Abstract
Background
Published data suggest worse outcomes in acute coronary syndrome (ACS) patients and concurrent coronavirus disease 2019 (COVID-19) infection. Mechanisms remain unclear.
Objectives
The purpose of this study was to report the demographics, angiographic findings, and in-hospital outcomes of COVID-19 ACS patients and compare these with pre–COVID-19 cohorts.
Methods
From March 1, 2020 to July 31, 2020, data from 55 international centers were entered into a prospective, COVID-ACS Registry. Patients were COVID-19 positive (or had a high index of clinical suspicion) and underwent invasive coronary angiography for suspected ACS. Outcomes were in-hospital major cardiovascular events (all-cause mortality, re–myocardial infarction, heart failure, stroke, unplanned revascularization, or stent thrombosis). Results were compared with national pre–COVID-19 databases (MINAP [Myocardial Ischaemia National Audit Project] 2019 and BCIS [British Cardiovascular Intervention Society] 2018 to 2019).
Results
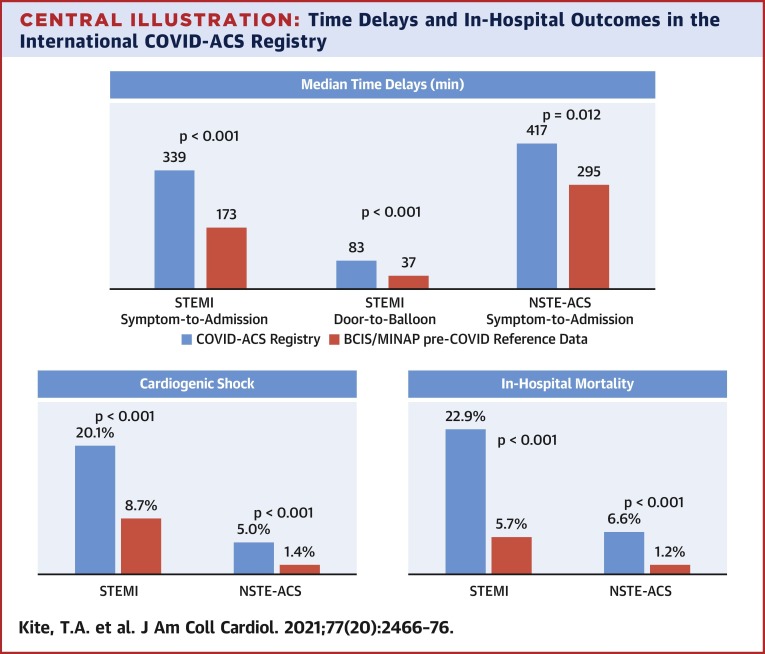
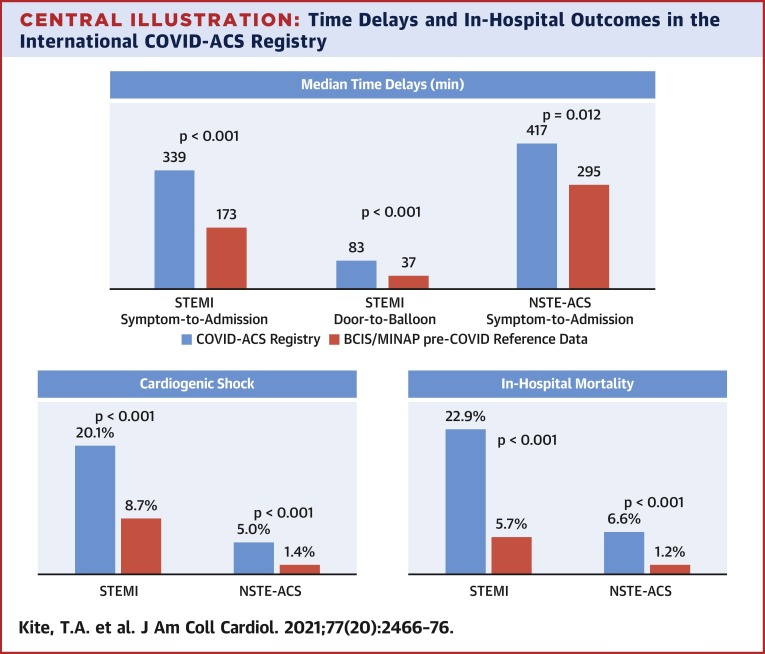
In 144 ST-segment elevation myocardial infarction (STEMI) and 121 non–ST-segment elevation acute coronary syndrome (NSTE-ACS) patients, symptom-to-admission times were significantly prolonged (COVID-STEMI vs. BCIS: median 339.0 min vs. 173.0 min; p < 0.001; COVID NSTE-ACS vs. MINAP: 417.0 min vs. 295.0 min; p = 0.012). Mortality in COVID-ACS patients was significantly higher than BCIS/MINAP control subjects in both subgroups (COVID-STEMI: 22.9% vs. 5.7%; p < 0.001; COVID NSTE-ACS: 6.6% vs. 1.2%; p < 0.001), which remained following multivariate propensity analysis adjusting for comorbidities (STEMI subgroup odds ratio: 3.33 [95% confidence interval: 2.04 to 5.42]). Cardiogenic shock occurred in 20.1% of COVID-STEMI patients versus 8.7% of BCIS patients (p < 0.001).
Conclusions
In this multicenter international registry, COVID-19–positive ACS patients presented later and had increased in-hospital mortality compared with a pre–COVID-19 ACS population. Excessive rates of and mortality from cardiogenic shock were major contributors to the worse outcomes in COVID-19 positive STEMI patients.
Key Words: acute coronary syndrome, cardiogenic shock, COVID-19, non–ST-segment elevation myocardial infarction, ST-segment elevation myocardial infarction
Abbreviations and Acronyms: ACS, acute coronary syndrome; COVID-19, coronavirus disease 2019; MI, myocardial infarction; NSTE-ACS, non–ST-segment elevation acute coronary syndrome; N-STEMI, non–ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction
Central Illustration
Since its outbreak in Hubei Province, China in December 2019, the novel severe acute respiratory syndrome coronavirus 2 has spread rapidly, resulting in a worldwide pandemic from this multisystem disease (1). The effect on ACS is 2-fold.
First, viral infections such as influenza have been reported to exacerbate ACS (2). Multiple hypotheses for the higher incidence and greater adverse outcomes in ACS have been proposed, including arterial (macrovascular and microvascular) and venous thrombosis mediated by an endothelial inflammatory response, microvascular dysfunction, sepsis hypoxia, sympathetic nervous system overactivity, and cytokine and possible bradykinin release (3). Indeed, early reports suggest spontaneous thrombus development in the pulmonary and peripheral vasculature (4) and excess coronary thrombus formation may be causes for high mortality rates (5). However, nonobstructed epicardial coronary arteries with microthrombi or cellular inflammatory processes have also been observed (6), as have cases of myocarditis masquerading as ACS (7).
Second, early reports also demonstrated a marked decline in ACS admissions during the coronavirus disease 2019 (COVID-19) pandemic, together with a definite increase in mortality compared with non-COVID ACS patients (8, 9, 10). Although the proinflammatory nature of COVID-19 and its subsequent complex interaction with the cardiovascular system make this an essential area of investigation, many of the clinical findings could be explained by patients’ perception of potential harm in attending the hospital (COVID-19 fear). We proposed that the poorer outcomes in COVID-19–positive ACS patients were in part due to the logistical consequences of such understandable concerns.
We therefore established the International COVID-ACS Registry to document the demographic, procedural, and angiographic characteristics and in-hospital clinical outcomes of COVID-19–positive (or high index suspicion) patients admitted with ACS, paying particular attention to delays in standard management. We posited whether there was a link between previously published rates of increased mortality and factors, such as delayed presentation, that could affect adverse outcomes.
Methods
Study design
The University Hospitals of Leicester (UHL) NHS Trust, in collaboration with the University of Glasgow Clinical Trials Unit, developed an online, web-hosted remote data entry system, allowing colleagues from international centers to prospectively enter anonymized data on patients who met the registry inclusion criteria. After seeking regulatory advice from the UHL Clinical Audit Department, the study was registered as a health survey audit. No formal ethical approval was required. Each center entered its own data according to a site-specific user account with no patient identifiable data collected. Data transfer agreements were established between UHL, University of Glasgow, and sites as required. The inclusion criteria for the study were: 1) COVID-19 positive or a high index of clinical suspicion; and 2) invasive coronary angiography undertaken for suspected ACS. High-index clinical suspicion was defined as clinical status plus chest x-ray (CXR) or computed tomography (CT) findings suggestive of COVID-19 infection (11). The study comprised 55 centers located across 5 continents, with data collected from March 1, 2020, to July 31, 2020.
Data collection
Patient demographics, including age, sex, and body mass index (BMI), were documented. Users recorded co-morbidities based on the International Classification of Diseases-10th Revision codes, including cardiovascular disease (hypertension, hyperlipidemia, diabetes mellitus, previous myocardial infarction [MI], previous percutaneous coronary intervention [PCI], and congestive cardiac failure), smoking status, and history of lung disease. Procedural and angiographic characteristics were noted, along with requirements for intensive care admission, inotropic/vasopressor support, invasive ventilation, and mechanical support. Timing data including symptom-to-admission, door-to-balloon, and door-to-angiography were also recorded. Symptom onset time was defined as the start of patient-reported cardiovascular symptoms (i.e., chest pain or dyspnea, but not cough or fever). Thrombotic occlusion at the time of angiography was graded using the TIMI (Thrombolysis In Myocardial Infarction) Thrombus Grade Score.
Outcomes
The primary endpoint was in-hospital all-cause mortality. Secondary endpoints included in-hospital repeat MI (Fourth Universal Definition of Myocardial Infarction) (12); heart failure, unplanned revascularization, and stroke (2017 Cardiovascular Endpoint Definitions for Clinical Trials Consensus Report) (13); cardiogenic shock (CGS) (systolic blood pressure <90 mm Hg for >30 min with signs of hypoperfusion, or need for inotropes); bleeding (Bleeding Academic Research Consortium criteria) (14); and stent thrombosis (Academic Research Consortium-2 Consensus Document) (15). We also reported total length of hospital stay.
Comparative groups
COVID-ACS registry patients were subdivided into: 1) ST-segment elevation myocardial infarction (STEMI); and 2) non–ST-segment elevation acute coronary syndrome (NSTE-ACS) (including non–ST-segment elevation myocardial infarction [NSTEMI] and unstable angina). Because a key aim was to investigate possible delays in presentation to hospital and reperfusion therapy, we excluded type 2 MI COVID-ACS registry patients, as clinical outcomes in this group are not influenced by invasive coronary angiography and expeditious revascularization. Comparisons were thus performed between type 1 MI patients from our registry and pre-COVID STEMI and NSTE-ACS data from the U.K.-based British Cardiovascular Intervention Society (BCIS) National PCI Audit (April 1, 2018, to March 31, 2019), and English data from the Myocardial Ischaemia National Audit Project (MINAP) (2019) databases. All patients undergoing an invasive strategy for ACS in the United Kingdom are submitted to these robust and internationally acknowledged databases. The optimal comparative databases were BCIS for the STEMI population and MINAP for the NSTE-ACS population. We chose not to use concurrent COVID-19–negative ACS patients as control subjects, because we recognized that systems of care were severely disrupted at this time and would not represent the pre-COVID standard. Furthermore, in-hospital events in BCIS and MINAP are similar to other internationally recognized national databases (16,17) and offer a reliable benchmark with which to compare outcomes in the COVID-ACS registry.
Statistical analyses
Descriptive statistics were presented for baseline demographics and characteristics. Frequency and percentage were reported for categorical variables, and mean ± SD or median (interquartile range) were reported for continuous variables depending on their distributions. To compare the characteristics between the COVID-ACS and MINAP/BCIS datasets, Fisher exact test or chi-square tests were performed for categorical variables, and Student’s t-test or Mann-Whitney U tests were used for continuous variables according to their distributions. To account for confounding factors and balance any differences in patient characteristics between the COVID-STEMI cohort and the BCIS STEMI database, a propensity score was derived using logistic regression to predict whether patients were from COVID-ACS or BCIS, including age, sex, hypertension, hyperlipidemia, and diabetes. A propensity score–based inverse probability treatment weights method was then used to calculate the difference in mortality between patients recorded in the COVID-STEMI subgroup and BCIS STEMI databases, further adjusted for CGS status and ischemia time. A propensity score was not derived to compare NSTE-ACS cohorts due to the low number of clinical events observed in the registry subgroup.
Results
In total, 316 hospitalized patients from 55 international centers across 5 continents were included: 238 (75.3%) from Europe, 35 (11.1%) from South America, 21 (6.6%) from Asia, 15 (4.7%) from Africa, and 7 (2.2%) from North America (Supplemental Table 1). Demographic variables and comorbidities for the combined STEMI/NSTE-ACS cohort are shown in Table 1 .
Table 1.
Baseline Characteristics of Combined STEMI/NSTE-ACS COVID-ACS Registry Cohort (N = 265)∗
| Mean age, yrs | 64.9 ± 12.9 |
| Male | 75.5 (200/265) |
| Hypertension | 66.2 (174/263) |
| Hyperlipidemia | 54.1 (131/242) |
| BMI, kg/m2 | 27.5 ± 4.7 |
| Diabetes | 36.2 (92/265) |
| Smoking status | |
| Current smoker | 27.1 (62/229) |
| Ex-smoker | 27.1 (62/229) |
| Nonsmoker | 45.8 (105/229) |
| Heart failure | 19.3 (49/254) |
| Previous MI | 20.2 (57/258) |
| Previous PCI | 17.5 (46/263) |
| Chronic kidney disease (stages 3–5) | 14.6 (38/260) |
| Lung disease | 16.5 (42/254) |
| Previous stroke | 7.2 (19/265) |
| COVID-19 positive | 74.3 (197/265) |
| COVID-19 high index suspicion | 25.7 (68/265) |
| Killip class III/IV on admission | 17.4 (46/265) |
| Out-of-hospital cardiac arrest | 5.3 (14/265) |
| Admission lactate, mmol/l | 4.1 ± 7.3 |
| Admission lactate >2.0 mmol/l | 61.7 (58/94) |
| Presentation symptoms typical of ACS | 81.4 (214/263) |
| Full PPE worn during procedure | 90.9 (209/230) |
Values are mean ± SD or % (n/N). Denominators not equal to n = 265 are due to incomplete data.
ACS = acute coronary syndrome; BMI = body mass index; COVID-19 = coronavirus disease 2019; MI = myocardial infarction; NSTE-ACS = non–ST-segment elevation acute coronary syndrome; PCI = percutaneous coronary intervention; PPE = personal protective equipment; STEMI = ST-segment elevation myocardial infarction.
Excludes patients with type 2 myocardial infarction (see Figure 1).
Baseline characteristics
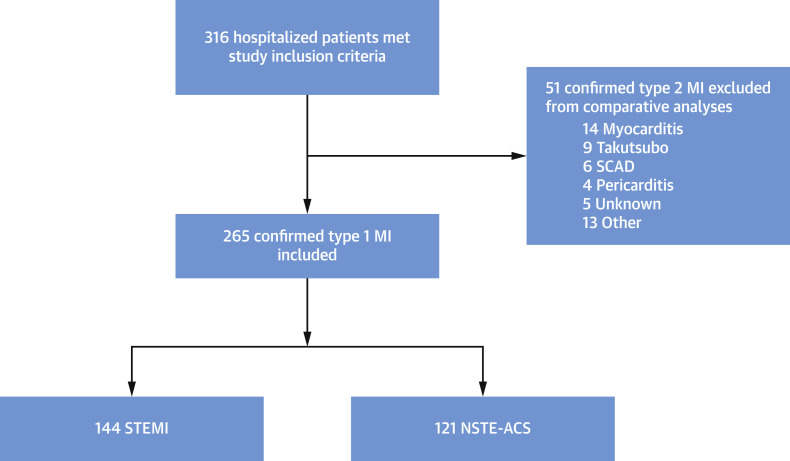
Of the 316 patients, 144 (54.3%) were diagnosed with STEMI and 121 (45.6%) with NSTE-ACS. These 2 groups formed the basis of the comparative analyses with MINAP/BCIS data. The study profile is outlined in Figure 1 .
Figure 1.
Patient Selection for the International COVID-ACS Registry
Flow diagram detailing patients enrolled in the International COVID-ACS Registry. A total of 51 patients with type 2 myocardial infarction were excluded from comparative analyses with pre-COVID-19 BCIS/MINAP (British Cardiovascular Intervention Society/Myocardial Ischaemia National Audit Project) reference data. ACS = acute coronary syndrome; MI = myocardial infarction; NSTE-ACS = non–ST-segment elevation acute coronary syndrome; SCAD = spontaneous coronary artery disease; STEMI = ST-segment elevation myocardial infarction.
The mean age of the STEMI/NSTE-ACS combined cohort was 64.9 ± 12.9 years; 75.5% were men; 66.2% had hypertension, 54.1% hyperlipidemia, 36.2% diabetes mellitus, 20.2% a previous MI, 19.3% prior history of heart failure, and 14.6% chronic kidney disease stage 3 to 5; and 27.1% were current smokers.
In total, 74.3% of patients tested positive for COVID-19 infection, with viral polymerase chain reaction testing used in 98.9% of these cases. An additional 25.7% were defined as COVID-19 suspected (treated as positive despite a negative PCR test) due to a high index of clinical suspicion (clinical status plus CXR or CT findings compatible with COVID-19). On admission, 17.4% of patients were defined as Killip heart failure class III/IV, 61.7% had a serum lactate level >2.0 mmol/l, and 5.3% had experienced an out of hospital cardiac arrest.
Demographics, comorbidities, procedural characteristics, and post-procedural support requirements in the COVID-STEMI subgroup are shown in Table 2 . Compared with non-COVID STEMI patients (BCIS cohort), our COVID-STEMI subgroup was younger, with significantly more hypertension, hyperlipidemia, diabetes, heart failure, previous PCI, and renal dysfunction. Numerical but nonsignificant differences in cardiac troponin T and I were noted, although these analyses are limited by small numbers due to use of differing troponin assays at international centers (high-sensitivity vs. contemporary, troponin I vs. troponin T), whereas BCIS/MINAP collect only high-sensitivity troponin data.
Table 2.
Baseline Demographics/Procedural Characteristics of COVID-STEMI and BCIS STEMI Subgroups
| COVID-STEMI Total (n = 144) | BCIS 2018–2019 (n = 24,961) | p Value | |
|---|---|---|---|
| Mean age, yrs | 63.1 ± 12.6 | 65.6 ± 13.4 | 0.018 |
| Male | 77.8 (112/144) | 72.2 (17,972/24,961) | 0.14 |
| Hypertension | 64.8 (92/142) | 44.8 (9,456/24,961) | <0.001 |
| Hyperlipidemia | 46.0 (58/126) | 28.9 (6,039/24,961) | <0.001 |
| BMI, kg/m2 | 27.3 ± 4.5 | 27.8 ± 5.5 | 0.18 |
| Diabetes | 34.0 (49/144) | 20.9 (4,926/24,961) | <0.001 |
| Current smoker | 31.7 (39/123) | 33.7 (7,645/24,961) | 0.77 |
| Heart failure | 19.0 (27/142) | 2.8 (569/24,961) | <0.001 |
| Previous MI | 16.4 (23/140) | 13.0 (2747/24,961) | 0.056 |
| Previous PCI | 13.9 (20/144) | 10.2 (2,129/24,961) | 0.034 |
| Chronic kidney disease (stage 3–5) | 9.9 (14/141) | 3.6 (739/24,961) | <0.001 |
| Lung disease | 11.8 (16/135) | 13.4 (2,763/24,961) | 0.78 |
| Stroke | 7.6 (11/144) | 5.7 (1,178/24,961) | 0.11 |
| COVID-19 positive | 76.4 (110/144) | N/A | |
| COVID-19 suspected | 23.6 (34/144) | N/A | |
| SBP at admission, mm Hg | 119.5 ± 26.8 | 131.9 ± 27.5 | <0.001 |
| Heart rate at admission, beats/min | 86.0 ± 22.0 | 78.5 ± 20.1 | <0.001 |
| Troponin T, ng/l | 2224.0 (58.0–7,449.5) | 899.0 (100.0–3,745.0) | 0.15 |
| Troponin I, ng/l | 762.0 (50.0–23,037.0) | 61.4 (14.6–1,118.4) | 0.19 |
| LVEF, % | 39.7 ± 12.5 | N/A | |
| Procedure | |||
| Symptom onset to admission, min | 339.0 (175.0–1,481.5) | 173.0 (107.0–387.0) | <0.001 |
| Door-to-balloon time, min | 83.0 (37.0–336.0) | 37.0 (31.0–109.0) | <0.001 |
| Transradial access | 74.3 (107/144) | 87.4 (19,611/22,442) | <0.001 |
| Nonobstructive CAD | 2.8 (4/144) | N/A | |
| SYNTAX score | 16.5 ± 9.1 | N/A | |
| Thrombotic occlusion (TIMI grade 5) | 37.5 (54/144) | N/A | |
| Use of aspiration thrombectomy | 12.5 (18/144) | 17.1 (3,754/21,915) | 0.15 |
| Complete revascularization | 45.8 (66/144) | N/A | |
| Post-procedure | |||
| ICU admission | 45.8 (66/144) | N/A | |
| Ventilation | 20.8 (30/144) | 3.8 (863/22,442) | <0.001 |
| Pressor support | 27.1 (39/144) | 4.6 (1,001/21,720) | <0.001 |
| Mechanical support device, % | 5.6 (8/144) (ECMO = 3, IABP = 5) | 2.1 (459/21,720) | 0.012 |
Values are mean ± SD, % (n/N), or median (interquartile range). Denominators not equal to n = 144 are due to incomplete data. Incomplete timing data was recorded in 9% (13 of 144) of COVID-STEMI patients. Bold p values indicate statistical significance.
CAD = coronary artery disease; ECMO = extracorporeal membrane oxygenation; IABP = intra-aortic balloon pump; ICU = intensive care unit; N/A = data unavailable; TIMI = Thrombolysis In Myocardial Infarction; other abbreviations as in Table 1.
Likewise, our COVID NSTE-ACS subgroup (Table 3 ) had a greater comorbidity burden with a significantly lower mean age than non-COVID NSTE-ACS patients from the MINAP cohort. Again, significantly higher incidences of hypertension, hyperlipidemia, diabetes, heart failure, and renal dysfunction were observed.
Table 3.
Baseline Demographics/Procedural Characteristics of COVID NSTE-ACS and MINAP NSTE-ACS Subgroups
| COVID NSTE-ACS Total (n = 121) | MINAP 2019 (n = 46,389) | p Value | |
|---|---|---|---|
| Mean age, yrs | 66.9 ± 12.9 | 70.2 ± 13.3 | 0.005 |
| Male | 79.3 (88/111) | 65.5 (30,388/46,389) | 0.002 |
| Hypertension | 68.3 (82/120) | 57.8 (24,359/46,389) | <0.001 |
| Hyperlipidemia | 62.9 (73/116) | 33.6 (13,895/46,389) | <0.001 |
| BMI, kg/m2 | 27.8 ± 4.9 | 28.2 ± 6.0 | 0.37 |
| Diabetes | 38.8 (47/121) | 31.1 (14,101/46,389) | 0.048 |
| Current smoker | 21.7 (23/106) | 20.4 (8,834/46,389) | 0.46 |
| Heart failure | 18.8 (22/117) | 9.6 (3,968/46,389) | <0.001 |
| Previous MI | 24.6 (29/118) | 29.1 (12,181/46,389) | 0.75 |
| Previous PCI | 21.8 (26/119) | 18.7 (7,684/46,389) | 0.14 |
| Chronic kidney disease (stage 3–5) | 20.2 (24/119) | 10.2 (4,214/46,389) | <0.001 |
| Lung disease | 21.8 (26/119) | 19.2 (7,908/46,389) | 0.18 |
| Stroke | 6.6 (8/121) | 10.0 (4,121/46,389) | 0.52 |
| COVID-19 positive | 71.9 | N/A | |
| COVID-19 high index suspicion | 28.1 | N/A | |
| SBP at admission, mm Hg | 122.0 ± 29.2 | 142.4 ± 27.3 | <0.001 |
| Heart rate at admission, beats/min | 80.2 ± 18.7 | 79.6 ± 20.0 | 0.73 |
| Troponin T, ng/l | 60.0 (1.0-288.0) | 144.0 (47.0-460.0) | 0.37 |
| Troponin I, ng/l | 171.0 (39.75–1,279.0) | 276.6 (47.1–1,371.4) | 0.48 |
| LVEF, % | 48.6 ± 13.3 | N/A | |
| Procedure | |||
| Symptom onset to admission, min | 417.0 (157.0–2,904.0) | 295.0 (130.0–1,021.0) | 0.012 |
| Door-to-angiography time, h | 48.5 (12.2–132.4) | 57.7 (25.1–105.3) | 0.49 |
| Transradial access | 77.7 (94/121) | 88.0 (29,777/33,833) | 0.002 |
| Nonobstructive CAD | 18.2 (22/121) | N/A | |
| SYNTAX score | 19.3 ± 11.7 | N/A | |
| Thrombotic occlusion (TIMI grade 5) | 5.0 (6/121) | N/A | |
| Use of aspiration thrombectomy | 0.0 (0/121) | 2.41 (804/33,250) | 0.12 |
| Complete revascularization | 42.7 (32/75) | N/A | |
| Post-procedure | |||
| ICU admission | 33.9 (41/121) | N/A | |
| Ventilation | 11.6 (14/121) | 0.4 (138/33,833) | <0.001 |
| Pressor support | 19.0 (23/121) | 0.9 (306/32,666) | <0.001 |
| Mechanical support device, % | 0.8 (1/121) (IABP = 1) | 0.6 (203/32,666) | 0.52 |
Procedural characteristics
Symptom onset to admission and door-to-balloon times were more than double in our COVID-STEMI subgroup compared with BCIS (Table 3). Incomplete timing data was recorded in 9% (13 of 144) of COVID-STEMI patients. Admission systolic blood pressure was significantly lower and admission heart rate was higher. Transradial access use was noted to be lower. Only 2.8% of this group was found to have nonobstructive coronary disease, with 37.5% reporting TIMI grade 5 intracoronary thrombus; 45.8% required intensive care admission and 20.8% mechanical ventilation—in some the indications were likely respiratory and not cardiac. The need for pressor support was 6-fold greater than in the pre-COVID national database, with twice as many requiring mechanical support devices.
Similarly, in the COVID NSTE-ACS subgroup, symptom onset to admission times were prolonged, and admission systolic blood pressure was lower. However, no significant delays in admission to angiography time were observed compared with the MINAP data, with a nonsignificant trend toward shorter in-hospital waits for the catheter laboratory noted (48.5 h vs. 57.7 h; p = 0.49). Post-procedural support requirement differences were also higher but were not required as frequently as with the COVID-STEMI subgroup.
In-hospital outcomes
Overall, in-hospital mortality in the study cohort was 15.5%. Among COVID-STEMI patients, the in-hospital mortality was 24.5% in those who were COVID-19 positive versus 18.2% in those with a high index of clinical suspicion (p = 0.49) (Supplemental Table 2). In-hospital mortality more than quadrupled in our COVID-STEMI subgroup (22.9% vs. 5.7%; p < 0.001) with higher rates of CGS (20.1% vs. 8.7%; p < 0.001) (Table 4 ). Rates of stroke (2.1% vs. 0.1%; p = 0.002) and bleeding (2.8% vs. 0.3%; p < 0.001) were also significantly elevated. Inpatient stay was twice as long in the COVID-STEMI patients (6.4 days vs. 3.0 days; p < 0.001) compared with BCIS.
Table 4.
In-Hospital Outcomes of COVID-STEMI and BCIS STEMI Subgroups
| COVID-STEMI Total (n = 144) | BCIS 2018–2019 | p Value | |
|---|---|---|---|
| Death | 22.9 (33/144) | 5.7 (1,232/21,675) | <0.001 |
| Myocardial infarction | 5.6 (8/144) | N/A | |
| Heart failure | 23.6 (34/144) | N/A | |
| Stent thrombosis | 1.4 (2/144) | N/A | |
| Bleeding (Bleeding Academic Research Consortium 3–5) | 2.8 (4/144) | 0.26 (36/13,913) | <0.001 |
| Stroke | 2.1 (3/144) | 0.14 (32/21,994) | 0.002 |
| Cardiogenic shock | 20.1 (29/144) | 8.7 (1,898/21,972) | <0.001 |
| In-patient stay, days | 6.4 (2.7–12.7) | 3.0 (2.0–5.0) | <0.001 |
Values are % (n/N) or median (interquartile range). Bold p values indicate statistical significance.
Abbreviations as in Table 1.
For the COVID NSTE-ACS group, mortality was more than 4-fold greater compared with the pre-COVID MINAP NSTE-ACS cohort (6.6% vs. 1.2%; p < 0.001) (Table 5 ). For NSTE-ACS, in-hospital mortality was 5.7% in COVID-19–positive patients versus 8.8% in those with a high index of clinical suspicion (p = 0.69) (Appendix 2). Higher incidences of CGS (5.0% vs. 1.4%; p = 0.007) and bleeding (2.5% vs. 0.1%; p = 0.006) were also noted in the COVID NSTE-ACS group versus the MINAP NSTE-ACS reference cohort, as well as a significant prolongation in total hospital stay (6.9 days vs. 5.0 days; p < 0.001).
Table 5.
In-Hospital Outcomes of COVID NSTE-ACS and MINAP NSTE-ACS Subgroups
| COVID NSTE-ACS Total (n = 121) | MINAP 2019 | p Value | |
|---|---|---|---|
| Death | 6.6 (8/121) | 1.2 (378/32546) | <0.001 |
| Myocardial infarction | 4.1 (5/121) | N/A | |
| Heart failure | 19.0 (23/121) | N/A | |
| Stent thrombosis | 0.0 (0/121) | N/A | |
| Bleeding (Bleeding Academic Research Consortium 3–5) | 2.5 (3/121) | 0.12 (28/22,445) | 0.006 |
| Stroke | 0.8 (1/121) | 0.05 (18/33,352) | 0.067 |
| Cardiogenic shock | 5.0 (6/121) | 1.4 (461/33,342) | 0.007 |
| In-patient stay, days | 6.9 (3.4–18.4) | 5.0 (3.0–8.0) | <0.001 |
Values are % (n/N) or median (interquartile range). Bold p values indicate statistical significance.
Abbreviations as in Table 1.
In terms of raw unadjusted data, for CGS patients, mortality was 58.6% in the combined COVID-ACS data and 32.8% in MINAP/BCIS, whereas for non-CGS patients, mortality was 13.9% in the combined COVID-ACS data and 3.0% in MINAP/BCIS. Table 6 lists the reported cause of death, associated incidence of CGS, and related time delays.
Table 6.
Causes of Death Association With Cardiogenic Shock and Ischemia Times
| Cause of Mortality (STEMI/NSTE-ACS) | Incidence of Cardiogenic Shock | Ischemia Time, min STEMI Only |
||
|---|---|---|---|---|
| CGS (n = 19∗) | no CGS (n = 106∗) | |||
| Cardiovascular | 58.5 (24/41) | 75.0 (18/24) | 1271.0 (355.0–2,760.0) | 440.5 (208.0–1,701.0) |
| Respiratory | 31.7 (13/41) | 23.1 (3/13) | ||
| Neurological | 4.9 (2/41) | 0.0 (0/2) | ||
| Unknown | 4.9 (2/41) | 0.0 (0/2) | ||
Values are % (n/N) or median (interquartile range).
CGS = cardiogenic shock; IQR = interquartile range; other abbreviations as in Table 1.
n = 19, n = 106 due to incomplete data.
Multivariable propensity-based analyses
Adjustment using propensity score analyses for age, sex, hypertension, diabetes, and hyperlipidemia demonstrated that COVID-STEMI patients in our registry still had increased overall mortality compared with the reference patients (odds ratio [OR]: 3.33; 95% confidence interval [CI]: 2.04 to 5.42) (Table 7 ). Separate analyses stratified by CGS status show that, in patients with CGS, risk of mortality for COVID-ACS registry patients is greater compared with BCIS reference patients (OR: 1.83; 95% CI: 0.80 to 4.19), yet this is greatly increased in patients without CGS (OR: 4.16; 95% CI: 2.33 to 7.44).
Table 7.
Multivariate Propensity Analyses Comparing COVID-STEMI Patients With the BCIS Database
| COVID-STEMI vs. BCIS | All Patients | CGS | Non-CGS |
|---|---|---|---|
| Overall mortality∗ | 3.33 (2.04–5.42) | 1.83 (0.80–4.19) | 4.16 (2.33–7.44) |
| Total ischemia time (for every 10 min)† | 1.10 (1.01–1.19) | 1.25 (1.09–1.45) | 1.04 (0.94–1.15) |
| CGS‡ | 1.48 (1.27–1.72) |
Values are odds ratio (95% confidence interval). COVID-STEMI and BCIS were matched for age, sex, hypertension, diabetes, and hyperlipidemia using a propensity score. Total ischemic time (symptom-to-admission plus admission-to-balloon) was right skewed, therefore a logarithm transformation with base 10 was performed.
Overall mortality: this adjusts for age, sex, hypertension, hyperlipidemia, diabetes, ischemia time, and CGS.
Mortality related to ischemia time.
Mortality related to presence of CGS.
Correcting for the potential confounders listed in the previous text, we also demonstrate that for every 10-min delay in total ischemia time (symptom-to-admission plus door-to-balloon), a 10% mortality risk increase is observed (OR: 1.10; 95% CI: 1.01 to 1.19). The confidence interval remained >1.0 for those with CGS (OR: 1.25; 95% CI: 1.09 to 1.45), whereas in those without CGS, this crosses the line of unity (OR: 1.04; 95% CI: 0.94 to 1.15). A further separate analysis showed a 48% increase in death in patients diagnosed with CGS (OR: 1.48; 95% CI: 1.27 to 1.72).
Discussion
This international registry describes the demographics, procedural characteristics, and outcomes of COVID-19 ACS patients undergoing invasive coronary angiography and compares these to historical cohorts. It provides mechanistic information on the excess mortality observed in COVID-19 ACS patients.
Compared with the pre-COVID era, we report: 1) significantly prolonged delays in patients seeking medical care, and longer door-to-balloon times in COVID-STEMI patients; 2) significantly higher rates of CGS, and requirement for intensive care unit admission and ventilatory and/or hemodynamic support; and 3) quadrupling of in-hospital mortality compared with our pre-COVID cohort databases. Moreover, both COVID-ACS subgroups were found to be younger and carried a greater burden of comorbidity.
To date, reports on concomitant COVID-19 infection in patients who present with ACS are limited to small observational studies of STEMI patients (6,18,19) (the largest included 78 patients), with a paucity of data in NSTE-ACS. In the most robust study, a single-center observation of 39 consecutive COVID-19–positive STEMI cases reported in-hospital mortality of 17.9% compared with 6.5% in COVID-19–negative control subjects. This was statistically nonsignificant, likely due to small numbers; however, higher thrombus burden was suggested for the increased mortality, which is notable as symptom-to-admission and door-to-balloon times did not differ (5).
Hence, contemporary data thus far have principally described the effects of the pandemic on COVID-19–negative ACS patients. The largest registry to date of 6,090 patients undergoing PCI (of whom 2,419 were in 2020) documented higher mortality (6.8% vs. 4.9%) and longer ischemia times in those treated during the COVID era (20). However, only 62 patients in this study were COVID-19 positive (in-hospital mortality 29.0%), with no further details provided. Our study focused on COVID-19–positive ACS cases, including time to treatment and potential mechanisms driving the elevated mortality rates in these patients.
Symptom-to-admission times and STEMI door-to-balloon times in our registry were significantly greater than the pre-COVID cohort and should be considered in the context of decreases in absolute hospitalizations for ACS during the COVID-19 pandemic (8,10,21)—most likely due to public fear of viral contagion (22). We assert that the delays seen in door-to-balloon time data may be due to restructured “COVID-19 pathways” and time spent donning appropriate PPE, which was utilized in more than 90% of cases from our registry. The nonsignificant trend to accelerated door-to-angiography times in our NSTE-ACS group is likely due to widespread suspension of elective catheter laboratory work (23), thus creating availability for acute cases.
Our data support the notion that prolonged ischemia times were associated with poor outcomes, with a 10% increase in mortality for the COVID-ACS patients for every 10-min delay. This was exacerbated in those with CGS (25% increase/10 min), with the association still present in those without CGS (4%/10 min). For the STEMI cohort (ACS and reference database, COVID-19 positive and COVID-19 negative combined), experiencing CGS increased mortality by 48%.
Given the strong relationship between prolonged ischemia time and poorer outcomes in STEMI, the increased incidence of CGS is an important contributor to the higher rates of adverse outcomes and supports reported data of excess deaths due to CGS during the pandemic (22). Historical ACS longitudinal data describe the incidence of CGS as approximately 7% (24), one-half of the 13.2% in our study. The relationship of presentation times and onset of CGS is intuitive, but is not robustly reported. It is therefore reasonable to assert that prolonged ischemia times in our population were responsible for the high incidence of CGS, although consideration must be given to the hypothesis that higher CGS incidence could also be related to COVID-19 infection and potential pro-thrombotic mechanisms.
Higher rates of hypertension, hyperlipidemia, diabetes mellitus, heart failure, and chronic kidney disease all may contribute to an elevated risk of major adverse cardiovascular events in COVID-19 ACS patients, consistent with other recent cohorts (25). However, our data suggest that these factors did not play a major role, because correcting for them still resulted in excess mortality. In a separate analysis stratified by CGS correcting for these confounders, the absolute differences in mortality between all STEMI patients who were either COVID-19 positive or negative with CGS was 25.8%, whereas it was only 10.9% in those without CGS. However, the relative risk of mortality with concomitant COVID-19 infection in patients without CGS was 4.16, but was 1.83 for those with CGS. Thus, COVID-19 significantly increases risk of death in patients without CGS, but in those who experience CGS, it is CGS that is the major determinant of mortality.
Thus, discriminating between the effects of acute MI and acute COVID-19 infection remains a significant challenge. However, the results from our International COVID-ACS Registry go further than previous studies and provide novel insights to support a hypothesis of potential COVID-19 fear and a consequent reluctance to go to hospital, a reluctance that appears to have led to an increase in deaths from ischemic heart disease during the pandemic (26). This multicomorbid population presented to hospitals significantly later and received less timely reperfusion therapy, thereby resulting in significantly higher rates of CGS and in-hospital mortality. This is supported by our data that suggest of those who died of cardiovascular causes, CGS was a key determinant, and CGS was associated with prolonged presentation times.
Study limitations
Due to its observational design, we cannot exclude the presence of unknown confounding factors and selection bias for patients entered to the registry, given the ratio of number of patients enrolled to number of centers is relatively low. ACS patients who did not reach the catheter laboratory and those medically treated were not included. A total of 29.1% of patients enrolled tested negative for COVID-19 on viral RT-PCR testing, yet were treated as highly suspicious for COVID-19 due to CXR or CT findings supporting severe acute respiratory syndrome coronavirus 2 infection. Rates of false-negative COVID-19 RT-PCR results of up to 38% are well recognized (27); therefore, we considered it important that these patients were included in the study. Furthermore, there were no significant differences in mortality between these groups. We also acknowledge that COVID-19 can present heterogeneously. The results of propensity analysis confirm that delays in presenting to hospital and CGS were the main factors determining outcomes—however, other mechanisms such as impact of the COVID-19 virus itself on the cardiovascular system cannot be discounted. However, we did record overall mortality and perceived causes of death. Furthermore, we recognize that these are short-term data and that there is a lack of a concurrent COVID-19–negative control group—systems of care during the pandemic were disrupted at this time and would therefore not represent the pre-COVID standard. Moreover, our control group comprises only U.K. data and should not be considered truly reflective of practice at the international sites that participated in the study. However, over 75% of patients enrolled in the registry were from centers in Europe, and thus these data, which report similar in-hospital outcomes compared with other respected European databases (16,17), are likely to offer one of the best historical comparisons available.
Conclusions
This large multinational, observational study of COVID-19 ACS patients demonstrates novel mechanistic data indicating that these patients present later and have increased in-hospital mortality compared with a pre-COVID ACS population (Central Illustration ). Importantly, COVID-19 ACS patients have excess rates of CGS, and adverse outcomes appear to be driven by delays in seeking medical care and timely reperfusion therapy, supporting yet again the concept of “time is muscle.” We should recognize that in patients with 2 diseases, differentiating one from the other may be difficult—thus, clear and simple public health messages for patients to present expeditiously to the hospital when they first experience symptoms of ACS are required during this and future pandemics.
Central Illustration.
Time Delays and In-Hospital Outcomes in the International COVID-ACS Registry
When compared with pre–coronavirus disease 2019 (COVID-19) reference data from the British Cardiovascular Intervention Society (BCIS) and Myocardial Ischaemia National Audit Project (MINAP) databases, patients enrolled in the International COVID-ACS registry were found to experience significant delays in presentation to hospital and time to reperfusion therapy, excess rates of cardiogenic shock, and greater in-hospital mortality. These novel data suggest 1 potential mechanism for the poorer outcomes observed in patients with acute coronary syndrome (ACS) and COVID-19, and yet again support the concept of “time is muscle” in myocardial infarction. Public health messaging during this and future pandemics should be clear—patients who experience cardiovascular symptoms should not delay in seeking medical attention. NSTE-ACS = non–ST-segment elevation acute coronary syndrome; STEMI = ST-segment elevation myocardial infarction.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: ACS in patients with COVID-19 is associated with a poor prognosis, particularly when medical intervention is delayed.
TRANSLATIONAL OUTLOOK: More research is needed to elucidate the mechanisms that trigger acute coronary syndromes in patients with COVID-19, their impact on the incidence and outcomes of cardiogenic shock, and implications for management.
Funding Support and Author Disclosures
The study was supported by the Clinical Trials Unit at The University of Glasgow. Dr. Gale has received personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, and Vifor Pharma; and has received grants from Abbott and Bristol Myers Squibb. Dr. Sabate has received personal fees from Abbott Vascular and IVascular. Dr. Sinagra has received personal fees from Biotronik, Boston Scientific, AstraZeneca, and Novartis. Dr. Savonitto has received personal fees from Bayer and Abbott. Dr. Curzen has received grants, personal fees, and nonfinancial support from Boston Scientific, Haemonetics, HeartFlow, and Abbott; has received grants from Beckmann Coulter; and has received nonfinancial support from Biosensors and Medtronic. Dr. Berry is supported by the British Heart Foundation (grant reference RE/18/6134217). Dr. Stone has received personal fees from Terumo, Cook, TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Matrizyme, Miracor, Neovasc, V-wave, Abiomed, Shockwave, MAIA Pharmaceuticals, and Vectorious; has received equity/options in Applied Therapeutics, Biostar, MedFocus, Aria, Cardiac Success, and Cagent; and has received personal fees and equity/options from SpectraWave, Valfix, Ancora, Orchestra Biomed, Qool Therapeutics, and Cardiomech. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Jonathan Gibb and Dionne Russell at the Glasgow Clinical Trials Unit for their expertise in establishing and maintaining the study database.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version for this paper.
Appendix
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:2540–2541. doi: 10.1056/NEJMc1805679. [DOI] [PubMed] [Google Scholar]
- 3.Libby P., Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudry F.A., Hamshere S.M., Rathod K.S. High thrombus burden in patients with covid-19 presenting with ST-elevation myocardial infarction. J Am Coll Cardiol. 2020;76:1168–1176. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanini G.G., Montorfano M., Trabattoni D. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mafham M.M., Spata E., Goldacre R. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J., Mamas M., Rashid M. Patient response, treatments and mortality for acute myocardial infarction during the COVID-19 pandemic. Eur Heart J Qual Care Clin Outcomes. 2020 Jul 30 doi: 10.1093/ehjqcco/qcaa062. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Filippo O., D'Ascenzo F., Angelini F. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in northern Italy. N Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson S., Kay F.U., Abbara S. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging. 2020;35:219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2018;72:223164. [Google Scholar]
- 13.Hicks K.A., Mahaffey K.W., Mehran R. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;137:961–972. doi: 10.1161/CIRCULATIONAHA.117.033502. [DOI] [PubMed] [Google Scholar]
- 14.Mehran R., Rao S.V., Bhatt D.L. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Garcia H.M., McFadden E.P., Farb A. standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 Consensus Document. Circulation. 2018;137:2635–2650. doi: 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- 16.Puymirat E., Simon T., Cayla G. Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation. 2017;136:1908–1919. doi: 10.1161/CIRCULATIONAHA.117.030798. [DOI] [PubMed] [Google Scholar]
- 17.Jernberg T., Johanson P., Held C. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA. 2011;305:1677–1684. doi: 10.1001/jama.2011.522. [DOI] [PubMed] [Google Scholar]
- 18.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamadeh A., Aldujeli A., Briedis K. Characteristics and outcomes in patients presenting with COVID-19 and ST-segment elevation myocardial infarction. Am J Cardiol. 2020;131:1–6. doi: 10.1016/j.amjcard.2020.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biondi-Zoccai G.G.L., Abbate A., Agostoni P. Long-term benefits of an early invasive management in acute coronary syndromes depend on intracoronary stenting and aggressive antiplatelet treatment: a metaregression. Am Heart J. 2005;149:504–511. doi: 10.1016/j.ahj.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Piccolo R., Bruzzese D., Mauro C. Population trends in rates of percutaneous coronary revascularization for acute coronary syndromes associated with the COVID-19 Outbreak. Circulation. 2020;141:2035–2037. doi: 10.1161/CIRCULATIONAHA.120.047457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J., Mamas M.A., Mohamed M.O. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart. 2021;107:113–119. doi: 10.1136/heartjnl-2020-317912. [DOI] [PubMed] [Google Scholar]
- 23.Einstein A.J., Shaw L.J., Hirschfeld C. International impact of COVID-19 on the diagnosis of heart disease. J Am Coll Cardiol. 2021;77:173–185. doi: 10.1016/j.jacc.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg R.J., Samad N.A., Yarzebski J., Gurwitz J., Bigelow C., Gore J.M. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med. 1999;340:1162–1168. doi: 10.1056/NEJM199904153401504. [DOI] [PubMed] [Google Scholar]
- 25.Rashid M., Wu J., Timmis A. Clinical characteristics and outcomes of COVID-19 positive acute coronary syndrome patients; a multisource electronic healthcare records study from England. medRxiv. 2020 Aug 22 doi: 10.1111/joim.13246. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadhera R.K., Shen C., Gondi S., Chen S., Kazi D.S., Yeh R.W. Cardiovascular deaths during the COVID-19 pandemic in the United States. J Am Coll Cardiol. 2021;77:159–169. doi: 10.1016/j.jacc.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucirka L.M., Lauer S.A., Laeyendecker O., Boon D., Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.