFC regions under both GO/PFA and PFA fixations. Quantification of these images further showed that GO/PFA fixation resulted in brighter signals for both RPA194 IF and 5′ ETS-1 FISH than those by PFA fixation (Supplemental Fig. S6D). Together, these observations suggest that the denaturation ability of GO has little effect on the ultrastructure of RNA-based NBs.
FIGURE 4.
Glyoxal/PFA fixation enables the covisualization of different types of nuclear bodies with minimal impact on their ultrastructures. (A) GO/PFA fixation shows a better preservation of the cell morphology than PFA alone, shown by fewer membrane blebbing (red arrows, left). BF, bright field. Scale bar: 15 µm. (B) Structured illumination microscopy (SIM) reveals that GO/PFA fixation enables a better preservation of the shell of paraspeckles labeled by NEAT1-5′ + 3′ RNA FISH than PFA alone. Scale bar: 200 nm. (C) SIM reveals that GO/PFA fixation enables a better preservation of the ultrastructure (the DFC regions) of nucleoli labeled by the CRISPR/Cas9-mediated EGFP knock-in at the endogenous FBL locus. Scale bar: 2 µm. (D,E) GO/PFA fixation enables the covisualization of different types of nuclear bodies. Nucleoli were labeled by the CRISPR/Cas9-mediated EGFP knock-in at the endogenous FBL locus (D) or CRISPR/Cas9-mediated EGFP knock-in at the endogenous RPA194 locus (E); paraspeckles were labeled by lncRNA NEAT1 FISH; perinucleolar compartments (PNC) were labeled by the lncRNA PNCTR FISH; telomeres were labeled by lncRNA TERRA FISH; splicing speckles were labeled by anti-SC35 antibodies; Cajal bodies were labeled by anticoilin antibodies. Nuclei were marked by dotted line. Scale bar: 5 µm.
Next, we fixed the cells carrying EGFP KI at the endogenous FBL or RPA194 locus with the GO/PFA method and combined RNA smFISH and immunostaining to visualize different NBs simultaneously. We covisualized PNCs, paraspeckles, nuclear speckles, and nucleoli by labeling the lncRNA PNCTR, the lncRNA NEAT1, and the protein SC35 in FBL-EGFP KI HeLa cell lines (Fig. 4D), or covisualized telomeres, paraspeckles, Cajal bodies, and nucleoli by labeling the lncRNA TERRA, the lncRNA NEAT1, and the protein coilin in RPA194-EGFP KI HeLa cell lines (Fig. 4E). These SIM results showed that all examined NBs could be readily imaged by the combination of multiple staining methods upon GO/PFA fixation (Fig. 4D,E).
DISCUSSION
NBs are involved in important regulatory processes in cells (Dundr 2012; Stanek and Fox 2017). Imaging is one of the most powerful tools to study the organization, structure and assembly of NBs. However, due to their unique dense nature and composition of a variety of RNAs and proteins, simultaneous imaging of multiple contents of NBs has remained technically challenging. Inspired by the previous report demonstrating GO in combination with ethanol as an alternative to PFA in immunostainings (Richter et al. 2018), we evaluated whether GO could facilitate imaging of covisualization of RNAs and proteins in NBs. By introducing low-concentration GO into PFA, we identified a simple fixation method that improved RNA imaging (Figs. 1, 2), preserved protein fluorescence and immunostainings (Fig. 3) as well as enabled covisualization of RNAs and proteins in different types of NBs (Fig. 4), indicating that the fixation method theoretically would allow for staining of both RNA and protein targets in a single NB, which warrants further investigations. Nonetheless, addition of GO to PFA increased the cell permeability, enhanced the probe accessibility (Fig. 2) and promoted the visibility of details of NB structures (Fig. 3). Importantly, the addition of GO did not cause strong autofluorescence (Fig. 1C,D), thus achieving a high signal-to-background ratio.
GO-mediated fixation has been used in immunostainings and RNA labeling (Richter et al. 2018). However, the combination of highly concentrated ethanol (∼20%) in this reported method (Richter et al. 2018) would potentially lead to RNA sedimentation, thus affecting the ultrastructure of RNA-enriched NBs. Indeed, we found the GO/EtOH (3% GO + 20% ethanol) fixation was not suitable for NB staining (Fig. 3A; Supplemental Figs. S1B, S4A) and showed weak capability in ultrastructure preservation (Supplemental Fig. S6B,C), which limits its application in fixation of NBs. Since the PFA has been commonly used in imaging NBs (West et al. 2016; Fei et al. 2017; Xing et al. 2017; Wang et al. 2018; Yao et al. 2019b), here, we examined PFA rather than EtOH as the main fixative and introduced GO to enhance RNA FISH signals without losing protein immunostaining signals. We also included methanol in the GO/PFA formula given the function of alcohol as an accelerator in the GO fixation (Richter et al. 2018), but methanol was supplied at a minimal concentration of 0.1% (Supplemental Fig. S1A) to prevent the RNA sedimentation, thus minimizing the effect on NB morphology. Indeed, such fixation preserves the ultrastructure of NBs to the maximum extent in examined conditions (Figs. 3, 4; Supplemental Fig. S6). In addition, our method does not have a complicated formulation, nor does it require further pH adjustment, which facilitates its application in the daily experimental procedures.
We mainly focused on the fixation and staining for the imaging of condensates in the nucleus in this study, although our preliminary data showed that GO addition also enhanced signals for several examined cytoplasmic RNAs (Fig. 1A,E; Supplemental Fig. S2). These results suggest that GO fixation could also be used for imaging of cytoplasmic RNPs and structures. Meanwhile, our GO/PFA method appears to be a strong fixation procedure, which would theoretically minimize loss of cytoplasmic materials and thus provides a possibility for a more precise quantitative imaging. Further, we observed that GO/PFA fixation resulted in a lower noise and a more uniform background in the most commonly used channels (Fig. 1C,D; Supplemental Fig. S1C,E). In principle, a lower noise and a more uniform background would be beneficial for nanoscopy. It will be of great interest to investigate whether the GO/PFA fixation would improve nanoscopic imaging such as STORM and STED.
In conclusion, we provide a simple and effective fixation method for RNA and protein imaging, particularly in NBs. GO/PFA fixation achieves a balanced performance in different staining methods including RNA FISH, immunostaining, and FP fixation to visualize multiple components in NBs simultaneously.
MATERIALS AND METHODS
Cell culture and cell transfection
Human HeLa and HEK293 cell lines were cultured using standard protocols from the American Type Culture Collection (ATCC; http://www.atcc.org). Transfection of plasmid was carried out with Lipofectamine 3000 Transfection Reagent (Invitrogen) according to the manufacturer's protocol.
Knock-in fluorescent protein by CRISPR/Cas9
The generation of mEGFP-KI HeLa cell lines were performed as described (Yao et al. 2019b). Briefly, 1 × 106 cells per well were seeded in a six-well plate with supplemented DMEM + 10% FBS at 37°C, 5% CO2. The following day, transfection was carried out using the bicistronic nuclease plasmid with the corresponding donor plasmid at the ratio of 2 to 1 and a total 2.5 µg plasmid were transfected as described above. One day later, puromycin (1 µg/mL) was added to the cells to increase the KI efficiency. Three days later, the cells were inspected by fluorescence microscopy and positive cells were sorted into a new 10 cm dish using FACSAria (BD Biosciences). Within ∼2 to 3 wk after single-cell sorting, positive single colonies were picked up and transferred into 24-well plates.
Sample fixation
The different sample fixation solutions were prepared according to the following protocol:
PFA fixation: 4% w/v paraformaldehyde (PFA) in DPBS;
GO/PFA fixation: 4% w/v PFA + 0.4% v/v glyoxal + 0.1% v/v methanol in DPBS (GO was freshly added into 4% PFA for an immediate use because it is known to readily oxidize to glyoxylic acid);
GA/PFA fixation: 4% w/v PFA + 0.1% v/v glutaraldehyde in DPBS;
AA/PFA fixation: 3.6 w/v % PFA + 10% v/v acetic acid in DPBS;
GO/EtOH fixation: 3% v/v glyoxal + 20% v/v EtOH (GO/EtOH) + 0.75% v/v acetic acid in ddH2O, pH = 4, from a recent study (Richter et al. 2018).
All cells were seeded on High Performance no.1.5 18 × 18 mm glass coverslips and were fixed with different solutions for 10 min at room temperature.
Single molecule RNA fluorescent in situ hybridization (smFISH)
All single molecule RNA FISH probes were designed via Stellaris Probe Designer and labeled with cy3 or cy5 on the 3′ ends. RNA FISH was carried out as described before (Raj and Tyagi 2010). Briefly, cells were fixed for 10 min, followed by permeabilization with 0.5% Triton X-100 for 5 min. Cells were incubated in 10% formamide/2× SSC for 10 min at room temperature follow by hybridization at 37°C for 16 h. After hybridization, the cells were blocked and incubated with antibodies as described above to visualize proteins. Samples were mounted in VECTASHIELD antifade mounting medium (Vector Lab). For samples labeled with Cy5, ProLong Diamond antifade reagent (Thermo Fisher) was used.
RNA FISH with nick-translation probes
To detect NEAT1 and SPA1 RNA, cells were rinsed briefly in PBS and then fixed in PFA or GO/PFA for 10 min at room temperature. Cells were permeabilized in PBS containing 0.5% Triton X-100 and 5 mM vanadyl ribonucleoside complex (Invitrogen) for 5 min. Cells were washed in PBS 3 × 10 min and rinsed once in 2× SSC prior to hybridization. Hybridization was carried out using nick-translated cDNA probes (nick-translation kit; Abbott) in a moist chamber at 37°C for 12–16 h. After hybridization, samples were mounted in VECTASHIELD antifade mounting medium (Vector Lab).
Protein visualization
To detect protein localization by immunofluorescence in fixed cells, cells were seeded on High Performance no.1.5 18 × 18 mm glass coverslips and were fixed for 10 min, followed by permeabilization with 0.5% Triton X-100 for 5 min. Then cells were blocked with 1% BSA for 1 h at room temperature. Primary antibodies were diluted with 1% BSA (SC35 sigma S4045 1:400, RPA194 Santa Cruz sc-48385 1:200, coilin sigma C1862 1:400) and incubated for 1 h at room temperature. After washing with 1× DPBS three times, fluorescent secondary antibodies (Invitrogen A-21424 or A-21236) were 1:1000 diluted in 1% BSA and incubated for 1 h at room temperature. Samples were mounted in VECTASHIELD antifade mounting medium (Vector Lab).
To detect EGFP -tagged proteins in fixed cells, cells were seeded on High Performance no.1.5 18 × 18 mm glass coverslips and were fixed for 10 min at room temperature. Samples were mounted with VECTASHIELD antifade mounting medium (Vector Lab).
For live-cell imaging, cells were seeded on 35 mm no.1.5 glass bottom dishes (Cellvis) 1 d prior to imaging. Cells were washed once with PBS and the medium was replaced by FluoroBrite DMEM (Gibco) supplemented with 10% FBS and placed back in the incubator for 1 h. All images were obtained at 37°C with 5% CO2 condition.
Widefield microscopy procedure
All widefield microscopy images were performed on a DeltaVision Elite imaging system equipped with a 60×/1.42 NA Plan Apo oil-immersion objective, or a 100×/1.40 NA Plan Apo oil-immersion objective (Olympus), as well as the CoolSnap HQ2 camera (Photometrics) equipped with the live-cell imaging environment control system (Live Cell Instrument). Raw data of all presented figures were deconvoluted by softWoRx 6.5 using the enhanced ratio method.
Structured illumination microscopy (SIM) procedure
All SIM experiments were performed on a DeltaVision OMX V4 system (GE Healthcare) equipped with a 60×/1.42 NA Plan Apo oil-immersion objective (Olympus) and six laser beams (405, 445, 488, 514, 568, and 642 nm; 100 mW) or a DeltaVision OMX SR system (GE Healthcare) equipped with a 60×/1.42 NA Plan Apo oil-immersion objective (Olympus) and four laser beams (405, 488, 568, and 642 nm; 100 mW). The microscope was routinely calibrated with a special image registration slide and algorithm provided by GE healthcare. To obtain optimal images, immersion oil with refractive indices of 1.516 was used at 25°C room temperature and 1.520 for 37°C. SIM image stacks were captured with a z-distance of 0.125 µm and with five phases, three angles, 15 raw images per plane. The raw data were reconstructed with channel specific OTFs and a Wiener filter was set to optimum value by using softWoRx 6.5 package (GE Healthcare). Images were registered with alignment parameters obtained from calibration measurements with 100 nm diameter TetraSpeck Microspheres with four colors (Molecular Probes).
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
Supplementary Material
ACKNOWLEDGMENTS
We thank Guang Xu for reading the manuscript and discussion. This work was supported by the Shanghai Municipal Commission for Science and Technology (20JC1410300), the National Natural Science Foundation of China (NSFC) (31830108, 31821004, 31725009), the Chinese Academy of Sciences (CAS) (XDB19020104), and the HHMI International Program (55008728) to L.-L.C. L.-L.C. acknowledges support from the Xplorer Prize.
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.078671.120.
REFERENCES
- Broude NE, Budowsky EI. 1971. The reaction of glyoxal with nucleic acid components. 3. Kinetics of the reaction with monomers. Biochim Biophys Acta 254: 380–388. 10.1016/0005-2787(71)90868-9 [DOI] [PubMed] [Google Scholar]
- Dunagin M, Cabili MN, Rinn J, Raj A. 2015. Visualization of lncRNA by single-molecule fluorescence in situ hybridization. Methods Mol Biol 1262: 3–19. 10.1007/978-1-4939-2253-6_1 [DOI] [PubMed] [Google Scholar]
- Dundr M. 2012. Nuclear bodies: multifunctional companions of the genome. Curr Opin Cell Biol 24: 415–422. 10.1016/j.ceb.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei J, Jadaliha M, Harmon TS, Li ITS, Hua B, Hao Q, Holehouse AS, Reyer M, Sun Q, Freier SM, et al. 2017. Quantitative analysis of multilayer organization of proteins and RNA in nuclear speckles at super resolution. J Cell Sci 130: 4180–4192. 10.1242/jcs.206854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebinger J, Spindler J, Holl KJ, Koos B. 2018. Quantification of protein mobility and associated reshuffling of cytoplasm during chemical fixation. Sci Rep 8: 17756. 10.1038/s41598-018-36112-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Schmitz KM, Li J, Grummt I, Santoro R. 2006. Intergenic transcripts regulate the epigenetic state of rRNA genes. Mol Cell 22: 351–361. 10.1016/j.molcel.2006.03.028 [DOI] [PubMed] [Google Scholar]
- McMaster GK, Carmichael GG. 1977. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci 74: 4835–4838. 10.1073/pnas.74.11.4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron E, Oldenkamp R, Brown JM, Pinto DMS, Xu CS, Faria AR, Shaban HA, Rhodes JDP, Innocent C, de Ornellas S, et al. 2020. Chromatin arranges in chains of mesoscale domains with nanoscale functional topography independent of cohesin. Sci Adv 6: eaba8811. 10.1126/sciadv.aba8811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya K, Takenaka O, Horinishi H, Shibata K. 1968. Reactions of glyoxal with nucleic acids. Nucleotides and their component bases. Biochim Biophys Acta 161: 23–31. 10.1016/0005-2787(68)90290-6 [DOI] [PubMed] [Google Scholar]
- Paavilainen L, Edvinsson A, Asplund A, Hober S, Kampf C, Ponten F, Wester K. 2010. The impact of tissue fixatives on morphology and antibody-based protein profiling in tissues and cells. J Histochem Cytochem 58: 237–246. 10.1369/jhc.2009.954321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, Tyagi S. 2010. Detection of individual endogenous RNA transcripts in situ using multiple singly labeled probes. Method Enzymol 472: 365–386. 10.1016/S0076-6879(10)72004-8 [DOI] [PubMed] [Google Scholar]
- Richter KN, Revelo NH, Seitz KJ, Helm MS, Sarkar D, Saleeb RS, D'Este E, Eberle J, Wagner E, Vogl C, et al. 2018. Glyoxal as an alternative fixative to formaldehyde in immunostaining and super-resolution microscopy. EMBO J 37: 139–159. 10.15252/embj.201695709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DD, Bensch K, Barrnett RJ. 1963. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol 17: 19–58. 10.1083/jcb.17.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer IA, Sturgill D, Dundr M. 2019. Membraneless nuclear organelles and the search for phases within phases. Wiley Interdiscip Rev RNA 10: e1514. 10.1002/wrna.1514 [DOI] [PubMed] [Google Scholar]
- Shah S, Takei Y, Zhou W, Lubeck E, Yun J, Eng CL, Koulena N, Cronin C, Karp C, Liaw EJ, et al. 2018. Dynamics and spatial genomics of the nascent transcriptome by intron seqFISH. Cell 174: 363–376.e316. 10.1016/j.cell.2018.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector DL, Lamond AI. 2011. Nuclear speckles. Cold Spring Harb Perspect Biol 3: a000646. 10.1101/cshperspect.a000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek D, Fox AH. 2017. Nuclear bodies: news insights into structure and function. Curr Opin Cell Biol 46: 94–101. 10.1016/j.ceb.2017.05.001 [DOI] [PubMed] [Google Scholar]
- Strohner R, Nemeth A, Jansa P, Hofmann-Rohrer U, Santoro R, Langst G, Grummt I. 2001. NoRC–a novel member of mammalian ISWI-containing chromatin remodeling machines. EMBO J 20: 4892–4900. 10.1093/emboj/20.17.4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umlas J, Tulecke M. 2004. The effects of glyoxal fixation on the histological evaluation of breast specimens. Hum Pathol 35: 1058–1062. 10.1016/j.humpath.2004.04.013 [DOI] [PubMed] [Google Scholar]
- Wang Y, Hu SB, Wang MR, Yao RW, Wu D, Yang L, Chen LL. 2018. Genome-wide screening of NEAT1 regulators reveals cross-regulation between paraspeckles and mitochondria. Nat Cell Biol 20: 1145–1158. 10.1038/s41556-018-0204-2 [DOI] [PubMed] [Google Scholar]
- West JA, Mito M, Kurosaka S, Takumi T, Tanegashima C, Chujo T, Yanaka K, Kingston RE, Hirose T, Bond C, et al. 2016. Structural, super-resolution microscopy analysis of paraspeckle nuclear body organization. J Cell Biol 214: 817–830. 10.1083/jcb.201601071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Yin QF, Luo Z, Yao RW, Zheng CC, Zhang J, Xiang JF, Yang L, Chen LL. 2016. Unusual processing generates SPA lncRNAs that sequester multiple RNA binding proteins. Mol Cell 64: 534. 10.1016/j.molcel.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Xing YH, Yao RW, Zhang Y, Guo CJ, Jiang S, Xu G, Dong R, Yang L, Chen LL. 2017. SLERT regulates DDX21 rings associated with Pol I transcription. Cell 169: 664–678.e616. 10.1016/j.cell.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Yao RW, Wang Y, Chen LL. 2019a. Cellular functions of long noncoding RNAs. Nat Cell Biol 21: 542–551. 10.1038/s41556-019-0311-8 [DOI] [PubMed] [Google Scholar]
- Yao RW, Xu G, Wang Y, Shan L, Luan PF, Wang Y, Wu M, Yang LZ, Xing YH, Yang L, et al. 2019b. Nascent pre-rRNA sorting via phase separation drives the assembly of dense fibrillar components in the human nucleolus. Mol Cell 76: 767–783.e711. 10.1016/j.molcel.2019.08.014 [DOI] [PubMed] [Google Scholar]
- Yin QF, Yang L, Zhang Y, Xiang JF, Wu YW, Carmichael GG, Chen LL. 2012. Long noncoding RNAs with snoRNA ends. Mol Cell 48: 219–230. 10.1016/j.molcel.2012.07.033 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Grummt I. 2005. The PHD finger/bromodomain of NoRC interacts with acetylated histone H4K16 and is sufficient for rDNA silencing. Curr Biol 15: 1434–1438. 10.1016/j.cub.2005.06.057 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.