Abstract
Herein, we present microwave-assisted AlCl3 catalyzed oxidation of bile acid hydroxyl groups in the presence of Oxone® in water media. Significant rate enhancements were observed for Wolff–Kishner reduction of synthesized bile acids oxo derivatives to the 5β-cholanic acid. Reaction of amidation of the simplest bile acid and aminolysis of the deoxycholic acid was accomplished in the absence of solvent and catalysts under sealed vessel microwave conditions. Because 5β-cholanic acid reportedly modulates glucocorticoid receptor signaling in cell models of Parkinson's disease, we tested the affinity of 5β-cholanic acid and deoxycholic acid derivatives for the glucocorticoid receptor in vitro using a yeast-based fluorescent screen. Treatment of GR-expressing yeast with prednisolone resulted in a dose-dependent increase in fluorescence; whereas 5β-cholanic acid binds to the glucocorticoid receptor with more moderate affinity. Similarly, molecular docking also suggests that 5β-cholanic acid can bind to the glucocorticoid receptor, with similar geometry to known GR ligands.
Green synthesis of bile acids derivatives and 5β-cholanic acid was achieved under microwave irradiation, and the binding affinity for the ligand binding domain of the glucocorticoid receptor was measured.
Introduction
Microwave-assisted organic synthesis is increasingly used in a range of scientific applications. Because of numerous advantages, microwave (MW) irradiation has been employed in organic synthesis, materials sciences and nanotechnology.1–4 It has become an invaluable tool for organic synthetic chemistry and drug discovery applications, since it dramatically reduces reaction times, increases product yields and enhances product purity by reducing unwanted side reactions compared to conventional heating methods. Several studies have reported the use of MW irradiation for more efficient synthesis of bile acids (BAs) derivatives.5–9
Arising interest in bile acid chemistry over the years have attracted considerable attention in the pharmaceutical science.10,11 In addition, BA derivatives have a wide range of applications in the treatment of numerous diseases.12–15 Thus, organic synthetic research has focused on development of efficient reaction conditions for chemical transformations of hydroxyl and carboxyl groups in BAs, including oxidation to the corresponding oxo derivatives and further reduction to a methylene group, as well as synthesis of bile acid esters and amides.5,12,13
Traditional oxidation methods require large quantities of transition-metals, noxious oxidants, organic solvents, long reaction times or severe reaction conditions.6,16 From an environmental and economic perspective, these procedures are also sub-optimal because the majority suffers from low atom efficiency and large amounts of waste products. Oxone® is a triple salt, with the formula 2KHSO5·KHSO4·K2SO4, where the active oxidizing agent is potassium peroxymonosulfate, KHSO5.17,18 It is an inexpensive and stable oxidizing reagent, which compares favorably with hydrogen peroxide and bleach. Oxone® has been used in a wide variety of applications.19–24 As a non-toxic reagent, Oxone® is suitable for work in teaching laboratories, and results in reduced hazardous waste byproducts.25,26 Wu et al. reported27 that in water media the Lewis acid, AlCl3, plays a dominant role in oxidation of hydroxyl groups in the presence of Oxone®. Additionally, the Huang-Minlon28 modification of the Wolff–Kishner reaction has replaced the original procedure. Application of this modified Wolff–Kishner method affords reduction of steroidal oxo groups in excellent yields.29,30 Moreover, the long reaction times required have been significantly decreased through application of MW irradiation.31,32 Thus, in the present study we combine MW-assisted Oxone® mediated BA oxidation with modified Wolff–Kishner reduction of the resulting BA oxo derivatives. To the best of our knowledge, there have been no reports on MW-assisted BA oxidation by Oxone® and Wolff–Kishner reduction of BA oxo derivatives.
For conjugation at the BA carboxyl group, amide bond linkers are an attractive option for synthetic chemists since amides appear in many naturally-occurring (e.g., peptides and proteins) and pharmacologically active compounds. Various methods for the synthesis of carboxamides exist, but in general amides are formed from activated carboxylic acids and amines.33,34 Although adequate results are obtained using traditional methods, procedures are time consuming and not atom efficient. To improve efficiency and reduce waste production, milder methods to prepare amides directly from non-activated carboxylic acids and amines in the absence of coupling reagents and solvent are desirable. A more convenient and simpler route for amide preparation could be direct condensation of carboxylic acids and amines. Nevertheless, such an amidation process requires very harsh conditions (T > 100 °C) in order to avoid formation of unreactive carboxylate–ammonium salts and obtain the desired amide bond.35–37
We have found only one recent report of amidation carried out in a dedicated MW reactor: Ahonen et al. presented a simple, solvent-free MW-assisted synthetic procedure for the synthesis of lithocholyl amides.38
Herein, we describe the use of MW irradiation for fast and efficient BA oxidation and Wolff–Kishner reduction of the resulting oxo derivatives. To introduce green chemistry concepts39,40 and decrease production of hazardous waste, a new method has been developed in our laboratory for BA oxidation by Oxone® in water as the sole solvent. We showed that MW energy is advantageous for Wolff–Kishner reduction and synthesis of 5β-cholanic acid. In addition, direct solvent-free amidation of 5β-cholanic acid and synthesis of deoxycholic acid amides was accomplished in the absence of solvent and catalysts under sealed vessel microwave conditions.
5β-Cholanic acid and structurally similar BA compounds have been reported to interact with glucocorticoid receptor signaling.41,42 The relative binding affinities of our BA derivatives for the glucocorticoid receptor using a fluorescent screen in yeast cells have been evaluated. The assay was previously optimized for newly synthesized steroid derivatives with estrogen or androgen receptor binding properties.43 In the present study, the ligand binding domain (LBD) of the glucocorticoid receptor (GR) was expressed “in-frame” with yellow fluorescent protein in the yeast Saccharomyces cerevisiae.44 Addition of GR ligands to yeast expressing the glucocorticoid receptor is expected to result in a dose-dependent increase in fluorescence intensity, enabling estimation of receptor binding affinities by fluorimetry or fluorescence microscopy.
Results and discussion
Chemical synthesis
In water media, the Oxone®/AlCl3 system has produced excellent results for BA oxidation under MW irradiation. Oxone®, is an inexpensive and stable oxidizing reagent, that is highly soluble in water and insoluble in common organic solvents. Oxone®, in conjunction with MW radiation, afforded oxo derivatives of deoxycholic acid (DCA), chenodeoxycholic (CDCA), cholic and lithocholic acid (CA and LCA) with high yields and purity in very short reaction times at 50 °C (Scheme 1).
Scheme 1. Reagents and conditions: (a) Oxone®/AlCl3, H2O, MW.
Scheme 1 shows that synthesis of compound 1 using a temperature-controlled mode of MW reactor (50 °C, 12 min) was significantly shorter compared to result obtained by conventional method (rt, 180 min). The benefits of fast and uniform microwave heating are illustrated through comparison of bile acid oxidation under conventional and MW heating. A higher degradation rate of Oxone® was observed during conventional heating at 50 °C, presumably due to the slower and nonuniform heating of the reaction mixture. Additional chemical experiments in our laboratory revealed no significant influence of increased reaction temperature on product yield, although faster decomposition of Oxone® was observed. The obvious advantages of the present protocol for BA oxidation include green reaction media, since deionized water is the sole solvent. This environmentally friendly method produced negligible quantities of hazardous waste. Notably, this new oxidation system afforded the desired products in good to excellent yields following only a few minutes of MW exposure.
Reduction of reaction times was also achieved using MW irradiation for subsequent synthetic steps, hydrogenation of BA oxo derivatives. For this study the reduction of compound 4 under conventional heating (200 °C, 14 h, 65%) was chosen as the model transformation. Applying the Huang-Minlon modification, MW-assisted Wolff–Kishner reduction of BA oxo derivatives (1, 3 and 4) afforded 5β-cholanic acid 5 in high yields at 240 °C (Scheme 2). Compared to conventional methods MW-assisted reaction times were decreased from hours to only a few minutes.
Scheme 2. Reagents and conditions: (a) NH2NH2·H2O, HOCH2CH2OH, KOH, MW, 240 °C, 15 min, 92% starting from 1, 67% starting from 3 and 73% starting from 4.
Irreversible thermal reduction was accompanied by accumulation of volatile reaction components in the headspace of the batch reactor, limiting the reaction temperature. As expected, combined high-temperature/high-pressure reduction conditions resulted in significantly higher conversion to 5β-cholanic acid 5: 73% starting from 3-oxo-5β-cholanic acid, 92% by reduction of 3, 12-dioxo-5β-cholanic acid and 67% starting from 3,7,12-trioxo-5β-cholanic acid. Our results show that the use of microwave heating for chemical transformation of BA and finally synthesis of 5β-cholanic acid 5 essentially mimics conventional heating experiments under carefully controlled reaction conditions.
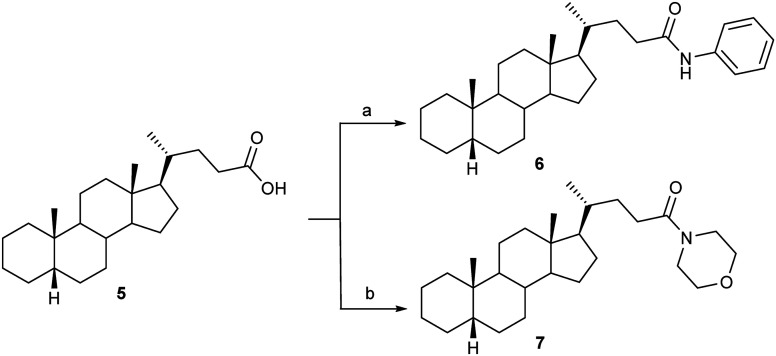
Next, we devoted our attention to the synthesis of potentially biologically active amide derivatives of 5β-cholanic acid 5. Uncatalyzed amidation of the simplest BA 5 was accomplished by MW-assisted solvent-free synthesis at 200 °C. Because the reaction involves thermolysis of the previously formed ammonium salt, and is promoted by nucleophilic addition of the amine to the carbonyl moiety, high-temperature MW heating was necessary to achieve high yields of N-phenyl-5β-cholan-24-amide 6 and N-morpholine-5β-cholan-24-amide 7 (Scheme 3).
Scheme 3. Reagents and conditions: MW, 200 °C, 1 h (a) C6H5NH2, 69% (b) O(CH2CH2)2NH, 78%.
We were pleased to find that this new protocol, using MW energy to rapidly reach the desired reaction temperature, enables extension of the method from secondary heterocyclic to less reactive primary aromatic amines. Due to steric hindrance of the tetracyclic carboxylic acid, the reaction is characterized by slower formation of ammonium salt. Consequently, longer irradiation times are necessary.
Similarly, favorable results were achieved for synthesis of aliphatic and aromatic amide BA derivatives. We chose DCA as a model BA. Our attempts to produce compound 9 (Scheme 4) had an interesting outcome. Early, detailed experiments revealed very slow direct condensation of the carboxylic acid and amine to give the corresponding amide. Increasing the reaction temperature or prolonging heating of the reaction mixture failed to improve yields to satisfactory levels, so esterification of DHA was necessary. Deoxycholic acid methyl ester 8 was synthesized under high-temperature/high-pressure reaction conditions after 30 seconds of MW irradiation.
Scheme 4. (a) abs. MeOH, cc H2SO4, MW, 140 °C, 30 s, 85%; MW, 200 °C, 1 h (b) C6H5NH2, 35% (c) n-C3H7NH2, 54%.
In the present study, DCA amides 9 and 10 were obtain at 200 °C in a single-mode MW reactor (Scheme 4). Aminolysis of the DCA methyl ester was performed in the absence of solvent and catalysts, in a closed MW reactor system under heating.
Biological evaluation
Bile acid derivatives 5–7, 9 and 10 were tested for glucocorticoid receptor (GR)-binding affinity using a modified fluorescent screen in yeast cells. The assay was previously optimized for identification of steroid hormone derivatives with estrogen or androgen receptor binding properties.43 For expression of GR in yeast cells, a galactose-inducible plasmid (pRF4-6-rGR LBD-EYFP) encoding the ligand binding domain (LBD) of the glucocorticoid receptor (GR) fused to enhanced yellow fluorescent protein (EYFP) was used.44 Expression of GR-LBD-EYFP was induced by adding galactose to the growth media and confirmed by fluorescence microscopy. The dose-dependent binding affinity of prednisolone, a known GR ligand, was then determined by measuring fluorescence changes in yeast cells upon addition of increasing concentrations of prednisolone (25, 50, 100, 250, 500 and 1000 μM) (Fig. 1).
Fig. 1. Dose-dependent changes in the fluorescence/optical density (F/OD) ratio of yeast cells expressing GR LBD-YFP upon addition of the glucocorticoid receptor ligand, prednisolone, at six different concentrations (25, 50, 100, 250, 500 and 1000 μM) following 15 h exposure.
Because assay sensitivity was highest at a final prednisolone concentration of 100 μM, bile acid derivatives 5–7, 9 and 10 were each tested at 100 μM. The fluorescence intensity of intact yeast cells was then determined by fluorimetry and visualized by fluorescence microscopy. Ligand binding affinity was evaluated as the fold fluorescence change between ligand-treated and cells treated with a negative control steroid, estradiol, which has negligible affinity for GR. Based on this assay, bile acid derivatives 5 and 9 appear to have moderate affinity for the ligand binding domain of the glucocorticoid receptor (Fig. 2). For comparison, whole cell fluorescence intensities were also observed by fluorescence microscopy using a FITC filter and 40× objective (Fig. 3). As can be seen, treatment of yeast cells with the positive control GR ligand, prednisolone results in a dramatic increase in fluorescence. Similarly, treatment of cells with 5 results in increased fluorescence intensity vs. negative control cells. However, compound 9 treated cells appear somewhat less fluorescent under the microscope than expected based on fluorimetry results.
Fig. 2. Bile acid derivatives 5 and 9 have moderate affinity for the ligand binding domain of glucocorticoid receptor based on a fluorescent assay in GR LBD-YFP expressing yeast cells. Ligand binding affinity was expressed as the fold fluorescence change between ligand-treated cells and cells treated with estradiol (negative control), measured by fluorimetry in 96-well plates. Recombinant yeast cells were exposed to test compounds at a final concentration of 100 μM for 15 h.
Fig. 3. Recombinant yeast cells expressing GR LBD-YFP were treated with 100 μM estradiol (negative control), prednisolone (positive control), or bile acid derivative 5 or 9 for 15 h and visualized by fluorescence microscopy using a FITC filter and 40× objective. Whole cell fluorescence intensities observed by microscopy correlate with results obtained by fluorimetry.
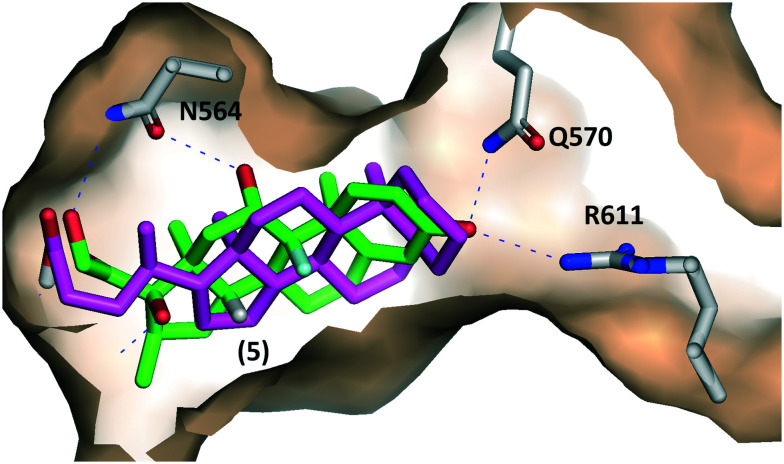
Compound 5 (also known as 5β-cholanic acid or ursocholanic acid) was previously reported to rescue mitochondrial dysfunction in patient-derived cell models of Parkinson's disease.41 Furthermore, this mitochondrial rescue effect of ursocholanic acid 5 depended on activation of the glucocorticoid receptor in a neuronal model of Parkinson's disease.41 These findings coupled with results for chemically related derivatives42 and our present results suggest that ursocholanic acid 5 directly binds and activates the GR. To predict the possible binding modes of compound 5 in complex with the GR-LBD, we conducted molecular docking simulations in the program Autodock Vina.45 Coordinates from the X-ray crystal structure of human GR-LBD in complex with dexamethasone46 (PDB 1M2Z) were used as the receptor for docking studies. As can be seen in Fig. 4, compound 5 is predicted by Autodock Vina to bind with lower affinity (−8.1 kcal mol−1) but similar geometry vs. dexamethasone (−13.2 kcal mol−1), including conservation of hydrogen bonding interactions with N564. Similar results were also obtained using Autodock 4.2, which is an independently developed docking program that utilizes a different algorithm:47 in this case the predicted binding energy of compound 5 for GR was −12.31 kcal mol−1vs. −13.82 kcal mol−1 for dexamethasone. Because compound 5 lacks many of the hydrophilic groups present in dexamethasone and prednisolone, less hydrogen bonding interactions are possible, providing a structural explanation for the reduced GR binding affinity we observed experimentally for 5vs. prednisolone in our yeast assay. Compounds 6, 7, 9 and 10 were not predicted to bind to GR-LBD based on either Autodock or Autodock Vina simulations.
Fig. 4. Possible binding mode of compound 5 in complex with GR-LBD predicted by Autodock Vina (magenta sticks). The experimental binding geometry of dexamethasone (green sticks) present in the X-ray crystal structure of GR (PDB ID 1M2Z) is shown for comparison.
Conclusions
We report efficient synthesis of BA oxo derivatives in water media with this new oxidation system Oxone®/AlCl3, under MW irradiation. Considerable reduction of reaction time was achieved by the MW-assisted Wolff–Kishner reduction of BA oxo derivatives, affording 5β-cholanic acid 5 in high yield. Reaction of amidation of the simplest BA 5 and aminolysis of the DCA was accomplished in the absence of solvent and catalysts, in a closed MW reactor system under high pressure heating. We believe that this environmentally friendly method can be applied to the synthesis of a wide range of bioactive BA amide derivatives. In addition, the bioactivity of the synthesized compounds was supported by assays that measure relative affinities for the ligand binding domain of the glucocorticoid receptor. Compound 5 is predicted by molecular docking to bind to the glucocorticoid receptor in a manner similar to other GR ligands.
Experimental part
Fluorescent screen in yeast
Recombinant expression of the ligand binding domain of the glucocorticoid receptor in yeast
The auxotrophic yeast strain (Saccharomyces cerevisiae FY250 strain, MATα, ura3-52, his32Δ00, leu2Δ1, trp1Δ6) and plasmid (pRF4-6-rGR LBD-EYFP) used in this study, were gifts from Dr. Blake Peterson, University of Kansas S. S. Muddana et al.48 For GR expression in FY250 yeast cells, plasmid pRF4-6-rGR LBD-EYFP was used: encoding the ligand binding domain (LBD) of the rat glucocorticoid receptor encompassing residues 497–795 (GR) fused to enhanced YFP (EYFP) under control of a galactose-inducible promoter. Lithium acetate polyethylene glycol-mediated transformation of yeast was performed according to Gietz et al.49 Yeast cells were transformed with pRF4-6-rGR LBD-EYFP, which carries a functional TRP1 gene, and selected on synthetic drop out agar plates lacking tryptophan for 3 days at 30 °C. Recombinant yeast cells were grown in selection medium with 2% raffinose in a Biosan orbital shaker-incubator ES-20/60 overnight. Saturated yeast cultures were diluted in fresh selection medium with 2% raffinose and incubated under identical conditions. Growth was monitored spectrophotometrically at 600 nm (OD600nm) using a Nicolet Evolution 100 UV-vis spectrophotometer. Expression of GR-LBD-EYFP was induced in mid-logarithmic phase of growth (OD600 nm ∼ 0.5) by adding galactose to a final concentration of 2%.
Dose response of prednisolone binding to the ligand binding domain of the glucocorticoid receptor in yeast
Dose-dependent binding of a synthetic glucocorticoid receptor ligand, prednisolone, was determined in order to optimize the assay for bile acid derivatives. Prednisolone was freshly dissolved in DMSO at six different concentrations (25, 50, 100, 250, 500, 1000 μM) and added to yeast simultaneously along with galactose. Because assay sensitivity was highest at a final prednisolone concentration of 100 μM, bile acid derivatives 5–7, 9 and 10 were each tested at 100 μM. Estradiol was used as negative control GR ligand at the same concentration.
Fluorescence spectroscopy
Recombinant cells expressing GR LBD-YFP were exposed to test and control compounds during 15 h incubation at room temperature in the dark. The fluorescence intensity of intact yeast cells was determined by two detection methods: fluorimetry and fluorescence microscopy. For fluorimetry readings on a Fluoroskan Ascent FL fluorometer, aliquots of 150 μl cell suspension were added in triplicate to 96-well microplates. Wells containing only selection medium were used for blank correction. Fluorescence intensity was recorded using excitation and emission wavelengths of 485 and 538 nm, respectively. Ligand binding affinity was expressed as the fold fluorescence change between ligand-treated and cells in the presence of negative control ligand, estradiol. Error bars were obtained by propagation of standard errors of the mean. To normalize obtained fluorescence intensities for the number of cells present in the sample, fluorescence/OD ratio (F/OD) was calculated. Dose-dependent curves and histograms, representing the relative binding affinities of the tested compounds, were created in Origin Pro 8.
Fluorescence microscopy
Differences between the fluorescence intensity distribution of recombinant yeast cells expressing GR LBD-YFP treated with bile acid derivatives or control compounds were also visualized by fluorescence microscopy using an Olympus BX51 fluorescence microscope equipped with an FITC filter.
Molecular docking
Preparation of ligand structural coordinates for docking
Structural coordinates for 5 were obtained from Pubchem (CID 92803, https://pubchem.ncbi.nlm.nih.gov/) and used to create 3D models of compounds 5–7 the program AVOGADRO 1.0.3 (Avogadro: an open-source molecular builder and visualization tool. Version 1.0.3 http://avogadro.openmolecules.net/). Coordinates for 7, 9 and 10 were modeled from 3D coordinates of deoxyglycocholic acid available under pubchem CID 22833539. Hydrogen atoms were added and ligand geometries were optimized (MMFF94 force field: 500 steps of conjugate gradients with a convergence setting of 10e-7) using the program AVOGADRO 1.0.3. Non-polar hydrogens were merged and Gasteiger partial charges were added in VEGAZZ 3.1.0 using the ‘ligand.c’ script. The resulting ligand coordinate files were saved in PDBQT format for use in Autodock Vina.
AutoDock Vina
Structural coordinates for the GR-LBD were obtained from the PDB (1M2Z). Coordinates for the receptor were converted to PDBQT format in the program VegaZZ using the ‘receptor.c’ scripts. Docking simulations in Autodock Vina were conducted with the following parameters: exhaustiveness = 8, center_x = 31.7, center_y = 6.9, center_z = 13.1. Results were visualized in the program PyMol (v0.99).
Synthetic procedures
All reagents and solvents were obtained from commercial suppliers and used without further purification. Microwave-assisted reactions were carried out in a CEM Discover BenchMate single-mode microwave reactor (300 W max magnetron power output) in 10 mL sealed process Pyrex vials with magnetic stirring. The microwave-assisted reaction time is the hold time at the designated temperature. Reaction temperatures were monitored by an external infrared (IR) sensor. The pressure-management device does not measure pressure. Due to the “snap-on” cap design, automatic venting is feasible when the internal pressure exceeds 20 bar (IntelliVent technology). Reaction cooling is performed by compressed air automatically after the heating period has elapsed. Reactions were monitored by thin layer chromatography (TLC) on silica gel plates (silica gel 60 F254). Purification of products was carried out by flash column chromatography using Kieselgel 60 (0.040–0.063, Merck). IR spectra were recorded with an FTIR Nexus 670 spectrophotometer (Thermo-Nicolet) and the band positions (λmax) are reported in cm−1. NMR spectra were recorded on a Bruker AC 250 E spectrometer operating at 250 MHz (1H) and 62.5 MHz (13C). Chemical shifts are expressed as ppm downfield from TMS using CDCl3 as solvent. For determination of multiplicity in 13C NMR, APT experiment was used. The letters s, d, t, q, and m are used to indicate singlet, doublet, triplet, quadruplet, and multiplet. All organic extracts were dried with anhydrous Na2SO4. Organic solutions were concentrated in a rotary evaporator under reduced pressure at a bath temperature above 30 °C.
3,12-Dioxo-5β-cholanic acid (1)
Method A
Starting compound 3α,12α-dihydroxy-5β-cholanic acid (39 mg, 0.1 mmol) was placed in a round bottom flask with Oxone (148 mg, 0.24 mmol), AlCl3·6H2O (65 mg, 0.27 mmol) and water (2 mL). The reaction mixture was stirred vigorously at room temperature for 180 min and the resulting reaction mixture was poured into water and extracted with ethyl acetate. Combined organic extracts were dried, solvent removed and the resulting crude product was purified by flash column chromatography (7 g silica gel, chloroform : acetone : glacial acetic acid = 6 : 4 : 0.1) affording pure compound 1, as white crystals (35 mg, 91%).
Method B
3α,12α-Dihydroxy-5β-cholanic acid (39 mg, 0.1 mmol), Oxone (147 mg, 0.24 mmol), AlCl3·6H2O (65 mg, 0.27 mmol) and water (2 mL) were placed into a 10 mL microwave process vial equipped with a magnetic stir bar. The reaction mixture was heated in a microwave reactor at 50 °C for 12 min. After the reaction time elapsed, mixtures were cooled by gas jet cooling and extracted with ethyl acetate. After removing the solvent under vacuo, the residue was purified by flash column chromatography (7 g silica gel, chloroform : acetone : glacial acetic acid = 6 : 4 : 0.1) affording pure compound 1, as a white crystals (31 mg, 81%). 1H NMR (CDCl3, δ, ppm): 0.83 (d, 3H, H-21); 1.04 (s, 3H, H-18); 1.09 (s, 3H, H-19); 1.29–2.57 (m, 26H, CH, CH2). 13C NMR (CDCl3, δ, ppm): 11.6 (C-18); 18.4 (C-21); 22.0 (C-19); 24.2; 25.3; 26.4; 27.3; 30.1; 31.2; 35.3; 35.4; 35.5; 36.6; 36.7; 38.2; 41.9; 43.6; 44.1; 46.3; 57.4; 58.4; 179.7 (CO2H); 212.3 (C-3); 214.2 (C-12).
3,7-Dioxo-5β-cholanic acid (2)
3α,7α-Dihydroxy-5β-cholanic acid (39 mg, 0.1 mmol), Oxone (147 mg, 0.24 mmol), AlCl3·6H2O (65 mg, 0.27 mmol) and water (2 mL) were placed into a 10 mL microwave process vial equipped with a magnetic stir bar. The reaction mixture was heated in a microwave reactor at 50 °C for 12 min or 10 min. After the reaction time elapsed, mixtures were cooled by gas jet cooling and extracted with ethyl acetate. After removing the solvent under vacuo, the residue was purified by flash column chromatography (7 g silica gel, chloroform : acetone : glacial acetic acid = 6 : 4 : 0.1) affording pure compound 2, as white crystals (32 mg, 83%). 1H NMR (CDCl3, δ, ppm): 0.69 (s, 3H, H-18); 0.92 (d, 3H, H-21); 1.14 (s, 3H, H-19); 1.25–2.55 (m, 25H, CH, CH2); 2.85 (m, 1H, H-6α). 13C NMR (CDCl3, δ, ppm): 12.0 (C-18); 18.3 (C-21); 22.0 (C-19); 22.3; 24.7; 28.1; 30.6; 30.9; 35.1; 35.3; 36.6; 38.8; 42.6; 42.8; 42.8; 44.9; 47.7; 48.7; 49.5; 54.7; 179.9 (CO2H); 210.4 and 211.2 (C-3 and C-7).
3,7,12-Trioxo-5β-cholanic acid (3)
3α,7α,12α-Trioxo-5β-cholanic acid (41 mg, 0.1 mmol), Oxone (295 mg, 0.48 mmol), AlCl3·6H2O (130 mg, 0.54 mmol) and water (2 mL) were placed into a 10 mL microwave process vial equipped with a magnetic stir bar. The reaction mixture was heated in a microwave reactor for 15 min at 50 °C. After the reaction time elapsed, mixtures were cooled by gas jet cooling and extracted with ethyl acetate. After removing the solvent under vacuo, the residue was purified by flash column chromatography (6 g silica gel, chloroform : acetone : glacial acetic acid = 6 : 4 : 0.1) to obtain pure compound 3 as white crystals (19 mg, 47%). 1H NMR (CDCl3, δ, ppm): 0.85 (d, 3H, H-21); 1.08 (s, 3H, H-18); 1.25 (s, 3H, H-19); 1.28–2.91 (m, 24H, CH, CH2). 13C NMR (CDCl3, δ, ppm): 11.8 (C-18); 18.5 (C-21); 21.8 (C-19); 25.1; 27.5; 29.6; 30.1; 31.1; 35.4; 35.9; 36.4; 38.6; 42.7; 44.9; 45.5; 45.6; 46.8; 48.9; 51.7; 56.8; 179.2 (CO2H); 208.7, 209.1 and 212.0 (C-3, C-7 and C-12).
3-Oxo-5β-cholanic acid (4)
3α-Hydroxy-5β-cholanic acid (57 mg, 0.15 mmol) was added to a solution of Oxone (111 mg, 0.18 mmol) and AlCl3·6H2O (34 mg, 0.14 mmol) in MeCN (0.5 mL) and water (1.5 mL). The reaction mixture was placed in a 10 mL microwave process vial equipped with a magnetic stir and irradiated in a microwave reactor for 6 min at 50 °C. Upon cooling to room temperature, the reaction mixture was extracted with ethyl-acetate. After removing the solvent under vacuo, the residue was purified by flash column chromatography (7 g silica gel, chloroform : acetone : glacial acetic acid = 6 : 4 : 0.1) to afford pure compound 4 as white crystals (51 mg, yield 90%). 1H NMR (CDCl3, δ, ppm): 0.67 (s, 3H, H-18); 0.91 (d, 3H, H-21); 1.00 (s, 3H, H-19); 1.03–2.68 (m, 28H, CH, CH2); 9.50 (br. s, 1H, CO2H). 13C NMR (CDCl3, δ, ppm): 11.9 (C-18); 18.1 (C-21); 21.1 (C-19); 22.5; 24.0; 25.6; 26.5; 28.0; 30.6; 30.9; 34.7; 35.2; 35.4; 36.9; 37.0; 39.9; 40.6; 42.2; 42.6; 44.2; 55.8; 56.3; 180.2 (CO2H); 213.8 (C-3).
5β-Cholanic acid (5)
Method A
3-Oxo-5β-cholanic acid (228 mg, 0.61 mmol) was dissolved in ethylene glycol (2.74 mL). Hydrazine-hydrate (121 μL, 2.44 mmol) and KOH (276 mg, 4.92 mmol) was added. The flask was heated gradually to 100 °C within 1.5 h, and then stirred at 100 °C for 2.5 h. Reflux condenser was replaced with a still head and condenser for downward distillation until the temperature reached 200 °C. After distillation of volatile compounds the reaction mixture was heated for additional 10 h. The solution was cooled to room temperature and pH adjusted to 2. After extraction with diethyl-ether (3 × 30 mL) and washing with 4% NaCl the solvent was removed under vacuo and the residue was purified by flash column chromatography (7 g silica gel, chloroform : acetone : glacial acetic acid = 8 : 2 : 0.1) to obtain pure compound 5 as white crystals (142 mg, 65%).
Method B
3-Oxo-5β-cholanic acid (228 mg, 0.61 mmol), 3,12-dioxo-5β-cholanic acid (200 mg, 0.52 mmol) or 3,7,12-trioxo-5β-cholanic acid (200 mg, 0.50 mmol) were placed in a 10 mL microwave process vial equipped with a magnetic stir bar with ethylene glycol (2.74 mL), hydrazine-hydrate (121 μL, 2.44 mmol; 204 μL, 4.12 mmol or 296 μL, 5.97 mmol, respectively) and KOH (276 mg, 4.92 mmol). The reaction mixture was heated in a microwave reactor for 15 min at 240 °C. After the reaction time elapsed, mixtures were cooled by gas jet cooling and pH adjusted to 2. Resulting mixtures were extracted with diethyl-ether (3 × 20 mL) and washed with 4% NaCl. After solvent removal under vacuo, the residue was purified by flash column chromatography (7 g silica gel, chloroform : acetone : glacial acetic acid = 8 : 2 : 0.1) to obtain pure compound 5 as white crystals (160 mg, 73% starting from 4; 170 mg, 92% starting from 1 or 120 mg, 67% starting from 3). IR (KBr, νmax, cm−1): 2928; 2858; 1707; 771. 1H NMR (CDCl3, δ, ppm): 0.65 (s, 3H, CH3-18); 0.92 (s, 3H, CH3-19); 0.94 (d, 3H, CH3-21); 1.00–2.68 (m, 30H, CH, CH2). 13C NMR (CDCl3, δ, ppm): 12.0 (CH3-18); 18.2 (CH3-21); 20.8 (CH3-19); 21.3; 24.2; 24.2; 26.5; 27.0; 27.2; 27.5; 28.1; 30.7; 30.9; 35.3; 35.4; 35.8; 37.5; 40.2; 40.4; 42.7; 43.7; 55.9; 56.5; 197.1 (CO2H). CAUTION: Hydrazine hydrate should be treated with extreme caution. It is potentially explosive in its pure and undiluted form. In addition, it is toxic by inhalation or contact with skin or eyes. Hydrazine is likely to be present under these high temperature and pressure conditions, therefore particular care should be taken. All reactions should be carried out in an efficient fume hood and a safety blast shield should be used. In cases where reaction vial pressure exceeded 20 bar, an IntelliVent sensor allowed for controlled venting of the pressure and subsequently automatically reseals the vial to maintain optimum safety.
General procedure for preparation of compounds 6 and 7
5β-Cholanic acid (300 mg, 0.83 mmol) and aniline (1.54 mL, 16.67 mmol), or morpholine (1.44 mL, 16.67 mmol) were added to a 10 mL microwave process vial equipped with a magnetic stir bar. The reaction mixture was heated in a microwave reactor for 1 h at 200 °C. After the reaction time elapsed, mixtures were cooled by gas jet cooling and extracted with methylene chloride (3 × 10 mL). Combined organic extracts were washed with 5% NaHCO3 (2 × 10 mL), 1 M HCl (2 × 10 mL), water (2 × 15 mL) and dried. The resulting crude product was purified by flash column chromatography (7 g silica gel, petroleum ether : ethyl acetate = 6 : 4) to obtain pure compound 6 or 7, respectively.
N-Phenyl-5β-cholan-24-amide (6)
Compound 6 was obtained as a yellow oil (250 mg, 69%). IR (KBr, νmax, cm−1): 3299, 2929, 2861, 1660, 755. 1H NMR (CDCl3, δ, ppm): 0.65 (s, 3H, CH3-18); 0.93 (d, 3H, CH3-21); 0.95 (s, 3H, CH3-19); 1.06–1.92 (m, 30H, CH, CH2); 7.25–7.56 (m, 5H, Ar–H); 7.93 (s, 1H, NH). 13C NMR (CDCl3, δ, ppm): 12.0 (CH3-18); 18.3 (CH3-21); 20.7 (CH3-19); 21.2; 24.1; 24.2; 26.5; 26.9; 27.1; 27.4; 28.2; 31.6; 34.5; 35.2; 35.5; 35.8; 37.5; 40.2; 40.4; 42.7; 43.6; 56.0; 56.5; 119.9, 123.9, 128.8 and 138.1 (Ar–C); 172.2 (C O).
N-Morpholine-5β-cholan-24-amide (7)
Compound 7 was obtained as a yellow oil (280 mg, 78%). IR (KBr, νmax, cm−1): 2928, 2860, 1649, 1449, 754. 1H NMR (CDCl3, δ, ppm): 0.59 (s, 3H, CH3-18); 0.86 (s, 3H, CH3-19); 0.89 (d, 3H, CH3-21); 1.04–1.83 (m, 30H, CH, CH2); 3.42–3.67 (m, 8H, morpholine ring); 7.30 (s, 1H, NH). 13C NMR (CDCl3, δ, ppm): 12.0 (CH3-18); 18.4 (CH3-21); 20.7 (CH3-19); 21.2; 24.1; 24.2; 26.4; 26.9; 27.1; 27.4; 28.2; 30.0; 31.2; 35.2; 35.5; 35.8; 37.5; 40.2; 40.4; 42.7; 43.6; 55.9; 56.5; 41.7 and 46.0 (CH2NCH2); 66.6 and 66.8 (CH2OCH2); 172.2 (C O).
Methyl 3α,12α-dihydroxy-5β-cholan-24-oate (8)
cc H2SO4 (10 μL, 0.15 mmol) was added to a solution of 3α,12α-dihydroxy-5β-cholanic acid (589 mg, 1.5 mmol) in absolute methanol (3.08 mL) and the reaction mixture was heated under microwave irradiation at 140 °C for 30 seconds. After the reaction time elapsed, mixtures were cooled by gas jet cooling, poured into water (60 mL) and extracted with chloroform (3 × 20 mL). Combined organic extracts were washed with a saturated solution of NaHCO3 (3 × 25 mL) and water (3 × 25 mL) and dried. The resulting crude product was purified by flash column chromatography (9 g silica gel, chloroform : acetone = 6 : 4) to obtain pure compound 8 as a yellow oil (519 mg, 85%). IR (KBr, νmax, cm−1): 3395, 2930, 1736, 757. 1H NMR (CDCl3, δ, ppm): 0.65 (s, 3H, CH3-18); 0.88 (s, 3H, CH3-19); 0.93 (d, 3H, CH3-21); 1.04–2.72 (m, 28H, CH, CH2 and 2H, OH); 3.57 (s, 1H, H-3); 3.64 (s, 3H, OCH3); 3.96 (s, 1H, H-12). 13C NMR (CDCl3, δ, ppm): 12.6 (CH3-18); 17.1 (CH3-21); 23.0 (CH3-19); 23.6; 26.0; 27.0; 27.4; 28.5; 30.2; 30.8; 31.0; 33.4; 34.0; 35.1; 35.2; 35.9; 36.3; 41.9; 46.3; 47.1; 48.0; 51.4 (OCH3); 71.5 (C-3); 73.0 (C-12); 174.6 (C O).
N-Phenyl-3α,12α-dihydroxy-5β-cholan-24-amide (9)
Methyl 3α,12α-dihydroxy-5β-cholan-24-oate (541 mg, 1.33 mmol) and aniline (2.43 mL, 26.6 mmol) were placed in a 10 mL microwave process vial equipped with a magnetic stir bar. The reaction mixture was heated in a microwave reactor for 90 min at 200 °C. After cooling, the mixture was poured into water (3 mL) and extracted with ethyl-acetate (3 × 10 mL). The resulting crude product was purified by flash column chromatography (7 g silica gel, chloroform : acetone = 6 : 4) to obtain pure compound 9, as a white crystals (215 mg, 35%). IR (KBr, νmax, cm−1): 3432, 2933, 1665, 753. 1H NMR (CDCl3, δ, ppm): 0.68 (s, 3H, CH3-18); 0.91 (s, 3H, CH3-19); 0.90 (d, 3H, CH3-21); 1.20–2.50 (m, 28H, CH, CH2 and 2H, OH); 3.60 (m, 1H, H-3); 4.00 (s, 1H, H-12); 6.50–7.60 (m, 5H, Ar–H and 1H, NH). 13C NMR (CDCl3, δ, ppm): 12.7 (CH3-18); 17.4 (CH3-21); 23.1 (CH3-19); 23.6; 26.1; 27.1; 27.5; 28.6; 29.6; 30.5; 31.3; 33.6; 34.1; 34.2; 35.1; 35.2; 36.0; 36.4; 42.0; 46.5; 46.7; 46.9; 48.2; 76.5 (C-12); 113.9–138.1 (Ar–C); 171.9 (C O).
N-Propyl-3α,12α-dihydroxy-5β-cholan-24-amide (10)
Methyl 3α,12α-dihydroxy-5β-cholan-24-oate (406 mg, 1 mmol) and N-propylamine (850 μL, 20 mmol) were placed into a 10 mL microwave process vial equipped with a magnetic stir bar. The reaction mixture was heated in a microwave reactor for 90 min at 200 °C. After cooling, the mixture was poured into water (3 mL) and extracted with ethyl-acetate (3 × 10 mL). The resulting crude product was purified by flash column chromatography (7 g silica gel, methylene chloride : acetone = 7 : 3) to obtain pure compound 10, as white crystals (234 mg, 54%). IR (KBr, νmax, cm−1): 3307, 2935, 1645, 755. 1H NMR (CDCl3, δ, ppm): 0.67 (s, 3H, CH3-18); 0.88 (s, 3H, CH3-19); 0.90 (d, 3H, CH3-21); 0.91–2.17 (m, 28H, CH, CH2 and 2H, OH); 3.18 (m, 2H, CH2N); 3.60 (m, 1H, H-3); 3.97 (s, 1H, H-12); 5.71 (s, 1H, NH). 13C NMR (CDCl3, δ, ppm): 11.3 (CH3-18); 12.7 (CH3-21); 17.3 (C̲H3CH2CH2N); 22.8 (CH3-19); 23.1; 23.6; 26.1; 27.1; 27.4; 28.5; 30.4; 31.6; 33.4; 33.5; 34.0; 35.1; 35.9; 36.3; 42.0; 46.4; 47.0; 48.1; 71.7 and 73.1 (C-3 and C-12); 173.63 (C O).
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors acknowledge financial support of the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451-03-68/2020-14/200125).
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0md00311e
References
- Cini E. and Taddei M., in Green Synthetic Processes and Procedures, ed. R. Ballini, RSC Green Chemistry, 2019, Chater 10: Microwave Dielectric Heating for Solvent-free Organic Transformations, pp. 216–244 [Google Scholar]
- Green M. Chen X. J. Materiomics. 2019;5:503. doi: 10.1016/j.jmat.2019.07.003. [DOI] [Google Scholar]
- Zeng X. Cheng X. Yu R. Stucky G. D. Carbon. 2020;168:606. doi: 10.1016/j.carbon.2020.07.028. [DOI] [Google Scholar]
- Kumar A. Kuang Y. Liang Z. Sun X. Mater Today Nano. 2020;11:100076. doi: 10.1016/j.mtnano.2020.100076. [DOI] [Google Scholar]
- Grbović L. J. Pavlović K. Jovanović-Šanta S. Vasiljević B. Curr. Org. Chem. 2019;23:256. doi: 10.2174/1385272823666190213114104. [DOI] [Google Scholar]
- Kuhajda K. Kevrešan S. Kandrač J. Fawceti J. Mikov M. Eur. J. Drug Metab. Pharmacokinet. 2006;31:179. doi: 10.1007/BF03190713. [DOI] [PubMed] [Google Scholar]
- Dayal B. Rao K. Salen G. Steroids. 1995;60:453. doi: 10.1016/0039-128X(95)00004-A. [DOI] [PubMed] [Google Scholar]
- Cravottoa G. Binello A. Boffaa L. Rosatib O. Boccalinic M. Chimichic S. Steroids. 2006;71:469. doi: 10.1016/j.steroids.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Huonga N. T. T. Klimkovaa P. Sorrenti A. Mancinib G. Drašarc P. Steroids. 2009;74:715. doi: 10.1016/j.steroids.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Copple B. L. Li T. Pharmacol. Res. 2016;104:9. doi: 10.1016/j.phrs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. J. Med. Chem. 2016;59:6553. doi: 10.1021/acs.jmedchem.5b00342. [DOI] [PubMed] [Google Scholar]
- Mishra R. Mishra S. Steroids. 2020;159:108639. doi: 10.1016/j.steroids.2020.108639. [DOI] [PubMed] [Google Scholar]
- Blanchet M. Brunel J. M. Curr. Med. Chem. 2018;30:3613. doi: 10.2174/0929867325666180309113737. [DOI] [PubMed] [Google Scholar]
- Faustino C. Serafim C. Rijo P. Reis C. P. Expert Opin. Drug Delivery. 2016;13:1133. doi: 10.1080/17425247.2016.1178233. [DOI] [PubMed] [Google Scholar]
- Sharma R. Long A. Gilmer J. F. Curr. Med. Chem. 2011;18:4029. doi: 10.2174/092986711796957266. [DOI] [PubMed] [Google Scholar]
- Popadyuk I. I. Salomatina O. V. Salakhutdinov N. F. Russ. Chem. Rev. 2017;86:388. doi: 10.1070/RCR4683. [DOI] [Google Scholar]; , and references therein
- Sigma-Aldrich catalog, https://www.sigmaaldrich.com/catalog/product/sial/911356?lang=en®ion=RS, (accessed January 2020)
- Narsaiah A. V. Synlett. 2002;7:1178. doi: 10.1055/s-2002-32601. [DOI] [Google Scholar]
- Frigerio M. Santagostino M. Sputore S. J. J. Org. Chem. 1999;64:4537. doi: 10.1021/jo9824596. [DOI] [Google Scholar]
- Yakura T. Konishi T. Synlett. 2007;5:765. doi: 10.1055/s-2007-970758. [DOI] [Google Scholar]
- McCarthy J. R. Matthews D. P. Paolini J. P. Org. Synth. 1995;72:209. doi: 10.15227/orgsyn.072.0209. [DOI] [Google Scholar]
- Travis B. R. Sivakumar M. Hollist G. O. Borhan B. Org. Lett. 2003;5:1031. doi: 10.1021/ol0340078. [DOI] [PubMed] [Google Scholar]
- Broshears W. C. Esteb J. J. Richter J. Wilson A. M. J. Chem. Educ. 2004;81:1018. doi: 10.1021/ed081p1018. [DOI] [Google Scholar]
- Carreňo M. C. González-López M. Urbano A. Angew. Chem., Int. Ed. 2006;45:2737. doi: 10.1002/anie.200504605. [DOI] [PubMed] [Google Scholar]
- Lang P. T. Harned A. M. Wissinger J. E. J. Chem. Educ. 2011;88:652. doi: 10.1021/ed100853f. [DOI] [Google Scholar]
- Sundar M. Easwaramoorthy D. Rani S. K. Palanichamy M. J. Solution Chem. 2007;36:1129. doi: 10.1007/s10953-007-9169-7. [DOI] [Google Scholar]
- Wu S. Ma H. Lei Z. Tetrahedron. 2010;66:8641. doi: 10.1016/j.tet.2010.09.035. [DOI] [Google Scholar]
- Minlon H. J. Am. Chem. Soc. 1946;68:2487. doi: 10.1021/ja01216a013. [DOI] [Google Scholar]
- Dangate P. S. Salunke C. L. Akamanchi K. G. Steroids. 2011;76:1397. doi: 10.1016/j.steroids.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Giovannini P. P. Grandini A. Perrone D. Pedrini P. Fantin G. Fogagnolo M. Steroids. 2008;73:1385. doi: 10.1016/j.steroids.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Jaisankar P. Pal B. Giri V. S. Synth. Commun. 2002;32:2569. doi: 10.1081/SCC-120005941. [DOI] [Google Scholar]
- Znidar D. O'Kearney-McMullan A. Munday R. Wiles C. Poechlauer P. Schmoelzer C. Dallinger D. Kappe C. O. Org. Process Res. Dev. 2019;23:2445. doi: 10.1021/acs.oprd.9b00336. [DOI] [Google Scholar]
- Lanigan R. M. Sheppard T. D. Eur. J. Org. Chem. 2013:7453. doi: 10.1002/ejoc.201300573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunetz J. R. Magano J. Weisenburger G. A. Org. Process Res. Dev. 2016;20:140. doi: 10.1021/op500305s. [DOI] [Google Scholar]
- Perreux L. Loupy A. Volatron F. Tetrahedron. 2002;58:2155. doi: 10.1016/S0040-4020(02)00085-6. [DOI] [Google Scholar]
- Gooßen L. J. Ohlmann D. M. Lange P. P. Synthesis. 2009;1:160. doi: 10.1055/s-0028-1083277. [DOI] [Google Scholar]
- Charville H. Jackson D. A. Hodges G. Whiting A. Wilson M. R. Eur. J. Org. Chem. 2011;30:5981. doi: 10.1002/ejoc.201100714. [DOI] [Google Scholar]
- Ahonen K. V. Lahtinen M. K. Valkonen A. M. Dračinsky M. Kolehmainen E. T. Steroids. 2011;76:261. doi: 10.1016/j.steroids.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Anastas P. T. and Warner J. C., Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 [Google Scholar]
- Erythropel H. C. Zimmerman J. B. de Winter T. M. Petitjean L. Melnikov F. Lam C. H. Lounsbury A. W. Mellor K. E. Janković N. Z. Tu Q. Pincus L. N. Falinski M. M. Shi W. Coish P. Plata D. L. Anastas P. T. Green Chem. 2018;20:1929. doi: 10.1039/C8GC00482J. [DOI] [Google Scholar]
- Mortiboys H. Aasly J. Bandmann O. Brain. 2013;136:3038. doi: 10.1093/brain/awt224. [DOI] [PubMed] [Google Scholar]
- Sharma R. Prichard D. Majer F. Byrne A. Kelleher D. Long A. Gilmer J. J. Med. Chem. 2011;54:122. doi: 10.1021/jm100860s. [DOI] [PubMed] [Google Scholar]
- Bekić S. Marinović M. A. Petri E. T. Sakač M. N. Nikolić A. R. Kojić V. V. Ćelić A. S. Steroids. 2018;130:22. doi: 10.1016/j.steroids.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Muddana S. Peterson B. ChemBioChem. 2003;4:848. doi: 10.1002/cbic.200300606. [DOI] [PubMed] [Google Scholar]
- Trott O. Olson A. J. Comput. Chem. 2010;31:455. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe R. Montana V. G. Stanley T. B. Delves C. J. Apolito C. J. McKee D. D. Consler T. G. Parks D. J. Stewart E. L. Willson T. M. Lambert M. H. Moore J. T. Pearce K. H. Xu H. E. Cell. 2002;110:93. doi: 10.1016/S0092-8674(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Morris G. Huey R. Lindstrom W. Sanner M. Belew R. Goodsell D. Olson A. J. Comput. Chem. 2009;16:2785. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddana S. S. Peterson B. R. ChemBioChem. 2003;4:848. doi: 10.1002/cbic.200300606. [DOI] [PubMed] [Google Scholar]
- Gietz D. Jean A. S. Woods R. A. Schiestl R. H. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.