Abstract
A common feature associated with Fetal Alcohol Spectrum Disorders is the inability to concentrate on a specific task while ignoring distractions. Human Continuous Performance Tasks (CPT), measure vigilance and cognitive control simultaneously while these processes are traditionally measured separately in rodents. We recently established a touchscreen 5-Choice CPT (5C-CPT) that measures vigilance and cognitive control simultaneously by incorporating both target and non-targets and showed it was sensitive to amphetamine-induced improvement in humans and mice. Here, we examined the effects of moderate prenatal alcohol exposure (PAE) in male and female mice on performance of the 5-Choice Serial Reaction Time Task (5-CSRTT), which contained only target trials, and the 5C-CPT which incorporated both target and non-target trials. In addition, we assessed gait and fine motor coordination in behavioral naïve PAE and control animals. We found that on the 5-CSRTT mice were able to respond to target presentations with similar hit rates regardless of sex or treatment. However, on the 5C-CPT PAE mice made significantly more false alarm responses versus controls. Compared to control animals, PAE mice had a significantly lower sensitivity index, a measure of ability to discriminate appropriate responses to stimuli types. During 5C-CPT, female mice, regardless of treatment, also had increased mean latency to respond when correct and omitted more target trials. Gait assessment revealed no significant differences in PAE and SAC mice on any measure. These findings suggest that moderate exposure to alcohol during development can have long lasting effects on cognitive control unaffected by gross motor alterations.
Introduction
Moderate alcohol use during pregnancy is estimated to occur in ~5–20% of births and results in serious long-term effects on the fetus characterized as Fetal Alcohol Spectrum Disorders (FASD). The most extreme end of this spectrum, resulting from heavy alcohol use during pregnancy, is characterized as Fetal Alcohol Syndrome (FAS) and occurs in ~2–4% of births (Popova et al., 2018). While individuals lower on the FASD spectrum, such as with Alcohol Related Neurological Disorder (ARND), lack the physical hallmarks of FAS (i.e. craniofacial dysmorphology), they still have behavior and learning impairments that persist across the lifespan (Mattson et al., 2011). These include deficits in cognitive control, behavioral flexibility, working memory, attention, and response inhibition that can have severe negative impacts on quality of life (Green et al., 2009b, Streissguth et al., 1991a).
Problems with attention are a common feature of FASD, with studies reporting comorbidities as high as 95% between the disorder and Attention-Deficit Hyperactivity Disorder (ADHD) (Fryer et al., 2007, Peadon & Elliott, 2010). Some of this overlap may be due to issues with diagnosis. Lack of the cranial facial characteristics seen in FAS, FASD diagnosis commonly relies on documentation of maternal drinking, which may be difficult or impossible to obtain (May & Gossage, 2001), leading to either missed diagnosis or misdiagnosis as ADHD (Chasnoff et al., 2015, Stoler & Holmes, 1999). Previous studies have shown that 40–75% of children with FASD are diagnosed initially as having ADHD before complete psychiatric evaluation (Chasnoff et al., 2015, Chasnoff et al., 2010, Fryer et al., 2007, Rasmussen et al., 2012). While individuals with FASD often display characteristics associated with the inattention subtype of ADHD, several studies also demonstrate developmental alcohol exposure can lead to long lasting alterations in response inhibition similar to those seen in hyperactive/impulsive subtype (Infante et al., 2015, Mattson et al., 2006, Mattson et al., 2011, Olson et al., 1998). A recent meta-analysis found that compared to ADHD, FASD was most strongly associated with deficits in shifting of attention, but was also accompanied by impairments in measures of vigilance and response inhibition (Kingdon et al., 2016).
In human studies, attention is typically measured using continuous performance tasks (CPT). CPTs require subjects to rapidly respond to briefly presented stimuli (targets) and inhibit responding to non-targets (Riccio et al., 2002). These paradigms can therefore assess attention, but also cognitive control via measurement of successful response inhibition during presentation of non-target trials. FASD clinical populations have difficulty with attention and inhibition when tested on these paradigms (Green et al., 2009a, Infante et al., 2015, Olson et al., 1998, Streissguth et al., 1991a, Streissguth et al., 2004) although the mechanism(s) underlying these difficulties is still not well understood.
Rodent models of developmental insult, including prenatal alcohol exposure (PAE), have become an important tool for investigating the mechanism underlying the long term behavioral impairments seen in FASD (Marquardt & Brigman, 2016). Models of developmental alcohol exposure in the rodent have particular translational relevance (Squire et al., 2013) as studies utilizing PAE have been shown to have blood alcohol content (BAC) congruent to those seen during maternal drinking in humans and rodent PAE studies have been able to model deficits seen in persons with FASD across multiple behavioral domains (Driscoll et al., 1990). For example, recent studies have shown that PAE in rodents leads to long-term deficits in measures of executive control including choice learning, value updating, and control over reward seeking across a range of alcohol doses, administration routes, and assessment modality (Atalar et al., 2016, Marquardt et al., 2019, Olguin et al., 2019, Thomas et al., 2004, Waddell & Mooney, 2017). While few studies have examined the impact of PAE on attention and response inhibition using CPT measures, developmental alcohol exposure has been shown to impair response inhibition on passive avoidance and taste aversion as well as slow acquisition of auditory go/no go discriminations (Mihalick et al., 2001, Riley et al., 1979a). More recently, high dose PAE was found to impair accurate responding to the shortest stimulus durations on the 5-choice serial reaction time task (5-CSRTT) as well as dysregulate firing in the medial prefrontal cortex (Louth et al., 2016).
Touchscreen automated paradigms have become an important tool for ensuring translation between clinical and pre-clinical models (Hvoslef-Eide et al., 2016). We have recently shown that a well-validated murine CPT assay, the 5-choice Continuous Performance Task (5C-CPT) can be successfully adapted to touchscreen systems. In addition, we were able to demonstrate pharmacological predictive validity of this task as D-amphetamine increased target responsivity in both humans and mice (Macqueen et al., 2018). The 5C-CPT has also been shown to require parietal cortical function in both humans and mice (Young et al., 2019) which is particularly important given the recent focus on utilizing behavioral approaches that recruit similar regions and circuits in rodents and humans. Although the 5C-CPT has proven useful in detecting behavioral alterations in animal models of schizophrenia (Barnes et al., 2012, Turner et al., 2013, Young et al., 2017, Young et al., 2013), and bipolar disorder (Young et al., 2019) to-date no studies have determined the effects of PAE on attention and response inhibition utilizing this approach.
Here, we assessed the effect of a moderate PAE paradigm on performance of a traditional 5-CSRTT and the 5C-CPT. Next, in order to examine whether any alterations in task performance seen by PAE were mediated by subtle alterations in motor function, we assessed a behaviorally naive cohort of PAE and control animals on a measure of gait and fine motor coordination. We hypothesized that while moderate PAE might negatively impact attention on the 5-CSRTT, it would have more robust effects when animals were required to discriminate target and non-targets on the 5C-CPT.
Methods
All experimental procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee
Prenatal Alcohol Exposure (PAE) Model.
Male and female C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), were used in a limited access PAE paradigm as previously described (Brady et al., 2012, Brady et al., 2013; Figure 1A). Briefly, two hours into the dark cycle, female mice were given access to either 0.066% (w/v) saccharin or an alcohol solution (5% w/v for five days, then 10% w/v) sweetened with 0.066% (w/v) saccharin for four hours (from 1000 to 1400 hr). After one week of drinking 10% alcohol or the saccharin control solution, individual females were placed into the cage of a singly-housed male for two hours immediately following the drinking period. Females continued to consume alcohol or saccharin solutions throughout the five-day mating period. Pregnancy was positively determined by monitoring weight gain every 3–4 days to minimize handling stress as previously described (Brady et al., 2012, Marquardt et al., 2019, Marquardt et al., 2014, Olguin et al., 2019). Access to alcohol was withdrawn beginning on post-natal day 0 using a step-down procedure over a 6-day period and offspring were weaned at approximately 23 days of age. We have shown this protocol reliably produces blood alcohol concentrations of 80–90 mg/dL at the end of the 4-hour drinking period with no negative effects on pup survival, weight, or litter size (Brady et al., 2012, Brady et al., 2013). Offspring were housed in groupings of 2–3 per cage in a temperature- and humidity- controlled vivarium under a reverse 12 h light/dark cycle (lights off 0800 h) and tested during the dark phase. To control for litter effects, operant behavior was conducted on no more than 2 adult male and female offspring per litter (1.75±0.75 mice per litter, n=9–11 per sex/treatment; ~8 weeks at onset of testing). Similarly, gait analysis was conducted in a separate cohort of no more than 2 PAE and saccharin SAC male and female animals per litter (1.50±0.50 mice per litter, n=6–7 per sex/treatment; ~12 weeks at onset of testing). While all males weighed significantly more at age of testing than females, there was no significant effect of treatment on pup size (PAE male=21.9g±0.44g SAC male=22.5g±0.77g; PAE female=20.5g±0.23g; SAC female=20.9g±0.31g).
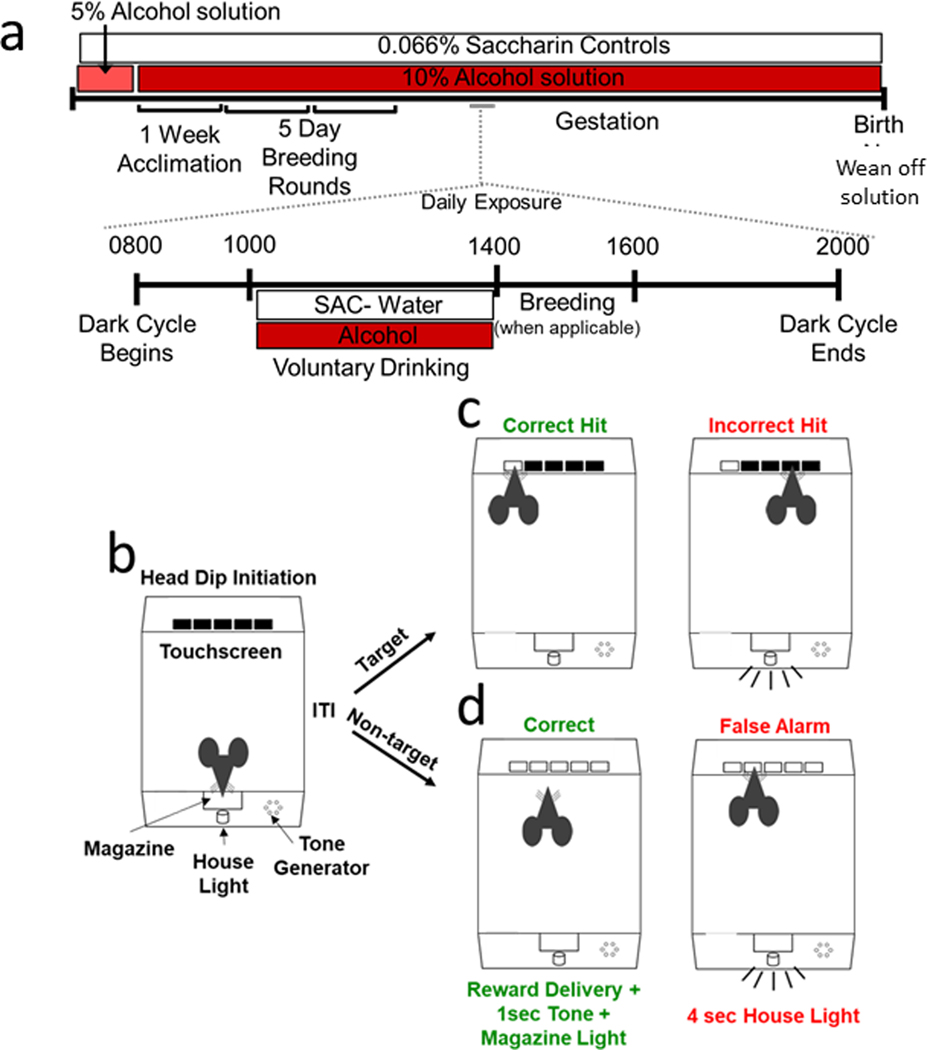
Figure 1.
Limited Access Moderate Prenatal Alcohol Model and Touchscreen 5-Choice Continuous Performance Task Method. In the New Mexico Alcohol Research Center mouse model of moderate prenatal alcohol exposure (a) female mice are acclimated to either the saccharin (0.066% w/v) control solution or the saccharin sweetened alcohol solution for five days (saccharin 0.066% w/v in 5% w/v alcohol) followed by acclimation to saccharin sweetened 10% w/v alcohol solution for 1 week, which they consume throughout gestation. For the following 5 days, females are placed with a single housed male for 2 hours after alcohol exposure for breeding rounds. On a day-by-day basis, two hours into the dark cycle water bottles are removed and replaced with either the saccharin alcohol solution or saccharin control solution for 4 hours after which they are removed and regular water bottles are returned. Methodology for the 5-choice continuous performance task begins with animal initiation of trials (b) by performing a head dip into the magazine followed by a 4 sec intra-trial interval (ITI). During 5C-SRT testing (c) presents only Target trials in which mice are rewarded (30uL strawberry milk) for responding to target trials (a single square) illuminated for a specific stimulus duration (20, 10, 8, 4, 2 sec). Touch at the incorrect location, response omission, or a premature touch led to a 4 sec. house light on timeout. During 5C-CPT testing (d), target trials were intermixed with non-target trials (all squares illuminated) at either a 2:1 or 5:1 target to non-target ratio. Mice could only obtain reward on non-target trials by withholding response (correct rejection) for the stimulus duration. A touch at any stimulus location (false alarm) resulted in a 4 sec. house light on timeout.
Operant Apparatus.
All operant behavior was conducted in a chamber measuring 21.6 × 17.8 × 12.7 cm (model # ENV-307W, Med Associates, St. Albans, VT, USA) housed within a sound- and light-attenuating box (Med Associates, St. Albans, VT), as previously described (Marquardt et al., 2019, Olguin et al., 2019). The standard grid floor of the chamber was covered with a solid acrylic plate to facilitate ambulation. A reward magazine, house light, and a tone generator was located at one end of the chamber. Liquid reward in the form of strawberry milk (Nesquik. S.A., Vevey, Switzerland, non-fat milk; 30μl) was delivered by peristaltic pump (Lafayette Instruments, Lafayette, IN) into a magazine in order to obtain large enough trial numbers (100–200) and avoid satiation from solid rewards as previously described (Young et al., 2019, Young et al., 2013). At the opposite end of the chamber there was a touch-sensitive screen (Conclusive Solutions, Sawbridgeworth, UK) covered by a black acrylic aperture plate allowing five active touch areas measuring 2.5 × 2.5 cm separated by 0.6 cm and located at a height of 1.6 cm from the floor of the chamber. Stimulus presentation in the response windows and touches were controlled and recorded by the K-Limbic Software Package (Conclusive Solutions, Sawbridgeworth, UK).
Pre-training.
Beginning at 6–8 weeks of age mice were slowly reduced and then maintained at 85% free-feeding body weight. Prior to training, mice were acclimated to the strawberry milk reward for 3 days by providing 3 drops of reward per mouse on a weigh boat in the home cage for 15 min or until consumed. After becoming acclimated to the reward mice were habituated to the operant chamber and retrieving reward from the magazine by being placed in the chamber for 30 min with 30uL of strawberry milk reward dispensed 30 sec after each reward collection (measured via head dip into the magazine). Mice that retrieved 10 rewards within 15 min were moved to touch training. Each touch training session consisted of 50 total trials or 30 min, whichever was achieved first. Touch training required mice to first initiate each trial with a head dip into the magazine, followed by a 4 sec intra-trial-interval (ITI), and then respond to presentation of a white square stimulus in 1 of the 5 response windows (spatially pseudorandomized; Figure 1B&C). The stimulus remained on the screen until a response was made either to the stimulus (correct) or in a blank response window (incorrect). Correct responses resulted in reward presentation, a 1 sec. tone, and illumination of the magazine until reward retrieval. After consuming reward, mice had to exit the magazine in order for the magazine light to be re-illuminated for initiation of the next trial. Incorrect responses resulted in a 10 sec. time out with the house light on to discourage indiscriminate responding. Mice could also respond during the ITI which results in a premature response with a 10 sec. time out with the house light on. Mice initiating and touching 50 times within 30 min were moved to 5-Choice Serial Reaction Task (5-CSRTT) with constant ITI of 4 sec.
5-Choice Serial Reaction Task (5-CSRTT).
This stage was identical to touch-training except that the stimulus duration became progressively shorter starting with 20 sec followed by a 2 sec limited hold in which the square is no longer illuminated, but a response can still be made. Failure to respond to a stimulus was counted as an omission. The stimulus duration was reduced to 10 sec, 8 sec, 4 sec, and 2 sec as criterion was met or six sessions were completed. Lastly, mice completed a 2 sec stimulus duration with a variable ITI of 3–7 sec. House light time out for incorrect, premature, and omissions was 4 sec. Criterion was ≥30% correct responses over a 120-trial session and a mean correct latency (MCL) of half the stimulus duration for two consecutive sessions as previously described (Macqueen et al., 2018). The MCL was defined as the time between stimulus presentation to when a response was made to the illuminated stimulus.
5-Choice Continuous Performance Task.
Following successful 5-CSRTT testing to train responses to target stimuli, mice were next tested on a 120-trial 5C-CPT paradigm as described above with the addition of non-target trials. As in training, target trials (Figure 1C) were defined as stimulus presentation of 2 sec followed by a 2 sec limited hold with a variable ITI of 3–7 sec. Non-target trials (Figure 1D) consisted of illumination of all 5 stimuli windows and withholding of a response was measured as a correct rejection (CR) and a reward was delivered. Response during a non-target trial was measured as a false alarm (FA) and resulted in a 4 sec time-out period. Mice initially started on a 2:1 ratio, which consisted of 2 target trials to 1 non-target trial for 5 sessions. Mice were then moved to a 5:1 ratio for 10 sessions.
Gait analysis.
A separate cohort of male and female PAE and SAC controls were tested for fine motor coordination and gait using the Catwalk XT system (Noldus Information Technology, Wageningen, Netherlands), as previously described (Thompson et al., 2015). Briefly, the mouse was placed in a start box at one end of a 1.3 m long runway consisting of a glass platform covered by a removable black tunnel and allowed to walk freely across the runway to reach their home-cage at the opposite end. The runway was illuminated from one side by reflected green fluorescent light. A high-speed camera recorded footprints on the glass from light reflected by downward pressure of individual footfalls. Three runway trials were recorded for each mouse during one testing session, with a trial being regarded as compliant by the software if the mouse crossed the recording area in under 5 sec and did not show a maximum speed variation > 60%. All trials marked as compliant were reviewed manually, if the mouse stopped or turned mid-run the trials were excluded.
Analysis was performed using Catwalk XT 8.1 Software. Gait and movement were analyzed for right fore (RF), left fore (LF), right hind (RH) and left hind (LH) paws on the following variables: paw print area (size of paw print area during a full stance), stride length (distance between 2 consecutive paw placements of the same paw), swing (time interval between 2 consecutive paw placements of the same paw), and swing speed (velocity of an individual paw between 2 consecutive placements). The percentage of step patterns categorized as cruciate, alternate or rotary was analyzed as previously described (Neumann et al., 2009).
Statistical Analysis
The following dependent measures were taken during all 5-CSRTT and 5C-CPT sessions: target correct responses, non-target correct rejections (CR), incorrect target responses, incorrect non- target false alarms (FA), premature responses, omissions, mean correct latency (MCL; time from stimulus presentation to correct response), and reward latency (time from correct response to reward retrieval). As well as the following calculations: percent omission, percent correct, Hit Rate (Correct/Correct+Omission), False Alarm Rate (FAR; FA/FA+CR). Based upon these basic parameters, a signal detection index was calculated to assess sensitivity index (SI): SI = HR − FAR / 2[HR + FAR] − [HR + FAR]2. For gait analysis, ANOVA was calculated for each gait analysis category, with paw and genotype or step category, sex and treatment as between-subject factors.
Results
Pre-training.
No significant differences between sex or treatment (Trt) and no interaction was seen on the number of sessions required to learn initiation and responding to one continuously presented stimuli on the touchscreen for reward [Sex: F(1,35)=1.77, p=0.19; Trt: F(1,35)=2.70, p=0.11; Sex x Trt: (F(1,35)=0.09, p=0.75]. Mice in each group (2 Male SAC; 1 Female SAC, 2 PAE Male; 2 PAE Female) failed to attain pre-training criterion within 25 testing sessions and were excluded from further testing.
5-Choice Serial Reaction Task.
When animals were tested on the 5-CSRTT all mice showed reduced hit rate as stimulus duration decreased (e.g. 20, 10, 8, 4, & 2 sec.) [Figure 2A; Session: F(5,170)=69.08, p=0.01] but groups did not significantly differ by sex or treatment with no interaction [Sex: F(1,35)=2.40, p=0.14; Trt: F(1,35)=1.73, p=0.20; Sex x Trt x Session: F(5,170)=0.78, p=0.56]. Similarly, all mice required more sessions to reach criteria as the duration of stimulus presentation decreased [Figure 2B; Session: F(5,170)=48.65, p=0.01]. However, no significant differences on sessions to reach criterion were seen between sex or treatment with no interaction [Sex: F(1,35)=2.65, p=0.12; Trt: F(1,35)=0.18, p=0.67; Sex x Trt x Session: F(5,170)=0.92, p=0.47]. As stimulus duration reduced, MCL also significantly reduced across groups [Figure 2C; Session: F(5,170)=147.28, p=0.01]. Across all durations, PAE mice regardless of sex had significantly faster mean correct latencies to respond to stimuli during 5-CSRTT testing [Sex: F(1,35)=0.62, p=0.43; Trt: F(1,35)=4.53, p=0.04] with no interaction [Sex x Trt x Session: F(5,170)=0.43, p=0.82]. Premature responses also significantly increased across groups as stimulus durations decreased [Figure 2D; Session: F(5,170)=15.53, p=0.01] but there were no significant differences or interactions across groups [Sex: F(1,35)=0.45, p=0.50; Trt: F(1,35)=0.02, p=0.89; Sex x Trt x Session: F(5,170)=0.42, p=0.83]. A similar pattern was seen for trials omitted [Figure 2E; Session: F(5,170)=79.86, p=0.01; Sex: F(1,35)=0.15, p=0.70; Trt: F(1,35)=0.72, p=0.40; Sex x Trt x Session: F(5,170)=0.65 p=0.66]. Finally, reward latency, a measure of motivation to retrieve reward, also significantly reduced concomitant with stimulus duration, and did not differ between sex or treatment groups with no interaction [Figure 2F; Session: F(5,170)=2.72, p=0.02; Sex: F(1,35)=1.92, p=0.18; Trt: F(1,35)=0.04, p=0.85; Sex x Trt x Session: F(5,170)=1.07, p=0.38].
Figure 2. PAE does not impair performance of the 5-choice serial reaction time task.
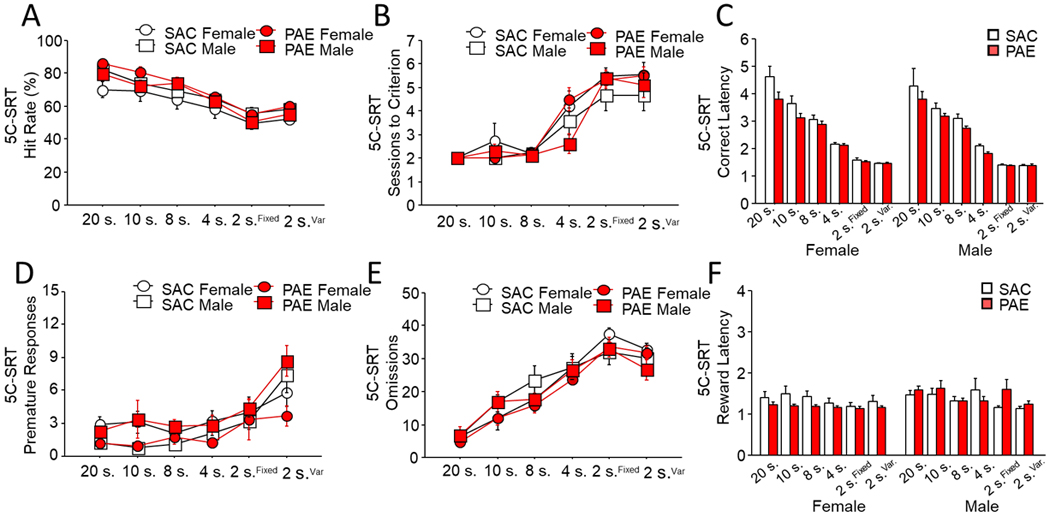
When required to respond to illuminated stimuli for rewards, no main effect of treatment, sex or their interaction was seen as stimulus durations were reduced as measured by (a) average hit rate or (b) sessions to criterion across stimulus durations. Average latency to respond on correct trials (c) reduced significantly concomitant with duration with no effect of sex, however PAE mice were significantly faster than controls across all stimulus durations. In addition, (d) premature responses and (e) omitted trials significantly increased as stimulus duration decreased, but did not differ by sex or treatment. Latency to (f) retrieve reward following a correct response decreased with stimulus duration but did not differ by sex or treatment. Data are group mean ± SEM.
5-Choice Continuous Performance Task.
When non-target trials were introduced for the 5C-CPT at a 2:1 ratio for 5 sessions (data not shown), no significant differences or interactions were seen between groups on hit rate [Sex: F(1,35)=1.23, p=0.27; Trt: F(1,35)=1.05, p=0.31; Sex x Trt: F(1,35)=1.51, p=0.23] or false alarm rate [Sex: F(1,35)=2.89, p=0.10; Trt: F(1,35)=0.25, p=0.62; Sex x Trt: F(1,35)=0.10, p=0.75] and there was no significant difference in sensitivity index [Sex: F(1,35)=0.01, p=0.99; Trt: F(1,35)=1.78, p=0.19; Sex x Trt: F(1,35)=0.69, p=0.41]. Groups did not significantly differ by sex or treatment on measures of premature responding [Sex: F(1,35)=2.79, p=0.11; Trt: F(1,35)=0.02, p=0.96; Sex x Trt: F(1,35)=1.71, p=0.20] or reward retrieval latency [Sex: F(1,35)=0.17, p=0.89; Trt: F(1,35)=0.11, p=0.74; Sex x Trt: F(1,35)=1.02, p=0.32]. However, in contrast to the treatment effect in 5-CSRTT, females consistently had a slower MCL regardless of treatment [Sex: F(1,35)=5.67, p=0.02; Trt: F(1,35)=0.21, p=0.65; Sex x Trt: F(1,35)=1.01, p=0.33] and also omitted significantly more trials [Sex: F(1,35)=4.70, p=0.03; Trt: F(1,35)=0.22, p=0.64; Sex x Trt: F(1,35)=0.36, p=0.55].
When tested on the less predictable and thus more challenging 5:1 variant of the 5C-CPT, PAE significantly altered behavior to non-target trials. While not affected by treatment group, female mice had significantly reduced hit rate during 5:1 CPT testing [Figure 3A; Sex: F(1,35)=5.46, p=0.03; Trt: F(1,35)=1.66, p=0.21] with no interaction [Sex x Trt: F(1,35)=1.83, p=0.18]. Analysis of false alarms found that PAE mice of both sexes were significantly more likely to respond to non-targets than SAC controls [Figure 3B; Sex: F(1,35)=1.31, p=0.26; Trt: F(1,35)=11.57, p=0.01] with no interaction [Sex x Trt: F(1,35)=0.08, p=0.78], indicative of poor response inhibition to these unpredictable stimuli. Similarly, PAE mice had a significantly reduced sensitivity index irrespective of sex [Figure 3C; Sex: F(1,35)=1.27, p=0.27; Trt: F(1,35)=6.59, p=0.01; Sex x Trt: F(1,35)=0.94, p=0.33] compared to control mice. Neither PAE or sex significantly altered the average number of premature responses made during 5:1 testing [Figure 3D; Sex: F(1,35)=0.82, p=0.27; Trt: F(1,35)=1.03, p=0.32]. While female PAE mice had on average a lower number of premature responses than any other group, this interaction did not reach significance [Sex x Trt: F(1,35)=3.37, p=0.07]. Consistent with results during 2:1 5C-CPT training, female mice, regardless of treatment, made significantly more omissions [Figure 3E; Sex: F(1,35)=6.12, p=0.02; Trt: F(1,35)=1.26, p=0.27; Sex x Trt: F(1,35)=0.95, p=0.34]. and had a significantly increased mean correct latency [Figure 3F; Sex: F(1,35)=6.06, p=0.02; Trt: F(1,35)=0.32, p=0.57; Sex x Trt: F(1,35)=2.52, p=0.12] compared to males. Latency to retrieve reward following a correct response did not differ across groups [Sex: F(1,35)=1.14, p=0.29; Trt: F(1,35)=0.54, p=0.81; Sex x Trt: F(1,35)=0.63, p=0.43].
Figure 3. PAE impaired cognitive control on the 5:1 variant of the 5-choice continuous performance task.
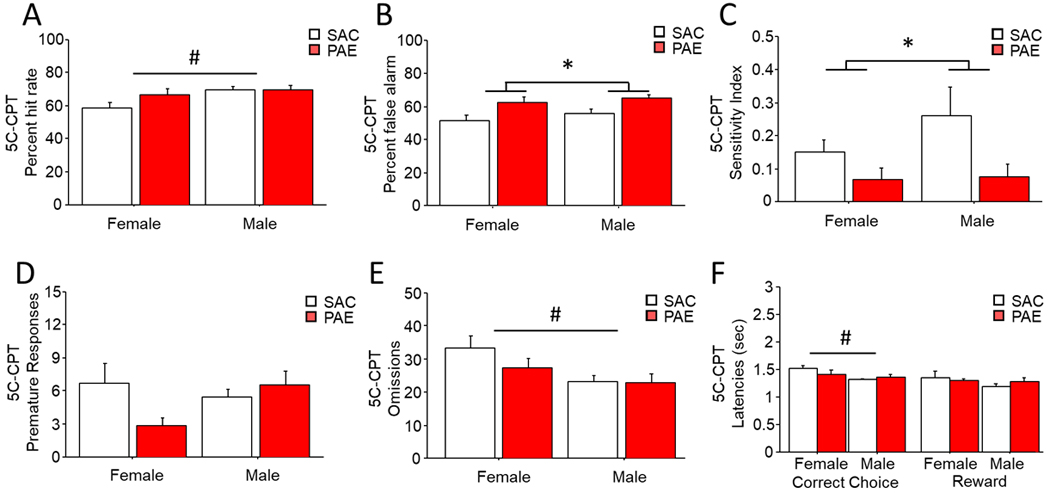
While there was a significant main effect of sex on hit rate (a) for target trials, hit rate did not significantly differ between treatments. During the 5:1 5C-CPT, PAE mice made significantly more (b) false alarms regardless of sex. PAE also significantly reduced (c) sensitivity index with no main effect of sex. While (d) premature responses did not differ by sex or treatment, female mice made significantly more (e) omissions regardless of treatment. Female mice also had significantly higher (f) mean correct latencies than males of either treatment, but latency to retrieve reward did not differ. * = <.05 main effect of treatment. # = <.05 main effect of sex. Data are group mean ± SEM.
Gait analysis.
Across groups, all mice completed sufficient compliant runs to be included in the analysis. There was no main effect of sex on any variable measured so males and females were combined for all measures. Analysis of swing for left and right fore- and hind-paws found no main effect of treatment or paw [Figure 4A; Trt: F(1,21)=1.46, p=0.55; Paw: F(3,21)=0.42, p=0.74]. Similarly swing speed did not significantly vary by treatment or limb [Figure 4B; Trt: F(1,21)=0.31, p=0.58; Limb: F(3,21)=0.27, p=0.99]. Treatment did not alter stride length, which also did not significantly vary by limb ([Figure 4C; Trt: F(1,21)=0.54, p=0.47; Limb: F(3,21)=1.08, p=0.32]. Analysis of print area revealed no main effect of treatment or paw [Figure 4D; Trt: F(1,21)=0.26, p=0.61; Paw: F(3,21)=0.08, p=0.97]. Finally, step pattern was examined by the 6 primary sequences within alternate (Aa: RF-RH-LF-LH; Ab: LF-RH-RF-LH), cruciate (Ca: RF-LF-RH-LH; Cb: LF-RF-LH-RH) and rotary (Ra: RF-LF-LH-RH; Rb: LF-RF-RH-LH) sequences. No animals in this cohort performed the rotary Ra sequence. There was a significant main effect of sequence type with the Ab alternate step pattern being the dominate sequence [Figure 4E; Step Pattern: F(4,21)=15.50, p=0.01]. However, there was no main effect of treatment on step pattern [Trt: F(1,21)=1.32, p=0.26] and no interaction found.
Figure 4. PAE did not alter fine motor movement or gait.
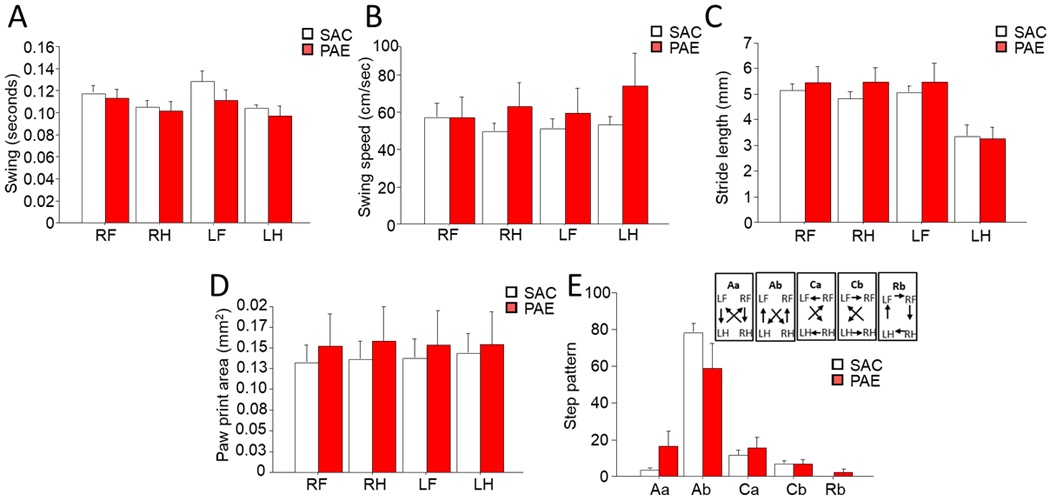
Analysis found no main effect of treatment on right fore (RF), left fore (LF), right hind (RH) or left hind (LH) paws on the following variables: (a) Swing (time interval between 2 consecutive paw placements of the same paw), (b) swing speed (velocity of an individual paw between 2 consecutive placements), (c) stride length (distance between 2 consecutive paw placements of the same paw), (d) paw print area (size of paw print area during a full stance). Lastly, the three categories of (e) step pattern were analyzed (cruciate: CA: RF-LF-RH-LH; CB: LF-RF-LH-RH; alternate: AA: RF-RH-LF-LH; AB: LF-RH-RF-LH; and rotary: RB: LF-RF-RH-LH) while alternate step pattern, AB, was significantly higher than the other step patterns, there is no effect of treatment. No main effect of sex or sex x treatment interaction was found on any measure of gait or fine motor movement. Data are group mean ± SEM.
Discussion
We found that moderate prenatal alcohol exposure (PAE) during the 1st and 2nd trimester equivalent did not interfere with attention as measured by a touchscreen 5-CSRTT that required rapid response to target trials alone. When tested on the more challenging 5C-CPT that included more rarely presented non-target trials, PAE mice exhibited a significant deficit in their withholding of responses (response disinhibition). Analysis of sensitivity index revealed that both male and female PAE animals did not distinguish between target and non-target trial types as readily as SAC controls.
In the current study we found that, regardless of sex, PAE did not alter hit rate, premature responses, or omissions on the 5-CSRTT. Nor did PAE increase the number of sessions required to proceed through each decreasing stimulus duration on the 5-CSRTT. These findings are in contrast to a recent report observing that PAE significantly increased omissions at short stimulus durations (1.2 and 1 second) on a 5-CSRTT paradigm, although there are several key methodological differences between studies (Louth et al., 2016). Louth and colleagues utilized a twice daily gavage of 4 g/kg/d alcohol from G12 to G18, resulting in maternal BAC of ~234.8 mg/dl, almost three times the levels reached using our exposure model. In addition, the previous study focused exclusively on the 5-CSRTT, and utilized a high criterion (>80% accuracy for 3 of 4 consecutive sessions) for each stimulus duration and tested lower stimulus durations (1.2 and 1 second (Louth et al., 2016). Our data suggest that that while high dose PAE may impair attention to targets at short durations, more moderate exposure may only reveal deficits when different trial types (target and non-target) introduce choice conflict.
Utilizing measures that can assay attention as well as response inhibition such as the 5C-CPT in models of developmental disorders, like FASD, may be particularly important, as alterations in attention per se are often accompanied or mediated by impaired cognitive control in disorders such as ADHD (Crosbie et al., 2008, Groman et al., 2009, Kuntsi et al., 2006). It is well-documented that individuals with FASD have difficulty with attention and response inhibition when tested on CPT assays (Infante et al., 2015, Olson et al., 1998, Streissguth et al., 2004, Streissguth et al., 1991b). Specifically, children with FASD have deficits with sustained attention as measured by increased response times, increased variable responding, and significantly more errors, especially errors of omission, on these paradigms (Kooistra et al., 2010). Children with FAS also perform more slowly, are more variable, and make more overall errors than children in the comparison group on fast-paced Go/No-Go tasks (Mattson et al., 2006). Both children and adults with confirmed alcohol exposure also show impairments in auditory attention, when task complexity is sufficiently high (Kerns et al., 1997, Mattson et al., 2006). Taken together these findings suggest that developmental exposure to alcohol can lead to deficits in sustained attention that are reliably revealed when task difficulty, and therefore attentional demand, is high. Studies using exposure models in mice and rats similar to those used in the current study further support this conclusion as studies utilizing the New Mexico Alcohol Research Center model have shown that this exposure generally spares learning processes including acquisition of instrumental responding, extinction of learned associations, visual discrimination, and acquisition of spatial responses. In contrast, these exposures impair performance when task demands are increased either via reinstatement of reward after extinction or reversal of a learned visual or spatial discrimination (Allan et al., 2014, Marquardt et al., 2014, Olguin et al., 2019). Rat studies using this model that yield similar BAC (~80 mg/dl) also generally spare learning yet impair performance when contingencies are changed or reversed (Hamilton et al., 2014, Riley et al., 1979b, Wainwright et al., 1990). Our current findings are in line with the general finding that more moderate alcohol exposures spare learning behaviors, as PAE mice of both sexes were able to perform a touchscreen 5-CSRTT at levels similarly to controls, and in fact had faster mean correct latencies. PAE mice were also unaffected when non-target trials were common on the 2:1 variant of the 5C-CPT. However, PAE animals were unable to withhold responding to non-targets when the task demands were increased during the 5:1 5C-CPT.
While our current findings suggest that moderate PAE is associated with deficits in cognitive control consistent with FASD, there is an increasing awareness that preclinical behavioral approaches are greatly enhanced if similarity in circuit recruitment can be established across rodents and humans. In humans, successful performance of attentional tasks recruit a network of cortical regions including the dorsolateral prefrontal cortex (DLPFC), parietal cortex, and posterior cingulate cortex (Squire et al., 2013). Studies in humans and non-human primates have demonstrated that prefrontal cortex loss or damage consistently impairs attentional control (Knight et al., 1995, Rueckert & Grafman, 1996, Wardak et al., 2006). In humans, the 5C-CPT specifically, has been shown to recruit similar regions, as electroencephalogram recordings in patients with schizophrenia have revealed decreased event-related potentials in the DLPFC as well as in the posterior cingulate cortex following a successful inhibition of response to a non-target stimulus compared to healthy controls (Young et al., 2017). Areas of the ventral striatum have also been implicated in the ability to withhold inappropriate responding during attentional tasks. While future studies are required to investigate how moderate PAE alters cognitive control during 5C-CPT, but spares attention during 5-CSRTT, previous work has found that parietal cortex is required to successfully perform 5-CSRTT in rodents (Muir et al, 1996). While, in human subjects the parietal cortex is functionally necessary to inhibit responding to non-targets in the 5C-CPT (Young et al, 2019). Recent findings in the mouse show that the firing rate of fast-spiking interneurons (FSI) in the nucleus accumbens negatively correlate with premature responses (Pisansky et al., 2019). Given strong evidence that PAE alters the activity and morphology of FSI (Delatour et al., 2019, Skorput et al., 2015, Skorput & Yeh, 2016) it is possible that PAE deficits in cognitive control in our animals are mediated by altered FSI activity in parietal cortex. Future studies are needed to determine whether the increase in inappropriate responses to non-target trials (i.e. false alarms) seen in our PAE mice may be driven by altered interneuron morphology and activity that is only revealed when mice must choose among multiple response options on the 5C-CPT.
While we previously reported that the PAE model used in the current study did not lead to gross alterations in physical health, sensory reflexes, or neurological functions (Marquardt et al., 2014) tasks such as the 5-CSRTT and the 5C-CPT have high demands on motor coordination which may be altered by PAE. Therefore, we investigated whether our moderate PAE model would alter general coordination and fine limb movement but found no significant effects of treatment or sex on limb and paw control or step pattern. Together, these findings support the hypothesis that individual limb movements, overall gait, and coordination are not altered by our moderate PAE model, and indicate that increases in false alarm rates seen during the challenging 5:1 CPT were not driven by gross motor deficit. However, it has been shown that PAE, including those similar to the model used in the current study, can lead to cerebellar defects including alterations in eye blink conditioning and rotarod (Valenzuela et al., 2012). While we have not detected alteration in any assay of motor behavior in this model to date, it is possible that subtle changes in motor sequences may underlie some of the deficits seen here. Future studies are needed to systematically assess patterns of motor sequences after moderate PAE.
Interestingly, while PAE mice showed faster latency to respond regardless of sex when presented with target only trials in 5-CSRTT, female mice showed significantly slower response to stimuli as measured by mean correct latency when non-target trials were incorporated. During 5C-CPT testing at both ratios slower MCL in females was accompanied by a small but significant increase in omitted trials. We have previously shown that females of both treatments required more trials and errors to learn a pairwise discrimination in behavioral flexibility experiments utilizing this moderate PAE model (Marquardt et al., 2014). Given differences in growth curves and total weight loss on percentage based reduced diets, this data suggest that motivation to perform complex behavioral tasks for food rewards may differ by sex, and may interact with other treatment conditions. This sex difference is potentially important given the common practice of using combined groups of male and female mice in behavioral studies of cognition.
In conclusion, we observed that moderate 1st and 2nd trimester equivalent prenatal alcohol exposure spared attention on the 5-CSRTT as measured by sessions to criterion and hit rate. However, PAE significantly impaired attention and cognitive control when rare non-target trials were intermixed with target trials in the more difficult 5:1 ratio 5C-CPT. During 5C-CPT female mice showed significantly slower response to stimuli as measured by mean correct latency regardless of treatment and this slowing was accompanied by a small but significant increase in omitted trials. Analysis of motor coordination suggest that these deficits during 5C-CPT were not due to gross changes in motor ability. Taken together, these data demonstrate that PAE can have a long lasting effect on cognitive control when animals are challenged to distinguish between stimuli types utilizing a highly-translatable touchscreen paradigm.
Acknowledgements
This work was supported by the National Institute on Alcohol Abuse and Alcoholism grants 2P50AA022534–06, 1R01AA025652–01, 5T32AA014127e13 and National Institute of Mental Health grant 1UH2-MH109168–01. Data available upon request from the authors.
References
- Allan AM, Goggin SL & Caldwell KK (2014) Prenatal alcohol exposure modifies glucocorticoid receptor subcellular distribution in the medial prefrontal cortex and impairs frontal cortex-dependent learning. PLoS One, 9, e96200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalar EG, Uzbay T. & Karakas S. (2016) Modeling Symptoms of Attention-Deficit Hyperactivity Disorder in a Rat Model of Fetal Alcohol Syndrome. Alcohol Alcohol, 51, 684–690. [DOI] [PubMed] [Google Scholar]
- Barnes SA, Young JW & Neill JC (2012) Rats tested after a washout period from sub-chronic PCP administration exhibited impaired performance in the 5-Choice Continuous Performance Test (5C-CPT) when the attentional load was increased. Neuropharmacology, 62, 1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ML, Allan AM & Caldwell KK (2012) A limited access mouse model of prenatal alcohol exposure that produces long-lasting deficits in hippocampal-dependent learning and memory. Alcohol Clin Exp Res, 36, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM & King L. (2015) Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics, 135, 264–270. [DOI] [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM, Telford E, Schmidt C. & Messer G. (2010) Neurodevelopmental functioning in children with FAS, pFAS, and ARND. J Dev Behav Pediatr, 31, 192–201. [DOI] [PubMed] [Google Scholar]
- Crosbie J, Perusse D, Barr CL & Schachar RJ (2008) Validating psychiatric endophenotypes: inhibitory control and attention deficit hyperactivity disorder. Neurosci Biobehav Rev, 32, 40–55. [DOI] [PubMed] [Google Scholar]
- Delatour LC, Yeh PW & Yeh HH (2019) Ethanol Exposure In Utero Disrupts Radial Migration and Pyramidal Cell Development in the Somatosensory Cortex. Cerebral Cortex, 29, 2125–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP & Riley EP (1990) Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicol Teratol, 12, 231–237. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP & Mattson SN (2007) Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics, 119, e733–741. [DOI] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Brien DC, Armstrong IT, Nikkel SM, Stade BC, Rasmussen C, Munoz DP & Reynolds JN (2009a) Oculomotor control in children with fetal alcohol spectrum disorders assessed using a mobile eye-tracking laboratory. Eur J Neurosci, 29, 1302–1309. [DOI] [PubMed] [Google Scholar]
- Green CR, Mihic AM, Nikkel SM, Stade BC, Rasmussen C, Munoz DP & Reynolds JN (2009b) Executive function deficits in children with fetal alcohol spectrum disorders (FASD) measured using the Cambridge Neuropsychological Tests Automated Battery (CANTAB). J Child Psychol Psychiatry, 50, 688–697. [DOI] [PubMed] [Google Scholar]
- Groman SM, James AS & Jentsch JD (2009) Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev, 33, 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, Bird CW, Davies S. & Savage DD (2014) Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behav Brain Res, 269, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvoslef-Eide M, Nilsson SR, Saksida LM & Bussey TJ (2016) Cognitive Translation Using the Rodent Touchscreen Testing Approach. Curr Top Behav Neurosci, 28, 423–447. [DOI] [PubMed] [Google Scholar]
- Infante MA, Moore EM, Nguyen TT, Fourligas N, Mattson SN & Riley EP (2015) Objective assessment of ADHD core symptoms in children with heavy prenatal alcohol exposure. Physiol Behav, 148, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns KA, Don A, Mateer CA & Streissguth AP (1997) Cognitive deficits in nonretarded adults with fetal alcohol syndrome. J Learn Disabil, 30, 685–693. [DOI] [PubMed] [Google Scholar]
- Kingdon D, Cardoso C. & McGrath JJ (2016) Research Review: Executive function deficits in fetal alcohol spectrum disorders and attention-deficit/hyperactivity disorder - a meta-analysis. J Child Psychol Psychiatry, 57, 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT, Grabowecky MF & Scabini D. (1995) Role of human prefrontal cortex in attention control. Adv Neurol, 66, 21–34; discussion 34–26. [PubMed] [Google Scholar]
- Kooistra L, Crawford S, Gibbard B, Ramage B. & Kaplan BJ (2010) Differentiating attention deficits in children with fetal alcohol spectrum disorder or attention-deficit-hyperactivity disorder. Dev Med Child Neurol, 52, 205–211. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, McLoughlin G. & Asherson P. (2006) Attention deficit hyperactivity disorder. Neuromolecular Med, 8, 461–484. [DOI] [PubMed] [Google Scholar]
- Louth EL, Bignell W, Taylor CL & Bailey CD (2016) Developmental Ethanol Exposure Leads to Long-Term Deficits in Attention and Its Underlying Prefrontal Circuitry. eNeuro, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen DA, Minassian A, Kenton JA, Geyer MA, Perry W, Brigman JL & Young JW (2018) Amphetamine improves mouse and human attention in the 5-choice continuous performance test. Neuropharmacology, 138, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K. & Brigman JL (2016) The impact of prenatal alcohol exposure on social, cognitive and affective behavioral domains: Insights from rodent models. Alcohol, 51, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, Cavanagh JF & Brigman JL (2019) Alcohol exposure in utero disrupts cortico-striatal coordination required for behavioral flexibility. Neuropharmacology, 162, 107832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, Sigdel R, Caldwell K. & Brigman JL (2014) Prenatal ethanol exposure impairs executive function in mice into adulthood. Alcohol Clin Exp Res, 38, 2962–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE & Lang AR (2006) Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology, 20, 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N. & Nguyen TT (2011) Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev, 21, 81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA & Gossage JP (2001) Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health, 25, 159–167. [PMC free article] [PubMed] [Google Scholar]
- Mihalick SM, Crandall JE, Langlois JC, Krienke JD & Dube WV (2001) Prenatal ethanol exposure, generalized learning impairment, and medial prefrontal cortical deficits in rats. Neurotoxicol Teratol, 23, 453–462. [DOI] [PubMed] [Google Scholar]
- Neumann M, Wang Y, Kim S, Hong SM, Jeng L, Bilgen M. & Liu J. (2009) Assessing gait impairment following experimental traumatic brain injury in mice. J Neurosci Methods, 176, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguin SL, Zimmerman A, Zhang HK, Allan A, Caldwell KC & Brigman JL (2019) Increased Maternal Care Rescues Altered Reinstatement Responding Following Moderate Prenatal Alcohol Exposure. Alcoholism-Clinical and Experimental Research, 43, 1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson HC, Feldman JJ, Streissguth AP, Sampson PD & Bookstein FL (1998) Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res, 22, 1998–2012. [PubMed] [Google Scholar]
- Peadon E. & Elliott EJ (2010) Distinguishing between attention-deficit hyperactivity and fetal alcohol spectrum disorders in children: clinical guidelines. Neuropsychiatr Dis Treat, 6, 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisansky MT, Lefevre EM, Retzlaff CL, Trieu BH, Leipold DW & Rothwell PE (2019) Nucleus Accumbens Fast-Spiking Interneurons Constrain Impulsive Action. Biol Psychiat, 86, 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova S, Lange S, Probst C, Gmel G. & Rehm J. (2018) Global prevalence of alcohol use and binge drinking during pregnancy, and fetal alcohol spectrum disorder. Biochem Cell Biol, 96, 237–240. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Kully-Martens K, Denys K, Badry D, Henneveld D, Wyper K. & Grant T. (2012) The effectiveness of a community-based intervention program for women at-risk for giving birth to a child with Fetal Alcohol Spectrum Disorder (FASD). Community Ment Health J, 48, 12–21. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Reynolds CR, Lowe P. & Moore JJ (2002) The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol, 17, 235–272. [PubMed] [Google Scholar]
- Riley EP, Lochry EA & Shapiro NR (1979a) Lack of response inhibition in rats prenatally exposed to alcohol. Psychopharmacology (Berl), 62, 47–52. [DOI] [PubMed] [Google Scholar]
- Riley EP, Lochry EA, Shapiro NR & Baldwin J. (1979. b) Response perseveration in rats exposed to alcohol prenatally. Pharmacol Biochem Behav, 10, 255–259. [DOI] [PubMed] [Google Scholar]
- Rueckert L. & Grafman J. (1996) Sustained attention deficits in patients with right frontal lesions. Neuropsychologia, 34, 953–963. [DOI] [PubMed] [Google Scholar]
- Skorput AGJ, Gupta VP, Yeh PWL & Yeh HH (2015) In Utero Ethanol Exposure Alters Interneuron Distribution and Inhibitory/Excitatory Balance in the Adult Mpfc. Alcoholism-Clinical and Experimental Research, 39, 255a–255a. [Google Scholar]
- Skorput AGJ & Yeh HH (2016) Chronic Gestational Exposure to Ethanol Leads to Enduring Aberrances in Cortical Form and Function in the Medial Prefrontal Cortex. Alcoholism-Clinical and Experimental Research, 40, 1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire RF, Noudoost B, Schafer RJ & Moore T. (2013) Prefrontal contributions to visual selective attention. Annu Rev Neurosci, 36, 451–466. [DOI] [PubMed] [Google Scholar]
- Stoler JM & Holmes LB (1999) Under-recognition of prenatal alcohol effects in infants of known alcohol abusing women. J Pediatr, 135, 430–436. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA & Smith DF (1991a) Fetal alcohol syndrome in adolescents and adults. JAMA, 265, 1961–1967. [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K. & Young JK (2004) Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr, 25, 228–238. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Randels SP & Smith DF (1991b) A test-retest study of intelligence in patients with fetal alcohol syndrome: implications for care. J Am Acad Child Adolesc Psychiatry, 30, 584–587. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M. & O’Neill TM (2004) Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol, 26, 35–45. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Josey M, Holmes A. & Brigman JL (2015) Conditional loss of GluN2B in cortex and hippocampus impairs attentional set formation. Behav Neurosci, 129, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KM, Young JW, McGrath JJ, Eyles DW & Burne TH (2013) Cognitive performance and response inhibition in developmentally vitamin D (DVD)-deficient rats. Behav Brain Res, 242, 47–53. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR & Topper L. (2012) Does moderate drinking harm the fetal brain? Insights from animal models. Trends Neurosci, 35, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J. & Mooney SM (2017) Choline and Working Memory Training Improve Cognitive Deficits Caused by Prenatal Exposure to Ethanol. Nutrients, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright PE, Ward GR, Winfield D, Huang YS, Mills DE, Ward RP & McCutcheon D. (1990) Effects of prenatal ethanol and long-chain n-3 fatty acid supplementation on development in mice. 1. Body and brain growth, sensorimotor development, and water T-maze reversal learning. Alcohol Clin Exp Res, 14, 405–412. [DOI] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel JR & Olivier E. (2006) Contribution of the monkey frontal eye field to covert visual attention. J Neurosci, 26, 4228–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Bismark AW, Sun Y, Zhang W, McIlwain M, Grootendorst I. & Light GA (2017) Neurophysiological Characterization of Attentional Performance Dysfunction in Schizophrenia Patients in a Reverse-Translated Task. Neuropsychopharmacology, 42, 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Halberstadt AL, van Enkhuizen J, Minassian A, Khan A, Perry W. & Eyler LT (2019) Convergent neural substrates of inattention in bipolar disorder patients and dopamine transporter-deficient mice using the 5-choice CPT. Bipolar Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Rissling AJ, Sharp RF, Eyler LT, Asgaard GL & Light GA (2013) Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Transl Psychiatry, 3, e324. [DOI] [PMC free article] [PubMed] [Google Scholar]