Abstract
Gastrointestinal (GI) cancers, including colorectal cancer, gastric cancer, and esophageal cancer, are a major medical and economic burden worldwide and have the largest number of new cancer cases and cancer deaths each year. Esophageal and gastric cancers are most common in developing countries, while colorectal cancer forms the major GI malignancy in Western countries. However, a great shift in the predominant GI-cancer type is happening in countries under economically transitioning and, at the same time, esophageal and gastric cancers are reigniting in Western countries due to the higher exposure to certain risk factors. The development of all GI cancers is highly associated with lifestyle habits and all can be detected by identified precancerous diseases. Thus, they are all suitable for cancer screening. Here, we review the epidemiological status of GI cancers in China, the USA, and Europe; the major risk factors and their distribution in these regions; and the current screening strategies.
Keywords: colorectal cancer, gastric cancer, esophageal cancer, epidemiology, cancer screening
Introduction
Cancer is the first or second leading cause of premature death (at ages 30–69 years) in most countries, and premature death caused by cancer accounted for 29.8% of deaths from non-communicable diseases with a total of 4.5 million in 2016 [1]. In 2020, it was estimated that there were 19.3 million new cancer cases and 9.9 million cancer deaths, rising from 14.1 and 8.2 in 2018 to 18.1 and 9.6 in 2019 [2–4]. Due to the growth and aging of the population and the inequality in cancer control, cancer has become more prominent as a cause of death and challenges the previously predominant ischemic cardiovascular diseases [5]. The burden of cancer is expected to grow worldwide, particularly in less developed countries [6].
Gastrointestinal (GI) cancers, mainly including the malignancies derived from esophageal, stomach, and colorectum, are among the most common cancers in humans. These cancers, which are derived from distinct but associated origins, have diverse clinical features but share some similar characteristics. According to the data available from GLOBOCAN 2020, GI cancers [colorectal cancer (CRC), gastric cancer, and esophageal cancer] accounted for 18.7% of new cancer cases and 22.6% of cancer deaths in 2020, which are both highest among all cancer types, and are a significant public health burden for most countries [4]. However, in terms of the geographic and temporal distribution, major risk factors, and prevention strategies of GI cancers, great differences exist between the West and the East. In this article, we review the epidemiological data from China, Europe, and the USA; risk factors; and current progress in the prevention and screening of GI cancers in these and other countries.
CRC
CRC is the third most commonly diagnosed malignancy in males and the second in females, and the second leading cause of cancer death in both sexes [3]. CRC comprises 10% (1.9 million) of global new cancer cases and 9.4% (0.9 million) of cancer deaths in 2020 [4]. The global disease burden in 2016 was estimated as 17.2 million disability-adjusted life years, of which 97% came from years of life lost due to premature mortality and 3% came from years of healthy life lost due to disability [1].
Epidemiology characteristics
The incidence of CRC is unbalanced throughout the world and varies greatly between high and low human development index (HDI) regions. The incidence is about 3-fold higher in high-HDI regions vs low-HDI regions, but the average case fatality is higher in low-HDI regions. Furthermore, there are also rapid rises in incidence in countries undergoing economic development and changes in diet and lifestyle [7]. The incidence of CRC exhibits a preference in populations with ‘Western’ lifestyles brought by industrialization and economic growth, since diet, physical activities, and obesity are major factors associated with CRC [8]. This pattern can be observed in the USA and Europe, which have both high HDI and high age-standardized incidence rates (ASRs) [9, 10]. Besides, in those countries that are economically transitioning, like China, the incidence rates of CRC are under rapid growth (Figure 1). In China, CRC was the fourth most common cancer type at the beginning of the twenty-first century [11]. However, as reported by the National Cancer Center, the incidence rate of CRC ranked third (ASR 17.8 per 100,000) among all cancer types in 2015 [12]. Furthermore, among the three areas (Eastern, Middle, Western), CRC was the second common cancer type in the Eastern area of China (19.9 per 100,000), which stands for the most developed region of China [11, 12]. Moreover, it has been estimated by GLOBOCAN to have been the second most prevalent cancer in 2020. In 2020, as estimated by GLOBOCAN, China had the largest number of CRC cases, while the USA ranked second. The incidence rate in China is close to that in the USA (23.9 vs 25.6 per 100,000), especially in males (28.6 vs 28.7 per 100,000), whereas the incidence rate in Europe was still way ahead (30.4 per 100,000) (Table 1).
Figure 1.
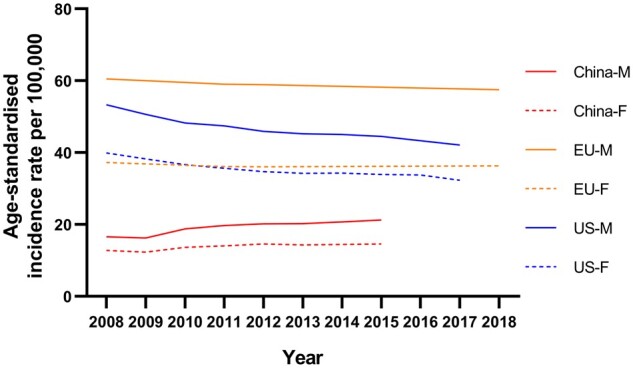
Time trends of incidence rates of colorectal cancer in men and women across China, the USA, and Europe. Data from National Cancer Center (China), Centers for Disease Control and Prevention (the USA), and European Cancer Information System (EU).
Table 1.
The estimated incidence and mortality (per 100,000) of gastrointestinal cancers by age groups in GLOBOCAN 2020
| China |
Europe |
USA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | Total | Male | Female | |
| All ages (ASRWa) | |||||||||
| Colorectal cancer | |||||||||
| Incidence | 23.9 | 28.6 | 19.5 | 30.4 | 37.9 | 24.6 | 25.6 | 28.7 | 22.9 |
| Mortality | 12.0 | 14.8 | 9.4 | 12.3 | 16.1 | 9.5 | 8.0 | 9.4 | 6.7 |
| Gastric cancer | |||||||||
| Incidence | 20.6 | 29.5 | 12.3 | 8.1 | 11.5 | 5.3 | 4.2 | 5.3 | 3.1 |
| Mortality | 15.9 | 22.8 | 9.5 | 5.5 | 7.9 | 3.5 | 1.7 | 2.2 | 1.3 |
| Esophageal cancer | |||||||||
| Incidence | 13.8 | 19.7 | 8.2 | 3.3 | 5.8 | 1.3 | 2.8 | 4.8 | 1.1 |
| Mortality | 12.7 | 18.3 | 7.4 | 2.7 | 4.9 | 1.0 | 2.4 | 4.2 | 0.8 |
| ≥50 years | |||||||||
| Colorectal cancer | |||||||||
| Incidence | 106.4 | 125.0 | 88.4 | 166.5 | 203.5 | 136.8 | 117.2 | 131.3 | 104.6 |
| Mortality | 56.8 | 66.6 | 47.3 | 80.3 | 97.2 | 66.8 | 42.7 | 47.8 | 38.2 |
| Gastric cancer | |||||||||
| Incidence | 92.1 | 131.3 | 54.2 | 43.1 | 59.8 | 29.7 | 20.2 | 26.3 | 14.7 |
| Mortality | 73.9 | 104.0 | 44.7 | 31.0 | 42.8 | 21.5 | 8.8 | 11.3 | 6.6 |
| Esophageal cancer | |||||||||
| Incidence | 64.9 | 90.0 | 40.8 | 16.9 | 28.9 | 7.3 | 14.9 | 24.7 | 6.0 |
| Mortality | 61.0 | 84.6 | 38.1 | 14.7 | 25.2 | 6.3 | 13.3 | 22.5 | 5.0 |
| <50 yearsb | |||||||||
| Colorectal cancer | |||||||||
| Incidence | 7.8 | 8.4 | 7.2 | 8.8 | 8.5 | 9.1 | 12.8 | 12.7 | 12.9 |
| Mortality | 2.5 | 2.8 | 2.2 | 2.3 | 2.3 | 2.2 | 3.1 | 3.4 | 2.8 |
| Gastric cancer | |||||||||
| Incidence | 6.4 | 7.6 | 5.1 | 2.7 | 3.1 | 2.4 | 1.9 | 1.9 | 1.8 |
| Mortality | 3.6 | 4.1 | 3.0 | 1.7 | 1.9 | 1.6 | 0.8 | 0.8 | 0.7 |
| Esophageal cancer | |||||||||
| Incidence | 2.5 | 3.9 | 1.0 | 1.0 | 1.5 | 0.4 | 0.6 | 1.0 | 0.3 |
| Mortality | 1.8 | 2.8 | 0.7 | 0.6 | 1.1 | 0.2 | 0.4 | 0.7 | 0.2 |
Age-standardized rate by world standard population (Segi’s population).
Including people aged 20–49 years.
In some countries with very high HDI, the incidence rates of CRC are now experiencing a decreasing trend, such as in the USA (Figure 1), Australia, and Japan. Arnold et al. [13] identified three categories based on temporal characteristics of incidence and mortality: group 1: increasing incidence and mortality (China, Brazil, Russia, Spain, etc.); group 2: increasing incidence and decreasing mortality (Canada, Denmark, UK, Singapore, etc.); group 3: decreasing incidence and mortality (USA, Australia, New Zealand, Iceland, etc.). China, for example, had rapidly increasing new CRC cases and the mortality rate has also been rising during recent decades, from an ASR per 100,000 of 6.18 in 2008 to 8.12 in 2015 [12, 14]. Moreover, the mortality rate of CRC in China was estimated to have overtaken that in the USA in 2020 (ASR per 100,000: 12.0 vs 8.0) [4]. In the USA, according to the Centers for Disease Control and Prevention (CDC), the mortality rate of CRC has been decreasing since 1999, from 20.9 in 1999 to 13.5 in 2017 (ASR per 100,000) [9]. The decrease in mortality can be attributed to the improvements in survival through the adoption of best practices in cancer treatment and management and increased screening leading to early detection. The decreasing incidence rates in group 3 countries may be due to the delayed effect of the screening programs. The introduction of screening programs may have initially increased the incidence rates, as there was more detection of early-stage CRC, but this has been proven to have resulted in lower incidence rates because of the removal of precancerous lesions by endoscopy [15].
The incidence and modality of CRC both increase rapidly after the age of 50 years [16, 17]. According to GLOBOCAN, in 2020, >90% of CRC cases and deaths occurring after this age. Although most CRC patients were diagnosed at an older age, an increasing trend for incidence in younger populations is emerging. While early-onset CRC has a familial component more often than late-onset disease, most cases are sporadic [18]. In the USA, increasing risk and incidence of CRC in those between 20 and 49 years old were found in sequential birth cohorts [19]. The incidence increased from 6.4 per 100,000 in 2002 to 8.3 per 100,000 in 2017 and was estimated to have reached 12.8 in 2020 [4, 9]. The incidence rate of early-onset CRC for the USA is higher than that for Europe, where the highest all-age incidence rate of CRC can be found (Table 1). These strong birth cohort effects may signal relatively recent changes in exposures that influence risk.
Higher incidence and mortality can be observed in men (Table 1). This may relate to a series of complicated factors. Several studies have indicated that men are more vulnerable to environmental factors in developing CRC [20, 21], and men also have higher exposure rates to the risk factors for CRC, such as alcohol intake, smoking, and obesity [22–24]. Moreover, men are inherently not protected by estrogen, which was known to be inversely associated with colorectal-cancer risk [25].
Risk factors
Genetic and environmental factors both play an important role in the etiology of CRC. Genetics contribute to individual risk, but environmental factors, including diet and lifestyle, affect the incidence in populations. About 75% of patients have a negative family history, suggesting that most CRCs are sporadic [20]. Several studies also indicated that, in the USA and Europe, ∼16%–71% of new CRC cases can be attributed to environmental factors [26, 27]. Thus, key behavior modifications and adherence to a healthy lifestyle could have avoided most CRC cases. Smoking is the most important lifestyle risk factor [28]. According to the continuous update project report by the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR), obesity, low physical activity, Western diet habits, and alcohol increase CRC risk [8].
Regarding genetic factors, the two most common hereditary colorectal-cancer syndromes are Lynch syndrome (hereditary nonpolyposis CRC) and familial adenomatous polyposis (FAP), and they together account for 5%–10% of CRC patients [29]. Lynch syndrome comprises 2%–4% of CRC cases and is caused by a mutation in one of the DNA mismatch-repair genes: MLH1, MSH2, MSH6, PMS2 or EPCAM [29]. Lynch syndrome increases the lifetime risk by ≤60%. In this setting, cancers evolve relatively quickly from adenomas or possibly even normal-appearing tissue and frequently elicit strong immunological responses [30]. FAP accounts for <1% of CRC cases and is caused by the adenomatous polyposis coli gene. Patients usually develop many adenomas at a young age, mainly in the distal colon. If the adenomas are not removed adequately, the risk of CRC would add up to 100% by 40 years of age [31].
Adenomas and serrated polyps are two major subtypes that are precursors to the majority of sporadic CRCs [32]. Approximately 85%–90% of sporadic CRCs evolve from adenomas. Advanced adenomas (≥1 cm in diameter, villous histology, or high-grade dysplasia) with or without multiplicity (more than three adenomas) have a significantly higher rate (30%–50%) of progress to CRC. Serrated polyps represent a group of heterogeneous lesions, including hyperplastic polyps, traditional serrated adenomas, and sessile serrated adenomas [33]. Patients with sessile serrated adenoma or traditional serrated adenoma are at increased risk of CRC and some would propose that the risk is similar to or higher than that for patients with conventional adenomas. The odd ratios (ORs) of CRC were 3.40 (2.35–4.91) for sessile serrated adenomas, 4.84 (2.36–9.93) for traditional serrated adenomas, and 2.51 (2.25–2.80) for conventional adenomas, compared with individuals without a history of polyps [34].
Now it has been recognized that the consumption of Western-type calorically-rich diets combined with chronic overnutrition and a sedentary lifestyle in Western societies evokes a state of chronic metabolic inflammation, which contributes to the development of CRC and other diseases, and this situation is also becoming more and prevalent in China [35].
Obesity is recognized as an established risk factor for CRC, and its effects are stronger in colon cancer than in rectal cancer [36]. With each unit increase in body mass index (BMI), the risk of CRC increases by 2%–3%. Moreover, this linear association was stronger in North American than European populations, but not significant in the Asian population. However, Asian individuals had a sharply increased risk from BMI < 23 kg/m2 to a relatively normal range (23–25 kg/m2), as each 5-kg/m2 increment was associated with an 18% increased risk [37]. This phenomenon may due to the difference in body-fat distribution between the West and the East. Recent studies suggest that waist circumference as a stronger risk factor [38], due to rising evidence on the effects of abdominal/visceral fatness on CRC [39, 40]. Abdominal fatness is more common in Asians than in Caucasians [41], which might contribute to the elevated risk of CRC in the Asian population with normal BMI.
Physical activity is well known for its potential for reducing cancer risk, but it is only established in colon cancers as an evidential risk factor [42], and the evidence in rectal cancer is not significant. Exercise may render its benefits mainly through its effect on weight loss [43], while other studies have also illustrated its benefits through enhancing gut motility, IL-6 redistribution, epinephrine release, and activating the immune system against tumors [44].
Dietary habits, both the healthy pattern and the unhealthy pattern, are well recognized as an important factor in the etiology of CRC [8, 45]. Intake of red meat and processed meat increases the risk of CRC by an estimated 1.16-fold per 100-g increase in daily intake [46]. By contrast, the consumption of milk, whole grains, fresh fruits, and vegetables, as well as an intake of fiber, multivitamins, and vitamin D, decreases the risk of CRC [47]. Of note, fiber supplements failed to protect against recurrent colorectal adenomas [48], which might imply overestimation in fiber supplements and the potential benefits of natural grains. As a result, dietary fiber was demoted to ‘probable evidence’ in the 2018 version of the continuous update project for CRC [8].
For calcium intake, each 300-mg/day increase was associated with an ∼9% reduced risk of CRC in a large observational study [49]. But randomized control trials failed to prove this finding with calcium supplements, even together with vitamin D [50, 51]. This may due to the calcium calculated in observational studies being mostly from dairy products and varying over a wider range. Nevertheless, calcium is considered to have potential against the development of CRC but, for now, the evidence is not sufficient to recommend calcium supplements for an anti-CRC purpose.
Moderate alcohol consumption (50 g per day) has been estimated to increase CRC risk by 38%, whereas even higher alcohol consumption (100 g per day) is associated with an ≤82% increased risk, and the association was stronger in Asians [52]. Cigarette smoking is significantly associated with CRC incidence and mortality. Smoking leads to a risk increase of 18% and an increase of 10.8 new cases per 100,000 person-years [53]. Interestingly, recent studies have shown that smoking is differentially associated with the risk of different molecular subtypes. Ever smoking was associated with microsatellite instability (MSI)-high and microsatellite stability (MSS)/MSI-low CRC, but the association was significantly stronger for ∼50% for MSI-high CRC [54], and cigarette smoking was also associated with the CIMP-positive and BRAF mutation-positive colorectal-cancer subtypes [55].
Inflammatory bowel disease (IBD) is also associated with an increased risk of CRC and the risk is higher when the history of IBD is longer [56]. However, it only explains ∼1% of all CRC in Western populations. Moreover, its risk of CRC seems to decreases over time due to the improved therapies for patients with IBD [57].
Prevention and screening
In addition to changing bad lifestyles, the application of aspirin has been extensively examined for its potential against CRC [7]. Aspirin was reported to protect patients from adenoma recurrence and the development of CRC [58, 59]. A long-term daily aspirin could reduce ∼25% incidence and 39% mortality. It was estimated that a minimum intake of 325 mg aspirin per week for ≥6 years might be required to obtain any benefit against CRC [60].
CRC is one of the cancers that can benefit most from screening, since the grossly visible lesions usually take 10 years to progress to CRC, leaving a wide time window for early diagnosis [61]. Screening can help to remove the precancerous lesions by colonoscopy or diagnose cancer at an earlier stage, which will reduce the incidence and mortality, and thus lower the burden of CRC. CRC is more suitable for population screening than any other malignancy owing to a combination of factors [62]. Major approaches for screening include a fecal occult blood test (FOBT), colonoscopy, and other tests based on stool samples.
The FOBT, or the fecal immunochemical test (FIT), is the most economical and easy-to-implement approach, so it has been recommended in several countries as a first-line population-screening approach [16, 63]. However, its accuracy is relatively lower and it must be followed by a colonoscopy to confirm the results [64]. A new method derived from FIT called multitarget stool DNA testing (DNA-FIT), which includes a series of hemoglobin and DNA mutations such as quantitative molecular assays for KRAS mutations, aberrant NDRG4, and BMP3 methylation. The FIT-DNA is more sensitive for detecting advanced precancerous lesions and CRC than FIT but has more false-positive results [65].
The colonoscopy, known as the golden standard for CRC screening and diagnosis, has high sensitivity and can perform excision for precancerous lesions, despite its higher costs. Thus, it can lower the incidence and mortality of CRC. After a 20-year follow-up of the US National Polyp Study cohort, CRC-specific mortality was ∼50% lower among subjects who at baseline had undergone endoscopic removal of adenomas than in an unscreened control cohort [66]. The results from a meta-analysis suggested a 40%–60% lower risk of incident CRC and death from CRC after screening colonoscopy [67].
Most European countries, the USA, and China have established CRC-screening guidelines and programs. Given the considerable rise in treatment costs, CRC screening is a cost-saving exercise in many countries. Most countries recommend FIT to those aged >50 years and with an average-risk population (China, Denmark, France, and Norway, etc.), while colonoscopy was only recommended in the USA, China, Germany, and Austria as a screening test [16]. As for frequency, most guidelines recommend that patients aged >50 years should take the FIT test once a year and once every 10 years for colonoscopy [16, 64, 68].
Gastric cancer
Gastric cancer is estimated to be the fourth most common cancer in both sexes, and was the fourth leading cause of death among all cancer types worldwide in 2020 and the third most common cause of cancer death in 2018 [1, 4]. In 2020, it was estimated that there were 1.0 million (5.6%) new gastric-cancer cases and 0.7 million (7.7%) cancer deaths [4]. Gastric cancer used to be one of the major causes of cancer-related death, but the breakthrough in understanding the causation of stomach cancer, namely a bacterium—Helicobacter pylori—has successfully helped to reduce the incidence and mortality over the last century. However, patients with gastric cancer are often diagnosed with advanced disease and survival is poor.
Epidemiology characteristics
Gastric cancer has very strong regional distribution differences, as Eastern Asia—China, Japan, and South Korea—contributes ∼65% of new global gastric-cancer cases each year [4]. In 2015, gastric cancer was the second most common cancer type in China (ASR 18.57 per 100,000) [12], whereas, in 2020, it was estimated to have been overtaken by CRC and became the third most common cancer [4]. Although its ASR is not the highest, China has the most gastric-cancer patients (∼478,000, 45% of new cases in 2020) all over the world [4]. Heterogeneities exist within European countries. Central and Eastern Europe have the second-highest incidence rate of gastric cancer in the world (ASR 11.3 per 100,000). On the contrary, the incidence is significantly lower in Northern and Western Europe (ASR 4.6–5.9 per 100,000), similar to that in the USA (ASR 4.2 per 100,000) [4]. The reasons for such differences are multiple and complex, and include genetic susceptibility, strains of H. pylori, hygiene, food preparation, and food preservation. Several migrant studies have shown that, after migration to low-incidence regions, the incidence for migrants was lowered for the first generation and was nearly similar to that for natives for the second generation, which suggests that environmental factors might be the main contributor to the development of gastric cancer [69]. Differently from CRC, improved living conditions associated with economic development have contributed to the reduction in incidence due to the clearance of H. pylori [11, 70]. In the USA and European countries, gastric cancer is expected to be a rare disease (defined as <6 per 100,000 person-years) by 2035, while the number of new cases will remain high and continue growing [71]. The incidence in China has also undergone a gradual decline in recent decades (Figure 2).
Figure 2.
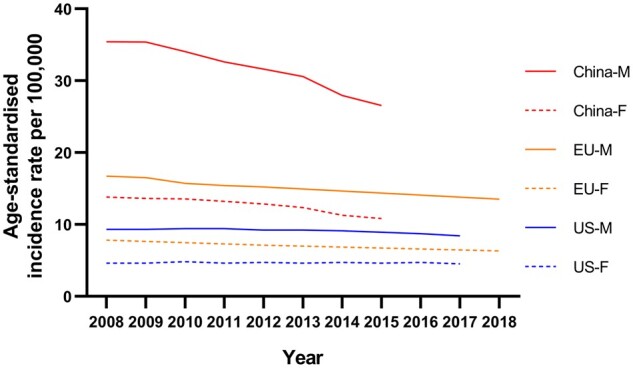
Time trends of incidence rates of gastric cancer in men and women across China, the USA, and Europe. Data from National Cancer Center (China), Centers for Disease Control and Prevention (the USA), and European Cancer Information System (EU).
Gastric cancer is more common in men than in women (about 2:1) and the most common age at diagnosis is ∼60 years [72]. Of note, incidence increases were seen in younger age groups (<50 years) in both low- and high-incidence populations, especially in low-incidence countries such as the UK and the USA—populations with a typically low prevalence of H. pylori infection [73], which may imply that changes in the prevalence of some lifestyle factors have contributed to the increases shown in more recent generations [74]. Mortality, due to the implementation of the screening program, has had a substantial reduction in recent years [75–77]. In China, gastric cancer has been the third most common cause of cancer deaths for decades but it has shown a remarkable decline in mortality. In 1990–1992, the ASR for the mortality of gastric cancer was 40.8 per 100,000 for males and 18.6 per 100,000 for females [78], while, in 2015, it was down to 18.6 for males and 7.53 for females [12], and is expected to continue to decline in the future. However, due to population growth and aging populations, the number of deaths is expected to continuously grow.
Risk factors
Helicobacter pylori infection is the most well-described risk factor for gastric cancer. Chronic infection of the gastric mucosa leads to stepwise progression from atrophic gastritis and intestinal metaplasia. Most H. pylori strains possess a cytotoxin-associated gene A (CagA) pathogenicity island—an oncoprotein that affects the expression of cellular signaling proteins [79]. Approximately 89% of non-cardia gastric cancers, representing ∼78% of all gastric cancers, can be attributable to H. pylori infection [80]. As estimated by a meta-analysis, there were ∼4.4 billion individuals with H. pylori infection worldwide in 2015 [70]. China had a prevalence estimate of 55.8% (95% CI: 51.8%–59.9%) and it was 35.6% (95% CI: 30.0%–41.1%) for the USA [70]. Consistently with the differences in the incidence rates in Europe, the prevalence estimates were highest in Eastern Europe (62.8%; 95% CI: 48.3%–77.2%) and lowest in Western Europe (34.3%; 95% CI: 31.3%–37.2%), and the prevalence estimates were 47.0% (95% CI: 41.8%–52.1%) for Europe as a whole [70]. In a study conducted in Japan, gastric cancer developed (over a mean follow-up of 7.8 years) in 2.9% of patients with peptic ulcer, dyspepsia, or gastric hyperplasia who had H. pylori infection, whereas no cases were detected in uninfected patients with these conditions [81]. In a Chinese cohort, the infection of H. pylori increased both cardia and non-cardia gastric-cancer risk compared to the non-infected population [82]. However, later studies have suggested that H. pylori infection is a risk factor only for non-cardia cancer and does not increase the risk of cardia cancer [83, 84]. As concluded by a meta-analysis, 5.9 is the best estimate of the relative risk of non-cardia cancer associated with H. pylori infection [84].
The Stomach Cancer Pooling Project, a consortium that included 23 epidemiological studies from Europe, North America, and Asia, found tobacco smoking was an important risk factor, no matter whether there was H. pylori infection or not [85]. Besides, the risk increased with the intensity and duration of smoking and decreased after smoking cessation. Other meta-analyses also concluded that smoking is the most important behavioral risk factor for gastric cancer, and increases the risk by ∼50% in males and 20% in females [86, 87]. Alcohol drinking is also recognized as a risk factor [88]. Drinking alcohol containing ethanol >10 g/day will increase the risk of gastric cancer and a dose–response meta-analysis showed a significant increase in risk in Asian males. Moreover, combined exposure to smoking and alcohol further increases the risk [87].
Foods preserved by salting, especially traditional Asian pickled foods, are considered to be associated with the development of gastric cancer. The consumption of foods preserved by salting increases the risk of gastric cancer. The WCRF/AICR found that people with a high intake of salt-preserved food had a 1.7-fold higher relative risk of gastric cancer compared to those with a low intake [88]. Another meta-analysis including 10 studies found that the population with the highest intake of salted vegetables had a 1.32 (95% CI: 1.10–1.58)-fold higher risk than those with the lowest intake [89]. Salted-food intake might increase the risk of H. pylori infection and could also act synergistically to promote the development of gastric cancer. Still, more research is needed to provide high-level evidence.
Obesity, which was not recognized as a risk factor for non-cardia gastric cancer, has a strong association with gastric cardia cancer [90]. As BMI increases by 5 kg/m2, the risk of gastric cardia cancer increases by 20%–30% [88, 91]. This finding corroborates the increasing obesity and numbers of gastric cardia cancers in the USA and Europe [2, 92, 93].
For other factors, such as low consumption of fruits and vegetables, results are mixed for both cardia and non-cardia gastric cancer [94–96]. For the consumption of processed meat, previous studies have shown that it was associated with an increased risk of gastric non-cardia cancer [97], but the results from newer cohort studies have shown that its effect was not significant [98, 99]. To reflect this contradiction, the WCRF/AICR have noted these factors as limited-suggestive [88].
Primary prevention and screening
As gastric cancer is an infection-associated malignancy, the main part of the primary prevention of gastric cancer is eradicating H. pylori. Since oral–oral and fecal–oral routes have been postulated to be involved in the transmission of H. pylori [80], the first possibility for preventing the associated stomach cancer consists of avoiding infection through personal hygiene, control of water supplies, food-quality control, and other measures [100]. For now, the best approach for the diagnosis of H. pylori infection is 13 C-UBT (urea breath test), with high sensitivity and specificity, and excellent performance [101]. Using regimens that contain two or three generic antibiotics plus a proton-pump inhibitor for 7–14 days can achieve ∼80% success in eliminating H. pylori infection. However, until now, there have been limited data from randomized trials on the effects of eradicating H. pylori. Earlier results from Japan have shown that, after the endoscopic resection of early gastric cancer, the eradication of H. pylori statistically significantly reduced the risk of metachronous gastric cancer [102]. Another randomized trial in China found a statistically significant 39% reduction in gastric-cancer risk after H. pylori eradication [95]. A study in South Korea found that eradicating H. pylori after the endoscopic resection of gastric tumors lowered the incidence of metachronous gastric carcinoma, although this result was not statistically significant [103]. It can be concluded that H. pylori treatment could have lowered the gastric-cancer incidence by 30%–40% [104], but the available data do not permit precise estimation of the overall benefits and possible adverse consequences, such as increased esophagitis [105].
Screening for gastric cancer in the population includes two aspects: screening for precancerous lesions—upper-gastrointestinal series (UGI), serum pepsinogen testing (PG), and endoscopy; and screening for H. pylori–H. pylori serology [106].
The UGI was a standard method for diagnosing gastric diseases, but has a relatively low sensitivity of ∼38% [107]. Studies conducted in Japan showed that screening by UGI series resulted in an ∼40% reduction in gastric-cancer mortality [106], so UGI is still recommended in the national gastric-cancer screening programs in Japan and Korea. PG testing is receiving wide recognition in Japan and China owing to its convenience, freedom from discomfort or risk, efficiency, and economy. PG I ≤ 70 ng/L and PG I/II ratio ≤ 3.0 are associated with an increased risk of gastric cancer [108]. In a meta-analysis assessing ∼300,000 people, the sensitivity and specificity of PG testing for gastric-cancer screening were 77% and 73%, respectively [109]. Endoscopy, which is the criterion for the diagnosis of gastric cancer, is the only method for direct visual examination of the gastric mucosa and it allows biopsy sampling so that histologic evaluation can be performed. Endoscopy is the primary method for gastric-cancer screening in Japan and South Korea, and is also highly recommended in China [110]. Endoscopy was reported with a sensitivity of 88%–95% and specificity of 85%–88% [106, 111]. A nested case–control study from Korea reported a 47% reduction in mortality from gastric cancer by using endoscopic screening [75].
Helicobacter pylori serology is used to detect the antibody of H. pylori, and the presence of H. pylori antibody is associated with the presence of gastric cancer or precancerous lesions [112]. Serology is cheap compared to other non-invasive test approaches; hence, serology was felt to be the best current option in the Asia–Pacific region for a population-based screening approach [113]. However, due to its low sensitivity and specificity, it is recommended to be combined with other screening approaches.
Right now, only Japan and South Korea have a comprehensive screening system for gastric cancer. To reflect recent studies and epidemiology reports, in 2015, the Japanese gastric screening guideline as adjusted the starting age of screening to 50 years and the screening interval to 2–3 years [106]. In Korea, people >40 years old receive either a UGI or endoscopy test for gastric-cancer screening every 2 years [114]. Chinese experts’ consensus on gastric-cancer screening recommends that people >40 years old and with at least one risk factor (with H. pylori infection, in a high-incidence-rate region, with a family history, with precancerous diseases, with other lifestyle risk factors) to start the serological biopsy for five biomarkers (PGI, PGII, PGI/PGII ratio, H. pylori antibody, and gastrin-17) as the first step for screening and stratifying [112]. By using risk stratification, patients with different risks underwent different screening projects based on endoscopy.
Esophageal cancer
Esophageal cancer ranks eighth in terms of incidence (604,100 new cases, 3.1%) and sixth in mortality (544,076 new deaths, 5.5%) overall [4]. The two histological types of esophageal cancer—esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC)—differ in populations and have completely distinct biological characteristics, geographical distributions, risk factors, and temporal trends [115]. Patients with either cancer are both diagnosed at an advanced stage due to the late occurrence of symptoms. Thus, the prognosis is usually poor, at ∼5%–34% [116]. Therefore, identifying its distributional characteristic and risk factors and promoting practical and accurate prevention and screening methods are crucial to reducing the global burden of esophageal cancer.
Epidemiology characteristics
The highest incidence rate of esophageal cancer can be found in Eastern Asia, with an ASR of 12.3 per 100,000 in 2020 [4]. However, esophageal cancers, both ESCC and EAC, are not a common cancer type in Europe and America (Table 1). Furthermore, the predominant histological type also varies from East to West [115].
ESCC comprises ∼90% of all cases [117]. ESCC has significant geographical-distribution differences. China has about half of all esophageal-cancer cases around the world, among which most cases are ESCC. In 2015, esophageal cancer was the sixth most common cancer in China, with ∼246,000 new cases (ASR 11.3 per 100,000), and it was estimated to have reached 13.8 per 100,000 in 2020 [4, 12]. The incidence rates for esophageal cancer in different regions can vary by ∼10-fold within China. Most of the ESCC cases are in the North Central region, especially the area around the Taihang Mountain, where the ASR reaches 80–110 per 100,000 in males and 40 per 100,000 in females [118], and Cixian, which is the most studied area for esophageal cancer in China, with the highest ASR in both males and females in the world (192.7 per 100,000 in males; 108.5 per 100,000 in females) [119]. ESCC has a relatively obvious sex difference. The incidence rate in men is three times that of women, which could be partly attributed to the unbalanced distribution of risk factors—the use of tobacco and alcohol between men and women. Because of the bad prognosis, mortality is relatively high. In 2015, esophageal cancer was the fourth most common cause of cancer death in China (188,000 deaths, ASR 8.36 per 100,000) [12]. Due to the implementation of screening, the control of risk factors in a large population, and advances in the clinical management of ESCC, the incidence and mortality of ESCC have been reduced in recent decades in China [3, 11, 120, 121] (Figure 3) and are predicted to continue decreasing in the coming years [122].
Figure 3.
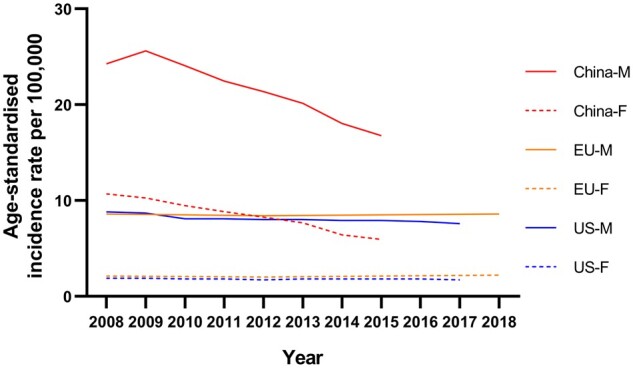
Time trends of incidence rates of esophageal cancer in men and women across China, the USA, and Europe. Data from National Cancer Center (China), Centers for Disease Control and Prevention (the USA), and European Cancer Information System (EU).
In 2012, an estimated 52,000 individuals (41,000 men and 11,000 women) developed EAC worldwide, resulting in a global incidence rate of 0.7 per 100,000 person-years (1.1 in men and 0.3 in women) [115]. Differently from ESCC, 53% of patients were from Europe, Northern America, or Oceania. The incidence of EAC has increased in many Western countries in recent decades [123]. Moreover, in some countries, including the UK and the USA, EAC has surpassed ESCC and become the predominant histological type of esophageal cancer [124]. It is expected to rise dramatically across high-income countries and will displace ESCC in more countries, such as Italy, Spain, and France, in the coming years [122]. Similar to ESCC, EAC also shows a striking male predominance in incidence. The highest sex difference can be found in the USA, as the males/females incidence ratio is 9:1 [115, 125].
Risk factors
ESCC and EAC have different etiological risk factors [116], which also reflect their difference in pathogenesis [126]. The pathophysiological pathway of ESCC is typically initiated by carcinogenic compounds in direct contact with the esophageal mucosa and thereby leads to esophageal squamous dysplasia. It is now widely recognized that smoking and alcohol overconsumption are both risk factors for ESCC [127], and the risk is higher when they are in combination [128]. Ever smokers had significantly higher risks of ESCC, with an OR of 2.8 as reported by a population-based case–control study [129]. An intensity-duration cumulative exposure effect was demonstrated by a study that, for equal pack-years, the mild intensity of smoking for a long time has a higher risk than stronger intensity for a shorter time [130]. Dose–response meta-analyses including six studies found a relative risk of 1.25 (95% CI: 1.12–1.41) per 10 g/day ethanol intake [127]. Another pooled analysis found that, when compared with no drinks, at least seven drinks per day had a relative risk of 9.62 (95% CI: 4.26–21.71) [131].
There are some other factors reported to be associated with ESCC, but at a relatively lower level of evidence, including the consumption of vegetables and fruits and processed meat. Most studies concluded that a higher intake of fruits and vegetables probably decreases the risk of ESCC [132]. One reported that 100 g/day of vegetable intake could reduce the risk of ESCC by 16%, but no significance was found between the highest vs lowest intakes [133]. This meta-analysis also reported that a 100-g/day consumption of fruits could reduce the risk by 39%, but with high heterogeneity (I2 = 90%). Regarding processed meat, which is often listed as a risk factor for GI cancers, studies found a 41% increase in risk in the highest-consumption population [134], but another meta-analysis found no significance [135]. Thus, the WCRF/AICR marked these factors as having limited evidence. Other factors, such as high-temperature drinks [136], HPV infection [137], and BMI [138], were reported to be related to ESCC, but the literature did not draw a consistent conclusion.
Differently from ESCC, alcohol was found not to be associated with EAC [131, 139], and the effect of smoking is also weaker than that in ESCC [127, 129], although it is still a strong risk factor for EAC [140, 141]. Furthermore, obesity and gastroesophageal reflux disease (GERD) are the distinct risk factors for EAC. Increasing BMI has been consistently associated with increased risk of EAC in a seemingly linear exposure–response pattern, with a relative risk of 2.7 (95% CI: 2.2–3.5) for patients with a BMI ≥ 30 kg/m2 [142, 143]. Similarly to CRC, predominantly central and intra-abdominal adiposity has a bigger influence than BMI alone [90, 144]. Obesity may explain partially the increase in EAC incidence in Western countries, especially in white people. Moreover, adiposity causes increased intra-abdominal pressure and facilitates reflux [145], which has been proven to be another important risk factor. GERD is a strong and dose-dependent risk factor for EAC, as confirmed in population-based studies [142, 146]. Results from a meta-analysis have shown that daily symptoms of reflux increased the odds of EAC by >7-fold [147]. Continuous GERD leads to the development of Barrett’s esophagus, which presents as metaplasia of the distal esophageal mucosa and is characterized as the precursor to EAC [148]. According to a population-based cohort study in Norway, weekly symptoms of GERD increased by ≥47% during 1995–2006 [149], which corresponded to the rapid increase in EAC incidence during this decade [115]. Interestingly, in contrast to its effects on gastric cancer, H. pylori infection may reduce the risk of EAC [150]. This phenomenon may be due to the gastric atrophy induced by H. pylori infection, which contributes to lesser gastric acid reflux and thereby lowers the risk [151]. Since the infection rate is continuously decreasing in Western countries, the effects of H. pylori on EAC and the individual eradication strategies need more thorough studies to confirm [152].
Prevention and screening
When the symptoms of both types of esophageal cancer start to surface, the cancer is often at an advanced stage and has a very poor prognosis, emphasizing the significance of prevention and early detection to lower the burden of esophageal cancer.
The proton-pump inhibitors (PPIs) [153, 154] and NSAID/aspirin [155] have shown protective effects for esophageal cancer. The PPIs were reported to decrease the risk of dysplasia and adenocarcinoma in patients with Barrett’s esophagus [156]. However, as the results were concluded from the observation of patients with GERD and Barrett’s esophagus after anti-reflux surgery, no reduction in the risk of EAC was found [157]. Thus, the PPIs are still not recommended conventionally for cancer prevention. The NSAID/aspirin was shown to reduce the risk of ESCC and EAC by 30%–40% [158]. However, results from a randomized trial showed that celecoxib did not affect the progression of both esophageal squamous dysplasia and Barrett's dysplasia [159, 160]. Given the additional preventive benefits of the use of aspirin for other cancer types and cardiovascular disease, these drugs may be good candidates for chemoprevention in groups at high risk [161].
Detection of esophageal cancer at an earlier, potentially curable stage is crucial to improving patient survival. Now, endoscopy is widely accepted as the best method for esophageal screening and diagnosis. Endoscopic screening for precursor lesions and endoscopic resection or ablation of the dysplastic lesions have been shown to reduce the risk of developing ESCC and dying from the disease [121]. For EAC, the current British Society of Gastroenterology and American College of Gastroenterology guidelines suggest screening in patients who have a history of GERD lasting >5 years and have multiple other risk factors, including male sex, Caucasian race, central obesity, and current or past history of smoking [162, 163]. For ESCC, there is currently no guideline for screening, but Chinese experts drafted a consensus on esophageal-cancer screening in 2014 [164]. The consensus recommends that people >40 years old and with at least one risk factor (from an esophageal-cancer-prevalent region, with upper-GI symptoms, with an esophageal familial history, with precursor diseases of esophageal cancer, with other high-risk factors) should undergo endoscopic screening. The following screening or treatment plans are decided based on the results of endoscopy and biopsy.
However, endoscopy is invasive and expensive, and, despite the rapid increase in incidence in recent decades, the low absolute numbers of esophageal-cancer cases in the West remain a barrier to the implementation of screening programs. Some other non-endoscopic screening methods have emerged in recent years and reported their preliminary results. For ESCC, the capsule-sponge methodology had a sensitivity and specificity of 100% and 97%, without extra safety problems and unsatisfied experience [165]. By using a minimally invasive cell-sampling device and immunohistochemical staining for Trefoil Factor 3, the sensitivity and specificity reached 87.2% and 92.4% in diagnosing patients with ≥3 cm of circumferential Barrett’s esophagus [166].
Discussion and conclusion
As concluded in Table 2, an unhealthy lifestyle, including overuse of alcohol and smoking, is the main risk factor for all GI cancers and, because of their direct contact with food, the dietary pattern is also highly associated with all GI cancers. Thus, primary prevention of GI cancer is the most efficient and cost-beneficial means of reducing the cancer burden. A healthy lifestyle can lower the risk of all GI cancers.
Table 2.
Characteristics of each gastrointestinal (GI) cancer
| GI cancers | Precancerous lesions | Environmental factors of strong level of evidence | Environmental factors of moderate level of evidence | Environmental factors of limited level of evidence | Primary prevention | Screening methods | Guidelines and screening programs |
|---|---|---|---|---|---|---|---|
| Colorectal cancer | Adenomas and serrated polyps |
Obesity (unfavor) Processed meat (unfavor) Alcohol (unfavor) Smoking (unfavor) Physical activity (favor) |
Read meat (unfavor) Dietary fiber (favor) Wholegrains (favor) Calcium intake (favor) |
Vegetable and fruits (favor) Vitamin C (favor) Vitamin D (favor) |
Risk-factor controls Aspirin |
FOBT/FIT DNA-FIT Colonoscopy |
USA, UK China Japan Germany Australia etc. |
| Gastric cancer | Atrophic gastritis and intestinal metaplasia |
H. pylori infection (unfavor) Smoking (unfavor) |
Alcohol (unfavor) Obesity (cardia) (unfavor) Salt-preserved food (unfavor) |
Processed meat (non-cardia) (unfavor) Fruits (favor) Fiber (favor), Vegetables (favor) |
Risk-factor controls Eradication of H. pylori NSAIDs/aspirin |
Upper-GI series Pepsinogen, H. pylori serology Endoscopy |
Japan South Korea |
| Esophageal squamous cell carcinoma | Esophageal squamous dysplasia |
Alcohol (unfavor) Smoking (unfavor) |
– |
Processed meat (unfavor) Vegetables and fruits (favor) Physical activity (favor) HPV infection (unfavor) |
Risk-factor controls NSAIDs/aspirin |
Endoscopy | – |
| Esophageal adenocarcinoma | Barrett’s esophagus |
Obesity (unfavor) GERD (unfavor) Smoking (unfavor) |
– |
Vegetables (favor) Physical activity (favor) Fiber (favor) |
Risk-factor controls PPIs NSAIDs/aspirin |
Endoscopy |
USA UK |
FOBT, fecal occult blood test; FIT, fecal immunochemical test; NSAIDs, non-steroidal anti-inflammatory drugs; GERD, gastroesophageal reflux disease; PPIs, proton-pump inhibitors.
These GI cancers are also similar in that they all have identified precursor diseases: adenomas and serrated polyps for CRC, atrophic gastritis and intestinal metaplasia caused by H. pylori infection for gastric cancer, and esophageal squamous dysplasia/Barrett’s esophagus for esophageal cancer. Thus, GI cancers can be diagnosed in a precancerous state and early treatment can reduce both incidence and mortality. However, there are only guidelines and screening programs for CRC and gastric cancer because of the geographical-distribution differences of cancers and the lack of cheap and relatively reliable approaches like FIT in CRC. Thus, future orientation will be the development of screening methods with both reliability and practicality, and the ability to recognize specific patients at high risk of GI cancer to perform personalized screening.
GI cancers are a major medical and economic burden worldwide. The last century has witnessed that, regardless of the level of economics, medical conditions, or public health, there is always a prevalent GI cancer that changes accordingly. Esophageal and gastric cancers are most common in developing countries, while CRC is the predominant GI malignancy in developed countries. In this article, we have reviewed the epidemiology, risk factors, prevention, and screening of these three cancers in China, the USA, and Europe. They share some common features, but also have distinct characteristics that imply the differences in population susceptibility and pathways to development malignancies. With the establishment of screening guidelines and the implantation of screening programs in more and more countries, the incidence and mortality rate of GI cancers are expected to decline in the future. However, due to the continuously increasing exposure to some risk factors such as obesity and the aging of the population, GI cancers will still be a great global health burden for a long time.
Authors’ Contributions
Y.M.X. drafted the manuscript; L.S. collected and analysed the data; X.S.H. conceived of the project and revised the manuscript; Y.X.L. revised the manuscript and worked on the final approval of the version to be published.
Funding
This work was supported by Sun Yat-Sen University Clinical Research 5010 Program [No. 2018026, YL] and the ‘Five Five’ Constructive Talent Project of the Sixth Affiliated Hospital of Sun Yat-Sen University [No. P20150227202010244, JW; No. P20150227202010251, YL].
Acknowledgements
None.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.WHO. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. Geneva: World Health Organization. https://www.who.int/healthinfo/global_burden_disease/en (25 January 2021, date last accessed). [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4. Ferlay J, Ervik M, Lam F. et al. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. https://gco.iarc.fr/today (30 January 2021, date last accessed). [Google Scholar]
- 5. Cao B, Bray F, Beltrán-Sánchez H. et al. Benchmarking life expectancy and cancer mortality: global comparison with cardiovascular disease 1981-2010. BMJ 2017;357:j2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bray F, Jemal A, Grey N. et al. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol 2012;13:790–801. [DOI] [PubMed] [Google Scholar]
- 7. Keum N, Giovannucci E.. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 2019;16:713–32. [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund International/American Institute for Cancer Research. Continuous update project report 2018: diet, nutrition, physical activity, and colorectal cancer. https://www.wcrf.org/dietandcancer/colorectal-cancer (18 January 2021, date last accessed).
- 9.U.S. Cancer Statistics Working Group. United States Cancer Statistics, Data Visualizations Tool, based on 2019 submission data (1999–2017). https://gis.cdc.gov/Cancer/USCS/DataViz. Released (15 June 2021, date last accessed).
- 10.European Cancer Information System. https://ecis.jrc.ec.europa.eu (18 January 2021, date last accessed).
- 11. Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- 12. Sun KX, Zheng RS, Zhang SW. et al. Report of cancer incidence and mortality in different areas of China. China Cancer 2015. 2019;28:1–11. [Google Scholar]
- 13. Arnold M, Sierra MS, Laversanne M. et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–91. [DOI] [PubMed] [Google Scholar]
- 14. Chen WQ, Zheng RS, Zhang SW. et al. Report of incidence and mortality in china cancer registries, 2008. Chin J Cancer Res 2012;24:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levin B, Lieberman DA, McFarland B, et al. ; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58:130–60. [DOI] [PubMed] [Google Scholar]
- 16. Ebell MH, Thai TN, Royalty KJ.. Cancer screening recommendations: an international comparison of high income countries. Public Health Rev 2018;39:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolf A, Fontham E, Church TR. et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–81. [DOI] [PubMed] [Google Scholar]
- 18. Silla IO, Rueda D, Rodríguez Y. et al. Early-onset colorectal cancer: a separate subset of colorectal cancer. World J Gastroenterol 2014;20:17288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siegel RL, Fedewa SA, Anderson WF. et al. Colorectal cancer incidence patterns in the United States. J Natl Cancer Inst 2017;109:1974–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graff RE, Möller S, Passarelli MN. et al. Familial risk and heritability of colorectal cancer in the Nordic Twin Study of Cancer. Clin Gastroenterol Hepatol 2017;15:1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mousavi SM, Fallah M, Sundquist K. et al. Age- and time-dependent changes in cancer incidence among immigrants to Sweden: colorectal, lung, breast and prostate cancers. Int J Cancer 2012;131:E122–8. [DOI] [PubMed] [Google Scholar]
- 22. Wilsnack RW, Wilsnack SC, Kristjanson AF. et al. Gender and alcohol consumption: patterns from the multinational GENACIS project. Addiction 2009;104:1487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imamura F, Micha R, Khatibzadeh S. et al. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Glob Health 2015;3:e132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Report on the Global Tobacco Epidemic, 2019. Geneva: World Health Organization, 2019. License: CC BY-NC-SA 3.0 IGO.
- 25. Murphy N, Strickler HD, Stanczyk FZ. et al. A prospective evaluation of endogenous sex hormone levels and colorectal cancer risk in postmenopausal women. JNCI J 2015;107:djv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aleksandrova K, Pischon T, Jenab M. et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med 2014;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Erdrich J, Zhang X, Giovannucci E. et al. Proportion of colon cancer attributable to lifestyle in a cohort of US women. Cancer Causes Control 2015;26:1271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liang PS, Chen TY, Giovannucci E.. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 2009;124:2406–15. [DOI] [PubMed] [Google Scholar]
- 29. Jasperson KW, Tuohy TM, Neklason DW. et al. Hereditary and familial colon cancer. Gastroenterology 2010;138:2044–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boland PM, Yurgelun MB, Boland CR.. Recent progress in Lynch syndrome and other familial colorectal cancer syndromes. CA Cancer J Clin 2018;68:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel SG, Ahnen DJ.. Familial colon cancer syndromes: an update of a rapidly evolving field. Curr Gastroenterol Rep 2012;14:428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Conteduca V, Sansonno D, Russi S. et al. Precancerous colorectal lesions [Review]. Int J Oncol 2013;43:973–84. [DOI] [PubMed] [Google Scholar]
- 33. Nagtegaal ID, Odze RD, Klimstra D. et al. ; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive tract. Histopathology 2020;76:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Erichsen R, Baron JA, Hamilton-Dutoit SJ. et al. Increased risk of colorectal cancer development among patients with serrated polyps. Gastroenterology 2016;150:895–902.e5. [DOI] [PubMed] [Google Scholar]
- 35. Christ A, Lauterbach M, Latz E.. Western diet and the immune system: an inflammatory connection. Immunity 2019;51:794–811. [DOI] [PubMed] [Google Scholar]
- 36. Dong Y, Zhou J, Zhu Y. et al. Abdominal obesity and colorectal cancer risk: systematic review and meta-analysis of prospective studies. Biosci Rep 2017;37:BSR20170945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ning Y, Wang L, Giovannucci EL.. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev 2010;11:19–30. [DOI] [PubMed] [Google Scholar]
- 38. Song M, Hu FB, Spiegelman D. et al. Long-term status and change of body fat distribution, and risk of colorectal cancer: a prospective cohort study. Int J Epidemiol 2016;45:871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Calle EE, Kaaks R.. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4:579–91. [DOI] [PubMed] [Google Scholar]
- 40. Keum N, Lee DH, Kim R. et al. Visceral adiposity and colorectal adenomas: dose-response meta-analysis of observational studies. Ann Oncol 2015;26:1101–9. [DOI] [PubMed] [Google Scholar]
- 41. Lim U, Ernst T, Buchthal SD. et al. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr & Diabetes 2011;1:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rezende L, Sá TH, Markozannes G. et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med 2018;52:826–33. [DOI] [PubMed] [Google Scholar]
- 43. Giovannucci E. An integrative approach for deciphering the causal associations of physical activity and cancer risk: the role of adiposity. J Natl Cancer Inst 2018;110:935–41. [DOI] [PubMed] [Google Scholar]
- 44. Ruiz-Casado A, Martín-Ruiz A, Pérez LM. et al. Exercise and the hallmarks of cancer. Trends Cancer 2017;3:423–41. [DOI] [PubMed] [Google Scholar]
- 45. Ezzati M, Riboli E.. Behavioral and dietary risk factors for noncommunicable diseases. N Engl J Med 2013;369:954–64. [DOI] [PubMed] [Google Scholar]
- 46. Song M, Garrett WS, Chan AT.. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015;148:1244–60.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dahm CC, Keogh RH, Spencer EA. et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst 2010;102:614–26. [DOI] [PubMed] [Google Scholar]
- 48. Alberts DS, Martínez ME, Roe DJ. et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas: Phoenix Colon Cancer Prevention Physicians' Network. N Engl J Med 2000;342:1156–62. [DOI] [PubMed] [Google Scholar]
- 49. Keum N, Aune D, Greenwood DC. et al. Calcium intake and colorectal cancer risk: dose-response meta-analysis of prospective observational studies. Int J Cancer 2014;135:1940–8. [DOI] [PubMed] [Google Scholar]
- 50. Wactawski-Wende J, Kotchen JM, Anderson GL. et al. ; Women's Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684–96. [DOI] [PubMed] [Google Scholar]
- 51. Lappe J, Watson P, Travers-Gustafson D. et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA 2017;317:1234–43. [DOI] [PubMed] [Google Scholar]
- 52. Fedirko V, Tramacere I, Bagnardi V. et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol 2011;22:1958–72. [DOI] [PubMed] [Google Scholar]
- 53. Botteri E, Iodice S, Bagnardi V. et al. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765–78. [DOI] [PubMed] [Google Scholar]
- 54. Carr PR, Alwers E, Bienert S. et al. Lifestyle factors and risk of sporadic colorectal cancer by microsatellite instability status: a systematic review and meta-analyses. Ann Oncol 2018;29:825–34. [DOI] [PubMed] [Google Scholar]
- 55. Limsui D, Vierkant RA, Tillmans LS. et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst 2010;102:1012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu XR, Zheng XB, Huang Y. et al. Risk factors for colorectal neoplasia in patients with underlying inflammatory bowel disease: a multicenter study. Gastroenterol Rep (Oxf) 2019;7:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jess T, Simonsen J, Jørgensen KT. et al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology 2012;143:375–81.e1. [DOI] [PubMed] [Google Scholar]
- 58. Cole BF, Logan RF, Halabi S. et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst 2009;101:256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rothwell PM, Wilson M, Elwin CE. et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741–50. [DOI] [PubMed] [Google Scholar]
- 60. Cao Y, Nishihara R, Wu K. et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol 2016;2:762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carethers JM, Jung BH.. Genetics and genetic biomarkers in sporadic colorectal cancer. Gastroenterology 2015;149:1177–90.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kuipers EJ, Rösch T, Bretthauer M.. Colorectal cancer screening--optimizing current strategies and new directions. Nat Rev Clin Oncol 2013;10:130–42. [DOI] [PubMed] [Google Scholar]
- 63.National Cancer Center (China), Expert Group of the Development of China Guideline for the Screening, Early Detection and Early Treatment of Colorectal Cancer. [ China guideline for the screening, early detection and early treatment of colorectal cancer (2020, Beijing)]. Chin J Oncol 2021;43:16–38. [DOI] [PubMed] [Google Scholar]
- 64. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 65. Imperiale TF, Ransohoff DF, Itzkowitz SH. et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014;370:1287–97. [DOI] [PubMed] [Google Scholar]
- 66. Zauber AG, Winawer SJ, O'Brien MJ. et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brenner H, Stock C, Hoffmeister M.. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Heisser T, Peng L, Weigl K. et al. Outcomes at follow-up of negative colonoscopy in average risk population: systematic review and meta-analysis. BMJ 2019;367:l6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kolonel LN, Hankin JH, Nomura AM.. Multiethnic studies of diet, nutrition, and cancer in Hawaii. Princess Takamatsu Symp 1985;16:29–40. [PubMed] [Google Scholar]
- 70. Hooi J, Lai WY, Ng WK. et al. Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017;153:420–9. [DOI] [PubMed] [Google Scholar]
- 71. Arnold M, Park JY, Camargo MC. et al. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020;69:823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ajani JA, Lee J, Sano T. et al. Gastric adenocarcinoma. Nat Rev Dis Primers 2017;3:17036. [DOI] [PubMed] [Google Scholar]
- 73. Anderson WF, Rabkin CS, Turner N. et al. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J Natl Cancer Inst 2018;110:608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Camargo MC, Anderson WF, King JB. et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut 2011;60:1644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jun JK, Choi KS, Lee HY. et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017;152:1319–28.e7. [DOI] [PubMed] [Google Scholar]
- 76. Kim H, Hwang Y, Sung H. et al. Effectiveness of gastric cancer screening on gastric cancer incidence and mortality in a community-based prospective cohort. Cancer Res Treat 2018;50:582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang X, Li M, Chen S. et al. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology 2018;155:347–54.e9. [DOI] [PubMed] [Google Scholar]
- 78. Sun X, Mu R, Zhou Y. et al. [1990–1992 mortality of stomach cancer in China]. Chin J Oncol 2002;24:4–8. [PubMed] [Google Scholar]
- 79. Chen SY, Zhang RG, Duan GC.. Pathogenic mechanisms of the oncoprotein CagA in H. pylori-induced gastric cancer [Review]. Oncol Rep 2016;36:3087–94. [DOI] [PubMed] [Google Scholar]
- 80.IARC Helicobacter pylori Working Group (2014). Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. Lyon: International Agency for Research on Cancer (IARC Working Group Reports, No. 8). http://www.iarc.fr/en/publications/pdfsonline/wrk/wrk8/index.php (18 January 2021, date last accessed).
- 81. Uemura N, Okamoto S, Yamamoto S. et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784–9. [DOI] [PubMed] [Google Scholar]
- 82. Limburg P, Qiao Y, Mark S. et al. Helicobacter pylori seropositivity and subsite-specific gastric cancer risks in Linxian, China. J Natl Cancer Inst 2001;93:226–33. [DOI] [PubMed] [Google Scholar]
- 83. Kamangar F, Dawsey SM, Blaser MJ. et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst 2006;98:1445–52. [DOI] [PubMed] [Google Scholar]
- 84.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001;49:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Praud D, Rota M, Pelucchi C. et al. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur J Cancer Prev 2018;27:124–33. [DOI] [PubMed] [Google Scholar]
- 86. Ladeiras-Lopes R, Pereira AK, Nogueira A. et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control 2008;19:689–701. [DOI] [PubMed] [Google Scholar]
- 87. Sjödahl K, Lu Y, Nilsen TI. et al. Smoking and alcohol drinking in relation to risk of gastric cancer: a population-based, prospective cohort study. Int J Cancer 2007;120:128–32. [DOI] [PubMed] [Google Scholar]
- 88.World Cancer Research Fund International/American Institute for Cancer Research. Continuous update project report 2018: diet, nutrition, physical activity, and stomach cancer. http://www.wcrf.org/dietandcancer/stomach-cancer (18 January 2021, date last accessed).
- 89. Ren JS, Kamangar F, Forman D. et al. Pickled food and risk of gastric cancer—a systematic review and meta-analysis of English and Chinese literature. Cancer Epidemiol Biomarkers Prev 2012;21:905–15. [DOI] [PubMed] [Google Scholar]
- 90. O'Doherty MG, Freedman ND, Hollenbeck AR. et al. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut 2012;61:1261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen Y, Liu L, Wang X. et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev 2013;22:1395–408. [DOI] [PubMed] [Google Scholar]
- 92. Jemal A, Center MM, DeSantis C. et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893–907. [DOI] [PubMed] [Google Scholar]
- 93. Finucane MM, Stevens GA, Cowan MJ. et al. ; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhou Y, Zhuang W, Hu W. et al. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011;141:80–9. [DOI] [PubMed] [Google Scholar]
- 95. Ma JL, Zhang L, Brown LM. et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012;104:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kamangar F, Karimi P.. The state of nutritional epidemiology: why we are still unsure of what we should eat. Arch Iran Med 2013;16:483–6. [PubMed] [Google Scholar]
- 97. González CA, Jakszyn P, Pera G. et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst 2006;98:345–54. [DOI] [PubMed] [Google Scholar]
- 98. Keszei AP, Schouten LJ, Goldbohm RA. et al. Red and processed meat consumption and the risk of esophageal and gastric cancer subtypes in The Netherlands Cohort Study. Ann Oncol 2012;23:2319–26. [DOI] [PubMed] [Google Scholar]
- 99. Cross AJ, Freedman ND, Ren J. et al. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol 2011;106:432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. De Flora S, Bonanni P.. The prevention of infection-associated cancers. Carcinogenesis 2011;32:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Malfertheiner P, Megraud F, O'Morain CA. et al. ; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- 102. Fukase K, Kato M, Kikuchi S. et al. ; Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008;372:392–7. [DOI] [PubMed] [Google Scholar]
- 103. Choi J, Kim SG, Yoon H. et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol 2014;12:793–800.e1. [DOI] [PubMed] [Google Scholar]
- 104. Ford AC, Forman D, Hunt RH. et al. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 2014;348:g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lee YC, Chen TH, Chiu HM. et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 2013;62:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hamashima C; Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol 2018;48:673–83. [DOI] [PubMed] [Google Scholar]
- 107. Choi KS, Jun JK, Park EC. et al. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS One 2012;7:e50041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Watabe H, Mitsushima T, Yamaji Y. et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut 2005;54:764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dinis-Ribeiro M, da Costa-Pereira A, Lopes C. et al. Validity of serum pepsinogen I/II ratio for the diagnosis of gastric epithelial dysplasia and intestinal metaplasia during the follow-up of patients at risk for intestinal-type gastric adenocarcinoma. Neoplasia 2004;6:449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cho E, Kang MH, Choi KS. et al. Cost-effectiveness outcomes of the national gastric cancer screening program in South Korea. Asian Pac J Cancer Prev 2013;14:2533–40. [DOI] [PubMed] [Google Scholar]
- 111. Hamashima C, Okamoto M, Shabana M. et al. Sensitivity of endoscopic screening for gastric cancer by the incidence method. Int J Cancer 2013;133:653–9. [DOI] [PubMed] [Google Scholar]
- 112. Tu H, Sun L, Dong X. et al. A serological biopsy using five stomach-specific circulating biomarkers for gastric cancer risk assessment: a multi-phase study. Am J Gastroenterol 2017;112:704–15. [DOI] [PubMed] [Google Scholar]
- 113. Talley NJ, Fock KM, Moayyedi P.. Gastric Cancer Consensus conference recommends Helicobacter pylori screening and treatment in asymptomatic persons from high-risk populations to prevent gastric cancer. Am J Gastroenterol 2008;103:510–4. [DOI] [PubMed] [Google Scholar]
- 114. Choi KS, Kwak MS, Lee HY. et al. Screening for gastric cancer in Korea: population-based preferences for endoscopy versus upper gastrointestinal series. Cancer Epidemiol Biomarkers Prev 2009;18:1390–8. [DOI] [PubMed] [Google Scholar]
- 115. Arnold M, Soerjomataram I, Ferlay J. et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381–7. [DOI] [PubMed] [Google Scholar]
- 116. Rustgi AK, El-Serag HB.. Esophageal carcinoma. N Engl J Med 2014;371:2499–509. [DOI] [PubMed] [Google Scholar]
- 117. Abnet CC, Arnold M, Wei WQ.. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 2018;154:360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cao XQ, Sun XB.. Incidence and trend of esophageal cancer. Chin J Clin Oncol 2016;43:932–6. [Google Scholar]
- 119. Murphy G, McCormack V, Abedi-Ardekani B. et al. International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann Oncol 2017;28:2086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Steevens J, Botterweck AA, Dirx MJ. et al. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur J Gastroenterol Hepatol 2010;22:669–78. [DOI] [PubMed] [Google Scholar]
- 121. Wei WQ, Chen ZF, He YT. et al. Long-term follow-up of a community assignment, one-time endoscopic screening study of esophageal cancer in China. JCO 2015;33:1951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Arnold M, Laversanne M, Brown LM. et al. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am J Gastroenterol 2017;112:1247–55. [DOI] [PubMed] [Google Scholar]
- 123. Lagergren J, Lagergren P.. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin 2013;63:232–48. [DOI] [PubMed] [Google Scholar]
- 124. Thrift AP, Whiteman DC.. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol 2012;23:3155–62. [DOI] [PubMed] [Google Scholar]
- 125. Xie SH, Lagergren J.. The male predominance in esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2016;14:338–47.e1. [DOI] [PubMed] [Google Scholar]
- 126. Reichenbach ZW, Murray MG, Saxena R. et al. Clinical and translational advances in esophageal squamous cell carcinoma. Adv Cancer Res 2019;144:95–135. [DOI] [PubMed] [Google Scholar]
- 127.World Cancer Research Fund International/American Institute for Cancer Research. Continuous update project report 2018: diet, nutrition, physical activity, and oesophageal cancer. https://www.wcrf.org/dietandcancer/esophageal-cancer (18 January 2021, date last accessed).
- 128. Prabhu A, Obi KO, Rubenstein JH.. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis. Am J Gastroenterol 2014;109:822–7. [DOI] [PubMed] [Google Scholar]
- 129. Pandeya N, Williams GM, Sadhegi S. et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008;168:105–14. [DOI] [PubMed] [Google Scholar]
- 130. Lubin JH, Cook MB, Pandeya N. et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett's Esophagus and Esophageal Adenocarcinoma Consortium. Cancer Epidemiol 2012;36:306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Freedman ND, Murray LJ, Kamangar F. et al. Alcohol intake and risk of oesophageal adenocarcinoma: a pooled analysis from the BEACON Consortium. Gut 2011;60:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jeurnink SM, Büchner FL, Bueno-de-Mesquita HB. et al. Variety in vegetable and fruit consumption and the risk of gastric and esophageal cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2012;131:E963–73. [DOI] [PubMed] [Google Scholar]
- 133. Liu J, Wang J, Leng Y. et al. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013;133:473–85. [DOI] [PubMed] [Google Scholar]
- 134. Qu X, Ben Q, Jiang Y.. Consumption of red and processed meat and risk for esophageal squamous cell carcinoma based on a meta-analysis. Ann Epidemiol 2013;23:762–70.e1. [DOI] [PubMed] [Google Scholar]
- 135. Zhu HC, Yang X, Xu LP. et al. Meat consumption is associated with esophageal cancer risk in a meat- and cancer-histological-type dependent manner. Dig Dis Sci 2014;59:664–73. [DOI] [PubMed] [Google Scholar]
- 136. Islami F, Pourshams A, Nasrollahzadeh D. et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ 2009;338:b929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhang SK, Guo LW, Chen Q. et al. Prevalence of human papillomavirus 16 in esophageal cancer among the Chinese population: a systematic review and meta-analysis. Asian Pac J Cancer Prev 2014;15:10143–9. [DOI] [PubMed] [Google Scholar]
- 138. Lindkvist B, Johansen D, Stocks T. et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the Me-Can project. BMC Cancer 2014;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ji J, Sundquist J, Sundquist K.. Associations of alcohol use disorders with esophageal and gastric cancers: a population-based study in Sweden. Eur J Cancer Prev 2017;26:119–24. [DOI] [PubMed] [Google Scholar]
- 140. Cook MB, Kamangar F, Whiteman DC. et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst 2010;102:1344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Coleman HG, Bhat S, Johnston BT. et al. Tobacco smoking increases the risk of high-grade dysplasia and cancer among patients with Barrett's esophagus. Gastroenterology 2012;142:233–40. [DOI] [PubMed] [Google Scholar]
- 142. Whiteman DC, Sadeghi S, Pandeya N, for the Australian Cancer Study et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut 2008;57:173–80. [DOI] [PubMed] [Google Scholar]
- 143. Turati F, Tramacere I, La Vecchia C. et al. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol 2013;24:609–17. [DOI] [PubMed] [Google Scholar]
- 144. Edelstein ZR, Farrow DC, Bronner MP. et al. Central adiposity and risk of Barrett's esophagus. Gastroenterology 2007;133:403–11. [DOI] [PubMed] [Google Scholar]
- 145. Jacobson BC, Somers SC, Fuchs CS. et al. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med 2006;354:2340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lagergren J, Bergström R, Lindgren A. et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31. [DOI] [PubMed] [Google Scholar]
- 147. Rubenstein JH, Taylor JB.. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Karamchandani DM, Zhang Q, Liao XY. et al. Inflammatory bowel disease- and Barrett’s esophagus-associated neoplasia: the old, the new, and the persistent struggles. Gastroenterol Rep (Oxf) 2019;7:379–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ness-Jensen E, Lindam A, Lagergren J. et al. Changes in prevalence, incidence and spontaneous loss of gastro-oesophageal reflux symptoms: a prospective population-based cohort study, the HUNT study. Gut 2012;61:1390–7. [DOI] [PubMed] [Google Scholar]
- 150. Whiteman DC, Parmar P, Fahey P, Australian Cancer Study et al. Association of Helicobacter pylori infection with reduced risk for esophageal cancer is independent of environmental and genetic modifiers. Gastroenterology 2010;139:73–83. quiz e11–2. [DOI] [PubMed] [Google Scholar]
- 151. Islami F, Sheikhattari P, Ren JS. et al. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma—a systematic review and meta-analysis. Ann Oncol 2011;22:754–60. [DOI] [PubMed] [Google Scholar]
- 152. Nyrén O, Blot WJ.. Helicobacter pylori infection: mainly foe but also friend. J Natl Cancer Inst 2006;98:1432–4. [DOI] [PubMed] [Google Scholar]
- 153. Kastelein F, Spaander MC, Steyerberg EW, ProBar Study Group et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2013;11:382–8. [DOI] [PubMed] [Google Scholar]
- 154. Nguyen DM, El-Serag HB, Henderson L. et al. Medication usage and the risk of neoplasia in patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Liao LM, Vaughan TL, Corley DA. et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012;142:442–52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Singh S, Garg SK, Singh PP. et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis. Gut 2014;63:1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tran T, Spechler SJ, Richardson P. et al. Fundoplication and the risk of esophageal cancer in gastroesophageal reflux disease: a Veterans Affairs cohort study. Am J Gastroenterol 2005;100:1002–8. [DOI] [PubMed] [Google Scholar]
- 158. Corley DA, Kerlikowske K, Verma R. et al. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47–56. [DOI] [PubMed] [Google Scholar]
- 159. Limburg PJ, Wei W, Ahnen DJ. et al. Randomized, placebo-controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology 2005;129:863–73. [DOI] [PubMed] [Google Scholar]
- 160. Heath EI, Canto MI, Piantadosi S. et al. ; Chemoprevention for Barrett's Esophagus Trial Research Group. Secondary chemoprevention of Barrett's esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst 2007;99:545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Chan AT, Detering E.. An emerging role for anti-inflammatory agents for chemoprevention. Recent Results Cancer Res 2013;191:1–5. [DOI] [PubMed] [Google Scholar]
- 162. Fitzgerald RC, di Pietro M, Ragunath K. et al. ; British Society of Gastroenterology. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7–42. [DOI] [PubMed] [Google Scholar]
- 163. Shaheen NJ, Falk GW, Iyer PG. et al. ; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol 2016;111:30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Endoscopology CSoD. [Expert consensus on screening and endoscopic diagnosis and treatment of early esophageal cancer in China (Beijing, 2014)]. Chin J Pract Intern Med 2015;35:320–37. [Google Scholar]
- 165. Roshandel G, Merat S, Sotoudeh M. et al. Pilot study of cytological testing for oesophageal squamous cell dysplasia in a high-risk area in Northern Iran. Br J Cancer 2014;111:2235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ross-Innes CS, Debiram-Beecham I, O'Donovan M. et al. ; on behalf of the BEST2 Study Group. Evaluation of a minimally invasive cell sampling device coupled with assessment of Trefoil Factor 3 expression for diagnosing Barrett's esophagus: a multi-center case-control study. PLoS Med 2015;12:e1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]


